Abstract
Pharyngeal dilator muscles are important in the pathophysiology of obstructive sleep apnoea syndrome (OSA). We have previously shown that during wakefulness, the activity of both the genioglossus (GGEMG) and tensor palatini (TPEMG) is greater in patients with OSA compared with controls. Further, EMG activity decreases at sleep onset, and the decrement is greater in apnoea patients than in healthy controls. In addition, it is known that the prevalence of OSA is greater in middle-aged compared with younger men. Thus, we had two goals in this study. First we compared upper airway muscle activity between young and middle-aged healthy men compared with men with OSA. We also explored the mechanisms responsible for the decrement in muscle activity at sleep onset in these groups. We investigated muscle activity, ventilation 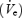 , and upper airway resistance (UAR) during wakefulness and sleep onset (transition from α to θ EEG activity) in all three groups. Measurements were obtained during basal breathing (BB) and nasal continuous positive airway pressure (CPAP) was applied to reduce negative pressure-mediated muscle activation). We found that during wakefulness there was a gradation of GGEMG and UAR (younger < older < OSA) and that muscle activity was reduced by the application of nasal CPAP (to a greater degree in the OSA patients). Although CPAP eliminated differences in UAR during wakefulness and sleep, GGEMG remained greater in the OSA patients. During sleep onset, a greater initial fall in GGEMG was seen in the OSA patients followed by subsequent muscle recruitment in the third to fifth breaths following the α to θ transition. On the CPAP night,
, and upper airway resistance (UAR) during wakefulness and sleep onset (transition from α to θ EEG activity) in all three groups. Measurements were obtained during basal breathing (BB) and nasal continuous positive airway pressure (CPAP) was applied to reduce negative pressure-mediated muscle activation). We found that during wakefulness there was a gradation of GGEMG and UAR (younger < older < OSA) and that muscle activity was reduced by the application of nasal CPAP (to a greater degree in the OSA patients). Although CPAP eliminated differences in UAR during wakefulness and sleep, GGEMG remained greater in the OSA patients. During sleep onset, a greater initial fall in GGEMG was seen in the OSA patients followed by subsequent muscle recruitment in the third to fifth breaths following the α to θ transition. On the CPAP night, 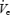 and GGEMG still fell further in the OSA patients compared with control subjects. CPAP prevented the rise in UAR at sleep onset along with the associated recruitment in GGEMG. Differences in TPEMG among the groups were not significant. These data suggest that the middle-aged men had upper airway function midway between that of young normal men and the abnormal airway of those with OSA. Furthermore it suggests that the initial sleep onset reduction in upper airway muscle activity is due to loss of a ‘wakefulness’ stimulus, rather than to loss of responsiveness to negative pressure, and that this wakefulness stimulus may be greater in the OSA patient than in healthy controls.
and GGEMG still fell further in the OSA patients compared with control subjects. CPAP prevented the rise in UAR at sleep onset along with the associated recruitment in GGEMG. Differences in TPEMG among the groups were not significant. These data suggest that the middle-aged men had upper airway function midway between that of young normal men and the abnormal airway of those with OSA. Furthermore it suggests that the initial sleep onset reduction in upper airway muscle activity is due to loss of a ‘wakefulness’ stimulus, rather than to loss of responsiveness to negative pressure, and that this wakefulness stimulus may be greater in the OSA patient than in healthy controls.
Obstructive sleep apnoea (OSA) is a common disorder (Young et al. 1993) characterized by repetitive collapse of the pharyngeal airway during sleep (Remmers et al. 1978) and is associated with important adverse consequences (Flemons & Tsai, 1997; Teran-Santos et al. 1999; Peppard et al. 2000). The pathogenesis of OSA is complex but it is likely to be due to a combination of an anatomically small pharyngeal airway (Haponik et al. 1983; Schwab et al. 1995) in conjunction with a sleep-related decline in upper airway dilator muscle activity (Remmers et al. 1978). Thus, understanding the mechanisms controlling upper airway muscle activity in normal individuals and patients with OSA is of considerable importance.
