Abstract
Hematopoietic stem cells (HSC) show heterogeneous behavior even when isolated as phenotypically homogeneous populations. The cellular and molecular mechanisms that control the generation of diversity (GOD) in the HSC compartment are not well understood, but have been the focus of much debate. There is increasing evidence that the most important HSC functions, self-renewal and differentiation, are epigenetically preprogrammed and therefore predictable. Indeed, recent data show that the adult HSC compartment consists of a limited number of functionally distinct subsets of HSC. This contradicts older models of HSC behavior, which postulated a single type of HSC that can be continuously molded into different subtypes of HSC. We propose a clonal diversity model where the adult HSC compartment consists of a fixed number of different types of HSC, each with epigenetically preprogrammed behavior. Aging or disease may change the overall function of the HSC population. The model predicts that these changes reflect the relative composition of the HSC subsets, rather than changes in individual HSC. This view has implications for using HSC in experimental and clinical settings. Selection for the appropriate subsets of HSC, rather than attempts to force HSC to adjust, should improve their utility in transplantation and gene transfer applications.
Keywords: hematopoietic stem cells, stem cell purification, stem cell subsets, deterministic, stochastic, epigenetic, lineage-bias, IL-7, niche, generation of diversity, aging
HETEROGENEITY IN THE HSC COMPARTMENT
Hematopoietic stem cells (HSC) differentiate to replenish all cells of all of the hematopoietic lineages, including lymphocytes, myeloid cells, and erythrocytes. Upon commitment to differentiation, HSC are thought to divide and generate one each of a lymphoid and a myeloid restricted progenitor. Each of these progenitors will then differentiate into precursors that have fewer and fewer lineage choices. Finally, unipotential precursors will give rise to mature cells. The astounding demand for mature cells in the periphery (more than 1011 leukocytes are generated every day in humans) creates a constant pressure on HSC to provide differentiated progeny. Yet, when HSC commit to differentiation, they lose self-renewal capacity and pluripotency and cease being HSC. To avoid depleting the HSC compartment, HSC will proliferate to generate more HSC. Thus, self-renewal and pluripotent differentiation capacity together are defining characteristics of HSC. As all adult stem cells, HSC are tissue specific: a HSC will give rise only extremely infrequently to cells of the nonhematopoietic lineages.1
The hematopoietic differentiation process accounts for most of the extensive heterogeneity of the hematopoietic tissues. Heterogeneity has also been demonstrated in the HSC compartment itself. HSC can differ in how rapidly they repopulate an ablated host after transplantation, how long they can produce mature cells, and how much they can self-renew.2-16 The molecular and cellular mechanisms that generate such diversity in the HSC compartment have been much debated, but have been difficult to assess experimentally.
Many theories have been developed to explain the generation of diversity (GOD) in the HSC compartment. The major themes of these theories are intrinsic vs. instructive. The instructive models,17-19 postulate that the microenvironment educates HSC to change. The models of intrinsic regulation of HSC diversity can be subdivided into stochastic and deterministic theories. These hypothesize respectively that HSC behavior is either not predictable (stochastic) or predictable (deterministic).20-26 For a comprehensive overview of models see the (review by Viswanathan and Zandstra; ref. 27). Without fail, all currently accepted theories assume the existence of a single type of mother-of-all-HSC (or most primitive HSC) in adult bone marrow (BM). Such HSC will continuously generate diversity by producing functionally heterogeneous daughter HSC. Data from our group challenge this concept. We summarize evidence that the HSC compartment, at least in the adult mouse, consists of a limited number of HSC types—each with predictable behavior. Based on these data, we sketch a model of GOD for HSC in a deterministic system.
THE BOLTS AND NUTS OF DETECTING HSC
Most of the properties of a HSC can be detected in vivo in transplantation assays.3 HSC will find all signals necessary for self-renewal and differentiation in the host environment. The injected HSC and the ablated hosts are chosen from strains of mice that are congenic for easily distinguishable markers such as the cell surface antigen CD45 or the intracellular transgene green fluorescent protein. Mature cells derived from the injected (donor type) HSC therefore can be enumerated by immunofluorescence in a few drops of blood. The white blood cells are also stained with markers specific for the lymphocyte or myeloid-erythroid lineages to ascertain that the injected cell is pluripotent, i.e., gives rise to all hematopoietic lineages. Because this assay requires little blood, hosts can be tested repeatedly and the kinetics with which HSC repopulate the periphery with differentiated cells can be measured. For the studies described here, criteria were chosen that allowed the detection of HSC but excluded non-HSC (progenitors and precursors) from the analysis.
