Abstract
This study examined the effect of attention in infants on the ERP changes occurring during the recognition of briefly presented visual stimuli. Infants at ages 4.5, 6 and 7.5 months were presented with a Sesame Street movie that elicited periods of attention and inattention, and computer-generated stimuli were presented overlaid on the movie for 500 ms. One stimulus was familiar to the infants and was presented frequently, a second stimulus was familiar but presented infrequently, and a set of 14 novel stimuli were presented infrequently. An ERP component labeled the ‘Nc’ (Negative Central, about 450–550 ms after stimulus onset) was larger during attention than inattention and increased in magnitude over the three testing ages during attention. Late slow waves in the ERP (from 1000 to 2000 ms post-stimulus onset) consisted of a positive slow wave in response to the infrequent familiar stimulus at all three testing ages. The late slow wave in response to the infrequent novel stimulus during attention was a positive slow wave for the 4.5-month-old infants, to a positive-negative slow wave for the 6-month-old infants and a negative slow wave for the 7.5-month-old infants. These results show attention facilitates the brain response during infant recognition memory and show that developmental changes in recognition memory are closely related to changes in attention.
Introduction
The study of recognition memory in young infants has been aided by the use of measures of scalp electrical activity, the electroencephalogram (EEG) and event-related potentials (ERP). The EEG is spontaneously varying electrical potential on the scalp and the ERP is EEG activity that is time-locked to the onset of stimulus presentation. The ERP has positive and negative electrical waves, components, such as the P1 (or ‘P100’), N1, P2, N2, P3 (or ‘P300’) and various slow waves (see de Haan & Nelson, 1997; or Nelson & Monk, 2001, for a discussion of these components in infants; see Fabiani, Gratton & Coles, 2000; Hillyard, Mangun, Woldroff & Luck, 1995; or Swick, Kutas & Neville, 1994, for a discussion of these components in adults). Infants’ responses to familiar and novel stimuli may be distinguished by averaged ERP activity occurring at about 500 to 700 ms following stimulus onset (Courchesne, 1977, 1978; Courchesne, Ganz & Norcia, 1981). Slow waves in the ERP response, occurring from 750 ms to about 1500 ms following stimulus onset, may distinguish stimulus presentation probability and stimulus novelty (Nelson & Collins, 1991, 1992; Nelson & deRegnier, 1992; Nelson & Salapatek, 1986). The current study shows that infant attentiveness mediates ERP responses during the presentation of familiar and novel stimuli.
Several studies have examined scalp-recorded ERP as a response to frequently and infrequently presented visual stimuli of very brief duration (~500 ms) in young infants. Nelson and his colleagues (Nelson & Collins, 1991, 1992; Nelson & deRegnier, 1992; Nelson & Salapatek, 1986; also see reviews by Nelson, 1994; Nelson & Dukette, 1998; Nelson & Monk, 2001; Nelson & Webb, 2003) and others (Courchesne, 1977, 1978; Courchesne et al., 1981; Karrer & Ackles, 1987, 1988; Karrer & Monti, 1995) have used an ‘oddball’ paradigm in which one stimulus is presented relatively frequently (‘standard stimulus’) and a second stimulus is presented infrequently (‘oddball stimulus’ or ‘rare stimulus’). These studies report a large negative ERP component occurring about 400–800 ms after stimulus onset located primarily in the frontal and central EEG leads. This has been labeled the Nc component (Nc is ‘Negative’ ‘central’; Courchesne, 1977, 1978; Courchesne et al., 1981). In many studies the Nc component is larger to the rare stimulus and is thought to represent a general attentive or alerting to the presence of a novel stimulus, i.e. the infrequently presented rare stimulus.
Nelson (1994; Nelson & Dukette, 1998; Nelson & Monk, 2001) argues that the Nc is part of a general orienting system that is not related to stimulus novelty per se. The oddball procedure presents two stimuli that are novel at the beginning of the stimulus presentation. Over the course of stimulus presentations in young infants the standard stimulus becomes more familiar than the rare stimulus due to the difference in frequency of stimulus exposure. Therefore, stimulus novelty is confounded with presentation probability over the course of stimulus presentations. Nelson modified this procedure by familiarizing the infant with two stimuli before beginning the brief presentations. One familiar stimulus becomes the standard stimulus in the brief presentations, ‘frequent familiar’, and the other familiar stimulus becomes the rare stimulus, ‘infrequent familiar’. Therefore, the ERP responses to these stimuli are a function of presentation probability rather than stimulus familiarity. If the standard and rare stimuli are already familiar to the infant the Nc component does not differ (Nelson & Collins, 1991, 1992). A series of novel stimuli also are presented infrequently (‘infrequent novel’) and may be compared with the infrequently presented familiar stimuli to assess stimulus novelty. The Nc component also does not differ in this procedure to the infrequently presented novel stimuli. The Nc therefore is an ERP component that must be a type of general orienting to the stimulus related to infant attention rather than infant memory.
One goal of the present study was to examine the effect of infant attentiveness on the Nc ERP component. If the Nc component reflects the infants orienting to the brief stimulus presentation, then this response should be larger during periods of attentiveness than during periods of inattentiveness. Infant attentiveness may be measured with heart rate changes elicited by visual stimulus presentation. There is a sudden deceleration of heart rate in response to interesting visual stimuli that continues as long as the infant is attentive to the stimulus (Richards, 2001, 2003; Richards & Casey, 1992). When the infant becomes disinterested in the stimulus the heart rate returns to its prestimulus level even though fixation may continue. Thus, heart rate changes distinguish attention engagement and disengagement in young infants. Richards (2001, 2003) has argued that these heart rate changes index a brain system controlling arousal that affects a wide variety of stimulus modalities and cognitive functions. Stimulus orienting should be enhanced when this arousal system is active compared to periods when the arousal system is not engaged. Therefore, the Nc component should be enhanced when the infant’s heart rate has decelerated in response to a stimulus presentation or remains below prestimulus level due to an ongoing arousing stimulus.
These studies of infants’ responses to briefly presented visual stimuli also report later components in the ERP. The ERP in infants in response to the brief visual stimuli has slowly changing positive or negative potential shifts from about 800 to 1500 ms following stimulus presentation. Nelson and Collins (1991) reported that in 6-month-old infants the slow wave differs as a function of presentation type. First, the infrequently presented familiar stimulus resulted in a larger positive slow wave in the ERP than when the familiar stimulus was presented frequently. Second, stimuli with which the infant was not familiar and were presented infrequently, infrequent novel, resulted in a larger negative slow wave in the ERP than the frequent familiar stimulus. Thus, infants in this procedure are sensitive to the relative probability of stimulus occurrence (frequent and infrequent familiar stimuli) as well as stimulus novelty (infrequent familiar and novel stimuli).
A second goal was to examine the effect of attention on the late slow waves. Infant’s attentiveness affects recognition memory during acquisition and retrieval periods. Richards (1997) and Frick and Richards (2001) presented infants with novel stimuli for brief periods of time (2.5, 5.0, 7.5 s) when infants were attentive or inattentive. If these stimuli were presented when the infant was attentive, the memory for these briefly presented stimuli was nearly at the same levels as stimuli presented for 20 s in a typical accumulated-fixation presentation method. This suggests that the acquisition of stimulus information during familiarization occurs primarily during attention in young infants. If the negative late slow wave in the ERP response to briefly presented stimuli represents the infants’ recognition of novelty and the beginning of stimulus processing, it may be enhanced when the infant is aroused (Richards, 2001, 2003). Similarly, infants may exhibit signs of recognition memory when in an attentive state. Infants shift their fixation back and forth between the novel and familiar stimulus during the test phase of the paired-comparison recognition memory procedure, but spend more time looking at the novel than the familiar stimulus during attention (Richards & Casey, 1990). Alternatively, during inattentiveness infants spend equal amounts of time looking at the novel and familiar stimulus. The positive slow wave in the ERP response to briefly presented stimuli occurs in response to the infrequently presented familiar stimuli. This activity has been interpreted as recognition of the stimulus and an updating of stimulus information in memory (Nelson & Dukette, 1998). Thus, the positive slow wave may be enhanced during attention if it measures recognition processes in this procedure.
