Abstract
Language lateralization in the brain is dependent on family history of handedness, personal handedness, pathology, and other factors. The influence of age on language lateralization is not completely understood. Increasing left lateralization of language with age has been observed in children, while the reverse has been noted in healthy young adults. It is not known whether the trend of decreasing language lateralization with age continues in the late decades of life and at what age the inflection in language lateralization trend as a function of age occurs. In this study, we examined the effect of age on language lateralization in 170 healthy right‐handed children and adults ages 5–67 using functional MRI (fMRI) and a verb generation task. Our findings indicate that language lateralization to the dominant hemisphere increases between the ages 5 and 20 years, plateaus between 20 and 25 years, and slowly decreases between 25 and 70 years. Hum Brain Mapp, 2005. © 2005 Wiley‐Liss, Inc.
Keywords: language lateralization, handedness, age, fMRI
INTRODUCTION
Modern studies of language localization and lateralization began with the first reports by Broca and Wernicke, who introduced the concept of unilateral left hemispheric control of language functions [Broca,1861; Wernicke,1911]. At the same time, John Hughlings Jackson and Pierre Marie proposed “holistic or noetic models” of language localization; they postulated that speech is the result of the processing that occurs in the entire brain [Basso,2003]. Since then, the localization of language processing in healthy and diseased brains has become the subject of intense research. The “classical model” of language organization based on data from aphasic patients with brain lesions, popularized in the 19th century, remains in common use [Geschwind and Galaburda,1985; Loring et al.,1990; Rasmussen and Milner,1977]. The general principle of this “classical model” is supported by studies of individuals who have lost language secondary to focal brain lesions. Although unilateral cerebral lesions provide a useful approach to the study of early and late hemispheric specialization and offer a context for the investigation of language plasticity [Booth et al.,2000; Borod et al.,1985; Liegeois et al.,2004; Naeser and Borod,1986], they are not a good model for studying normal development of language functions.
In health, handedness and language lateralization are interdependent. Even early studies of language development in children postulated association between language dominance and handedness and/or eye preference [Belmont and Birch,1965; Benton and Kemble,1960]. A study of right–left discrimination found that children with the lowest scores on a sequential reading test had frequent right–left body confusion [Belmont and Birch,1965]. Based on these findings, the authors concluded that left–right disorientation had a significant association with reading performance and that non‐dextrality was associated with abnormal language development. Another study of healthy children found that early language development starts with phonological awareness, letter recognition, and visual symbolic memory processes at the age of 5 and is followed by holistic recognition skills at the age of 6 and analytic visual skills at the age of 7 [Ellis and Large,1988].
Handedness or hand dominance, a feature unique to humans, is subject to genetic and environmental influences [Annett,1967,1972; Corbalis,1997; Ellis et al.,1998; Finch,1941]. It is also postulated that language lateralization and handedness are related [Annett,1972] but a clear genetic link proving this relationship is still missing [Corbalis,1997; Jones and Martin,2000]. A study in primates found that there is no clear hand‐preference and that hand (a)symmetry has a normal (Gaussian) distribution that is attributed to chance: 25% of animals are right‐, 50% mixed, and 25% left‐limbed [Annett,1967; Finch,1941]. This animal study is in clear contrast to the human studies estimating left‐handedness at 6–16% depending on age [Gilbert and Wysocki,1992]. Annett and Alexander explain this skewed distribution of handedness in humans using the “right‐shift theory” [Annett,1972,1998; Annett and Alexander,1996]. According to them, the presence of a single dominant gene called “RS” causes bias towards left cerebral advantage/dominance. Such shift could be a by‐product of the mechanisms which induce left hemisphere advantage for speech and for language functions [Annett,1998]. While the presence of the dominant RS gene and, therefore, left‐hemispheric dominance for language is expected in about 80% of healthy subjects, approximately 20% lack this gene and have about a 50% chance of becoming either left‐ or right‐handed. This explains the 6–16% incidence of left‐handedness and ambidextrality in population; this theory is supported by empirical data from studies involving normal families with a history of sinistrality [Jones and Martin,2000]. The right‐shift theory of language lateralization is at least partially supported by experimental fMRI/transcranial Doppler studies of language distribution in healthy subjects [Knecht et al.,2000b; Szaflarski et al.,2002b] and by leftward asymmetries in the motor hand area of the precentral gyrus found in an anatomical study [Foundas et al.,1998].
It is widely accepted that language is predominantly a left‐hemispheric function. Lesional studies as well as studies in epilepsy patients undergoing presurgical evaluation provided estimates of language localization in a healthy population [Borod et al.,1985; Rasmussen and Milner,1977]. Subsequently, PET, transcranial Doppler, and fMRI studies evaluated language localization and lateralization in healthy subjects [Knecht et al.,2000b,2003; Pujol et al.,1999; Springer et al.,1999; Szaflarski et al.,2002b; Tzourio et al.,1998]. These studies, among other findings, reported that language lateralization in healthy subjects depends on personal handedness, but it may also be influenced by other factors, e.g., family history of handedness or age [Holland et al.,2001; Springer et al.,1999; Szaflarski et al.,2002b].
Recent noninvasive neuroimaging studies in healthy adults demonstrated that approximately 95% of dextrals have left hemispheric language dominance and that there is no correlation between the degree of right‐handedness and the strength of left hemispheric dominance for language [Knecht et al.,2000a; Springer et al.,1999]. In healthy non‐dextrals the incidence of atypical language lateralization is higher than in right‐handed subjects, with about 20–27% of left‐handed healthy subjects exhibiting atypical (symmetric or right‐hemispheric) language dominance [Knecht et al.,2000b; Pujol et al.,1999; Szaflarski et al.,2002b]. Studies on healthy right‐ and left‐handed subjects have also shown that the nondominant hemisphere contributes to language processing, though to a varying degree in different subjects [Chee et al.,1998; Knecht et al.,2000a,b,2003; Tzourio et al.,1998; Tzourio‐Mazoyer et al.,2004]. Furthermore, these and other studies have also examined the factors that influence language lateralization. There is a growing body of evidence that age may affect language lateralization in a significant way, but the exact time course of language lateralization changes across age is unclear [Brown et al.,2005; Holland et al.,2001; Schlaggar et al.,2002; Springer et al.,1999; Szaflarski et al.,2002b].
Various cognitive tasks have been used in neuroimaging studies of language. These include semantic decision/tone decision [Springer et al.,1999; Szaflarski et al.,2002b], verb generation [Benson et al.,1999; Holland et al.,2001; Liegeois et al.,2004; Petersen et al.,1988; Wise et al.,1991], or word generation [Hertz‐Pannier et al.,1997; Pujol et al.,1999; Wood et al.,2004]. Other tasks, e.g., story listening, semantic decision, or covert reading have been used with good success in healthy children and adults [Gaillard et al.,2000,2001,2003; Lehericy et al.,2000]. Some of the studies, in addition to assessing language localization in healthy subjects, evaluated the agreement between functional MRI (fMRI) language tasks and the intracarotid amobarbital procedure (Wada test) or direct cortical stimulation and found a strong correlation between the procedures, suggesting that fMRI is an excellent tool in evaluating language localization in health and disease [Benson et al.,1999; Gaillard et al.,2002; Lehericy et al.,2000; Liegeois et al.,2002; Ojemann et al.,2002; Springer et al.,1999]. In the fMRI studies, the choice of the fMRI task is dictated by the primary research question and by the ability of the subjects to cooperate with the procedure. To allow us to scan children as young as 5 years of age, we chose a relatively easy‐to‐perform verb generation task that was initially used in a PET study [Petersen et al.,1988] and later employed by our group to evaluate language in children and adults [Holland et al.,2001; Szaflarski et al.,2002a]. This task involves naming verbs associated with a given noun. This or similar tasks have shown robust activation in PET, fMRI, and transcranial Doppler sonography studies in the left inferior frontal and lateral temporal regions with less activation in the right homologs [Hertz‐Pannier et al.,1997; Knecht et al.,2000b; Petersen et al.,1988].
