Abstract
VirB8-like proteins are essential components of type IV secretion systems, bacterial virulence factors that mediate the translocation of effector molecules from many bacterial pathogens into eukaryotic cells. Based on cell biological, genetic, and x-ray crystallographic data, VirB8 was proposed to undergo multiple protein–protein interactions to mediate assembly of the translocation machinery. Here we report the results of a structure–function analysis of the periplasmic domain of VirB8 from the mammalian pathogen Brucella suis, which identifies amino acid residues required for three protein–protein interactions. VirB8 variants changed at residues proposed to be involved in dimerization, and protein–protein interactions were purified and characterized in vitro and in vivo. Changes at M102, Y105, and E214 affected the self-association as measured by analytical ultracentrifugation and gel filtration. The interaction with B. suis VirB10 was reduced by changes at T201, and change at R230 inhibited the interaction with VirB4 in vitro. The in vivo functionality of VirB8 variants was determined by complementation of growth in macrophages by a B. suis virB8 mutant and by using a heterologous assay of type IV secretion system assembly in Agrobacterium tumefaciens. Changes at Y105, T201, R230, and at several other residues impaired the in vivo function of VirB8, suggesting that we have identified interaction sites of relevance in the natural biological context.
Keywords: membrane proteins, type IV secretion, VirB proteins, virulence
Type IV secretion systems (T4SSs) are important virulence factors used by many Gram-negative pathogenic bacteria to transfer toxins or other effectors into diverse eukaryotic hosts, ranging from mammals through arthropods to plants. T4SS-carrying pathogens live in different intra- and extracellular environments, translocate a variety of effector molecules, and cause diseases as diverse as brucellosis, cat scratch disease, crown gall disease, Legionnaire's disease, whooping cough, or stomach ulcers (1, 2). T4SSs serve as effector conduits, which can be exchanged between bacteria and adapted to their host environment (3). Due to their importance in bacterial virulence, T4SSs are studied in different organisms, and common features have emerged.
T4SSs are multiprotein assemblies spanning the bacterial envelope. Up to 12 individual proteins are used to build a T4SS, and they can be classified into three groups. Three proteins with Walker A nucleotide-binding sites (VirB4, VirB11, and VirD4) recognize substrates and energize translocation and T4SS assembly (4–7). They interact with each other and transfer the substrate to the core elements of the translocation machinery (VirB6, VirB8, VirB9, and VirB10), which span the cell envelope and presumably form the translocation channel (8–13). The last group of T4SS components is that of surface-exposed proteins. VirB2 is the major pilus component (14, 15) and VirB5 the minor component (16), and VirB7 and VirB3 also associate with the pilus or its components (17, 18). Whereas most mechanistic studies have been performed with the model Agrobacterium tumefaciens, it is clear that there is some variation among the T4SSs from different organisms. Nevertheless, the core components are universally conserved, and VirB8 stands out with a central role as assembly factor.
VirB8 is a bitopic inner membrane protein with a short cytoplasmic N terminus and a mainly periplasmic C terminus (19, 20). VirB8 provides the structural and functional link between the cytoplasmic and inner membrane-associated energizing proteins, the other core elements, and the outer membrane-localized pilus assembly complex (7, 21, 22). Several interaction partners of VirB8 (VirB1, VirB4, VirB5, VirB9, VirB8, VirB10, and VirB11) have been discovered by using crosslinking, immunoelectron microscopy, and the yeast two-hybrid system (7, 20, 23, 24). The recent finding that VirB8 nucleates T4SS assembly at the pole of A. tumefaciens emphasizes the role of its interactions (10). In addition, VirB8 was crosslinked to the nucleoprotein substrate in A. tumefaciens, suggesting it is a component of the translocation pore (9).
The description of the x-ray structure of the periplasmic domain of Brucella suis VirB8 (named in the following VirB8sp for suis periplasmic) was a key discovery on the path to unravel its function (25). The structure comprises a large extended β-sheet of four antiparallel strands juxtaposed against five α-helices. It resembles known folds, e.g., that of the nuclear transport factor NTF2, which undergoes several transient interactions (26). VirB8sp crystallizes with five molecules in an asymmetric unit, and each of the five chains was found to dimerize. This was suggestive of dimer formation, and the recently described x-ray structure of the periplasmic domain of the A. tumefaciens VirB8 homolog (named in the following VirB8ap for Agrobacterium periplasmic) is in accord with this notion (27). Both x-ray structures suggest potential interaction hotspots, and functionally important residues have been previously identified by random mutagenesis (22).
In this work, we performed structure-based site-directed mutagenesis to introduce changes into VirB8sp at residues potentially involved in dimerization and protein–protein interactions. VirB8sp variants were first characterized in vitro with biochemical methods, identifying residues required for interaction with VirB4 and VirB10 and for dimerization. Subsequent functional analysis of VirB8sp variants in B. suis and A. tumefaciens demonstrated that these residues are important for protein function in vivo. The experiments described here suggest that key protein–protein interaction sites have been identified and shed light on the activity of VirB8 as T4SS assembly factor.
Results
Mutational Analysis of VirB8sp.
