Abstract
Here, we report the isolation and characterization of an endogenous peptide ligand of GPR103 from rat brains. The purified peptide was found to be the 43-residue RF-amide peptide QRFP. We also describe two mouse homologues of human GPR103, termed mouse GPR103A and GPR103B. QRFP binds and activates the human GPR103, as well as mouse GPR103A and GPR103B, with nanomolar affinities in transfected cells. Systematic in situ hybridization analysis in mouse brains showed that QRFP is expressed exclusively in the periventricular and lateral hypothalamus, whereas the two receptor mRNAs are distinctly localized in various brain areas without an overlap to each other. When administered centrally in mice, QRFP induced feeding behavior, accompanied by increased general locomotor activity and metabolic rate. QRFP-induced food intake was abolished by preadministration of BIBP3226, a specific antagonist for the Y1 neuropeptide Y receptor. Hypothalamic prepro-QRFP mRNA expression was up-regulated upon fasting and in genetically obese ob/ob and db/db mice. Central QRFP administration also evoked highly sustained elevation of blood pressure and heart rate. Our findings suggest that QRFP and GPR103A/B may regulate diverse neuroendocrine and behavioral functions and implicate this neuropeptide system in metabolic syndrome.
Keywords: grooming, hypothalamus, QRFP, wakefulness, metabolic syndrome
G protein-coupled receptors (GPCRs) are members of a large protein family that share common structural motifs, including seven transmembrane helices, and play pivotal roles in cell-to-cell communications and in the regulation of cell functions. A large number of GPCRs still remain as “orphan receptors” whose cognate ligands have yet to be identified. Identification of ligands for orphan GPCRs provides a basis for understanding the physiological roles of those GPCRs and their ligands, which can involve the central nervous, endocrine, reproductive, cardiovascular, immune, inflammatory, digestive, and metabolic systems.
GPR103 (also referred to as SP9155 or AQ27) is an orphan GPCR that shows similarities with orexin, neuropeptide FF, and cholecystokinin receptors. Its mRNA has been detected predominantly in the brain including the cerebral cortex, pituitary, thalamus, hypothalamus, basal forebrain, midbrain, and pons in humans (1). Through bioinformatics approaches, two groups reported putative ligands for GPR103 as a part of a directed effort to identify the precursor genes for a novel RF-amide peptide and its receptor (2, 3). They identified a gene encoding a preproprotein that can be processed into several potential peptides, including a 26-aa (termed P518) and a 43-aa RF-amide peptide (termed QRFP) (2, 3). Both of these peptides activate GPR103, but the 43-aa QRFP exhibited more potent agonistic activity. When intravenously injected into rats, QRFP (43-aa) stimulates aldosterone release (3). The 26-aa RF-amide peptide (termed 26RFa) was independently purified from frog brain by monitoring NPFF-like immunoreactivity (4), and it exhibits orexigenic activity in mice when centrally administered. Although these predicted peptides have the agonistic effects on GPR103, the structure of the native ligand(s) in the mammalian brain has yet to be defined.
In an effort to identify ligands for orphan GPCRs by means of reverse pharmacology, we purified an endogenous ligand for GPR103 from rat brain extracts. This peptide was identical to QRFP (3). Intracerebroventricular (i.c.v.) administration of QRFP increased activity, intensified grooming response, and influenced blood pressure in addition to the effect on food consumption. The orexigenic action of QRFP is at least partly mediated through the neuropeptide Y (NPY) pathway, and QRFP mRNA level was increased in genetically obese ob/ob and db/db mice. These observations are in accord with our data on neuroanatomical distribution of QRFP and its two receptors, and show that the peptide is involved in modulating a range of functions in the CNS.
Results
Identification of Endogenous GPR103 Ligand from Rat Brain.
