Abstract
Whether masked words can be processed at a semantic level remains a controversial issue in cognitive psychology. Although recent behavioral studies have demonstrated masked semantic priming for number words, attempts to generalize this finding to other categories of words have failed. Here, as an alternative to subliminal priming, we introduce a sensitive behavioral method to detect nonconscious semantic processing of words. The logic of this method consists of presenting words close to the threshold for conscious perception and examining whether their semantic content modulates performance in objective and subjective tasks. Our results disclose two independent sources of modulation of the threshold for access to consciousness. First, prior conscious perception of words increases the detection rate of the same words when they are subsequently presented with stronger masking. Second, the threshold for conscious access is lower for emotional words than for neutral ones, even for words that have not been previously consciously perceived, thus implying that written words can receive nonconscious semantic processing.
Keywords: consciousness, emotion, visual masking
How deeply can visual stimuli be processed without being consciously perceived? With nonsymbolic stimuli such as emotional faces, several experiments using blindsight patients (1, 2) or subliminal masking in normal subjects (3, 4) have demonstrated a modulation of amygdala activity in the absence of conscious perception. Behavioral and neuroimaging evidence has led to the hypothesis of a fast subcortical pathway for the visual processing of phylogenetically relevant stimuli such as faces, snakes, or spiders (5–8). This pathway might have evolved to quickly extract low spatial frequencies (9, 10) and provides a fast nonconscious appraisal of stimuli critical to survival.
The situation is very different with symbolic stimuli such as written words. Evaluation of such stimuli cannot be subserved by an evolutionary ancient pathway but requires extensive cortical processing in the ventral visual pathway (11), followed by lexical and semantic access. Whether these pathways can be traversed by a subliminal word up to the semantic level remains controversial. A few studies of masked priming (12, 13) have suggested semantic processing under conditions of a demonstrable lack of conscious perception. These experiments reported semantic priming with faster responses or fewer errors when a target word is preceded by a semantically related nonconscious prime word. However, follow-up publications showed that many of these priming effects could be explained by direct motor specification (14). The prior exposure to a consciously perceived word creates a direct association between this stimulus and the corresponding motor response, bypassing semantic analysis. If the same word is subsequently used as masked prime, this direct link may facilitate, or interfere with, the processing of the upcoming targets. Abrams and Greenwald (14) demonstrated that their nonconscious priming was entirely explained by this prior conscious perception effect. Crucially, priming by novel primes that were never seen consciously was independent of their meaning and was obtained only inasmuch as some of their letter fragments matched a consciously perceived target word. For instance, after the correct conscious categorization of the words “smut” and “bile” as negative, the orthographically related subliminal prime “smile” primed the negative response, not the positive one. Thus, under those conditions, priming was based on orthographic proximity to previously seen targets rather than on subliminal semantic processing.
Currently, the only category of words for which a convincing series of reports has demonstrated nonconscious semantic priming, including generalization to novel primes, is the set of number words (15, 16). Nonconscious semantic processing for less stereotyped and limited sets of words remains uncertain. One recent line of study examined whether the emotional valence of words could be accessed nonconsciously. In a recent publication, Dijksterhuis and Aarts (17) showed that masked negative words could be categorized as negative above chance level, in contrast with positive words, even if subjects could not guess the meaning of the words. However, these results could be interpreted, at least in part, in terms of a systematic response bias that could not be ruled out in the absence of such detection measures as d′ (18). Finally, a demonstration of nonconscious semantic processing for emotional words came recently from intracranial recordings of amygdala (19). Using a masking paradigm, Naccache et al. (19) found that the emotional valence of masked words induced a reproducible modulation of amygdala activity while a total lack of consciousness was demonstrated. Yet those results were obtained from only three patients implanted with depth electrodes before surgery for long-lasting epilepsy, hence raising the issue of whether the patients could be generalized to the normal population.
Interestingly, in the Naccache et al. (19) study, although amygdala recordings gave evidence of subliminal semantic processing, there was no behavioral evidence of nonconscious processing of emotional valence, with no self-reported awareness of word presence and a null d′ in a forced-choice emotional categorization. This negative result suggests that behavioral methods may not be as sensitive as intracranial recordings. Indeed, there is at least one major problem with masked priming and forced-choice experiments (20): To ensure that conscious perception is impossible, such experiments typically use very short target-mask delays and verify that, even with focused attention, forced-choice measures of prime perception remain at chance level. There is a risk that such stringent experimental conditions also suppress all nonconscious semantic activation. Thus, previous priming experiments may have been insensitive to subliminal processing.
