Abstract
G3139, an 18-mer phosphorothioate antisense oligonucleotide targeted to the initiation codon region of the Bcl-2 mRNA, can induce caspase-dependent apoptosis via the intrinsic mitochondrial pathway in 518A2 and other melanoma cells. G3139-mediated apoptosis appears to be independent of its ability to down-regulate the expression of Bcl-2 protein, because the release of mitochondrial cytochrome c precedes in time the down-regulation of Bcl-2 protein expression. In this study, we demonstrate the ability of G3139 and other phosphorothioate oligonucleotides to bind directly to mitochondria isolated from 518A2 cells. Furthermore, we show that this interaction leads to the release of cytochrome c in the absence of a mitochondrial membrane permeability transition. Our data further demonstrate that there is an interaction between G3139 and VDAC, a protein that can facilitate the physiologic exchange of ATP and ADP across the outer mitochondrial membrane. Evidence from the electrophysiologic evaluation of VDAC channels reconstituted into phospholipid membranes demonstrates that G3139 is capable of producing greatly diminished channel conductance, indicating a closed state of the VDAC. This effect is oligomer length-dependent, and the ability of phosphorothioate homopolymers of thymidine of variable lengths to cause the release of cytochrome c from isolated mitochondria of 518A2 melanoma cells can be correlated with their ability to interact with VDAC. Because it has been suggested that the closure of VDAC leads to the opening of another outer mitochondrial membrane channel through which cytochrome c can transit, thus initiating apoptosis, it appears that VDAC may be an important pharmacologic target of G3139.
Keywords: cytochrome c, mitochondria, phosphorothioate
G3139 is an 18-mer phosphorothioate antisense oligonucleotide targeted to the initiation codon region of the Bcl-2 mRNA (1). The ability of G3139 to down-regulate the expression of the proapoptotic Bcl-2 protein has been demonstrated in a series of previous experiments (2–4), and this effect has been correlated with decreased resistance to cytotoxic chemotherapy in animal models (5). On the basis of favorable phase II clinical data (6), G3139 has recently entered phase III clinical trials in combination with dacarbazine for the treatment of advanced melanoma. However, the mechanism of action of G3139 remains somewhat uncertain. siRNA silencing of Bcl-2 protein expression in 518A2 melanoma cells, the preclinical model, did not lead to chemosensitization (3). In fact, G3139 induced extensive apoptosis in 518A2 and other melanoma cells, frequently in the absence of any discernable Bcl-2 protein down-regulation (3). Caspase-dependent apoptosis in melanoma cells in vitro occurred via activation of the intrinsic pathway and was characterized by cleavage of procaspase-3/7 to caspase-3 with subsequent cleavage of many intracellular proteins. In addition, Bid was processed to proapoptotic tBid in a caspase-3/7-dependent manner, without any activation of caspase-8 (7). The mitochondrial permeability transition occurred relatively late (8) in the apoptotic process (≈24 h after the initiation of the transfection with G3139).
The initiation of the apoptotic process via the intrinsic pathway can require the release of cytochrome c into the cytoplasm (9) from loosely bound sites in the intermembrane space of the mitochondrion (10). In the cytoplasm, cytochrome c combines with Apaf-1, dATP, and procaspase-9 to form the apoptosome (11), in which procaspase-9 is cleaved to caspase-9, which then activates caspase-3/7, resulting in intracellular demolition (12). We observed (3) that the G3139-induced release of cytochrome c from the mitochondria in intact melanoma cells occurred very early (9.5 h) after the initiation of the cellular transfection with G3139, much earlier, in fact, than what we typically observed with standard cytotoxic chemotherapy (20–24 h).
G3139 and phosphorothioate oligonucleotides can bind with high affinity to cell-surface heparin-binding proteins (13–15). Because of this ability, we postulated that the early release of cytochrome c from the mitochondria might also be occurring as a result of the interaction of G3139 with a heparin-binding protein present in the outer mitochondrial membrane.
VDAC is a channel-forming protein in the mitochondrial outer membrane responsible for metabolic flux (including nucleotide phosphates) through that membrane (16–18). VDAC has been widely implicated in the initiation of the mitochondrially mediated intrinsic pathway of apoptosis (19–21). Although the mechanism of action is still quite controversial (22), closure of the VDAC and the subsequent decrease in metabolic flux can precede, and may in fact cause, the permeabilization of the outer membrane to relatively small proteins, leading to apoptosis. The actual protein translocation channel would thus be generated by other, as yet unknown structures as a consequence of decreased metabolic flux. Because a variety of polyanions favor VDAC closure, we thus deemed it possible that G3139 could also induce VDAC closure, which would result in cellular apoptosis.
Results and Discussion
Phosphorothioate Oligonucleotides Bind to Mitochondria.
