Abstract
The Rpg1 gene confers resistance to many pathotypes of the stem rust fungus Puccinia graminis f. sp. tritici and has protected barley from serious disease losses for over 60 years. Rpg1 encodes a constitutively expressed protein with two tandem kinase domains. Fractionation by differential centrifugation and aqueous two-phase separation of the microsome proteins located Rpg1 mainly in the cytosol but also in the plasma membrane and intracellular membranes. Recombinant Rpg1 autophosphorylates in vitro intramolecularly only serine and threonine amino acids with a preference for Mn2+ cations and a Km of 0.15 and a Vmax of 0.47 nmol·min−1·mg−1 protein. The inability of wild-type Rpg1 to transphosphorylate a recombinant Rpg1 inactivated by site-directed mutation confirmed that Rpg1 autophosphorylation proceeds exclusively via an intramolecular mechanism. Site-directed mutagenesis of the two adjacent lysine residues in the ATP anchor of the two-kinase domains established that the first of the two tandem kinase domains is nonfunctional and that lysine 461 of the second domain is the catalytically active residue. Transgenic barley, expressing Rpg1 mutated in either the kinase 1 or 2 domains, were fully susceptible to P. graminis f. sp. tritici revealing requirement of both kinase domains for resistance. In planta-expressed Rpg1 mutant protein confirmed that mutation in domain 2, but not 1, rendered the protein incapable of autophosphorylation.
Keywords: cultivar, protein kinase 1 domain, protein kinase 2 domain
Disease resistance in plants is frequently governed by a gene-for-gene mechanism in which avirulence (Avr) gene products encoded by a pathogen are specifically recognized by plant disease resistance (R) gene products (1) and, thereafter, activate signaling cascades leading to resistance (2, 3). Predicted or identified subcellular locations of the R proteins and their corresponding Avr proteins indicate a clear display of spatial interdependency of both components. Among the ≈50 cloned R genes (3, 4), four encode serine/threonine (S/T) protein kinases. These Rgenes are the tomato Pto gene conferring resistance to the bacterium Pseudomonas syringae pv. tomato (4, 5), the Arabidopsis Pbs1 gene, conferring resistance to Pseudomonas syringae pv. phaseolicola, required for Rps5-mediated resistance (6), the gene for the rice receptor-like S/T kinase Xa21 with an extracellular leucine-rich repeat domain conferring resistance to Xanthomonas campestris pv. oryzae (7, 8), and the barley stem rust resistance gene Rpg1 with two tandem kinase domains. Rpg1 confers resistance to the stem rust fungus Puccinia graminis f. sp. tritici (9, 10).
The 164-aa AvrPto protein (11, 12) is delivered from the bacterium into the plant through the type III secretion pathway (13). It carries two different recognition regions, one interacting specifically with the Pto gene of tomato and another interacting with an R gene of tobacco as recognized by virulence-disrupting point mutations (14). AvrPto, synthesized in transgenic tobacco leaf cells with tetracycline-inducible gene expression, attached AvrPto to the plant cell plasma membrane by virtue of its myristoylation peptide (14). There, it interacts directly with the S/T kinase (15–17). Activity and autophosphorylation of the intracellular S/T kinase are required for resistance (18, 19), as is the presence of a functional Prf gene encoding an leucine-rich repeat domain and a nucleotide-binding site with a downstream role in the signal transduction chain (19). Pto interacts with several other proteins, a few of which also serve as substrates for Pto, including another S/T kinase and a family of transcription activators, ultimately leading to induction of defense-related genes, an oxidative burst, and localized cell death (20–23). Under in vitro conditions, Pto autophosphorylates multiple serine and threonine residues via an intramolecular mechanism. Among these residues, phosphorylation of T38 and S198 is essential for the hypersensitive response (18, 24). Pto belongs to the family of protein kinases, which have an arginine immediately preceding the conserved catalytic aspartate (RD kinases) and autophosphorylates its activation domain in vitro (18). The rice receptor-like Xa21 S/T kinase domain, a non-RD kinase, likewise undergoes intramolecular autophosphorylation but has different patterns of phosphorylation despite sharing catalytic domains and similar kinetic properties (8).
We cloned the Rpg1 gene by using a map-based approach (9). Rpg1 confers resistance to many pathotypes of Puccinia graminis f. sp. tritici (25). The functional identity of the gene was confirmed by haplotype sequencing and by transformation of a susceptible barley cultivar (cv.) Golden Promise, which was rendered immune to the stem rust pathogen (10). Rpg1 is constitutively expressed in all plant parts and developmental stages tested and produces numerous alternate mRNA splice forms, most of which are predicted to translate into aberrant proteins (26). One minor alternate splice form could encode a full-length protein with a potential transmembrane domain and a signal peptide by retaining the first intron (26). This form, found both in the resistant cv. Morex and in Golden Promise made resistant by transformation with a single copy of Rpg1, could play a critical role in the Rpg1 modular interplay from signal perception to transduction.