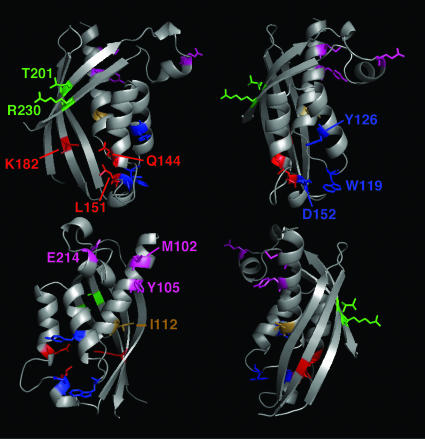
Based on the x-ray structure of VirB8sp, the gene was mutagenized (see Fig. 1 for location of mutations), and the StrepIIVirB8sp variants (StrepII indicating the affinity peptide for purification) were purified. The variants E214R, M102R, and Y105R carried changes at the putative dimer interface and were predicted to interfere with self-association. Variants R230D and T201A/Y carried changes of highly conserved residues on the solvent-exposed face of the β-sheet at the opposite side of the molecule. This region is close to a deep groove, which is an interaction hotspot in structural homologs such as nuclear transport factor 2 (25). The amino acids in the groove are not conserved, but changes K182E, L151R, and Q144R in that groove were predicted to interfere with binding. A patch of very high sequence identity was identified, and Y126E, D152R, and W119A were introduced to assess effects of changes in this region. The last variant was I112R, which is equivalent to a change in VirB8a (R107P) that was deficient in the interaction with VirB9a and VirB10a in the yeast two-hybrid system (22). CD spectroscopy measurements of StrepIIVirB8sp and variants showed that the amino acid changes did not affect the overall folding of the proteins (not shown).
Fig. 1.
Amino acids changed in this study shown in the B. suis VirB8sp x-ray structure. Ribbon representation showing a model of the VirB8sp structure in different orientations. Dimer interface residues E214, M102, and Y105 are shown in magenta; active-site groove residues K182, L151, and Q144 are shown in red; β-sheet's solvent-exposed face residues R230 and T201 are shown in green; very high-identity patch Y126, D152, and W119 are shown in blue; and I112 is shown in brown. The model was generated with macpymol (pymol.sourceforge.net) based on the VirB8sp Protein Data Bank file, www.pdb.org (PDB ID code 2BHM).
Self-Association of StrepIIVirB8sp and Changes at the Dimer Interface.
To directly test self-association, StrepIIVirB8sp (calculated molecular mass, 20 kDa) was incubated with increasing amounts of the crosslinking agent disuccinimidyl suberate (DSS). This led to successive formation of higher molecular mass complexes, which is in line with a dynamic equilibrium of monomers and multimers (Fig. 2A). Next, we performed size-exclusion chromatography with concentrations of the protein from 0.5 to 128 μM. StrepIIVirB8sp eluted from the column in fractions corresponding to a dimer at higher concentrations (peak at 10.8 ml, 40.6 kDa) and as a monomer (peak at 11.5 ml, 22.3 kDa) when lower amounts were separated (Fig. 2 B and C). The StrepIIVirB8sp variants were analyzed under similar conditions, and changes of the elution volume were observed in three cases, M102R, Y105R, and E214R (Fig. 2C). M102R and Y105R did not form dimers at high concentrations, but the molecular mass of 27.4–28.5 kDa also did not fit that of a monomer. The elution of E214R also differed from WT, but increases of the molecular mass to 32.2 kDa, which may correspond to a reduced degree of self-association or changes of the shape of the molecule, were observed at higher concentrations (Fig. 2C). These data suggested dimer formation of WT, and the self-association was reduced in the three variants.
Fig. 2.
Crosslinking, gel filtration, and analytical ultracentrifugation to assess dimer formation of VirB8sp. (A) StrepIIVirB8sp was incubated in the presence of increasing amounts of DSS before SDS/PAGE and Western blotting with VirB8sp-specific antisera. Arrowheads indicate the formation of higher molecular mass complexes, and molecular masses of reference proteins are shown on the right (in kDa). (B) Chromatogram showing elution of different concentrations of VirB8sp from a Superdex S75 column, 128 μM (black line), and 2 μM (gray line). The corresponding absorbance scales are indicated (mAU, milliabsorbance units at 280 nm). (C) Plot of the calculated molecular masses of StrepIIVirB8sp (rhombus), M102R (triangle), Y105R (square), and E214R (circle) obtained by size-exclusion chromatography as a function of the applied concentration. (D and E) Sedimentation equilibrium analysis of StrepIIVirB8sp and M102R, respectively. The lower graphs show a representative fit of the experimental data to a monomer/dimer model in case of StrepIIVirB8sp and to a single species (= monomer) model in case of M102R. The upper graphs show the residuals of the fit. The representative fits shown here were taken from the data obtained for proteins at concentration of A280 0.3 and rotor speeds of 20,000, 24,000, and 28,000 rpm in a Beckman Coulter XL-A analytical ultracentrifuge.
To assess self-association under conditions excluding effects of protein shape, we next performed sedimentation equilibrium analysis by analytical ultracentrifugation. First, StrepIIVirB8sp was analyzed at three concentrations and rotor speeds. Global analysis of the data resulted in an optimum fit for a monomer/dimer model (Fig. 2D) and less optimal fits for monomer/trimer and monomer/tetramer models. The apparent dissociation constant was calculated to be 116 μM. Second, the sedimentation equilibrium studies were carried out with M102R, Y105R, and E214R, and the data fit only for single ideal species (= monomer) models (Fig. 2E and Fig. 6, which is published as supporting information on the PNAS web site). These results are in accord with those of the gel filtration, showing self-association of VirB8sp, which was abolished by changes at the dimer interface. Interactions with other VirBs proteins were tested next.
