Abstract
Human erythrovirus is a minute, single-stranded DNA virus causing many diseases, including erythema infectiosum, arthropathy, and fetal death. After primary infection, the viral genomes persist in solid tissues. Besides the prototype, virus type 1, two major variants (virus types 2 and 3) have been identified recently, the clinical significance and epidemiology of which are mostly unknown. We examined 523 samples of skin, synovium, tonsil, or liver (birth year range, 1913–2000), and 1,640 sera, by qualitative and quantitative molecular assays for the DNA of human erythroviruses. Virus types 1 and 2 were found in 132 (25%) and 58 (11%) tissues, respectively. DNA of virus type 1 was found in all age groups, whereas that of type 2 was strictly confined to those subjects born before 1973 (P < 0.001). Correspondingly, the sera from the past two decades contained DNA of type 1 but not type 2 or 3. Our data suggest strongly that the newly identified human erythrovirus type 2 as well as the prototype 1 circulated in Northern and Central Europe in equal frequency, more than half a century ago, whereafter type 2 disappeared from circulation. Type 3 never attained wide occurrence in this area during the past ≥70 years. The erythrovirus DNA persistence in human tissues is lifelong and represents a source of information about our past, the Bioportfolio, which, at the individual level, provides a registry of one’s infectious encounters, and at the population level, a database for epidemiological and phylogenetic analyses.
Keywords: epidemiology, gene therapy, parvovirus, phylogeny, single-stranded DNA
Parvovirus B19, of the erythrovirus genus, is the prototypic human pathogen of the Parvoviridae family, encapsidating within its minute (≈20 nm) nonenveloped icosahedral protein shell a single-stranded DNA genome of 5,596 bp (1–4). The virus multiplies restrictively in the erythroid precursor cells of the bone marrow (5), giving rise to high-titer viremia, which, according to sensitive techniques, subsides more slowly than thought previously (6–10). The viral DNA genome was long considered highly stable and the species, phylogenically monolithic (11). Because of recent discoveries, however, we now recognize three major types, the prototype (genotype 1) and two variants (genotypes 2 and 3), diverging from each other in sequence by ≈10% and in the promoter region by >20% (12–15). The molecular biology and clinical significance of the newly recognized erythrovirus types are under active study (13, 15–17). In line with the difficulties in detecting these viruses (16, 18, 19), two previously undescribed human parvoviruses have been identified recently among symptomatic patients (20, 21). Indeed, the evolution rate of these mammalian single-stranded DNA viruses has turned out to be exceptionally high, comparable with that of RNA viruses (22, 23).
After primary infection, the erythroviral genomic DNA remains detectable in human tissues, in both symptomatic and asymptomatic subjects (24–26). Serodiagnostics (i.e., IgG seropositivity; IgG avidity, IgG epitope type specificity, and the absence of IgM ruling out recent primary infection) verified the specificity of the original findings and showed the DNA persistence in synovium to be long (24, 27). Besides revolutionizing the diagnostic criteria of parvovirus arthropathy (28, 29), the tissue persistence has evoked wide interest in the possible etiopathogenic role of these viruses in inflammatory and other chronic diseases (30–36). However, the substantial span of the viral genome persistence as well as its cellular and molecular mechanisms remained undefined.
In the present work, we have determined the extent and duration of persistence of genomic DNA of the different erythrovirus types in a large number of tissue samples and patient sera from the past two decades. We found that erythrovirus genome persistence in human tissues is ubiquitous and lifelong and represents an entity, named the Bioportfolio, which indicates that the newly discovered virus type 2 was actually “older” in occurrence in Central and Northern Europe than the virus prototype and that the type 3 never attained wide circulation in the area during the 70-year observation period from the 1930s to the present day.
Results and Discussion
In our previous studies with a small number of samples, the genomic DNA of erythrovirus type 2 was found in skin but not in synovium (12). We consequently determined the tissue type-specific occurrence of the previously known and the recently discovered virus types 2 and 3 by studying a large number of solid-tissue and serum samples with qualitative and quantitative molecular assays for DNA of all three erythrovirus types.