The best-studied upper airway muscle is the genioglossus, which has a pattern of EMG activity (GGEMG) that is typically inspiratory phasic (greater activity on inspiration) and functions as a dilator of the pharyngeal airway. The tensor palatini is another important upper airway dilator muscle whose activity (TPEMG) is typically more constant throughout the respiratory cycle (Fogel et al. 2004). Control of upper airway muscle activity is complex, and the changes that occur at sleep onset are not completely understood. Factors that may affect GGEMG and TPEMG include direct input from the brainstem respiratory central pattern generator (CPG) (Bianchi et al. 1995), chemoreceptive inputs (Onal et al. 1981a, b), vagal input due to changes in lung volume (Bartlett & St John, 1988) and a tonic ‘wakefulness’ drive that is present in the respiratory system (Orem, 1990). Finally, substantial evidence supports the presence of an intrapharyngeal negative pressure reflex (NPR) in animals (Mathew et al. 1982a, b) and humans (Horner et al. 1991a, b). It is well known that the application of negative pressure to the pharyngeal airway in animals and humans leads to a substantial increase in the activity of the genioglossus as well as other upper airway muscles (Horner, 1991; Malhotra et al. 2000b; Akahoshi et al. 2001; Fogel et al. 2001).
During wakefulness, patients with OSA have augmented activity of the genioglossus (GGEMG) muscle and others such as the tensor palatini (TPEMG) (Mezzanotte et al. 1992; Fogel et al. 2001). The mechanisms driving this increased upper airway muscle activity may include activation of the negative pressure reflex in the upper airway, increased CPG input or an increased ‘wakefulness’ drive. This increased activity is thought to represent a neuromuscular compensatory mechanism for an anatomically small and more collapsible pharyngeal airway. At sleep onset, this augmented upper airway dilator muscle activity is diminished or lost in association with pharyngeal collapse.
As OSA is a state-related disorder, understanding the factors controlling upper airway muscle activity at sleep onset is important. In normal individuals, we have shown that the transition from α to θ EEG (wakefulness to sleep) is associated with an abrupt decline in ventilation 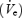 , a rise in upper airway resistance (UAR) and a fall in GGEMG and TPEMG (Worsnop et al. 1998). Further, genioglossus activity is recruited after the initial fall in GGEMG, such that during stable NREM (non-rapid eye movement) sleep genioglossal activity is similar to that seen during wakefulness. No such recruitment is seen in the TPEMG such that activity continues to decline as sleep deepens. We have recently demonstrated that during wakefulness, upper airway muscle activation is greater in middle-aged compared with younger men and that this difference was eliminated with the application of nasal CPAP, which also equalized UAR in the two groups (Fogel et al. 2003b). We also found that the fall in upper airway muscle activity was identical in these two groups of men at sleep onset, suggesting that the sleep onset effect is not different as healthy subjects age.
, a rise in upper airway resistance (UAR) and a fall in GGEMG and TPEMG (Worsnop et al. 1998). Further, genioglossus activity is recruited after the initial fall in GGEMG, such that during stable NREM (non-rapid eye movement) sleep genioglossal activity is similar to that seen during wakefulness. No such recruitment is seen in the TPEMG such that activity continues to decline as sleep deepens. We have recently demonstrated that during wakefulness, upper airway muscle activation is greater in middle-aged compared with younger men and that this difference was eliminated with the application of nasal CPAP, which also equalized UAR in the two groups (Fogel et al. 2003b). We also found that the fall in upper airway muscle activity was identical in these two groups of men at sleep onset, suggesting that the sleep onset effect is not different as healthy subjects age.