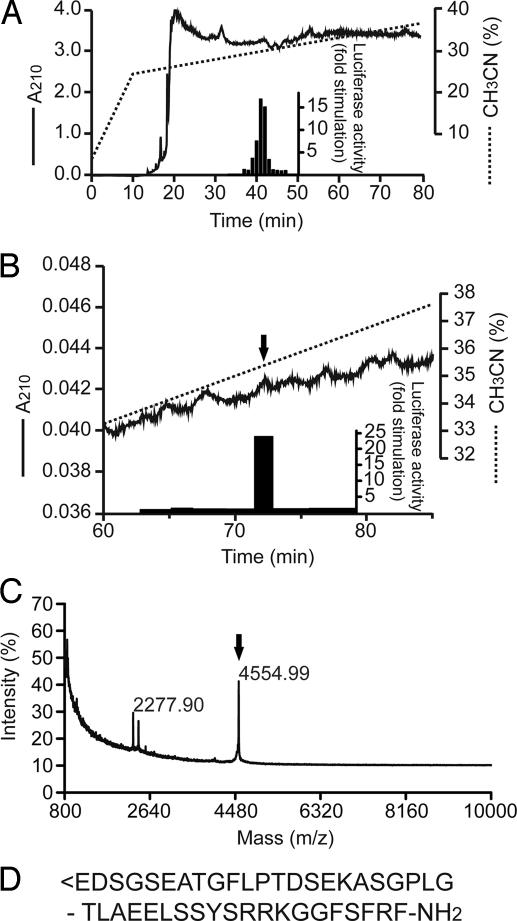
Jurkat cells cotransfected with the human GPR103 cDNA and a luciferase reporter driven by nuclear factor of activated T cells (NFAT) were used to monitor the ligand activities. NFAT acts as a downstream effecter of Gαq protein-coupled receptors in lymphoid cells (5). This system exhibited very low background signals; Jurkat cells appear to express a relatively limited number of GPCRs that are activated by substances in the crude tissue extracts. We found that two reverse-phase HPLC fractions of rat brain extracts can elicit increased NFAT-luciferase activity in Jurkat cells expressing GPR103 (Fig. 1A; the two activity peaks not well separated in this example). The major activity was purified to homogeneity from acid-acetone extracts of rat brains by collecting active fractions after eight steps of HPLC. The final purified material, corresponding to the absorbance peak indicated at the arrow in Fig. 1B, was subjected to structural analysis by N-terminal sequencing, MALDI-TOF mass spectrometry (Fig. 1C), and peptide mass fingerprint analysis after limited trypsin digestions. The active substance was found to be a 43-aa peptide of 4,554 Da, with an N-terminal pyroglutamyl residue and C-terminal amidation (Fig. 1D). This structure and molecular mass were identical to rat QRFP, which was previously predicted as a potential ligand for GPR103 by means of the bioinformatics approach (3). Also identified and purified was another activity that was a 43-aa peptide with the same sequence but with a free N-terminal glutamine lacking pyroglutamylation. No activities other than these two peaks were detected, suggesting that the only endogenous ligand for GPR103 detectable in the rat brain is the QRFP. This finding also indicates that this peptide receives no posttranslational modifications other than N-terminal pyroglutamylation and C-terminal amidation.
Fig. 1.
Purification of endogenous GPR103 ligand from rat brain extracts. (A) Chromatogram showing the activation of human GPR103 in transfected Jurkat cells by reverse-phase HPLC fractions of crude peptide extracts from rat brain. Specific NFAT reporter responses induced by fractions were detected in Jurkat cells through the increase of luciferase activity (filled bars). (B) Final purification of the active fraction by C18 reverse-phase HPLC column. The arrow indicates the final QRFP peak. (C) MALDI-TOF mass spectrum of the peak indicated by the arrow in B. The spectrum shows a protonated molecular ion of m/z 4,554.99. (D) A 43-aa peptide of 4,554 Da, with an N-terminal pyroglutamyl residue and C-terminal amidation.
Two Mouse Orthologues of Human GPR103.
A search of the mouse genome sequence with human GPR103 as the query found two putative homologues. These are here called the mouse GPR103A and GPR103B (GenBank accession nos. NP_937835 and AX665940, respectively). We cloned the complete coding regions of these receptors from mouse brain mRNA. The deduced amino acid sequences of GPR103A and GPR103B are aligned in Fig. 7, which is published as supporting information on the PNAS web site. Human GPR103 shares 83% amino acid identity with mouse GPR103A and 79% with GPR103B, while GPR103A and GPR103B share 75% amino acid identity with each other.
In Vitro Pharmacology of QRFP on GPR103A and GPR103B.
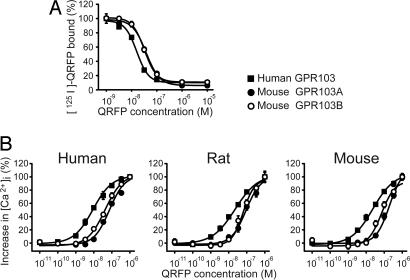
Competition binding assays were performed by using [125I-Tyr-32]-QRFP on monolayers of COS-7 cells transiently transfected with the GPR103A or GPR103B cDNA. Orexin receptor type 2 (OX2R)-transfected COS-7 cells did not exhibit detectable levels of specific binding of the radioligand (data not shown). The radioligand binding was inhibited by nanomolar concentrations of unlabeled synthetic mouse QRFP in a dose-dependent manner (Fig. 2A). The concentration of unlabeled QRFP required to displace 50% of specific radioligand binding (IC50) was 16, 38, and 32 nM for human GPR103, mouse GPR103A and GPR103B, respectively. In HEK293 cells transiently transfected with human GPR103, mouse GPR103A, or mouse GPR103B cDNA, rat QRFP induced a transient increase of intracellular Ca2+ levels ([Ca2+]i) in a dose-dependent manner, with EC50 values of 23, 91, and 63 nM, respectively (Fig. 2B). The peptide failed to induce detectable [Ca2+]i transients in mock- or OX2R-transfected HEK293 cells (data not shown). Mouse GPR103A and GPR103B responded similarly to human QRFP (EC50 of 73 and 42 nM, respectively) and mouse QRFP (EC50 of 194 and 70 nM, respectively) (Fig. 2B).
Fig. 2.