Up till now, the course of language lateralization changes across all ages has not been established. One study examining language lateralization in healthy children ages 7–18 found that with increasing age language becomes more lateralized to the left hemisphere [Holland et al.,2001]. An fMRI study of right and left‐handed children (6–15 years of age) did not find any correlation between language lateralization and age but approximately 19% of subjects in that study were left‐handed, which may have affected the results [Wood et al.,2004]. Another fMRI study compared brain activation patterns of children and adults using a verbal fluency task and found that children had a more robust blood oxygenation‐level dependent (BOLD) signal response than adults, with somewhat more right‐hemisphere and inferior frontal gyrus activation. However, the differences were not significant, which was probably due to a low number of subjects (16 children and 29 adults) [Gaillard et al.,2003].
In contrast to the above, recent fMRI studies of children and adults using word processing tasks found differences in brain activation patterns between children and adults [Brown et al.,2005; Schlaggar et al.,2002]. In adults, a correlation between age and language lateralization in the left‐ and right‐handed healthy adults was noted, but the available studies focused on young adults only (ages 18 to ∼40), and, therefore, it is not clear whether the trend of decreasing language lateralization with age continues in the late decades of life [Springer et al.,1999; Szaflarski et al.,2002b]. In summary, the effects of age on language lateralization across age are not fully understood and require further investigation.
The goals of this study were to evaluate the changes in language lateralization in the brain of right‐handed children and adults between ages 5 and 70 using a verb generation task and fMRI and to establish the age at which a change in the trajectory of language lateralization may occur.
SUBJECTS AND METHODS
Healthy right‐handed children and adults (as measured by the Edinburgh Handedness Inventory (EHI) score of ≥ 50) [Oldfield,1971] were recruited by word of mouth and through newspaper and TV advertising. All children had normal neurological and psychometric examination and no history of head trauma or neurological/general medical conditions. Adults were included only if they had no history of previous or current neurological or general medical conditions. We enrolled only right‐handed subjects to avoid the potential effects of different language lateralization on the language developmental curves in left‐handed and ambidextrous subjects [Knecht et al.,2000b,2003; Pujol et al.,1999; Springer et al.,1999; Szaflarski et al.,2002b]. Although in subjects who fall within the EHI range of 50–100 the degree of dextrality may vary, subjects with EHI ≥ 50 are considered dextral for research and social purposes; EHI is an acceptable and “…sufficient means of assessment of handedness aspect” [Oldfield,1971, p. 110] in right‐ and left‐handed subjects [Ellis et al.,1998; Knecht et al.,2000b; Oldfield,1971; Springer et al.,1999; Tzourio et al.,1998; Tzourio‐Mazoyer et al.,2004]. Furthermore, a choice of this range of EHI scores was dictated by the fact that studies of healthy subjects utilizing EHI as a measure of dextrality did not find any correlation between the degree of right‐handedness and language lateralization [Knecht et al.,2000a; Springer et al.,1999].
All subjects signed informed consent approved by the Institutional Review Board of the Cincinnati Children's Hospital Medical Center; all adult subjects also signed informed consent approved by the IRB at the University of Cincinnati Medical Center. Assent was obtained from children and consent from their parents.
fMRI Verb Generation Task
This block‐design task was originally described by Petersen and later used by many others, including our group [Petersen et al.,1988]. Briefly, during the activation period a series of concrete nouns (e.g., chair, oven, spoon) was presented binaurally at the frequency of one noun per 5 s. The subject was required to covertly generate verbs that are associated with each noun. Five activation periods of 30 s each were separated by resting periods. An initial 30 s rest period was used to allow for the MRI signal to reach T1 equilibrium but the data from this interval was later discarded during postprocessing. During the six resting periods the subject was instructed to perform bilateral finger‐tapping in response to a target tone presented every 5 s (FM tones centered on 400 Hz with 25% modulation). Engagement in performance of this task was monitored in two ways. First, as mentioned, the finger‐tapping control interval was monitored visually to ensure that the subject was following the block periodic sequence of the task. Second, to assess engagement in the verb generation task a noun recall test was administered outside the scanner immediately after the scanning was complete [Chiu et al., 2004].
MRI Procedure and MRI Data Analysis
Subjects were scanned without sedation using a 3T Bruker Biospec 30/60 MRI scanner (n = 172; Bruker Biospin, Karlsruhe, Germany) or 4T Varian MRI scanner (n = 6; ages 34, 41, 56, 56, 66, and 66; Oxford Magnet Technology, Oxford, UK). Foam padding and head restraint were used to control head movement. The description of the MRI procedure is provided for the Bruker MRI scanner and it is followed by the description of the differences between Bruker and Varian procedures. All MRI studies were reviewed by a board‐certified neuroradiologist. fMRI data on subjects with any anatomical abnormalities were excluded from analysis.
Bruker
After the subjects were positioned in the scanner the scanner was automatically shimmed to provide a homogeneous magnetic field for image acquisition. An initial alignment scan was done in three orthogonal planes simultaneously using a fast gradient echo sequence developed for the scanner. This scan takes approximately 9 s and provides a quick view of the subject's head position. From this, the location of the 32 axial planes to be imaged in the fMRI procedures was identified. The subjects underwent the fMRI scan sequence during which they were asked to perform the active and control tasks. The specific protocol for these scans was T2*‐weighted gradient‐echo, echo planar imaging (EPI) pulse sequence: TR/TE = 3000/38 ms, FOV = 25.6 × 25.6 cm, matrix 64 × 64 pixels, slice thickness = 5 mm, flip angle = 90°.
Thirty‐two image slices were acquired at each time point. Synchronization of the high‐field fMRI scans with the audio stimuli was fully automated so that when the presentation commences on the Apple Macintosh computer, the scanner is triggered to begin acquisitions [Holland and Marchevsky,1999]. Finally, a high‐resolution T1‐weighted 3D anatomical scan was obtained using a modified driven equilibrium Fourier transform (MDEFT) protocol: TR = 15 ms, TI = 550 ms, TE = 4.3 ms, FOV = 25.6 × 19.2 × 16.2, flip angle = 20° to provide images for anatomical localization of the activation maps [Duewell et al.,1996; Wansapura et al.,1999]. This acquisition took approximately 9 min and yielded spatial resolution of 1 × 1.5 × 1.5 mm with sufficient signal‐to‐noise ratio and contrast between gray and white matter for manual and semiautomated segmentation of regional brain volumes [Wilke et al.,2002]. Thirty‐two fMRI scan planes were extracted from this 3D anatomical data set by interpolation for use as an anatomical underlay for activation maps.
