Abstract
Pituitary adenylate cyclase-activating polypeptide (PACAP) has been reported to decrease ischemic neuronal damage and increase IL-6 secretion in rats. However, the mechanisms underlying neuroprotection are still to be fully elucidated. The present study was designed to investigate the role played by PACAP and IL-6 in mediating neuroprotection after ischemia in a null mouse. Infarct volume, neurological deficits, and cytochrome c in cytoplasm were higher in PACAP+/− and PACAP−/− mice than in PACAP+/+ animals after focal ischemia, although the severity of response was ameliorated by the injection of PACAP38. A decrease in mitochondrial bcl-2 was also accentuated in PACAP+/− and PACAP−/− mice, but the decrease could be prevented by PACAP38 injection. PACAP receptor 1 (PAC1R) immunoreactivity was colocalized with IL-6 immunoreactivity in neurons, although the intensity of IL-6 immunoreactivity in PACAP+/− mice was less than that in PACAP+/+ animals. IL-6 levels increased in response to PACAP38 injection, an effect that was canceled by cotreatment with the PAC1R antagonist. However, unlike in wild-type controls, PACAP38 treatment did not reduce the infarction in IL-6 null mice. To clarify the signaling pathway associated with the activity of PACAP and IL-6, phosphorylated STAT (signal transducer and activator of transcription) 3, ERK (extracellular signal-regulated kinase), and AKT levels were examined in PACAP+/− and IL-6 null mice after ischemia. Lower levels of pSTAT3 and pERK were observed in the PACAP+/− mice, whereas a reduction in pSTAT3 was recorded in the IL-6 null mice. These results suggest that PACAP prevents neuronal cell death after ischemia via a signaling mechanism involving IL-6.
Keywords: bcl-2, extracellular signal-regulated kinase, ischemia, pituitary adenylate cyclase-activating polypeptide-specific receptor, signal transducer and activator of transcription 3
Pituitary adenylate cyclase-activating polypeptide (PACAP), first isolated from ovine hypothalamus, belongs to the secretin/glucagon/vasoactive intestinal peptide (VIP) superfamily and exists in two amidated forms, PACAP38 and PACAP27 (1). PACAP is considered to play an important role in mediating antiinflammatory responses (2, 3) as well as vasodilation and bronchodilation (4–6) through three specific receptors: PACAP receptor 1 (PAC1R) and two VIP/PACAP receptors (7). PACAP has also been shown to have neuroprotective effects at very low concentrations, with intracerebroventricular (i.c.v.) infusion of PACAP in the rat preventing neuronal loss in the hippocampus and decreasing the extent of infarction after global and focal ischemia (8–11). Given that PACAP can cross the blood–brain barrier by a saturable mechanism (12, 13), its i.v. injection has also been demonstrated to prevent ischemic neuronal damage in transient global and focal ischemia (8, 11). Various in vitro studies involving exposure to β-amyloid (14), ceramide (15, 16), hydroxyl peroxide (17), and ethanol (18, 19) have indicated that the neurotrophic PACAP signaling pathway is increased or activated by means of phosphorylation of the extracellular signal-regulated kinase (ERK) type of mitogen-activated protein kinase (15–17), phosphatidylinositol 3′-OH kinase (18), and protein kinase A (18, 20) but is decreased or inhibited by means of the phosphorylation of cJun N-terminal kinase (JNK) (15) and the caspases cascade (14, 17). PACAP suppresses cell death indirectly by the induction of BDNF in neuronal culture by glutamate toxicity (21, 22). Moreover, PACAP injection has been demonstrated to increase IL-6 levels in cultured astrocytes, folliculo stellate cells, and the pituitary (1, 23, 24). Similarly, we have previously reported that i.c.v. injection of PACAP induces IL-6 secretion in cerebrospinal fluid (CSF) and inhibits JNK and p38 phosphorylation in the hippocampus after cardiac arrest in the rat (25). However, the role of endogenous PACAP in mediating neuroprotection after ischemia in vivo, together with the underlying regulatory mechanisms, remains to be elucidated.
IL-6, a pleiotrophic cytokine, regulates both innate and acquired immunity and inflammatory responses (26–28). IL-6 and its receptor (IL-6R) are expressed in neurons after ischemia (29–31), and it has been shown that i.c.v. injection of IL-6 in rats decreases the infarct volume (32), and endogenous IL-6 also plays a neuroprotective role through thermoregulation (33). More recently, IL-6R antibody injection increased infarct volume after ischemia (34). Together, these findings suggest that IL-6 plays a pivotal role in mediating neuronal survival. IL-6 binds to the IL-6R and gp130 heterodimer, which is involved in the activation of phosphatidylinositol 3′-OH kinase/AKT (the AKT8 virus oncogene cellular homolog), Janus kinase/STAT (signal transducers and activators of transcription), and ERK pathways, which are known to participate in antiapoptosis (27, 34).
Although numerous papers have reported a neuroprotective role for PACAP and IL-6, there is still a lack of direct evidence. In this study, we first investigated the involvement of endogenous PACAP that exists in the brain on neuroprotection by using a PACAP null mouse. We then demonstrated, by using IL-6 null mice, that PACAP is involved in the generation of IL-6 through PAC1R and that PACAP decreases ischemic neuronal cell death in association with the IL-6 signaling pathway.
Results
PACAP Lessens Severity of Ischemic Neuronal Cell Death.