Threshold Measurement as an Indicator of Nonconscious Processing
In the present paper, we introduce an alternative behavioral method to demonstrate nonconscious semantic processing. We presented words under a range of experimental conditions that varied from easily seen to undoubtedly subliminal presentation, obtained by varying target-mask stimulus onset asynchrony (SOA). We then measured the subjects’ ability to identify the hidden word, and we examined whether performance differed for words carefully matched in their perceptual and lexical characteristics (length, frequency, etc.) but belonging to different semantic categories (here, we contrasted emotional vs. neutral words). Better performance for one category of words would indicate that the two categories received differential semantic processing. Because this difference in semantic processing would be causally involved in the modulation of the conscious threshold, it would necessarily occur before conscious access.
An interesting property of this method is that it can provide evidence for nonconscious processing with words that are presented at long target-mask intervals, close to the threshold for conscious access. Hence, the method’s sensitivity to nonconscious processes is likely to be superior to masked priming measures, in which shorter SOAs must be used to ensure that, on all trials, no conscious perception occurs.
A seminal paper by Anderson and Phelps (21) made partial use of this method in an attentional blink paradigm with emotional words. In this paradigm, the processing of a first target T1 in a rapid stream of stimuli can suppress conscious perception of a second target T2. Anderson demonstrated that when T2 was an emotionally negative word, it was more often reported by subjects during the blink than if it was a neutral word. This emotional effect was actually better explained by arousal than by valence per se (22, 23), but for the present purposes, this distinction is not critical as arousal and valence are both semantic attributes of words.
Crucially, this result could not be accounted for by a systematic bias to report emotional words, regardless of the hidden word’s nature: in fact, subjects tended to report more neutral words than emotional words. Furthermore, Anderson (22) confirmed that identification was better for negative arousal words than for orthographically similar but neutral words (e.g., danger vs. ranger), even if the report of the negative similar word was scored as a good response for these neutral words. Zeelenberg et al. (24) obtained converging evidence using a different paradigm. Subjects were required to choose which of two alternatives (a target and a foil) corresponded to a briefly flashed sample word. This two-alternative forced-choice task allowed the disentanglement of perceptual enhancement from response bias. Emotional targets were better identified than neutral targets, and this advantage for emotional words resulted from a genuine perceptual enhancement effect rather than from a bias for emotional stimuli.
Although neither Anderson and Phelps (21, 22) nor Zeelenberg et al. (24) discussed this point, do their experiments imply nonconscious semantic processing? There is one potential confounding factor that complicates this interpretation. The prior conscious presentation of emotional words might lead to their superior detection in the absence of nonconscious semantic processing. In the attentional blink studies of Anderson and Phelps (21, 22), word visibility was randomized. Thus, before their presentation at lags where the blink occurs, the same words could have been consciously perceived at lags where no blink occurs. Similarly in Zeelenberg’s paradigm, the masked target words were also used as visible foils. It has been repeatedly shown that subsequent memory is enhanced for consciously perceived emotional words (25–28). Furthermore, feedback from the amygdala to the visual ventral pathway (29) leads to long-lasting improvements in the subsequent visual processing of stimuli associated with a strong emotional experience (30–32). Thus, prior exposure to emotional words might enhance their perception and memory and thus lower their detection threshold without implying semantic mediation.
The Logic of Our Approach
The main purpose of the present work is to demonstrate that negative emotional words do enjoy a better access to consciousness because subliminal semantic processing of emotional content can occur before conscious access. For each subject, we estimated the conscious access threshold for negative emotional words in comparison with neutral words. Furthermore, we examined whether the prior conscious perception of words could modulate this threshold. Crucially, we studied the interaction between these two effects. In particular, we examined whether emotional words that had not been perceived consciously before nevertheless had a lower threshold for conscious access.
We studied the threshold for conscious access during masking. We modulated conscious perception by varying the SOA between each word and a subsequent backward mask. Words were presented from high masking to low masking condition. Half of the words were negative in valence and high in arousal, and the other half were emotionally neutral.
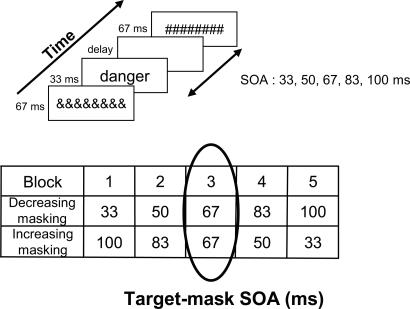
To independently manipulate the prior conscious perception of those words, we further separated these words into two lists, which were randomly assigned to one of two conditions: increasing masking or decreasing masking. Across blocks, some words were first presented in the high masking condition and were progressively unmasked (decreasing masking); whereas the others were first presented in the low masking condition and were progressively masked (increasing masking; see Fig. 1). Importantly, in the central block, all words were presented at the same intermediate SOA of 67 ms, and all words had been presented equally often previously, yet half of them were likely not to have been consciously identified before (those in the decreasing masking condition), whereas the other half were likely to have been identified earlier (those in the increasing masking condition). Thus, on that critical block, our experiment implemented a 2 × 2 factorial design which allowed us to study separately the effects of emotional valence and of prior conscious perception as well as their interaction.
