Abstract
Type IV collagen is a predominant component of basement membranes, and glomeruli of a kidney filter ≈70–90 liters of plasma every day through a specialized glomerular basement membrane (GBM). In Alport syndrome, a progressive disease primarily affecting kidneys, mutations in GBM-associated type IV collagen genes (COL4A3, COL4A4, or COL4A5) lead to basement membrane structural defects, proteinuria, renal failure, and an absence of all three GBM collagen triple helical chains because of obligatory posttranslational assembly requirements. Here, we demonstrate that transplantation of wild-type bone marrow (BM) into irradiated COL4A3−/− mice results in a possible recruitment of BM-derived progenitor cells as epithelial cells (podocytes) and mesangial cells within the damaged glomerulus, leading to a partial restoration of expression of the type IV collagen α3 chain with concomitant emergence of α4 and α5 chain expression, improved glomerular architecture associated with a significant reduction in proteinuria, and improvement in overall kidney histology compared with untreated COL4A3−/− mice or irradiated COL4A3−/− mice with BM from adult COL4A3−/− mice. The α3(IV) collagen produced by BM-derived podocytes integrates into the GBM and associates with other α-chains to form type IV collagen triple helical networks. This study demonstrates that BM-derived stem cells can offer a viable strategy for repairing basement membrane defects and conferring therapeutic benefit for patients with Alport syndrome.
Keywords: Alport syndrome, bone marrow transplantation, glomerular basement membrane, type IV collogen α3 chain, glomeruli
Type IV collagen is a major structural component of all basement membranes, including the kidney glomerular basement membrane (GBM) in vertebrates and nonvertebrates (1, 2). Alport syndrome is characterized as an hereditary progressive disease of GBMs resulting from mutations in the basement membrane (type IV) collagen COL4A3, COL4A4, and COL4A5 genes (1, 3). All genetic forms of Alport syndrome (X-linked, autosomal recessive, and dominant) manifest typically in young adults and are associated with macroscopic hematuria, proteinuria, and progressive loss of glomerular filtration, leading to end-stage renal failure in the second or third decade of life. Patients frequently also develop neurosensory deafness and anterior lenticonus.
Type IV collagen consists of six distinct α-chains: namely, α1–α6 encoded by the COL4A1–COL4A6 genes (4). All α-chains are composed of three domains, the N-terminal 7 S domain, the middle triple helical domain, and the C-terminal globular noncollagenous (NC1) domain (5). These α-chains are assembled as triple helices, which form a three-dimensional network to provide a scaffold for other macromolecules of the basement membrane. This network is essential for tissue function in providing structural support, serving as ligands for cell receptors. In the GBM, the predominant type IV collagen chains are α3, α4, and α5 polypeptides, and they associate with each other to form viable triple helical and other suprastructural molecules. Therefore, deletion of any one of these three chains leads to compound loss of all three chains in the GBM because of impaired posttranslational assembly.
The GBM, a specialized form of basement membrane, is a principal component of the filtration barrier and is interposed between the endothelial and epithelial cell layers of the glomerular capillary wall (6). Mice that are deficient in the COL4A3 gene serve as a mouse model for human Alport syndrome (3, 7, 8). These mice develop progressive renal failure with decreased glomerular filtration capacity, leading to uremia, impaired GBM and glomerular architecture, proteinuria, and eventual renal failure. Transmission EM analysis reveals focal multilaminated thickening and thinning of the GBM beginning soon after birth. At ≈14 weeks of age, mice develop end-stage renal disease and die at ≈20–23 weeks of age (3, 7).
The current therapy of choice for Alport patients is kidney transplantation, or hemodialysis while waiting for a kidney allograft. Here, we demonstrate that allogenic bone marrow transplantation (BMT) [WT bone marrow (BM) transplanted into COL4A3−/− mice] leads to reversal of kidney disease. This study demonstrates the capacity of BM-derived progenitor cells in the repair of extracellular basement membrane structural defects caused by the recruitment of resident glomerular cells that have the ability to synthesize extracellular matrix/basement membrane.
Results
Eight-week-old COL4A3−/− mice irradiated with 10 Gy of a 137cesium gamma source were rescued 24 h later by i.v. administration of unfractionated BM cells (2–5 × 106 cells) derived from ROSA26/LacZ+ mice (LacZ mice).
BMT and Recruitment of Glomerular Visceral Epithelial Cells (Podocytes) and Mesangial Cells.