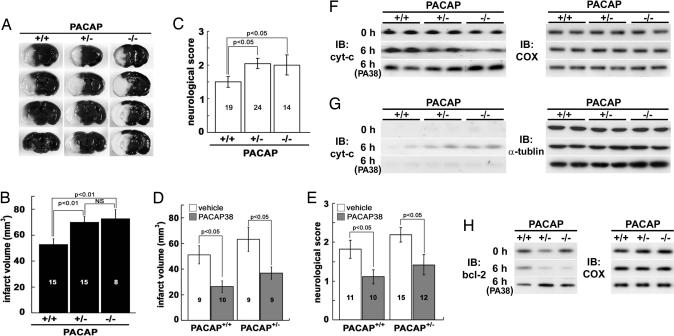
To evaluate the role of endogenous PACAP during ischemia in the mouse, the infarct volume at 24 h and the neurological deficit score at 2 h after permanent middle cerebral artery occlusion (pMCAO) were determined in wild-type, heterozygous, and PACAP null (PACAP+/+, PACAP+/−, and PACAP−/−, respectively) mice. The infarct volume in both the PACAP+/− and PACAP−/− mice (P < 0.01) was significantly larger than that in the PACAP+/+ mice (Fig. 1A and B), with the former groups also recording larger neurological deficit scores (Fig. 1C). To confirm the effects of endogenous PACAP on the brain, PACAP38 (1 pmol) was administrated into the lateral ventricle as compensation for endogenous PACAP in PACAP+/+ and PACAP+/− mice immediately after pMCAO. As shown in Fig. 1 D and E, both the infarct volume and neurological deficit score improved significantly in the PACAP38-treaed groups.
Fig. 1.
PACAP null mice exhibit ischemic neuronal damage that is suppressed by infusion of PACAP38. (A) Areas of brain infarction in PACAP+/+, PACAP+/−, and PACAP−/− mice 24 h after pMCAO appear white. (B) Infarct volumes 24 h after pMCAO in PACAP+/− and PACAP−/− mice are larger than those of PACAP+/+ mice. (C) PACAP+/− and PACAP−/− mice display significantly larger neurological deficit scores than PACAP+/+ mice (P < 0.05). (D and E) Infarct volume (D) and neurological deficit (E) scores after pMCAO are improved by i.c.v. injection of PACAP38 (P < 0.05 for each). Each value is the mean ± SE. (F and G) Cytochrome c (cyt-c) levels in mitochondrial (F) and cytoplasmic (G) fraction revealed by immunoblotting (IB) before (0 h) and 6 h after pMCAO in PACAP null mice (n = 5–6 per time point). Most of the cyt-c signals are detected in mitochondrial fraction at 0 h (top rows). PACAP+/− and PACAP−/− mice exhibit a cyt-c level increase in cytoplasmic fraction (middle rows) compared with PACAP+/+ mice 6 h after pMCAO. The increase is suppressed by PACAP38 (PA38) injection (bottom rows). (H) Bcl-2 levels in the mitochondrial fraction in PACAP+/− and PACAP−/− mice are lower than those in PACAP+/+ mice at 6 h (middle rows) after pMCAO (n = 4 per time point). The decreases in bcl-2 are cancelled by PACAP38 injection (bottom rows). Cytochrome oxidase subunit I and α-tubulin served as internal controls for the mitochondrial and cytoplasmic fractions, respectively (F–H Right).
Cytochrome c Release and bcl-2 Levels After MCAO.
Recently, the release of cytochrome c into cytoplasm has been suggested as a key event of cell death induction, and bcl-2 is shown to have a role in the regulation of the switching as an antiapoptotic factor (35–37). To investigate the pathway underlying cell death, cytochrome c release from mitochondria to cytoplasm and bcl-2 level in mitochondria before (0 h) and 6 h after pMCAO were determined by immunoblotting after cell fractionation (Fig. 1 F–H). Cytochrome c signals were localized mostly in the mitochondrial fraction, and few were detected in cytoplasmic fraction at 0 h (Fig. 1F). However, 6 h later, the cytochrome c levels in the cytoplasmic fraction of PACAP+/− and PACAP−/− mice were higher than those in PACAP+/+ mice (Fig. 1G; n = 5–6). Expressions of bcl-2 were observed in the mitochondrial fraction for each genotype of PACAP null mice before ischemia (Fig. 1H). The bcl-2 levels in PACAP+/− and PACAP−/− mice decreased at 6 h after ischemia, and the bcl-2 levels in PACAP−/− mice decreased significantly in comparison with those in PACAP+/+ mice (n = 4; data not shown). These effects were abolished by administration of PACAP38 (1 pmol i.c.v.) immediately after pMCAO (Fig. 1 G and H).
IL-6 Expression in Response to PACAP After MCAO.
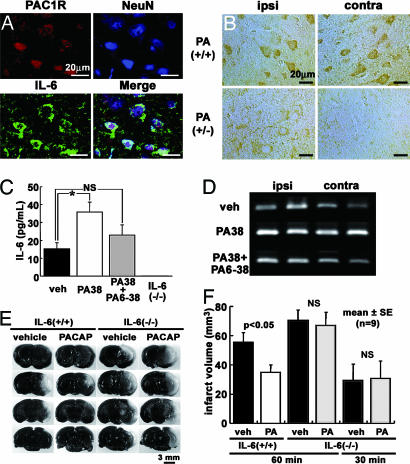
Previously, we have suggested that neuroprotective pathways of PACAP are involved in the regulation of IL-6 secretion through PAC1R after ischemia (25). To investigate whether the neuroprotective effect of PACAP involved the regulation of IL-6, costaining for PAC1R and IL-6 with a neuronal marker, NeuN, was performed. Little IL-6 immunoreactivity (ir) was observed in the brain at 0 h. However, IL-6 ir increased throughout both hemispheres after an ischemic insult (data not shown). As illustrated in Fig. 2A, both PAC1R- and IL-6 ir were colocalized with NeuN stained neurons 4 h after pMCAO. Furthermore, the intensity of IL-6 ir in PACAP+/− mice was less than that in PACAP+/+ mice in the neocortex of both hemispheres (Fig. 2B).