Varian
From the initial scout images, 30 axial planes to be imaged in the fMRI procedures were identified. The specific protocol for the gradient‐echo EPI scans was: TR/TE = 3000/25 ms, FOV 25.6 × 25.6 cm, matrix 64 × 64 pixels, slice thickness = 4 mm, flip angle array: 85/180/180/90, and for the anatomical scans was TR = 13 ms, TE = 6 ms, FOV = 25.6 × 19.2 × 15.0, flip angle array of 3: 22/90/180 with the voxel size of 1 × 1 × 1 mm.
The fMRI image postprocessing was performed with CCHIPS (Cincinnati Children's Hospital Image Processing System) software that runs in the IDL software environment (IDL 5.4; Research Systems, Boulder, CO). CCHIPS generates statistical parametric maps from fMRI data with options for spatial or temporal filtering for mapping data onto stereotactic coordinates [Talairach and Tournoux,1988], and for generating composite activation maps across multiple subjects [Schmithorst and Dardzinski,2000]. A Hamming filter was applied to raw EPI data prior to reconstruction to reduce the truncation artifacts at the edges of k‐space and to reduce high‐frequency noise in the images [Lowe and Sorenson,1997]. Geometric distortion was corrected for via the multiecho reference method [Schmithorst et al.,2001]. Data were then coregistered to further reduce the effects of motion artifact using a previously developed pyramid coregistration algorithm [Thevenaz and Unser,1998]. The median voxel displacement across frames was stored as a measure of subject motion. Data with excessive motion artifact were discarded, using the criterion of median voxel displacement >2 mm. High quality data were then transformed into Talairach space. On a voxel‐by‐voxel basis, the average percent change between resting and active conditions was computed, with the first three frames after each transition discarded in order to account for the delay in the hemodynamic response. The first 10 frames were discarded in the analysis in order to allow the spins to reach relaxation equilibrium.
Laterality Index Estimations
The regions of interest (ROIs) were designed based on activation regions defined from the global composite map (Fig. 2) for the verb generation task (i.e., Broca, Wernicke ROIs), using the criterion of Z > 6 (P < 0.001 Bonferroni‐corrected for multiple voxel comparisons) for significant activation. The activations from the composite maps of children and adults performing the verb generation task are similar to the activations that were used to generate ROIs in our previous study; for a detailed description of the areas included in the ROIs, see the legend to Figure 2 and our previous article [Holland et al.,2001]. The largest areas of the activation found in the inferior frontal lobe (Broca's area) and in the lateral posterior temporal lobe (Wernicke's area) on the left were mirrored onto the contralateral side and the Talairach coordinates of all pixels encompassed within these regions were stored. ROIs designed this way correspond to classically recognized areas associated with language. The centroids of activation were located with maximum over the middle and superior temporal gyri (Talairach coordinates −55, −33, 5; BA 21/22/42) for the Wernicke's area ROI and with maximum over the inferior and middle frontal gyri (Talairach coordinates −46, 19, 20; BA 44/45/10) for the Broca's area ROI. We also designed a Global Language ROI, which is a sum of activations in the lateral frontal and lateral temporal areas. This Global Language ROI was designed to facilitate evaluation of the global language lateralization changes associated with age and to allow us to compare the results of our study to the results of other studies focusing on hemispheric language localization and lateralization in right‐handed subjects. However, it should also be understood that this ROI is not an independent region in the statistical analysis.
Figure 2.
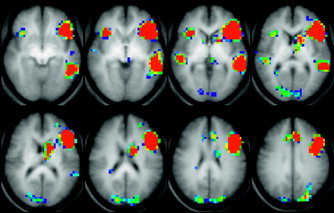
Average activation map showing areas of higher BOLD contrast during the verb generation task in 170 children and adults in radiological convention. Spatial coordinates for the Broca's ROI centroid (x = −46; y = 19; z = 20) correspond predominantly to the left inferior frontal gyrus (BA 44 and 45; also included in the ROI are parts of the middle frontal gyrus and inferior part of the precentral gyrus; BA 6, 9, and 46); spatial coordinates for the Wernicke's ROI (x = −54; y = −33; z = 5) correspond predominantly to the medial/superior temporal gyri (BA 21, 22, 42; also included is posterior part of the inferior temporal gyrus; BA 37).
Laterality indices for each ROI were calculated for each subject based on the individual percentage change maps in the following manner. A threshold was determined by calculating the mean value of the t‐statistics for all pixels within the ROIs. The number of pixels exceeding this threshold was counted for both the left‐ and right‐side ROI. The lateralization index was calculated as follows: LI = (ΣNL‐ΣNR)/(ΣNL+ΣNR), where ΣNL and ΣNR represented the sum of the fMRI pixels that exceeded the threshold for the left and right hemispheric ROI, respectively. Calculating the LI in this manner avoided the biases introduced by arbitrary thresholding and clustering schemes, as well as possible differences in BOLD contrast‐to‐noise ratio between the two scanners operating at different field strength. Both features were crucial for the present study to ensure the consistency in our between‐group data analysis.
This approach yields LIs that range between −1 (right‐sided activation only/maximum right hemispheric dominant) and 1 (left‐sided activation only/maximum left dominant). Values close to “0” (i.e., |LI|<0.1) define bilateral language distribution [Benson et al.,1999; Holland et al.,2001]. A subject with LI > 0.1 is categorized as left dominant, while a subject with LI < −0.1 is categorized as right‐side dominant.
Data Analysis
Age was measured in years. We also used a binary variable, age group, to distinguish between children (ages 5–17; reference category) and adults (18 years and older).
LI data analysis: We computed descriptive statistics for the total sample, including bivariate tests (t‐tests) for gender differences in the laterality indices and the differences between children and adults in the Edinburgh Handedness Inventory (EHI) and the Family Handedness Inventory (FHI) scores. We also computed bivariate correlations (Pearson's r) for all the variables of interest. Next, we used ordinary‐least‐square (OLS) regression to try to explain the variation in LIs by age. We regressed LIs on age separately for the children and the adult subsamples, adjusting for gender, EHI, and FHI only if their correlations with LIs or age were significant. For each subsample, we reported slope coefficients (b) with standard errors (SE) and the variance explained by each model (R2). To test for differences in the slopes for age between children and adults, we used the full sample to run the regression models with added interaction terms between age group (children vs. adults) and age. A significant interaction coefficient (P < 0.05) indicated a statistically significant difference in the effect of age on LI between children and adults.
RESULTS
Demographics and Bivariate Results
Between the years 2000 and 2004, 178 right‐handed subjects were enrolled in the study. Data from eight subjects (three female, ages 5, 8, and 9; five male, ages 10, 14, 18, 18, and 58; all scanned at 3T) were excluded due to excess motion (median voxel displacement >2 mm). The final sample consists of 115 children (63 female; 52 male) ages 5–17 and 55 adults (37 female; 18 male) ages 18–67. In 2001 we began administering a postscan recall quiz to the pediatric subjects entering the study to test their attention to the fMRI verb generation task (n = 51). This quiz consists of testing the recall of nouns given during the active part of the task. There were no significant differences between younger and older children in noun recall (P = 0.18); the recall accuracy was 82% for children 7–8 years old and of 88% for children 10–18 years old [Chiu et al., 2004].
