Abstract
The highly pathogenic simian immunodeficiency virus/HIV type 1 (SHIV) chimeric virus SHIVDH12R induces a systemic depletion of CD4+ T lymphocytes in rhesus monkeys during the initial 3–4 weeks of infection. Nonetheless, high levels of viral RNA production continue unabated for an additional 2–5 months. In situ hybridization and immunohistochemical analyses revealed that tissue macrophage in the lymph nodes, spleen, gastrointestinal tract, liver, and kidney sustain high plasma virus loads in the absence of CD4+ T cells. Quantitative confocal immunofluorescence analysis indicated that greater than 95% of the virus-producing cells in these tissues are macrophage and less than 2% are T lymphocytes. Interestingly, the administration of a potent reverse transcriptase inhibitor blocked virus production during the early T cell phase but not during the later macrophage phase of the SHIVDH12R infection. When interpreted in the context of HIV-1 infections, these results implicate tissue macrophage as an important reservoir of virus in vivo. They become infected during the acute infection, gradually increase in number over time, and can be a major contributor to total body virus burden during the symptomatic phase of the human infection.
With the exception of some long-term nonprogressors, HIV-1 infections invariably result in fatal immunodeficiencies associated with marked depletions of the CD4+ subset of T lymphocytes. The institution of highly active antiretroviral therapy has resulted in a major reduction of virus loads in individuals tolerating the regimen, a stabilization of the clinical course, and a significant decline in mortality/morbidity (1, 2). Nonetheless, currently available combination antiretroviral therapy fails to eliminate HIV-1 from infected persons, indicating the existence of a refractory reservoir(s) of virus in these individuals (3–6).
During the past few years, the persistence of latent HIV-1 in resting CD4+ memory T lymphocytes has received considerable attention (7–9). It is currently thought that the virus that invariably emerges after the cessation of highly active antiretroviral therapy emanates from this source (4–6). However, the clinical significance of this virus reservoir has been recently questioned by reports indicating (i) a lack of correlation between the frequency of resting CD4+ T cells carrying inducible virus and the kinetics of the HIV-1 appearing in plasma after cessation of therapy; and (ii) genotypic discordance between the HIV-1 resting memory cell pool and the rebounding viral RNA (10–12). These discrepancies suggest that resting CD4+ T cells may not be the principal source of the emerging HIV-1 and that other reservoirs of virus, such as tissue macrophage, must be seriously entertained.
Materials and Methods
Virus, Animal Inoculations, and Plasma Viral RNA Measurements.
The source and preparation of the tissue culture-derived SHIVDH12R stock [4.1 × 105 tissue culture 50% infective dose (TCID50)/ml measured in MT-4 cells] has been described (13). We also reported that SHIVDH12R, like its HIVDH12 parent, utilizes both CCR5 and CXCR4 chemokine receptors (13). The rhesus macaques used in this study were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (14). They were housed in a biosafety level 2 facility; biosafety level 3 practices were followed. Phlebotomies, virus inoculations, and intramuscular 9-[2(R)-(phosphonomethoxy)propyl]adenine (PMPA) administration were performed with animals anesthetized with Tiletamine⋅HCl and Zolazepam⋅HCl (Telazol; Fort Dodge Laboratories, Fort Dodge, IA). Plasma viral RNA levels were determined by real time PCR (ABI Prism 7700 Sequence Detection System, Perkin–Elmer) by using primers derived from the gag gene sequence of SIVmac239 as described (15).
Lymphocyte Immunophenotyping and in Situ Hybridization (ISH).
Immunophenotyping of EDTA-treated blood samples was carried out by using fluorochrome-conjugated mAbs (CD3-fluorescein isothiocyanate, PharMingen; CD4-allophycocyanin, CD8-peridinin chlorophyll protein, and CD20-phycoerythrin, Becton Dickinson Immunocytometry Systems) as described (16). Formalin-fixed, paraffin-embedded tissues were subjected to the ISH assay as reported (17).
Immunostaining of Fixed Tissue with an Anti-CD4 mAb.
Fixed embedded tissue sections were stained with an anti-human CD4 mouse mAb (NCL-CD4; Novocastra Laboratories, Newcastle upon Tyne, U.K.). Sections were rehydrated and processed for 6–8 min in a Presto pressure cooker (National Presto Industries, Eau Claire, WI) in 1 mM EDTA (pH 8.0) to unmask CD4 antigen. The samples were sequentially treated with PBS, aqueous hydrogen peroxide, serum block (3% normal goat serum/1% nonfat milk/0.5% BSA), and the anti-CD4 mAb (1:40) for 1 h. The reaction was visualized by using the Vectastain Mouse-IgG Peroxidase ABC kit (Vector Laboratories) and diaminobenzidine followed by 10 s of treatment in DAB Enhancing Solution (Vector Laboratories). Samples were then rinsed in distilled water and counterstained with hematoxylin.