Whether a HSC had self-renewed can be measured by serial transplantation of the donor type HSC, by injecting these into cohorts of secondary, tertiary and quaternary hosts. If the secondary hosts are repopulated by donor type cells, one can conclude that the original donor HSC had self-renewed in the primary host. As an aside, methods other than transplantation are notoriously unreliable for the detection of HSC that had been transplanted previously.28,29 Overall, repopulation patterns are highly informative for HSC function and the functional diversity of HSC is resolvable through the behavior of clonal HSC in repopulation experiments.
Why is it necessary to perform clonal analysis of HSC behavior? Decisions about self-renewal and differentiation are made on the level of a single HSC. If one tests many HSC at the same time, one will inevitably detect the composite behavior of the HSC in the pool. The contribution of individual HSC will be obscured, making it difficult to derive meaningful information on the GOD of HSC. Our group used limiting dilution strategies together with streamlined experimental and statistical approaches to examine HSC on the clonal level. This approach allowed us to examine a large number of clonally derived HSC.2-4,30-32 So far we have followed the repopulation behavior of 106 individual HSC clones for at least seven months. The data provided strong evidence that much of the behavior of HSC is predetermined.
HOW MANY DIFFERENT TYPES OF HSC EXIST IN THE HSC COMPARTMENT?
We asked whether our set of clonal HSC contained all possible patterns of repopulation kinetics.3 Since donor type cells were enumerated every other month for at least seven months, each repopulation curve was comprised of at least three segments. The individual segments were symbolized so that a positive slope was labeled with a “+”, a negative with a “−“ and a flat slope as “∼”. The symbolized repopulation curves were then compared to each other and sorted into groups if they were of identical shape.31 We used the relative Hamming distance,33 a method from quantum mechanics, to compare the shapes of the symbolized curves.3,31 Interestingly, less than 30% of all possible kinetics were found and there is a vanishingly low probability that the analysis of additional HSC would yield new types of repopulation curves.3 The most likely interpretation of this finding is that the HSC compartment consists of a limited number of different subsets of HSC.
Previous models of GOD in the HSC compartment predicted that HSC would constantly create new heterogeneity. However, this is not the case. We found that individual HSC do not regenerate the heterogeneity seen in the HSC compartment. Rather, individual HSC self-renew to give rise to daughter HSC which are very similar to each other in their kinetics of repopulation. Moreover, the daughter HSC gave rise to mature cells with the same lineage ratios.4 Thus, HSC in the adult can be classified into a small number of HSC types, each with predetermined and predictable proliferation and differentiation abilities.
To reveal the full potential of the compartment, HSC clones were obtained from unseparated BM. Next, we asked whether different purification methods would enrich similarly for all subsets of HSC. We compared the clonal repopulation curves from two populations of HSC with a different, albeit partially overlapping phenotype (Fig. 1). HSC enriched as Lineage−Rhodaminelow Side Population cells were sorted and tested in Vancouver and the data have been published previously.3,34 Lineage-Sca-1+ckit+ (LSK) cells were sorted in our laboratory and injected in limiting numbers into ablated hosts (unpublished). All repopulation curves derived from both populations of sorted cells fit into the groups defined already by HSC from unseparated BM and no new kinetics were identified. Thus, the HSC classifications are remarkably robust, since they held true when data from two different laboratories, generated with three different methods were compared. Interestingly, the purification methods appear to select for different, albeit overlapping, subsets of HSC (Fig. 1). For example, the LSK cells do not generate repopulation curves with steadily declining levels of donor type cells (− − −). In contrast, Rholow cells are enriched for such − − − kinetics.3 Relatively few repopulation curves were examined, suggesting that additional analysis might fill in some of the gaps (Fig. 1). Yet, it is unlikely that overall distribution will change much. Thus, different purification methods can unexpectedly enrich very different subsets of HSC.
Figure 1.
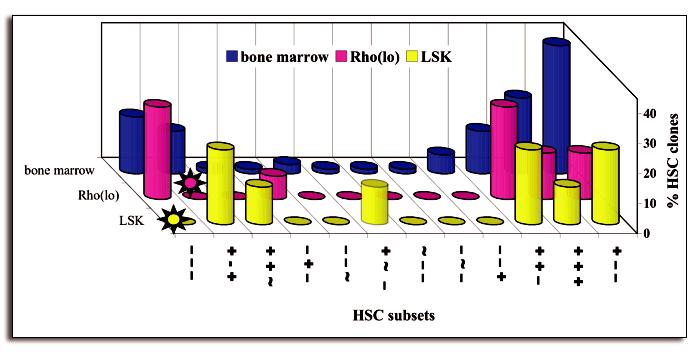
Different purification protocols enrich for different subsets of HSC (modified from ref. 2) Clonally derived HSC were classified according to their kinetics of repopulation as described.3,31 These represent distinct subsets of HSC (indicated on horizontal axis). The data are expressed as percent of HSC types found in each of the subsets. Three populations were tested: unseparated bone marrow (dark blue), or HSC purified as lineage− Rhodamine-123low Hoechst 33342− side population cells (Rho(low), shown in red) or Lin-Sca-1+ckit+ (LSK; shown in yellow). Bone marrow encompasses individual repopulation kinetics of 84 HSC, Rho(low) had 12, and LSK had 10 kinetics. Stars highlight the missing − − − subset in the LSK population and the missing + − + subset in the Ro(lo) population.