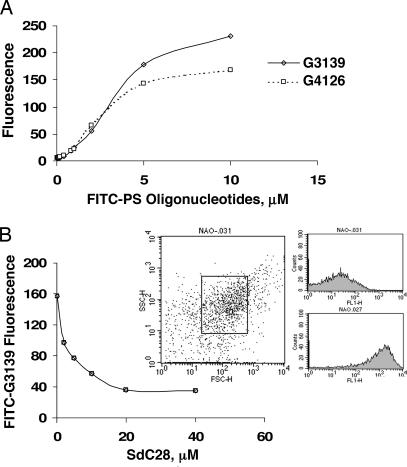
As mentioned previously, 518A2 melanoma cells treated with G3139 undergo apoptosis characterized by relatively rapid release of mitochondrial cytochrome c. We hypothesized that this release might be due to a direct interaction of G3139 with the mitochondrial membrane. Accordingly, we isolated mitochondria from 518A2 cells by differential centrifugation. The purity of the mitochondria was assessed flow cytometrically by nonyl acridine orange (NAO) staining, and subsequently only the NAO-gated population was analyzed. The flow cytometric results of treatment of isolated mitochondria at 4°C with increasing concentrations of either 5′-FITC-G3139 or -4126 (a two-base mismatch) are shown in Fig. 1A. The binding is essentially maximal by 30 min and remains undiminished for up to 2 h (data not shown). In contrast to what was observed with these two oligomers, the 5′-FITC-isosequential phosphodiester congener of G3139 (5′-FITC-PO-G3139) did not bind to the mitochondria. This difference in binding affinity between a phosphodiester (low) and an isosequential phosphorothioate (high) oligonucleotide has been long recognized (13).
Fig. 1.
Binding of oligonucleotides to isolated mitochondria. (A) Isolated mitochondria from 518A2 melanoma cells were incubated with 5′-FITC-G3139 and -G4126 at various concentrations (10 nM to 10 μM), as described in Materials and Methods, and the binding was assessed flow cytometrically. (B) The binding of 5′-FITC-G3139 can be competed by SdC28 in a concentration-dependent manner (2–20 μM). (Inset) Mitochondria were gated flow cytometrically by NAO staining, which specifically stained the mitochondrial cardiolipin.
In addition, as shown in Fig. 1B, the binding occurs substantially on the basis of a charge interaction, because it can be ≈75% competed by SdC28, a longer phosphorothioate oligomer that is a homopolymer of cytidine. Moreover, the ability of the binding to be competed indicates that it occurs predominately at the outer mitochondrial membrane, as opposed to within the organelle itself.
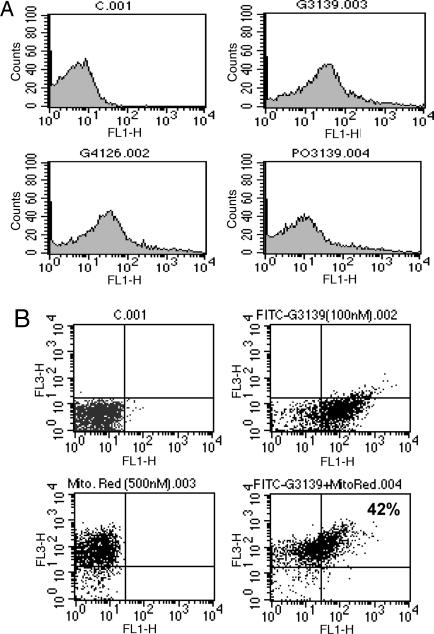
We then treated intact 518A2 cells with 100 nM 5′-FITC-G3139/Lipfectamine 2000 (1.9 μg/ml) and other oligonucleotides for 5 h, and then isolated mitochondria. As shown in Fig. 2A, substantial mitochondrial staining was observed flow cytometrically in the NAO-gated population after either treatment with 5′-FITC-G3139 or the two-base mismatch 5′-FITC-G4126. In contrast, treatment with the 5′-FITC-phosphodiester analog of G3139 produced only a small increase in mitochondrial staining. We confirmed these observations by treating intact cells with 5′-FITC-G3139 as above and the specific mitochondrial stain MitoTracker Red. Again, flow-cytometric analysis clearly demonstrated that a substantial proportion (42%) of the mitochondrial population stained with both dyes (Fig. 2B).
Fig. 2.
Binding of oligonucleotides to mitochondria isolated from intact cells. (A) 518A2 melanoma cells treated with 100 nM 5′-FITC-oligonucleotides/Lipofectamine 2000 (1.9 μg/ml) for 5 h were fractionated, and the staining of the mitochondria with FITC-oligonucleotides was assessed by flow cytometry. A minimum of 10,000 mitochondria per sample were analyzed as described in Materials and Methods. A >8-fold increase in FL-1 fluorescence was observed in both FITC-G3139 and -G4126-treated samples, whereas FITC-PO-3139 yielded only a very small increase (≈2-fold) (Table 1). (B) Intact 518A2 melanoma cells were treated with 100 nM 5′-FITC-G3139/Lipfectamine 2000 (1.9 μg/ml) for 5 h and stained with 500 nM MitoTracker Red. As analyzed flow cytometrically, ≈42% of the mitochondria were costained with FITC-G3139 and MitoTracker Red, as shown in the dot plot.
The binding of phosphorothioate oligonucleotides to the mitochondrial membrane does not result in a membrane permeability transition. This permeability transition was assessed flow cytometrically by JC-1 staining in mitochondria isolated from 518A2 cells that were then treated with either 20 or 40 μM G3139.
Treatment of Isolated Mitochondria with Phosphorothioate Oligonucleotides Causes Release of Cytochrome c.