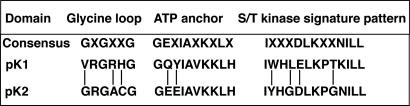
The predicted Rpg1 protein is a unique receptor-like kinase containing a weak putative transmembrane domain and a C-terminal farnesylation site but lacking a specific signal peptide sequence. It is a non-RD kinase and is characterized by the presence of dual kinase domains. Although the two domains, designated pK1 and pK2, share 58.4% amino acid sequence homology, only pK2 has all of the functional features of a typical S/T kinase. pK1 has critical residues altered in the glycine loop, in the binding site of the ATP anchor, in the S/T kinase active signature motif, and in the ATP phosphotransfer domain (Fig. 1). The nearly invariant aspartic acid involved in phosphotransfer is substituted by glutamic acid in the pK1 domain.
Fig. 1.
Key variant amino acids distinguish the Rpg1 two-kinase domains. The pK2 domain conforms strictly to the consensus of all functional features of an S/T kinase (27), whereas the pK1 domain has altered residues in the three depicted critical motifs. The nearly invariant aspartic acid involved in phosphotransfer in the pK2 domain is substituted by glutamic acid in pK1.
In the presented experiments, we have investigated the subcellular location of the Rpg1 protein, the phosphorylation capacity of the two kinase domains, and whether both kinase domains are required for resistance to P. graminis f. sp. tritici.
Results
Rpg1 Is Located in the Cytosol, Plasma Membrane, and Endomembranes.
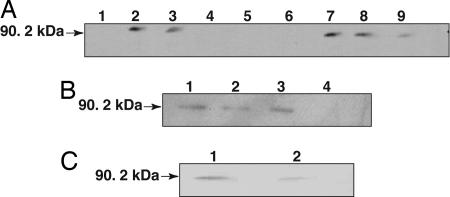
Total barley seedling leaf protein extract was fractionated by centrifugation, and the fractions were subjected to Western blot analysis by using Rpg1-specific antibody. A majority of the Rpg1 protein was recovered in the 100,000 × g supernatant, whereas a minor fraction was present in the 100,000 × g pellet (Fig. 2A). The 100,000 × g pellet retained Rpg1 after proteinase K and high pH treatment, indicating that the microsomes are intact and Rpg1 is integral with the inner face of the membrane vesicles (Fig. 2B). Further separation of the microsomal fraction by an aqueous two-phase system into plasma and intracellular membrane fractions revealed that Rpg1 is associated with both the plasma membrane and intracellular membranes (Fig. 2C). Identity of the plasma membrane and exclusion of significant contamination with intracellular membranes was verified by marker enzymes: ≈90% of the ATPase activity in the upper phase plasma membrane preparation was inhibited by 50 μM vanadate (Na3VO4). The endoplasmic reticulum NADPH-dependent cytochrome c reductase was distributed with 401.3 and 19.4 μmoles·min−1·mg−1 to the intracellular and plasma membranes, respectively. The mitochondrial cytochrome c oxidase was distributed correspondingly with 643.7 and 19.4 μmoles·min−1·mg−1 to the intracellular and plasma membrane fractions.
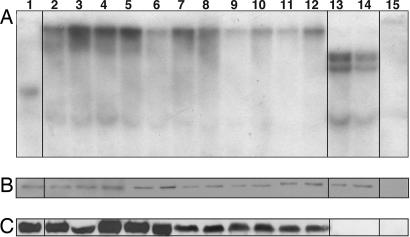
Fig. 2.
Immunoblot analysis of subcellular localization of Rpg1. (A) Crude Rpg1 protein extracted from 10-day-old Morex seedling leaves was fractionated by centrifugation and visualized by immunoblotting. Lane 1, cell wall debris retained by Mira cloth filter; lane 2, total extract after filtration; lane 3, 3,000 × g × 15 min supernatant; lane 4, 3,000 × g × 15 min pellet; lane 5, 15,000 × g × 15 min pellet; lane 6, empty; lane 7, 15,000 × g × 15 min supernatant; lane 8, 100,000 × g × 2 h supernatant; lane 9, 100,000 × g × 2 h pellet. Approximately 25 μg of total protein were loaded per lane. (B) The 100,000 × g pellet was treated with either 100 mM Na2CO3 (pH 11.5) or proteinase K (100 μg/ml) at 0°C for 30 min. The reaction mixtures were centrifuged at 14,000 × g, and the supernatant and precipitate were subjected to SDS/PAGE followed by Western blot analysis. Lanes 1 and 2, Na2CO3-treated microsomal pellet and supernatant; lanes 3 and 4, proteinase K-treated microsomal pellet and supernatant. (C) The 100,000 × g pellet was separated by two-phase aqueous partitioning (28), and the products were analyzed by SDS/PAGE and immunoblotting. Assays of marker enzymes for plasma membrane, endoplasmic reticulum, and mitochondria were as described in ref. 29. Lane 1, intracellular membranes; lane 2, plasma membrane.