Changes at the Solvent-Exposed Face of the β-Sheet Abolish Interactions with VirB4s and VirB10sp.
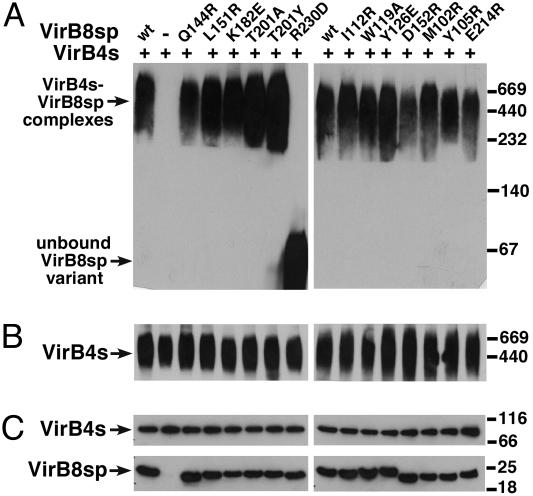
We described previously that StrepIIVirB4s forms oligomers and, after coexpression with VirB8sp from a bicistron construct, both proteins cofractionated during blue native electrophoresis (BNE) (7). We here adapted this assay to test the interaction of StrepIIVirB4s with StrepIIVirB8sp and its variants by mixing the two separately purified components, followed by BNE and Western detection. Similar to previous findings, StrepIIVirB4s and StrepIIVirB8sp cofractionated in a high molecular mass complex >440 kDa (Fig. 3A and B). The analysis of most other StrepIIVirB8sp variants gave similar results with the exception of R230D, which was detected exclusively in the lower molecular mass range (Fig. 3A). When StrepIIVirB8sp and its variants were separated by BNE, they were all detected in the low molecular mass range like R230D (not shown). These results show that fractionation in a high-molecular-mass complex depended on StrepIIVirB4s, and the R230D variant was not able to undergo this interaction.
Fig. 3.
BNE to analyze the interaction of VirB8sp and its variants with VirB4s. StrepIIVirB4s-enriched extract was mixed with StrepIIVirB8sp and variants, followed by BNE and Western blotting with VirB8sp-specific (A) and StrepII-specific (B) antisera for detection of StrepIIVirB4. The migration of high-molecular-mass StrepIIVirB4s-StrepIIVirB8sp complexes and of the unbound R230D variant in the low-molecular-mass portion of the gel are indicated. (C) Results of SDS/PAGE and Western blotting of samples from the same experiment as protein-loading controls. Molecular masses of reference proteins are shown on the right (in kDa).
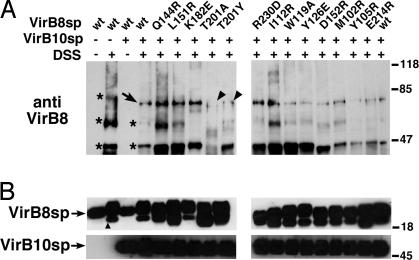
Crosslinking with DSS was used next to test interactions with StrepIIVirB5sp, StrepIIVirB9sp, and StrepIIVirB10sp. The proteins were mixed with StrepIIVirB8sp and its variants in the presence of DSS. We did not detect reproducible changes between the crosslinking patterns with StrepIIVirB5sp and StrepIIVirB9sp (not shown). However, when StrepIIVirB10sp (calculated molecular mass: 38 kDa, migrates as 45-kDa protein in SDS/PAGE) was incubated with StrepIIVirB8sp (20 kDa) and its variants in the presence of DSS; the formation of a 70-kDa complex was detected in most cases, which likely corresponds to a heterodimer (Fig. 4). Over the course of several repetitions, we reproducibly observed a reduction of the formation of this complex only in case of changes in the β-sheet at T201A and T201Y, suggesting that those residues are important for the interaction (Fig. 4). We next determined the consequences of changes of the VirB8s sequence in the natural biological context of the T4SS.
Fig. 4.
Analysis of interaction between StrepIIVirB8sp variants and StrepIIVirB10sp by crosslinking. (A) StrepIIVirB8sp and variants were mixed with StrepIIVirB10sp and incubated with 0.1 mM DSS followed by SDS/PAGE and Western blotting with VirB8s-specific antiserum. Crosslinked products of StrepIIVirB8sp in the absence of StrepIIVirB10sp are indicated by ∗, the occurrence of a novel product in the presence of StrepIIVirB10sp is indicated by an arrow, and arrowheads indicate reduced amounts of this presumptive heterodimer in case of two variants as compared with WT (representative results of three repetitions are shown). (B) Western blots of SDS gels from crosslinked samples as loading controls. An additional VirB8sp-specific signal of lower molecular mass (indicated by a triangle) was observed in the presence of DSS that may correspond to an internal crosslink causing the change of migration. Molecular masses of reference proteins are shown on the right (in kDa).