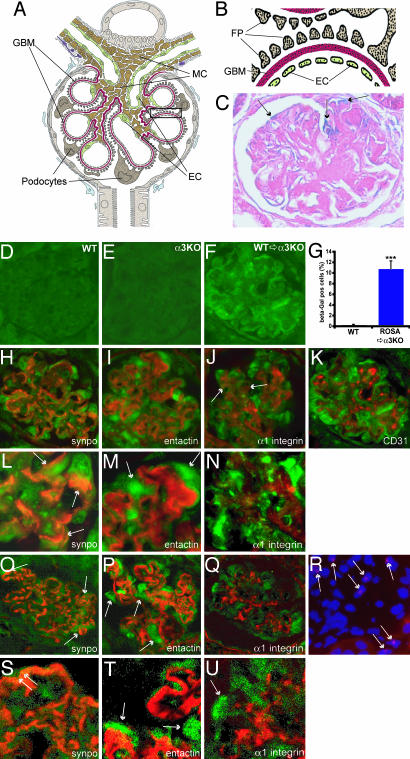
The COL4A3−/− mice are born normal, but, starting ≈8 weeks of age, they develop proteinuria and significant signs of histological kidney disease and eventually die of renal failure between 20 and 23 weeks. In the present study, transplantation of WT BM into 8-week-old COL4A3−/− mice leads to recruitment of podocytes and mesangial cells by 12 weeks of age (Fig. 1). A schematic illustration of a glomerular capsule with all of the constituent cells is shown in Fig. 1 A and B. Glomerular filtration of the blood to generate urine takes place across three layers with the support of the perivascular mesangial cells. The three layers include the fenestrated glomerular endothelial cells, the GBM, and the foot processes and slit diaphragms of the glomerular podocytes. In Alport syndrome, because of severe defects in the GBM, the podocyte architecture is compromised, and, collectively, such abnormalities in the glomerular apparatus lead to proteinuria and eventual renal failure.
Fig. 1.
Schematic illustration of a normal glomerulus and recruitment of BM-derived cells. (A and B) Schematic illustration of a normal glomerulus. (A) The filtration barrier is composed of three layers of fenestrated endothelial cells (EC) at the inner aspect, the interposed GBM (red), and epithelial cells (podocyte) at the outer aspect with larger processes and interdigitating foot processes (FP). MC, mesangial cells. (B) At higher magnification, the characteristic three-layer assembly of the filtration barrier is illustrated (the glomerular schema was kindly provided by W. Kriz, University of Heidelberg, Heidelberg, Germany). (C) β-Gal labeling of kidney sections after BMT. In glomeruli from COL4A3−/− mice transplanted with LacZ BM, β-gal-positive cells (arrows, blue) are detected. (D–Q) Fluorescence microscopy analysis of β-gal. The specificity of this β-gal labeling was confirmed by immunohistochemistry using an antibody to β-gal. Although normal WT mice transplanted with WT BM (D) or COL4A3−/− mice transplanted with COL4A3−/− BM (E) did not show any positive labeling for β-gal antibody, COL4A3−/− mice transplanted with WT LacZ BM revealed labeling for β-gal (F). (G) Quantitation of β-gal-positive cells revealed 6.2% β-gal-positive cells per glomerulus at 12.5 weeks of age (data not shown and Fig. 6X), with an increase to 10.8% β-gal-positive cells per glomerulus at 21 weeks. Results are shown as mean ± SEM. Means were compared by using the Mann–Whitney rank sum test. ∗∗∗, P < 0.001. (H–N) Double fluorescence analysis of mouse kidney cortex. We could demonstrate colocalization of β-gal labeling with synaptopodin (synpo, a gift from Peter Mundel, Mount Sinai School of Medicine, New York), a specific marker for podocyte foot processes (arrows in L and O), and some transplanted cells showed colocalization with α1-integrin (J and N) as a mesangial cell marker (arrows in J). No colocalization with CD31-positive endothelial cells at 12.5 weeks (data not shown and Fig. 6O) and 21 weeks (K) of age could be detected. (I and M) After double labeling with entactin (a GBM marker), at high magnification (×600, M), β-gal labeling is abundantly clear at the outer aspect of the GBM, corresponding to epithelial cells (arrows in M). (O–Q and S–U) Confocal laser scanning double fluorescence analysis. Confocal double fluorescence analysis confirmed the colocalization of β-gal labeling with synaptopodin (arrows in O and S) and emphasized an epithelial localization of the β-gal labeling at the outer aspect of the GBM (arrows in P and T) as revealed by double labeling with entactin. Minimal colocalization was found with α1-integrin (Q and U, arrow in Fig. 6P). At high magnification (×1,000, S and T) colocalization of the β-gal labeling with synaptopodin becomes undeniable, as well as the epithelial localization of the β-gal labeling at the outer aspect of the GBM. (R) FISH analysis for the Y chromosome was performed on 4-μm cryosections from kidneys (male WT BM was transplanted into female COL4A3−/− mice) by using the starFISH kit. Y chromosomes appear as red dots (arrows) in a subset of nuclei in the glomerulus. FISH analysis further confirmed that male BM-derived stem cells are recruited into the damaged glomeruli of female COL4A3−/− mice. Y chromosome cannot be detected in nuclei of female COL4A3−/− mice (data not shown and Fig. 6W). Photomicrographs were taken by using a Zeiss Axioscop 2plus fluorescence microscope or Zeiss/Bio-Rad MRC 1024ES UV laser scanning microscope. (Magnification: D and E, H–K, and O–R, ×400; L–N, ×600; S–U, ×1,000.)