There may be age changes in the first year in the ERP response to these briefly presented stimuli. The Nc component may be found in infants as young as 4–6 weeks of age (Karrer & Ackles, 1987; Karrer & Monti, 1995) but a different response to frequent and infrequent stimuli is not apparent as late as 4 months of age (Courchesne et al., 1981; Nelson & Collins, 1991). In studies showing a difference between the response to the standard and rare stimuli, the first age at which ERP responses differ is 6 months (Courchesne et al., 1981; Karrer & Ackles, 1987, 1988). One study showed that the magnitude of the Nc component continued to increase through 18 months of age (Karrer & Ackles, 1987, 1988). This component also occurs in later childhood ages (Courchesne, 1977, 1978). A similar pattern of development has been found for the late slow waves. Similar to the Nc component, at 4 months of age there was no difference between the later occurring slow waves (Nelson & Collins, 1992), whereas by 6 months of age (Nelson & Collins, 1991) or 8 or 12 months (Nelson & Collins, 1992; Nelson & deRegnier, 1992) these three stimulus presentation procedures resulted in differing ERP potential shifts. These changes in the ERP components suggest that attention and memory changes occur over the first year of life.
Infants were tested at 4.5, 6 and 7.5 months of age. These ages were chosen to cover a range of ages over which there are changes in the ERP responses to briefly presented stimuli. Infants were presented with a Sesame Street movie that elicited periods of attention and inattention (Frick & Richards, 2001; Richards, 1997), and computer-generated stimuli were overlaid on (replaced) the movie for 500 ms. The stimuli were a familiar stimulus that occurred on 60% of the presentations (frequent familiar), a familiar stimulus that occurred on 20% of the presentations (infrequent familiar) and a series of novel stimuli that occurred on 20% of the presentations (infrequent novel). The combination of these stimuli distinguishes infant responses to the probability of stimulus presentation (frequent familiar vs. infrequent familiar) and the novelty of the stimulus (infrequent familiar vs. infrequent novel). Scalp-recorded ERP was used and the Nc and late slow waves in the ERP were quantified. Attentiveness was assessed with heart-rate-defined attention phases. One goal of the study was to determine if the Nc component was affected by the infant’s attentiveness. The second goal of the study was to determine if the late slow waves distinguishing novelty processing were affected by infant attentiveness.
Method
Participants
Infants were recruited from the Columbia, South Carolina area. There were 48 infants sampled cross-sectionally at 20 (N = 16, M = 140.7 days, SD = 3.78, 8 M/8 F), 26 (N = 16, M = 184.6 days, SD = 4.45, 10 M/6 F) or 32 (N = 16, M = 225.6 days, SD = 3.55, 11 M/5 F) weeks of age. The infants were full-term (birthweight greater than 2500 grams, gestational age at least 38 weeks based on mother’s report of her last menstrual cycle). All infants were in good health at the time of the recording. An additional 10 infants were tested (2 at 20 weeks, 4 at 26 weeks, 4 at 32 weeks) who became fussy or sleepy during testing.
Apparatus
Infants were held on a parent’s lap approximately 55 cm from the center of a black and white 49 cm (19 in) monitor. The plane of the TV was parallel to the infant’s eyes and subtended a 44° visual angle. The area surrounding the TV and infants was covered in black cloth. A video camera was centered above the TV to allow an observer in an adjacent room to judge infant fixations and allowed for recording of the sessions with a time code to synchronize physiological and experimental information for analysis.
The stimuli were a 2° blinking square, a Sesame Street movie (Follow that Bird ) and computer-generated visual stimuli. The blinking square was used to attract fixation. The Sesame Street movie played on the center monitor without audio. The stimuli to test recognition memory were 16 computer-generated visual stimuli consisting of static visual patterns (same as in Frick & Richards, 2001, or Richards, 1997) presented in a 30° square centered on the monitor. Each subject had one of the computer-generated stimuli randomly chosen as the ‘frequent familiar’ stimulus, one randomly chosen as the ‘infrequent familiar’ stimulus and the remaining 14 stimuli served as the ‘infrequent novel’ stimuli.
Procedure
There were three phases of the experimental trials. The first phase was a familiarization phase designed to give the infant 20 s of exposure to the stimuli designated as the frequent familiar and infrequent familiar stimuli. One stimulus was presented on the monitor. The stimulus remained on for 5 s of accumulated looking time as judged by an on-line observer. This stimulus was then replaced with the other stimulus for 5 s of accumulated looking time. This procedure was repeated four times for each stimulus, resulting in 20 s of accumulated looking time in the familiarization phase for each stimulus. The physiological variables were not analyzed from this phase.
The second phase consisted of pre-experimental presentations of the frequent familiar and infrequent familiar stimuli alternately for 5 presentations with timing similar to the experimental trials. A blinking square was presented on the monitor. When the infant was judged to be looking at the blinking square, one of the two ‘familiar’ stimuli was presented for 500 ms. This was followed by an interstimulus interval ranging from 1.5 to 2.0 s and the presentation of the other familiar stimulus. These 10 presentations were done only if the on-line observer judged the infant to be looking at the monitor. If the infant was not looking at the monitor when the stimulus was to be presented, a delay of up to 10 s was given followed by the presentation of the blinking square and accompanied if necessary by an experimenter tapping the monitor or otherwise attracting fixation to the monitor. The ERP was analyzed from this phase to insure an equivalent response to the stimuli that had been arbitrarily designated as the frequent familiar and the infrequent familiar.
The experimental trials consisted of the presentation of the Sesame Street program and the frequent familiar, infrequent familiar and infrequent novel stimuli. Each experimental trial began with the presentation of the blinking square. When the infant was looking at the blinking square, one of the three memory stimuli was presented. The stimulus was presented for 500 ms followed by a blank screen for a 1.5 to 2.0 s interval. The stimulus was followed by the presentation of the Sesame Street movie. The movie was presented to elicit the attention phases. At intervals of approximately 3.5 to 6.0 s the recognition memory stimuli were presented. This was done by replacing the ongoing Sesame Street movie with a memory stimulus for 500 ms, followed by a 1.5 to 2.0 s blank screen, followed by the resumption of the Sesame Street movie. The Sesame Street movie remained on for 1.5 to 4 s, followed by another memory stimulus. The memory stimulus presentations were done only if the infant was judged to be looking at the monitor. This presentation period continued for 60 s, at which time the screen was blanked for 5 s, and then the procedure was repeated.
The first memory stimulus presentation on each experimental trial was equally divided between the three memory stimuli. Otherwise, on 60% of the presentations the frequent familiar stimulus was presented, on 20% of the presentations the infrequent familiar stimulus was presented, and the remaining 20% of the presentations consisted of the infrequent novel stimuli. This presentation sequence was done by presenting three frequent familiar, one infrequent familiar and one infrequent novel stimulus randomly ordered in 5-stimulus blocks. There was an attempt to present the three stimuli types equally in attentive and inattentive periods. This was done by evaluating the heart rate changes on-line and rearranging the stimulus types on-line in the 5-stimulus blocks to obtain equal numbers of presentations of the stimulus types in the attentive and the inattentive phases. The trials were continued as long as the infants were not fussy in order to obtain as many trials as possible. The mean number of experimental trials was 9.66 (SD = 1.43, range from 5 to 13). The number of memory stimulus presentations for each infant ranged from 43 to 100 (M = 71.5, SD = 13.53). Over all participants, the number of frequent familiar stimulus presentations in stimulus orienting, sustained attention and inattentiveness was 695, 982 and 466 (56% of total), the number of infrequent familiar stimulus presentations was 290, 374 and 205 (23% of total), and the number of infrequent novel stimulus presentations was 252, 367 and 188 (21% of total).