VirB8sp Changes Impact Intracellular Survival in Macrophages.
An in-frame deletion of virB8 was engineered into the chromosome of the WT strain B. suis 1330 according to established procedures to generate strain BS1008 (ΔvirB8) (G.P., Zhong Qi, Gisele Bourg, C.B., and D.O., unpublished work). Next, the fragments encoding VirB8sp variants were cloned into a broad host range vector under control of the B. suis virB promoter to generate in-frame fusions to a fragment encoding the nonmodified N terminus. To measure T4SS functionality, J774 macrophage cultures were infected with B. suis 1330 WT, BS1008, and BS1008 complemented with plasmids encoding VirB8s and its variants, and intracellular growth was monitored 48 h after infection (Fig. 5A). The absence of virB8 strongly impaired the ability of B. suis to grow intracellularly, in a manner similar to that seen with polar virB mutants (28). A plasmid encoding VirB8s restored intracellular replication of strain BS1008 to almost WT levels, whereas the VirB8s variants complemented to differing levels. Changes at the β-sheet face (T201A, T201Y, and R230D) strongly reduced intracellular growth (1,000-fold), and BS1008-producing Y216E was as attenuated as noncomplemented BS1008. In contrast, changes D152R and those at the dimer interface (M102R, Y105R, and E214R) were less attenuated, and their growth was 10- to 100-fold reduced. VirB8s carrying other changes (Q144R, L151R, K182E, I112R, and W119A) complemented intracellular growth similar to WT. The levels of VirB8s in the B. suis strains grown on minimal medium under virulence gene-inducing conditions were comparable (Fig. 7A, which is published as supporting information on the PNAS web site), showing that the effects were not due to reduced stability. We next used an alternative B. suis T4SS assay to assess the functionality of VirB8s variants.
Fig. 5.
Functionality of VirB8sp variants in B. suis and in the heterologous T4SS assembly assay in A. tumefaciens. (A) J774 macrophages were infected with B. suis 1330 (wt), BS1008 (ΔvirB8), and BS1008 carrying plasmids for expression of VirB8s and its variants, and intracellular growth was quantified 48 h after infection (logarithmic scale, geometric mean ± standard deviation from 3–12 independent repetitions). Complementation by pIN38 (VirB8s wt) is indicated by the dashed line. (B) The recipient UIA143 carrying pTrcB1+3–12 (wt), pTrcB1+3–12ΔvirB8 (ΔvirB8), and strains complemented with pTrcPVirB8s and variants were cocultivated with the donor A348 pLS1. The transconjugants (TC) were identified by growth on selective agar, and the pLS1 transfer efficiency (transconjugants per recipient, TC/R) into UIA143 carrying pTrcB1+3–12ΔvirB8, and pTrcPVirB8s was set to 100% (indicated by dashed line) (error bars show standard deviation from four repetitions, and bacterial titer is given in a linear scale).
Impact of VirB8sp Changes on T4SS Assembly.
We showed recently that the production of a subset of the B. suis VirB proteins (VirB1 and VirB3-12) in A. tumefaciens increased the recipient competence for IncQ plasmid pLS1 in conjugation experiments (29). This system serves to analyze B. suis T4SS assembly without the need for work under biosafety level 3 conditions, and it was here applied to measure complementation. We first engineered a virB8 deletion into plasmid pTrcB1+3–12, and genes encoding full-length VirB8s and its variants were engineered in the compatible vector pTrcP200. Plasmid pTrcB1+3–12, its deletion derivative pTrcB1+3–12ΔvirB8, and pTrcP200 derivatives encoding VirB8s and its variants were introduced into the recipient A. tumefaciens UIA143, the strains were cultivated with the donor A348 pLS1, and the efficiency of conjugative plasmid transfer was determined. Western blots of cell lysates showed that levels of VirB8s and of its interaction partner VirB5s were similar in all cases where the genes were present (Fig. 7B), but the conjugation efficiency was reduced to <1% of the WT in UIA143 carrying pTrcB1+3–12ΔvirB8 (Fig. 5B and Table 1, which is published as supporting information on the PNAS web site). VirB8s was therefore essential for T4SS assembly, but the difference (338-fold reduction in the absence of VirB8s) was not as pronounced as in B. suis (5-log reduction in the absence of VirB8s). Complementation with a plasmid directing VirB8s production stimulated pLS1 uptake competence to 167-fold as compared with the WT level (388-fold stimulation), and expression of the 13 variants had differential effects. Variants with changes at the dimer interface (M102R, Y105R, and E214R), at the solvent-exposed face of the β-sheet (T201A and T201Y), and of strongly conserved residues (Y126E and D152A) only partly complemented (15- to 62-fold stimulation), and these results are in agreement with those in B. suis. Similar to B. suis, variants K182E, Q144R, and W119A were not or were only weakly attenuated, and the result was similar in the case of R230D (116- to 171-fold stimulation). In addition, variants I112R and L151R complemented with reduced efficiency (68- and 62-fold stimulation) only in the heterologous assay, showing that the assays give different results in some cases. Nevertheless, the results of both in vivo assays show that changes at the dimer interface and the β-sheet face and of the conserved residue Y126 reduce VirB8s functionality during the type IV secretion process.