In contrast to these findings in normal individuals, we have previously observed the fall in GGEMG and TPEMG in the first two breaths following the α to θ transition to be greater in patients with OSA than in normal subjects (Mezzanotte et al. 1996). However, whether this decrement in upper airway muscle activity is due to loss of NPR input to GGEMG, or is secondary to other sleep onset processes in patients with sleep apnoea could not be determined in this previous study. We thus studied sleep onset in a group of men with OSA both on and off nasal CPAP to determine the importance of changes in negative pressure responsiveness in the decrement in the upper airway muscle activation. We also compared these results with our data in normal controls that were both younger and middle-aged. We had several underlying hypothesizes. First, although we had previously seen increased muscle activity in middle-aged subjects, by comparing them with age-matched OSA patients, we would find that upper airway muscle activity in the OSA patients would be greater than either control group during wakefulness, and that the healthy middle-aged men would have muscle activity that was intermediate between the younger men and those with OSA. Second, that nasal CPAP would cause a greater reduction in upper airway muscle activity in the OSA patients. Third, that by eliminating differences in upper airway resistance and negative pressure-mediated muscle activation between these groups with CPAP that sleep onset effects would be identical in the three groups, demonstrating a consistent wakefulness effect on upper airway muscle activity and ventilatory drive.
Methods
Subjects
Twelve healthy young men between ages 18 and 25 (mean age 22.75 ± 0.39 (s.e.m.) years and mean body mass index (BMI) 22.87 ± 0.57 kg m−2), 13 older men between ages 45 and 65 (mean age 51.00 ± 1.8 years and mean BMI 23.66 ± 0.30 kg m−2) and 12 men with OSA (mean age 50.58 ± 1.55 years and mean BMI 34.75 ± 3.46 kg m−2) participated in the study. The controls were healthy and without sleep complaints. The subjects with OSA were required to be compliant with CPAP (> 6 h per night by history) for at least 3 months. The controls were taking no medications, while the subjects with OSA could be on stable antihypertensive therapy only. Informed consent was obtained from each subject, with the protocol conforming to the principles outlined in the Declaration of Helsinki and having the prior approval of the Human Subjects Committee of the Brigham and Women's Hospital. Each subject was studied for two nights separated by at least 1 week. One older subject was unable to sleep during the protocol, and another was found to have prominent central apnoea at sleep onset. Thus data from 12 young, 11 older and 12 men with OSA were analysed. Some of the data in the older and younger control subjects has been previously reported (Fogel et al. 2003b).
Equipment and techniques
The laboratory procedures, EMG recordings, and measurement of ventilation and resistance were conducted as previously described (Wheatley et al. 1993). In order to assess sleep–wake state, subjects were instrumented with two channels of electroencephalography (EEG), two channels of electro-oculography (EOG) and chin EMG.
Airway mechanics
Subjects wore a nasal mask (Respironics, Inc., Murraysville, PA, USA) connected to a non-rebreathing valve, a pneumotachometer (model 3700A, Hans Rudolph Inc., Kansas City, MO, USA) and differential pressure transducer (Validyne Corp., Northridge, CA, USA), to measure airflow. Subjects breathed exclusively through the nose and were monitored by video camera. During sleep, the mouth was taped shut, in order to minimize mouth breathing. Mask leak and end tidal PCO2 (PetCO2) were measured (Capnograph Monitor, BCI, Waukesha, WI, USA) as previously described (Fogel et al. 2003b).
Pressures were monitored in the mask with an open catheter attached to a pressure transducer (Validyne Corp.) and in the airway at the level of the epiglottis using a pressure-tipped catheter (MPC-500, Millar, Houston, TX, USA). Any drift in the pressure catheters was corrected on a breath-by-breath basis by an automated computer program that defined end-inspiration and end-expiration by identifying the point of zero flow and then correcting any offset in the pressure signals. Minute ventilation and upper airway resistance (measured at a fixed flow rate (0.2 l s−1) and as average inspiratory resistance) were calculated on a breath-by-breath basis. As minute ventilation is proportional to body weight, it was corrected by dividing by the body weight where appropriate. Our automated analysis program was modified to allow analysis of breaths with complete airway obstruction (as occurred in some OSA patients on the normal breathing night). The algorithm initially used the flow signal to define the beginning and end of each inspiration. However, if respiratory-related variations in pressure were detected in the absence of variations in flow, the computer then used the epiglottic pressure signal to define the beginning and end of inspiration. In addition, on breaths with extremely low flow rates, resistance values were set at a maximum of 99 cmH20 l−1 s−1.