Combined ISH and Immunohistochemistry.
Formalin-fixed, paraffin-embedded tissues were hybridized with a digoxigenin riboprobe as outlined above. The hybridized probe was detected with a monoclonal sheep anti-digoxigenin peroxidase conjugate followed by biotinylated tyramide (NEN) and streptavidin-Alexa 488 (Molecular Probes). These samples were then stained with either an anti-CD3 (Dako) or anti-macrophage HAM56 (Dako) antibodies and then with streptavidin Cyanine-5 (NEN). These double-labeled samples were observed with a Leica Confocal microscope and the images were digitally photographed.
Results
SHIVDH12R Induces a Rapid, Irreversible, and Systemic Depletion of CD4+ T Cells.
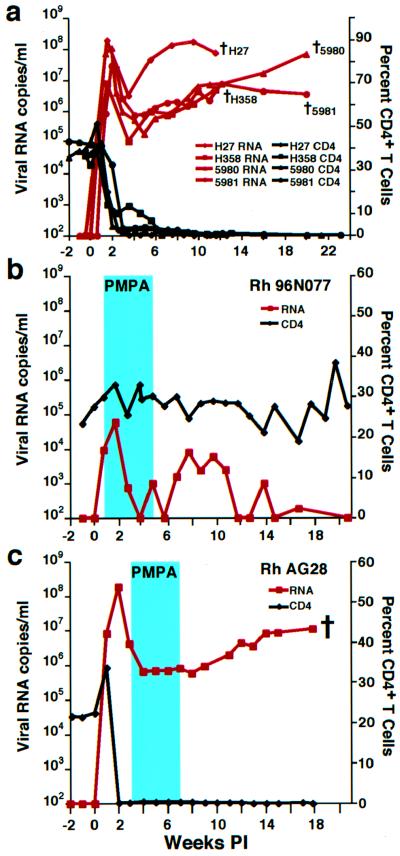
We have described a highly pathogenic SHIV, designated SHIVDH12R (13), which induces an irreversible loss of circulating CD4+ T lymphocytes within 3 weeks of infection (Fig. 1a). Plasma viremia in these infected monkeys peaks at 1 × 107 to 2 × 108 RNA copies per ml 10–17 days after inoculation, declines somewhat between weeks 3 and 5, and gradually increases again over the next 2–4 months, at which point the macaques are killed because of opportunistic infections, anorexia, cachexia, and weight loss (15).
Figure 1.
SHIV and CD4+ T lymphocyte levels in infected rhesus monkeys. (a) Viral RNA loads (red) and CD4+ T cell levels (black) in rhesus macaques inoculated intravenously with 4.1 × 105 (Rh H27 and Rh H358), 16,400 (Rh 5980), and 656 (Rh 5981) TCID50 of the SHIVDH12R stock virus are shown. The black crosses indicate the times of animal killing because of their deteriorating clinical condition. (b and c) Plasma viral RNA (red) and CD4+ T cell levels (black) in SHIVDH12R-infected rhesus macaques administered PMPA (30 mg per kg) for 28 days beginning 5 days (b) or 21 days (c) postinfection (PI) (blue rectangles). The cross indicates the time when monkey AG28 was killed.