Fig. 2.
PACAP decreases ischemic neuronal cell death in association with the IL-6 generation pathway through PAC1R. (A) IL-6 ir (green) is colocalized with PAC1R (red) in neurons (blue) identified by using the neuronal marker NeuN. (B) Intensity of IL-6 ir in PACAP+/− (PA+/−) mice is less than that in PACAP+/+ mice (PA+/+) in ipsilateral (ipsi) and contralateral (contra) hemispheres. (C) IL-6 levels in CSF as determined by B9 bioassay are significantly increased after PACAP38 (PA38) infusion (i.v.) compared with vehicle (veh) infusion. This response is suppressed by administration of PACAP38 plus PACAP6-38 (PA38+PA6-38). No effect was observed in IL-6−/− mice. (D) IL-6 mRNA expression at 4 h after MCAO, as determined by RT-PCR, is higher in the brain after PACAP38 infusion than after vehicle infusion and is reduced by administration of PACAP plus PACAP6-38. (E) Images of the brain in IL-6+/+ and IL-6−/− mice 24 h after tMCAO. (F) Infarct volume in IL-6+/+ mice treated with PACAP38 is less than that in animals treated with vehicle. In contrast, no PACAP38-mediated neuroprotection is apparent in IL-6 −/− mice at either 30 or 60 min tMCAO.
It has been reported that PACAP38 can cross the blood–brain barrier (12, 13) and that i.v. application of PACAP38 also decreases neuronal cell death (8, 11). Generation of IL-6 after administration of PACAP38 by i.v. injection was evaluated in wild-type (IL-6+/+) mice 4 h after transient MCAO (tMCAO). When measured in CSF with a B9 bioassay (Fig. 2C), the IL-6 levels in PACAP38-treated mice were found to be significantly higher than those in vehicle-treated animals. Coinfusion of PACAP38 with the PAC1R antagonist, PACAP6-38, abolished this effect. No response was observed in IL-6 null (IL-6−/−) mice. PACAP38 treatment also increased IL-6 mRNA expression in the brain, as determined by RT-PCR, whereas cotreatment with PACAP6-38 partially suppressed this response (Fig. 2D).
Effect of PACAP38 Treatment on Infarct Volume in IL-6 Knockout Mice.
Having shown that PACAP was involved in the expression of IL-6 after ischemia, we next evaluated whether this effect was the basis for the neuroprotective effect of PACAP (Fig. 2 E and F). i.v. injection of PACAP38 in IL-6+/+ mice significantly decreased the infarct volume after tMCAO compared with the response in vehicle-treated animals (31.8 ± 21.4 vs. 59.7 ± 21.3 mm3; P < 0.01). However, when IL-6−/− mice were treated with PACAP38, no reduction of infarct volume was observed at either 0.5 or 1 h after ischemia. To check whether vasodilatation caused by PACAP38 was a contributory factor in this outcome, regional cerebral blood flow was evaluated in IL-6+/+ and IL-6−/− mice with or without PACAP38 treatment, revealing no significant differences (data not shown). Thus, the neuroprotective effect of PACAP appeared to be mediated by means of the regulation of IL-6.
Contribution of PACAP and IL-6 to the Intracellular Signaling Pathway.
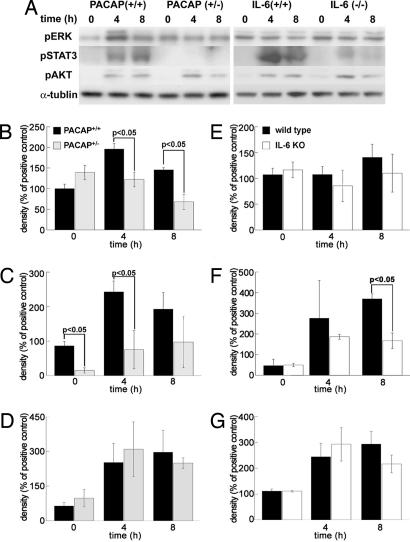
To evaluate the role of PACAP and IL-6 in antiapoptotic signaling pathways, phosphorylated (p) STAT3, ERK, and AKT levels in the brain of PACAP+/− or IL-6−/− mice were compared with those in wild-type animals after pMCAO (Fig. 3).
Fig. 3.
PACAP and IL-6 regulate antiapoptotic signals. (A) Representative immunoblotting signals before and after tMCAO in PACAP (Left) or IL-6 (Right) null mice. (B–G) Effect of PACAP and IL-6 on pERK (B and E), pSTAT3 (C and F), and pAKT (D and G) after pMCAO. In PACAP+/− mice (gray bars), pERK (B) and pSTAT3 (C) are significantly reduced compared with levels in PACAP+/+ mice (black bars). In IL-6−/− mice (white bars), pSTAT3 (F) levels are less than those in IL-6+/+ mice (black bars). There are no significant differences in pAKT (D and G). Each value is the mean ± SE (n = 4–8).
In PACAP+/− mice, pSTAT3 and pERK levels were significantly decreased, whereas there was no significant change in the level of pAKT (Fig. 3 A–D). In contrast, in IL-6−/− mice, the level of pSTAT3 was significantly lower than that in IL-6+/+ animals, but pAKT and pERK levels were not significantly different (Fig. 3 A and E–G).
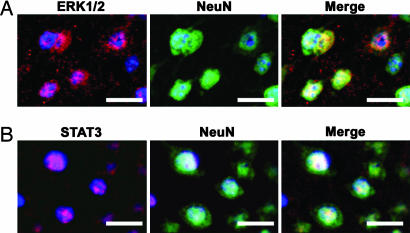
The localization of pERK and pSTAT3 was then determined by costaining with a neuronal marker, NeuN. As shown in Fig. 4, both the pERK ir and pSTAT3 ir were clearly observed in neuronal nuclei.