Muscle activation
The GGEMG and TPEMG were measured with a pair of unipolar intramuscular electrodes referenced to a single ground, producing a bipolar recording. Two stainless steel Teflon-coated 30-gauge wire electrodes were inserted 15–20 mm into the body of the genioglossal muscle using our previously described techniques (Malhotra et al. 2000a; Fogel et al. 2001, 2003a). To confirm TP electrode placement, the following respiratory manoeuvres, which have been shown previously to activate the TP muscle, were performed: sucking, blowing and swallowing (Malhotra et al. 2000b). Diaphragm EMG (DiaEMG) was obtained from electrodes placed at the right sixth to eighth intercostals spaces adjacent to the costal margin.
The raw EMG was amplified to provide an easily visible phasic inspiratory signal during quiet breathing (Grass Instruments, Quincy, MA, USA), band pass filtered (between 30 and 1000 Hz), and stored for subsequent data analysis by computer software. Sections of the recording containing movement or other artefacts were removed before analysis. In addition, sections of the DiaEMG containing QRS complexes (100–160 ms duration) were removed and replaced with the immediately preceding and succeeding sections. The raw EMG signal for all muscles were then integrated using a 100 ms moving time average (MTA). Several values were calculated on a breath-by-breath basis for each muscle. The first was the tonic level. Activity in the expiratory phase was divided into 10 equal segments and the level of tonic activity of the muscle was defined as the activity of the lowest segment. The second was peak activity, which was defined as the highest value that occurred during inspiration. The third was phasic activity, which was defined as the difference between peak activity and tonic activity. For the tensor palatini (which does not typically show such phasic activity) peak activity is reported. In order to allow comparison between subjects and between the CPAP and control nights, the GGEMG and TPEMG were quantified as a per cent of the total inspiratory activity observed during swallowing as previously described (Fogel et al. 2003b). This was done as this manoeuvre was readily reproducible, produced a consistent level of activation in each individual across each of the two study nights and was almost always equivalent to the maximal activation for both upper airway muscles. To define the level of EMG activity during a swallow, the peak activity for all swallows from a night for each individual were averaged, yielding a single value for each muscle, for each condition, and for each individual. The average number of swallows on the normal and CPAP nights were 56 and 35 for the younger subjects, 65 and 36 for the older subjects, and 68 and 32 for the OSA subjects, respectively. The tonic, phasic and peak EMG activity for each breath were then expressed as a percentage of this value. The diaphragm was recorded in arbitrary units, which were then normalized to the baseline α (wakeful) level on each night.
Nasal CPAP application
On one of the two nights (order randomised) subjects were studied on nasal CPAP. Initially, 5–10 min of data were recorded during quiet wakefulness with CPAP in place in order to estimate the level of GGEMG/TPEMG. In the control subjects, nasal CPAP was applied (BIPAP S/T-D, Respironics, Inc., Murraysville, PA, USA) at 5 cmH2O and increased to a maximum of 10 cmH2O, or until the minimal level of GGEMG was obtained. The level of CPAP producing this minimal GGEMG for that subject was then used throughout the protocol (see below). In the OSA patients, CPAP was initially applied at their prescribed pressure, and if flow limitation was noted during sleep, this pressure was adjusted as necessary.
Protocol
Each subject reported to the laboratory at approximately 9 p.m., having fasted for at least 4 h. After obtaining informed consent, all recording equipment was attached. Subjects subsequently lay supine with eyes open, and were allowed to acclimatise to the equipment. The order of the study nights (CPAP or basal breathing (BB)) was randomised. After recording data during wakefulness, subjects were allowed to fall asleep (remaining in the supine posture). In order to obtain multiple sleep onsets, subjects were woken if they had slept for five consecutive minutes without spontaneous awakenings, and were thereafter allowed to fall asleep again. This procedure was repeated until approximately 4 h of data had been collected on each night.