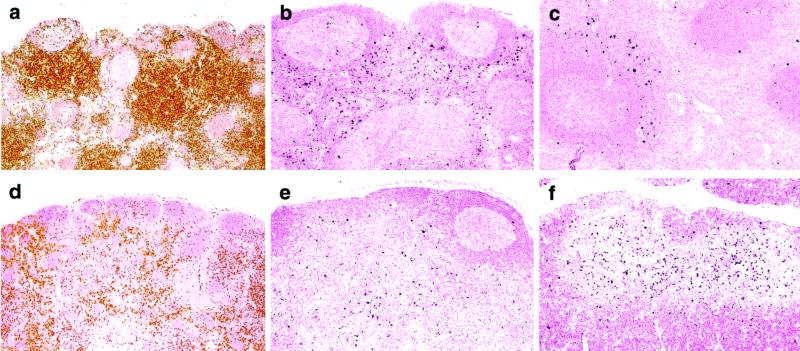
In a study to be described in detail elsewhere (T.I., C.B., A.B.-W., R.P., V.H., and M.A.M., unpublished work), the rapid and unremitting CD4+ T cell depletion in rhesus macaques inoculated with 1–4 × 105 TCID50 of SHIVDH12R was evaluated systemically by performing serial necropsies at multiple time points during the first 3 weeks of the primary infection (14). DNA PCR and ISH analyses of tissues collected from those animals revealed that SHIVDH12R infections of lymphocytes in axillary and mesenteric lymph nodes, gastrointestinal tract-associated lymphoid tissue, spleen, and thymus increased synchronously, peaking slightly earlier (between days 10 and 14) than virus replicating in circulating peripheral blood mononuclear cells (between days 14 and 21). CD4+ T cells in lymphoid tissues, detected by anti-human CD4 staining of formalin-fixed specimens, declined rapidly in the SHIVDH12R-infected monkeys, becoming virtually undetectable by day 21. A representative experiment from that study shows that CD4+ T lymphocytes in the paracortical areas of mesenteric lymph nodes were the principal targets of the SHIVDH12R infection and significantly declined in number by day 14 (Fig. 2 a, b, d, and e). Vigorous virus replication was also observed on day 10 in CD4+ T lymphocytes located in the periarteriolar lymphoid sheath region of the spleen (Fig. 2c) and in the thymus medulla (Fig. 2f), where mature, single-positive T lymphocytes primarily reside.
Figure 2.
Virus replication and CD4+ T cell depletion in lymphoid tissues during primary SHIVDH12R infections of rhesus monkeys. Animals inoculated with 1.0 × 105 TCID50 of SHIVDH12R were killed on day 10 [Rh AH5E (a–c)] or day 14 [Rh AE56 (d–f)]. Mesenteric lymph nodes (a, b, d, and e), spleen (c), and thymus (f) were examined by ISH (b, c, e, and f; visualized as dark blue) and immunohistochemistry for CD4 staining (a and d; visualized as brown). (Original magnifications: a, ×5; b, ×10; c, ×10; d, ×4; e, ×10; and f, ×10.)
In the Absence of CD4+ T Cells, Tissue Macrophage Sustain Virus Production.
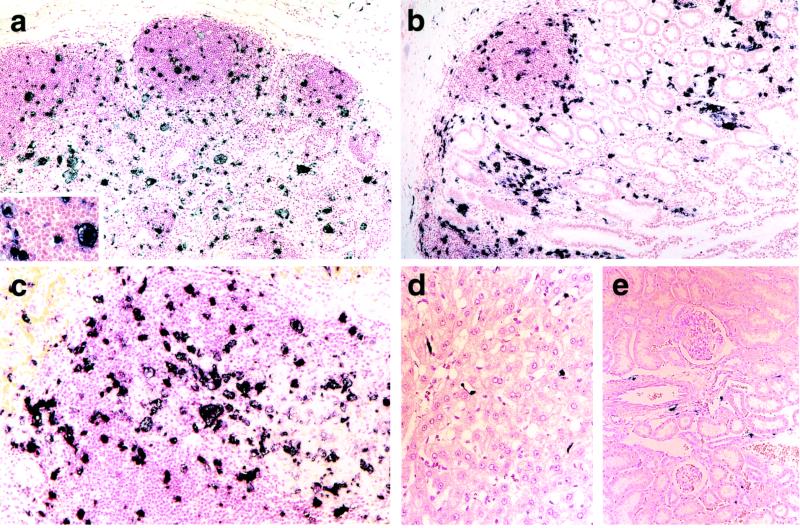
The rapid and overwhelming SHIVDH12R infection results in the loss of nearly all of the CD4+ T cell population from the blood and lymphoid tissues of the inoculated rhesus monkeys. However, as indicated in Fig. 1, these animals, and several other similarly SHIVDH12R-infected macaques, survive for an additional 3–6 months and continue to produce extremely high virus loads (106-108 viral RNA copies per ml) despite the absence of significant numbers of circulating and tissue-associated CD4+ T lymphocytes. As the first step in identifying the cell type sustaining these robust levels of viremia, tissues were collected from one animal (Rh H27; Fig. 1) at the time of its necropsy on week 12.6 and examined for viral RNA synthesis by ISH. Large (20–60 μm in diameter) multinucleated virus-producing cells, with a morphology typical of tissue macrophage, were scattered throughout the lymph nodes (Fig. 3a). In the gastrointestinal tract and spleen, numerous large ISH-positive cells also prominently stood out against a background of smaller nonreactive cells, presumably of lymphocyte lineage (Fig. 3 b and c). This late-stage pattern of ISH is to be contrasted with that observed during the acute phase of the infection when T lymphocytes were the principal sites of virus replication in these same lymphoid tissues (compare with Fig. 2). Equally surprising was the presence of virus-positive cells in organs not known to be prominent sources of HIV-1 and SIV production such as liver and kidney. Flattened nonhepatocytes, which lined sinusoids in the liver and possess the morphology of Kupffer cells, were virus positive in the liver (Fig. 3d). Within the kidney, interstitial cells, distinct from tubules, glomeruli, and blood vessels, and presumably of macrophage origin, were synthesizing viral RNA (Fig. 3e). Similar virus-positive cells have been observed in SIV-infected macaques, which rapidly progress to AIDS in the absence of a SIV-specific immune response (18). However, these SIV-infected monkeys do not generally experience the severe depletions of circulating and tissue-associated CD4+ lymphocytes observed in the SHIVDH12R-infected animals.