Discussion
VirB8 is an essential core component of all T4SSs, and previous work showed that this assembly factor undergoes multiple protein–protein interactions (7, 10, 21, 23, 24). The x-ray structures of the periplasmic domains of both the B. suis and A. tumefaciens homologs VirB8sp and VirB8ap have been solved (25, 27). Both studies suggest dimer formation, which is further supported by results obtained with the yeast two-hybrid system. The dimer interface of VirB8sp identified in the x-ray structure spans a large surface area of 1,700 Å2, and residues mainly in the α1- and α4-helix (Y98, V101, M102, K104, Y105, L218, P221, L222, and E214) are involved in building this surface (25). We created variants with changes M102R (α1-helix), Y105R (α1-helix), and E214R (α4-helix) to test their contribution to dimer formation (Fig. 1). Using gel filtration and analytical ultracentrifugation, we showed self-association of the WT, and it was reduced or abolished in case of these variants. Moreover, using two independent assays for B. suis T4SS functionality and assembly in vivo, we demonstrated that the ability of these variants to complement a nonpolar virB8 deletion was reduced. Also, the residue corresponding to Y105 in VirB8a (A100) was found to be important for complementation in bacterial virulence assays (22).
These data strongly support a model implying that dimer formation of VirB8s may be important in the natural biological context. The apparent dissociation constant of StrepIIVirB8sp was determined to be 116 μM, which reflects a weak interaction suggesting that VirB8-like proteins may undergo a cycle of association and dissociation. It is interesting to note that nuclear transport factor 2, a structural homolog of VirB8sp, undergoes transient interactions with nuclear pore components and with Ran-GDP at different sites of the molecule. Its homodimer formation is also relatively weak (Kd = 1 μM), and dissociation was proposed to contribute to Ran-GDP release in the nucleus (26). Although it is unlikely that VirB8 moves in the T4SS in the way nuclear transport factor 2 moves across the nuclear pore, it does interact with several other VirB proteins and the translocated substrate. It is reasonable to postulate that most of these interactions may be transient and follow a sequence during T4SS assembly. It will be a challenge of future research to develop quantitative methods to measure these interactions and to determine whether VirB8 associates with other VirB proteins in a sequential manner, and whether dissociation of the dimer plays a role. In addition to the periplasmic domain analyzed here, it is possible that the short inner membrane and/or the cytoplasmic domain may contribute to the association of VirB8, so that the actual dimer formation in vivo may be strong. This possibility has to be addressed as well to understand its contribution to T4SS function.
The second critical region for VirB8 function identified here is the β-sheet interface located opposite the self-association domain. The highly conserved R230 and T201 were required for interaction with StrepIIVirB4s and StrepIIVirB10sp, respectively. Both residues localize close to a deep groove of the molecule between β4 at the edge of the β3-sheet and helix α3, which was postulated to be involved in protein–protein interactions. Our results are well in accord with this notion, but changes of amino acids in the groove (K182E, L151R, and Q144R) did not have substantial effects, suggesting they are not important for VirB8 functionality. The surface-exposed β-sheet may therefore bind multiple interaction partners, and it will be interesting to follow up on these findings, e.g., by changing additional residues in this region and by competing the interactions with VirB8-derived peptides.
The functionality of the Y126E and D152R variants was substantially reduced in both in vivo assays, showing that these conserved residues are important for VirB8s function, but the changes did not affect interactions in in vitro assays. These residues may therefore be required for binding to one of the other putative interaction partners identified by yeast two-hybrid analysis (VirB1 and VirB11) (24), or they may be required for a VirB8s function not tested here, e.g., substrate translocation (9). Alternatively, they may play a role only in the dynamic context of the T4SS, or the variants may undergo nonproductive interactions and thereby block translocation.
Apart from the in vitro assays for VirB protein interactions, one of the main advances of our recent work was the development of a heterologous assay of B. suis T4SS assembly in A. tumefaciens (29). This assay exploits the ability of a subset of B. suis T4SS components (VirB1 and VirB3–VirB12) to increase the recipient competence of A. tumefaciens for T4SS-mediated plasmid transfer, and similar findings were reported for the Agrobacterium VirB T4SS (30). The heterologous T4SS assembly assay was here directly compared with T4SS function in its native environment in B. suis. Deletion of the virB8 gene reduced the recipient competence 100-fold, in a similar way to the attenuation of virulence seen with the virB8 mutant, and both could be complemented in trans by a WT virB8 gene. When the effects of changes in VirB8s on B. suis T4SS function in A. tumefaciens and in its native host were compared, it became evident that most had qualitatively similar effects. The requirement for full assembly and substrate translocation in B. suis or the absence of VirB2 in the heterologous system may also explain the different effects of changes R230D, I112R, and L151R in the two in vivo assays. Nevertheless, most changes had comparable effects. The heterologous assay can therefore be applied for structure–function analysis of B. suis VirB proteins and for biochemical approaches to characterize this system, which would not be feasible under biosafety level 3 conditions required for work with Brucella.
The work presented here exploits information provided by x-ray analysis of VirB8sp, and biochemical approaches detected residues required for its protein–protein interactions. The functional importance of most of the residues identified here (dimerization site and β-sheet residues) was confirmed by using two in vivo assays. Identifying interaction sites of VirB8-like proteins from B. suis is a substantial contribution to understanding the role of this assembly factor to T4SS function. Future research will aim to determine the minimal interaction surface required for the binding of VirB8 to other VirB components and the sequence of its transient interactions. This information will be a stepping stone for creating peptide aptamers (31) or small molecules to inhibit the interactions, which could be applied as tools for functional studies or as leads for the design of T4SS-inhibiting drugs.