Virus type 1 DNA was found in all tissue types (skin, synovia, tonsil, and liver), with detection rates varying from 16% in tonsils to 35% in synovia. The universal distribution held also for virus type 2, albeit at a lower frequency (Table 1). Also, the DNA copy levels of the two virus types were similar (data not shown). Virus type 3 was absent from all of the tissues studied. Two tonsils contained erythroviral DNA with a melting point of 63°C (18), which, by sequencing, turned out to be a subvariant of virus type 1. Altogether, these large-scale PCR findings ruled against tissue specificity of any of the erythrovirus types.
Table 1.
Numbers of tissue samples and serum pools studied and of erythrovirus types (DNA) found
| Tissue | Genotype |
Negative | Total | ||
|---|---|---|---|---|---|
| 1 | 2 | 3 | |||
| Skin | 43 (31) | 24 (17) | ND | 73 (52) | 140 |
| Synovia | 30 (35) | 7 (8) | ND | 49 (57) | 86 |
| Tonsil | 36 (16) | 3 (1) | 0 (0) | 181 (82) | 220 |
| Liver | 25 (32) | 24 (31) | ND | 28 (36) | 77 |
| Serum pools | 28 (17) | 0 (0) | 0 (0) | 136 (83) | 164 |
ND, not determined. Numbers in parentheses are percentages.
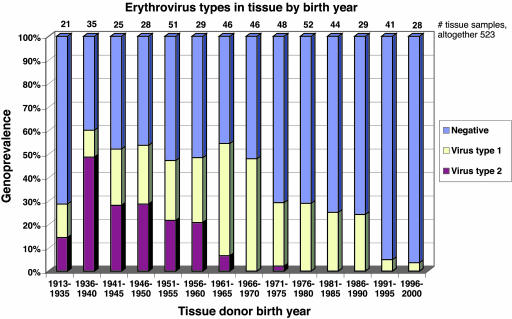
We next grouped the sample donors according to their birth years (Fig. 1). Whereas virus type 1 was seen almost uniformly in subjects of all ages (except small children), virus type 2 was strictly confined to the older age groups (P < 0.001). Among those born in the 1950s or earlier, the genoprevalences of virus types 1 and 2 were similar: 22% (41 of 189) and 28% (53 of 189), respectively. By contrast, among those born in the 1960s, virus type 2 occurred in merely 3% (3 of 92); and among those born in the 1970s, virus type 2 was present in only a single individual (1%; 1 of 100). Among children born during the 1990s, the overall erythrovirus genoprevalence was low (4%; 3 of 69), and all were of virus type 1.
Fig. 1.
Altogether, 303 samples of skin, synovium, or liver were assayed for DNA of erythrovirus types 1 and 2, and 220 samples of tonsil were assayed for DNA of erythrovirus types 1, 2, or 3. The DNA findings were plotted according to the corresponding year of birth, and the arithmetic mean of which for virus type 1 was 1965 and for virus type 2 was 1945. Virus type 3 was not found.
In the sera collected during 1983–1997 from patients with a variety of symptoms, erythrovirus DNA was detected in 17% of the serum pools. According to both melting curve analysis and type-specific PCR, all of the findings were of virus type 1, whereas virus types 2 and 3 were absent from all of the 1,640 sera.
In the literature, the recently discovered erythrovirus variants (types 2 and 3) have been encountered infrequently. Of 120,000 Danish and 140,000 Finnish contemporary blood donors, many had virus type 1 in plasma, but none had type 2 or 3 (13, 18). Also, in the sera of symptomatic European patients, the occurrence of virus type 2 has been sporadic (9, 13–16). Furthermore, in plasma-derived coagulation factor concentrates, type 2 DNA has been detected rarely, yet more often in older preparations (37). Virus type 3 was recently encountered, at a low level, in blood donor samples from Ghana, but not from Malawi, South Africa, or the United Kingdom (38). Occasional French or Brazilian patients carried virus type 3 in their blood or bone marrow (14, 17).