Fig. 4.
Coimmunostaining of pERK (A) and pSTAT3 (B) with the neuron marker NeuN after pMCAO. Coimmunostaining for pERK and pSTAT3 with NeuN reveals that phosphorylation of the intracellular signaling factors are localized in nuclei of neurons after pMCAO. Blue is DAPI staining as counterstaining of nuclei.
Discussion
In this study, we have shown by using PACAP null mice that (i) endogenous PACAP plays a critical role in the prevention of ischemic neuronal cell death in vivo and that (ii) PACAP suppresses cytochrome c release by means of the regulation of bcl-2 in mitochondria. Furthermore, we determined by using both PACAP and IL-6 null mice that (iii) PACAP regulates pERK directly and pSTAT3 indirectly by the generation of IL-6 through PAC1R in neurons.
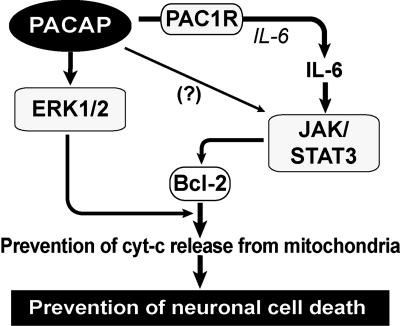
It has previously been shown that a very low concentration of exogenously applied PACAP suppresses neuronal cell death in rat global and local brain ischemia models (8–11). However, to our knowledge, the role of endogenous PACAP and its effect in a mouse ischemia model had not been addressed. In this study, both PACAP null mice and PACAP+/− animals subjected to pMCAO displayed a significant increase in neuronal damage, as evaluated by neurological deficit score and infarct volume. However, this deterioration could be ameliorated in PACAP null mice by PACAP38 treatment. These results indicate that endogenous PACAP participates in the prevention of neuronal damage. Previously, the targets of PACAP in relation to neuroprotection have been poorly understood, although the involvement of some signaling and neurotrophic factors has been assumed based on in vitro paradigms. Recently, it has been suggested that the release of cytochrome c into cytoplasm plays a central role in cell death induction and that the bcl-2 family regulates the switching of life or death (35–37). In this present study, we clearly demonstrated that bcl-2 levels in mitochondria decreased dramatically in PACAP+/− and PACAP−/− mice after ischemia but recovered in response to PACAP38 treatment. Moreover, the cytochrome c level in cytoplasm after ischemia was increased in the PACAP null mice but was reduced after administration of PACAP38. These findings were explored further in vivo to elucidate the underlying mechanism, focusing on the possible involvement of IL-6, a known neurotrophic cytokine (27, 32) that, as we previously demonstrated, is released in response to PACAP (10, 23, 25). IL-6 ir was shown to colocalize with PAC1R ir in neurons, with the intensity of IL-6 ir in PACAP+/− mice being less than that in PACAP+/+ animals. IL-6 levels were increased after PACAP38 injection, a response that was inhibited in the presence of a PAC1R antagonist. However, PACAP38 injection in IL-6−/− mice did not decrease the infarct volume as greatly as it did in IL-6+/+ mice. Taken together, these results strongly suggested that the neuroprotective effect of PACAP is associated with IL-6 signaling through PAC1R, leading us to investigate the phosphorylation of IL-6 signaling factors such as STAT3, ERK, and AKT in PACAP and IL-6 null mice. In IL-6 null mice, only pSTAT3 was decreased, whereas in PACAP null mice, both pSTAT3 and pERK levels were lower. Recently, blocking the IL-6 receptor has been shown to decrease levels of pSTAT3 (34) but not pERK and pAKT. Moreover, injection of leukemia inhibitory factor, a member of the IL-6 family, leads to an increase in pSTAT3, but not pERK or pAKT, after MCAO (38). pSTAT3 has been shown to increase the expression of bcl-2 (27, 39). Thus, PACAP might express bcl-2 by regulating STAT3 indirectly via the IL-6 signaling pathway. In contrast, we could not observe a direct contribution of pERK on suppression of neuronal damage because the effect of PACAP38 was canceled in IL-6−/− mice. However, a number of studies have shown that pERK is involved in augmenting bcl-2 phosphorylation and serves as an antiapoptotic factor (40, 41). It is presumed that bcl-2 expression decreases in IL-6−/− mice, given the decrease in pSTAT3 in these animals and the fact that bcl-2 phosphorylation by pERK is weakened by the reduction in bcl-2. Thus, both the ERK and STAT3 signaling pathways might be acting synergistically to enhance bcl-2 expression and phosphorylation, thus reducing cytochrome c release from mitochondria and preventing neuronal cell death (Fig. 5).
Fig. 5.
Schematic diagram illustrating PACAP-mediated neuroprotection after ischemia. PACAP is involved in the expression of IL-6 through PAC1R. PACAP phosphorylates ERK directly and STAT3 indirectly by means of IL-6. STAT3 and ERK increase and phosphorylate bcl-2, suppressing cytochrome c release from the mitochondria to the cytoplasm, thereby preventing neuronal cell death.
In this study, we could not demonstrate the participation of astrocytes in the neuroprotective pathways at acute periods (several hours after ischemia) because PAC1R-positive reaction localized in neurons, but not in astrocytes, at these periods. However, we have shown previously that PAC1R expresses in activated astrocytes several days after stab wounding (42) and that PACAP application increases IL-6 in cultural astrocytes (24). Moreover, it has been reported that PACAP prevented delayed neuronal cell death even if PACAP infusion was begun several hours after the onset of ischemia (8, 9, 11). These previous results imply that PACAP might have other mechanisms of neuroprotection via astrocytes at chronic periods of ischemia. The role and action of PACAP and IL-6 at chronic periods of ischemia still must be clarified. The findings that PACAP can stimulate bcl-2 expression, perhaps through multiple pathways, and the elucidation of the pathways of PACAP involved in the association with IL-6 by means of PAC1-R have important bearings on the neuroprotectant potential of PACAP in clinical applications.