In an attempt to identify the protein translated from the alternate spliced transcript form in the microsomal fraction, we resorted to MALDI-TOF analysis. The tryptic digests of the protease-resistant microsomal fragment of Rpg1, when subjected to MALDI-TOF analysis, failed to identify a peptide of 2,485.18 m/z (MMVRLAAKSCCCWLLHGLICLFFLWGSQRRMVMK), predicted to result from digestion of Rpg1 translated from the alternatively spliced transcript with the retained first intron. On the other hand, the peptides expected from correctly spliced Rpg1 transcripts were identified. In a second approach to identify a protein with the translated intron sequence, an antibody was raised to the synthetic LHGLICLFFLWGSQ peptide encoded in the first intron. Although the antibodies recognized the peptide by ELISA, they failed to identify the Rpg1 alternate spliced form in the microsomal fraction.
Rpg1 Is a Functional Kinase.
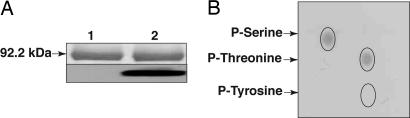
Full-length Rpg1 was expressed in Pichia pastoris as a C-terminal His-tagged recombinant protein by using the pPICZB plasmid (Invitrogen) and purified by nickel chelate affinity chromatography. The purified Rpg1 protein catalyzed autophosphorylation with [γ-32P]ATP (Fig. 3A). Two-dimensional thin layer electrophoresis of the hydrolyzed phosphoprotein and autoradiography showed serine and threonine, but not tyrosine, to be phosphorylated (Fig. 3B).
Fig. 3.
In vitro autophosphorylation of serine and threonine residues by Rpg1. (A) Purified recombinant Rpg1 was incubated with [γ-32P]ATP, separated by gel electrophoresis, stained with Coomassie blue (Upper), and autoradiographed (Lower). Lane 1, control; lane 2, incubated with [γ-32P]ATP. (B) γ-32P-labeled Rpg1 was hydrolyzed with HCl and subjected to two-dimensional thin layer electrophoresis and autoradiography. The radioactive spots correspond to phosphoserine and phosphothreonine. Expected position of phosphotyrosine is circled.
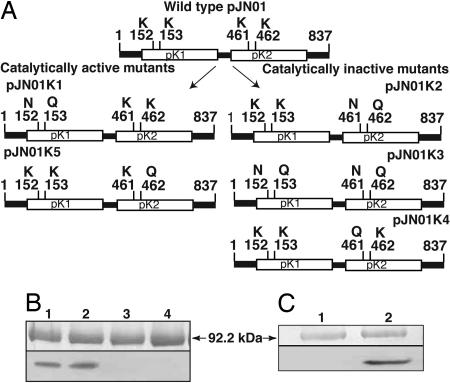
To determine whether one or both kinase domains are active, several mutants were generated by site-specific mutagenesis. Lysines 152 and 153 of the pK1 and 461 and 462 of the pK2 domain in the ATP anchor were substituted by asparagine and glutamine separately in each domain and in both domains simultaneously (Fig. 4). Autophosphorylation assays revealed that the pK1 mutant retained an intrinsic catalytic activity, whereas the pK2 mutant and the double kinase mutant completely lost their ability to autophosphorylate. This loss indicated that the pK1 domain is not functional and that the pK2 domain is functional for autophosphorylation (Fig. 4B). Further, the K461Q mutant completely lost autokinase activity, whereas the K462Q mutant was fully active, indicating that the lysine at position 461 is the catalytic residue (Fig. 4C). These results also demonstrated that phosphorylation of the fusion protein was not due to contaminating yeast proteins, because pK2-K461Q and the double mutant lost their activity completely.
Fig. 4.
Site-directed mutations and their catalytic activity. (A) Lysines 152 and 153 of the pK1 and 461 and 462 of the pK2 domain in the ATP anchor were substituted by asparagine and glutamine separately in each domain and in both domains simultaneously. (B) The pK1 mutant (pJN01K1) retained autophosphorylation activity (lane 2), whereas the pK2 mutant (pJN01K2) and the double mutant (pJN01K3) lost their catalytic activity (lanes 3 and 4). Lysine 461 or 462 was mutated to glutamine in pJN01K4 and pJN01K5, respectively. (C) The pJN01K4 construct lost phosphorylation activity (lane 1), whereas the pJN01K5 retained it (lane 2), identifying lysine 461 as the catalytic residue. Wild-type phosphorylation is presented (B, lane 1), and numbering of the amino acids is according to GenBank (no. AF509747) (9).
Function of both Kinase Domains Is Required for Disease Resistance.
The Rpg1 gene was mutated to encode asparagine and glutamine instead of the two adjacent lysines in the ATP anchor of the pK1 and pK2 kinase domains (cf. Figs. 1 and 4). The two plasmids carrying the mutated pK1 or pK2 domains were transformed into cv. Golden Promise lacking the Rpg1 gene. Eleven T2 progeny plants analyzed from a transformant containing the mutated pK1 transgene with a single copy insertion (Fig. 5A) were all susceptible with infection type 3 when inoculated with pathotype MCC of P. graminis f. sp. tritici (Fig. 6). Two T1 transformants containing the mutated pK2 transgene with three insertion copies (Fig. 5A) were likewise completely susceptible (Fig. 6). The mutated Rpg1 gene was transcribed and translated in all transformants as evidenced by Western blot analysis (Fig. 5B) and real-time PCR with an average value of 71.2 ± 3.1, by using wild-type transgene set at 100 and the Golden Promise host as 0. Autophosphorylation assays of the in planta-expressed Rpg1 mutant proteins revealed that all pK1 transformants retained their ability to autophosphorylate, whereas the pK2 transformants completely lost this ability (Fig. 5C).