Since the subsets of HSC differ noticeably in function, it would not be surprising if each had a different expressed gene program. Indeed, there is evidence that the subsets of short-term and long-term repopulating HSC differ in that respect.35 Thus, it will be important to define the clonal composition of different populations of HSC that are obtained with different selection methods. Perhaps, a different composition of HSC types in different purified populations could reconcile some of the strikingly discrepant results in HSC research. For example, when different groups catalogued the expressed gene program of HSC there was little overlap in the genes identified.36-38
LINEAGE-BIASED HSC—A PARADIGM OF EPIGENETICALLY FIXED HSC BEHAVIOR
Another unexpected finding from the clonal analysis of HSC was the discovery of lineage-biased HSC.2,4 All HSC can be classified by the ratio of lymphoid to myeloid cells that they generate upon differentiation. Three classes of HSC have been defined: balanced HSC that repopulate peripheral white blood cells in the same ratio of myeloid to lymphoid cells as seen in unmanipulated mice (about 15% myeloid and 85% lymphocytes). Myeloid-biased (My-bi) HSC give rise to too few lymphocytes, and lymphoid-biased (Ly-bi) HSC generate too few myeloid cells.2 All three types are normal HSC in that they have self-renewal capacity and can regenerate all hematopoietic lineages (pluripotency). Strikingly, the lineage bias is preserved through multiple rounds of serial transplantation: balanced HSC self-renew to give rise to daughter HSC that are also balanced, My-bi HSC give rise to My-bi daughter HSC, and Ly-bi produce Ly-bi daughter HSC. There is no precursor-progeny relationship between the three types of HSC and they do not represent stages of differentiation. Rather, these are three classes of HSC, each with an epigenetically fixed differentiation program.
How could epigenetic imprinting on the level of the HSC affect the generation of downstream differentiated progeny? My-bi HSC were studied in more detail to define the mechanism(s) that account for the lineage bias. We found that My-bi HSC give rise to normal myeloid progeny but their lymphoid progeny showed a blunted response to Interleukin-7.2 This lymphokine is central for the differentiation and homeostatic proliferation of lymphoid cells.39,40 Analogously, Ly-bi HSC have an impaired ability to generate myeloid progeny.2 It is likely that each type of HSC expresses a different epigenetically fixed set of genes that changes downstream expression of genes necessary for differentiation.
We reexamined the groups of HSC classified according to their repopulation kinetics3 and found that balanced, My-bi and Ly-bi HSC tend to segregate into different repopulation groups although there is some overlap (unpublished). This agrees with the observation that the classification of the kinetics predicted to some extent self-renewal capacity.3 For example, kinetics with a steady increase (+ + +) were more likely to self-renew than HSC with other kinetics. Similarly, My-bi HSC generally have more self-renewal capacity than other types of HSC and + + + kinetics are the most frequent repopulation patterns for My-bi HSC.2 Thus, both self-renewal and differentiation behavior of individual HSC is predictable and therefore preprogrammed on the level of the HSC. If adult HSC are capable of generating heterogeneous daughter HSC, this must be a very infrequent event.
A CLONAL DIVERSITY MODEL OF THE GOD OF HSC
Our data are not consistent with the older models of how heterogeneity is generated in the HSC compartment. The best fit for the data is a model where the adult HSC compartment consists of functionally discrete subsets of HSC, and the GOD is largely complete. Since many HSC are active at any given time point,5,6,41,42 the steady state hematopoietic system will be derived from the combined functions of these different types of HSC. However, changes in the HSC compartment can be induced—for example through diseases, chemotherapy, transplantation, cytokine treatment, and aging.43,34,44-53 After such events the HSC compartment can become pauciclonal.