Fig. 1.
Experimental paradigm. (Upper) Trial structure. Subjects were asked to name emotionally negative or neutral words flashed for 33 ms. Each target word was preceded by a 67-ms mask and followed by a variable blank and a 67-ms postmask. The SOA between the target and the subsequent mask varied from 33 to 100 ms. (Lower) Design of Experiment 1. Subjects were presented with five blocks of words. Each block included one presentation of each of the 60 words. For half of the words, masking strength increased across blocks, whereas it decreased for the other half. These two subsets of words were counterbalanced between subjects. The circle highlights the central block in which all words were presented with a 67-ms target-mask SOA. By comparing words from the decreasing and increasing masking conditions, this critical block allowed the disentanglement of the effects of emotional valence and of prior conscious perception.
The primary task was to attempt to name all of the words. All responses were recorded, including errors. After each word presentation, subjects were also asked to rate the word’s visibility by placing a cursor on a quasi-continuous horizontal visual scale ranging from “not seen” at left to “seen” at right (33, 34).
Results
Experiment 1.
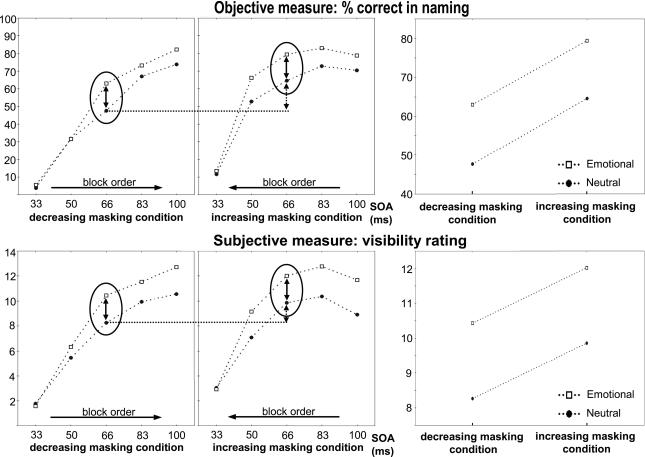
Identification rates varied from 9 to 76% across conditions with a main effect of SOA [F(4,44) = 131, P < 0.0001; see Fig. 2] and of emotion [F(1,11) = 23.10, P < 0.0005], emotional words being identified better than neutral words. An analysis restricted to the central SOA of 67 ms showed a mean identification rate of 64% (see Fig. 2). There was a main effect of emotion [F(1,11) = 33.51, P < 0,0001] as well as a main effect of masking order [F(1,11) = 16.52, P = 0.0019], with a higher identification rate for previously less masked words. No interaction was found between the emotion effect and this prior conscious perception effect [F(1,11) < 0.01, P > 0.9]. To restrict the analysis to words that would never have been previously identified, we tested the effect of emotion at the central SOA of 67 ms only for words that had been previously presented at a short SOA and had not been previously named. The emotion effect was still significant [F(1,11) = 6.35, P = 0.0284], as emotional words were perceived better than neutral words (55.2 vs. 33.4% correct).
Fig. 2.
Effects of emotional valence and masking order in Experiment 1. Correct identification rates as a function of SOA are plotted for emotional words (squares) and neutral words (circles) and in the decreasing masking condition (Upper Left) and in the increasing masking condition (Upper Center). The vertical ellipse highlights data from the central block with a 67-ms SOA. In this block, there was an effect of emotional valence (solid arrow) and an effect of prior conscious perception (dashed arrow), with no interaction of those two effects (Upper Right). The same pattern was observed with subjective rating for word visibility (Lower).
Analyses of subjective reports of visibility confirmed those findings. At the central value of SOA, the mean visibility rating was affected by main effects of emotion [F(1,11) = 13.88, P = 0.0033] and of masking order [F(1,11) = 7.58, P = 0.0188], indicating that subjects judged the words as more visible both when they were emotional and when they had been consciously seen previously. Again, there was no interaction between these two main effects [F(1,11) = 2.02, P = 0.1828]. Restricting analysis to words that had never been previously identified confirmed the main effect of emotion [F(1,11) = 20.59, P = 0.0008].