Fig. 5.
Transformants of susceptible barley cv. Golden Promise with mutated domain pK1 or pK2. The two lysines in the ATP anchor were mutated to N and Q. (A) Southern blot of HindIII-digested barley DNA hybridized with the 4.5-kb genomic probe, RSB228. Lane 1, cv. Morex; lanes 2–12, T2 transformants containing a single copy of the mutated pK1 transgene of pK1; lanes 13 and 14, T1 transformants containing three copies of the mutated pK2 transgene of pK2; lane 15, cv. Golden Promise. (B) Western blot demonstrating that all lines except cv. Golden Promise express the Rpg1 protein. (C) Autophosphorylation of in planta synthesized Rpg1. Rpg1 was immunoprecipitated from the same leaf samples scored for disease and subjected to autophosphorylation. Rpg1 from the mutated pK1 transformants exhibited autocatalytic activity (lanes 2–12), whereas Rpg1 of the mutated pK2 transformants lost the ability to autophosphorylate (lanes 13 and 14).
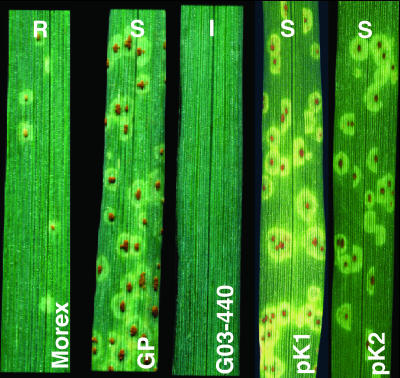
Fig. 6.
Susceptible disease reaction of mutated pK1 or pK2 domain transgenic seedlings compared with resistant Morex (R), susceptible Golden Promise (S), and highly resistant transformant (G03-440) with the wild-type Rpg1 gene (I).
Rpg1 Autophosphorylation Activity Requires the Presence of Either Mn2+ or Mg2+ but Is Inactive in the Presence of Ca2+ Ions.
A linear increase in the enzymatic activity of Rpg1 was observed in the range of 1.25–40 mM of MgCl2 and 1.25–20 mM of MnCl2 (Fig. 8 A–C, which is published as supporting information on the PNAS web site). The kinase activity of Rpg1 was higher in the presence of MnCl2 than of MgCl2 and inactive with CaCl2. His-Rpg1 exhibited a standard Michaelis–Menten kinetics with respect to ATP (Fig. 9, which is published as supporting information on the PNAS web site). The Km and Vmax values for ATP, determined by a double reciprocal plot, were 0.15 and 0.47 nmol·min−1·mg−1 protein, respectively.
Rpg1 Autophosphorylation Occurs Through an Intramolecular Mechanism.
To test whether autophosphorylation proceeds via an intra- or intermolecular mechanism, autophosphorylation was carried out in the presence of increasing concentrations of His-Rpg1. The relative phosphorylation reaction followed first-order kinetics, suggesting an intramolecular mechanism (Fig. 10A, which is published as supporting information on the PNAS web site). The van’t Hoff analysis of autophosphorylation (logarithm of phosphorylation rate versus logarithm of enzyme concentration) illustrated a slope of 1.04 ± 0.02 and a correlation coefficient of 0.99 for linear regression (Fig. 10 B and C). Further, the inability of the recombinant wild-type Rpg1 to transphosphorylate a recombinant kinase inactivated by mutation of K461N and K462Q in the pK2 ATP anchor confirmed that Rpg1 autophosphorylation proceeds exclusively via an intramolecular mechanism (Fig. 7).
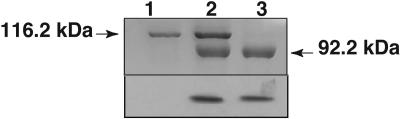
Fig. 7.
Purified His-tagged active Rpg1 expressed in P. pastoris failed to transphosphorylate the purified GST-tagged K461N + K462Q inactive mutant Rpg1 expressed in E. coli. Proteins were analyzed by SDS/PAGE, Coomassie stained (Upper), and autoradiographed (Lower). Lane 1, GST-tagged mutant Rpg1; lane 2, equal mixture of wild-type Rpg1 and mutant Rpg1; lane 3, wild-type Rpg1.
Discussion
Rpg1 is a functionally active S/T kinase with two tandem kinase domains. By site-directed mutagenesis of the two adjacent lysines in the ATP-anchor, we demonstrated that the N-terminal domain does not autophosphorylate, whereas the C-terminal domain is active in autophosphorylation of serine and threonine residues in vitro. The Rpg1 gene, mutated in both kinase domains individually, was expressed in barley transformants and tested for their ability to provide rust resistance to a cultivar lacking the Rpg1 gene. The transformants with the mutated pK1 domain retained the ability to autophosphorylate, whereas the mutated pK2 domain transformants lost this ability. However all transformants expressed the transgene at the mRNA and protein level but were susceptible to the pathogen, demonstrating the requirement of both kinase domains for rust resistance. Mammalian Jak2 also contains two tyrosine kinases in tandem, one lacking critical amino acids for kinase function followed C-terminally by a domain with all requisite residues. Removal of the “pseudodomain” increased kinase activity 8-fold, leading to IFN ligand independent activation of STAT5, one of the signal transducers and transcription activators of the pathway (30). It remains to be determined what control is exerted by the “pseudokinase” domain of Rpg1.