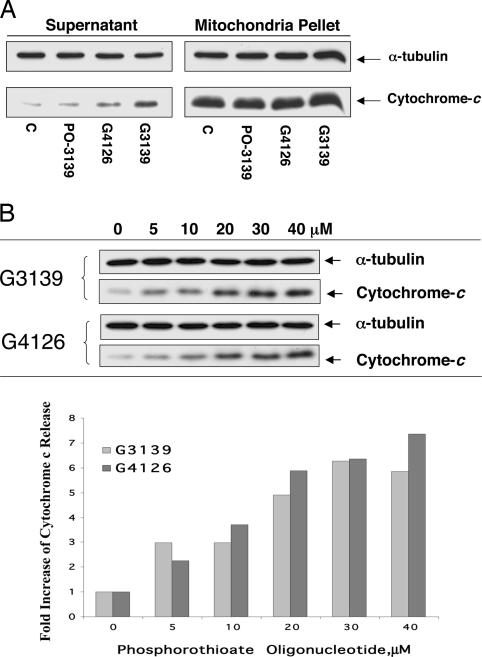
When isolated mitochondria were directly treated with 20 μM G3139 (Fig. 3A) in energizing buffer B with or without 100 μM Mg2+ for 2 h at 10°C, cytochrome c was released into the supernatant, as demonstrated by Western blotting. This release was identical whether mitochondrial were isolated either by low-speed differential centrifugation or ultracentrifugation. The release of cytochrome c is concentration-dependent and Mg2+-independent (Fig. 3B) but can easily be observed at a concentration of as low as 5 μM, also by Western blotting. Estimates of the intracellular concentration of 5′-FITC-G3139, based on the data in Figs. 1A and 2A, indicate that it may be at least as high as 2–3 μM. This is despite the much lower media concentration of 100 nM, a value that nevertheless has questionable significance because the oligonucleotide, when complexed with Lipofectamine 2000, is particulate and essentially precipitates on the cells in culture at the bottom of the wells. The ability of G4126 to cause concentration-dependent release of cytochrome c from isolated mitochondria was almost identical to that of G3139.
Fig. 3.
Release of cytochrome c from isolated mitochondria. (A) Isolated mitochondria from 518A2 melanoma cells were treated with 20 μM oligonucleotides (including PO-3139, G4126, and G3139) for 2 h in buffer B, and the supernatants were collected. Cytochrome c protein levels in both mitochondrial pellets and supernatant were analyzed by Western blotting as described in Materials and Methods. As shown, both phosphorothioate oligonucleotides G3139 and, to a lesser extent, G4126 caused a significant release of cytochrome c from the mitochondria. However, PO-3139 did not lead to any detectable release of cytochrome c from the mitochondria. (B) Western blot of the concentration-dependent profile of cytochrome c release by G3139 and G4126 from mitochondria isolated from 518A2 cells.
As mentioned above, the release of cytochrome c from mitochondria in response to G3139 or G4126, but not PO-G3139 treatment, is not accompanied by a mitochondria permeability transition. Simultaneous treatment with up to 20 μM MPTP inhibitor cyclosporin A, which binds (23) to cyclophilin D [a PTP regulatory protein (24)], also does not alter the concentration-dependent increase of cytochrome c release by G3139.
The release of cytochrome c from the mitochondria is not accompanied by inner mitochondrial membrane permeabilization. Fifty-kilodalton fumarase is an enzyme present in the mitochondrial matrix (25), and its release is considered normative evidence of damage/disruption of the inner mitochondrial membrane (26). Treatment of isolated mitochondria with Triton X-100 led to rupture of the mitochondrial membranes and release of fumarase activity, whereas treatment with up to 80 μM G3139 did not lead to the release of any detectable fumarase activity. In addition, treatment of isolated mitochondria also did not lead to release of MnSOD, also a mitochondrial matrix protein. Dextran sulfate (500 kDa; 500–1,000 μg/ml, 2 h, 10°C) and Koenig’s polyanion (50–100 μg/ml) are both agents that favor closure of the voltage-dependent anion selective channel (VDAC), a mitochondrial outer membrane protein (27, 28). Treatment of isolated mitochondria did lead, under the same conditions, to the release of cytochrome c, but this release occurred only at the highest concentrations. The release of 14-kDa cytochrome c from the mitochondria is also customarily associated with the release of other, relatively low molecular weight proteins [e.g., Smac/DIABLO, Htra2/Omi, AIF, and endonuclease G (29)].
These data suggested that the mitochondrial outer membrane target of the phosphorothioate oligonucleotides may be VDAC. These are the proteins that form a large-diameter (≈3 nm) channel that permits the physiologic exchange of anionic metabolites (e.g., ATP and ADP) across the outer mitochondrial membrane when they are in the open configuration (22, 30). Although controversial, it is believed that antiapoptotic proteins such as Bcl-2 and Bcl-xL maintain this channel in the open configuration (21).
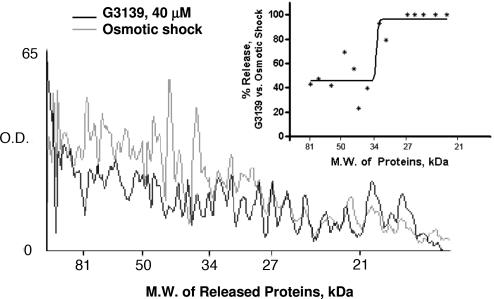
However, as shown in Fig. 4, there appears to be a cut-off in the molecular masses of the proteins released from the mitochondria after they are treated by G3139. The relative lack of release of proteins of molecular mass greater than ≈35 kDa here [and 60 kDa in the work of Siskind et al. (26)] suggests the formation of another channel in the mitochondrial membrane subsequent to VDAC closure. The nature of this channel has been previously postulated to be of oligomerized ceramide (26), but little is actually known about it.
Fig. 4.
Release of proteins from the mitochondrial intermembrane space was characterized by Coomassie blue staining of the supernatants collected either from the osmotic shock of isolated mitochondria in ddH2O or by treatment with 40 μM G3139. G3139-induced protein release was normalized to the total release induced by osmotic shock; peaks representing 14 proteins from the histogram were randomly chosen and quantitated. The profile of protein release by G3139 displayed an approximate 35-kDa molecular-mass cutoff, as shown in the scatter plot (Inset).