In summary, both objective and subjective measures demonstrated additive effects of emotion and of prior conscious perception on conscious access. In fact, objective and subjective measures probably addressed the same underlying process. Across trial types and subjects, objective identification rates and subjective visibility ratings were highly correlated (r2 = 0.889, P < 0.001). We also used both measures to extract a quantitative estimate of the consciousness threshold. As shown in Fig. 2, both measures followed a sigmoid curve as a function of SOA. Thus, we used nonlinear regression to fit the curve with a sigmoid defined as f (x) = α1 + α2/(1 + e−α3(x − α4)) where the αi are free parameters, x is the SOA, and f(x) is the identification rate or the subjective score. The access threshold was defined as the SOA value for which f(x) = 50%. When calculated in this way, the objective threshold correlated very tightly across subjects with the subjective threshold (mean thresholds were 34 and 33 ms, respectively; r2 = 0.808, P < 0.01). This suggests that both measures were indicators of the same threshold for conscious access.
We also analyzed the errors made by subjects. On 27.1% of trials, the subject’s response was confined to a simple “don’t know,” but on 19.3% of trials, subjects made a naming response that differed from the word target. An observer blind to the actual target classified these responses as being either emotional or neutral. As in previous reports in attentional blink (22), a liberal criterion was used, with responses classified as emotionally significant (for arousal and/or valence) even if they were only mildly or ambiguously so (e.g., dollar, error, race). If subjects had a strong bias to respond with emotional words, they might have fallen upon the correct response to an emotional word purely by chance. Such a bias might therefore artificially elevate the identification rate of emotional words and might create an apparent lowering of the conscious threshold for emotional words in the absence of any actual semantic processing of the target words. However subjects were actually biased in the opposite direction; overall, only 32.5% of their errors were emotional words whereas 67.5% of their errors were neutral, a proportion significantly different from the proportion of 50% used in the actual experimental lists (P = 0.0022). Most interestingly, this percentage of emotional responses was significantly higher when the target word was itself emotional than when it was neutral [39.2% vs. 26.5%, t(11 df) = 3.53, P = 0.0048]. Thus, even when subjects failed to identify the target, some information about its semantic emotional content occasionally influenced the verbal response (e.g., target “war”, response “danger”; target “bomb”, response “death”). This finding further supports the hypothesis of a nonconscious semantic processing of masked words.
Experiment 2.
In a replication experiment, we used the same lists of words and the same paradigm but with only three values of SOA (including the same central 67-ms SOA used in Experiment 1). We also introduced a new set of words presented only once and exclusively in the critical central block. This set comprised pairs of orthographic neighbors, i.e., words differing only by one letter but with contrasting emotional content [neutral vs. negative in valence and high in arousal, e.g., “couleur” vs. “douleur” (color vs. pain)]. Enhanced perception of emotional words in this list would definitely rule out the possibility that low-level visual features contribute to the change in threshold.
Separate analyses were conducted for the two lists of words. On the replication list, all of the results obtained in Experiment 1 were replicated, namely main effects of emotion and masking order and a main effect of emotion for words that had not been consciously perceived previously (all P values were <0.003 on the objective measure and <0.02 on the subjective measures). Crucially, there was again no interaction between masking order and emotion (P > 0.6 both for objective and subjective measures). No bias favoring the report of emotional words was observed. Rather, errors were biased toward neutral words. Overall, only 34.2% of errors were emotional words, whereas 65.8% were neutral, a proportion significantly different from the actual proportion of 50% in experimental lists (P < 0.0001). This percentage of emotional responses was significantly higher when the target word was itself emotional than when it was neutral (39.6% vs. 29.8%, P = 0.0065).
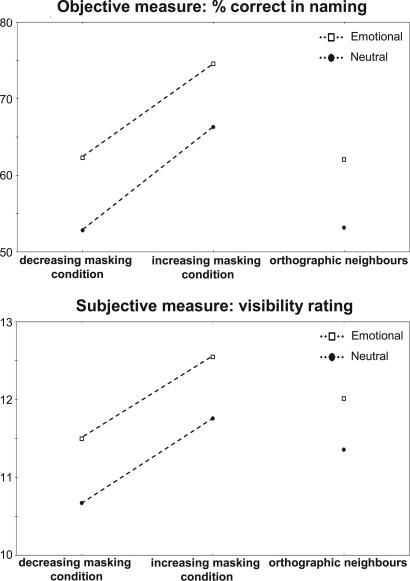
Crucially, analyses on the second list of emotional and neutral words differing by a single letter again revealed a main effect of emotion [F(1,23) = 8.04, P = 0.0094 for identification rate; F(1,23) = 4.2, P = 0.0517 for subjective ratings] (Fig. 3). To provide a more liberal assessment of the influence of orthographic features (22), in a second analysis we reclassified a response correct if the observer reported either the actual target or its visually similar counterpart. For example, when presented with the word COULEUR (COLOR), report of DOULEUR (PAIN) was now scored as correct. The effect of emotion remained significant [F(1,23) = 5.13, P = 0.0333 for identification rate]. Finally, when we excluded these error responses, the effect of emotion still remained significant [F(1,23) = 7.38, P = 0.0123 for objective identification rate; F(1,23) = 4.74, P = 0.0399 for subjective ratings].