Our model predicts that such functional changes of the HSC population are caused by the depletion of some subsets of HSC with the concurrent enrichment in other subsets. Rather than changing the function of individual HSC as has been proposed by previous hypotheses, we predict a shift in the composition of the HSC subsets. One way this might be achieved is if different types of HSC would be more sensitive to hematological toxicity than other types. Alternatively, depletion or enrichment of HSC could come from the different life spans of the HSC.2 Short-lived HSC could simply be depleted for and long-lived HSC may be enriched after hematological stress or aging. Indeed, preliminary data from our laboratory suggest that in aged BM, Ly-bi HSC are depleted while My-bi HSC are enriched (unpublished). An HSC compartment depleted of the rapidly repopulating Ly-bi could account, at least in part, for the defects in immunity seen in the aged. The clonal diversity model could also explain why increases in the number of HSC are beneficial for transplantation patients. The higher the dose of HSC transplanted the greater the probability that all types of HSC will be replenished to create a healthy diversity of the HSC compartment in the host.
The lack of heterogeneity in the daughter HSC after clonal self-renewal2,4 indicates that in vivo niches do not play a role in the GOD. Thus, the microenvironment is permissive, rather than instructive. The clonal diversity model makes the prediction that extrinsic signals can modulate predetermined HSC behaviors, but cannot change them fundamentally. For example, a putative factor that supports HSC self-renewal might enhance the self-renewal divisions of a HSC that is already primed to self-renew. However, that same factor will not help a HSC that is not in self-renewal mode.
Epigenetic imprinting of the expressed gene program would provide a molecular basis for deterministic HSC behavior. The dynamical changes in the epigenome during differentiation are increasingly well understood.54 HSC (analyzed as a population) show open chromatin configurations for lineage specific genes and HSC seem to express lineage specific genes promiscuously.54-57 Differentiation is accompanied by the expression of genes, such as Pax5, that suppress genes needed for the development into other lineages.58 There is evidence that removing or interfering with epigenetic imprinting will protect HSC maintenance, even if the culture conditions favor differentiation.59 This is consistent with the interpretation that differentiation causes restrictions of a multipotent gene program. Nevertheless, it is unclear whether the “promiscuity” is derived from the heterogeneity of the analyzed population rather than the indiscriminate gene expression pattern of a single type of HSC.
The epigenetic fixation of HSC described here must be more stable and less dynamic than the processes involved in differentiation. At the same time, it is likely that different gene networks are responsible for differentiation and epigenetic fixation of HSC function. The preprogrammed lineage potential of HSC is likely a reflection of an epigenetically fixed set of genes that interferes with the expression of genes necessary for a robust response to lineage specific signals in the progeny of the HSC. The expression patterns of such a set of genes are “inherited” to all daughter HSC. In contrast, differentiation associated changes in the epigenome are not maintained on the HSC level. Thus, the epigenetic events that control HSC behavior are likely upstream of those that control differentiation.
An obvious extension of the clonal diversity model is that epigenetic imprinting must have happened during development. In analogy to the earliest differentiation events in the fertilized oocyte and embryonic stem cells,60,61 HSC in early development might be unrestricted in their (hematopoietic) gene program. This would allow maximal adaptation to the rapidly changing environments when the developing HSC migrate from the aorta-gonadmesonephros (AGM) to the fetal liver to the BM during embryonic and fetal development.62,63 HSC might become increasingly epigenetically restricted during development, concurrently with a lessening of the demand for proliferation to fill the growing hematopoietic tissues. There is evidence that the frequency of preleukemic clones is much higher in newborns than the corresponding disease in children.64 It is tempting to speculate that restricting the potential of HSC in the adult might also limit the survival of preleukemic HSC clones and thus protect against full leukemic transformation.65
The model that the adult HSC compartment consists of a mixture of preprogrammed HSC has some immediate implications for the clinical application of HSC. It is not unreasonable to assume that different applications would benefit from using different types of HSC. For example, one might consider selectively transplanting Ly-bi HSC for a severe case of lymphopenia. Similarly, an HSC graft for a gene therapy application would benefit from preselecting long-lived HSC. This would increase the efficiency of gene transfer specifically into long-lived HSC and would assure a life-long supply of HSC carrying the transgene. In contrast, it is essential that rapidly repopulating HSC are present in HSC transplants given as supportive therapy after marrow ablative treatment. So far, attempts to tailor HSC to specific applications have been based on the concept that HSC can be molded to the application. For example, cytokine cocktails are used to try to manipulate HSC ex vivo. Perhaps the limited success of such approaches is the strongest support for a clonal diversity model and the idea that HSC should be selected according to their preprogrammed potential to fit the application.
ACKNOWLEDGEMENTS
Work cited from this laboratory was supported by grants from the National Institute for Health AG023197 and DK48015. We thank Dr. Becky Adkins, University of Miami Medical School, for critical discussion of this manuscript and for naming the new model.