For the entire sample, the laterality index for the Broca ROI ranged from −0.64 to 0.9, for Wernicke from −0.79 to 0.84, and for Global Language ROI from −0.59 to 0.77. No gender differences in laterality indices (LIs) for Broca, Wernicke, and Global Language ROIs were found: all P ≥ 0.25. As expected, 94.7% patients had Broca's ROI activation lateralized to the left hemisphere, 1.8% to the right hemisphere, and 3.5% had fairly symmetric activation, for Wernicke's ROI these percentages were: 82.9, 5.3, and 11.8, and for Global Language LI they were: 95.9, 0.6, and 3.5, respectively. There were no differences between children and adults in the Edinburgh Handedness Inventory (EHI) scores (mean score 89 vs. 92; P = 0.21) or in the Family Handedness Inventory (FHI) scores (mean score 84 vs. 87; P = 0.31).
Positive and significant relationships (P < 0.001) were observed for the three laterality indices. The bivariate Pearson correlations ranged from 0.580 for the Broca and Wernicke LIs to 0.700 and 0.955, respectively, for the Wernicke and Global Language LIs and the Broca and Global Language LIs. Note that the Global Language ROI fully encompasses the other two ROIs and is therefore not independent. Significant correlations were also observed between age and Broca LI, Wernicke LI, and Global Language LI (r = −0.152, r = −0.216, and r = −0.173, respectively; all P < 0.05). There were no correlations between gender, EHI, and FHI and the Broca, Wernicke, and Global Language LIs (all r ≤ 0.098, P ≥ 0.205).
Regression Results for Language Lateralization and Age
In children ages 5–17 the relationships between the LIs and age were all positive and two of them were statistically significant. The effects of age were as follows: b = 0.018 for Broca LI (SE = 0.005, P = 0.001; R2 = 0.100); b = 0.003 for Wernicke LI (SE = 0.006, P = 0.601; R2 = 0.002); and, b = 0.014 for Global Language LI (SE = 0.005, P = 0.003; R2 = 0.075). These results indicate that 5 years of development is associated with an average of 0.09 point increase in Broca LI (increasingly left‐dominant) and with an average of 0.07 point increase in Global Language LI.
In contrast to children, age had negative and significant associations with all LIs in adults: b = −0.006 for Broca LI (SE = 0.003, P = 0.036; R2 = 0.080); b = −0.007 for Wernicke LI (SE = 0.003, P = 0.035; R2 = 0.081); and, b = −0.006 for Global Language LI (SE = 0.003, P = 0.033; R2 = 0.083). These results indicate that, over the period of 5 years, the LIs in adults decrease by an average of 0.03 points for Broca LI and Global Language LI and by an average of 0.04 points for Wernicke LI.
The results from the interaction model for the full sample show two of the three apparent differences in the effect of age on LI between children and adults to be statistically significant. The effects of age on Broca LI and Global Language LI are different between children and adults as indicated by significant regression coefficients (b's) for the interactions between age group (children vs. adults) and age (P < 0.001 and P = 0.001, respectively, for Broca LI and Global Language LI). The difference in the effect of age on Wernicke LI is not significant (P = 0.194). Figure 1 illustrates the linear relationships between Global Language ROI and age for children and adults and a quadratic curve fit for all subjects. Figure 2 shows a composite map of brain activation related to the verb generation task for all subjects included in this report as it was used for generation of the ROIs.
Figure 1.
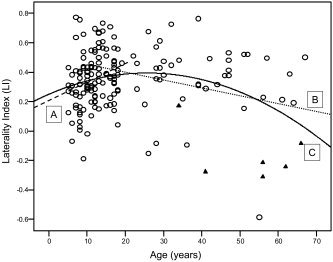
Scatterplot of hemispheric language lateralization as a function of age in children (A: dashed line), adults (B: dotted line), and all subjects (C). Circles: data obtained at 3T, triangles: at 4T.
DISCUSSION
This study examines language lateralization changes with age in a large sample of healthy right‐handed children and adults. The three main findings of the study are: 1) Language became more lateralized to the left hemisphere with increasing age in children and adolescents; 2) The strongest left language lateralization was observed between the ages of 20 and 25; and 3) Thereafter, language lateralization decreased with age—a trend that continued until the 7th decade. The pattern of increasing language lateralization with age seen in children and adolescents is similar to that reported previously [Holland et al.,2001]; the pattern seen in adults is comparable to studies that utilized different language fMRI tasks in young adults [Springer et al.,1999; Szaflarski et al.,2002b].
Recently, left hemispheric lateralization of language function, as observed by fMRI, was reported in infants from 0–3 months of age, suggesting that the neural substrates supporting language are in place and that they are left‐dominant even at birth [Dehaene‐Lambertz et al.,2002]. The presence of language lateralization at such an early age, even before infants are exposed to environmental stimuli for a prolonged period of time, suggests that language dominance is genetically determined. Furthermore, the finding of language lateralization at such an early age has important implications for future studies. Research has shown that phonological awareness develops in children 3–4 years old [Carroll et al.,2003] and that language development progresses from alphabet and phonological awareness in 5‐year‐olds to analytic visual perceptual analysis in 7‐year‐olds [Ellis and Large,1988]. Given the above facts and the fact that the tests of oral fluency show a behavioral trend towards an increased number of words generated with age [Spreen and Strauss,1998], it is possible that the increasing degree of language lateralization to the dominant hemisphere in this group is related to the improving linguistic skills. However, this notion was negated by two recent fMRI and behavioral studies that compared language task‐related BOLD signal changes in children and adults while controlling for performance [Brown et al.,2005; Schlaggar et al.,2002]. Therefore, the shift in language lateralization to the dominant hemisphere in this age group more likely represents age‐related increases in specialization of the left hemisphere for language processing than a simple maturation process. Schlaggar et al. [2002] found clear age‐related/performance‐independent increases in left frontal regions (average age of the comparison groups 9 vs. 25; BA 9 and 44). A follow‐up study by the same group identified a clear effect of increasing age on activation in the left dorsolateral frontal and left parietal cortices with 50% of maturational increases seen by the age of 12 and 75% by the age of 15. At the same time, they noticed maturational decreases in activation in the medial brain regions (medial frontal, cingulate, medial parietal, and occipital cortices) and in right dorsolateral frontal cortex [Brown et al.,2005] which also contribute to the increasing language lateralization to the dominant hemisphere. The results of our study agree with these findings.
In contrast to the above findings derived from behavioral and imaging studies, in 48 children studied with verb generation and orthographic lexical retrieval tasks, Wood et al. [2004] found that the degree of language lateralization was not associated with age. Rather, better performance on the orthographic lexical retrieval task was associated with increased number of activated voxels in the left lateral frontal areas. Unfortunately, this study used a high proportion of left‐handed subjects (9/48), and therefore the findings of lack of effect of age on language lateralization cannot be accepted as dogmatic in view of several studies showing clear association between handedness and the degree of language lateralization [Knecht et al.,2000a,b; Pujol et al.,1999; Springer et al.,1999; Szaflarski et al.,2002b; Wood et al.,2004], which, in turn, affects the strength of language lateralization [Springer et al.,1999; Szaflarski et al.,2002b]. Gaillard et al. [2003] did not find the association between increasing age and change in left‐hemispheric activation. In particular, they found no significant differences in localization or laterality of activation between children and adults except for a slightly increased number of activated pixels in the left inferior and middle frontal gyri. Unfortunately, subjects' performance was not monitored, hence the lack of differences in language localization and lateralization between adults and children in that and another study by the same group could be potentially related to differences in performance [Gaillard et al.,2000,2003].