Fig. 3.
Effects of emotional valence and masking order in Experiment 2. Correct identification rates at the central SOA (67-ms) are plotted for emotional words (squares) and for neutral words (circles) and in the decreasing masking condition (left side of Upper) and in the increasing masking condition (middle of Upper). As in Experiment 1, a main effect of emotion was observed on a subset of orthographic neighbors (right side of Upper), i.e., words differing only by one letter but with contrasting emotional content [e.g., couleur vs. douleur (color vs. pain)]. The same pattern was observed with subjective rating for word visibility (Lower).
Discussion
Our experiments disclosed two independent sources of modulation of the threshold for access to consciousness. First, we found that prior conscious perception of words increases the detection rate of the same words when they are subsequently presented with stronger masking. Second, we also demonstrated that the threshold for conscious access is lower for emotional words than for neutral ones. The lack of any interaction between these two effects suggests that they are of independent origin and, in particular, that genuine nonconscious semantic access to the emotional content of words can occur even for words that were never seen before. If the emotional effect had been limited to words that were previously perceived consciously, the emotional modulation of the conscious threshold could have been explained entirely by nonsemantic factors such as enhanced memory or perceptual facilitation. On the contrary, the persistence of an identical emotional effect for words that were never previously perceived consciously in the experiment should be interpreted as a demonstration of nonconscious semantic process.
The present method departs considerably from previous studies which have mostly attempted to demonstrate semantic priming in the presence of a null d′ in identification or categorization tasks (12, 13). A difficulty with these prior studies is that very short SOAs were needed to ensure that d′ would remain essentially zero. As a result, the priming effects were very small. By contrast, the present method works at long SOAs (e.g., 67 ms) at which d′ would certainly be positive, given that many words could be identified. Nevertheless, the significantly higher identification of emotional words compared with neutral words provides evidence that some differential semantic processing of those two categories must have occurred. Our work also shows that objective and subjective measures of the consciousness threshold can be used equivalently to demonstrate this effect. Nevertheless, the objective naming task may provide more reliable results that are less susceptible to bias effects because it allows a direct and objective characterization of the subject’s conscious contents. In that respect, it is particularly interesting to note that, even on trials in which subjects did not have enough information to accurately name the target word, error analysis revealed that partial information about its emotional contents was still available. This finding is congruent with other observations of partial semantic transmission of word content, as suggested with masked words in normal subjects (35) and demonstrated in the form of semantic paraphasias in neuropsychological patients with deep dyslexia or deep dysphasia (36, 37).
What could be the mechanism for the observed emotional enhancement? Low-level features such as length, frequency of words, letters, bigrams, trigrams, quadrigrams, and number of orthographic neighbors were matched for emotional and neutral words. Furthermore, the effect of emotion was replicated with words that differed only by one letter, thus ruling out a contribution of low-level visual features idiosyncratic to emotional words. Still, it remains possible that, as a result of prior reading experience, word recognition in ventral visual cortex differs for emotional and neutral words. According to this hypothesis, the emotional experience concomitant with the reading of high-arousal words would enhance their perceptual learning. Back-projections from the amygdala to high-level visual areas and even primary visual cortex (29–32) might indeed enhance the cortical representation of emotional words during the acquisition of reading. Accordingly, in an magnetoencephalography study, Ortigue et al. (38) found an early right occipito-temporal modulation (100–140 ms) for emotional words. The strength of this study is however limited by the small sample of eight emotional words which were using repeatedly during the experiment. Furthermore, the perceptual enhancement hypothesis is questioned by an attentional blink experiment in adults with amygdala lesions (21), which showed a lack of emotional enhancement for patients with left but not right anterior temporo-medial lobectomy. If emotional enhancement was based on visual cortex alone, amygdala lesions occurring after the acquisition of reading should not have disrupted it. As an alternative account, we therefore hypothesize that the better report of emotional words may reflect a nonconscious activation of the amygdala by emotionally relevant semantic attributes of words. The nonconscious extraction of the meaning of emotional words would lead to an amplification of cortical processing, thus increasing the probability of crossing a minimal threshold of neuronal activation needed for conscious access. Consistent with this view, intracranial recordings show a subliminal modulation of amygdala activity by masked emotional words (19). Furthermore, abundant connections link the cortico-subcortical networks mediating emotion to a fronto-parietal cortical network thought to subserve conscious access (34, 39–42). Recent neuroimaging studies have revealed brain structures common to those two systems, especially the anterior cingulate and orbitofrontal cortex (43–45). Thus, connections between the amygdala, the anterior cingulate, and the orbitofrontal cortex may play a crucial role in the present effect. Connections from the amygdala to high-level visual areas and primary visual cortex (29–32) could also enhance the perceptual representations of emotional stimuli once their emotional content has been retrieved.