Figure 3.
Virus replication during the “macrophage phase” of SHIVDH12R infection. Rhesus macaque Rh H27 was infected with SHIVDH12R (4.1 × 105 TCID50) and killed at week 12.6. Specimens from mesenteric lymph node (a), ileum (b), spleen (c), liver (d), and kidney (e) were evaluated for virus replication by ISH. (Original magnifications: a, ×10; Inset, ×30; b, ×10; c, ×20; d, ×20; and e, ×10.)
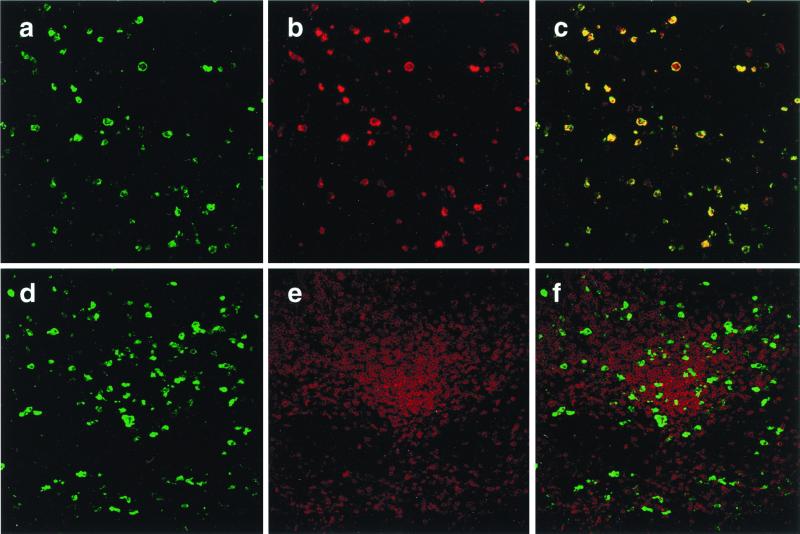
To more definitively identify and quantitate the cell type(s) actively producing viral RNA in the absence of CD4+ T cells, formalin-fixed axillary, mesenteric, and colonic lymph node specimens were subjected to ISH, immunostained with antibodies that recognize (i) a digoxigenin-conjugated riboprobe specific for SHIV sequences (green) or (ii) macrophage or T lymphocytes (both red), and analyzed by confocal immunofluorescent microscopy. As shown in Fig. 4 a–c, the vast majority of the ISH-positive cells stain with the macrophage-specific mAb and the immunofluorescent images colocalize. In contrast, minimal colocalization was observed when the lymph node was stained with an antibody specific for T cells (Fig. 4 d–f). A similar pattern was observed in screens of 10 different sections prepared from axillary, mesenteric, and colonic lymph nodes. Quantitative confocal immunofluorescence analysis indicated that more than 95% of the virus-producing cells in the lymph nodes collected at week 12.6 were macrophage, 1–2% were T lymphocytes, and 3–5% were of unknown lineage.
Figure 4.
Identification of virus-producing lymph node cells during the “macrophage phase” of SHIVDH12R infection. Two individual mesenteric lymph node sections (a–c or d–f) were subjected to combination ISH using a riboprobe recognizing SHIV sequences (a and d; visualized in green) and immunostaining specific for macrophage (b; visualized in red) or CD3+ T lymphocytes (e; visualized in red). (c) Superimposed image of a with b. (f) Superimposed image of d and e.
Virus-Infected Macrophage in Infected Monkeys Are Refractory to a Potent Reverse Transcriptase (RT) Inhibitor.