Glomerular morphometric analysis demonstrates that ≈10% of the resident glomerular cells are from the BM in the transplanted COL4A3−/− mice at 21 weeks of age (13 weeks after transplantation) (Figs. 1 C and G and 2 H–J; see also Fig. 6 A–V and X, which is published as supporting information on the PNAS web site). The LacZ-positive BM-derived cells from WT mice were detected by immunohistochemistry using an anti-LacZ antibody and colocalized with specific glomerular cell markers to ascertain the location of the transplanted cells. Entactin was used as a GBM marker, synaptopodin was used as a podocyte foot process marker, anti-integrin α1 antibody was used as a mesangial cell marker, and CD31 was used as a glomerular endothelial cell marker (Figs. 1 H–K and 6 M–P). Our data demonstrate that the transplanted cells are podocytes (above the GBM and along the synaptopodin labeling) and mesangial cells (colocalized with anti-integrin α1 antibody) (Figs. 1 H–J and L–N and 6 A–F). Confocal laser scanning double fluorescence analysis confirmed the colocalization of β-gal labeling with synaptopodin (arrows in Fig. 1 O and S) and emphasized an epithelial localization of the β-gal labeling at the outer aspect of the GBM (arrows in Fig. 1 P and T) as revealed by double labeling with entactin. Minimal colocalization was also found with α1-integrin (Figs. 1 Q and U and 6 G–L and Q–V). In this study, transplanted endothelial cells were not detected. Untreated control WT mice or mice transplanted with WT BM did not reveal localization of transplanted cells, suggesting that inflamed/injured glomeruli specifically provide necessary cues for the recruitment of BM-derived stem cells (Figs. 1 D and 6 M–P). Using Y chromosome FISH analysis (Fig. 1R), we further demonstrate that BM transplanted cells can be detected in the injured glomeruli (female COL4A3−/− mice receiving male LacZ BM) and that such Y chromosome localization is not detected in untransplanted COL4A3−/− female mice (Fig. 6W).
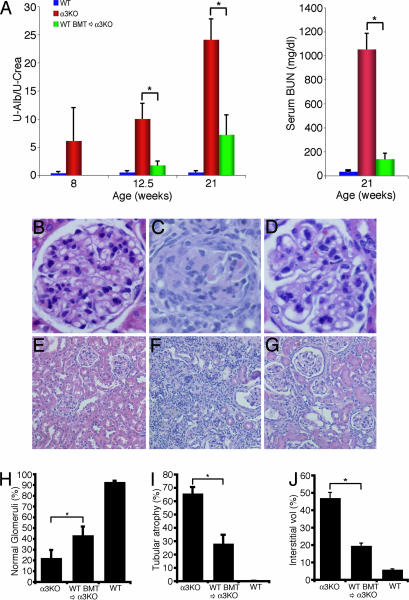
Fig. 2.
Time course of renal function. (A) (Left) Urinary albumin and creatinine concentration were estimated by using a colorimetric assay. Urine albumin excretion was estimated as the quotient of urine albumin and urine creatinine as described. Bar graphs show urine albumin excretion of 8-, 12.5-, and 21-week-old WT mice (blue bars), COL4A3−/− mice (red bars), and COL4A3−/− mice transplanted with WT BM (green bars). Recruitment of BM-derived progenitor cells into the damaged glomeruli upon BMT with WT BM leads to reduced proteinuria. No statistically significant difference could be detected between the BMT groups at 12.5 and 21 weeks (P = 0.323). (Right) BUN was measured by using the Sigma BUN 20 Endpoint kit. Bar graph shows a tremendous reduction of serum BUN in COL4A3−/− mice transplanted with WT BM (green bar) compared with untreated COL4A3−/− mice (red bar) at 21 weeks. Results are shown as mean ± SEM. ∗, P < 0.05. (B–D) Quantitation of normal glomeruli. (B) Histological analysis of a representative glomerulus from 21-week-old WT mice transplanted with WT BM shows normal histology in light microscopy [×400 magnification; hematoxylin/eosin (H&E) labeling]. (C) In COL4A3−/− mice transplanted with COL4A3-deficient BM at 21 weeks of age, fibrous crescent was seen in the glomerulus, and glomerular capillary tufts were collapsed (×400 magnification; H&E labeling). (D) BMT of COL4A3−/− mice with BM from WT mice improves the glomerular damage (×400 magnification; H&E labeling). (E–H) Tubular damage and interstitial fibrosis. (E) Light-microscopic analysis of representative areas of H&E-labeled kidney sections from 21-week-old WT mice transplanted with WT BM (×100 magnification) shows normal tubular and interstitial compartments. (F) In COL4A3−/− mice transplanted with COL4A3-deficient BM at 21 weeks, the interstitium is expanded with separation of tubules by fibrosis and tubular atrophy (×100 magnification; H&E labeling). (G) Treatment of COL4A3−/− mice with BMT from WT mice inhibits progression of tubular damage and interstitial fibrosis (×100 magnification; H&E labeling). (H) Morphometric analysis of the portion of normal glomeruli. BMT of COL4A3−/− mice with BM from WT mice significantly increased the percentage of normal glomeruli at 12.5 (E) and 21 (F) weeks of age. (I and J) Quantitation of tubular damage and interstitial fibrosis. BMT of COL4A3−/− mice with BM from WT mice results in significantly decreased tubular damage (I) as well as decreased relative interstitial volume (J) compared with untreated COL4A3−/− mice. ∗, P < 0.05.
Improvement of Renal Function and Histology upon BMT.
In COL4A3−/− mice, urine protein excretion (estimated as urine albumin/creatinine ratio) was elevated at 8 weeks of age (at the time point of BMT), and, in the untreated COL4A3−/− mice, proteinuria continued to increase until their death at an age of ≈20–23 weeks (Fig. 2A Left). Transplantation of COL4A3−/− mice with BM from WT LacZ mice significantly attenuated the levels of urine albumin excretion when evaluated at 12.5- and 21-week time points (4.5 and 13 weeks after BMT, respectively) (Fig. 2A). A significant reduction of serum blood urea nitrogen (BUN) was also observed in COL4A3−/− mice transplanted with WT BM compared with untreated COL4A3−/− mice at 21 weeks (Fig. 2A Right).