ABBREVIATIONS
- HSC
hematopoietic stem cells
- GOD
generation of diversity
- BM
bone marrow
- My-bi
myeloid-biased
- Ly-bi
lymphoid biased
- IL-7
interleukin-7
References
- 1.Orkin SH, Zon LI. Hematopoiesis and stem cells: Plasticity versus developmental heterogeneity. Nat Immunol. 2002;3:323–8. doi: 10.1038/ni0402-323. [DOI] [PubMed] [Google Scholar]
- 2.Muller-Sieburg CE, Cho RH, Karlsson L, Huang JF, Sieburg HB. Myeloid-biased hematopoietic stem cells have extensive self-renewal capacity but generate diminished lymphoid progeny with impaired IL-7 responsiveness. Blood. 2004;103:4111–8. doi: 10.1182/blood-2003-10-3448. [DOI] [PubMed] [Google Scholar]
- 3.Sieburg HB, Cho RH, Dykstra B, Uchida N, Eaves CJ, Muller-Sieburg CE. The hematopoietic stem compartment consists of a limited number of discrete stem cell subsets. Blood. 2005 doi: 10.1182/blood-2005-07-2970. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muller-Sieburg CE, Cho RH, Thoman M, Adkins B, Sieburg HB. Deterministic regulation of hematopoietic stem cell self-renewal and differentiation. Blood. 2002;100:1302–9. [PubMed] [Google Scholar]
- 5.Jordan C, Lemischka I. Clonal and systemic analysis of long-term hematopoiesis in the mouse. Genes Dev. 1990;4:220–32. doi: 10.1101/gad.4.2.220. [DOI] [PubMed] [Google Scholar]
- 6.Keller G, Snodgrass R. Life span of multipotential hematopoietic stem cells in vivo. J Exp Med. 1990;171:1407–18. doi: 10.1084/jem.171.5.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keller G. Clonal analysis of hematopoietic stem cell development in vivo. Curr Top Microbiol Immunol. 1992;177:41–57. doi: 10.1007/978-3-642-76912-2_4. [DOI] [PubMed] [Google Scholar]
- 8.Abkowitz JL, Golinelli D, Harrison DE, Guttorp P. In vivo kinetics of murine hemopoietic stem cells. Blood. 2000;96:3399–405. [PubMed] [Google Scholar]
- 9.Smith L, Weissman I, Heimfeld S. Clonal analysis of hematopoietic stem-cell differentiation in vivo. Proc Natl Acad Sci USA. 1991;88:2788–92. doi: 10.1073/pnas.88.7.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenechea G, Gan OI, Dorrell C, Dick JE. Distinct classes of human stem cells that differ in proliferative and self-renewal potential. Nat Immunol. 2001;2:75–82. doi: 10.1038/83199. [DOI] [PubMed] [Google Scholar]
- 11.Mazurier F, Gan OI, McKenzie JL, Doedens M, Dick JE. Lentivector-mediated clonal tracking reveals intrinsic heterogeneity in the human hematopoietic stem cell compartment and cultureinduced stem cell impairment. Blood. 2004;103:545–52. doi: 10.1182/blood-2003-05-1558. [DOI] [PubMed] [Google Scholar]
- 12.Zhong R, Astle C, Harrison D. Distinct developmental patterns of short-term and long-term functioning lymphoid and myeloid precursors defined by competitive limiting dilution analysis in vivo. J Immunol. 1996;157:138. [PubMed] [Google Scholar]
- 13.Rebel VI, Miller CL, Thornbury GR, Dragowska WH, Eaves CJ, Lansdorp PM. A comparison of long-term repopulating hematopoietic stem cells in fetal liver and adult bone marrow from the mouse. Experimental Hematology. 1996;24:638–48. [PubMed] [Google Scholar]
- 14.Rosendaal M, Hodgson G, Bradley T. Organization of haemopoietic stem cell: The generation-age hypothesis. Cell Tissue Kinet. 1979;12:17–29. doi: 10.1111/j.1365-2184.1979.tb00110.x. [DOI] [PubMed] [Google Scholar]
- 15.Glimm H, Eisterer W, Lee K, Cashman J, Holyoake TL, Nicolini F, Shultz LD, von Kalle C, Eaves CJ. Previously undetected human hematopoietic cell populations with short-term repopulating activity selectively engraft NOD/SCID-beta2 microglobulin-null mice. J Clin Invest. 2001;107:199–206. doi: 10.1172/JCI11519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Spangrude G, Weissman IL. Mature T cells generated from single thymic clones are phenotypically and functionally heterogeneous. J Immunol. 1988;141:1877–90. [PubMed] [Google Scholar]