The Interaction Between G3139 and the VDAC.
To unambiguously determine whether phosphorothioate and other oligonucleotides directly influence the properties of VDAC, evaluations were performed on pure VDAC channels reconstituted into phospholipids membranes. VDAC channels were purified from rat liver mitochondria and inserted into planar phospholipid membranes. Positive and negative 50-mV voltage steps were applied to the membrane, and ion permeability induced by the channels in the membrane was measured as current flow across the membrane. VDAC channels are voltage-dependent and thus the voltage steps induce the opening and closing of the channels. VDAC channels exist primarily in the open state at 0 mV and low voltages, and tend to close at voltages above ±20 mV. Closing is a slow process and is thus well resolved in a time scale of 1 s (Fig. 5). The opening process occurs in a millisecond time scale and thus seems to occur instantly in the figure. Note that the closed state of VDAC, although impermeable to ATP, allows substantial flux of both K+ and Cl− ions; thus, the current drops only to 50–60% of the open-state current.
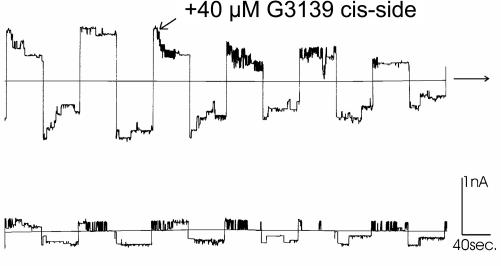
Fig. 5.
A few VDAC channels were reconstituted into a planar phospholipid membrane as described in Materials and Methods. The voltage was held at either +50 mV or −50 mV and alternated every 50 s. The long horizontal line indicates zero current. The 40 μM G3139 was added where indicated. The upper and lower traces are a continuous record without any breaks.
When 40 μM G3139 was added to the cis side of the chamber (Fig. 5), VDAC channels flickered between the open and closed states at high negative voltages (more than −30 mV). Note that the negative potential draws VDAC’s voltage sensor to the cis side (31); this voltage sensor may interact with G3139. No flickering was observed under positive potentials where the sensor would be driven toward the opposite, trans side. This flickering effect indicates that there is a direct interaction between G3139 and VDAC. The isosequential phosphodiester G3139, at the identical concentration, does not produce flickering of VDAC (data not shown), demonstrating the importance of the phosphorothioate backbone for the interaction.
The time scale of the flickering is ≈1 ms, and the amplitude of the conductance fluctuations is close to the conductance of a single VDAC channel. This amplitude is far greater than the conductance difference between the open state and the normal closed states of VDAC (Fig. 6). Thus, G3139 is able to induce unusually low levels of VDAC conductance and even a loss of total conductance (note the periods of very low conductance in between the periods of rapid flickering). In Fig. 5, most channels, except for one single channel, completely lose their conductance. Some channels, in fact, fail to reopen. On the other hand, some channels merely flicker without achieving full closure. This variation may be related to the existence of different forms of VDAC [isoforms (32) or splice variants (33), or other modified versions].
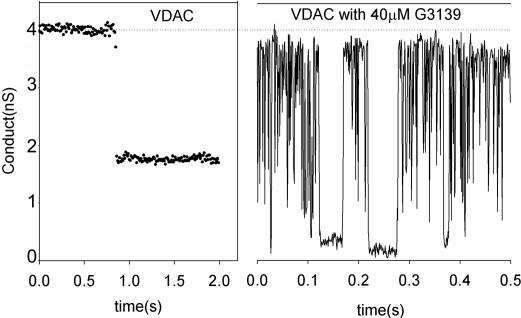
Fig. 6.
A comparison of VDAC closure resulting from voltage gating (Left) and the rapid conductance fluctuations (flickering) resulting from the addition of G3139. The applied voltage was −48 mV on the cis side, the same side to which G3139 was added.
Correlation Between Levels of VDAC1 Expression and Induction of Cytochrome c Release by G3139.
VDAC1 protein expression was determined in several melanoma and prostate cancer cell lines by Western blotting. In Fig. 7, the correlation between these levels, the concentrations of G3139 needed to release cytochrome c in mitochondria isolated from these cells, and the amount of cytochrome c released are correlated. Note that the PC3 and DU145 prostate cancer cell lines do not undergo apoptosis (34), except perhaps at very high G3139 concentrations. These two cell lines also have the lowest amount of mitochondrial membrane VDAC1. On the other hand, LNCaP cells, which do undergo apoptosis in response to G3139 treatment (4), have levels of VDAC1 similar to those found in 518A2 cells.
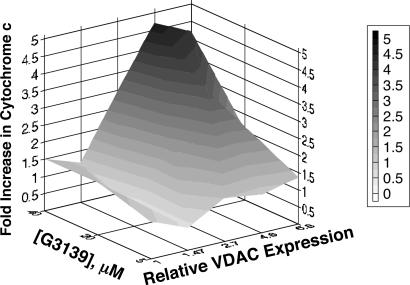
Fig. 7.
Correlation of G3139-induced cytochrome c release from isolated mitochondria to the relative VDAC expression from various prostate cancer and melanoma cell lines from which the mitochondria were derived (listed in Table 2). G3139 caused significant release of cytochrome c from the mitochondria derived from cell lines containing more VDAC protein expression level, in a concentration-dependent manner.
Sequence Dependence of the Potency of Phosphorothioate Oligonucleotides.