Pharmacological characterization of QRFP on GPR103 using synthetic QRFP. (A) Competitive radioligand binding assays. Displacement of [125I-Tyr-32]-mouse QRFP binding to cells expressing human GPR103 (filled squares), mouse GPR103A (filled circles), and GPR103B (open circles) by increasing concentrations of unlabeled, mouse QRFP was determined in triplicate. The level of nonspecific binding was ≈10% of the binding in the absence of competitor. (B) QRFP stimulates [Ca2+]i transients in HEK293 cells transiently transfected with human GPR103 (filled squares), GPR103A (filled circles), or GPR103B (open circles) cDNA. Increases in [Ca2+]i after exposure to designated concentrations of human, mouse, or rat QRFP were monitored by FDSS3000 and represented as percentages of maximum responses (mean ± SE, n = 4).
Tissue Expression Profile of QRFP and Its Receptors.
Quantitative real-time RT-PCR was used to examine the mRNA distribution of QRFP, GPR103A, and GPR103B in 15 different mouse tissues. QRFP mRNA was detected in CNS, eye, and testis, whereas both GPR103 mRNAs were found in CNS, eye, testis, and adrenal gland (Fig. 8, which is published as supporting information on the PNAS web site).
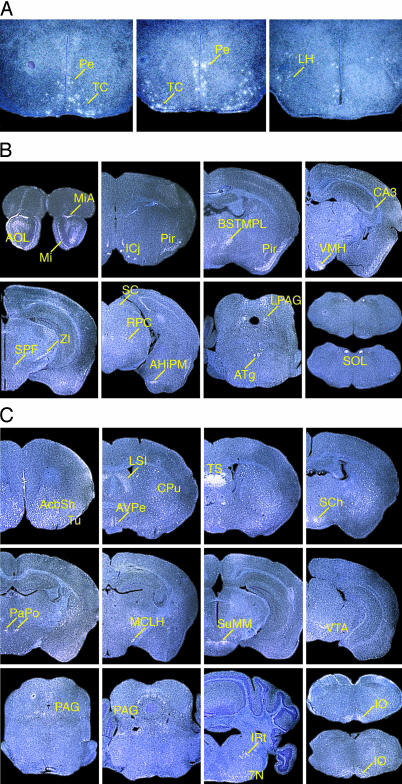
Using in situ hybridization, the precise localization of the prepro-QRFP, GPR103A, and GPR103B mRNAs was further studied through the entire mouse brain (Fig. 3). The prepro-QRFP mRNA was distinctly localized in limited areas of the brain, namely the periventricular hypothalamic nucleus and lateral hypothalamic areas (LH) (Fig. 3A). GPR103A and GPR103B mRNAs were differentially distributed in multiple regions of the brain. Notably, the mRNA distributions were virtually without an overlap with each other or the ligand mRNA. Particularly strong expression for GPR103A was observed in the mitral cell layer of the olfactory bulb, accessory olfactory bulb, island of Calleja, and nucleus of the solitary tract (Fig. 3B). In contrast, GPR103B mRNA was detected in the caudate-putamen, triangular septal nuclei, paraventricular hypothalamic nucleus, magnocellular nucleus of the lateral hypothalamus, medial supramammillary nucleus, rostral part of the facial nerve nucleus, and retroventrolateral reticular nucleus (Fig. 3C). In addition, a subset of strongly positive neurons was evident in the olfactory tubercle, extending caudally into both the accumbens core and shell of the ventral pallidum and dorsally into the caudate-putamen (striatum) (Fig. 3C and Table 1, which is published as supporting information on the PNAS web site). No signal was detected in tissues hybridized with the sense probes for QRFP, GPR103A, or GPR103B (data not shown).
Fig. 3.
In situ hybridization of prepro-QRFP, mouse GPR103A, and mouse GPR103B mRNAs on mouse brain sections. (A) Visualization of neurons containing prepro-QRFP mRNA in adult mouse brain. Shown are coronal section hybridized with 35S-labeled antisense riboprobes indicating that QRFP mRNA is distributed in the periventricular hypothalamic nucleus (Pe), lateral hypothalamic area (LH), and tuber cinereum area (TC) of the subthalamic area. (B) GPR103A mRNA in mouse brain. Mi, mitral cell layer of olfactory bulb; MiA, mitral cell layer of accessory olfactory bulb; AOL, anterior olfactory nucleus, lateral; Pir, piriform; ICj, island of Calleja; BSTMPL, bed nucleus of stria terminalis, medial, posterior lateral; CA3, CA3 field of hippocampus; VMH, ventromedial thalamic nucleus central, ventrolateral; ZI, zona incerta ventral; SPF, subparafascicular nucleus; AHiPM, amygdalohippocampal area postmedial part; RPC, red nucleus, parvicellular part; SC, optic nerve layer of superior colliculus; ATg, anterior tegmental nucleus; LPAG, lateral periaqueductal gray; SOL, solitary tract. (C) GPR103B mRNA in mouse brain. Tu, olfactory tubercle; AcbSh, accumbens shell; CPu, caudate putamen area; LSI, lateral septal nucleus; AVPe, anteroventral periventricular nucleus; TS, triangular septal nucleus; SCh, suprachiasmatic nucleus; PaPo, paraventricular hypothalamic nucleus, posterior; MCLH, magnocellular nucleus of lateral hypothalamus; SuMM, supramammillary nucleus, medial; PAG, periaqueductal gray; VTA, ventral tegmental area; 7N, facial nucleus; IRt, intermediate reticular nucleus; IO, inferior olive.