The constitutively expressed mammalian tyrosine-specific Janus kinases (Jak1, Jak2, Jak3, and Tyk2) are of special interest in connection with our findings. Contrary to earlier suggestions for their cytosolic location, Jak1 and Jak2 have now been unequivocally located to the plasma membrane and the endoplasmic reticulum by subcellular fractionation, immunofluorescence, and confocal microscopy of yellow fluorescence protein-tagged Jaks (31). The reason for earlier misinterpretations were the use of antibodies recognizing only the targeted Jak protein and underrepresentation of the membrane-located Jaks by depletion of adhering membranes in the step removing the nuclei. Jak 1 remains a membrane-localized protein after cytokine stimulation, suggesting that the cytokine receptor-Jak kinase complexes are receptor tyrosine kinases.
The barley stem rust resistance protein Rpg1 was determined to reside primarily in the cytosol with smaller but significant amounts associated with membranes. In light of the Jak kinase findings, further investigations are necessary to refine the subcellular localization of Rpg1.
The corresponding Avr gene of the fungus P. graminis f. sp. tritici has not been cloned, and thus the delivery and location within the cell is not known. Rust pathogen filaments grow among the mesophyll cells and then penetrate the host cell walls to form haustoria; however, the haustoria remain separated from the cytoplasm of host cells. Flax rust Avr genes encoding AvrL proteins with a size of 127 aa that are incompatible with the rust resistance genes L5, L6, and L7 were isolated from Melampsora lini (32, 33). The encoding genes are transcribed in the haustoria. Transient intracellular expression of the AvrL proteins in the presence of corresponding R genes in the host elicited a strong cell death response, whereas plants transgenic for AvrL proteins crossed with plants expressing incompatible L genes gave lethal progeny. These results imply that the Avr proteins are secreted from haustoria into the extrahaustorial matrix and recognized by the intracellularly expressed L proteins, either by their translocation into the host cell or by interaction at the plasma membrane. The differential centrifugation procedure developed for Rpg1 can be applied to infected leaf segments and used to determine the proteins that bind with Rpg1 by using Blue native electrophoresis for isolation of membrane complexes (34, 35) possibly by combination with cross-linking procedures for enzymatic active complexes (36). This procedure can supplement the yeast two-hybrid approach and is essential to identify both the avirulence protein and the initial components of the signal transduction chain activated by the Rpg1 protein.
In conclusion, the elucidated functions of the Rpg1 serine and threonine kinase providing durable resistance to stem rust in barley show significant parallels to the complex signal pathways recognized in analysis of the Pto gene providing resistance to bacterial speck. It also has remarkable similarity to the cytokine receptor-signaling pathway of mammals.
Methods
Antibody Production.
Polyclonal antibody was raised against a synthetic peptide NKLTATPLEEKSRSC, representing a unique region of the predicted Rpg1 protein in rabbits (Alpha Diagnostics International, San Antonio, TX). Affinity-purified antibodies were used for Western blot analysis. The peptide LHGLICLFFLWGSQ, encoded by the first intron in the proposed alternate splice form, was synthesized by Alpha Diagnostics and solubilized in DMSO. Polyclonal antibodies were raised in New Zealand White rabbits at the Washington State University animal facility by using Ribi adjuvant according to manufacturer’s recommendation (Corixa, Hamilton, MT).
SDS/PAGE and Immunoblotting.
SDS/PAGE gels consisted of 10% (wt/vol) acrylamide:bisacrylamide (29:1) (pH 8.8) in the resolving phase and 5% (pH 6.8) in the stacking phase. Protein extracts were mixed with Laemmli’s loading buffer (37) and boiled for 3 min at 95°C before fractionation, followed by transfer to poly(vinylidene difluoride) membrane. The membrane was incubated with Rpg1 antiserum (dilution: 1:3000) in PBS containing 10% (wt/vol) nonfat dry milk, and the antigen-antibody complexes were visualized with horseradish peroxidase-conjugated goat antibody against rabbit IgG by using the Nu Glo-Enhanced chemiluminescent detection system from Alpha Diagnostics International (San Antonio, TX) according to manufacturer’s recommendations.
MALDI-TOF.
The proteinase K-resistant Rpg1 in microsomal membranes was excised from a silver-stained SDS/PAGE gel and subjected to in-gel digestion with trypsin (38). The tryptic peptides were then subjected to MALDI-TOF, and data was analyzed by mascot or proteinprospector software.
Synthesis and Purification of 6×His-Tagged Rpg1 with P. pastoris Vector pPICB (Invitrogen).