The COL4A3−/− mice are characterized by initial glomerulonephritis, leading to progressive tubulointerstitial disease and renal fibrosis starting at ≈8 weeks of age. We killed COL4A3−/− mice at 8, 12.5, and 21 weeks to establish the kinetics of disease progression (Fig. 2 B–J).
At 12.5 and 21 weeks of age, COL4A3−/− mice reveal fibrous crescents in glomeruli, glomerular capillary collapse, tubular atrophy, and interstitial fibrosis; BMT ameliorates the renal damage, resulting in an increased percentage of normal glomeruli (1.3- and 1.9-fold increase of normal glomeruli at 12.5 and 21 weeks of age, respectively; Fig. 2 B–D and H) and significantly healthy tubulointerstitial compartment in treated mice (Fig. 2 E–G, I, and J). Morphometric analysis of the glomerular and tubulointerstitial compartments reveals that, compared with control mice, the treated mice exhibit a significant decrease in glomerular and relative interstitial volume (2.6- and 2.3-fold decrease of tubular atrophy at 12.5 and 21 weeks, respectively; 2.2- and 2.4-fold decrease in relative interstitial volume at 12.5 and 21 weeks, respectively) when compared with controls (Fig. 2 I and J).
These results strongly suggest that transplantation of WT BM containing stem cells producing the α3 chain of type IV collagen is essential for therapeutic realization. Immune modulation caused by irradiation and BMT procedures are not determining factors in therapy, as established by the lack of significant efficacy in experiments involving irradiated COL4A3−/− mice receiving BM from COL4A3−/− mice.
Ultrastructural Analysis Reveals That BMT Repairs GBM and Podocyte Defects in COL4A3−/− Mice.
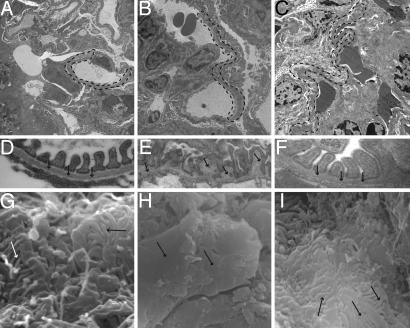
EM was used to examine kidney tissue at 12.5 and 21 weeks of age with particular emphasis on GBM architecture and podocyte integrity. Transmission EM analysis of WT mice showed normal GBM pattern (Fig. 3A and D), and COL4A3−/− mice at 8, 12.5, and 21 weeks showed a significant progressive damage to the GBM, with characteristic splitting, multilaminations, thinning, and thickening with breaks, as observed in human Alport syndrome (9) (Fig. 3 B and E and data not shown). In the COL4A3−/− mice with a transplant of COL4A3-deficient BM, the defects remained the same, but COL4A3−/− mice transplanted with WT lacZ BM revealed significant repair of the GBM at 12.5 and 21 weeks of age (4.5 and 13 weeks after BMT, respectively) (Fig. 3 C and F and data not shown). Scanning EM of podocytes from COL4A3−/− mice revealed foot process effacement (flattening) and microvillous transformation when compared with WT control mice. In WT mice, upon BMT with WT BM, the foot processes interdigitated between two adjacent podocytes to form fern-leaf structures (Fig. 3G). The defects remained the same (as in COL4A3−/− mice) in COL4A3−/− mice that received BM from COL4A3−/− mice (Fig. 3H). COL4A3−/− mice transplanted with WT lacZ BM revealed significant repair of podocyte integrity with reduction in effacement at 12.5 and 21 weeks of age (4.5 and 13 weeks after BMT, respectively) (Fig. 3I and data not shown).
Fig. 3.
Ultrastructural analysis of GBM. (A–F) Transmission EM. (A) WT mice (21 weeks old) show normal GBM structure (dashed line in A and arrows in D). (D) Between the foot processes, the slit diaphragma can be seen. At low (B) and high (E) magnification, GBM of untreated COL4A3−/− mice shows splitting, a basket-weave appearance (arrows in E), lamination, and thinning (dashed line in B). Podocytes show microvillous transformation and foot process effacement. (C) BMT of COL4A3−/− mice with COL4A3+/+ BM from WT ROSA26 mice inhibits foot process effacement and reconstitutes damaged GBM structure (dashed line in C and arrows in F). At high magnification (F), intact slit diaphragm after α3 chain restoration can be seen. (G–I) Scanning EM. (G) Normal WT mice, upon BMT with WT BM, show a complex interdigitation (arrows) of podocyte foot processes between adjacent podocytes forming the filtration barrier. (H) In COL4A3−/− mice with BMT with COL4A3-deficient BM, the interdigitation of foot processes is lost (arrows) and forms sheet-like structures. (I) BMT of COL4A3−/− mice with WT COL4A3+/+ BM recovers foot process interdigitation (arrows). (Magnification: A–C, ×4,400; D and E, ×54,800; F, ×82,000; G, ×30,000; H, ×8,000; I, ×15,000.)
Expression of α3, α4, and α5 Chains of Type IV Collagen in COL4A3−/− Mice After BMT.