Although studies focusing on the maturation of the central nervous system indicate that emergence of cognitive function is dependent on the density of synaptic connections, of which the maximum is attained within the first few postnatal months, reaching the fully mature capacity likely depends on the elimination of the unnecessary connections that occurs in late teenage and early adult years [Goldman‐Rakic,1987]. The stable number and density of the synapses is reached in the late teenage/early adult years, and this number remains constant throughout the rest of the adult life [Goldman‐Rakic,1987; Huttenlocher,1979]. Finally, in the human brain microscopic and diffusion‐tensor imaging studies confirm that myelination in areas such as frontal cortex continues through the teenage years (possibly into the mid‐ and late‐twenties), during which period synaptic density decreases minimally [Klingberg et al.,1999; Schmithorst et al.,2002; Yakovlev and Lecours,1967] and a corresponding increase in the white matter volume is noted [Sowell et al.,1999,2002; Wilke et al.,2002]. This suggests that not only the density of the synaptic connections but also myelination may influence language lateralization [Gaillard et al.,2000; Klingberg et al.,1999]. These microanatomic changes are also associated with changes in glucose metabolism. PET studies in children have shown higher baseline resting brain glucose metabolism and blood flow than in adults [Chugani et al.,1987]; resting glucose metabolism is a reflection of synaptic density and activity, and it is usually coupled with cerebral blood flow. While these studies illustrate the microanatomic and physiologic changes occurring in the maturing brain, they also support the findings of our study of increasing language lateralization to the left hemisphere as a result of these changes. It should be noted here that because our findings are reported as a lateralization index, which is a ratio of the fMRI signal in ROIs on the left and right side of the brain, the results should be largely immune to age dependence of the fMRI signal changes or differences in BOLD signal scanners of different field strengths [Schapiro et al.,2004].
All studies examining language lateralization in the pediatric population provide similar incidence rates for language lateralization to the dominant hemisphere in right‐handed subjects, but the data regarding the effect of age on language lateralization is conflicting. Some authors noted increasing lateralization of language functions to the left hemisphere with age [Holland et al.,2001], while other studies did not confirm that finding [Gaillard et al.,2000,2003; Lee et al.,1999; Wood et al.,2004]. There may be multiple explanations for the discrepancy between studies: 1) differences in language tasks used in these studies; 2) differences in task presentation (auditory vs. visual; verb generation task used in this study yields more consistent language lateralization when compared to visual presentation of the same task [Holland et al.,1999]); and 3) limited age span or low number of subjects, or combining right‐ and left‐handed subjects in data analysis of studies that did not confirm the relationship between age and language lateralization, or subject performance.
The study by Holland et al. [2001] did not measure subjects' performance during or after the scanning and, therefore, it is not clear whether the correlation between language lateralization and age is related to performance; other studies did not find a correlation between performance and language lateralization [Gaillard et al.,2003; Wood et al.,2004], although an increasing number of activated voxels in the left frontal regions was noted in one of these studies [Wood et al.,2004]. Since we have not seen any differences in performance between younger and older children (which may reflect potential ceiling effect for noun memory in older children), the finding of increasing language lateralization with age in children appears to be true and age‐related.
The finding of decreasing language lateralization in adults is not surprising in view of the previous studies of language localization in left‐ and right‐handed adults that used different language activation paradigms [Springer et al.,1999; Szaflarski et al.,2002b]. Also, the magnitude of the correlation between age and LIs is similar between our study and the aforementioned studies, supporting the validity of our results. It is somewhat surprising that these trends, instead of reaching a plateau at a certain age, continue through the 7th decade of life and the high incidence of right‐hemispheric LI in subjects over 50 (4/14; 29%). This is somewhat counterintuitive since the incidence of aphasia in left medial cerebral artery stroke patients does not decrease with age, nor is there an improvement in the poststroke aphasia recovery in older adults. The presence of age‐related reorganization in language lateralization could allow the elderly subjects to maintain cognitive performance [Grady,1996], though our study was not designed to test for that. The findings of changing language LI with age in our study are also not thought to be related to the demands of the language task itself, since the same verb generation task was administered to all subjects. This task was designed to be relatively simple, based on nouns found in the lexicon of the average 5‐year‐old, and it can be performed easily by all ages included in this study [Byars et al.,2002; Chiu et al., 2004]. Furthermore, increase in the demands of a word retrieval task did not lead to changes in language lateralization in a previous functional transcranial Doppler sonography study [Drager and Knecht,2002].
It is unlikely that our findings of decreasing asymmetry of the LI with age in adults is related to anatomical differences between young and old subjects, e.g., cerebral atrophy. All scans were reviewed by a board‐certified neuroradiologist and scans with any anatomical abnormalities were excluded from the study. Stebbins et al. [2002] evaluated the issue of age‐related changes in brain anatomy and fMRI activation using a word‐encoding task and found no age effect on the volume of activated tissue.
There may be other explanations for the decreasing language lateralization in elderly. Lexical decision, as in the verb‐generation task used in this study, consists of multiple processes that include word encoding, lexical access, stimulus decision, response selection, and response execution [Allen et al.,2004], which all can be affected by aging that leads to changes in hemispheric lateralization of language functions. While there are no clear timing, size, and distribution of lexical associative effects between young and old subjects as tested with event‐related potentials (ERP), higher‐order language processes are delayed in elderly [Federmeier et al.,2003]. This results in decrease in lexical retrieval in elderly as compared to younger subjects [Connor et al.,2004; Mackay et al.,2002] with the rate of 2% per decade. This, in turn, could also be either partially explained or even caused by the presence of stroke risk factors, e.g., diabetes or hypertension that are omnipresent in the elderly [Brady et al.,2001]. We minimized this possibility by screening our subjects for the presence of any medical conditions and included in the study only healthy subjects. However, we did not specifically obtain the medical records of the subjects from their family physicians to corroborate the histories, so there could be unknown risk factors in our population. Another ERP study examined the effects of age on speech perception [Bellis et al.,2000]. These authors found left‐hemispheric lateralization for responses to speech stimuli in healthy children and adults but symmetric responses in elderly. Finally, the changes in language laterality could be related to the changes in memory encoding necessary for the processing of the verb‐generation task utilized in this study [Allen et al.,2004; Stebbins et al.,2002]. Stebbins et al. [2002] observed age‐related decreases in the left inferior frontal gyrus (BA 44/45) BOLD signal changes which lead to more symmetric lateralization indices for word encoding in the elderly. Since the fMRI activations observed in the present study partially involve the inferior frontal gyrus, the decreases in global and frontal LIs may in part be related to the changes in lateralization of memory encoding. Therefore, our finding of decreasing language lateralization with age, although surprising, is in agreement with previous ERP, behavioral, and fMRI studies.
Other factors that could be associated with changes in hemispheric lateralization are: decreasing with age white matter diffusivity and fractional anisotropy and atrophy of the corpus callosum [Laissy et al.,1993; O'Sullivan et al.,2001a,b]. Changes in the mean diffusivity and fractional anisotropy that could be present in elderly subjects even before the appearance of the T2‐weighted signal changes may lead to disruption of functional relationships between various cortical regions, e.g., the Broca/Wernicke areas and nondominant hemisphere homologs, and lead to the loss of coordination of the whole neural response that would imply loss of functional connectivity [O'Sullivan et al.,2001a,b]. Age‐related decreases in the size of corpus callosum with age [Laissy et al.,1993] could further contribute to the degraded communication between hemispheres and affect the interhemispheric information transfer, as seen in an evoked response potential (ERP) study of competing speech tasks [Greenwald and Jerger,2001]. These factors could lead to increased activation in the nondominant hemisphere homologs that could be related to disconnection rather than activation of new regions responsible for language. Such interpretation of the findings of this study could explain the presence of significant aphasia after left hemispheric stroke in patients who are left‐dominant for language despite the finding of more symmetric language distribution with increasing age. This is, of course, speculative since the neuroimaging techniques provide only indirect evidence of cerebral connectivity and depend on the assumption of a linear relationship between BOLD signal and hemodynamic response that may not be present in the elderly subjects.