Measurement and quantification of physiological variables
The electrocardiogram (ECG) was recorded with Ag-AgCl electrodes placed on the infant’s chest. The ECG was digitized on-line at 1000 Hz (1 ms). The R-wave of the ECG was identified and inter-beat intervals were computed as the interval between the occurrences of the R-wave. Three attention phases were defined. Stimulus orienting was defined as the period before a heart rate deceleration occurred, which usually lasted 2 to 5 s. Sustained attention was defined as beginning at the onset of a significant heart rate deceleration. A significant heart rate deceleration was defined as five successive beats with inter-beat intervals each longer than the median of the five prestimulus beats (i.e. sustained attention; Richards, 1997; Richards & Casey, 1991). Inattentiveness was defined as beginning when heart rate returned to its prestimulus level following a significant heart rate deceleration. The return of heart rate to its prestimulus level was defined as five beats with inter-beat intervals shorter than median of the five prestimulus beats (i.e. attention termination, or inattentiveness; Richards, 1997; Richards & Casey, 1991). The attention phases were evaluated on-line by examining the acquired inter-beat intervals. For the off-line analyses, if the infant looked away for at least 2 s from the monitor during the 60-s presentation periods the attention phases were defined again at the next look onset beginning with another stimulus orienting phase.
The horizontal electrooculogram (EOG) was recorded with 6-mm Ag-AgCl electrodes that were placed posterior to the outer canthus of each eye using disposable electrode collars. The EOG was digitized at 1000 Hz (each ms) with a microcomputer. The EOG was amplified at 2K and a DC-recording was made. Saccades were identified in the horizontal EOG recording (see Richards & Hunter, 1997). The vertical EOG recording was used to identify blinks in the recording. Stimulus presentations with eye movements or blinks were not used in the analyses.
The electroencephalogram (EEG) was recorded from 20 locations with non-polarizable electrodes mounted in an elastic cap (ElectroCap International) and located at standard center, left and right hemisphere positions spanning the scalp according to the International 10/20 recording system, and non-10/20 electrode, OZ (Jasper, 1958; Pivik, Broughton, Coppola, Davidson, Fox & Nuwer, 1993). These sites and the right mastoid were measured relative to a left mastoid reference electrode, and the EEG waveforms were algebraically rereferenced to the average of the left and right mastoids after the recording. This linked mastoid reference was used to keep the ERP analyses comparable with past research in this area. The EEG was recorded with a Grass Neuro-data Acquisition system with bandpass filters set at 0.1 and 100 Hz, amplified by 20K, with a 60 Hz notch filter, and was digitized at 250 Hz (every 4 ms). The EEG was digitally filtered with a 0.1 to 30 Hz bandpass filter following acquisition. Three caps were used with the cap size determined by the circumference of the infant’s head (38 to 42 cm, 42 to 46 cm, 46 to 50 cm). Each electrode location was filled with Omni-Prep, a light intensity rub was done, and then the electrode was filled with a separate recording gel. The electrodes were adjusted until impedance for all electrodes was < 5K ohms. This preparation took 10–12 min and a second experimenter entertained the infants with toys, a child ‘busy box’, clown faces, etc. Following recommendations for infants and human subjects concerns (Pivik et al., 1993; Putnam, Johnson & Roth, 1992), the scalp was not abraded, making this a non-critical recording situation.
The event-related potentials (ERPs) were obtained from the EEG recordings. The EEG recordings were first inspected for artifacts (e.g. change in EEG > 100 μV) or poor recordings and individual channels or locations within trials were eliminated from the analyses if these occurred. Trials or portions of trials containing saccades or blinks were eliminated from the analyses. The ERP averages were made from the 4-ms interval (250 Hz) EEG recording. The ERP averages to stimulus onset were calculated from 100 ms before stimulus onset through 2 s after onset, and calculated as the difference between the prestimulus baseline lasting 100 ms. The EEG was averaged for individual infants for each attention phase (stimulus orienting, sustained attention, inattentiveness), stimulus type (frequent familiar, infrequent familiar, infrequent novel) and electrode combination. The basic data for the statistical analysis therefore consisted of the 2.1 s (525 4-ms intervals) of EEG for an infant for the 9 cells in the experimental design (attention phase × stimulus type) and the 20 electrodes.
Topographical ERP scalp potential maps were calculated for some of the effects. For the topographical maps, the scalp potentials were plotted with interpolations using a third-order spherical spline technique (Ganis, Kutas & Sereno, 1995; Nunez, 1990; Perrin, Bertrand & Pernier, 1987; Perrin, Pernier, Bertrand & Echallier, 1989). The scalp potential maps show the distribution of the scalp potentials averaged over a selected interval and are useful in visualizing the ERP data shown in figures.
Looking judgments
A single observer judged the infant’s fixation during the experiment in an adjacent room on a TV monitor to control the experimental protocol. The 60-s presentation periods were begun only when the infant was judged as looking at the blinking square. The Sesame Street recording was played for the entire 60 s regardless of infant looks. However, the stimulus presentations were not done unless the on-line observer judged the infant to be looking at the monitor. Each session was also judged off-line. A time code recorded on the videotapes allowed the judgment to be made with ms accuracy, though resolution was limited to a single video scan (0.5 × total frame length = ~16 ms). The observer judged whether the infant was looking at the monitor or not. Stimulus presentations were used only if the observer judged the infant to be looking at the stimulus.
Design for statistical analysis
The design for the study included the experimental factors of testing age (3: 20, 26, 32 weeks) as a between-subjects factor and attention phase (3: stimulus orienting, sustained attention, inattentiveness) and stimulus type (3: frequent familiar, infrequent familiar, infrequent novel) as repeated-measures factors. For the ERP recordings, the 20 recording electrodes (20: 19 10–20 electrodes and OZ) were used as a repeated measure.
The time-dependent changes in the ERP were analyzed in two ways. First, the data collected in the 4-ms samples were analyzed with a factor representing ‘intervals’ as a repeated-measures factor. This analysis should reveal different patterns of response in the ERP as a function of the experimental factors or electrode location and retains the temporal character of the time-series data (Jennings & Stine, 2000). The intervals effects (4-ms samples) were analyzed with repeated-measures ANOVA, adjusting the intervals effects with the Huynh-Feldt ɛ-adjustment to the degrees of freedom to control for inflated error rates with psychophysiological measures (Huynh & Feldt, 1970; Jennings & Wood, 1976; Keselman & Keselman, 1988; Pivik et al., 1993). Second, for the Nc component, the amplitude of the ERP at the time of the peak of the grand average was analyzed. This is the typical measure of the Nc as an ERP component and should therefore be comparable with past work in this area. Specifically, this was estimated as the 80 ms average of the ERP centered at 510 ms following stimulus onset, which was the maximum point of the ERP response (Figures 1 and 2). The statistical tests for this measure used the error terms derived from the related intervals effects analyses, Scheffe-type methods to control for inflation of testwise error rate, and all significant tests that are reported occurred at p < .05. Finally, there was a significant change in the ERP in the occipital electrodes around 700 ms following stimulus onset. Because there was no ERP component predicted from prior work in that scalp location or time, peak ERP component analysis was not done. The late slow waves were analyzed only with intervals effects analysis because the latencies and directional change of the slow wave effects has not been consistent in prior work in this area.
Figure 1.
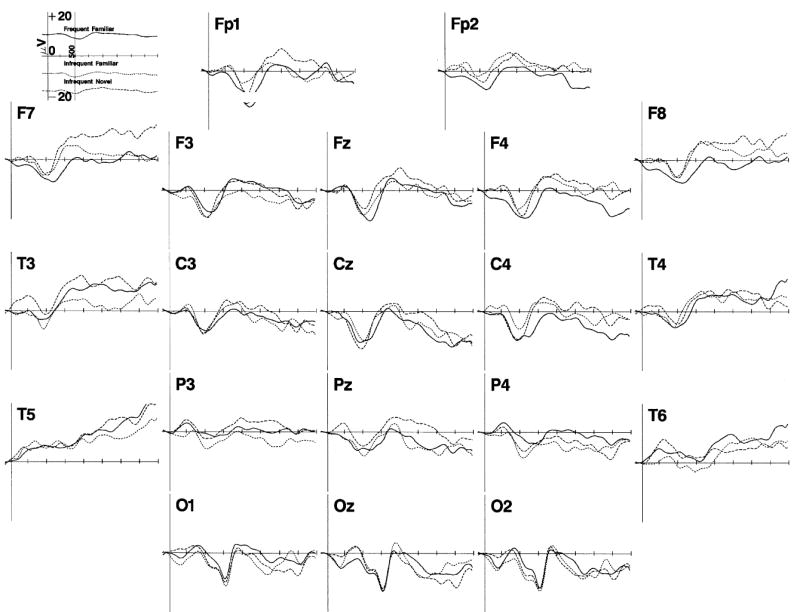
The ERP responses to the memory stimulus onset. The responses are presented separately for the 20 recording electrodes, and for the frequent familiar (solid line), infrequent familiar (small dots), and infrequent novel (dashes) stimuli, averaged across the three testing ages. The data are presented from 100 ms prior to stimulus onset through 2 s following stimulus onset. The Nc component can be seen about 450 to 550 ms post-onset primarily in the frontal and central leads. Late slow wave activity occurred from about 1 to 2 s post-stimulus onset.