In conclusion, we have demonstrated a neuroprotective mechanism mediated by PACAP in vivo after ischemia. PACAP decreases neuronal cell death by suppressing cytochrome c release by means of bcl-2 regulation. Moreover, a synergistic effect of bcl-2 is also suggested by the increases in pERK and pSTAT3 associated with IL-6.
Methods
Mice.
All experimental procedures involving animals were approved by the Institutional Animal Care and Use Committee of Showa University (regulation nos. 04093, 04096, 03020, and 02098). PACAP null (C57/B6J) mice are described in ref. 43, and IL-6−/− (BALB/c) mice are described in ref. 44. The IL-6+/+ mice were generated from the same chimeric founder, and experiments were performed in age- and weight-matched animals. Because PACAP null mice showed a high rate of lethality at 1–2 weeks after birth (43), in the present study, some experiments were performed only in PACAP+/− and wild-type mice.
MCAO Production and PACAP38 Injection.
Mice were anesthetized with 2.0% sevoflurane in N2O/O2, after which, they were subjected to pMCAO or tMCAO according to an intraluminal filament technique by using a monofilament nylon suture as described (45). IL-6+/+ and IL-6−/− mice were briefly reanesthetized at 0.5 or 1.0 h after MCAO, and the suture was withdrawn, allowing reperfusion to occur (tMCAO). Animals that did not show grade 1 neurological symptoms 1 h after ischemia or those that hemorrhaged were excluded from the experiment.
After MCAO, some PACAP+/+, PACAP+/−, and PACAP−/− mice were fixed to a stereotaxic frame and bolus-injected into the lateral ventricle of the contralateral hemisphere with 1 μl of either PACAP38 (1 pmol/μl, Peptide Institute, Osaka) or vehicle (artificial CSF) for 8 min.
In contrast, some IL-6+/+ and IL-6−/− mice were bolus-injected into the jugular vein with vehicle (0.1% BSA/saline), PACAP38 (5 nmol/kg), or PACAP38 plus PACAP6-38 (50 nmol/kg; Peptide Institute, Osaka). A PE10 polyethylene tube connected to a miniosmotic pump (0.5 μl/h; Alza) that was filled vehicle, PACAP38 (32 pmol/μl), or PACAP plus PACAP6-38 (320 pmol/μl) was then inserted for continuous administration. All doses were chosen on the basis of our previous in vivo rat studies (8–10).
Evaluation of Cell Death After Ischemia.
Cell death after MCAO was evaluated by neurological deficit (2 h) and infarct volume as reported (45, 46). Briefly, neurological deficits were evaluated based on five grades (grades 0–4) as follows: 0 (normal), no observable neurological deficit; 1 (mild), failure to extend the contralateral forepaw upon lifting the animal by the tail; 2 (moderate), circling to the contralateral side; 3 (severe), leaning to the contralateral side when at rest; and 4 (heavy), no spontaneous motor activity. One hour after ischemia, any animal that was not displaying at least grade 1 deficits was excluded from the experiment.
Calculation of Infarct Volume.
Infarct volume 24 h after ischemia was determined by using nih image software analysis after 2,3,5-triphenyltetrazolium chloride (TTC) staining as described (47). Briefly, the animal was decapitated, and the brain was sliced into four 2-mm coronal sections by using a mouse brain matrix. The brain slices were then stained with 2% TTC at 37°C for 30 min and photographed on the anterior surface of each section with a scale bar. The areas of infarction were delineated on the basis of the relative lack of staining in the ischemic slice and measured by using nih image software.
Western Blot Analysis.
Cerebrums at 0 h (sham-operated) and 4 and 8 h after MCAO were removed from decapitated animals and divided into the ipsilateral and contralateral hemispheres. The samples were then homogenized in lysis buffer as described (48) before being centrifuged at 12,000 × g for 30 min at 4°C. Supernatant was collected as total cell lysate for the detection of phospho-specific antibodies. Mitochondrial and cytosolic proteins were fractionated by using the Mitochondria/Cytosol Fractionation Kit (BioVision Research Products, Mountain View, CA) to evaluate cytochrome c and bcl-2 levels. The cell fractionations were confirmed by immunoblotting for cytochrome oxidase subunit I (a mitochondrial marker) and α-tubulin (a cytoplasmic marker) and by assaying for the enzymes succinate dehydrogenase (a mitochondrial protein) and lactate dehydrogenase (a cytoplasmic marker; data not shown). Protein concentrations were determined by the Bradford method (Bio-Rad).
The total cell lysate, as well as the mitochondrial and cytoplasmic samples, was loaded onto a 10–15% SDS/PAGE. After transfer, the membrane was blocked with 1% skim milk/PBS for 1 h and probed with rabbit polyclonal antibody to cytochrome c (1:2,000; Santa Cruz Biotechnology), pERK (1:1,000; Cell Signaling Technology, Beverly, MA), or pSTAT3 (1:1,000; Cell Signaling Technology) or with mouse monoclonal antibodies to cytochrome oxidase subunit I (1:1,000; Molecular Probes), α-tubulin (1:4,000; Sigma), bcl-2α (1:200; Sigma), or pAKT (1:1,000; Cell Signaling Technology) for 1.5 h. The membranes were then incubated with horseradish peroxidase-conjugated anti-rabbit IgG (1:3,000; Amersham Pharmacia) or anti-mouse IgG (1:2,000; Amersham Pharmacia) for 1 h, and protein bands were visualized by using a chemiluminescent system (SuperSignal West Dura Extended Duration Substrate, Pierce). The films were scanned, and the densities of the signals were quantified with the nih image 1.62 image analyzer.