The Rpg1 cDNA pNRG072 (10) was amplified by PCR with the primers listed in Table 1, which is published as supporting information on the PNAS web site, and cloned into the NotI cloning site of the vector to yield pJN01. Insert orientation and sequence was confirmed by using primers provided by the manufacturer and gene specific primers (Table 2, which is published as supporting information on the PNAS web site). Purified pJN01 DNA was linearized at the unique BstXI restriction site and transformed into P. pastoris GS115 by the lithium acetate method (39).
Cultures (500 ml in 2-liter flasks) were grown in minimal medium (13.4 g/liter yeast nitrogen base without amino acids/400 μg/liter biotin/0.1 M Mes-NaOH, pH 6.5) containing 1% (vol/vol) glycerol at 28°C with vigorous shaking (250 rpm). After the cultures reached saturation (48–72 h), cells were harvested by centrifugation and resuspended in 0.5 volume of minimal medium containing 0.5% (vol/vol) methanol. The incubation was continued for another 72 h, during which time additional methanol (5 ml/liter) was added at 24 and 48 h. Cells were harvested by centrifugation, washed once with ice-cold distilled water, and stored at −80°C. For purification, cells were thawed, resuspended in lysis buffer (final concentration 25 mM Tris·HCl, pH 7.5/500 mM NaCl/0.2% Triton X-100/10 mM imidazole), EDTA-free protease inhibitor tablets (Roche Molecular Biochemicals) and incubated on ice for 30 min. PMSF was added to a final concentration of 1 mM immediately before lysis by sonication (4 × 15 s with cooling on ice). The lysate was cleared by centrifugation at 40,000 × gfor 25 min. The supernatant was subjected to nickel chelate affinity chromatography according to manufacturer’s instructions (Sigma). Fractions of 1 ml were collected, and those fractions containing Rpg1 as determined by spectrophotometric monitoring (A280) and SDS/PAGE were dialyzed at 4°C for at least 4 h against buffer containing 25 mM Tris·HCl, pH 7.5/100 mM NaCl/1 mM EDTA/1 mM DTT. Protein samples were stored in aliquots at −80°C.
Site-specific mutagenesis was carried out by using the QuikChange system (Stratagene) with primers specified in Table 1. The site-directed mutations, as well as the absence of undesired mutations, were confirmed by DNA sequencing. The plasmids with the mutated Rpg1 were expressed in P. pastoris as described for wild-type Rpg1, and the purified protein was tested for autophosphorylation by using [γ-32P]ATP.
The Rpg1 mutant constructs were generated by cloning a 3.1-kb HindIII--ClaI fragment containing the pK1 and pK2 domain of the genomic clone pNRG028 (9) into the respective sites of pBluescript II KS± (Stratagene). The plasmids were subjected to site-specific mutagenesis. The inserts with the confirmed desired mutations were used to replace the equivalent fragment in the wild-type Rpg1 gene in the plasmid pNRG040, which is a derivative of the Agrobacterium vector pJH260 (10).
Autophosphorylation and Phosphoamino Analysis.
For assays, 0.5–1 μg of His-Rpg1 was incubated with 5 μCi (1 Ci = 37 GBq) of [γ-32P]ATP in 50 mM Tris (pH 7.0), 1 mM DTT, and 10 mM MnCl2 and 20 mM ATP at room temperature for 20 min. The proteins were precipitated with trichloroacetic acid (28% final concentration) for 30 min on ice. The pellets obtained after centrifugation were washed three times with ice-cold acetone, solubilized, and subjected to SDS/PAGE and autoradiography. For phosphoamino analysis, autophosphorylated protein was hydrolysed with 6 M HCl for 90 min at 110°C. The hydrolysate was dried and washed twice with distilled water. The residue was dissolved in 10 μl of 2.2% formic acid and 7.8% acetic acid. The samples were loaded onto a TLC plate (Analtech) and subjected to electrophoresis horizontally at pH 1.9 and vertically at pH 3.5. The γ-32P-labeled amino acids were detected by autoradiography and compared with authentic standards of phosphothreonine, phosphoserine, and phosphotyrosine (Sigma).
In ATP-dependent autophosphorylation, 0.5 μg of Rpg1 protein was incubated with 5–50 μM nonradioactive ATP containing 5 μCi of [γ-32P]ATP in a total volume of 30 μl kinase buffer and stopped after 20 min by adding 10 μl Laemmli buffer (37). ATP incorporation was calculated based on the radioactive counts and concentration of cold ATP. Km and Vmax were estimated by double reciprocal plot and linear regression analysis.
Intramolecular autophosphorylation assays were performed according to Sessa et al. (18). Increasing concentrations of the fusion proteins from 0.32–18 μM were incubated with 5 μCi of [γ-32P]ATP. The reactions were stopped after 20 min by adding an equal volume of 75 mM H3PO4. One half of the reaction was spotted onto P-81 phosphocellulose paper and washed six times with 0.75 M phosphoric acid (10 min per wash). The filters were washed with 200 ml of acetone at RT for 5 min and dried at RT. The incorporated 32P was measured by using a scintillation counter and subjected to linear regression analysis.
Purification of Rpg1 Fusion Protein in Escherichia coli and Transphosphorylation Assay.