Some potent RT inhibitors, whether administered alone or in combination with other antiretroviral agents, effectively reduce virus loads in vivo to undetectable levels. One of these inhibitors, PMPA, lowers SIV RNA levels by >97% in chronically infected animals and can even prevent the establishment of viral infections in macaque monkeys if given shortly after virus inoculation (19–21). To ascertain whether this drug could control the early T cell and/or the late macrophage phases of SHIVDH12R infections in vivo, PMPA was administered intramuscularly (30 mg per kg) for 28 days to two animals, starting on day 5 or 21 postinfection. In contrast to untreated macaques (Fig. 1a), the peak plasma virus load in macaque Rh 96N077, which received PMPA beginning on day 5, was reduced 50- to 1,000-fold (Fig. 1b). Furthermore, after cessation of PMPA treatment on week 5, the levels of viral RNA in the blood gradually declined even further and became undetectable by week 20. Most significantly, the rapid control of the plasma viremia protected monkey Rh 96N077 from a decline of its circulating CD4+ T cells. At week 80 postinfection, this animal remains healthy with undetectable plasma RNA and 38% CD4+ T lymphocyte levels. Although PMPA therapy did not prevent infection, it markedly reduced plasma virus loads and blocked the SHIVDH12R-induced irreversible CD4+ T cell loss when administered for 4 weeks during the T cell phase of infection. We have observed a similar salutary effect in other rhesus monkeys when PMPA treatment was initiated on day 5 postinfection (Y.E., A.B.-W., R.P., N.B., and M.A.M., unpublished work).
In contrast, no demonstrable antiviral effect was observed when PMPA was administered to animal Rh AG28 for 4 weeks during the macrophage phase of its virus infection (Fig. 1c). The levels of virus in the plasma remained high and were virtually indistinguishable from those measured in untreated SHIVDH12R-infected macaques (compare with Fig. 1a). This lack of response not only differed from the potent antiviral activity of PMPA observed during the very early phase of SHIVDH12R infection, but also contrasted with the effects of PMPA administered in a similar fashion to cynomolgus monkeys chronically infected with SIV (20). In the latter study, the SIV-infected macaques had experienced only modest reductions of their CD4+ T cell counts (a decline to the 520–970 cells per μl range at 19 weeks postinfection), although the 4-week course of PMPA therapy lowered SIV loads by >97%. Taken together, these results imply that a potent RT inhibitor, such as PMPA, will effectively reduce plasma virus loads as long as there are sufficient numbers of CD4+ T cell targets to support new rounds of virus infection. When this lymphocyte subset becomes severely depleted, as is the case for chronically SHIVDH12R-infected monkeys, long-lived chronically infected tissue macrophage are not only refractory to RT inhibitors but are able to sustain high levels of virus production.
Discussion
The resistance of rhesus monkey AG28 to PMPA antiviral therapy was not totally unanticipated. DNA copies of the SHIVDH12R inoculum should have been generated and integrated into the chromosomal DNA of virus-producing macrophage at least 2 weeks before the institution of PMPA treatment. Because of the reported noncytopathic nature of HIV-1 infections of macrophage compared with CD4+ T lymphocytes (22–24), the former would remain viable and be refractory to the antiviral effects of administered RT inhibitors. The sensitivity of cultured human monocyte-derived macrophage (MDM) to a variety of potent antiretroviral agents has, in fact, been extensively evaluated (25–29). These studies indicate that MDM are as sensitive to nucleoside RT inhibitors as peripheral blood mononuclear cells during acute infections. However, chronically infected MDM are completely resistant to 3′-azido-3′-deoxythymidine (AZT), 2′,3′-dideoxyinosine (ddI), and 2′,3′-dideoxycytidine (ddC) in assays measuring progeny virus production or de novo viral RNA synthesis. It is also worth noting that the activity of HIV-1 protease inhibitors (not evaluated in this study) was reduced ≈100-fold in persistently infected MDM compared with their effects during acute infections of both peripheral blood mononuclear cells and MDM; complete inhibition of virus production was never achieved (30).
It must be stressed that the extraordinarily rapid and irreversible decline of CD4+ T cells within 3–4 weeks of infection in macaques inoculated with a highly pathogenic SHIV such as SHIVDH12R differs significantly from the response of humans after exposure to HIV-1. Extremely rapid CD4+ T lymphocyte depletion is also rarely observed in SIV-infected monkeys, although one SIV variant, SIVPBj14, causes a fulminant diarrheal syndrome with death in 7–10 days, but little if any T cell loss (31). Thus, whereas one might view the disease induced by highly pathogenic SHIVs such as SHIVDH12R, SHIV89.6P, and SHIVKU-1 as a laboratory contrivance (13, 32, 33), the targeting and elimination of the identical T cell subset affected by HIV-1 coupled with the appearance of opportunistic infections and other signs of immunodeficiency 3–6 months later is consistent with an accelerated model of HIV-1-induced AIDS in humans. At the very least, the SHIVDH12R/macaque system described shares many features with the late stages of HIV-1 infections in which CD4+ T lymphocytes have been severely depleted and clinical symptoms are prominent.