Previous studies have demonstrated subliminal emotional processing for visual stimuli such as faces (1–3, 7, 46, 47). Fear conditioning has also been demonstrated for subliminal masked faces (48–50) and is also easier for fear-relevant natural categories such as snakes or spiders than for neutral ones (6, 51). These results have been interpreted in terms of evolutionary constraints (5), but fear conditioning can also be modulated by cultural factors, as recently discussed in the context of comparison of racial groups (52). Our results further underline this cultural dimension by showing how the emotional content of learned strings of letters can modulate conscious access.
We close by noting that the observed enhancement of conscious access for negative words goes opposite to the repression hypothesis of psychoanalytic theory (53), which would rather predict a reduced reportability. Although tachistoscopic presentation of words initially suggested a lower recognition rate for taboo words (54), in agreement with Freud’s repression hypothesis, further studies demonstrated that this so-called perceptual defense was explained by response inhibition rather than by a lack of conscious perception (55). We demonstrate here that nonconscious emotional processes increase rather than decrease the probability of conscious perception. This modified threshold for access to consciousness may be advantageous to social cognition because it enhances the probability of a conscious cognitive appraisal of emotionally relevant stimuli.
Methods
Experiment 1.
Visual words were presented for 33 ms on a computer screen (Courier New 14, 4° angle; see Fig. 1). We modulated conscious perception by varying the SOA between each word and a subsequent backward mask (consisting of a series of eight # characters). Words were presented in five conditions of masking, from high masking (SOA = 33 ms) to low masking condition (SOA = 100 ms) (see Fig. 1). Sixty words were used, half of them negative in valence and high in arousal and the other half emotionally neutral. To avoid any difference in low-level features, the two categories were matched in terms of number of letters, gender, frequency of letters, bigrams, trigrams, quadrigrams, and words and number of orthographic neighbors (all P values were >0.2).
Twelve subjects were included in this experimental procedure after giving informed consent.
Experiment 2.
We used the same lists of words and the same paradigm but with only three values of SOA (50, 67, and 83 ms). We also introduced a new set of 40 words presented only once and exclusively in the critical central block. This set comprised pairs of orthographic neighbors, i.e., words differing only by one letter, but with contrasting emotional content [neutral vs. negative in valence and high in arousal, e.g., “couleur” vs. “douleur” (color vs. pain)]. The two categories were also similar in terms of frequency of letters (P = 0.8), bigrams (P = 0.5), trigrams (P = 0.1), quadrigrams (P = 0.2), and words (P = 0.9).
Twenty-four subjects were included in this experimental procedure after giving informed consent.
Abbreviation
- SOA
stimulus onset asynchrony.
Footnotes
Conflict of interest statement: No conflicts declared.