Figure 2.
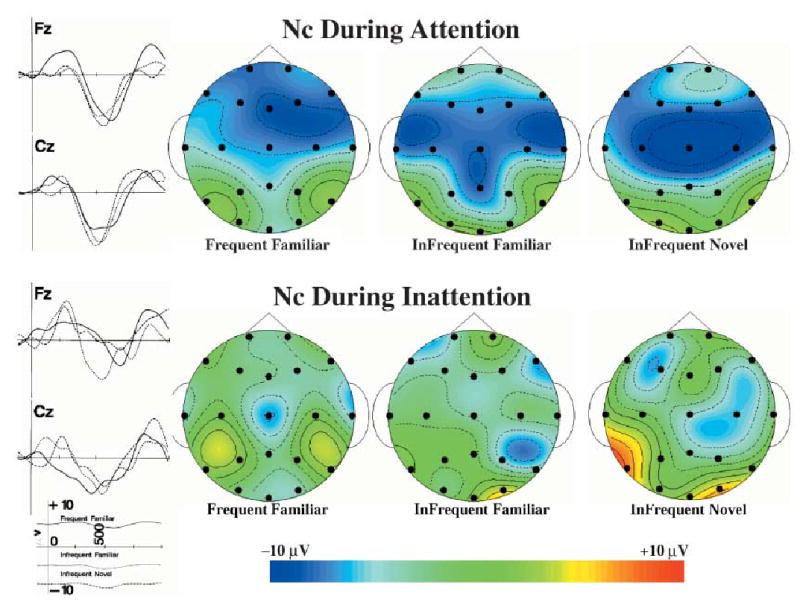
The Nc component during attention and inattention. The ERP recording from 100 ms prior to stimulus onset through 1 s following stimulus is shown for the FZ and CZ electrodes for attentive (top figures) and inattentive (bottom figures) periods, combined over the three testing ages. The topographical scalp potential maps show the distribution of this component for the three memory stimulus types in attention and inattention. The topographical maps represent an 80-ms average of the ERP for the Nc component at the maximum point of the ERP response. The data are plotted with a cubic spline interpolation algorithm and represent absolute amplitude of the ERP.
The ANOVAs for the analyses were done with a general linear models approach using non-orthogonal design because of the unequal distribution of the number of trials in the cells of the stimulus types × attention phases × subjects factorial design (see Hocking, 1985; Searle, 1971, 1987). The sums of squares (hypothesis and error) for the nested effects in the design were estimated using ‘subjects’ as a class and nesting repeated measures (attention phase, stimulus type, electrode location) within this class variable. The ‘PROC GLM’ of SAS was used for the computations.
Results
Experimental presentations
The ERP to the presentations of the memory stimuli in the experimental trials was analyzed. First, the EEG changes in response to the three stimuli types were examined by plotting the ERP data as a function of electrode site and memory stimulus type. Figure 1 shows the ERP for 100 ms prior to the stimulus presentation through 2 s following stimulus presentation, averaged across the three testing ages and three attention phases. There were three obvious ERP components in the results. First, the ‘Nc’ component occurring about 450 to 550 ms following stimulus onset was shown as a large negative ERP change located primarily in the frontal and central electrodes. It appears in Figure 1 that this component did not differ for the three memory stimuli. Second, there was a large negative deflection at about 750 ms following stimulus onset occurring primarily in the occipital electrodes. This change occurred as an additional negative potential to the ongoing Nc component, and did not differ for the three stimulus types. Third, there were late slow waves in the period from about 1 s to 2 s following stimulus onset. The late slow waves were primarily negative across all electrodes, and appear to differ for the three memory stimulus types. Given these changes, and the concern of previous research with the Nc and late slow waves, the ERP data were analyzed in three segments: Nc latency from 250 to 750 ms following stimulus onset, the occipital negative from 650 to 850 ms following stimulus onset, and the late slow waves from 1 to 2 s following stimulus onset.
The Nc component
The ERP data from the intervals from 250 to 750 ms following stimulus onset were analyzed to determine the effects of the attention phase and the memory stimulus type on the Nc component. The 4-ms ERP data from this interval were analyzed with an Age (3) × Stimulus Type (3) × Attention Phase (3) × Electrode Location (20) × Intervals (126: 4-ms intervals from 250 to 750 ms) ANOVA. There was a significant main effect of intervals, F(4, 213, ɛ = .038) = 10.42, p < .0001, and an interaction of intervals and electrode location, F(90, 4051, ɛ = .038) = 8.50, p < .0001. The intervals main effect and the interaction between intervals and electrode location was due to significant and different ERP changes occurring at different electrode locations in response to the stimulus presentations (Figure 1). The attention phase factor interacted with several experimental factors. There were significant interactions between attention phase and intervals, F(9, 427, ɛ = .038) = 2.16, p = .0213, and attention phase, intervals and electrode location, F(180, 3238, ɛ = .038) = 4.44, p = .0001.
These effects were examined with the peak amplitude, the Nc ERP component. The Nc amplitude at the frontal-central electrodes (e.g. FZ and CZ) and several frontal-lateral electrodes (e.g. F3, F4, F7, F8) were the same for stimulus orienting and sustained attention, but the Nc amplitude of these frontal-central and frontal-lateral electrodes differed between these two attention conditions and the inattentiveness phases. For example, the mean value of the ERP representing the Nc amplitude for the CZ electrode was −8.68 and −7.55 μV for the stimulus orienting and sustained attention phases, respectively, and was −3.12 μV for the inattentive phase. Alternatively, the ERP amplitude at the posterior electrode sites (parietal and occipital) did not differ at the maximum point of the ERP response between the three attention phases.
Figure 2 shows the ERP from the FZ and CZ electrodes for the two attentive phases combined (top half) and the inattentive phase (bottom half ), combined over the three testing ages. The ERP changes for these two electrodes were larger for the attentive than the inattentive phases. Figure 2 also shows topographical ERP scalp potential maps of the Nc component separately for the three memory stimulus types, separately for the attentive and inattentive phases, combined over the three testing ages. There was a large widespread negativity in the ERP during attention that did not differ for the three memory stimulus types. The ERP activity during inattention was more focal (e.g. CZ electrode in frequent familiar) and smaller in amplitude for the three memory stimulus types.
The age and memory stimulus type factors did not interact with the intervals factor. The main effect of age was statistically significant, F(2, 45) = 5.55, p = .0070, and the interaction of age and electrode location was significant, F(38, 845) = 1.65, p = .0082. The amplitude representing the Nc ERP component was examined in relation to age and electrode location. There was an increase in the Nc amplitude over the three testing ages during attention, but not during inattention. For example, during attention the Nc amplitude at the FZ electrode was − 4.72, −7.26 and −6.71 μV for the 20-, 26- and 32-week-old infants, respectively, and −8.00, −8.50 and −9.27 μV at the CZ electrode. Alternatively, during inattention the Nc amplitude for the 20-, 26- and 32-week-old infants was 1.50, −5.47 and −3.89 μV for the FZ electrode and −1.66, −2.42 and −2.40 μV for the CZ electrode. The age differences during attention occurred primarily in the frontal-central electrodes, but the differences between the ERP during attention and inattention became more widespread across the three testing ages.
Figure 3 shows the ERP from the FZ and CZ electrodes combined over the three stimulus types, and topographical ERP scalp potential maps for the Nc component for the three ages. A small increase in the amplitude of the Nc component can be seen in the ERP from the FZ and CZ electrodes. This increase was reflected in the topographical maps as an increase in amplitude of the Nc component over the three testing ages as well as an increase in the number of electrodes across which this effect was found. The main effect of stimulus type was not significant, and there were no interactions of stimulus type with the other experimental factors.