Macrophage have long been known to be targets of HIV-1 in infected individuals. Macrophage and microglia in the central nervous system of patients with AIDS dementia complex were identified as one of the first nonlymphocyte cell lineages that supported virus replication (34, 35). It is now appreciated that macrophage throughout the body become exposed to virus very early in the infection (36–38). This is also the case for SIV-infected rhesus monkeys, in which 10–12% of virus-infected lymph node cells were shown to be of macrophage lineage 7–12 days after infection (39). In SHIVDH12R-infected macaques, tissue macrophage appear to be refractory to the cytopathic effects of virus and sustain the production of 106-107 copies of RNA per ml of plasma for several months after CD4+ T cell depletion (Fig. 1a). Whereas this level of progeny virion generation is quite substantial, it is still 5- to 20-fold lower than that measured at the peak of the T cell phase of infection, 2 weeks postinoculation. These values, nonetheless, are consistent with reports of p24 production by MDM cultures after infection with HIV-1 macrophage-tropic isolates [160 and 700 ng per ml reported for HIV-1BAL and HIV-1AD8, respectively; (40, 41)]. Furthermore, using empirical equations relating the time to peak virus production to the input multiplicity of infection, we previously calculated the burst size of infectious particles released from human T cell lines and peripheral blood mononuclear cells to be 100–300 per cell (42). A similar analysis of human MDM, infected with the macrophage-tropic HIV-1AD8 isolate, resulted in values of 5–10 infectious virions per cell (D. Dimitrov and M.A.M., unpublished work).
In HIV-1-seropositive persons, the number of virus-producing macrophage, relative to infected CD4+ T lymphocytes, is probably extremely low early in the infection, but over time, this fraction of infected cells may increase substantially. In this regard, an analysis of HIV-1 decay, after the institution of highly active antiretroviral therapy in patients with moderately low CD4+ T cell numbers (mean cell counts of 212 per μl), indicated that 1–7% of the plasma virus was produced by long-lived infected cells, putatively defined as tissue macrophage (43). At much later times, when CD4+ T cell levels in AIDS patients fall to the same range (<50 cells per μl) observed in SHIVDH12R-infected rhesus monkeys, HIV-1 also becomes detectable in several nonlymphoid organs such as lung, colon, brain, liver, and kidney (44, 45). In addition, the coexistence of opportunistic infections has also been reported to greatly augment both the number of productively HIV-1-infected monocyte/macrophage and viral RNA copies per cell (24). The SHIVDH12R/macaque monkey system described here may be useful for monitoring primate lentiviral dynamics in tissue macrophage, quantitating the contribution of this cell type to the levels of circulating virus, and identifying novel therapies capable of eradicating the infected macrophage reservoir.
Acknowledgments
We thank M. Eckhaus for assisting with the histopathologic analyses, R. Byrum for his participation in the animal experiments, and O. Schwartz and S. Bour for image processing.
Abbreviations
- HIV-1
HIV type 1
- SHIV
simian immunodeficiency virus (SIV)/HIV-1 chimeric virus
- TCID50
tissue culture 50% infective dose
- PMPA
9-[2(R)-(phosphonomethoxy)propyl]adenine
- ISH
in situ hybridization
- RT
reverse transcriptase
- MDM
monocyte-derived macrophage
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.021551798.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.021551798
References
- 1.Gulick R M, Mellors J W, Havlir D, Eron J J, Gonzalez C, McMahon D, Richman D D, Valentine F T, Jonas L, Meibohm A, et al. N Engl J Med. 1997;337:734–739. doi: 10.1056/NEJM199709113371102. [DOI] [PubMed] [Google Scholar]