Our approach to investigate single-stranded DNA virus genomes naturally stored in live human tissues, with storage capacity here shown to extend over decades, permitted us to view lifelong variations in the circulation of the three virus types. We found that the “new” variant (virus type 2) is, in fact, “older” than the prototype (virus type 1); the subjects persistently carrying the former were born on average 20 years earlier than those carrying the latter. Therefore, assuming for virus type 2 preferential acquisition during childhood and adolescence, as holds for the virus prototype (39, 40), both types have circulated in Northern and Central Europe widely, and in equal frequency, from the 1930s to the 1950s. However, type 2 appears to have disappeared from wide circulation by the 1970s and remained absent thereafter, as confirmed by our patient sera drawn during the 1980s and 1990s. The absence of virus type 3 from the samples studied (220 tonsils and 1,640 sera) rules out widespread occurrence of this variant in Northern Europe for the past ≥70 years. The occurrence of virus type 3 endemically in Ghana (38) and occasionally in the two Western countries (France and Brazil) with a significant influx of people of West African origin (14, 17) is in line with the absence of type 3 DNA from the tissues and sera of our Nordic population with only small-scale African immigration.
An entirely different explanation for the occurrence of DNA of virus type 2 exclusively among the elderly could be their selective susceptibility for primary infections of this type, as opposed to the preferential susceptibility of children and young adults for the prototype virus (39, 40). However, such an “inverse chronological tropism” is highly unlikely because it (i) would imply continued circulation of virus type 2, a condition ruled out by the present study; and (ii) has not been disclosed for any human virus known.
Indeed, our data indicate that human tissues possess, regarding the genomes of single-stranded DNA viruses, a storage mechanism of lifelong (≥70 years) capacity. For this concept, we propose the term Bioportfolio.
Because of the absence of type 2 genomes from both the sera and tissues of the recent decades, the Bioportfolio cannot be maintained by exogenous replenishment (reinfection). Also, the fact that the genoprevalence of erythroviral DNA was not diminished in the older generations (Fig. 1) points to extreme permanence in human tissue, either since primary infection or by endogenous replenishment. Should the latter storage mechanism be operational, it would need to be nonviremic and nontransmitting, as shown by the absence of virus type 2 from our sera over the past two decades and from the tissues of the young. On the other hand, lifelong DNA persistence without any replication is not entirely easy to envision, in light of the rapidity of in vivo turnover of human cells of most types (41). Indeed, exceptionally slow erythrovirus DNA replication was recently documented in a nonerythroid human cell line (42). In light of the infrequency of human parvovirus reinfections in general, virus types 2 and 3 have been encountered in blood of immunodeficient individuals conspicuously often (13, 14, 16, 17), suggesting that they might have been released from tissue persistence. In accordance with this view, the maintenance of the Bioportfolio could involve a dynamic interplay between viral replication and the immune surveillance of the host, as has been discussed for the occasional erythrovirus persistence in blood (38, 10). We nevertheless believe that an immunological difference does not explain the restrictive occurrence of type 2 viral DNA in tissues of the elderly because of the similarity among the three virus types (15, 43) and the paucity of viremic (15, 16) type 2 infections during recent years, as shown here.
Because of its remarkable longevity, the Bioportfolio has interesting potential utilities. As shown here, at the level of an individual patient, it provides a lifelong registry of one’s infectious encounters. At the global and epidemiological level, it provides a database for analysis of the occurrence and circulation of viruses and their variants. Moreover, in light of the well preserved integrity and full-length coding potential of the persistent macromolecular viral DNA genomes (27), the Bioportfolio might provide the desired long-term permanence for gene therapy vectors, which, in the future, could be designed in accordance with this innate characteristic of the human body.
Methods
Tissue and Serum Samples.