Materials and Methods
Strains, Plasmids, Infection, and Growth Conditions.
The strains and plasmids used in this study are given in Table 2, which is published as supporting information on the PNAS web site. Escherichia coli strains JM109 (32) and GJ1158 (33) were used as hosts for cloning and protein overproduction, respectively. A. tumefaciens strain A348 was used as pLS1 donor and strain UIA143 as host for the heterologous expression of the B. suis T4SS, plasmid propagation, and cell cultivation, which were conducted as described (29). For detection of VirBs protein production, B. suis strains were cultivated in minimal medium (34), and J774 murine macrophage-like cells were infected with a standard gentamicin protection assay (28). Proteins in cell lysates were detected by SDS/PAGE (35) and Western blotting with VirBs protein-specific antisera, as described (7). For detection in B. suis, the antisera were diluted 1:100 (VirB8s and VirB9s) or 1:500 (VirB12s).
Plasmid Construction.
Site-directed mutagenesis of the virB8 genes in was pT7–7StrepIIVirB8sp performed via inverse PCR by using overlapping primers (Table 3, which is published as supporting information on the PNAS web site) that carried the desired mutation (36). Plasmid pTrcP200 was constructed for expression of virB8 genes (WT and mutants) under the control of the trc promoter in a kanamycin resistance-determining vector compatible with pTrcB1+3–12. To this end, a 2.2-kb NcoI-NspHI fragment in pSDM1661 was replaced by a BspHI-NspHI DNA-linker containing a SpeI site, and the 2.7-kb region containing the lacI and multiple cloning site from pTrc200 was PCR-amplified and inserted into the SpeI site. For construction of pTrcB1+3–12ΔvirB8, a 4.9-kb PstI/XbaI fragment containing virB7-virB12 was subcloned into pUC19 to generate pUCvirB7–12. A 0.9-kb ApaI/SacII fragment was then cloned into pBluescriptSK+, which led to a virB8-fragment with 220 flanking base pairs (pBluevirB8). Using inverse PCR with overlapping primers (36), the virB8 deletion was created, and the resulting plasmid was pBlueΔvirB8. Subsequent subcloning of the 220-bp fragment from pBlueΔvirB8 to pUCvirB7–12 and then to pTrcB1+3–12 led to pTrcB1+3–12ΔvirB8. For complementation of the virB8 defect in A. tumefaciens, full-length virB8 was PCR-amplified from pTrcB1+3–12 and cloned in pBluescriptSK+ into the KpnI/BamHI sites leading to pBlueVirB8s. EcoRV-PstI subclones from pT7–7StrepIIVirB8sp variants were subcloned into pBlueVirB8s to introduce the site-directed mutants into the full-length gene. The virB8 gene was PCR-amplified from pTrcB1+3–12 and cloned into KpnI/BamHI-restricted pTrcP200, and similarly the different virB8 alleles were subsequently subcloned.
For complementation of the virB8 defect in B. suis, plasmid pIN38 (G.P. et al., unpublished work) containing virB8 downstream of the B. suis virB promoter was used (37). The pIN40–52 plasmids encoding VirB8s variants were obtained by replacing the 3′ part of the WT virB8 gene corresponding to the periplasmic part by the DNA fragments from pT7–7StrepIIVirB8s variants. For this purpose, a silent mutation creating a unique Mlu1 restriction site was introduced into codon 76 of virB8 and the alleles were PCR-amplified and cloned into pIN38 via Mlu1/BglII.
Protein Purification and CD Spectroscopy.
StrepIIVirB8sp and its variants and StrepIIVirB5sp, StrepIIVirB9sp, and StrepIIVirB10sp were purified as described (7). StrepIIVirB4s was overproduced in E. coli GJ1158 (7), cells were lysed by bead beating in buffer W (0.1 M Tris·HCl, pH 8.0/0.15 M NaCl/1 mM EDTA, pH 8) with 0.5 mM PMSF and protease inhibitor mixture (Roche Applied Science, Indianapolis), followed by enrichment via a 1-ml StrepTactin Superflow column and elution with 2.5 mM desthiobiotin. Folding of StrepIIVirB8sp and it variants was analyzed by CD spectroscopy on an Aviv (Lakewood, NJ) spectropolarimeter by measuring the molar ellipticity from 260 to 195 nm.
Large-Zone Chromatography and Analytical Ultracentrifugation.
The molecular masses of purified StrepIIVirB8sp and of its variants were determined by gel filtration by using frontal (large-zone) elution (38). Five hundred-microliter aliquots with protein concentrations ranging from 0.5 to 128 μM were applied to a Superdex 75 HR FPLC column (Amersham Pharmacia Biosciences) in buffer W at 4°C. The void volume of the column was determined with Blue Dextran 2000, and the calibration curve was determined by using the Low Molecular Weight Gel Filtration Calibration Kit (Amersham Pharmacia Biosciences).