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Morris J. S., DeGelder B., Weiskrantz L., Dolan R. J. Brain. 2001;124:1241–1252. doi: 10.1093/brain/124.6.1241. [DOI] [PubMed] [Google Scholar]
- 2.Pegna A. J., Khateb A., Lazeyras F., Seghier M. L. Nat. Neurosci. 2005;8:24–25. doi: 10.1038/nn1364. [DOI] [PubMed] [Google Scholar]
- 3.Whalen P. J., Rauch S. L., Etcoff N. L., McInerney S. C., Lee M. B., Jenike M. A. J. Neurosci. 1998;18:411–418. doi: 10.1523/JNEUROSCI.18-01-00411.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Morris J. S., Ohman A., Dolan R. J. Proc. Natl. Acad. Sci. USA. 1999;96:1680–1685. doi: 10.1073/pnas.96.4.1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohman A., Mineka S. Psychol. Rev. 2001;108:483–522. doi: 10.1037/0033-295x.108.3.483. [DOI] [PubMed] [Google Scholar]
- 6.Mineka S., Ohman A. Biol. Psychiatry. 2002;52:927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- 7.Whalen P. J., Kagan J., Cook R. G., Davis F. C., Kim H., Polis S., McLaren D. G., Somerville L. H., McLean A. A., Maxwell J. S., Johnstone T. Science. 2004;306:2061. doi: 10.1126/science.1103617. [DOI] [PubMed] [Google Scholar]
- 8.Carretie L., Hinojosa J. A., Mercado F., Tapia M. Neuroimage. 2005;24:615–623. doi: 10.1016/j.neuroimage.2004.09.009. [DOI] [PubMed] [Google Scholar]
- 9.Vuilleumier P., Armony J. L., Driver J., Dolan R. J. Nat. Neurosci. 2003;6:624–631. doi: 10.1038/nn1057. [DOI] [PubMed] [Google Scholar]
- 10.Johnson M. H. Nat. Rev. Neurosci. 2005;6:766–774. doi: 10.1038/nrn1766. [DOI] [PubMed] [Google Scholar]
- 11.Cohen L., Dehaene S. Neuroimage. 2004;22:466–476. doi: 10.1016/j.neuroimage.2003.12.049. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald A. G. Science. 1996;273:1699–1702. doi: 10.1126/science.273.5282.1699. [DOI] [PubMed] [Google Scholar]
- 13.Dehaene S., Naccache L., Le Clec H. G., Koechlin E., Mueller M., Dehaene-Lambertz G., van de Moortele P. F., Le Bihan D. Nature. 1998;395:597–600. doi: 10.1038/26967. [DOI] [PubMed] [Google Scholar]
- 14.Abrams R. L., Greenwald A. G. Psychol. Sci. 2000;11:118–124. doi: 10.1111/1467-9280.00226. [DOI] [PubMed] [Google Scholar]
- 15.Naccache L., Dehaene S. Cognition. 2001;80:223–237. doi: 10.1016/s0010-0277(00)00139-6. [DOI] [PubMed] [Google Scholar]
- 16.Greenwald A., Abrams R., Naccache L., Dehaene S. J. Exp. Psychol. Learn. Mem. Cognit. 2003;29:235–247. doi: 10.1037/0278-7393.29.2.235. [DOI] [PubMed] [Google Scholar]
- 17.Dijksterhuis A., Aarts H. Psychol. Sci. 2003;14:14–18. doi: 10.1111/1467-9280.t01-1-01412. [DOI] [PubMed] [Google Scholar]
- 18.Labiouse C. L. Psychol. Sci. 2004;15:364–365. doi: 10.1111/j.0956-7976.2004.00685.x. [DOI] [PubMed] [Google Scholar]
- 19.Naccache L., Gaillard R., Adam C., Hasboun D., Clemenceau S., Baulac M., Dehaene S., Cohen L. Proc. Natl. Acad. Sci. USA. 2005;102:7713–7717. doi: 10.1073/pnas.0500542102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merikle P. M. Am. Psychol. 1992;47:792–796. doi: 10.1037//0003-066x.47.6.792. [DOI] [PubMed] [Google Scholar]
- 21.Anderson A. K., Phelps E. A. Nature. 2001;411:305–309. doi: 10.1038/35077083. [DOI] [PubMed] [Google Scholar]
- 22.Anderson A. K. J. Exp. Psychol. Gen. 2005;134:258–281. doi: 10.1037/0096-3445.134.2.258. [DOI] [PubMed] [Google Scholar]
- 23.Keil A., Ihssen N. Emotion. 2004;4:23–35. doi: 10.1037/1528-3542.4.1.23. [DOI] [PubMed] [Google Scholar]
- 24.Zeelenberg R., Wagenmakers E. J., Rotteveel M. Psychol. Sci. 2006;17:287–297. doi: 10.1111/j.1467-9280.2006.01700.x. [DOI] [PubMed] [Google Scholar]
- 25.Kensinger E. A., Corkin S. Mem. Cognit. 2003;31:1169–1180. doi: 10.3758/bf03195800. [DOI] [PubMed] [Google Scholar]