Our study is subject to several possible limitations. The discussion presented above reflects the association between age and global laterality index. One could suppose that presenting the results as a relationship between the frontal language ROI (e.g., Broca) and age would reflect the changes in the age/LI relationship more accurately. In fact, many previous studies either used tasks that predominantly activate the frontal regions or used the frontal activation as the measure of language lateralization [Lehericy et al.,2000; Petersen et al.,1988; Pujol et al.,1999; Wise et al.,1991]. This is similar to our findings, where the age/LI relationship in children is significant for Broca's and global ROIs but not significant for Wernicke's ROI and significant for all three regions in adults. Therefore, inclusion of other regions would weaken rather than strengthen the relationship between age and global LI noted in this study if language lateralization was more closely related to frontal than to other brain regions. The data presented here support the model that language lateralization is predominantly driven by frontal brain regions and that the correlation between age and LI is stronger for the frontal ROI (i.e., Broca's) rather than temporal ROI (i.e., Wernicke's).
Another limitation of this study is the use of LI as a measure of dynamic changes in brain plasticity. Calculating the LI presents some limitations: 1) classification of LI may differ based on the threshold criteria; 2) generating ROI boundaries based on the distribution of activated voxels across many subjects (which may exclude certain language areas in subjects with less typical language function localization); and 3) the relationship between fMRI‐derived map of language localization and the functional significance of such areas [Liegeois et al.,2002]. Although we cannot entirely exclude the first possibility, our analysis was designed to circumvent such bias by calculating laterality indices based on the average percent change activation for all voxels within the ROI, and by avoiding arbitrary thresholding and clustering schemes that could potentially affect the language lateralization (this also addresses the concern that the decreasing language lateralization with age in adults is related to the differences between scanners (3T vs. 4T) and the fact that only subjects older than 30 were imaged at 4T). To avoid the second bias, we used generous boundaries for the ROIs (Fig. 2), which led us to include area of greater functional relevance. Finally, although we did not directly compare the accuracy of language localization between fMRI with verb‐generation task and cortical mapping (as the gold standard for language localization), this was done by other groups with good success [Benson et al.,1999; Liegeois et al.,2002; Ojemann et al.,2002], with clear advantage of verb‐generation task over object‐naming and single‐word‐reading tasks shown by Benson et al. [1999].
We were unable to assess performance inside the scanner due to the covert nature of the task, which is another limitation of the present study. We are therefore unable to reject the alternative hypothesis that our results may, to some extent, represent age‐related differences in performance. It is not known whether and to what extent language performance is related to laterality of activation, although in the most recent study that collected the performance data there was no correlation between age and language lateralization [Wood et al.,2004]. Further research, possibly using an overt task with a specially designed scan sequence with silent intervals enabling the subjects' responses to be heard, will be necessary to overcome this limitation [Schmithorst and Holland,2004]. Since the control condition was not a baseline resting condition, but rather a bilateral finger‐tapping task, the possibility also remains that our results might be influenced by activation resulting from the finger‐tapping, as increases in activation with age for a finger‐tapping task has been previously shown [Schapiro et al.,2004]. To minimize this possibility the analysis was restricted to ROIs with very high significance for activation from the language task.
Acknowledgements
This work was presented in part at the 56th annual meeting of the American Academy of Neurology, San Francisco, CA, 4/2004. The authors thank Dr. Magdalena Szaflarski for help with the statistical analysis and Dr. Marian Annett for comments on an earlier version of the manuscript.
REFERENCES
- Allen PA, Murphy MD, Kaufman M, Groth KE, Begovic A (2004): Age differences in central (semantic) and peripheral processing: the importance of considering both response times and errors. J Gerontol B Psychol Sci Soc Sci 59: P210–219. [DOI] [PubMed] [Google Scholar]
- Annett M (1967): The binomial distribution of right, mixed and left handedness. Q J Exp Psychol 19: 327–333. [DOI] [PubMed] [Google Scholar]
- Annett M (1972): The distribution of manual asymmetry. Br J Psychol 63: 343–358. [DOI] [PubMed] [Google Scholar]
- Annett M (1998): Handedness and cerebral dominance: The right shift theory. J Neuropsych 10: 459–469. [DOI] [PubMed] [Google Scholar]
- Annett M, Alexander MP (1996): Atypical cerebral dominance: predictions and tests of the right shift theory. Neuropsychologia 34: 1215–1227. [DOI] [PubMed] [Google Scholar]
- Basso A (2003): Aphasia and its therapy. New York: Oxford University Press. [Google Scholar]
- Bellis TJ, Nicol T, Kraus N (2000): Aging affects hemispheric asymmetry in the neural representation of speech sounds. J Neurosci 20: 791–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont L, Birch HG (1965): Lateral dominance, lateral awareness, and reading disability. Child Dev 36: 57–71. [PubMed] [Google Scholar]
- Benson RR, FitzGerald DB, LeSueur LL, Kennedy DN, Kwong KK, Buchbinder BR, Davis TL, Weisskoff RM, Talavage TM, Logan WJ, Cosgrove GR, Belliveau JW, Rosen BR (1999): Language dominance determined by whole brain functional MRI in patients with brain lesions. Neurology 52: 798–809. [DOI] [PubMed] [Google Scholar]
- Benton AL, Kemble JD (1960): Right‐left orientation and reading disability. Psychiatr Neurol (Basel) 139: 49–60. [DOI] [PubMed] [Google Scholar]
- Booth JR, MacWhinney B, Thulborn KR, Sacco K, Voyvodic JT, Feldman HM (2000): Developmental and lesion effects in brain activation during sentence comprehension and mental rotation. Dev Neuropsychol 18: 139–169. [DOI] [PubMed] [Google Scholar]
- Borod JC, Carper M, Naeser M, Goodglass H (1985): Left‐handed and right‐handed aphasics with left hemisphere lesions compared on nonverbal performance measures. Cortex 21: 81–90. [DOI] [PubMed] [Google Scholar]
- Brady CB, Spiro A 3rd, McGlinchey‐Berroth R, Milberg W, Gaziano JM (2001): Stroke risk predicts verbal fluency decline in healthy older men: evidence from the normative aging study. J Gerontol B Psychol Sci Soc Sci 56: P340–346. [DOI] [PubMed] [Google Scholar]
- Broca P (1861): Remarques sur le siege de la faculte du langage articule; suivies d'une observation d'aphemie. Bull Soc Anat Paris 6: 398–407. [Google Scholar]