The Rpg1 cDNA clone pNRG072 was mutated in the pK2 domain by substituting K461 and 462 with N and Q by using the primers described earlier and cloned as a N-terminal fusion protein with a glutatione S-transferase (GST) tag in pGEX6P-1 (pJN01K2-GS) and expressed in E. coli strain BL21. Protein expression was induced with 0.1 mM isopropyl β-d-thiogalactoside at 16°C, and the protein was purified by using glutathione agarose Hi-TRAP column from Amersham Pharmacia. Transphosphorylation ability of wild-type Rpg1 was assayed by mixing equal amounts of active His-Rpg1 and kinase deficient GST-Rpg1 and incubating the mixture at 20°C for 20 min.
Plant Transformation, Southern blot analysis, real-time PCR, and phenotyping for disease resistance were carried out as described in refs. 10, 25, and 40.
Autophosphorylation of in Planta-Expressed Rpg1.
For immunoprecipitations, ≈500 micrograms of total plant protein was combined with 30 μl of affinity purified Rpg1 antisera in 500 μl of extraction buffer and 500 μl of 2× immunoprecipitation buffer (1M KCl, 0.02M EDTA, and 2 mM PMSF and rotated end-over-end at 4°C for overnight). The immune complexes were precleared with 30 μl of protein A agarose on ice for 1 h, washed thrice with 1 ml of ice-cold 1× immunoprecipitation buffer, and subjected to autophosphorylation as described under Autophosphorylation.
Supplementary Material
Acknowledgments
We thank Gwen M. Anderson, Stephanie Dahl, and Tamas Szinyei for technical assistance. Research was supported by National Research Initiative of the United States Department of Agriculture Cooperative State Research, Education, and Extension Service Grant 2004-35301-14635 (to A.K. and B.J.S.). This is scientific paper 0503-05 from the College of Agriculture, Human, and Natural Resource Sciences, Washington State University, Project 0196.
Abbreviations
- Avr
avirulence
- cv.
cultivar
- pK1
protein kinase 1 domain
- pK2
protein kinase 2 domain
- R
resistance
- S/T
serine and threonine
- RD
arginine and aspartate.
Footnotes
Conflict of interest statement: No conflicts declared.
References
- 1.Flor H. H. Annu. Rev. Phytopathol. 1971;9:275–296. [Google Scholar]
- 2.Hammond-Kossack K. E., Jones J. D. G. Annu. Rev. Plant. Physiol. Plant Mol. Biol. 1997;48:575–607. doi: 10.1146/annurev.arplant.48.1.575. [DOI] [PubMed] [Google Scholar]
- 3.Hulbert S. H., Webb C. A., Smith S. M., Sun Q. Annu. Rev. Phytopathol. 2001;39:285–312. doi: 10.1146/annurev.phyto.39.1.285. [DOI] [PubMed] [Google Scholar]
- 4.Martin G. B., Bogdanove A. J., Sessa G. Annu. Rev. Plant Biol. 2003;54:23–61. doi: 10.1146/annurev.arplant.54.031902.135035. [DOI] [PubMed] [Google Scholar]
- 5.Martin G. B., Brommonschenkel S. H., Chunwongse J., Frary A., Ganal M. W., Spivey R., Wu T., Earle E. D., Tanksley S. D. Science. 1993;262:1432–1436. doi: 10.1126/science.7902614. [DOI] [PubMed] [Google Scholar]
- 6.Swiderski M. R., Innes R. W. Plant J. 2001;26:101–112. doi: 10.1046/j.1365-313x.2001.01014.x. [DOI] [PubMed] [Google Scholar]
- 7.Song W.-Y., Wang G. L., Chen L., Kim H., Pi L., Gardner J., Wang B., Holsten T., Zhai W., Zhu L., et al. Science. 1995;270:661–667. doi: 10.1126/science.270.5243.1804. [DOI] [PubMed] [Google Scholar]
- 8.Liu G. Z., Pi L. Y., Walker J. C., Ronald P. C., Song W. Y. J. Biol. Chem. 2002;277:20264–20269. doi: 10.1074/jbc.M110999200. [DOI] [PubMed] [Google Scholar]
- 9.Brueggeman R., Rostoks N., Kudrna D., Kilian A., Han F., Chen J., Druka A., Steffenson B. J., Kleinhofs A. Proc. Natl. Acad. Sci. USA. 2002;99:9328–9333. doi: 10.1073/pnas.142284999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horvath H., Rostoks N., Brueggeman R., Steffenson B. J., von Wettstein D., Kleinhofs A. Proc. Natl. Acad. Sci. USA. 2003;100:364–369. doi: 10.1073/pnas.0136911100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ronald P. C., Salmeron J. M., Carland F. M., Staskawicz B. J. J. Bacteriol. 1992;174:1604–1611. doi: 10.1128/jb.174.5.1604-1611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salmeron J. M., Staskawicz B. J. Mol. Gen. Genet. 1993;239:6–16. doi: 10.1007/BF00281595. [DOI] [PubMed] [Google Scholar]