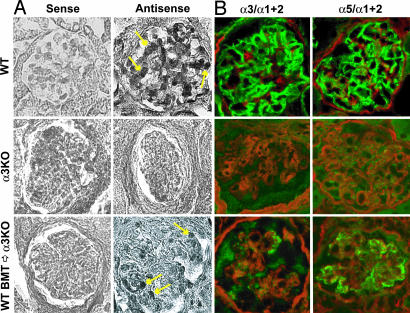
In WT mice, glomerular capillary basement membrane is composed of triple helical type IV collagen molecules comprising α3, α4, and α5 chains. Several studies suggest that α3 chain is produced by podocytes and glomerular endothelial cells (10). To further evaluate whether the repair of GBM architecture was caused by de novo synthesis of α3 chain by the transplanted podocytes derived from the BM in the COL4A3−/− mice, in situ hybridization for COL4A3 transcript was performed (Fig. 4A). The mRNA for type IV collagen α3 chain was expressed in podocytes of glomeruli from WT mice (arrows, Fig. 4A Right). A complete lack of type IV collagen α3 mRNA expression was noted in the COL4A3−/− mice. BMT from WT mice revealed COL4A3 mRNA expression in newly recruited podocytes in the glomeruli of COL4A3−/− mice (arrows, Fig. 4A Right).
Fig. 4.
In situ hybrization for COL4A3 mRNA and immunofluorescence analysis of type IV collagen α3 and α5 chain expression. (A) In situ hybridization for COL4A3 mRNA was performed on blocks of formalin-fixed, paraffin-embedded tissue. The mRNA for type IV collagen α3 chain was expressed in podocytes (arrows) of glomeruli from WT mice, whereas a complete lack of type IV collagen α3 mRNA expression was noted in COL4A3−/− mice. BMT from WT mice induced type IV collagen α3 chain expression (arrows) in newly recruited podocytes in glomeruli of COL4A3−/− mice. (Right) “Antisense” probe labeling. (Left) In all cases, “sense” probe did not reveal any specific labeling. (B) Immunofluorescence analysis of type IV collagen α3 and α5 chain expression. Frozen kidney sections were labeled with antibodies to α3 or α5 chain of type IV collagen, and labeling was visualized by using FITC-conjugated anti-rabbit IgG antibodies. The same sections were double-labeled with an antibody against α1 and α2 chains of type IV collagen and rhodamine-conjugated anti-goat IgG. Normal kidneys from 21-week- old WT mice displayed a strong linear immunolabeling for α3 and α5 chain (both green) along the GBM. COL4A3−/− mice showed the absence of α3 chain and α5 chain of type IV collagen. Kidneys from COL4A3−/− mice treated with BMT with BM from WT mice show a stippled or segmental linear labeling of type IV collagen α3 chain (green). After BMT of COL4A3−/− mice transplanted with BM from WT mice, α5 chain of type IV collagen was recovered to almost normal levels (green). Red color shows labeling for α1 and α2 chain of type IV collagen. (Magnification: ×200.)
To evaluate whether the presence of mRNA for α3 chain of type IV collagen leads to deposition of this protein in the GBM of transplanted COL4A3−/− mice, we performed immunofluorescence experiments by using chain-specific type IV collagen antibodies described in ref. 11. In glomeruli of WT mice, α1 and α2 chain isoforms of type IV collagen are preferentially localized to the mesangial matrix, and the α3 and α5 chains reveal specific expression in the GBM (Fig. 4B). Loss of α3 chain of type IV collagen in the GBM leads to a transition of α1 and α2 chain of type IV collagen from the mesangial matrix (normal distribution) to the capillary GBM (abnormal localization), as described (11) (Fig. 4B). As expected, in COL4A3−/− mice, the α3 chain of type IV collagen is absent in the GBM, which further leads to the absence of α4 and α5 chain because of the assembly requirements of forming viable triple helical molecules (Fig. 4B). Upon WT BMT, COL4A3−/− mice reveal patchy, speckled, and some linear labeling patterns for α3 chain and significant recovery of α5 chain expression in the GBM (Fig. 4B). Despite partial recovery of α3 chain in the GBM, α1 and α2 chain still exhibits abnormal expression in the GBM (Fig. 4B), suggesting that all features of altered matrix expression in these mice are not restored.
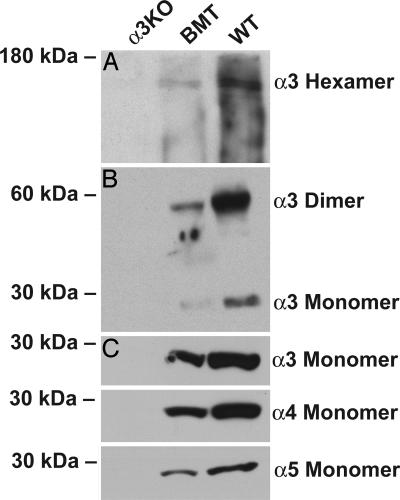
To evaluate whether reexpression of type IV collagen α3 chain mRNA and protein led to appropriate incorporation of this chain in the type IV collagen network and reinduced the incorporation of α4 and α5 chains, renal basement membranes from snap-frozen kidneys of control and experimental mice were isolated by using salt and detergent extraction, as described. The cell-free basement membrane extract was subsequently subjected to collagenase digestion to degrade triple helical collagen networks, liberating the noncollagenous domains. Among this protein mixture is also the C-terminal globular hexamer, which represents assembled type IV collagen protomers. Immunoblotting with α3 antibodies reveals the presence of α3NC1 collagen chain in the hexamers of control mice and also COL4A3−/− mice with WT BMT but not the COL4A3−/− mice, although the levels of α3NC1 protein are lower in BMT mice, further supporting the results observed in immunofluorescence experiments (Fig. 5A). The α3NC1 could also be detected as dimers and monomers in the COL4A3−/− BMT mice compared with control WT mice (Fig. 5B). Such expression of α3 chain in the GBM type IV collagen network was associated with reemergence of α4 and α5NC1 protein expression in the type IV collagen NC1 hexamers, as determined by disulfide bond-reducing SDS/PAGE analysis, when compared with the COL4A3−/− mice (Fig. 5C). To assess for protein loading in all immunoblotting experiments, the transfer membranes were labeled with Coomassie brilliant blue before immunoincubation (Fig. 7, which is published as supporting information on the PNAS web site).