To quantitatively compare the ability of different phosphorothioate oligonucleotides to close VDAC channels, multichannel membranes (>20 channels) were used to average out the channel-to-channel variation. In addition, the dose was reduced to 5 μM. Even at this concentration, G3139 still caused VDAC channels to flicker, but full channel closure was somewhat less pronounced (data not shown).
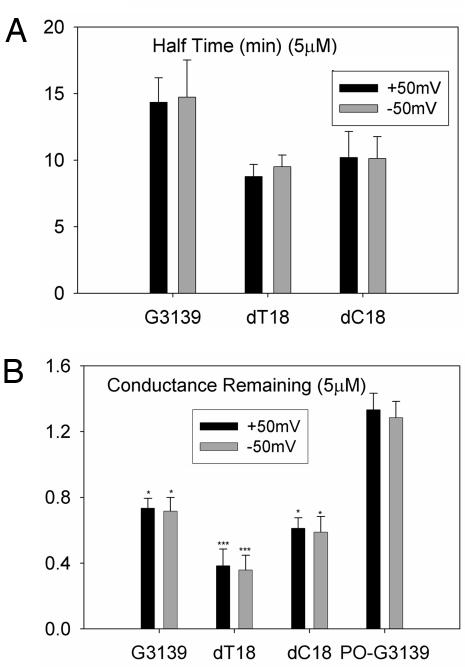
The identical pulse sequence was used as in Fig. 5. The conductance before channel closure was used as the measure of oligonucleotide activity. The addition of phosphorothioate oligonucleotide caused a reduction in this parameter; this corresponds to some of the VDAC channels remaining closed. The fractional drop in this parameter after it reached steady state (Fig. 8A) and the half-life of this decay (Fig. 8B) are shown for the various oligonucleotides tested. SdT18 demonstrated the strongest ability to close VDAC channels and the most rapid effect (half-life of 10 min). G3139 resulted in a 20–30% reduction in conductance, with a half-life of 15 min. 5′-truncated variants of G3139 as small as 10 mer in length had the same level of potency but also had a more rapid rate of onset. PO-G3139 at the identical concentration could not close VDAC channels at all. The reproducible increase in conductance was the result of channel insertion.
Fig. 8.
Fractional conductance remaining (A) and half time of the response (B) after treatment with 5 μM oligonucleotides. The conditions were the same as those in Fig. 1. Each result is the average of three experiments, except dT18, which is an average of six experiments. Statistical tests indicate significance in the reduction of the conductance. ∗, P < 0.05; ∗∗∗, P < 0.005.
Length Dependence of Phosphorothioate Oligonucleotides on the VDAC Interaction.
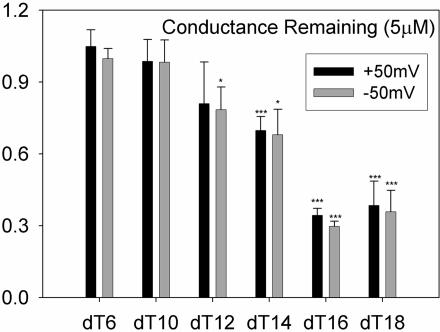
The importance of the length of the oligonucleotide on VDAC closure was tested by using a series of phosphorothioate homopolymers of thymidine of different lengths. Once again, multichannel membranes were used. Fig. 9 demonstrates the changes in membrane conductance after addition of 5 μM SdT6, -10, -12, -14, -16, or -18. The three longest oligonucleotides (SdT18, -16, and -14) caused significant losses of conductance and also caused flickering of VDAC channels (data not shown). SdT12, on the other hand, had only small and variable effects on VDAC, while shorter oligomers were not active. These data obtained from isolated VDAC channels parallel the length dependence of the release of cytochrome c from isolated mitochondria after treatment with G3139, as described previously. The cytochrome c release induced by SdT18 and SdT14 is almost equivalent to the release induced by G3139. The release induced by SdT12 is somewhat diminished, but little or no release was observed with either SdT10 or SdT8.
Fig. 9.
Fractional conductance remaining after treatment with 5 μM phosphorothioate homopolymers of thymidine of different lengths. The conditions were the same as those in Fig. 6. Each result is the average of six experiments, except dT6 and dT16, which are averages of three experiments. Statistical tests indicate significance in the reduction of the conductance. ∗, P < 0.05; ∗∗∗, P < 0.005.
Conclusions
The apoptosis that we have previously observed (3) in melanoma cells being treated with G3139 occurs as a result of activation of the intrinsic pathway, which can be initiated by release of cytochrome c from the mitochondria. This release is related to the closure of VDAC as a direct result of the binding to G3139, but it should also be recognized that VDAC is most likely not the only mitochondrial outer membrane surface protein to which this oligomer can bind with high affinity.