Biopsies of synovial tissue (n = 86) were obtained in Finland during arthroscopy from healthy adults (birth year range, 1931–1992; mean ± SD, 1964 ± 15) with joint trauma and simultaneously from skin of the arthroscopy wound edge (hereafter referred to as skin–synovial tissue pairs). Biopsies of skin (n = 54) were obtained from patients with B19-unrelated dermatological lesions and from healthy hospital or laboratory staff (range, 1913–1991; mean, 1951 ± 19). Biopsies of tonsillar tissue (n = 220) were obtained during tonsillectomy from patients (range, 1929–2000; mean, 1979 ± 15) with tonsillitis or tonsillar hypertrophy (44).
Biopsies of liver tissue (n = 77; range, 1915–1981; mean, 1948 ± 14) were collected in Germany for diagnostic purposes and treated as described in ref. 26. Of these tissues, 53 had been tested previously for virus type 1 (26) and were studied here for virus type 2. Additionally, 19 specimens were obtained in transplantation from the explanted livers and 5 from diagnostic biopsies.
The study included 1,640 sera (donor birth year range, 1907–1993; mean, 1966 ± 19) collected in Finland for virus diagnosis. The specimens comprised (i) 1,393 sera collected during 1983–1997 from 1,393 patients with rash, fever, or other constitutional symptoms, initially studied with negative results for rubella and measles (45), Sindbis virus (46), or hantavirus disease (47); and (ii) 247 sera collected during 1992–1993 from 247 patients with serologically confirmed erythema infectiosum (48, 49). The sera were studied in pools of 10 (10 μl each), taking 20 μl per pool for DNA purification.
Plasmid Clones.
The plasmid clone of virus type 1 (nucleotides 180-5416) has been described by Brunstein et al. (50). Virus type 2 from skin was amplified in five overlapping areas by PCR and cloned (18). After several restriction and ligation steps, a single clone covering nucleotides 202-5147 was constructed. Virus type 3 clone (AJ249437) covering nucleotides 282-5314 was kindly provided by A. Garbarg-Chenon (51). These clones were used for validation of and as positive controls in the PCRs. Nucleotide numbering is according to the GenBank sequence AY504945.
DNA Purification and PCR.
DNA from the skin biopsies, the skin–synovial sample pairs (collected before 2003), and from the serum pools was isolated by proteinase K digestion followed by phenol–chloroform extraction and ethanol precipitation and finally was resuspended into 20 μl of water. DNA from the tonsillar and liver biopsies and from the skin–synovial tissue pairs (collected from 2003 to date) was isolated with the QIAamp DNA Mini kit (Qiagen, Hilden, Germany). All of the DNA preparations were studied by PCR undiluted and at 1:10 dilution.
As shown in Table 2, the DNA preparations from skin, synovia, tonsils, and sera were studied first by nested or non-nested VP1-PCRs detecting all three virus types. The preparations from liver were studied for virus type 1 with nested PCR as described in refs. 37 and 53. In the PCR-positive dermal, synovial, liver, and serum preparations, virus type 2 was identified by the K71-PCR (12). Virus types 1, 2, and 3 in the VP1-PCR-positive tonsillar and serum preparations were distinguished by the Real Art Parvo B19 LC PCR (Artus, Hilden, Germany) followed by melting curve analysis (18). Besides identification, the method quantifies the three virus types.
Table 2.