Immunohistochemistry.
Mice were fixed with 2% paraformaldehyde in PBS under sodium pentobarbital (50 mg/kg i.p.) anesthesia at 4 h after MCAO. Their brains were removed, postfixed, embedded in 20% sucrose, and cryosectioned (8 μm) for immunohistochemical staining.
For IL-6 staining alone, sections were pretreated with 0.3% H2O2 and 2.5% normal horse serum followed by incubation in a rat monoclonal anti-mouse IL-6 antibody (1:50; BioSource International, Camarillo, CA). After subsequent incubation with biotinylated goat anti-rat IgG (Vector Laboratories) for 1.5 h, the sections were visualized by using avidin–biotin complex/diaminobenzidine as a chromogen (Vector Laboratories).
Costaining for IL-6 and PAC1R with NeuN was performed to identify the distribution of IL-6. After pretreatment with 2.5% normal horse serum, sections were coincubated with anti-IL-6 antibody, rabbit polyclonal anti-PAC1R antibody (1:500), and mouse monoclonal anti-NeuN antibody (1:1,000; Chemicon). The rabbit polyclonal anti-PAC1R antibody was raised by using the N-terminal residue as an antigen (42). After incubation, the sections were immersed in Alexa Fluor 350-, 488-, and 546-labeled anti-mouse, anti-rat, or anti-rabbit IgG secondary antibodies (1:400; Molecular Probes). To identify the cellular sources of intracellular signaling, costaining for pERK or pSTAT3 and NeuN was also performed, following a similar procedure to that described above, except for a pretreatment step with 1 mM EDTA (pH 8.0) for 15 min at 85°C and the use of rabbit polyclonal antibodies for pERK (1:200) or pSTAT3 (1:250) and anti-NeuN. At the end of the staining periods, the sections were counterstained with DAPI. All of the above procedures were carried out on three to four brains per condition. Sections were observed with the aid of a fluorescence microscope (Keyence, Tokyo).
B9 Bioassay for IL-6 in CSF.
IL-6 bioactivity was measured by using proliferation of the mouse IL-6-dependent hybridoma subclone B9 as described (24). Briefly, CSF was carefully collected from cisterna magna (n = 8) under sodium pentobarbital (50 mg/kg i.p.) anesthesia 4 h after tMCAO. This time point was chosen based on our preliminary studies to check IL-6 bioactivity in plasma and CSF. Samples and standard were pipetted into 96-well plates in six consecutive 1:1 dilutions. B9 cells were added to the wells, and the plates were incubated for 72 h. At the end of the incubation period, proliferation in the cells was determined colorimetrically by using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Sigma) at 570 nm. PACAP and PACAP6-38 did not influence proliferation of B9 cells when added at concentrations identical to those used in these experiments.
RT-PCR of IL-6.
Brains were removed under barbiturate anesthesia at 4 h after tMCAO and immediately separated on ice into contralateral and ipsilateral hemispheres. The samples were then snap-frozen by using liquid nitrogen and stored at −80°C until assayed. Total mRNA was extracted by using the acid phenol-guanidinium thiocianate method (49).
cDNA was synthesized from RNA (1.0 μg) as described (45). PCR amplification of cDNA was carried out with primers specific for IL-6 with β-actin (data not shown) used as a housekeeping gene. Primers used for PCR were determined as reported (50). DNA fragments were amplified as follows: 95°C for 1 min and then 35 (IL-6) and 20 (β-actin) cycles of 60°C for 30 s and 72°C for 1 min. The number of cycles ensuring nonsaturating PCR conditions was established in preliminary studies. PCR products were analyzed by electrophoresis on a 2% agarose gel containing ethidium bromide.
Statistical Analysis.
Data are expressed as mean ± SEM. Statistical comparisons were made by one-way ANOVA after Dunnett’s post-hoc tests compared with the sham-operated control (0 h) or the vehicle-treated mice. P < 0.05 was considered statistically significant.
Acknowledgments
This work was supported by a Showa University Grant-In-Aid for Innovative Collaborative Research Projects and a Special Research Grant-In-Aid for Development of Characteristic Education from the Ministry of Education, Culture, Sports, Science, and Technology (to H.O. and S.S.).
Abbreviations
- CSF
cerebrospinal fluid
- ERK
extracellular signal-regulated kinase
- i.c.v.