- Brown TT, Lugar HM, Coalson RS, Miezin FM, Petersen SE, Schlaggar BL (2005): Developmental changes in human cerebral functional organization for word generation. Cereb Cortex 15: 275–290. [DOI] [PubMed] [Google Scholar]
- Byars AW, Holland SK, Strawsburg RH, Bommer W, Dunn RS, Schmithorst VJ, Plante E (2002): Practical aspects of conducting large‐scale functional magnetic resonance imaging studies in children. J Child Neurol 17: 885–890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JM, Snowling MJ, Hulme C, Stevenson J (2003): The development of phonological awareness in preschool children. Dev Psychol 39: 913–923. [DOI] [PubMed] [Google Scholar]
- Chee MW, Buckner RL, Savoy RL (1998): Right hemisphere language in a neurologically normal dextral: an fMRI study. Neuroreport 9: 3499–502. [DOI] [PubMed] [Google Scholar]
- Chiu C, Schmithorst J, Brown R, Holland S, Dunn R (2005): Making memories: a cross‐sectional investigation of episodic memory encoding in children using fMRI. Dev Neuropsychol (in press). [DOI] [PubMed] [Google Scholar]
- Chugani DC, Phelps ME, Mazziotta JC (1987): Positron emission tomography study of human brain functional development. Ann Neurol 22: 487–497. [DOI] [PubMed] [Google Scholar]
- Connor LT, Spiro A 3rd, Obler LK, Albert ML (2004): Change in object naming ability during adulthood. J Gerontol B Psychol Sci Soc Sci 59: P203–209. [DOI] [PubMed] [Google Scholar]
- Corbalis M (1997): The genetics and evolution of handedness. Psychol Rev 104: 714–727. [DOI] [PubMed] [Google Scholar]
- Dehaene‐Lambertz G, Dehaene S, Hertz‐Pannier L (2002): Functional neuroimaging of speech perception in infants. Science 298: 2013–2015. [DOI] [PubMed] [Google Scholar]
- Drager B, Knecht S (2002): When finding words becomes difficult: is there activation of the subdominant hemisphere? Neuroimage 16: 794–800. [DOI] [PubMed] [Google Scholar]
- Duewell S, Wolff SD, Wen H, Balaban RS, Jezzard P (1996): MR imaging contrast in human brain tissue: assessment and optimization at 4 T. Radiology 199: 780–786. [DOI] [PubMed] [Google Scholar]
- Ellis N, Large B (1988): The early stages of reading: a longitudinal study. Appl Cogn Psychol 2: 47–76. [Google Scholar]
- Ellis SJ, Ellis PJ, Marshall E, Windridge C, Jones S (1998): Is forced dextrality an explanation for the fall in the prevalence of sinistrality with age? A study in northern England. J Epidemiol Community Health 52: 41–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federmeier KD, Van Petten C, Schwartz TJ, Kutas M (2003): Sounds, words, sentences: age‐related changes across levels of language processing. Psychol Aging 18: 858–872. [DOI] [PubMed] [Google Scholar]
- Finch G (1941): Chimpanzee handedness. Science 94: 117–118. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Hong K, Leonard CM, Heilman KM (1998): Hand preference and magnetic resonance imaging asymmetries of the central sulcus. Neuropsychiatry Neuropsychol Behav Neurol 11: 65–71. [PubMed] [Google Scholar]
- Gaillard WD, Hertz‐Pannier L, Mott SH, Barnett AS, LeBihan D, Theodore WH (2000): Functional anatomy of cognitive development: fMRI of verbal fluency in children and adults. Neurology 54: 180–185. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Pugliese M, Grandin CB, Braniecki SH, Kondapaneni P, Hunter K, Xu B, Petrella JR, Balsamo L, Basso G (2001): Cortical localization of reading in normal children: an fMRI language study. Neurology 57: 47–54. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B et al. (2002): Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology 59: 256–265. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B et al. (2003): Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp 18: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geschwind N, Galaburda AM (1985): Cerebral lateralization. Biological mechanisms, associations, and pathology. I. A hypothesis and a program for research. Arch Neurol 42: 428–459. [DOI] [PubMed] [Google Scholar]
- Gilbert AN, Wysocki CJ (1992): Hand preference and age in the United States. Neuropsychologia 30: 601–608. [DOI] [PubMed] [Google Scholar]
- Goldman‐Rakic PS (1987): Development of cortical circuitry and cognitive function. Child Dev 58: 601–622. [PubMed] [Google Scholar]
- Grady CL (1996): Age‐related changes in cortical blood flow activation during perception and memory. Ann N Y Acad Sci 777: 14–21. [DOI] [PubMed] [Google Scholar]
- Greenwald RR, Jerger J (2001): Aging affects hemispheric asymmetry on a competing speech task. J Am Acad Audiol 12: 167–173. [PubMed] [Google Scholar]
- Hertz‐Pannier L, Gaillard WD, Mott SH, Cuenod CA, Bookheimer SY, Weinstein S, Conry J, Papero PH, Schiff SJ, Le Bihan D et al. (1997): Noninvasive assessment of language dominance in children and adolescents with functional MRI: a preliminary study. Neurology 48: 1003–1012. [DOI] [PubMed] [Google Scholar]
- Holland S, Marchevsky JI (1999): An electronic counter/monitor for synchronization of fMRI scans with audio/video stimuli. ISMRM 7th Annual Meeting and Exhibition, Philadelphia, PA.
- Holland S, Strawsburg R, Weber A, Schmithorst J, Dunn R, Ball W (1999): Differences in language activation patterns between audio and visual presentation of the same verb generation task in pediatric epilepsy patients. ISMRM 7th Scientific Meeting, Philadelphia, PA.
- Holland SK, Plante E, Weber Byars A, Strawsburg RH, Schmithorst VJ, Ball WS Jr (2001): Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage 14: 837–843. [DOI] [PubMed] [Google Scholar]
- Huttenlocher P (1979): Synaptic density in human frontal cortex: developmental changes and effects of age. Brain Res 163: 195–205. [DOI] [PubMed] [Google Scholar]
- Jones GV, Martin M (2000): A note on Corballis (1997) and the genetics and evolution of handedness: developing a unified distributional model from the sex‐chromosomes gene hypothesis. Psychol Rev 107: 213–218. [DOI] [PubMed] [Google Scholar]
- Klingberg T, Vaidya CJ, Gabrieli JD, Moseley ME, Hedehus M (1999): Myelination and organization of the frontal white matter in children: a diffusion tensor MRI study. Neuroreport 10: 2817–2821. [DOI] [PubMed] [Google Scholar]
- Knecht S, Deppe M, Drager B, Bobe L, Lohmann H, Ringelstein E, Henningsen H (2000a): Language lateralization in healthy right‐handers. Brain 123: 74–81. [DOI] [PubMed] [Google Scholar]
- Knecht S, Drager B, Deppe M, Bobe L, Lohmann H, Floel A, Ringelstein EB, Henningsen H (2000b): Handedness and hemispheric language dominance in healthy humans. Brain 123(Pt 12): 2512–2518. [DOI] [PubMed] [Google Scholar]
- Knecht S, Jansen A, Frank A, van Randenborgh J, Sommer J, Kanowski M, Heinze HJ (2003): How atypical is atypical language dominance? Neuroimage 18: 917–927. [DOI] [PubMed] [Google Scholar]
- Laissy JP, Patrux B, Duchateau C, Hannequin D, Hugonet P, Ait‐Yahia H, Thiebot J (1993): Midsagittal MR measurements of the corpus callosum in healthy subjects and diseased patients: a prospective survey. AJNR Am J Neuroradiol 14: 145–154. [PMC free article] [PubMed] [Google Scholar]
- Lee BC, Kuppusamy K, Grueneich R, El‐Ghazzawy O, Gordon RE, Lin W, Haacke EM (1999): Hemispheric language dominance in children demonstrated by functional magnetic resonance imaging. J Child Neurol 14: 78–82. [DOI] [PubMed] [Google Scholar]