Figure 3.
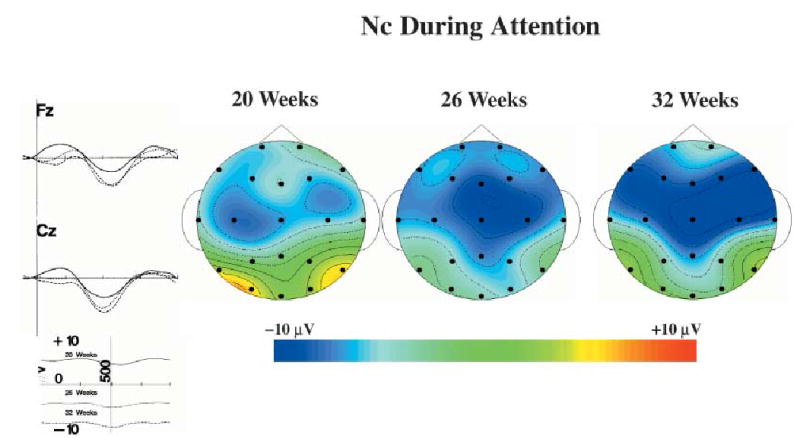
The Nc component during attention for the three testing ages. The ERP recording from 100 ms prior to stimulus onset through 1 s following stimulus is shown for the FZ and CZ electrodes for the 20-week-old, 26-week-old and 32-week-old infants. The topographical scalp potential maps show the distribution of this component for the three ages during attention.
The occipital response
The ERP data from the intervals from 650 to 850 ms following stimulus onset were analyzed to determine the effects of the attention phase and the memory stimulus type on the large negative response that occurred primarily in the occipital leads. The 4-ms ERP data from this interval were analyzed with an Age (3) × Stimulus Type (3) × Attention Phase (3) × Electrode Location (20) × Intervals (51) ANOVA. The only significant effect was an interaction of intervals and electrode location, F(54, 2456, ɛ = 0.072) = 11.56, p < .0001. The interaction between intervals and electrode location was due to the large negative ERP change that occurred in the occipital leads at around 750 ms following stimulus onset that was not occurring in most of the other leads. There were no main effects or interactions involving age, memory stimulus type or attention phase on the ERP data from this period.
The late slow wave
The ERP data from the intervals from 1 s to 2 s following stimulus onset were analyzed as the late slow waves in the ERP response. Because the main hypothesis of interest in the late slow waves was the difference between the frequent familiar and the other stimuli, the infrequent familiar and infrequent novel stimuli were analyzed as a difference from the ERP response to the frequent familiar stimulus. This was done by first averaging for an infant the ERP from this interval for any presentation of the frequent familiar stimulus, and subtracting that average from the infant’s ERP responses to the infrequent stimuli. Second, the data were calculated as a change from the ERP values at 950 to 1000 ms in order to show the changing slow waves over this interval rather than just differences from the responses to the frequent stimuli. The 4-ms ERP data from this interval were analyzed with an Age (3) × Stimulus Type (2; infrequent familiar, infrequent novel) × Attention Phase (3) × Electrode Location (20) × Intervals (251) ANOVA. There were several main effects and interactions, including the four-way interaction between age, stimulus type, attention phase and intervals, F(13, 21619, ɛ = 0.026) = 5.60, p = .0001.
To examine this interaction, the ERP responses were examined for the three testing ages, separately for the attentive and inattentive phases, and for the infrequent familiar and infrequent novel stimuli. The ERP responses during the inattentive phases were quite variable and did not follow a regular pattern. There were no significant statistical effects involving stimulus type or age for the inattentive phase.
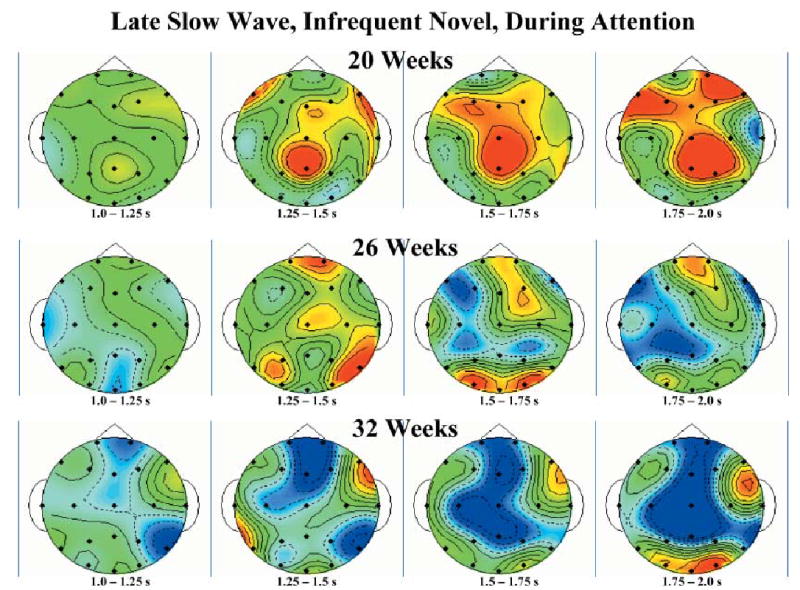
The late slow wave activity during attention showed a regular change over the three testing ages for the infrequent familiar and the infrequent novel stimuli. The post-hoc tests on the data from the ERP responses to the infrequent novel stimulus during attention showed a significant interaction of age and intervals, and significant intervals effects at each age. The ERP to the infrequent novel stimulus from the FZ, CZ and PZ electrodes are shown for the three testing ages in Figure 4. Post-hoc tests showed a positive slow wave for the 20-week-old infants from 1 to 2 s following stimulus onset, a positive then negative slow wave for the 26-week-old infants and a negative slow wave for the 32-week-old infants. Topographical scalp potential maps for this effect shown in Figure 5 show that these changes were generally located over central and parietal leads near the midline but also included activity in the frontal region near the midline. This was true of both the 32-week-olds’ negative slow wave activity and the 20-week-olds’ positive slow wave activity.
Figure 4.
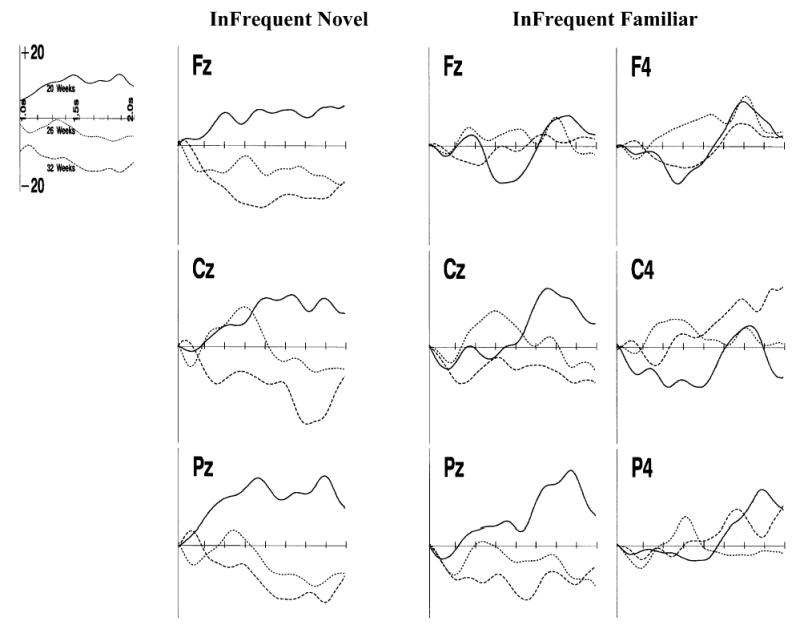
The late slow wave responses in the ERP. This figure shows the ERP from the FZ, CZ and PZ electrodes for the infrequent novel stimulus presentations (left figures), the center and center-right electrodes for the infrequent familiar stimulus presentations, separately for the three testing ages. The data are presented from 1 s to 2 s following stimulus onset. The data are presented as the difference from the ERP on the frequent familiar stimulus presentations.