Fig. 5.
Western blot analysis of native type IV collagen α3–α5 NC1 domains. PAGE was performed on 10% polyacrylamide gels. (A) After nondenaturing PAGE α3-containing hexamers in WT mice and COL4A3−/− mice with WT BMT (BMT) could be detected but not in untreated COL4A3−/− mice (α3KO). (B) Nonreducing SDS/PAGE revealed α3 monomers and α3 dimer formation in WT mice and COL4A3−/− mice with WT BMT but not in untreated COL4A3−/− mice. The two dots at ≈50 kDa (lane 2) appear to be nonspecific. (C) Denaturing SDS/PAGE revealed type IV collagen α3, α4, and α5 monomers (28 kDa) in WT and COL4A3−/− mice with WT BMT but not in COL4A3−/− mice without treatment. Labeling of the identical membranes with Coomassie brilliant blue demonstrates the same protein loading and separation (data not shown and Fig. 7).
Discussion
Our results demonstrate a successful transplantation of COL4A3−/− mice, a mouse model of human Alport syndrome (1, 3), with BM-derived COL4A3+/+ cells. These cells incorporate into the damaged glomeruli as podocytes and mesangial cells, which leads to a clear improvement in renal function and a lucid amelioration of glomerular architecture defects. The repair of GBM architecture and glomerular integrity is associated with expression of α3 chain of type IV collagen and restoration of α4 and α5 chain expression, with viable triple helical molecules and network organization of type IV collagen in the renal basement membranes.
The use of stem cell therapy in human diseases remains a controversial issue because of ethical concerns of using human embryonic stem cells (12). Alternatively, in the last few years, several reports have suggested the ability of BM cells to implant and differentiate into different tissues, including the kidney (13–19). Further, BM-derived cells localize to inflamed tissues of various organs. (For further discussion, see Supporting Text, which is published as supporting information on the PNAS web site.)
In this study, BMT protocol was performed after kidney injury was established in the organ. This report shows how stem cells can be used to repair extracellular/basement membrane defects. Our study unequivocally demonstrates that soluble factors liberated by the putative stem cells cannot explain the therapeutic effect; instead, BM-derived cells are required to become resident glomerular cells and provide the missing type IV collagen chain to enable repair and reversal of GBM defects and recovering of renal function. Alternatively, BM-derived cells could fuse with the existing glomerular cells and provide therapeutic benefit or transfer their nuclei to damaged podocytes and enable repair. Further experiments are required to understand the specific mechanisms. The issue of immune cells and other cells from the BM getting trapped in the glomeruli and providing nonspecific benefit by liberating collagens does not arise, because immune cells do not produce matrix proteins. Transplantation of mice with BM cells did not result in any Ig deposition along the GBM, suggesting a lack of humoral response toward the newly synthesized GBM at the time points evaluated (data not shown).
Interestingly, our study demonstrates that inflamed/injured glomeruli recruit cells that are BM stem cell-derived, suggesting that inflamed glomeruli (with respect to growth factors and other attractants) provide a molecular recognition sink for BM-derived cells that is not provided by normal glomeruli. We believe that such recruitment is caused by the special environment of the damaged glomeruli. Moreover, podocytes are present along the outer aspect of the GBM, and, hence, recruitment of transplanted podocytes in this model could be specific to the nature of this disease with respect to GBM defects and breaks, providing significant gaps that enable the migration of BM-derived cells to the outer aspects of the GBM. We demonstrate that ≈10% of the cells in COL4A3−/− mice with BMT are transplanted cells, suggesting that, for therapeutic benefit, a small but significant number of cells is required versus total replacement of all damaged cells and complete repair of GBM. Finally, our results offer an opportunity for patients with Alport syndrome: a treatment of their inherited renal failure that is an alternative to lifelong dependence on hemodialysis or the prospect of repeated kidney transplantation starting at young adolescence.
Materials and Methods
Mice.
Transgenic mice constitutively expressing lacZ in all tissues [TgR(ROSA26)26Sor; C57BL/6 background] and WT C57BL/6 mice were obtained from The Jackson Laboratory. Type IV collagen α3 chain knockout (COL4A3−/−) mice are described in refs. 3 and 7 and were obtained from The Jackson Laboratory and backcrossed into C57BL/6 mice. Mice were maintained at the Beth Israel Deaconess Medical Center animal facility under standard conditions. All animal studies were reviewed and approved by the animal care and use committee of Beth Israel Deaconess Medical Center.