- 2.Palella F J, Jr, Delaney K M, Moorman A C, Loveless M O, Fuhrer J, Satten G A, Aschman D J, Holmberg S D. N Engl J Med. 1998;338:853–860. doi: 10.1056/NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 3.Natarajan V, Bosche M, Metcalf J A, Ward D J, Lane H C, Kovacs J A. Lancet. 1999;353:119–120. doi: 10.1016/s0140-6736(05)76156-0. [DOI] [PubMed] [Google Scholar]
- 4.Furtado M R, Callaway D S, Phair J P, Kunstman K J, Stanton J L, Macken C A, Perelson A S, Wolinsky S M. N Engl J Med. 1999;340:1614–1622. doi: 10.1056/NEJM199905273402102. [DOI] [PubMed] [Google Scholar]
- 5.Zhang L, Ramratnam B, Tenner-Racz K, He Y, Vesanen M, Lewin S, Talal A, Racz P, Perelson A S, Korber B T, et al. N Engl J Med. 1999;340:1605–1613. doi: 10.1056/NEJM199905273402101. [DOI] [PubMed] [Google Scholar]
- 6.Dornadula G, Zhang H, VanUitert B, Stern J, Livornese L, Jr, Ingerman M J, Witek J, Kedanis R J, Natkin J, DeSimone J, et al. J Am Med Assoc. 1999;282:1627–1632. doi: 10.1001/jama.282.17.1627. [DOI] [PubMed] [Google Scholar]
- 7.Chun T W, Stuyver L, Mizell S B, Ehler L A, Mican J A, Baseler M, Lloyd A L, Nowak M A, Fauci A S. Proc Natl Acad Sci USA. 1997;94:13193–13197. doi: 10.1073/pnas.94.24.13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Finzi D, Hermankova M, Pierson T, Carruth L M, Buck C, Chaisson R E, Quinn T C, Chadwick K, Margolick J, Brookmeyer R, et al. Science. 1997;278:1295–1300. doi: 10.1126/science.278.5341.1295. [DOI] [PubMed] [Google Scholar]
- 9.Wong J K, Hezareh M, Gunthard H F, Havlir D V, Ignacio C C, Spina C A, Richman D D. Science. 1997;278:1291–1295. doi: 10.1126/science.278.5341.1291. [DOI] [PubMed] [Google Scholar]
- 10.Davey R T, Jr, Bhat N, Yoder C, Chun T W, Metcalf J A, Dewar R, Natarajan V, Lempicki R A, Adelsberger J W, Miller K D, et al. Proc Natl Acad Sci USA. 1999;96:15109–15114. doi: 10.1073/pnas.96.26.15109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chun T W, Davey R T, Jr, Ostrowski M, Shawn Justement J, Engel D, Mullins J I, Fauci A S. Nat Med. 2000;6:757–761. doi: 10.1038/77481. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Chung C, Hu B S, He T, Guo Y, Kim A J, Skulsky E, Jin X, Hurley A, Ramratnam B, et al. J Clin Invest. 2000;106:839–845. doi: 10.1172/JCI10565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Igarashi T, Endo Y, Englund G, Sadjadpour R, Matano T, Buckler C, Buckler-White A, Plishka R, Theodore T, Shibata R, et al. Proc Natl Acad Sci USA. 1999;96:14049–14054. doi: 10.1073/pnas.96.24.14049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Committee on the Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals, revised Ed. Department of Health and Human Services, Washington, DC: National Institutes of Health; 1985. , publication no. (NIH) 85–23. [Google Scholar]
- 15.Endo Y, Igarashi T, Nishimura Y, Buckler C, Buckler-White A, Plishka R, Dimitrov D S, Martin M A. J Virol. 2000;74:6935–6945. doi: 10.1128/jvi.74.15.6935-6945.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shibata R, Maldarelli F, Siemon C, Matano T, Parta M, Miller G, Fredrickson T, Martin M A. J Infect Dis. 1997;176:362–373. doi: 10.1086/514053. [DOI] [PubMed] [Google Scholar]
- 17.Hirsch V M, Dapolito G, Johnson P R, Elkins W R, London W T, Montali R J, Goldstein S, Brown C. J Virol. 1995;69:955–967. doi: 10.1128/jvi.69.2.955-967.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirsch V, Adger-Johnson D, Campbell B, Goldstein S, Brown C, Elkins W R, Montefiori D C. J Virol. 1997;71:1608–1620. doi: 10.1128/jvi.71.2.1608-1620.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsai C C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 20.Tsai C C, Follis K E, Beck T W, Sabo A, Bischofberger N, Dailey P J. AIDS Res Hum Retroviruses. 1997;13:707–712. doi: 10.1089/aid.1997.13.707. [DOI] [PubMed] [Google Scholar]
- 21.Tsai C C, Emau P, Follis K E, Beck T W, Benveniste R E, Bischofberger N, Lifson J D, Morton W R. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gartner S, Markovits P, Markovitz D M, Kaplan M H, Gallo R C, Popovic M. Science. 1986;233:215–219. doi: 10.1126/science.3014648. [DOI] [PubMed] [Google Scholar]