Figure 5.
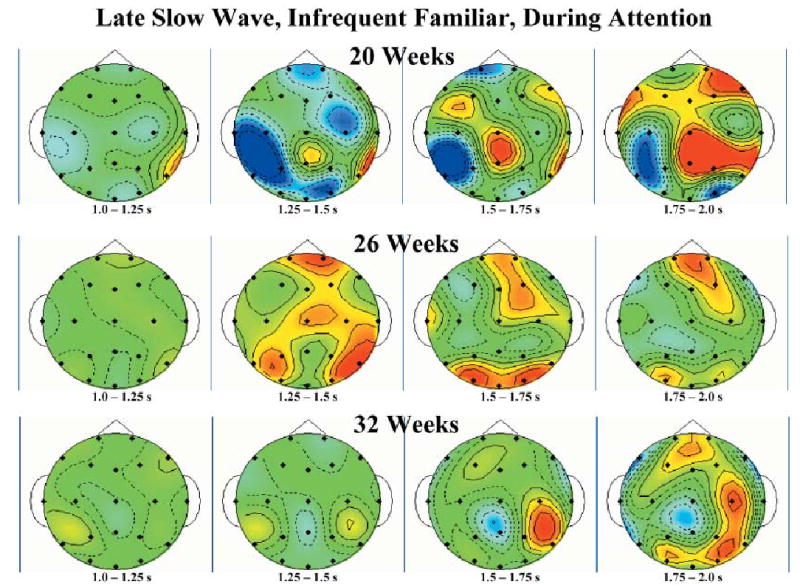
Topographical scalp potential maps representing the late slow wave responses to the infrequent novel stimulus separately for the three testing ages and for 250-ms intervals from 1 to 2 s following stimulus presentation. The color scale is the same as presented in Figures 2 and 3.
The post-hoc tests on the data from the ERP responses to the infrequent familiar stimulus during attention showed significant intervals effects at each age, but the intervals effect did not differ at the three testing ages. The ERP to the infrequent familiar stimulus is shown in Figure 4 for the three center electrodes (FZ, CZ, PZ) and the three right-center (F4, C4, P4) electrodes, and topographical scalp potential maps are shown in Figure 6. Unlike the response to the infrequent novel stimulus, the infants at all three ages showed a similar response over this late interval, which was a positive slow wave over the entire interval. The ERP plots in Figure 4 show this gradual positive change in the ERP, and the topographical maps in Figure 6 show an irregular scalp distribution with the overall positive change. This response was predominant over the right hemisphere scalp leads. It tended to occur over posterior locations (e.g. P4).
Figure 6.
Topographical scalp potential maps representing the late slow wave responses to the infrequent familiar stimulus separately for the three testing ages and for 250-ms intervals from 1 to 2 s following stimulus presentation. The color scale is the same as presented in Figures 2 and 3.
Pre-experimental presentations
The ERP to the presentations of the two stimuli designated as the frequent familiar and infrequent familiar stimuli when presented in the pre-experimental trials was analyzed. This was analyzed to insure that the stimuli elicited similar ERP changes after familiarization when presented equally often. The 4-ms ERP data from 250 to 750 ms following stimulus onset representing the Nc component were analyzed with an Age (3) × Stimulus Type (2: frequent familiar, infrequent familiar) × Electrode Location (20) × Intervals (126) ANOVA. There was a main effect of intervals, F(9, 404, ɛ = 0.077) = 6.52, p < .0001, an interaction of intervals and electrode location, F(182, 7411, ɛ = 0.077) = 3.66, p < .0001, an interaction of intervals and age, F(19, 404, ɛ = 0.077) = 3.00, p < .0001, but no significant effects involving stimulus type. The intervals main effect and the interaction between intervals and electrode location were due to significant ERP changes occurring at different electrode locations in response to the stimulus presentations. These responses were similar to what occurred with the frequent familiar stimulus in the experimental trials and so are not detailed here. The interaction of age and intervals corresponds to the age effect found with the experimental trials (Figure 3). The lack of main effects or interactions involving the memory stimulus type shows that the ERP changes were similar for the two stimuli arbitrarily designated frequent familiar and infrequent familiar when they were presented equally often.
The late slow wave in the ERP in the pre-experimental trials was analyzed. The 4-ms ERP data from 1 to 2 s following stimulus onset representing the late slow wave were analyzed with an Age (3) × Stimulus Type (2: frequent familiar, infrequent familiar) × Electrode Location (20) × Intervals (251) ANOVA. There was a main effect of intervals, F(15, 808, ɛ = 0.062) = 6.52, p = .003, and an interaction of intervals and electrode location, F(294, 14726, ɛ = 0.062) = 1.65, p < .0001, but no significant effects involving age or stimulus type. The lack of main effects or interactions involving the memory stimulus type shows that the late slow waves were the same for the stimuli arbitrarily designated frequent familiar and infrequent familiar when they were presented equally often.
Discussion
The first goal of the study was to examine the Nc ERP component under different attention levels. The Nc response was a widespread negative response over the central area of the scalp that was larger when the infant was attending to the stimulus presentation than when the infant was looking toward the stimulus but was unengaged. The Nc response during attention was similar for the frequent familiar, infrequent familiar and infrequent novel stimuli. This indicates this response was a semi-obligatory orienting response unrelated to stimulus probability or stimulus novelty. There was an increase over the three testing ages in the amplitude of the Nc response during attention. The second goal was to examine the effect of attentiveness on the late slow waves in the ERP in response to stimulus novelty. These late slow waves were virtually indistinguishable in the three presentation conditions if the infant was inattentive. The effects of stimulus probability (frequent vs. infrequent familiar) occurred during attention as a more positive late slow wave for the infrequent stimulus. The effect of stimulus novelty (frequent familiar [or infrequent familiar] vs. infrequent novel) occurred during attention and changed over the three testing ages. The infants at 4.5 months showed a positive late slow wave that was similar for the familiar and novel stimuli, whereas at 6 months and to a greater extent at 7.5 months the infrequently presented novel stimuli elicited a negative late slow wave.
There was a close relation between the infant’s attentiveness during the brief stimulus presentation and the size of the Nc component. Attentiveness in this experiment was measured by changes in heart rate indicating sustained attention. The occurrence of the heart rate deceleration and the prolongation of the heart rate changes during sustained attention index a general arousal system in the brain (Richards, 2001, 2003). The Nc response during sustained attention was larger than during periods of inattention (e.g. Figure 2). This close association between attention and the Nc component suggests that the cognitive and brain processes underlying this ERP component are related to attention rather than to memory per se. Nelson (1994; Nelson & Dukette, 1998; Nelson & Monk, 2001) has suggested that this component reflects a general orienting to the brief stimulus when it is presented. This orienting is necessary for the evaluation of stimulus novelty or stimulus probability but does not constitute a cognitive process intrinsic to memory.
A second finding in this study implies the Nc component is not related to stimulus novelty. The Nc component was similar for the frequent familiar, infrequent familiar and infrequent novel stimuli. These three presentations allow the distinction between a response to stimulus novelty or stimulus probability. A novelty effect would be shown by a difference between the infrequent familiar and infrequent novel stimuli. A probability effect would be shown by a difference between the frequent familiar and infrequent familiar stimuli. Neither of these comparisons resulted in a difference in this study for the Nc component. The close association of attention and the amplitude of the Nc component, and the lack of difference in the Nc component for the three stimulus presentation methods, implies that the Nc response is an orienting response that is greater during attention but is not closely related to stimulus novelty or recognition memory. The Nc response is larger when the general arousal system is energized (‘sustained attention’) than when the arousal system is not active (‘inattention’).