- 17.Trentin JJ. Determination of bone marrow stem cell differentiation by stromal hemopoietic inductive microenvironments (HIM) Am J Pathol. 1971;65:621–8. [PMC free article] [PubMed] [Google Scholar]
- 18.Wolf NS, Trentin JJ. Hemopoietic colony studies. V. Effect of hemopoietic organ stroma on differentiation of pluripotent stem cells. J Exp Med. 1968;127:205–14. doi: 10.1084/jem.127.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schofield R. The relationship between the spleen colony-forming cell and the haemopoietic stem cell. Blood Cells. 1978;4:7–25. [PubMed] [Google Scholar]
- 20.Rosendaal M, Hodgson GS, Bradley TR. Haemopoietic stem cells are organised for use on the basis of their generation-age. Nature. 1976;264:68–9. doi: 10.1038/264068a0. [DOI] [PubMed] [Google Scholar]
- 21.Blackett N, Gordon M. “Stochastic” -40 years of use and abuse. Blood. 1999;93:3148–9. [PubMed] [Google Scholar]
- 22.Enver T, Heyworth CM, Dexter TM. Do stem cells play dice? Blood. 1998;92:348–51. discussion 352. [PubMed] [Google Scholar]
- 23.Metcalf D. Lineage commitment and maturation in hematopoietic cells: The case for extrinsic regulation. Blood. 1998;92:345–7. discussion 352. [PubMed] [Google Scholar]
- 24.Abkowitz J, Catlin S, Guttorp P. Evidence that hematopoiesis may be a stochastic process in vivo. Nat Med. 1996;2:190–7. doi: 10.1038/nm0296-190. [DOI] [PubMed] [Google Scholar]
- 25.Quesenberry PJ, Colvin GA, Lambert JF. The chiaroscuro stem cell: A unified stem cell theory. Blood. 2002;100:4266–71. doi: 10.1182/blood-2002-04-1246. [DOI] [PubMed] [Google Scholar]
- 26.Roeder I, Loeffler M. A novel dynamic model of hematopoietic stem cell organization based on the concept of within-tissue plasticity. Exp Hematol. 2002;30:853–61. doi: 10.1016/s0301-472x(02)00832-9. [DOI] [PubMed] [Google Scholar]
- 27.Viswanathan S, Zandstra P. Towards predictive models of stem cell fate. Cytotechnology. 2003;41:75–92. doi: 10.1023/A:1024866504538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spangrude G, Brooks D, Tumas D. Long-term repopulation of irradiated mice with limiting numbers of purified hematopoietic stem cells: In vivo expansion of stem cell phenotype but not function. Blood. 1995;85:1006–16. [PubMed] [Google Scholar]
- 29.Bertoncello I. Status of high proliferative potential colony-forming cells in the hematopoietic stem cell hierarchy. Curr Top Microbiol Immunol. 1992;177:83–94. doi: 10.1007/978-3-642-76912-2_7. [DOI] [PubMed] [Google Scholar]
- 30.Cho RH, Muller-Sieburg CE. High frequency of long-term cultureinitiating cells retain in vivo repopulation and self-renewal capacity. Exp Hematol. 2000;28:1080–6. doi: 10.1016/s0301-472x(00)00507-5. [DOI] [PubMed] [Google Scholar]
- 31.Sieburg HB, Muller-Sieburg CE. Classification of short kinetics by shape. Silico Biology. 2004;4:0018. Epub ahead of print. [PubMed] [Google Scholar]
- 32.Sieburg HB, Cho RH, Muller-Sieburg CE. Limiting dilution analysis for estimating the frequency of hematopoietic stem cells: Uncertainty and significance. Exp Hematol. 2002;30:1436–43. doi: 10.1016/s0301-472x(02)00963-3. [DOI] [PubMed] [Google Scholar]
- 33.Hamming R. Coding And Information Theory. 2nd Prentice-Hall, Inc.; Upper Saddle River, NJ, USA: 1986. [Google Scholar]
- 34.Uchida N, Dykstra B, Lyons KJ, Leung FY, CJ E. Different in vivo repopulating activities of purified hematopoietic stem cells before and after being stimulated to divide in vitro with the same kinetics. Exp Hematol. 2003;12:1338–47. doi: 10.1016/j.exphem.2003.09.001. [DOI] [PubMed] [Google Scholar]
- 35.Forsberg EC, Prohaska SS, Katzman S, Heffner GC, Stuart JM, Weissman IL. Differential expression of novel potential regulators in hematopoietic stem cells. PLoS Genet. 2005;1:e28. doi: 10.1371/journal.pgen.0010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ivanova NB, Dimos JT, Schaniel C, Hackney JA, Moore KA, Lemischka IR. A stem cell molecular signature. Science. 2002;298:601–4. doi: 10.1126/science.1073823. [DOI] [PubMed] [Google Scholar]