Data recording and analyses
All signals (GGEMG, TPEMG and DiaEMG (raw and an electronically derived moving time average), airway pressure (mask and epiglottic), PET,CO2, EKG, sleep staging and inspiratory flow were recorded on a 16-channel Grass model 78 polygraph (Grass Instruments, Astro-Medical, Inc., West Warwick, RI, USA). Certain signals (GGEMG, TPEMG and DiaEMG, airway pressures, EEG, inspiratory flow and EKG) were also recorded onto computer using data acquisition software (Spike 2; version 3.17, Cambridge Electronic Design, Ltd, Cambridge, UK).
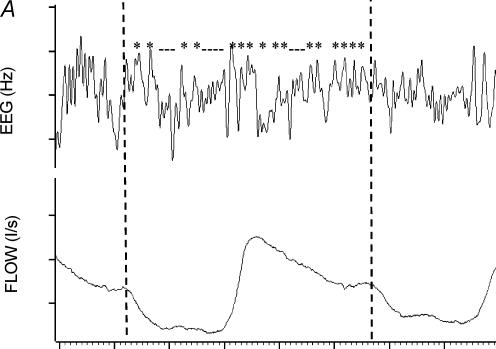
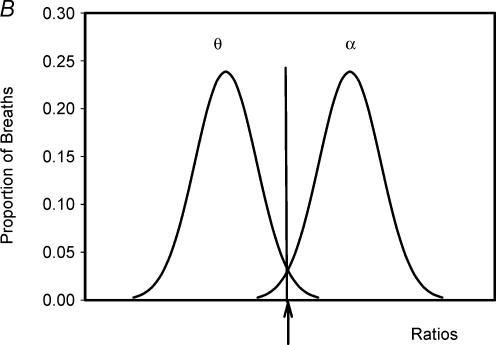
For each individual, the occipital EEG during each breath was assessed as being predominantly α or θ, as previously described (Worsnop et al. 1998, 2000). Briefly, for each subject, 10 min of α and 10 min of θ were visually identified. For each breath in these two periods, the frequency characteristics of the corresponding EEG were determined using a peak-to-peak procedure. Intervals in the 0.3–50 Hz range were determined and these intervals were divided into those > 8 Hz (shorter than 0.125 s) and those < 8 Hz. For each breath, a ratio of the number of EEG intervals > 8 Hz to the total number of intervals was calculated. The distributions of EEG ratios for the breaths in the selected 10 min of α and θ were plotted. The point of intersection between these two distributions was identified, and the ratio corresponding to this point of intersection became the criterion ratio for that subject as previously described. Thus any breath with a ratio less than the criterion ratio was classified as an α breath while any breath with a ratio greater than the criterion ratio was classified as θ. This procedure was used to classify every breath for each subject as occurring either during α or θ EEG activity (see Fig. 1 and Worsnop et al. (1998, 2000) for further description of this procedure).
Figure 1. Calculation of the criterion ratio for analysis of sleep onset.
A single breath from a period of θ activity. The dotted lines define the beginning and end of this breath. Peak-to-peak intervals in the θ range (> 0.125 s) are shown with * and those in the α range are designated with _. The ratio of α range intervals to total intervals for this breath is 10/26 or 0.38. A ratio such as this is calculated for every breath in a 10 min period of typical θ and a similar period of typical α. B, a diagram of a plot of frequency distributions of ratios for the breaths in these two periods in a hypothetical individual. The arrow marks the point which separates these two distributions. This is the criterion ratio.
Analysis of sleep-onset effects
Once each breath had been classified as α or θ, computer software was used to identify periods of α to θ transition. An adequate α–θ transition was defined by having at least three consecutive α breaths followed by at least two consecutive θ breaths. Each breath in the transition was then assigned a position relative to the transition from −5 to +5 as previously described by Worsnop et al. (2000). For each subject, the following parameters were averaged at each breath position: 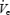 , UAR, GGEMG and TPEMG (as percentages of swallow activity) and DiaEMG (arbitrary units). In addition, for each variable the mean α level (−5 to −1) and the mean θlevel (+1 to +2) was also calculated.