Among the many ways of initiating apoptosis, blocking the exchange of metabolites across the outer mitochondrial membrane may be sufficient. VDAC closure can occur as a result of growth factor withdrawal or by treatment with polyanions. Normal VDAC closure results in a selective drop in the flux of anions such as phosphocreatine, ATP4−, and HPO42−. VDAC permeability to cations can be retained, but as shown here after treatment with G3139, that too is also lost. It is possible that these disturbances in metabolic flux across the outer mitochondrial membrane may lead to the formation of a protein-conductive pathway across the outer membrane allowing cytochrome c and other proteins to be released from at least some mitochondria, which hence promotes the initiation of apoptosis. However, a permeability transition does not occur until relatively late in the process of apoptosis (24 h after the initiation of the transfection) compared with the early release of cytochrome c (9 h after the initiation of the transfection). In fact, as demonstrated by Bossy-Wetzel et al. (8), the release of mitochondrial cytochrome c does not require mitochondrial membrane depolarization. Only a relatively small percentage of the total cytochrome c found in the mitochondrial intermembrane space is actually released in the early phases of apoptosis initiation, but because of the amplifying nature of the process, cellular demolition and diminished Δψm is the inevitable result. The role of at least some of the Bcl-2-family proteins seems to be minimal in preventing G3139-induced apoptosis. Bcl-2 and, more convincingly, Bcl-xL have been shown to favor the open configuration of VDAC. This would thus permit small anion exchange, which would prevent disruption of normal mitochondrial physiological processes that could lead to the release of cytochrome c. However, silencing of either Bcl-2 or Bcl-xL by an siRNA strategy (35) produce neither spontaneous apoptosis nor increased sensitivity to a variety of cytotoxic agents. It also did not produce any alteration in the ability G3139 to induce apoptosis in melanoma cells. Furthermore, Bax has been reported to dock with VDAC1 (36), and we also observed release of this proapoptotic protein into the supernatants of G3139-treated mitochondria, as might be expected after VDAC1 closure. This observation, however, is not inconsistent with other work (37) that demonstrated that the electrophysiologic properties of VDAC channels reconstituted into planar phospholipid membranes are not affected by addition of either monomeric or oligomeric forms of Bax.
Although polyanions, in general, are known to favor VDAC closure, phosphorothioate oligodeoxynucleotides (and, we anticipate, siRNAs that contain a high percentage of phosphorothioate linkages) seem to be particularly potent, suggesting a relatively selective interaction. However, although there is a clear length dependence to the ability of a phosphorothioate oligonucleotide to induce VDAC closure [which closely parallels results observed with other, heparin-binding proteins such as basic fibroblast growth factor (13, 15)], the sequence and phosphorothioate content dependency is not as yet fully understood. Nevertheless, because of the wide variety of sequences, chemistries, and structures that can be investigated, the judicious application of phosphorothioate linkages in synthetic oligonucleotides may be a promising way of generating molecular and potentially therapeutic tools to control the initiation of apoptosis.
Materials and Methods
Cell Culture and Oligonucleotide Transfections.
The mycoplasma-free human melanoma cell line 518A2 was a kind gift from Volker Wacheck (University of Vienna). Cells were grown in DMEM supplemented with 10% heat-inactivated FBS and 100 units/ml penicillin G sodium and 100 μg/ml streptomycin sulfate. Stock cultures of all cells were maintained at 37°C in a humidified 5% CO2 incubator.
Cells were seeded the day before the experiment in six-well plates at a density of 15 × 104 cells per well, to be 60–70% confluent on the day of the experiment. All transfections were performed in OptiMEM medium (Invitrogen) plus complete media without antibiotics, as described in refs. 2 and 3. The incubation time for oligonucleotide/Lipofectamine 2000 complexes was 5 h. The total incubation time before cell lysis and protein isolation was from 24 to 72 h at 37°C.
Reagents.
The anti-cytochrome c monoclonal antibody was purchased from Santa Cruz Biotechnology. The anti-α-tubulin monoclonal antibody was from Sigma-Aldrich. Lipofectamine 2000 was from Invitrogen. MitoTracker Red was from Molecular Probes. Oligonucleotides were kindly supplied by Genta (Berkeley Heights, NJ).
Subcellular Fractionation and Oligonucleotide Treatment of Isolated Mitochondria.
Cells were harvested by trypsinization and washed with cold PBS. Cell pellets were resuspended in 300 μl of buffer A (250 mM sucrose/10 mM Tris·HCl, pH 7.4/1 mM EGTA/50 μg/ml Pefabloc/15 μg/ml leupeptin, aprotinin and pepstatin). Cells were then homogenized on ice in a Dounce homogenizer until ≈90% of cells were disrupted, as judged by Trypan blue staining. Crude lysates were centrifuged at 1,000 × g for 10 min at 4°C twice to remove nuclei and unbroken cells. The supernatant was collected and subjected to centrifugation at 10,000 × g for 30 min at 4°C. The supernatant was collected as the cytosolic fraction, and the mitochondrial pellets were resuspended in 30 μl of buffer A. In some experiments, mitochondria were highly purified by ultracentrifugation through a Percoll gradient at 100,000 × g.
Western Blot Analysis and Coomassie Blue Staining.
Cells treated with oligonucleotide–lipid complexes were extracted in lysis buffer at 4°C for 1 h. Aliquots of cell extracts, containing 25–40 μg of protein, were resolved by SDS/PAGE and then transferred to Hybond ECL filter paper (Amersham Pharmacia). After treatment with the appropriate primary and secondary antibodies, ECL was performed. The typical margin of error for Western blotting is at least 20–25%.
For Coomassie blue staining, gels after electrophoresis were stained with 0.025% Coomassie blue R250 in 40% methanol/7% acetic acid (vol/vol) for 1 h, followed by washing with destaining solution [7% acetic acid/5% methanol (vol/vol)]. The stained gel was then scanned by laser-scanning densitometry, and the bands were quantitated.
Labeling of Mitochondria with Fluorescent G3139 and MitoTracker Red.
Cells were transfected with 100 nM FITC-G3139/Lipofectamine 2000 complexes for 5 h as described in ref. 2 and 3 and incubated for an additional 19 h before staining with MitoTracker Red, a mitochondrion-selective probe. For live-cell staining, the cell culture medium was replaced with 500 nM MitoTracker Red in prewarmed DMEM, and the cells were incubated for 30 min under standard growth conditions. Then, the stained cells were washed twice with 1× PBS before analysis by flow cytometrically.
Binding of Fluorescinated Oligonucleotides to Mitochondria.