QRFP-Induced Feeding Behavior Is, in Part, Mediated by NPY.
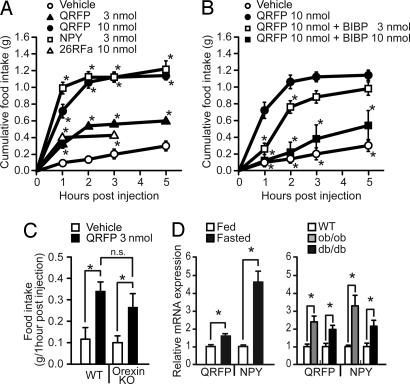
The distribution of QRFP in the LH suggested that QRFP may be involved in the regulation of feeding behavior. To address this hypothesis, synthetic rat QRFP peptide was administered acutely into the lateral ventricle of male mice through preimplanted indwelling catheters. When QRFP was administered in bolus i.c.v. in the early light period, QRFP stimulated food consumption within 1 h (Fig. 4A) that lasted for 2 h. The magnitude of stimulation with 3 and 10 nmol of QRFP at the 1-h time point was 3- and 7-fold, respectively, and the total amount of food intake during the interval from 1–2 h postinjection was increased ≈8.5-fold compared with vehicle controls, which was equivalent to that of 0.3 nmol of porcine NPY. Ten nanomoles of 26RFa (4), the 26-aa fragment of QRFP, also stimulated feeding behavior, but cumulative food intake was 70% less compared with that of 43-aa QRFP at the 1-h time point. Three nanomoles of 26RFa did not appreciably increase food intake. During the dark period, there was no obvious change in food intake between QRFP-treated and vehicle control-treated mice (data not shown).
Fig. 4.
The effect of QRFP on feeding behavior and the regulation of prepro-QRFP mRNA in mice. (A) Stimulation of food consumption by i.c.v. injection of QRFP in freely fed mice. Indicated amount of synthetic rat QRFP, porcine NPY, or rat 26RFa was i.c.v. injected at 2 h into light phase. Cumulative food intake was measured during the periods indicated (n = 5 per group). ∗, P < 0.05. (B) Inhibition of QRFP-induced food consumption by NPY-Y1 receptor antagonist BIBP3226. Mice were pretreated with BIBP3226 (3 or 10 nmol i.c.v.) or saline and then synthetic rat QRFP (10 nmol) was i.c.v. injected. ∗, P < 0.05 compared with QRFP alone (n = 5 per group). (C) Effect of QRFP on food intake in orexin knockout mice. Rat QRFP (3 nmol) or saline was i.c.v. injected into orexin knockout mice (n = 12) and their littermates (n = 8). Food consumption during a 1-h period was measured for each mouse. (D) Relative amounts of hypothalamic prepro-QRFP mRNA expression in male C57BL/6J mice under either fed or fasted conditions (n = 8 each) (Left) and ob/ob (n = 4), db/db (n = 4), or their control littermates (n = 5) (8 weeks of age) on an ad libitum chow diet (Right). C57BL/6J male mice (10 weeks of age) were on an ad libitum chow diet before the study and then either fed ad libitum or fasted for 48 h (n = 8 per group). Mice were killed at 10 a.m., and the thalamic/hypothalamic prepro-QRFP or NPY mRNA expression was determined by quantitative real-time RT-PCR as described in Supporting Materials and Methods. ∗, P < 0.01 compared with the value in fed (Left) or control littermate (Right) mice.
NPY has been established as one of the positive regulators of feeding behavior (6). NPY neurons are localized in the hypothalamic arcuate nucleus and project to the paraventricular nucleus, the dorsomedial and ventromedial hypothalamic nuclei (7–12). Using a specific NPY-Y1 receptor antagonist, BIBP3226 (13), we examined whether QRFP-induced feeding behavior is mediated through NPY. Pretreatment with 10 nmol of BIBP3226 profoundly inhibited QRFP-induced food consumption (Fig. 4B), indicating that QRFP-induced feeding behavior requires the activation of the Y1 receptor. These data also suggest that the QRFP-containing neurons may interact with NPY neurons. We also examined whether QRFP-induced food intake was mediated through orexin, a lateral hypothalamic neuropeptide implicated in the regulation of wakefulness and feeding behavior (14). The effect of QRFP was evaluated in orexin knockout mice (15). QRFP-induced food consumption in orexin knockout mice is similar to that in wild-type mice, indicating that QRFP-induced feeding behavior is independent of the orexin signaling pathway (Fig. 4C).
Prepro-QRFP mRNA Is Up-Regulated in the Fasted Condition and in Genetically Obese ob/ob and db/db Mice.