The conclusion that the Nc response is a general orienting response and not related to stimulus novelty is consistent with some previous findings but inconsistent with other findings. Studies that familiarize the infants with the stimuli before the test phase find no difference in the response to the infrequently presented stimulus and the frequently presented stimulus (Nelson & Collins, 1991, 1992; Nelson & deRegnier, 1992; Nelson & Salapatek, 1986). Studies that do not familiarize the infants with the stimuli report that the Nc component is larger in response to the infrequently presented rare stimulus than the frequently presented standard stimulus (Courchesne et al., 1981; Karrer & Ackles, 1987, 1988; Karrer & Monti, 1995). An explanation for the difference between these studies involves the familiarization given before the testing. Without a familiarization period, the rare and standard stimuli are ‘novel’ at the beginning of stimulus presentation. Over the course of stimulus presentation the standard stimulus becomes familiar to the infant at a faster rate than the rare stimulus due to the frequency of presentations. Indeed, the Nc component diminishes in magnitude over the course of the repeated presentations in 6-month-old infants (Nikkel & Karrer, 1994). The difference in the Nc response between the rare and standard stimulus therefore would be related to the changing familiarity of the two stimuli over the course of stimulus presentation. In one sense this is a distinction on the infant’s part between a ‘novel’ and a ‘familiar’ stimulus. However, according to this explanation, novelty, familiarity, presentation probability and the number of stimulus exposures are inextricably confounded without a familiarization period preceding the test phase.
The presentation of the brief stimuli overlaid on a background pattern in the present study highlights the association between stimulus orienting and the Nc response. A sudden change in the environment elicits a phase of attention called ‘stimulus orienting’ (Berg & Richards, 1997; Richards, 2001, 2003; Richards & Casey, 1992). In the current study the brief visual stimuli were presented by replacing an ongoing background Sesame Street movie. The Sesame Street movie has constantly changing characters, action and scenes, and elicits a strong heart rate response indicating attention engagement. The response to any briefly presented stimulus different than the background presentation was therefore an unexpected change in background event and equally elicited the stimulus orienting system, shown by the Nc component. The typical protocol for this procedure involves the presentation of the brief stimuli without other background stimulation. In this procedure without a familiarization period the standard stimulus may become the ‘background’ stimulus over the course of repeated stimulus presentation and the Nc response to this stimulus would habituate (Nikkel & Karrer, 1994). The rare stimulus in this case represents a change in the background stimulation that would elicit stimulus orienting and a larger Nc response. This should occur irrespective of the infant’s past experience with the stimulus, i.e. should occur to the sudden appearance of a rare but familiarized stimulus or to a rare and completely novel stimulus. Thus in the studies showing a different Nc response for standard and rare stimuli (Courchesne et al., 1981; Karrer & Ackles, 1987, 1988; Karrer & Monti, 1995; Nikkel & Karrer, 1994) the response to the rare stimuli represents stimulus orienting in the presence of the background of the standard stimulus presentations. Alternatively, the familiarization of the standard and rare stimuli before the brief stimulus presentations (e.g. Nelson & Collins, 1991, 1992) leads to no difference in the Nc amplitude for the frequent familiar, infrequent familiar and infrequent novel stimuli. This implies that the familiarization procedure, with equal presentations of the stimuli later to be presented frequently or infrequently, results in the effective background stimulus being the presentation of any stimulus, leading to a Nc response for all briefly presented stimuli but no difference in this response. Or, the presence of an explicit background stimulus (i.e. Sesame Street in the present study) would result in a Nc response for all briefly presented stimuli. This explanation could be tested by a study in which the three presentation methods (frequent familiar, infrequent familiar, infrequent novel) were done without a familiarization period. In this case, without an explicit background one would expect that the frequently presented standard stimulus would have a smaller Nc than either the infrequently presented familiar stimulus or the infrequently presented novel stimuli. Alternatively, the three presentation methods should result in an equivalent Nc response in the presence of an explicit background stimulus even without a familiarization period preceding the brief stimulus presentations. Alternative background stimuli that did not change continuously like Sesame Street or did not readily engage attention might not affect whether a familiarization period was included.
The age changes in the ERP components in this study are consistent with previous findings and the results from this study clearly distinguish changes in attention from changes in memory processes over this age range. There was an increase over the three testing ages in the amplitude of the Nc response during attention but not during inattention. Prior studies have shown an overall increase in the size of the Nc component from about 4–6 weeks of age until 18 months of age (Courchesne et al., 1981; Karrer & Ackles, 1987, 1988; Karrer & Monti, 1995). Additionally, in the studies without a familiarization procedure, the differential response to frequent and infrequent stimuli is not present at 4–6 weeks of age (Karrer & Ackles, 1987; Karrer & Monti, 1995) or 4 months of age (Courchesne et al., 1981; Nelson & Collins, 1991), occurs at 6 months of age (Courchesne et al., 1981; Karrer & Ackles, 1987, 1988) and seems to be even larger at 18 months of age (Karrer & Ackles, 1987, 1988). The interpretation of this paper is that the larger response to the rare stimulus represents stimulus orienting in the presence of habituation to the standard stimulus. And, the amplitude of the Nc component was closely associated with attention in the current study. These imply that the age changes found in these studies reflect changes in infant attentiveness rather than changes in memory processes. The implication of this finding is that sustained attention, measured by heart rate changes and reflecting a general arousal / attention system in the brain (Richards, 2001, 2003; Richards & Casey, 1992), shows development throughout the first year of life and may continue developing in the first part of the second year of life.
Alternatively, there are changes in age in memory processes reflected in the changes occurring in the late slow waves of the ERP. The positive slow wave response to the infrequently presented familiarized stimulus, relative to the frequently presented familiarized stimulus, represents the response to a stimulus existing in memory for which an updating of the stimulus is necessary because of the infrequent presentations (Nelson, 1994). However, in the present study this positive slow wave was found in the 4.5-month-old infants for both infrequently presented stimuli. This finding is similar to a study showing a late positive slow wave in 3-month-old infants to a novel face in a habituation paradigm (Pascalis, de Haan, Nelson & de Schonen, 1998). Thus it may be interpreted as a response to relatively infrequent stimuli regardless of familiarity, i.e. a stimulus probability effect (cf. Nelson & Collins, 1992). By 6 months of age the slow wave activity distinguishes stimulus probability (frequent vs. infrequent familiar) and stimulus novelty (infrequent familiar vs. infrequent novel) (Figure 4, this study; also Nelson & Collins, 1991). Finally, the 7.5-month-old infants show a clear and consistent negative slow wave for the infrequent novel stimuli relative to the frequent familiar or infrequent familiar stimuli. These findings suggest that the updating of memory of the familiarized stimulus (Nelson, 1994; Nelson & Dukette, 1998), or the differential response to frequent and infrequent stimuli, is a response shown by infants at fairly young testing ages. There is a gradual increase in the recognition of stimulus novelty over the testing ages used in this study. Although these changes occurred primarily for stimuli presented during attention, they represent changes in memory per se rather than simply changes in attentiveness over this age range.
The results of this study highlight the important role that attention plays in infant recognition memory. The heart rate changes distinguishing attention and inattentiveness represent a brain system that controls general arousal (Richards, 2001, 2003). This arousal system affects a wide variety of cognitive processes. The enhancement of the Nc component during attention found in this study implies that the first phase of information processing in recognition memory is a nonspecific orienting to the stimulus. This stimulus orienting and the general arousal that enhances orienting show changes over the first year of life and perhaps well into the second year. In addition to the effect of attention on the Nc, which represents an orienting process, this general arousal system enhances the specific memory processes occurring during the recognition of low frequency stimuli and the recognition of novel stimuli. The positive slow waves and the negative slow waves found in this study occurred primarily when sustained attention was occurring. The slow wave activity represents specific memory processes, such as recognition by the 6- and 7.5-month-old infants of the novelty of the infrequently presented novel stimuli. The enhancement of the slow waves during attention demonstrates how the general brain system invigorates specific brain areas occurring during recognition memory. The facilitative effect of attention on infant recognition memory (e.g. Richards, 1997; Frick & Richards, 2001) may occur because specific brain areas responsible for information acquisition or recognition are enhanced during attention.
Acknowledgments
This research was supported by a grant from the National Institute of Child Health and Human Development, #R01-HD18942.
References
- Berg, W.K., & Richards, J.E. (1997). Attention across time in infant development. In P.J. Lang, R.F. Simons & M.T. Balaban (Eds.), Attention and orienting: Sensory and motivational processes (pp. 347–368). Mahwah, NJ: Erlbaum.