- 23.Meltzer M S, Nakamura M, Hansen B D, Turpin J A, Kalter D C, Gendelman H E. AIDS Res Hum Retroviruses. 1990;6:967–971. doi: 10.1089/aid.1990.6.967. [DOI] [PubMed] [Google Scholar]
- 24.Orenstein J M, Fox C, Wahl S M. Science. 1997;276:1857–1861. doi: 10.1126/science.276.5320.1857. [DOI] [PubMed] [Google Scholar]
- 25.Aquaro S, Balestra E, Cenci A, Francesconi M, Calio R, Perno C F. J Biol Regul Homeost Agents. 1997;11:69–73. [PubMed] [Google Scholar]
- 26.Crowe S M, McGrath M S, Elbeik T, Kirihara J, Mills J. J Med Virol. 1989;29:176–180. doi: 10.1002/jmv.1890290306. [DOI] [PubMed] [Google Scholar]
- 27.Perno C F, Yarchoan R, Cooney D A, Hartman N R, Gartner S, Popovic M, Hao Z, Gerrard T L, Wilson Y A, Johns D G, et al. J Exp Med. 1988;168:1111–1125. doi: 10.1084/jem.168.3.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perno C F, Aquaro S, Rosenwirth B, Balestra E, Peichl P, Billich A, Villani N, Calio R. J Leukocyte Biol. 1994;56:381–386. doi: 10.1002/jlb.56.3.381. [DOI] [PubMed] [Google Scholar]
- 29.Aquaro S, Perno C F, Balestra E, Balzarini J, Cenci A, Francesconi M, Panti S, Serra F, Villani N, Calio R. J Leukocyte Biol. 1997;62:138–143. doi: 10.1002/jlb.62.1.138. [DOI] [PubMed] [Google Scholar]
- 30.Perno C F, Newcomb F M, Davis D A, Aquaro S, Humphrey R W, Calio R, Yarchoan R. J Infect Dis. 1998;178:413–422. doi: 10.1086/515642. [DOI] [PubMed] [Google Scholar]
- 31.Fultz P N, McClure H M, Anderson D C, Switzer W M. AIDS Res Hum Retroviruses. 1989;5:397–409. doi: 10.1089/aid.1989.5.397. [DOI] [PubMed] [Google Scholar]
- 32.Reimann K A, Li J T, Veazey R, Halloran M, Park I W, Karlsson G B, Sodroski J, Letvin N L. J Virol. 1996;70:6922–6928. doi: 10.1128/jvi.70.10.6922-6928.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joag S V, Li Z, Foresman L, Stephens E B, Zhao L J, Adany I, Pinson D M, McClure H M, Narayan O. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiley C A, Schrier R D, Nelson J A, Lampert P W, Oldstone M B. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Koenig S, Gendelman H E, Orenstein J M, Dal Canto M C, Pezeshkpour G H, Yungbluth M, Janotta F, Aksamit A, Martin M A, Fauci A S. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 36.Davis L E, Hjelle B L, Miller V E, Palmer D L, Llewellyn A L, Merlin T L, Young S A, Mills R G, Wachsman W, Wiley C A. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 37.Appleman M E, Marshall D W, Brey R L, Houk R W, Beatty D C, Winn R E, Melcher G P, Wise M G, Sumaya C V, Boswell R N. J Infect Dis. 1988;158:193–199. doi: 10.1093/infdis/158.1.193. [DOI] [PubMed] [Google Scholar]
- 38.Sidtis J J, Price R W. Neurology. 1990;40:323–326. doi: 10.1212/wnl.40.2.323. [DOI] [PubMed] [Google Scholar]
- 39.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus K A, Reimann K A, Reinhart T A, Rogan M, Cavert W, Miller C J, et al. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 40.Willey R L, Theodore T S, Martin M A. J Virol. 1994;68:4409–4419. doi: 10.1128/jvi.68.7.4409-4419.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arthos J, Rubbert A, Rabin R L, Cicala C, Machado E, Wildt K, Hanbach M, Steenbeke T D, Swofford R, Farber J M, et al. J Virol. 2000;74:6418–6424. doi: 10.1128/jvi.74.14.6418-6424.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dimitrov D S, Willey R L, Sato H, Chang L J, Blumenthal R, Martin M A. J Virol. 1993;67:2182–2190. doi: 10.1128/jvi.67.4.2182-2190.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Nature (London) 1997;387:188–191. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 44.Donaldson Y K, Bell J E, Ironside J W, Brettle R P, Robertson J R, Busuttil A, Simmonds P. Lancet. 1994;343:383–385. doi: 10.1016/s0140-6736(94)91222-x. [DOI] [PubMed] [Google Scholar]
- 45.Cao Y Z, Dieterich D, Thomas P A, Huang Y X, Mirabile M, Ho D D. AIDS. 1992;6:65–70. doi: 10.1097/00002030-199201000-00008. [DOI] [PubMed] [Google Scholar]