Isolated mitochondria were resuspended in 20 μl of energizing buffer B (250 mM sucrose/10 mM Tris-HCl, pH 7.4/1 mM EGTA/50 μg/ml Pefabloc/10 mM KCl/3 mM KH2PO4/5 mM succinate/100 μM ADP/15 μg/ml leupeptin, aprotinin, and pepstatin), which contained increasing concentrations of fluorescent-labeled oligonucleotides (1–40 μM). In some experiments, a competitor of G3139 binding to the mitochondrial surface, a 28-mer phosphorothioate homopolymer of cytidine (SdC28; 20 μM), was added. Incubation was carried out on ice for 10 min before washing twice with cold 1× PBS. After centrifugation at 10,000 × g, the pellet was resuspended in 200 μl of blank buffer B before flow-cytometric analysis.
Planar Phospholipid Membrane Studies.
The planar phospholipid membrane was generated according to standard methods (38). The membrane was formed of phospholipid monolayers consisting of diphytanoylphosphatidylcholine asolectin (soybean phospholipid polar extract) and cholesterol (1:1:0.1 mass ratio). The aqueous solution generally contained 1.0 M KCl, 5 mM CaCl2, 1 mM EDTA, and 5 mM Hepes (pH 7.2). All experiments were performed at ≈25°C.
VDAC was purified from mitochondria isolated from rat liver (39, 40). It was then solubilized in 2.5% Triton X-100, and a 1- to 3-μl aliquot was stirred into 4–6 ml of aqueous solution on the cis side of the chamber. The opposite, trans side was held at virtual ground by the voltage clamp.
Statistical Analysis.
Data are expressed as mean ± SD, and significance was determined by Student’s t test.
Table 1.
Fluorescence mean channel from Fig. 2A
| Samples | Fluorescence mean channel |
|---|---|
| C | 6.71 |
| G4126 | 55.84 |
| G3139 | 55.55 |
| PO-3139 | 15.19 |
Table 2.
Relative VDAC1 expression in human tumor cell lines
| Cell line | Relative VDAC1 expression |
|---|---|
| PC3 | 1 |
| DU145 | 1.5 |
| LNCap | 2.7 |
| 518A2 | 6.8 |
| 346.1 | 5.7 |
| 201.2 | 4.8 |
Abbreviation
- NAO
nonyl acridine orange.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Klasa R., Gillum A., Klem R., Frankel S. Antisense Nucleic Acid Drug Dev. 2002;12:193–213. doi: 10.1089/108729002760220798. [DOI] [PubMed] [Google Scholar]
- 2.Raffo A., Lai J., Miller P., Stein C. A., Benimetskaya L. Clin. Cancer Res. 2004;10:3195–3206. doi: 10.1158/1078-0432.ccr-03-0287. [DOI] [PubMed] [Google Scholar]
- 3.Lai J., Benimetskaya L., Khvorova A., Wu S., Hua E., Miller P., Stein C. Mol. Cancer Ther. 2005;4:305–315. [PubMed] [Google Scholar]
- 4.Leung S., Miyake H., Zellweger T., Tolcher A., Gleave M. Int. J. Cancer. 2001;91:846–850. doi: 10.1002/1097-0215(200002)9999:9999<::aid-ijc1131>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 5.Jansen B., Schlagbeuer-Wadl H., Brown B. D., Bryan R. N., van Elsas A., Muller M., Wolff K., Eichler H. G., Pehamberger H. Nat. Med. 1998;4:232–234. doi: 10.1038/nm0298-232. [DOI] [PubMed] [Google Scholar]
- 6.Jansen B., Wacheck V., Heere-Ress E., Schlagbauer-Wadl H., Hoeller C., Lucas T., Hoermann M., Hollenstein U., Wolff K., Pehamberger H. Lancet. 2000;356:1728–1733. doi: 10.1016/S0140-6736(00)03207-4. [DOI] [PubMed] [Google Scholar]
- 7.Slee E., Keogh S., Martin S. Cell Death Differ. 2000;7:556–565. doi: 10.1038/sj.cdd.4400689. [DOI] [PubMed] [Google Scholar]
- 8.Bossy-Wetzel E., Newmeyer D., Green D. EMBO J. 1998;17:37–49. doi: 10.1093/emboj/17.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu X., Kim C., Yang J., Jemmerson R., Wang X. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 10.Ott M., Roertson J., Gogvadze V., Zhivotovsky B., Orrenius S. Proc. Natl. Acad. Sci. USA. 2002;99:1259–1263. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Nijhawan D., Budihardjo I., Srinivasula S. M., Ahmad M., Alnemri E. S., Wang X. Cell. 1997;91:479–489. doi: 10.1016/s0092-8674(00)80434-1. [DOI] [PubMed] [Google Scholar]
- 12.Salvesen G. S., Dixit V. M. Cell. 1997;91:443–446. doi: 10.1016/s0092-8674(00)80430-4. [DOI] [PubMed] [Google Scholar]
- 13.Guvakova M. A., Yakubov L. A., Vlodavsky I., Tonkinson J. L., Stein C. A. J. Biol. Chem. 1995;270:2620–2627. doi: 10.1074/jbc.270.6.2620. [DOI] [PubMed] [Google Scholar]
- 14.Benimetskaya L., Loike J., Khaled Z., Loike G., Silverstein S., Cao L., El-Khoury J., Kai T.-Q., Stein C. A. Nat. Med. 1997;3:414–420. doi: 10.1038/nm0497-414. [DOI] [PubMed] [Google Scholar]