- Lehericy S, Cohen L, Bazin B, Samson S, Giacomini E, Rougetet R, Hertz‐Pannier L, Le Bihan D, Marsault C, Baulac M (2000): Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology 54: 1625–1633. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Salmond CH, Gadian DG, Vargha‐Khadem F, Baldeweg T (2002): A direct test for lateralization of language activation using fMRI: comparison with invasive assessments in children with epilepsy. Neuroimage 17: 1861–1867. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha‐Khadem F, Baldeweg T (2004): Language reorganization in children with early‐onset lesions of the left hemisphere: an fMRI study. Brain 127: 1229–1236. [DOI] [PubMed] [Google Scholar]
- Loring DW, Meador KJ, Lee GP, Murro AM, Smith JR, Flanigin HF, Gallagher BB, King DW (1990): Cerebral language lateralization: evidence from intracarotid amobarbital testing. Neuropsychologia 28: 831–838. [DOI] [PubMed] [Google Scholar]
- Lowe MJ, Sorenson JA (1997): Spatially filtering functional magnetic resonance imaging data. Magn Reson Med 37: 723–729. [DOI] [PubMed] [Google Scholar]
- Mackay AI, Connor LT, Albert ML, Obler LK (2002): Noun and verb retrieval in healthy aging. J Int Neuropsychol Soc 8: 764–770. [DOI] [PubMed] [Google Scholar]
- Naeser MA, Borod JC (1986): Aphasia in left‐handers: lesion site, lesion side, and hemispheric asymmetries on CT. Neurology 36: 471–488. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Ojemann GA, Lettich E (2002): Cortical stimulation mapping of language cortex using a verb generation task: effects of learning and comparison to mapping based on object naming. J Neurosurg 97: 33–38. [DOI] [PubMed] [Google Scholar]
- Oldfield RC (1971): The assessment and analysis of handedness: the Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Jones DK, Summers PE, Morris RG, Williams SC, Markus HS (2001a): Evidence for cortical “disconnection” as a mechanism of age‐related cognitive decline. Neurology 57: 632–638. [DOI] [PubMed] [Google Scholar]
- O'Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SC, Markus HS (2001b): Normal‐appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 57: 2307–2310. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME (1988): Positron emission tomographic studies of the cortical anatomy of single‐word processing. Nature 331: 585–589. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A (1999): Cerebral lateralization of language in normal left‐handed people studied by functional MRI. Neurology 52: 1038–1043. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B (1977): The role of early left‐brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci 299: 355–369. [DOI] [PubMed] [Google Scholar]
- Schapiro MB, Schmithorst VJ, Wilke M, Byars AW, Strawsburg RH, Holland SK (2004): BOLD fMRI signal increases with age in selected brain regions in children. Neuroreport 15: 2575–2578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaggar BL, Brown TT, Lugar HM, Visscher KM, Miezin FM, Petersen SE (2002): Functional neuroanatomical differences between adults and school‐age children in the processing of single words. Science 296: 1476–1479. [DOI] [PubMed] [Google Scholar]
- Schmithorst V, Dardzinski B (2000): CCHIPS/IDL enables detailed MRI analysis. http://www.researchsystems.com/AppProfile/idl_med_cchips.cfm.
- Schmithorst VJ, Holland SK (2004): Event‐related fMRI technique for auditory processing with hemodynamics unrelated to acoustic gradient noise. Magn Reson Med 51: 399–402. [DOI] [PubMed] [Google Scholar]
- Schmithorst VJ, Dardzinski BJ, Holland SK (2001): Simultaneous correction of ghost and geometric distortion artifacts in EPI using a multiecho reference scan. IEEE Trans Med Imaging 20: 535–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmithorst VJ, Wilke M, Dardzinski BJ, Holland SK (2002): Correlation of white matter diffusivity and anisotropy with age during childhood and adolescence: a cross‐sectional diffusion‐tensor MR imaging study. Radiology 222: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Batth R, Jernigan TL, Toga AW (1999): Localizing age‐related changes in brain structure between childhood and adolescence using statistical parametric mapping. Neuroimage 9: 587–597. [DOI] [PubMed] [Google Scholar]
- Sowell ER, Trauner DA, Gamst A, Jernigan TL (2002): Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Dev Med Child Neurol 44: 4–16. [DOI] [PubMed] [Google Scholar]
- Spreen O, Strauss E (1998): A compendium of neuropsychological tests: administration, norms, and commentary. New York: Oxford University Press. [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan PS, Brewer CC, Perry HM, Morris GL, Mueller WM (1999): Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain 122: 2033–2046. [DOI] [PubMed] [Google Scholar]
- Stebbins GT, Carrillo MC, Dorfman J, Dirksen C, Desmond JE, Turner DA, Bennett DA, Wilson RS, Glover G, Gabrieli JD (2002): Aging effects on memory encoding in the frontal lobes. Psychol Aging 17: 44–55. [DOI] [PubMed] [Google Scholar]
- Szaflarski J, Holland S, Shear P, Schmithorst V, Strakowski S, Privitera M (2002a): Using different fMRI language and memory tasks in healthy adults at 3T. Epilepsia 43: 55. [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA (2002b): Language lateralization in left‐handed and ambidextrous people: fMRI data. Neurology 59: 238–244. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P (1988): Co‐planar stereotaxic atlas of the human brain. New York: Thieme Medical. [Google Scholar]
- Thevenaz P, Unser M (1998): A pyramid approach to sub‐pixel registration based on intensity. IEEE Trans Image Process 7: 27–41. [DOI] [PubMed] [Google Scholar]
- Tzourio N, Crivello F, Mellet E, Nkanga‐Ngila B, Mazoyer B (1998): Functional anatomy of dominance for speech comprehension in left handers vs right handers. Neuroimage 8: 1–16. [DOI] [PubMed] [Google Scholar]
- Tzourio‐Mazoyer N, Josse G, Crivello F, Mazoyer B (2004): Interindividual variability in the hemispheric organization for speech. Neuroimage 21: 422–435. [DOI] [PubMed] [Google Scholar]
- Wansapura JP, Holland SK, Dunn RS, Ball WS Jr (1999): NMR relaxation times in the human brain at 3.0 Tesla. J Magn Reson Imaging 9: 531–538. [DOI] [PubMed] [Google Scholar]
- Wernicke C (1911): The symptom of complex aphasia In: Church AE, editor. Diseases of the nervous system. New York: Appleton; p 265–324. [Google Scholar]
- Wilke M, Schmithorst VJ, Holland SK (2002): Assessment of spatial normalization of whole‐brain magnetic resonance images in children. Hum Brain Mapp 17: 48–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R, Chollet F, Hadar U, Friston K, Hoffner E, Frackowiak R (1991): Distribution of cortical neural networks involved in word comprehension and word retrieval. Brain 114: 1803–1817. [DOI] [PubMed] [Google Scholar]
- Wood AG, Harvey AS, Wellard RM, Abbott DF, Anderson V, Kean M, Saling MM, Jackson GD (2004): Language cortex activation in normal children. Neurology 63: 1035–1044. [DOI] [PubMed] [Google Scholar]
- Yakovlev P, Lecours A (1967): The myelogenetic cycles of regional maturation of the brain In: Minkowski A, editor. Regional development of the brain in early life. Philadelphia: F.A. Davis; p 3–70. [Google Scholar]