PCR primers used and their ability to detect erythrovirus types
| PCR | Primers | Virus types detectable (sensitivity) | Skin | Synovium | Tonsil | Liver | Serum |
|---|---|---|---|---|---|---|---|
| Non-nested VP1-PCR (52) | p6: GGAGAATCATTTGTCGGAAG | 1, 2, and 3 (5 DNA copies each)* | X | X | |||
| p5: AGGCTTGTGTAAGTCTTCAC | |||||||
| Nested VP1-PCR (24) | p6: GGAGAATCATTTGTCGGAAG | 1, 2, and 3 (15 DNA copies each) | X | X | |||
| p3: CTTCTGCAGAATTAACTGAAGTC | |||||||
| p8: TGTGCTTACCTGTCTGGATTG | |||||||
| p5: AGGCTTGTGTAAGTCTTCAC | |||||||
| Nested K71-PCR (12) | of: TTTACTGAAGACAAATGGAAGT | 2 (1.5 DNA copies) | X | X | X | X | X |
| or: CACTGGGACAGTTTTGGCAATA | |||||||
| if: AGTGGATTTCAATCAATATACA | |||||||
| ir: TCATAATTTTGGCATAATAATAG | |||||||
| Genotype 1 PCR (53) | p1: AATACACTGTGGTTTTATGGGCCG | 1, 3 (1.5 and 15 copies, resp.) | X | ||||
| p6: CCATTGCTGGTTATAACCACAGGT | |||||||
| p2: AATGAAAACTTTCCATTTAATGATGTAG | |||||||
| p5: CTAAAATGGCTTTTGCAGCTTCTAC | |||||||
| Real Art Parvo B19 LC PCR (18) | VP1 area | 1, 2, and 3 (5 DNA copies each)† | X | X |
*For the type 3 variant D91.1., 500 DNA copies.
†For the type 3 variant D91.1., 5,000 DNA copies.
The detection sensitivities of all five of the PCRs were examined with the plasmid clones in a series of 10-fold dilutions and with constant template volumes of 1.5 and 5 μl for the nested and non-nested PCRs, respectively. All of the PCRs were observed to amplify their corresponding targets at a very high efficiency and with detection sensitivities within 1 log (Table 2). This finding, along with the highest sensitivity of the K71-PCR for virus type 2, rules out the possibility of a detection artifact caused by differences in sensitivity between detection of genotype 1 and 2 in tissues with very low viral load. Because of the high sensitivity of all of the PCRs, stringent precautions were taken to avoid contamination. As before (12, 24, 26), the samples and PCR mixtures were handled under laminar flow hoods in separate rooms, using disposable racks and aerosol-resistant tips. Water was used as a negative control during DNA isolation and in each PCR run.
DNA Sequences and Statistical Analysis.
For additional confirmation of virus typing, some of the PCR amplicons of this study (all from the livers) were sequenced by cycle sequencing at the Haartman Institute (University of Helsinki) core facility or the Institute of Medical Microbiology, Immunology, and Parasitology (University of Bonn), with ABI BigDye Terminator kits (Applied Biosystems). The reactions were run on an ABI 3100 capillary sequencer or ABI PRISM 3130 Genetic Analyzer, respectively.
Statistical analysis was performed with statxact (Cytel, San Diego) with a linear-by-linear trend test.
The tissue samples were obtained with informed consent, and the studies were approved by the Ethical Committee of the Helsinki University Central Hospital.
Acknowledgments