A Beckman Coulter XL-A analytical ultracentrifuge was used to perform sedimentation equilibrium studies. The protein samples were dialyzed twice in buffer W. Analysis was carried out by using three different concentrations of StrepIIVirB8sp and variants (A280 of 0.1, 0.3, and 0.5) in a total volume of 120 μl at 4°C at three different rotor speeds (20,000, 24,000, and 28,000 rpm). Concentration profiles were obtained by absorbance scans at 280 nm after 15 and 16 h with a radial step of 0.001 cm with five replicates per scan. The data were analyzed by using origin Version 6.0 (Microcal, Amherst, MA). The partial specific volumes of StrepIIVirB8sp and solvent density were calculated to be 0.7178 and 1.00925, respectively, by using the sednterp program, and the calculated molecular mass was 20,186 Da. The molar dissociation constant Kd was calculated by deriving the concentration-dependent association constant from the absorbance-based association constant calculated by origin 6.0 using the following equation: Ka(conc) = Ka(abs) (εl/2) [Ka(conc) is the molar association constant, Ka(abs) is the absorbance-based association constant, ε is the calculated molar extinction coefficient (37,820 cm−1·M−1), and l is the path length of the sample cell of 1.2 cm]. Kd was calculated by taking the inverse of Ka(conc) (39).
Analysis of Protein–Protein Interactions by Crosslinking and BNE.
Chemical crosslinking was performed as described (7). For BNE, 35 μl of enriched StrepIIVirB4s was mixed with 10 μl of StrepIIVirB8sp and its variants (0.01 μg/μl) and 10 μl of buffer W, or with 20 μl of buffer W as control. For the StrepIIVirB8sp controls, 5 μl of the proteins (0.01 μg/μl) was mixed with 15 μl of buffer W. The mixtures were incubated at room temperature for 1 h, and 13 μl was analyzed by SDS/PAGE Western blotting as loading controls. BNE was preformed in linear 12.5% acrylamide gels, as described (7).
Supplementary Material
Acknowledgments
We thank August Böck for support and discussions and Patricia C. Zambryski for the communication of results before publication. We also thank Raquel Epand and Mark Pereira for help with CD spectroscopy and analytical ultracentrifugation, respectively; Annette Vergunst [Institut National de la Santé et de la Recherche Médicale (INSERM), Nîmes, France] for providing pSDM1661; and Gisele Bourg (INSERM) for technical assistance. This work was supported by the Canadian Institutes of Health Research (Grant MOP-64300) and by grants from the Canada Foundation for Innovation and the Ontario Innovation Trust (to C.B.). A.P. was partly supported by a research fellowship from the Deutsche Forschungsgemeinschaft. The O'Callaghan laboratory is supported by INSERM, the Université de Montpellier 1 (BQR), la Région Languedoc Roussillon, and la Ville de Nîmes. R.B. and G.W. were funded by Wellcome Trust Grant 065932.
Abbreviations
- BNE
blue native electrophoresis
- DSS
disuccinimidyl suberate
- T4SS
type IV secretion system.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Christie P. J., Atmakuri K., Krishnamoorthy V., Jakubowski S., Cascales E. Annu. Rev. Microbiol. 2005;59:415–485. doi: 10.1146/annurev.micro.58.030603.123630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Christie P. J. Biochim. Biophys. Acta. 2004;1694:219–234. doi: 10.1016/j.bbamcr.2004.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baron C. FEMS Microbiol. Lett. 2005;253:163–170. doi: 10.1016/j.femsle.2005.09.030. [DOI] [PubMed] [Google Scholar]
- 4.Atmakuri K., Cascales E., Christie P. J. Mol. Microbiol. 2004;54:1199–1211. doi: 10.1111/j.1365-2958.2004.04345.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yeo H. J., Savvides S. N., Herr A. B., Lanka E., Waksman G. Mol. Cell. 2000;6:1461–1472. doi: 10.1016/s1097-2765(00)00142-8. [DOI] [PubMed] [Google Scholar]
- 6.Gomis-Ruth F. X., Moncalian G., Perez-Luque R., Gonzalez A., Cabezon E., de la Cruz F., Coll M. Nature. 2001;409:637–641. doi: 10.1038/35054586. [DOI] [PubMed] [Google Scholar]
- 7.Yuan Q., Carle A., Gao C., Sivanesan D., Aly K., Höppner C., Krall L., Domke N., Baron C. J. Biol. Chem. 2005;280:26349–26359. doi: 10.1074/jbc.M502347200. [DOI] [PubMed] [Google Scholar]
- 8.Cascales E., Christie P. J. Proc. Natl. Acad. Sci. USA. 2004;101:17228–17233. doi: 10.1073/pnas.0405843101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cascales E., Christie P. J. Science. 2004;304:1170–1173. doi: 10.1126/science.1095211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judd P. K., Kumar R. B., Das A. Proc. Natl. Acad. Sci. USA. 2005;102:11498–11503. doi: 10.1073/pnas.0505290102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Judd P. K., Kumar R. B., Das A. Mol. Microbiol. 2005;55:115–124. doi: 10.1111/j.1365-2958.2004.04378.x. [DOI] [PubMed] [Google Scholar]