- Courchesne E. Event-related brain potentials: comparison between children and adults. Science. 1977;197:589–592. doi: 10.1126/science.877575. [DOI] [PubMed] [Google Scholar]
- Courchesne E. Neurophysiological correlates of cognitive development: changes in long-latency event-related potentials from childhood to adulthood. Electroencephalography and Clinical Neurophysiology. 1978;45:468–482. doi: 10.1016/0013-4694(78)90291-2. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: a neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- Fabiani, M., Gratton, G., & Coles, M.G.H. (2000). Event-related brain potentials: methods, theory, and applications. In J.T. Cacioppo, L.G. Tassinary & G.G. Berntson (Eds.), Handbook of psychophysiology (pp. 53–84). New York: Cambridge University Press.
- Frick J, Richards JE. Individual differences in infants’ recognition of briefly presented visual stimuli. Infancy. 2001;2:331–352. doi: 10.1207/S15327078IN0203_3. [DOI] [PubMed] [Google Scholar]
- Ganis G, Kutas M, Sereno M. Freeing the ERPs: freeware for high quality spatial map construction and presentation. Psychophysiology. 1995;32:S33. [Google Scholar]
- Hillyard, S.A., Mangun, G.R., Woldroff, M.G., & Luck, S.J. (1995). Neural systems mediating selective attention. In M.S. Gazzaniga (Ed.), Cognitive neurosciences (pp. 665–682). Cambridge, MA: MIT Press.
- Hocking, R.R. (1985). The analysis of linear models Monterey, CA: Brooks/Cole Publishers.
- Huynh H, Feldt LS. Conditions under which the mean square ratios in repeated measurements designs have exact F distributions. Journal of the American Statistical Association. 1970;65:1582–1589. [Google Scholar]
- Jasper HH. The ten twenty electrode system of the International Federation. Electroencephalography and Clinical Neurophysiology. 1958;10:371–375. [PubMed] [Google Scholar]
- Jennings, J.R., & Stine, L.A. (2000). Salient method, design, and analysis concerns. In J.T. Cacioppo, L.G. Tassinary & G.G. Berntson (Eds.), Handbook of psychophysiology (pp. 870–899). New York: Cambridge University Press.
- Jennings JR, Wood CC. The ɛ-adjustment procedure for repeated-measures analyses of variance. Psychophysiology. 1976;13:277–278. doi: 10.1111/j.1469-8986.1976.tb00116.x. [DOI] [PubMed] [Google Scholar]
- Karrer, R., & Ackles, P.K. (1987). Visual event-related potentials of infants during a modified oddball procedure. In R. Johnson, J.W. Rohrbaugh & R. Parasuraman (Eds.), Current trends in event-related potential research (pp. 603–608). Amsterdam: Elsevier Science Publishers. [PubMed]
- Karrer, R., & Ackles, P.K. (1988). Brain organization and perceptual/cognitive development in normal and Down syndrome infants: a research program. In P. Vietze & H.G. Vaughan, Jr. (Eds.), The early identification of infants with developmental disabilities (pp. 210–234). Philadelphia: Grune & Stratton.
- Karrer R, Monti LA. Event-related potentials of 4–7-week-old infants in a visual recognition memory task. Electroencephalography and Clinical Neurophysiology. 1995;94:414–424. doi: 10.1016/0013-4694(94)00313-a. [DOI] [PubMed] [Google Scholar]
- Keselman HJ, Keselman JC. Comparing repeated measures means in factorial designs. Psychophysiology. 1988;25:612–618. doi: 10.1111/j.1469-8986.1988.tb01898.x. [DOI] [PubMed] [Google Scholar]
- Nelson, C.A. (1994). Neural correlates of recognition memory in the first postnatal year. In G. Dawson & K.W. Fischer (Eds.), Human behavior and the developing brain (pp. 269–313). New York: Guilford Press.
- Nelson CA, Collins PF. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain and Cognition. 1992;19:105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Nelson CA, deRegnier RA. Neural correlates of attention and memory in the first year of life. Developmental Neuropsychology. 1992;8:119–134. [Google Scholar]
- Nelson, C.A., & Dukette, D. (1998). In J.E. Richards (Ed.), Cognitive neuroscience of attention: A developmental perspective (pp. 327–362). Hillsdale, NJ: Lawrence Erlbaum.
- Nelson, C.A., & Monk, C.S. (2001). The use of event-related potentials in the study of cognitive development. In C.A. Nelson & M. Luciana (Eds.), Developmental cognitive neuroscience (pp. 125–136). Cambridge, MA: MIT Press.
- Nelson CA, Salapatek P. Electrophysiological correlates of infant recognition memory. Child Development. 1986;57:1483–1497. [PubMed] [Google Scholar]
- Nelson, C.A., & Webb, S. (2003). A cognitive neuroscience perspective on early memory development. In M. de Haan & M.H. Johnson (Eds.), The cognitive neuroscience of development (pp. 99–126). Hove, Sussex: Psychology Press.
- Nikkel L, Karrer R. Differential effects of experience on the ERP and behavior of 6-month-old infants: trends during repeated stimulus presentation. Developmental Neuropsychology. 1994;10:1–11. [Google Scholar]
- Nunez PL. Localization of brain activity with electroencephalography. Advances in Neurology. 1990;54:39–65. [PubMed] [Google Scholar]
- Pascalis O, de Haan M, Nelson CA, de Schonen S. Long-term recognition memory for faces assessed by visual paired comparison in 3- and 6-month-old infants. Journal of Experimental Psychology: Learning, Memory, and Cognition. 1998;24:249–260. doi: 10.1037//0278-7393.24.1.249. [DOI] [PubMed] [Google Scholar]
- Perrin F, Bertrand O, Pernier J. Scalp current density mapping: value and estimation from brain data. IEEE Transactions on Biomedical Engineering. 1987;34:283–288. doi: 10.1109/tbme.1987.326089. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Pivik RT, Broughton RJ, Coppola R, Davidson RJ, Fox N, Nuwer MR. Guidelines for the recording and quantitative analysis of electroencephalographic activity in research contexts. Psychophysiology. 1993;30:547–588. doi: 10.1111/j.1469-8986.1993.tb02081.x. [DOI] [PubMed] [Google Scholar]
- Putnam LE, Johnson R, Roth WT. Guidelines for reducing the risk of disease transmission in the psychophysiology laboratory. Psychophysiology. 1992;29:127–141. doi: 10.1111/j.1469-8986.1992.tb01676.x. [DOI] [PubMed] [Google Scholar]
- Richards JE. Effects of attention on infants’ preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Developmental Psychology. 1997;33:22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Richards, J.E. (2001). Attention in young infants: a developmental psychophysiological perspective. In C.A. Nelson & M. Luciana (Eds.), Developmental cognitive neuroscience (pp. 321–338). Cambridge, MA: MIT Press.
- Richards, J.E. (2003). The development of visual attention and the brain. In M. de Haan & M.H. Johnson (Eds.), The cognitive neuroscience of development (pp. 73–98). Hove, Sussex: Psychology Press.
- Richards JE, Casey BJ. Infant visual recognition memory performance as a function of heart rate defined phases of attention. Infant Behavior and Development. 1990;13:585. [Google Scholar]
- Richards JE, Casey BJ. Heart rate variability during attention phases in young infants. Psychophysiology. 1991;28:43–53. doi: 10.1111/j.1469-8986.1991.tb03385.x. [DOI] [PubMed] [Google Scholar]
- Richards, J.E., & Casey, B.J. (1992). Development of sustained visual attention in the human infant. In B.A. Campbell, H. Hayne & R. Richardson (Eds.), Attention and information processing in infants and adults (pp. 30–60). Mahway, NJ: Erlbaum.
- Richards JE, Hunter S. Peripheral stimulus localization by infants with eye and head movements during visual attention. Vision Research. 1997;37:3021–3035. doi: 10.1016/s0042-6989(97)00082-5. [DOI] [PubMed] [Google Scholar]
- Searle, S.R. (1971). Linear models New York: John Wiley.
- Searle, S.R. (1987). Linear models for unbalanced data New York: John Wiley.
- Swick, D., Kutas, M., & Neville, H.J. (1994). Localizing the neural generators of event-related brain potentials. In A. Kertesz (Ed.), Localization and neuroimaging in neuropsychology. Foundations of neuropsychology (pp. 73–121). San Diego: Academic Press.