intracerebroventricular
- ir
immunoreactivity
- MCAO
middle cerebral artery occlusion
- pMCAO
permanent MCAO
- tMCAO
transient MCAO
- PACAP
pituitary adenylate cyclase-activating polypeptide
- PAC1R
PACAP receptor 1
- STAT
signal transducers and activators of transcription.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Arimura A. Jpn. J. Physiol. 1998;48:301–331. doi: 10.2170/jjphysiol.48.301. [DOI] [PubMed] [Google Scholar]
- 2.Ganea D., Delgado M. Crit. Rev. Oral Biol. Med. 2002;13:229–237. doi: 10.1177/154411130201300303. [DOI] [PubMed] [Google Scholar]
- 3.Martinez C., Abad C., Delgado M., Arranz A., Juarranz M. G., Rodriguez-Henche N., Brabet P., Leceta J., Gomariz R. P. Proc. Natl. Acad. Sci. USA. 2002;99:1053–1058. doi: 10.1073/pnas.012367999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linden L. Pulm. Pharmacol. Ther. 1999;12:229–236. doi: 10.1006/pupt.1999.0209. [DOI] [PubMed] [Google Scholar]
- 5.Naruse S., Suzuki T., Ozaki T., Nokihara K. Peptides. 1993;14:505–510. doi: 10.1016/0196-9781(93)90139-8. [DOI] [PubMed] [Google Scholar]
- 6.Ohtaki H., Dohi K., Yofu S., Nakamachi T., Kudo Y., Endo S., Aruga T., Goto N., Watanabe J., Kikuyama S., et al. Regul. Pept. 2004;123:61–67. doi: 10.1016/j.regpep.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 7.Shioda S., Zhou C. J. I., Ohtaki H., Yada T. In: Pituitary Adenylate Cyclase-Activating Polypeptide. Vaudry H., Arimura A., editors. Boston: Kluwer; 2003. pp. 95–124. [Google Scholar]
- 8.Uchida D., Arimura A., Somogyvári-Vigh A., Shioda S., Banks W. A. Brain Res. 1996;736:280–286. doi: 10.1016/0006-8993(96)00716-0. [DOI] [PubMed] [Google Scholar]
- 9.Reglodi D., Tamas A., Somogyvári-Vigh A., Szanto Z., Kertes E., Lenard L., Arimura A. Peptides. 2002;23:2227–2234. doi: 10.1016/s0196-9781(02)00262-0. [DOI] [PubMed] [Google Scholar]
- 10.Dohi K., Mizushima H., Nakajo S., Ohtaki H., Matsunaga S., Aruga T., Shioda S. Regul. Pept. 2002;109:83–88. doi: 10.1016/s0167-0115(02)00190-8. [DOI] [PubMed] [Google Scholar]
- 11.Reglodi D., Somogyvari-Vigh A., Vigh S., Kozicz T., Arimura A. Stroke. 2000;31:1411–1417. doi: 10.1161/01.str.31.6.1411. [DOI] [PubMed] [Google Scholar]
- 12.Banks W.A., Uchida D., Arimura A., Somogyvári-Vigh A., Shioda S. Ann. N.Y. Acad. Sci. 1996;805:270–279. doi: 10.1111/j.1749-6632.1996.tb17489.x. [DOI] [PubMed] [Google Scholar]
- 13.Banks W.A., Kastin A. J., Komaki G., Arimura A. J. Pharmacol. Exp. Ther. 1993;267:690–696. [PubMed] [Google Scholar]
- 14.Onoue S., Endo K., Ohshima K., Yajima T., Kashimoto K. Peptides. 2002;23:1471–1478. doi: 10.1016/s0196-9781(02)00085-2. [DOI] [PubMed] [Google Scholar]
- 15.Vaudry D., Falluel-Morel A., Basille M., Pamantung T. F., Fontaine M., Fournier A., Vaudry H., Gonzalez B. J. J. Neurosci. Res. 2003;72:303–316. doi: 10.1002/jnr.10530. [DOI] [PubMed] [Google Scholar]
- 16.Falluel-Morel A., Aubert N., Vaudry D., Basille M., Fontaine M., Fournier A., Vaudry H., Gonzalez B. J. Neurochem. 2004;91:1231–1243. doi: 10.1111/j.1471-4159.2004.02810.x. [DOI] [PubMed] [Google Scholar]
- 17.Vaudry D., Pamantung T. F., Basille M., Rousselle C., Fournier A., Vaudry H., Beauvillain J. C., Gonzalez B. J. Eur. J. Neurosci. 2002;15:1451–1460. doi: 10.1046/j.1460-9568.2002.01981.x. [DOI] [PubMed] [Google Scholar]
- 18.Bhave S. V., Hoffman P. L. J. Neurochem. 2004;88:359–369. doi: 10.1046/j.1471-4159.2003.02167.x. [DOI] [PubMed] [Google Scholar]
- 19.Vaudry D., Rousselle C., Basille M., Falluel-Morel A., Pamantung T. F., Fontaine M., Fournier A., Vaudry H., Gonzalez B. J. Proc. Natl. Acad. Sci. USA. 2002;99:6398–6403. doi: 10.1073/pnas.082112699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Onoue S., Ohshima K., Endo K., Yajima T., Kashimoto K. FEBS Lett. 2002;522:65–70. doi: 10.1016/s0014-5793(02)02886-7. [DOI] [PubMed] [Google Scholar]
- 21.Frechilla D., Garcia-Osta A., Palacios S., Cenarruzabeitia E., Del Rio J. NeuroReport. 2001;12:919–923. doi: 10.1097/00001756-200104170-00011. [DOI] [PubMed] [Google Scholar]
- 22.Shintani N., Suetake S., Hashimoto H., Koga K., Kasai A., Kawaguchi C., Morita Y., Hirose M., Sakai Y., Tomimoto S., et al. Regul. Pept. 2005;126:123–128. doi: 10.1016/j.regpep.2004.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Tatsuno I., Somogyvári-Vigh A., Mizuno K., Gottschall P. E., Hidaka H., Arimura A. Endocrinology. 1991;129:1797–1804. doi: 10.1210/endo-129-4-1797. [DOI] [PubMed] [Google Scholar]