The stimulation of food intake by i.c.v. administrated QRFP suggested that QRFP may play a physiological role in the central regulation of feeding behavior. To evaluate the possibility that QRFP production is affected by nutritional state, the level of prepro-QRFP mRNA expression was compared in the hypothalami of fed and fasted wild-type mice with quantitative real-time RT-PCR analysis. The expression of NPY mRNA, known to be up-regulated in the fasted condition (14), was also examined as a positive control. In wild-type mice, hypothalamic prepro-QRFP mRNA was up-regulated 1.5-fold after 48 h fasting as compared with fed control animals (Fig. 4D Left), albeit to lesser extent than NPY mRNA.
We next examined whether prepro-QRFP mRNA expression is regulated by leptin signaling. NPY mRNA is known to increase in genetically obese ob/ob and db/db mice and contributes to the development of obesity (16). These models of obesity are characterized by leptin insufficiency (ob/ob) or leptin insensitivity (db/db) (17). QRFP may also contribute to the development of obesity in these genetic animal models. To evaluate this possibility, the mRNA expression was quantified in these mice. We found that QRFP mRNA was up-regulated 2- to 3-fold in both ob/ob and db/db mice as compared with age-matched control mice (Fig. 4D Right).
QRFP Increases Metabolic Rate, Locomotor Activity, and Grooming Behavior.
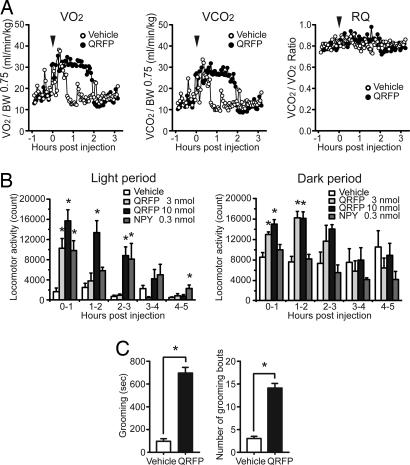
Many of the factors involved in the regulation of energy homeostasis affect metabolic rates and/or arousal states. To determine the effect of QRFP on energy expenditure, oxygen consumption was used as an index of metabolic rate. A bolus QRFP administration (3 nmol, i.c.v.) in the light period increased oxygen consumption, which stayed at elevated levels for 2 h after injection (Fig. 5A). The respiratory quotient (RQ) was not altered. Administration of QRFP (3 or 10 nmol) significantly stimulated locomotor activity during both the light and dark periods. NPY (0.3 nmol) increased locomotor activity during the light period but not during the dark period (Fig. 5B). In addition, i.c.v. QRFP caused a profound increase in time spent grooming and an increase in the number of grooming bouts (Fig. 5C). QRFP did not significantly exert anxiogenic or anxiolytic effects as assessed by using the elevated plus-maze (10 nmol per mouse, i.c.v.; n = 8) (Fig. 9, which is published as supporting information on the PNAS web site). These observations suggest that QRFP is involved not only in the regulation of feeding behavior, but also in the regulation of metabolic rate and physical activity, as well as response to stress.
Fig. 5.
The effect of QRFP on metabolic rate (A), locomotor activity (B), and grooming (C). (A) Oxygen consumption (VO2) and RQ were determined in male mice by indirect calorimetry after bolus i.c.v. administration (3 nmol). (B) Total locomotor activity was measured by beam breaks in light (Left) and dark (Right) period. ∗, P < 0.01 (n = 5 per group). (C) Effects of QRFP (3 nmol) on grooming duration (s) (Left) and number of grooming bouts (Right) recorded for a 2-h period immediately after peptide administration (3 nmol i.c.v.). Significant differences from vehicle-treated mice are shown. ∗, P < 0.01 (n = 6 per treatment group).
Central QRFP Administration Increases Blood Pressure.
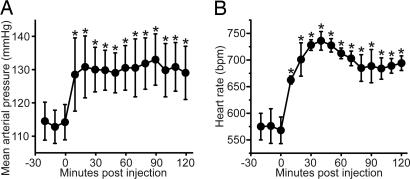
The possible involvement of QRFP in the response to stress and arousal prompted us to examine the effects of i.c.v. administered QRFP on mean arterial pressure and heart rate in conscious, unrestrained mice. Significant elevation of mean arterial pressure and heart rate was observed after bolus i.c.v. administration of 10 nmol of QRFP. A maximum increase in mean arterial pressure and heart rate was attained within 20–30 min after injection (Fig. 6). These effects were extremely long-lasting, with the arterial pressure and heart rate staying elevated even at 2 h postinjection. In these same mice, administration of a saline vehicle did not alter the mean arterial pressure or heart rate (data not shown).
Fig. 6.
Measurement of arterial blood pressure (A) and heart rate (B) in conscious, unrestrained mice (n = 5) centrally bolus administrated with QRFP (10 nmol). ∗, P < 0.01 compared with preinjection mean pressures.
Discussion
Structure of Endogenous Ligand for GPR103.