BMT.
Eight-week-old mice (COL4A3−/−, COL4A3+/−, and COL4A3+/+) were lethally irradiated with 10 Gy of a 137cesium gamma source. Mice were rescued by i.v. administration of 2–5 × 106 unfractionated BM cells 24 h after irradiation (13, 20, 21). BM cells were obtained aseptically by flushing femur, tibia, and humerus of ROSA26 or COL4A3−/− donor mice (13, 20, 21). After BMT mice were housed in autoclaved cages and received antibiotics for 3 weeks with drinking water [0.1% amoxicillin (Sigma) and 0.015% enrofloxacine (Fluka–Sigma)]. To study the possible influence of the BMT procedure itself (in particular, to exclude antiinflammatory effects caused by the irradiation process on the progression of the renal disease), COL4A3-deficient BM was transplanted into irradiated COL4A3−/− mice.
Proteinuria.
Urinary albumin and creatinine concentration were estimated by using a colorimetric assay according to the manufacturer's recommendation (Sigma). Urine albumin excretion was estimated as the quotient of urine albumin and urine creatinine as described in ref. 22.
BUN.
BUN was measured by using the BUN 20 Endpoint kit (Sigma) according to the manufacturer's recommendations.
Immunohistochemistry.
Immunofluorescence labeling was performed as described in refs. 3, 23, and 24; for details, see Supporting Text.
Histological Assessment of Renal Injury.
The extent of renal injury was assessed by morphometry of glomerular disease, tubular atrophy, and interstitial fibrosis as described; for details, see Supporting Text.
Confocal Microscopy.
Simultaneous confocal double fluorescence microscopy was performed as described in ref. 23 by using a Zeiss/Bio-Rad MRC 1024ES UV laser scanning microscope equipped with appropriate filters. Micrographs were digitally processed by using photoshop 7.0 (Adobe Systems, San Jose, CA).
EM.
Transmission EM and scanning EM were performed as described in refs. 23 and 25.
In Situ Hybridization.
In situ hybridization for type IV collagen α3 chain was performed on blocks of formalin-fixed, paraffin-embedded tissue as described in ref. 26.
FISH Analysis for the Mouse Y Chromosome.
In a subset of transplanted mice (n = 7), kidneys from BM transplanted mice (male WT BM was transplanted into female COL4A3−/− mice) were analyzed for the mouse Y chromosome. Cryosections (4 μm) were fixed in 100% ethanol for 20 min at −20°C. After dehydration in a graded alcohol series, FISH analysis was performed by using the starFISH kit (Cambio, Cambridge, U.K.) according to the manufacturer's recommendations.
Protein Purification of Native Type IV Collagen α3 NC1 Domain and Western Blotting.
Type IV collagen α3 NC1 domain was isolated from snap-frozen kidneys, and PAGE was performed as described in refs. 23 and 27; for details, see Supporting Text.
Statistical Analysis.
All values are expressed as mean ± SEM. ANOVA was used to determine statistical differences between groups with sigma-stat software (Jandel, San Rafael, CA). Further analysis was carried out by using Student's t test with Fisher's correction to identify significant differences. When the equal variance test failed, the Mann–Whitney rank sum test was used to identify significant differences. A level of P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
This work was supported primarily by a grant from the Emerald Foundation (to R.K.); partially by National Institutes of Health Grants DK55001 and DK62987 (to R.K.) and DK55000 (to D.C.); a research fund of the Center for Matrix Biology at Beth Israel Deaconess Medical Center; a fellowship from the Stop and Shop Pediatric Tumor Foundation (to H.S.); the Sigrid Juselius Fellowship and Foundation (M.S.); the Maud Kuistila Foundation (M.S.); the Finnish Medical Society Duodecim (M.S.); the Emil Aaltonen Foundation (M.S.); and Deutsche Forschungsgemeinschaft Grant MU 2298/2-1 (to T.M.M.).