- 24.Gottschall P. E., Tatsuno I., Arimura A. Brain Res. 1994;637:197–203. doi: 10.1016/0006-8993(94)91233-5. [DOI] [PubMed] [Google Scholar]
- 25.Shioda S., Ozawa H., Dohi K., Mizushima H., Matsumoto K., Nakajo S., Takaki A., Zhou C. J., Nakai Y., Arimura A. Ann. N.Y. Acad. Sci. 1998;865:111–117. doi: 10.1111/j.1749-6632.1998.tb11169.x. [DOI] [PubMed] [Google Scholar]
- 26.Taga T., Kishimoto T. Annu. Rev. Immunol. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- 27.Hirano T., Ishihara K., Hibi M. Oncogene. 2000;19:2548–2556. doi: 10.1038/sj.onc.1203551. [DOI] [PubMed] [Google Scholar]
- 28.Kishimoto T. Annu. Rev. Immunol. 2005;23:1–21. doi: 10.1146/annurev.immunol.23.021704.115806. [DOI] [PubMed] [Google Scholar]
- 29.Suzuki S., Tanaka K., Nagata E., Ito D., Dembo T., Fukuuchi Y. Neurosci. Lett. 1999;262:117–120. doi: 10.1016/s0304-3940(99)00051-8. [DOI] [PubMed] [Google Scholar]
- 30.Dihne M., Peters M., Block F. NeuroReport. 2001;12:3143–3148. doi: 10.1097/00001756-200110080-00032. [DOI] [PubMed] [Google Scholar]
- 31.Vollenweider F., Herrmann M., Otten U., Nitsch C. Neurosci. Lett. 2003;341:49–52. doi: 10.1016/s0304-3940(03)00136-8. [DOI] [PubMed] [Google Scholar]
- 32.Loddick S. A., Turnbull A. V., Rothwell N. J. J. Cereb. Blood Flow Metab. 1998;18:176–179. doi: 10.1097/00004647-199802000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Herrmann O., Tarabin V., Suzuki S., Attigah N., Coserea I., Schneider A., Vogel J., Prinz S., Schwab S., Monyer H., et al. J. Cereb. Blood Flow Metab. 2003;23:406–415. doi: 10.1097/01.WCB.0000055177.50448.FA. [DOI] [PubMed] [Google Scholar]
- 34.Yamashita T., Sawamoto K., Suzuki S., Suzuki N., Adachi K., Kawase T., Mihara M., Ohsugi Y., Abe K., Okano H. J. Neurochem. 2005;94:459–468. doi: 10.1111/j.1471-4159.2005.03227.x. [DOI] [PubMed] [Google Scholar]
- 35.Tsujimoto Y., Shimizu S. FEBS Lett. 2000;466:6–10. doi: 10.1016/s0014-5793(99)01761-5. [DOI] [PubMed] [Google Scholar]
- 36.Zamzami N., Kroemer G. Nat. Rev. Mol. Cell. Biol. 2001;2:67–71. doi: 10.1038/35048073. [DOI] [PubMed] [Google Scholar]
- 37.Chan P. H. Neurochem. Res. 2004;29:1943–1949. doi: 10.1007/s11064-004-6869-x. [DOI] [PubMed] [Google Scholar]
- 38.Suzuki S., Yamashita T., Tanaka K., Hattori H., Sawamoto K., Okano H., Suzuki N. J. Cereb. Blood Flow Metab. 2005;25:685–693. doi: 10.1038/sj.jcbfm.9600061. [DOI] [PubMed] [Google Scholar]
- 39.Lin Q., Lai R., Chirieac L. R., Li C., Thomazy V. A., Grammatikakis I., Rassidakis G. Z., Zhang W., Fujio Y., Kunisada K., et al. Am. J. Pathol. 2005;167:969–980. doi: 10.1016/S0002-9440(10)61187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tamura Y., Simizu S., Osada H. FEBS Lett. 2004;569:249–255. doi: 10.1016/j.febslet.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 41.Wu T. W., Wang J. M., Chen S., Brinton R. D. Neuroscience. 2005;135:59–72. doi: 10.1016/j.neuroscience.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 42.Suzuki R., Arata S., Nakajo S., Ikenaka K., Kikuyama S., Shioda S. Brain Res. Mol. Brain Res. 2003;115:10–20. doi: 10.1016/s0169-328x(03)00172-4. [DOI] [PubMed] [Google Scholar]
- 43.Hashimoto H., Shintani N., Tanaka K., Mori W., Hirose M., Matsuda T., Sakaue M., Miyazaki J., Niwa H., Tashiro F., et al. Proc. Natl. Acad. Sci. USA. 2001;98:13355–13360. doi: 10.1073/pnas.231094498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopf M., Baumann H., Freer G., Freudenberg M., Lamers M., Kishimoto T., Zinkernagel R., Bluethmann H., Kohler G. Nature. 1994;368:339–342. doi: 10.1038/368339a0. [DOI] [PubMed] [Google Scholar]
- 45.Ohtaki H., Funahashi H., Dohi K., Oguro T., Horai R., Asano M., Iwakura Y., Yin L., Matsunaga M., Goto N., et al. Neurosci. Res. 2003;45:313–324. doi: 10.1016/s0168-0102(02)00238-9. [DOI] [PubMed] [Google Scholar]
- 46.Hara H., Huang P. L., Panahian N., Fishman M. C., Moskowitz M. A. J. Cereb. Blood Flow Metab. 1996;16:605–611. doi: 10.1097/00004647-199607000-00010. [DOI] [PubMed] [Google Scholar]
- 47.Bederson J. B., Pitts L. H., Germano S. M., Nishimura M. C., Davis R. L., Bartkowski H. M. Stroke. 1986;17:1304–1308. doi: 10.1161/01.str.17.6.1304. [DOI] [PubMed] [Google Scholar]
- 48.Shioda S., Funahashi H., Nakajo S., Yada T., Maruta O., Nakai Y. Neurosci. Lett. 1998;243:41–44. doi: 10.1016/s0304-3940(98)00082-2. [DOI] [PubMed] [Google Scholar]
- 49.Chomczynski P., Sacchi N. Anal. Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 50.Overbergh L., Valckx D., Waer M., Mathieu C. Cytokine. 1999;11:305–312. doi: 10.1006/cyto.1998.0426. [DOI] [PubMed] [Google Scholar]