The exact structures of native peptide ligands, including posttranslational modifications cannot be determined by bioinformatics alone. For example, some of the biologically active peptides undergo an unusual proteolytic processing (e.g., endothelin) (18) and/or posttranslational modification (e.g., ghrelin) (19) and neuropeptide B (20), which may influence biological functions. In the current study, we demonstrated that QRFP, a mammalian RF-amide peptide, is actually present in rat brain in an N-terminally pyroglutamylated and C-terminally amidated form. During all of the purification steps, only two activity peaks for GPR103 were detected, which corresponded to the 43-residue peptide with and without N-terminal pyroglutamylation. The 43-residue peptide without N-terminal pyroglutamylation has a free glutamine as its N-terminal instead, suggesting that this peptide is a premature form of QRFP. Although the 26-aa residue peptide might be processed from the same prepro-QRFP (4), the current data indicate that the 43-residue peptide is the major processed form in mammals that exhibits full agonistic activity in vivo (Fig. 4).
QRFP Is Implicated in the Regulation of Feeding in Mice.
Leptin, an adipocyte-derived hormone, plays an important role in controlling food intake and energy expenditure (17). Pathways that stimulate food intake and promote weight gain (e.g., NPY, AgRP, orexin, and MCH) are inhibited by leptin and/or activated during fasting (17), whereas those pathways that inhibit food intake and reduce body weight (e.g., melanocortins, CART, TSH-releasing hormone, and CRH) are stimulated by leptin and down-regulated by fasting (17, 21). The observations that QRFP stimulates food intake and that hypothalamic QRFP mRNA expression is elevated in ob/ob and db/db mice suggest that QRFP may be regulated by leptin and involved in a highly integrated and redundant system of neurochemical pathways regulating energy homeostasis.
NPY is implicated in feeding behavior and functions as an anabolic neuropeptide that increases both the utilization of carbohydrates as energy substrate and the synthesis of fat from carbohydrate. NPY administration increases RQ while having no effect on energy expenditure or locomotor activity (22–25). QRFP administration stimulates food consumption in part via the NPY pathway (Fig. 4), but the locomotor activity and energy expenditure were also augmented, and there was no effect on RQ. These data suggest that QRFP may not be involved simply in anabolic action. This notion is supported by the findings that the continuous or repeated central administration of QRFP for 10 days period do not alter cumulative food intake and body weight gain (Fig. 10, which is published as supporting information on the PNAS web site), whereas continuous NPY administration leads to obesity (23, 26).
Both QRFP and orexin stimulate food intake robustly after acute i.c.v. injection in the light phase, and expression of mRNA encoding these peptides increases in response to fasting. In addition, locomotor activity and metabolic rate were up-regulated by both orexin and QRFP. However, regulation in obese animals is different: QRFP transcripts are up-regulated in genetically obese ob/ob and db/db mice, whereas those of orexin are down-regulated because of hyperglycemia in these mice (27). This observation suggests that increased expression of QRFP in these obese models may contribute to the increase in food intake, but it may also work to limit obesity by increasing metabolic rates in a manner similar to orexin (28, 29). Both orexin and QRFP induce arousal. Feeding behavior induced by exogenous orexin may well be largely due to its wake-promoting effect. Although the inhibition of QRFP-induced feeding behavior by an NPY antagonist suggests that QRFP may have a more specific effect on feeding, further studies are needed to determine whether the QRFP-induced feeding response is secondary to an increase arousal.
Intense grooming was observed in response to acute QRFP administration. This is a characteristic behavioral response to some neuropeptides, including corticotropin releasing hormone (CRH) and ACTH (30, 31). Grooming has been implicated in elevated stress levels (30). However, QRFP exerted no appreciable effect in elevated plus-maze tests, which is known to be sensitive to modulators of the hypothalamic–pituitary–adrenal axis such as CRH (31, 32), and to anxiolytic and anxiogenic drugs. This profile of neuroendocrine and behavioral effects distinguishes the effects of QRFP from those of CRH. In addition to its effect on arousal in conscious mice, we also revealed that acute central QRFP administration potently induces a long-lasting elevation of blood pressure. This may be accompanied by elevated stress levels as judged by the observation of intense grooming behavior. It is possible that QRFP neurons modulate stimulate sympathetic tone, which may contribute to the observed elevation of metabolic rate, arterial pressure, and heart rate. Taken in conjunction with the finding that QRFP expression is up-regulated in obese animals, it is tempting to speculate that QRFP is implicated in the hypertension of metabolic syndrome.