- 26.Kensinger E. A., Corkin S. Proc. Natl. Acad. Sci. USA. 2004;101:3310–3315. doi: 10.1073/pnas.0306408101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Strange B. A., Dolan R. J. Proc. Natl. Acad. Sci. USA. 2004;101:11454–11458. doi: 10.1073/pnas.0404282101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strange B. A., Hurlemann R., Dolan R. J. Proc. Natl. Acad. Sci. USA. 2003;100:13626–13631. doi: 10.1073/pnas.1635116100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amaral D. G., Behniea H., Kelly J. L. Neuroscience. 2003;118:1099–1120. doi: 10.1016/s0306-4522(02)01001-1. [DOI] [PubMed] [Google Scholar]
- 30.Krolak-Salmon P., Henaff M., Vigheto A., Bertrand O., Mauguière F. Neuron. 2004;42:665–676. doi: 10.1016/s0896-6273(04)00264-8. [DOI] [PubMed] [Google Scholar]
- 31.Sabatinelli D., Bradley M. M., Fitzsimmons J. R., Lang P. J. Neuroimage. 2005;24:1265–1270. doi: 10.1016/j.neuroimage.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Vuilleumier P., Richardson M. P., Armony J. L., Driver J., Dolan R. J. Nat. Neurosci. 2004;7:1271–1278. doi: 10.1038/nn1341. [DOI] [PubMed] [Google Scholar]
- 33.Sergent C., Dehaene S. Psychol. Sci. 2004;15:720–728. doi: 10.1111/j.0956-7976.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- 34.Sergent C., Baillet S., Dehaene S. Nat. Neurosci. 2005;8:1391–1400. doi: 10.1038/nn1549. [DOI] [PubMed] [Google Scholar]
- 35.Marcel A. J. Cognit. Psychol. 1983;15:197–237. doi: 10.1016/0010-0285(83)90009-9. [DOI] [PubMed] [Google Scholar]
- 36.Buchanan L., McEwen S., Westbury C., Libben G. Brain Lang. 2003;84:65–83. doi: 10.1016/s0093-934x(02)00521-7. [DOI] [PubMed] [Google Scholar]
- 37.Coltheart M., Patterson K., Marshall J. C., editors. Deep Dyslexia. 2nd Ed. London: Routledge; 1987. pp. 22–43. [Google Scholar]
- 38.Ortigue S., Michel C. M., Murray M. M., Mohr C., Carbonnel S., Landis T. Neuroimage. 2004;21:1242–1251. doi: 10.1016/j.neuroimage.2003.11.007. [DOI] [PubMed] [Google Scholar]
- 39.Dehaene S., Naccache L. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- 40.Panksepp J. Conscious Cogn. 2005;14:30–80. doi: 10.1016/j.concog.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Rolls E. T. The Brain and Emotion. Oxford: Oxford Univ. Press; 1999. [Google Scholar]
- 42.LeDoux J. The Emotional Brain. New York: Simon & Schuster; 1992. [Google Scholar]
- 43.Bush G., Luu P., Posner M. I. Trends Cogn. Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- 44.Vuilleumier P., Schwartz S. Neurology. 2001;56:153–158. doi: 10.1212/wnl.56.2.153. [DOI] [PubMed] [Google Scholar]
- 45.Killgore W. D., Yurgelun-Todd D. A. Neuroimage. 2004;21:1215–1223. doi: 10.1016/j.neuroimage.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 46.Williams M. A., Morris A. P., McGlone F., Abbott D. F., Mattingley J. B. J. Neurosci. 2004;24:2898–2904. doi: 10.1523/JNEUROSCI.4977-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Anderson A. K., Christoff K., Panitz D., De Rosa E., Gabrieli J. D. J. Neurosci. 2003;23:5627–5633. doi: 10.1523/JNEUROSCI.23-13-05627.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Esteves F., Parra C., Dimberg U., Ohman A. Psychophysiology. 1994;31:375–385. doi: 10.1111/j.1469-8986.1994.tb02446.x. [DOI] [PubMed] [Google Scholar]
- 49.Morris J. S., Friston K. J., Buchel C., Frith C. D., Young A. W., Calder A. J., Dolan R. J. Brain. 1998;121:47–57. doi: 10.1093/brain/121.1.47. [DOI] [PubMed] [Google Scholar]
- 50.Olsson A., Phelps E. A. Psychol. Sci. 2004;15:822–828. doi: 10.1111/j.0956-7976.2004.00762.x. [DOI] [PubMed] [Google Scholar]
- 51.Hygge S., Ohman A. J. Pers. Soc. Psychol. 1978;36:271–279. doi: 10.1037//0022-3514.36.3.271. [DOI] [PubMed] [Google Scholar]
- 52.Olsson A., Ebert J. P., Banaji M. R., Phelps E. A. Science. 2005;309:785–787. doi: 10.1126/science.1113551. [DOI] [PubMed] [Google Scholar]
- 53.Freud S. The Standard Edition of the Complete Psychological Works of Sigmund Freud. Vol. 1. London: HogarthM; 1966. pp. 117–128. [Google Scholar]
- 54.McGinnies E. Psychol. Rev. 1949;56:244–251. doi: 10.1037/h0056508. [DOI] [PubMed] [Google Scholar]
- 55.Zajonc R. B. J. Exp. Psychol. 1962;64:206–214. doi: 10.1037/h0047568. [DOI] [PubMed] [Google Scholar]