We thank the many volunteers for participation in this study; Heidi Bondén and Helena Service for cloning; Lea Hedman, Laura Pukkila, Maria Pesonen, Sesilja Aranko, and Ulrike Reber for assistance with the clinical samples; Seppo Sarna for statistical analysis; and Malcolm Richardson for language revision. The study was supported by Commission of the European Community Grant QLK2-CT-2001-00877, Finnish Academy Project 76132, the Jenny and Antti Wihuri Foundation, the Medical Society of Finland (FLS), the Finnish Technology Advancement Fund, and the Helsinki University Central Hospital Research and Education Fund.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Siegl G., Bates R. C., Berns K. I., Carter B. J., Kelly D., Kurstak E., Tattersall P. Intervirology. 1985;23:61–73. doi: 10.1159/000149587. [DOI] [PubMed] [Google Scholar]
- 2.Deiss V., Tratschin J. D., Weitz M., Siegl G. Virology. 1990;175:247–254. doi: 10.1016/0042-6822(90)90205-6. [DOI] [PubMed] [Google Scholar]
- 3.Weigel-Kelley K. A., Yoder M. C., Srivastava A. Blood. 2003;102:3927–3933. doi: 10.1182/blood-2003-05-1522. [DOI] [PubMed] [Google Scholar]
- 4.Kaufmann B., Simpson A. A., Rossmann M. G. Proc. Natl. Acad. Sci. USA. 2004;101:11628–11633. doi: 10.1073/pnas.0402992101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ozawa K., Kurtzman G., Young N. Science. 1986;233:883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- 6.Musiani M., Zerbini M., Gentilomi G., Plazzi M., Gallinella G., Venturoli S. J. Infect. Dis. 1995;172:1360–1363. doi: 10.1093/infdis/172.5.1360. [DOI] [PubMed] [Google Scholar]
- 7.Knöll A., Louwen F., Kochanowski B., Plentz A., Stussel J., Beckenlehner K., Jilg W., Modrow S. J. Med. Virol. 2002;67:259–266. doi: 10.1002/jmv.2216. [DOI] [PubMed] [Google Scholar]
- 8.Lindblom A., Isa A., Norbeck O., Wolf S., Johansson B., Broliden K., Tolfvenstam T. Clin. Infect. Dis. 2005;41:1201–1203. doi: 10.1086/444503. [DOI] [PubMed] [Google Scholar]
- 9.Enders M., Schalasta G., Baisch C., Weidner A., Pukkila L., Kaikkonen L., Lankinen H., Hedman L., Söderlund-Venermo M., Hedman K. J. Clin. Virol. 2006;35:400–406. doi: 10.1016/j.jcv.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 10.Lefrére J.-J., Servant-Delmas A., Candotti D., Mariotti M., Thomas I., Brossard Y., Lefrére F., Girot R., Allain J.-P., Laperche S. Blood. 2005;106:2890–2895. doi: 10.1182/blood-2005-03-1053. [DOI] [PubMed] [Google Scholar]
- 11.Hemauer A., von Poblotzki A., Gigler A., Cassinotti P., Siegl G., Wolf H., Modrow S. J. Gen. Virol. 1996;77:1781–1785. doi: 10.1099/0022-1317-77-8-1781. [DOI] [PubMed] [Google Scholar]
- 12.Hokynar K., Söderlund-Venermo M., Pesonen M., Ranki A., Kiviluoto O., Partio E. K., Hedman K. Virology. 2002;302:224–228. doi: 10.1006/viro.2002.1673. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen Q., Wong S., Heegard E., Brown K. Virology. 2002;301:374–380. doi: 10.1006/viro.2002.1585. [DOI] [PubMed] [Google Scholar]
- 14.Servant A., Laperche S., Lallemand F., Marinho V., De Saint Maur G., Meritet J. F., Garbarg-Chenon A. J. Virol. 2002;76:9124–9134. doi: 10.1128/JVI.76.18.9124-9134.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blümel J., Eis-Hübinger A. M., Stühler A., Bönsch C., Gessner M., Löwer J. J. Virol. 2005;79:14197–14206. doi: 10.1128/JVI.79.22.14197-14206.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liefeldt L., Plentz A., Klempa B., Kershaw O., Endres A. S., Raab U., Neumayer H. H., Meisel H., Modrow S. J. Med. Virol. 2005;75:161–169. doi: 10.1002/jmv.20251. [DOI] [PubMed] [Google Scholar]