Experimental Procedures
The methods are described in detail in Supporting Materials and Methods, which is published as supporting information on the PNAS web site. For purifying QRFP, acid/acetone extraction of frozen rat brains was performed as described in refs. 14 and 20. The details of the HPLC procedures are also described in Supporting Materials and Methods. Structural analysis of the purified native peptide was performed by using MALDI-TOF MS. All peptides were solid-phase-synthesized and purified; rat QRFP was synthesized with a free N-terminal Gln residue, and human and mouse QRFP were synthesized with an N-terminal pyroglutamate (33). Cytoplasmic calcium levels were measured by using an FDSS 3000 (Hamamatsu Photonics) with Fura-2/AM. Mice were maintained under controlled temperature (21–23°C) and light (light on 0800–2000 hours) with ad libitum access to food and water. Intracerebroventricular cannulae were stereotaxically implanted into the lateral ventricle under sterile conditions. Food intake was measured as described in ref. 14. Energy expenditure was measured with an O2/CO2 metabolism measuring system (MK-5000RQ; Muromachi, Kikai, Japan), and locomotor activity was measured by using an infrared ray passive sensor system (Supermex; Muromachi, Kikai, Japan) as described in refs. 20 and 28. All animal experiments were performed in accordance with Japanese Physiological Society guidelines for animal care.
All values are reported as means ± SEM. Results were analyzed by unpaired Student’s t test for comparison of two means or one-way ANOVA, followed by post hoc analysis of significance by Fisher’s multiple range test. A probability value of P < 0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Satomi Takahashi and Ruby Nagata for technical assistance. This work was supported through Exploratory Research for Advanced Technology by Japan Science and Technology Agency (Yanagisawa Orphan Receptor Project). J.S. is a recipient of funds from Special Coordination Fund for Science and Technology from Ministry of Education, Culture, Sports, Science, and Technology. M.Y. is an Investigator of the Howard Hughes Medical Institute.
Abbreviations
- GPCR
G protein-coupled receptor
- i.c.v.
intracerebroventricular(ly)
- LH
lateral hypothalamic area
- NPY
neuropeptide Y
- RQ
respiratory quotient.
Note Added in Proof.
Very recently, Moriya et al. (34) reported that chronic administration of QRFP can cause obesity in mice. QRFP-induced weight gain under normal chow diet was marginal in their data, which were basically consistent with ours. However, they further showed that QRFP profoundly enhances body weight gain under moderately high-fat diet, providing support for our speculation that QRFP may be involved in metabolic syndrome.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Lee D. K., Nguyen T., Lynch K. R., Cheng R., Vanti W. B., Arkhitko O., Lewis T., Evans J. F., George S. R., O’Dowd B. F. Gene. 2001;275:83–91. doi: 10.1016/s0378-1119(01)00651-5. [DOI] [PubMed] [Google Scholar]
- 2.Jiang Y., Luo L., Gustafson E. L., Yadav D., Laverty M., Murgolo N., Vassileva G., Zeng M., Laz T. M., Behan J., et al. J. Biol. Chem. 2003;278:27652–27657. doi: 10.1074/jbc.M302945200. [DOI] [PubMed] [Google Scholar]
- 3.Fukusumi S., Yoshida H., Fujii R., Maruyama M., Komatsu H., Habata Y., Shintani Y., Hinuma S., Fujino M. J. Biol. Chem. 2003;278:46387–46395. doi: 10.1074/jbc.M305270200. [DOI] [PubMed] [Google Scholar]
- 4.Chartrel N., Dujardin C., Anouar Y., Leprince J., Decker A., Clerens S., Do-Rego J.-C., Vandesande F., Llorens-Cortes C., Costentin J., et al. Proc. Natl. Acad. Sci. USA. 2003;100:15247–15252. doi: 10.1073/pnas.2434676100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boss V., Talpade D. J., Murphy T. J. J. Biol. Chem. 1996;271:10429–10432. doi: 10.1074/jbc.271.18.10429. [DOI] [PubMed] [Google Scholar]
- 6.Fetissov S. O., Kopp J., Hokfelt T. Neuropeptides. 2004;38:175–188. doi: 10.1016/j.npep.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 7.Bai F. L., Yamano M., Shiotani Y., Emson P. C., Smith A. D., Powell J. F., Tohyama M. Brain Res. 1985;331:172–175. doi: 10.1016/0006-8993(85)90730-9. [DOI] [PubMed] [Google Scholar]
- 8.Dube M. G., Xu B., Kalra P. S., Sninsky C. A., Kalra S. P. Brain Res. 1999;816:34–46. doi: 10.1016/s0006-8993(98)00985-8. [DOI] [PubMed] [Google Scholar]