- 37.Fortunel NO, Otu HH, Ng H-H, Chen J, Mu X, Chevassut T, Li X, Joseph M, Bailey C, Hatzfeld JA, Hatzfeld A, Usta F, Vega VB, Long PM, Libermann TA, Lim B. Comment on “ ‘Stemness’: Transcriptional Profiling of Embryonic and Adult Stem Cells” and “A Stem Cell Molecular Signature” (I) Science. 2003;302:393b. doi: 10.1126/science.1086384. [DOI] [PubMed] [Google Scholar]
- 38.Ramalho-Santos M, Yoon S, Matsuzaki Y, Mulligan RC, Melton DA. “Stemness”: Transcriptional profiling of embryonic and adult stem cells. Science. 2002;298:597–600. doi: 10.1126/science.1072530. [DOI] [PubMed] [Google Scholar]
- 39.Peschon JJ, Morrissey PJ, Grabstein KH, Ramsdell FJ, Maraskovsky E, Gliniak BC, Park LS, Ziegler SF, Williams DE, Ware CB, Meyer JD, Davison BL. Early lymphocyte expansion is severely impaired in interleukin 7 receptor-deficient mice. J Exp Med. 1994;180:1955–60. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Freeden-Jeffry U, Vieria P, Lucian L, McNeil T, Burdach S. Lymphopenia in interleukin- (IL-7) gene deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mintz B, Anthony K, Litwins S. Monoclonal derivation of mouse myeloid and lymphoid lineages from totipotent hematopoietic stem cells experimentally engrafted in fetal hosts. Proc Natl Acad Sci USA. 1984;8:7835–9. doi: 10.1073/pnas.81.24.7835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Harrison DE, Astle CM, Lerner C. Number and continuous proliferative pattern of transplanted primitive immunohematopoietic stem cells. Proc Natl Acad Sci USA. 1988;85:822–6. doi: 10.1073/pnas.85.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison D, Astle CM. Loss of stem cell repopulating ability with transplantation. Effects of donor age, cell number and transplant procedure. J Exp Med. 1982;156:1767–79. doi: 10.1084/jem.156.6.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harrison D, Lerner C. Most primitive hematopoietic stem cells are stimulated to cycle rapidly after treatment with 5-fluorouracil. Blood. 1991;78:1237–40. [PubMed] [Google Scholar]
- 45.Colvin GA, Lambert JF, Abedi M, Dooner MS, Demers D, Moore BE, Greer D, Aliotta JM, Pimentel J, Cerny J, Lum LG, Quesenberry PJ. Differentiation hotspots: The deterioration of hierarchy and stochasm. Blood Cells Mol Dis. 2004;32:34–41. doi: 10.1016/j.bcmd.2003.09.013. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Astle C, Harrison D. Development and aging of primitive hematopoietic stem cells in BALB/cBy mice. Exp Hematol. 1999;27:928–35. doi: 10.1016/s0301-472x(99)00018-1. [DOI] [PubMed] [Google Scholar]
- 47.Marley S, Lewis J, Davidson R, Roberts I, Dokal I, Goldman J, Gordon M. Evidence for a continuous decline in haematopoietic cell function from birth: Application to evaluating bone marrow failure in children. Br J Haematol. 1999;106:162–72. doi: 10.1046/j.1365-2141.1999.01477.x. [DOI] [PubMed] [Google Scholar]
- 48.Bender JG, Unverzagt K, Walker DE, Lee W, Smith S, Williams S, Van Epps DE. Phenotypic analysis and characterization of CD34+ cells from normal human bone marrow, cord blood, peripheral blood, and mobilized peripheral blood from patients undergoing autologous stem cell transplantation. Clin Immunol Immunopathol. 1994;70:10–8. doi: 10.1006/clin.1994.1003. [DOI] [PubMed] [Google Scholar]
- 49.Uchida N, He D, Friera AM, Reitsma M, Sasaki D, Chen B, Tsukamoto A. The unexpected G0/G1 cell cycle status of mobilized hematopoietic stem cells from peripheral blood. Blood. 1997;89:465–72. [PubMed] [Google Scholar]
- 50.Chen G, Zeng W, Maciejewski JP, Kcyvanfar K, Billings EM, Young NS. Differential gene expression in hematopoietic progenitors from paroxysmal nocturnal hemoglobinuria patients reveals an apoptosis/immune response in ‘normal’ phenotype cells. Leukemia. 2005;19:862–8. doi: 10.1038/sj.leu.2403678. [DOI] [PubMed] [Google Scholar]