Abbreviations
- BM
bone marrow
- BMT
BM transplantation
- GBM
glomerular basement membrane
- BUN
blood urea nitrogen
- H&E
hematoxylin/eosin
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Kalluri R., Shield C. F., Todd P., Hudson B. G., Neilson E. G. J. Clin. Invest. 1997;99:2470–2478. doi: 10.1172/JCI119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hudson B. G., Tryggvason K., Sundaramoorthy M., Neilson E. G. N. Engl. J. Med. 2003;348:2543–2556. doi: 10.1056/NEJMra022296. [DOI] [PubMed] [Google Scholar]
- 3.Cosgrove D., Meehan D. T., Grunkemeyer J. A., Kornak J. M., Sayers R., Hunter W. J., Samuelson G. C. Genes Dev. 1996;10:2981–2992. doi: 10.1101/gad.10.23.2981. [DOI] [PubMed] [Google Scholar]
- 4.Barker D. F., Hostikka S. L., Zhou J., Chow L. T., Oliphant A. R., Gerken S. C., Gregory M. C., Skolnick M. H., Atkin C. L., Tryggvason K. Science. 1990;248:1224–1227. doi: 10.1126/science.2349482. [DOI] [PubMed] [Google Scholar]
- 5.Timpl R., Wiedemann H., van Delden V., Furthmayr H., Kuhn K. Eur. J. Biochem. 1981;120:203–211. doi: 10.1111/j.1432-1033.1981.tb05690.x. [DOI] [PubMed] [Google Scholar]
- 6.Kalluri R. Nat. Rev. Cancer. 2003;3:422–433. doi: 10.1038/nrc1094. [DOI] [PubMed] [Google Scholar]
- 7.Miner J. H., Sanes J. R. J. Cell Biol. 1996;135:1403–1413. doi: 10.1083/jcb.135.5.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kalluri R., Cosgrove D. J. Biol. Chem. 2000;275:12719–12724. doi: 10.1074/jbc.275.17.12719. [DOI] [PubMed] [Google Scholar]
- 9.Kashtan C. E. Medicine (Baltimore) 1999;78:338–360. doi: 10.1097/00005792-199909000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Heidet L., Cai Y., Guicharnaud L., Antignac C., Gubler M. C. Am. J. Pathol. 2000;156:1901–1910. doi: 10.1016/S0002-9440(10)65063-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamano Y., Grunkemeyer J. A., Sudhakar A., Zeisberg M., Cosgrove D., Morello R., Lee B., Sugimoto H., Kalluri R. J. Biol. Chem. 2002;277:31154–31162. doi: 10.1074/jbc.M204806200. [DOI] [PubMed] [Google Scholar]
- 12.Anonymous. Nature. 2005;437:1065. [Google Scholar]
- 13.Bailey A. S., Jiang S., Afentoulis M., Baumann C. I., Schroeder D. A., Olson S. B., Wong M. H., Fleming W. H. Blood. 2004;103:13–19. doi: 10.1182/blood-2003-05-1684. [DOI] [PubMed] [Google Scholar]
- 14.Krause D. S., Theise N. D., Collector M. I., Henegariu O., Hwang S., Gardner R., Neutzel S., Sharkis S. J. Cell. 2001;105:369–377. doi: 10.1016/s0092-8674(01)00328-2. [DOI] [PubMed] [Google Scholar]
- 15.Orlic D., Kajstura J., Chimenti S., Jakoniuk I., Anderson S. M., Li B., Pickel J., McKay R., Nadal-Ginard B., Bodine D. M., et al. Nature. 2001;410:701–705. doi: 10.1038/35070587. [DOI] [PubMed] [Google Scholar]
- 16.Lagasse E., Connors H., Al-Dhalimy M., Reitsma M., Dohse M., Osborne L., Wang X., Finegold M., Weissman I. L., Grompe M. Nat. Med. 2000;6:1229–1234. doi: 10.1038/81326. [DOI] [PubMed] [Google Scholar]
- 17.Gussoni E., Soneoka Y., Strickland C. D., Buzney E. A., Khan M. K., Flint A. F., Kunkel L. M., Mulligan R. C. Nature. 1999;401:390–394. doi: 10.1038/43919. [DOI] [PubMed] [Google Scholar]
- 18.Gregory C. A., Ylostalo J., Prockop D. J. Sci. STKE. 2005;2005:pe37. doi: 10.1126/stke.2942005pe37. [DOI] [PubMed] [Google Scholar]
- 19.Lee R. H., Hsu S. C., Munoz J., Jung J. S., Lee N. R., Pochampally R., Prockop D. J. Blood. 2005;107:2153–2161. doi: 10.1182/blood-2005-07-2701. [DOI] [PubMed] [Google Scholar]
- 20.Rafii S., Meeus S., Dias S., Hattori K., Heissig B., Shmelkov S., Rafii D., Lyden D. Semin. Cell Dev. Biol. 2002;13:61–67. doi: 10.1006/scdb.2001.0285. [DOI] [PubMed] [Google Scholar]
- 21.Dwenger A., Rosenthal F., Machein M., Waller C., Spyridonidis A. Stem Cells. 2004;22:86–92. doi: 10.1634/stemcells.22-1-86. [DOI] [PubMed] [Google Scholar]
- 22.Sugimoto H., Hamano Y., Charytan D., Cosgrove D., Kieran M., Sudhakar A., Kalluri R. J. Biol. Chem. 2003;278:12605–12608. doi: 10.1074/jbc.C300012200. [DOI] [PubMed] [Google Scholar]
- 23.Mundel T. M., Heid H. W., Mahuran D. J., Kriz W., Mundel P. J. Am. Soc. Nephrol. 1999;10:435–443. doi: 10.1681/ASN.V103435. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto H., Shikata K., Matsuda M., Kushiro M., Hayashi Y., Hiragushi K., Wada J., Makino H. Diabetologia. 1998;41:1426–1434. doi: 10.1007/s001250051088. [DOI] [PubMed] [Google Scholar]
- 25.Cosgrove D., Rodgers K., Meehan D., Miller C., Bovard K., Gilroy A., Gardner H., Kotelianski V., Gotwals P., Amatucci A., Kalluri R. Am. J. Pathol. 2000;157:1649–1659. doi: 10.1016/s0002-9440(10)64802-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sayers R., Kalluri R., Rodgers K. D., Shield C. F., Meehan D. T., Cosgrove D. Kidney Int. 1999;56:1662–1673. doi: 10.1046/j.1523-1755.1999.00744.x. [DOI] [PubMed] [Google Scholar]
- 27.Gunwar S., Ballester F., Kalluri R., Timoneda J., Chonko A. M., Edwards S. J., Noelken M. E., Hudson B. G. J. Biol. Chem. 1991;266:15318–15324. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.