- 9.Sahu A., Kalra P. S., Crowley W. R., Kalra S. P. Brain Res. 1988;457:376–378. doi: 10.1016/0006-8993(88)90710-x. [DOI] [PubMed] [Google Scholar]
- 10.Sahu A., Dube M. G., Kalra S. P., Kalra P. S. Peptides. 1988;9:1269–1273. doi: 10.1016/0196-9781(88)90191-x. [DOI] [PubMed] [Google Scholar]
- 11.Dube M. G., Sahu A., Phelps C. P., Kalra P. S., Kalra S. P. Brain Res. Bull. 1992;29:865–869. doi: 10.1016/0361-9230(92)90157-s. [DOI] [PubMed] [Google Scholar]
- 12.Baker R. A., Herkenham M. J. Comp. Neurol. 1995;358:518–530. doi: 10.1002/cne.903580405. [DOI] [PubMed] [Google Scholar]
- 13.Rudolf K., Eberlein W., Engel W., Wieland H. A., Willim K. D., Entzeroth M., Wienen W., Beck-Sickinger A. G., Doods H. N. Eur. J. Pharmacol. 1994;271:R11–R113. doi: 10.1016/0014-2999(94)90822-2. [DOI] [PubMed] [Google Scholar]
- 14.Sakurai T., Amemiya A., Ishii M., Matsuzaki I., Chemelli R. M., Tanaka H., Williams S. C., Richardson J. A., Kozlowski G. P., Wilson S., et al. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- 15.Chemelli R. M., Willie J. T., Sinton C. M., Elmquist J. K., Scammell T., Lee C., Richardson J. A., Williams S. C., Xiong Y., Kisanuki Y., et al. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- 16.Erickson J. C., Hollopeter G., Palmiter R. D. Science. 1996;274:1704–1707. doi: 10.1126/science.274.5293.1704. [DOI] [PubMed] [Google Scholar]
- 17.Schwartz M. W., Baskin D. G., Kaiyala K. J., Woods S. C. Am. J. Clin. Nutr. 1999;69:584–596. doi: 10.1093/ajcn/69.4.584. [DOI] [PubMed] [Google Scholar]
- 18.Yanagisawa M., Kurihara H., Kimura S., Tomobe Y., Kobayashi M., Mitsui Y., Yazaki Y., Goto K., Masaki T. Nature. 1988;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- 19.Kojima M., Hosoda H., Date Y., Nakazato M., Matsuo H., Kangawa K. Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 20.Tanaka H., Yoshida T., Miyamoto N., Motoike T., Kurosu H., Shibata K., Yamanaka A., Williams S. C., Richardson J. A., Tsujino N., et al. Proc. Natl. Acad. Sci. USA. 2003;100:6251–6256. doi: 10.1073/pnas.0837789100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yaswen L., Diehl N., Brennan M. B., Hochgeschwender U. Nat. Med. 1999;5:1066–1070. doi: 10.1038/12506. [DOI] [PubMed] [Google Scholar]
- 22.Stanley B. G., Leibowitz S. F. Proc. Natl. Acad. Sci. USA. 1985;82:3940–3943. doi: 10.1073/pnas.82.11.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stanley B. G., Kyrkouli S. E., Lampert S., Leibowitz S. F. Peptides. 1986;7:1189–1192. doi: 10.1016/0196-9781(86)90149-x. [DOI] [PubMed] [Google Scholar]
- 24.Billington C. J., Briggs J. E., Grace M., Levine A. S. Am. J. Physiol. 1991;260:R321–R327. doi: 10.1152/ajpregu.1991.260.2.R321. [DOI] [PubMed] [Google Scholar]
- 25.Hwa J. J., Witten M. B., Williams P., Ghibaudi L., Gao J., Salisbury B. G., Mullins D., Hamud F., Strader C. D., Parker E. M. Am. J. Physiol. 1999;277:R1428–R1434. doi: 10.1152/ajpregu.1999.277.5.R1428. [DOI] [PubMed] [Google Scholar]
- 26.Zarjevski N., Cusin I., Vettor R., Rohner-Jeanrenaud F., Jeanrenaud B. Endocrinology. 1993;133:1753–1758. doi: 10.1210/endo.133.4.8404618. [DOI] [PubMed] [Google Scholar]
- 27.Yamanaka A., Beuckmann C. T., Willie J. T., Hara J., Tsujino N., Mieda M., Tominaga M., Yagami K., Sugiyama F., Goto K., et al. Neuron. 2003;38:701–713. doi: 10.1016/s0896-6273(03)00331-3. [DOI] [PubMed] [Google Scholar]
- 28.Hara J., Beuckmann C. T., Nambu T., Willie J. T., Chemelli R. M., Sinton C. M., Sugiyama F., Yagami K., Goto K., Yanagisawa M., Sakurai T. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- 29.Hara J., Yanagisawa M., Sakurai T. Neurosci. Lett. 2005;380:239–242. doi: 10.1016/j.neulet.2005.01.046. [DOI] [PubMed] [Google Scholar]
- 30.Dunn A. J., Guild A. L., Kramarcy N. R., Ware M. D. Pharmacol. Biochem. Behav. 1981;15:605–608. doi: 10.1016/0091-3057(81)90217-3. [DOI] [PubMed] [Google Scholar]
- 31.Jones D. N., Kortekaas R., Slade P. D., Middlemiss D. N., Hagan J. J. Psychopharmacology (Berlin) 1998;138:124–132. doi: 10.1007/s002130050654. [DOI] [PubMed] [Google Scholar]
- 32.Moreau J. L., Kilpatrick G., Jenck F. NeuroReport. 1997;8:1697–1701. doi: 10.1097/00001756-199705060-00027. [DOI] [PubMed] [Google Scholar]
- 33.Fields G. B., Noble R. L. Int. J. Pept. Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 34.Moriya R., Sano H., Umeda T., Ito M., Takahashi Y., Matsuda M., Ishihara A., Kanatani A., Iwaasa H. Endocrinology. 2006 Mar 16; doi: 10.1210/en.2005-1580. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.