- 51.Zeng W, Miyazato A, Chen G, Kajigaya S, Young NS. Maciejewski JP. Interferon-{gamma}-induced gene expression in CD34 cells: Identification of pathologic cytokine-specific signature profiles. Blood. 2006;107:167–75. doi: 10.1182/blood-2005-05-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li X, Plett PA, Yang Y, Hong P, Freie B, Srour EF, Orschell CM, Clapp DW, Haneline LS. Fanconi anemia type C-deficient hematopoietic stem/progenitor cells exhibit aberrant cell cycle control. Blood. 2003;102:2081–4. doi: 10.1182/blood-2003-02-0536. [DOI] [PubMed] [Google Scholar]
- 53.Luck L, Zeng L, Hiti AL, Weinberg KI, Malik P. Human CD34(+) and CD34(+)CD38(−) hematopoietic progenitors in sickle cell disease differ phenotypically and functionally from normal and suggest distinct subpopulations that generate F cells. Exp Hematol. 2004;32:483–93. doi: 10.1016/j.exphem.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 54.Bonifer C. Epigenetic plasticity of hematopoietic cells. Cell Cycle. 2005;4:211–4. [PubMed] [Google Scholar]
- 55.Miyamoto T, Iwasaki H, Reizis B, Ye M, Graf T, Weissman IL, Akashi K. Myeloid or lymphoid promiscuity as a critical step in hematopoietic lineage commitment. Dev Cell. 2002;3:137–47. doi: 10.1016/s1534-5807(02)00201-0. [DOI] [PubMed] [Google Scholar]
- 56.Hu M, Krause D, Greaves M, Sharkis S, Dexter M, Heyworth C, Enver T. Multilineage gene expression precedes commitment in the hemopoietic system. Genes Dev. 1997;11:774–85. doi: 10.1101/gad.11.6.774. [DOI] [PubMed] [Google Scholar]
- 57.Zhu J, Emerson SG. Hematopoietic cytokines, transcription factors and lineage commitment. Oncogene. 2002;21:3295–313. doi: 10.1038/sj.onc.1205318. [DOI] [PubMed] [Google Scholar]
- 58.Busslinger M, Nutt SL, Rolink AG. Lineage commitment in lymphopoiesis. Curr Opin Immunol. 2000;12:151–8. doi: 10.1016/s0952-7915(99)00065-5. [DOI] [PubMed] [Google Scholar]
- 59.Milhem M, Mahmud N, Lavelle D, Araki H, DeSimone J, Saunthararajah Y, Hoffman R. Modification of hematopoietic stem cell fate by 5aza 2'deoxycytidine and trichostatin A. Blood. 2004;103:4102–10. doi: 10.1182/blood-2003-07-2431. [DOI] [PubMed] [Google Scholar]
- 60.Murphy SK, Jirtle RL. Imprinting evolution and the price of silence. Bioessays. 2003;25:577–88. doi: 10.1002/bies.10277. [DOI] [PubMed] [Google Scholar]
- 61.Bedada FB, Gnther S, Kubin T, Braun T.Differentiation versus plasticity. Fixing the faye of undeterminined adult stem cells Cell Cycle 2005. This issue, (Once the issue is complete and page numbers have been assigned, the citation will change accordingly) [Google Scholar]
- 62.Kumaravelu P, Hook L, Morrison AM, Ure J, Zhao S, Zuyev S, Ansell J, Medvinsky A. Quantitative developmental anatomy of definitive haematopoietic stem cells/long-term repopulating units (HSC/RUs): Role of the aorta-gonad-mesonephros (AGM) region and the yolk sac in colonisation of the mouse embryonic liver. Development. 2002;129:4891–9. doi: 10.1242/dev.129.21.4891. [DOI] [PubMed] [Google Scholar]
- 63.Sanchez MJ, Holmes A, Miles C, Dzierzak E. Characterization of the first definitive hematopoietic stem cells in the AGM and liver of the mouse embryo. Immunity. 1996;5:513–25. doi: 10.1016/s1074-7613(00)80267-8. [DOI] [PubMed] [Google Scholar]
- 64.Mori H, Colman SM, Xiao Z, Ford AM, Healy LE, Donaldson C, Hows JM, Navarrete C, Greaves M. Chromosome translocations and covert leukemic clones are generated during normal fetal development. Proc Natl Acad Sci USA. 2002;99:8242–7. doi: 10.1073/pnas.112218799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bhalla KN. Epigenetic and chromatin modifiers as targeted therapy of hematologic malignancies. J Clin Oncol. 2005;23:3971–93. doi: 10.1200/JCO.2005.16.600. [DOI] [PubMed] [Google Scholar]