- 17.Sanabani S., Neto W. K., Pereira J., Sabino E. C. J. Clin. Microbiol. 2006;44:604–606. doi: 10.1128/JCM.44.2.604-606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hokynar K., Norja P., Laitinen H., Palomäki P., Garbarg-Chenon A., Ranki A., Hedman K., Söderlund-Venermo M. J. Clin. Microbiol. 2004;42:2013–2019. doi: 10.1128/JCM.42.5.2013-2019.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baylis S. A., Shah N., Minor P. D. J. Virol. Methods. 2004;121:7–16. doi: 10.1016/j.jviromet.2004.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Jones M. S., Kapoor A., Lukashov V. V., Simmonds P., Hecht F., Delwart E. J. Virol. 2005;79:8230–8236. doi: 10.1128/JVI.79.13.8230-8236.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allander T., Tammi M. T., Eriksson M., Bjerkner A., Tiveljung-Lindell A., Andersson B. Proc. Natl. Acad. Sci. USA. 2005;102:12891–12896. doi: 10.1073/pnas.0504666102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lopez-Bueno A., Mateu M. G., Almendral J. M. J. Virol. 2003;77:2701–2708. doi: 10.1128/JVI.77.4.2701-2708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shackelton L. A., Parrish C. R., Truyen U., Holmes E. C. Proc. Natl. Acad. Sci. USA. 2005;103:379–384. doi: 10.1073/pnas.0406765102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Söderlund M., von Essen R., Haapasaari J., Kiistala U., Kiviluoto O., Hedman K. Lancet. 1997;349:1063–1065. doi: 10.1016/S0140-6736(96)09110-6. [DOI] [PubMed] [Google Scholar]
- 25.Cassinotti P., Burtonboy G., Fopp M., Siegl G. J. Med. Virol. 1997;53:229–232. [PubMed] [Google Scholar]
- 26.Eis-Hübinger A. M., Reber U., Abdul-Nour T., Glatzel U., Lauschke H., Pütz U. J. Med. Virol. 2001;65:395–401. doi: 10.1002/jmv.2047. [DOI] [PubMed] [Google Scholar]
- 27.Hokynar K., Brunstein J., Söderlund-Venermo M., Kiviluoto O., Partio E. K., Konttinen Y., Hedman K. J. Gen. Virol. 2000;81:1017–1025. doi: 10.1099/0022-1317-81-4-1017. [DOI] [PubMed] [Google Scholar]
- 28.Kingsley G. Lancet. 1997;349:1038–1039. doi: 10.1016/S0140-6736(97)22015-5. [DOI] [PubMed] [Google Scholar]
- 29.Söderlund-Venermo M., Hokynar K., Nieminen J., Rautakorpi H., Hedman K. Pathol. Biol. 2002;50:307–316. doi: 10.1016/s0369-8114(02)00307-3. [DOI] [PubMed] [Google Scholar]
- 30.Takahashi Y., Murai C., Shibata S., Munakata Y., Ishii T., Ishii K., Saitoh T., Sawai T., Sugamura K., Sasaki T. Proc. Natl. Acad. Sci. USA. 1998;95:8227–8232. doi: 10.1073/pnas.95.14.8227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tolfvenstam T., Papadogiannakis N., Andersen A., Akre O. J. Gen. Virol. 2002;83:2321–2324. doi: 10.1099/0022-1317-83-9-2321. [DOI] [PubMed] [Google Scholar]
- 32.Wong S., Young N. S., Brown K. E. J. Infect. Dis. 2003;187:1581–1586. doi: 10.1086/374781. [DOI] [PubMed] [Google Scholar]
- 33.Mehraein Y., Lennerz C., Ehlhardt S., Remberger K., Ojak A., Zang K. D. Mod. Pathol. 2004;17:781–789. doi: 10.1038/modpathol.3800119. [DOI] [PubMed] [Google Scholar]
- 34.LaMonte A. C., Paul M. E., Read J. S., Frederick M. M., Erdman D. D., Han L. L., Anderson L. J. J. Infect. Dis. 2004;189:847–851. doi: 10.1086/381899. [DOI] [PubMed] [Google Scholar]