- 15.Rockwell P., O’Connor W., King K., Goldstein N., Zhang L. M., Stein C. A. Proc. Natl. Acad. Sci. USA. 1997;94:6523–6528. doi: 10.1073/pnas.94.12.6523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Colombini M. Nature. 1979;279:643–645. doi: 10.1038/279643a0. [DOI] [PubMed] [Google Scholar]
- 17.Rostovtseva T., Colombini M. Biophys. J. 1997;72:1954–1962. doi: 10.1016/S0006-3495(97)78841-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colombini M. Mol. Cell. Biochem. 2004;256/257:107–115. doi: 10.1023/b:mcbi.0000009862.17396.8d. [DOI] [PubMed] [Google Scholar]
- 19.Szabo I., Zoratti M. FEBS Lett. 1993;330:201–205. doi: 10.1016/0014-5793(93)80273-w. [DOI] [PubMed] [Google Scholar]
- 20.Cromptom M., Virji S., Ward J. Eur. J. Biochem. 1998;258:729–735. doi: 10.1046/j.1432-1327.1998.2580729.x. [DOI] [PubMed] [Google Scholar]
- 21.Vander Heiden M., Li X., Gottlieb E., Hill R., Thompson C., Colombini M. J. Biol. Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- 22.Vander Heiden M., Chandel N., Li X., Schumacker P., Colombini M., Thompson C. Proc. Natl. Acad. Sci. USA. 2000;97:4666–4671. doi: 10.1073/pnas.090082297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cesura A., Pinard E., Schubenel R., Goetschy V., Friedlein A., Langen H., Polcic P., Forte M., Bernardi P., Kemp J. J. Biol. Chem. 2003;278:49812–49818. doi: 10.1074/jbc.M304748200. [DOI] [PubMed] [Google Scholar]
- 24.Nicolli A., Basso E., Petronili V., Wenger R., Bernardi P. J. Biol. Chem. 1996;271:2185–2192. doi: 10.1074/jbc.271.4.2185. [DOI] [PubMed] [Google Scholar]
- 25.Tuboi S., Suzuki T., Sato M., Yoshida T. Adv. Enzyme Regul. 1990;30:289–304. doi: 10.1016/0065-2571(90)90023-u. [DOI] [PubMed] [Google Scholar]
- 26.Siskind L., Kolesnick R., Colombini M. J. Biol. Chem. 2002;277:26796–26803. doi: 10.1074/jbc.M200754200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colombini M., Yeung C., Tung J., Konig T. Biochim. Biophys. Acta. 1987;905:279–286. doi: 10.1016/0005-2736(87)90456-1. [DOI] [PubMed] [Google Scholar]
- 28.Mangan P., Colombini M. Proc. Natl. Acad. Sci. USA. 1987;84:4896–4900. doi: 10.1073/pnas.84.14.4896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Debatin K., Poncet D., Kroemer G. Oncogene. 2002;21:8786–8803. doi: 10.1038/sj.onc.1206039. [DOI] [PubMed] [Google Scholar]
- 30.Hodge T., Colombini M. J. Membr. Biol. 1997;157:271–279. doi: 10.1007/s002329900235. [DOI] [PubMed] [Google Scholar]
- 31.Sampson J., Ross L., Decker W., Craigen W. J. Biol. Chem. 1998;273:30482–30486. doi: 10.1074/jbc.273.46.30482. [DOI] [PubMed] [Google Scholar]
- 32.Song J., Midson C., Blachly-Dyson E., Forte M., Colombini M. Biophys. J. 1998;74:2926–2944. doi: 10.1016/S0006-3495(98)78000-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liberatori S., Canas B., Tani C., Bini L., Buonocore G., Godovac-Zimmermann J., Mishra O., Delivoria-Papadopoulos M., Bracci R., Pallini V. Proteomics. 2004;4:1335–1340. doi: 10.1002/pmic.200300734. [DOI] [PubMed] [Google Scholar]
- 34.Benimetskaya L., Wittenberger T., Stein C. A., Hofmann H.-P., Weller C., Lai J., Miller P., Gekeler V. Clin. Cancer Res. 2004;10:3678–3688. doi: 10.1158/1078-0432.CCR-03-0569. [DOI] [PubMed] [Google Scholar]
- 35.Benimetskaya L., Lai J., Wu S., Hua E., Khvorova A., Miller P., Stein C. A. Clin. Cancer Res. 2004;10:8371–8379. doi: 10.1158/1078-0432.CCR-04-1294. [DOI] [PubMed] [Google Scholar]
- 36.Shimizu S., Ide T., Yanagida T., Tsujimoto Y. J. Biol. Chem. 2000;275:12321–12325. doi: 10.1074/jbc.275.16.12321. [DOI] [PubMed] [Google Scholar]
- 37.Rostovtseva T., Antonsson B., Suzuki M., Youle R., Colombini M., Bezrukov S. J. Biol. Chem. 2004;14:13575–13583. doi: 10.1074/jbc.M310593200. [DOI] [PubMed] [Google Scholar]
- 38.Colombini M. Methods Enzymol. 1987;148:465–475. doi: 10.1016/0076-6879(87)48045-2. [DOI] [PubMed] [Google Scholar]
- 39.Freitan H., Benz R., Neupert W. Methods Enzymol. 1983;97:286–294. doi: 10.1016/0076-6879(83)97140-9. [DOI] [PubMed] [Google Scholar]
- 40.Blachly-Dyson E., Peng S., Colombini M., Forte M. Science. 1990;247:1233–1236. doi: 10.1126/science.1690454. [DOI] [PubMed] [Google Scholar]