- 13.Nimchuk Z., Marois E., Kjemtrup S., Leister T. R., Katagiri F., Dangl J. L. Cell. 2000;101:353–363. doi: 10.1016/s0092-8674(00)80846-6. [DOI] [PubMed] [Google Scholar]
- 14.Shan L. B., Thara V. K., Martin G. B., Zhou J. M., Tang X. Plant Cell. 2000;12:2323–2337. doi: 10.1105/tpc.12.12.2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Loh Y-T., Martin G. B. Plant Physiol. 1995;108:1735–1739. doi: 10.1104/pp.108.4.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scofield S. R., Tobias C. M., Rathjen J., Chang J. H., Lavelle D. T., Michelmore R. W., Staskawicz B. J. Science. 1996;274:2063–2065. doi: 10.1126/science.274.5295.2063. [DOI] [PubMed] [Google Scholar]
- 17.Tang X., Frederick R. D., Zhou J., Halterman D. A., Jia Y., Martin G. B. Science. 1996;274:2060–2063. doi: 10.1126/science.274.5295.2060. [DOI] [PubMed] [Google Scholar]
- 18.Sessa G., D’Ascenzo M., Martin G. B. EMBO J. 2000;19:2257–2269. doi: 10.1093/emboj/19.10.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rathjen J. P., Chang J. H., Staskawicz B. J., Michelmore R. W. EMBO J. 1999;18:3232–3240. doi: 10.1093/emboj/18.12.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bogdanove A. J., Martin G. B. Proc. Natl. Acad. Sci. USA. 2000;97:8836–8840. doi: 10.1073/pnas.97.16.8836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gu Y-Q., Yang C., Thara V. K., Zhou J., Martin G. B. Plant Cell. 2000;12:771–786. doi: 10.1105/tpc.12.5.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou J., Loh Y-T., Bressan R. A., Martin G. B. Cell. 1995;83:925–935. doi: 10.1016/0092-8674(95)90208-2. [DOI] [PubMed] [Google Scholar]
- 23.Zhou J., Tang X., Martin G. B. EMBO J. 1997;16:3207–3218. doi: 10.1093/emboj/16.11.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sessa G., D’Ascenzo M., Loh Y.-T., Martin G. B. J. Biol. Chem. 1998;273:15860–15865. doi: 10.1074/jbc.273.25.15860. [DOI] [PubMed] [Google Scholar]
- 25.Steffenson B. J. Euphytica. 1992;63:153–167. [Google Scholar]
- 26.Rostoks N., Steffenson B. J., Kleinhofs A. Physiol. Mol. Plant Pathol. 2004;64:91–101. [Google Scholar]
- 27.Hanks S. K., Quinn A. M., Hunter Y. Science. 1988;241:42–52. doi: 10.1126/science.3291115. [DOI] [PubMed] [Google Scholar]
- 28.Larsson C., Widell S., Kjellbom P. Methods Enzymol. 1987;148:558–568. [Google Scholar]
- 29.Briskin D. P., Leonard R. T., Hodges T. K. Methods Enzymol. 1987;148:543–558. [Google Scholar]
- 30.Saharinen P., Takaluamo K., Silvennoine O. Mol. Cell. Biol. 2000;20:3387–3395. doi: 10.1128/mcb.20.10.3387-3395.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Behrmann I., Smyczek T., Heinrich P. C., Schmitz-Van de Leur H., Komyod W., Giese B., Muller-Newen G., Haan C. J. Biol. Chem. 2004;279:35486–35493. doi: 10.1074/jbc.M404202200. [DOI] [PubMed] [Google Scholar]
- 32.Dodds P. N., Lawrence G. J., Catanzariti A. M., Ayliffe M. A., Ellis J. G. Plant Cell. 2004;16:755–768. doi: 10.1105/tpc.020040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Catanzariti A. M., Dodds P. N., Lawrence G. J., Ayliffe M. A., Ellis J. G. Plant Cell. 2006;18:243–256. doi: 10.1105/tpc.105.035980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schägger H., Von Jagow G. Anal. Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 35.Van Coster R., Smet J., George E., De Meirleir L., Seneca S., Van Hove J., Sebire G., Verhelst H., De Bleeker J., Van Vlem B., et al. Pediatr. Res. 2001;50:658–665. doi: 10.1203/00006450-200111000-00020. [DOI] [PubMed] [Google Scholar]
- 36.Aseeva E., Ossenbühl F., Eichacker L. A., Wanner G., Soll J., Vothknecht U. C. J. Biol. Chem. 2004;279:35535–35541. doi: 10.1074/jbc.M401750200. [DOI] [PubMed] [Google Scholar]
- 37.Laemmli U. K. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 38.Shevchenko A., Wilm M., Vorm O., Mann M. Anal. Chem. 1996;68:850–858. doi: 10.1021/ac950914h. [DOI] [PubMed] [Google Scholar]
- 39.Gietz R. D., Schiestl R. H., Williams A. R., Woods R. A. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 40.Kleinhofs A., Kilian A., Maroof M. A. S., Biyashev R. M., Hayes P., Chen F. O., Lapitan N., Fenwick A., Blake T. K., Kanzin V., et al. Theor. Appl. Genet. 1993;86:705–712. doi: 10.1007/BF00222660. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.