- 35.Poole B. D., Karetnyi Y. V., Naides S. J. J. Virol. 2004;78:7775–7783. doi: 10.1128/JVI.78.14.7775-7783.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuhl U., Pauschinger M., Noutsias M., Seeberg B., Bock T., Lassner D., Poller W., Kandolf R., Schultheiss H. P. Circulation. 2005;111:887–893. doi: 10.1161/01.CIR.0000155616.07901.35. [DOI] [PubMed] [Google Scholar]
- 37.Schneider B., Becker M., Brackmann H. H., Eis-Hübinger A. M. Thromb. Haemostasis. 2004;92:838–845. doi: 10.1160/TH04-04-0229. [DOI] [PubMed] [Google Scholar]
- 38.Candotti D., Etiz N., Parsyan A., Allain J. P. J. Virol. 2004;78:12169–12178. doi: 10.1128/JVI.78.22.12169-12178.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nunoue T., Okochi K., Mortimer P. P., Cohen B. J. J. Pediatr. 1985;107:38–40. doi: 10.1016/s0022-3476(85)80610-7. [DOI] [PubMed] [Google Scholar]
- 40.Adler S. P., Manganello A. M., Koch W. C., Hempfling S. H., Best A. M. J. Infect. Dis. 1993;168:361–368. doi: 10.1093/infdis/168.2.361. [DOI] [PubMed] [Google Scholar]
- 41.Spalding K. L., Bhardwaj R. D., Buchholz B. A., Druid H., Frisén J. Cell. 2005;122:133–134. doi: 10.1016/j.cell.2005.04.028. [DOI] [PubMed] [Google Scholar]
- 42.Munakata Y., Kato I., Saito T., Kodera T., Ishii K. K., Sasaki T. Virology. 2006;345:251–257. doi: 10.1016/j.virol.2005.09.040. [DOI] [PubMed] [Google Scholar]
- 43.Parsyan A., Kerr S., Owusu-Ofori S., Elliott G., Allain J.-P. J. Clin. Microbiol. 2006;44:1367–1375. doi: 10.1128/JCM.44.4.1367-1375.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen R. W., Waterboer T., Leivo I., Pawlita M., Vaheri A., Aaltonen L. M. J. Clin. Microbiol. 2005;43:1408–1410. doi: 10.1128/JCM.43.3.1408-1410.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Davidkin I., Valle M., Peltola H., Hovi T., Paunio M., Roivainen M., Linnavuori K., Jokinen S., Leinikki P. J. Infect. Dis. 1998;178:1567–1570. doi: 10.1086/314513. [DOI] [PubMed] [Google Scholar]
- 46.Brummer-Korvenkontio M., Vapalahti O., Kuusisto P., Saikku P., Manni T., Koskela P., Nygren T., Brummer-Korvenkontio H., Vaheri A. Epidemiol. Infect. 2002;129:335–345. doi: 10.1017/s0950268802007409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hedman K., Vaheri A., Brummer-Korvenkontio M. Lancet. 1991;338:1353–1356. doi: 10.1016/0140-6736(91)92235-t. [DOI] [PubMed] [Google Scholar]
- 48.Söderlund M., Brown K. E., Meurman O., Hedman K. J. Clin. Microbiol. 1992;2:305–311. doi: 10.1128/jcm.30.2.305-311.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Söderlund M., Brown C. S., Cohen B. J., Hedman K. J. Infect. Dis. 1995;171:710–713. doi: 10.1093/infdis/171.3.710. [DOI] [PubMed] [Google Scholar]
- 50.Brunstein J., Söderlund-Venermo M., Hedman K. Virology. 2002;274:284–291. doi: 10.1006/viro.2000.0460. [DOI] [PubMed] [Google Scholar]
- 51.Nguyen Q. T., Sifer C., Schneider V., Allaume X., Servant A., Bernaudin F., Auguste V., Garbarg-Chenon A. J. Clin. Microbiol. 1999;37:2483–2487. doi: 10.1128/jcm.37.8.2483-2487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Söderlund M., Ruutu P., Ruutu T., Asikainen K., Franssila R., Hedman K. Scand. J. Infect. Dis. 1997;29:129–135. doi: 10.3109/00365549709035872. [DOI] [PubMed] [Google Scholar]
- 53.Eis-Hübinger A. M., Sasowski U., Brackmann H. H., Kaiser R., Matz B., Schneweis K. E. Thromb. Haemostasis. 1996;76:1120. (lett.) [PubMed] [Google Scholar]