- 12.Jakubowski S. J., Cascales E., Krishnamoorthy V., Christie P. J. J. Bacteriol. 2005;187:3486–3495. doi: 10.1128/JB.187.10.3486-3495.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krall L., Wiedemann U., Unsin G., Weiss S., Domke N., Baron C. Proc. Natl. Acad. Sci. USA. 2002;99:11405–11410. doi: 10.1073/pnas.172390699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai E.-M., Kado C. I. J. Bacteriol. 1998;180:2711–2717. doi: 10.1128/jb.180.10.2711-2717.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eisenbrandt R., Kalkum M., Lai E. M., Lurz R., Kado C. I., Lanka E. J. Biol. Chem. 1999;274:22548–22555. doi: 10.1074/jbc.274.32.22548. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Eisenlohr H., Domke N., Angerer C., Wanner G., Zambryski P. C., Baron C. J. Bacteriol. 1999;181:7485–7492. doi: 10.1128/jb.181.24.7485-7492.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shamaei-Tousi A., Cahill R., Frankel G. J. Bacteriol. 2004;186:4796–4801. doi: 10.1128/JB.186.14.4796-4801.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sagulenko V., Sagulenko E., Jakubowski S., Spudich E., Christie P. J. J. Bacteriol. 2001;183:3642–3651. doi: 10.1128/JB.183.12.3642-3651.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorstenson Y. R., Zambryski P. C. J. Bacteriol. 1994;176:1711–1717. doi: 10.1128/jb.176.6.1711-1717.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das A., Xie Y.-H. J. Bacteriol. 2000;182:758–763. doi: 10.1128/jb.182.3.758-763.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar R. B., Xie Y.-H., Das A. Mol. Microbiol. 2000;36:608–617. doi: 10.1046/j.1365-2958.2000.01876.x. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R. B., Das A. J. Bacteriol. 2001;183:3636–3641. doi: 10.1128/JB.183.12.3636-3641.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Höppner C., Carle A., Sivanesan D., Hoeppner S., Baron C. Microbiology. 2005;151:3469–3482. doi: 10.1099/mic.0.28326-0. [DOI] [PubMed] [Google Scholar]
- 24.Ward D., Draper O., Zupan J. R., Zambryski P. C. Proc. Natl. Acad. Sci. USA. 2002;99:11493–11500. doi: 10.1073/pnas.172390299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Terradot L., Bayliss R., Oomen C., Leonard G., Baron C., Waksman G. Proc. Natl. Acad. Sci. USA. 2005;102:4596–4601. doi: 10.1073/pnas.0408927102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chaillan-Huntington C., Butler P. J., Huntington J. A., Akin D., Feldherr C., Stewart M. J. Mol. Biol. 2001;314:465–477. doi: 10.1006/jmbi.2001.5136. [DOI] [PubMed] [Google Scholar]
- 27.Bailey S., Ward D., Middleton R., Grossmann J. G., Zambryski P. Proc. Natl. Acad. Sci. USA. 2006;103:2582–2587. doi: 10.1073/pnas.0511216103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.O'Callaghan D., Cazevieille C., Allardet-Servent A., Boschiroli M. L., Bourg G., Foulongne V., Frutus P., Kulakov Y., Ramuz M. Mol. Microbiol. 1999;33:1210–1220. doi: 10.1046/j.1365-2958.1999.01569.x. [DOI] [PubMed] [Google Scholar]
- 29.Carle A., Höppner C., Aly K. A., Yuan Q., den Dulk-Ras A., Vergunst A., O‘Callaghan D., Baron C. Infect. Immun. 2006;74:108–117. doi: 10.1128/IAI.74.1.108-117.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bohne J., Yim A., Binns A. N. Proc. Natl. Acad. Sci. USA. 1998;95:7057–7062. doi: 10.1073/pnas.95.12.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoppe-Seyler F., Crnkovic-Mertens I., Tomai E., Butz K. Curr. Mol. Med. 2004;4:529–538. doi: 10.2174/1566524043360519. [DOI] [PubMed] [Google Scholar]
- 32.Yanisch-Perron C., Viera J., Messing J. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 33.Bhandari P., Gowrishankar J. J. Bacteriol. 1997;179:4403–4406. doi: 10.1128/jb.179.13.4403-4406.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulakov Y. K., Guigue-Talet P. G., Ramuz M. R., O'Callaghan D. Res. Microbiol. 1997;148:145–151. doi: 10.1016/S0923-2508(97)87645-0. [DOI] [PubMed] [Google Scholar]
- 35.Laemmli U. K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 36.Ansaldi M., Lepelletier M., Mejean V. Anal. Biochem. 1996;234:110–111. doi: 10.1006/abio.1996.0060. [DOI] [PubMed] [Google Scholar]
- 37.Boschiroli M. L., Ouahrani-Bettache S., Foulongne V., Michaux-Charachon S., Bourg G., Allardet-Servent A., Cazevieille C., Liautard J. P., Ramuz M., O'Callaghan D. Proc. Natl. Acad. Sci. USA. 2002;99:1544–1549. doi: 10.1073/pnas.032514299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Valdes R. J., Ackers G. K. Methods Enzymol. 1979;61:125–142. doi: 10.1016/0076-6879(79)61011-x. [DOI] [PubMed] [Google Scholar]
- 39.Hudson M. E., Nodwell J. R. J. Bacteriol. 2003;186:1330–1336. doi: 10.1128/JB.186.5.1330-1336.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.