, UAR, GGEMG and TPEMG (as percentages of swallow activity) and DiaEMG (arbitrary units). In addition, for each variable the mean α level (−5 to −1) and the mean θlevel (+1 to +2) was also calculated.
Statistical analyses
All statistical analyses were performed with commercially available software (Sigma Stat version 3.0 +, SPSS 12.01, SPSS Corp., Chicago). A 3 × 2 × 2 ANOVA for repeated measures (with the between group factor being subject type (young, old, OSA) and repeated measures breathing type (BB, CPAP), and sleep–wake state) was performed to determine the effects of CPAP, sleep–wake state and subject type, on muscle activation, airflow and upper airway resistance. When a significant effect was found, Tukey's test was used to determine which groups were significantly different. To determine if CPAP had an effect on the pattern of muscle recruitment following the transition to sleep, analyses of conditions (CPAP versus BB) by subject type (young, old, OSA) by breath number was performed. For all analyses, α was set at 0.05. Results are presented as means ± s.e.m.s.
Results
Complete data were obtained in all (35) individuals for 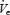 , UAR and GGEMG. In one young and one middle-aged control, technical problems with TPEMG made it impossible to obtain adequate TPEMG recordings and thus data are presented for 33 individuals for TPEMG.
, UAR and GGEMG. In one young and one middle-aged control, technical problems with TPEMG made it impossible to obtain adequate TPEMG recordings and thus data are presented for 33 individuals for TPEMG.
Muscle activation and ventilation during wakefulness
As can be seen in Table 1 and Fig. 2, during wakeful basal breathing, GGEMG was greater in patients with OSA than either control group (GGEMG peak, main effect of group, F= 8.29, P = 0.001). Similar effects were seen for tonic and phasic GGEMG (Table 1). While TPEMG tended towards being greater in the patients with OSA during BB, this effect was not significant (F= 1.52, P = n.s., Table 1 and Fig. 2). Pharyngeal resistance was greater during BB in the patients with OSA (UAR 0.2 l s−1, F= 5.88, P = 0.007). Subgroup analysis showed that resistance was higher in OSA patients than young controls (P = 0.005) but not than middle-aged controls (P = n.s.). There were no significant differences in 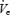 when corrected for weight among the three groups. Correlation analysis revealed a significant relationship between UAR during wakefulness and all measures of GGEMG during BB (e.g. UAR at 0.2 l s−1versus GGEMG peak, r= 0.56, P = 0.001). No correlation was seen between measures of UAR and TPEMG.
when corrected for weight among the three groups. Correlation analysis revealed a significant relationship between UAR during wakefulness and all measures of GGEMG during BB (e.g. UAR at 0.2 l s−1versus GGEMG peak, r= 0.56, P = 0.001). No correlation was seen between measures of UAR and TPEMG.
Table 1.
CPAP effect on muscle activation and UAR during wakefulness
| Young | Old | OSA | ||||
|---|---|---|---|---|---|---|
| BB | CPAP | BB | CPAP | BB | CPAP | |
| GGEMG (% swallow) | ||||||
| Tonic | 9.97 ± 1.8† | 6.74 ± 1.1† | 14.90 ± 6.1† | 10.52 ± 2.6* | 27.83 ± 6.1 | 24.35 ± 5.4* |
| Phasic | 12.45 ± 3.5† | 7.29 ± 1.6*† | 20.74 ± 4.7 | 9.84 ± 3.6* | 32.12 ± 4.8 | 17.98 ± 3.1* |
| Peak | 18.68 ± 3.2† | 12.64 ± 1.7*† | 28.10 ± 3.9† | 16.45 ± 4.6* | 44.90 ± 6.2 | 24.78 ± 3.9* |
| TPEMG (% swallow) | 13.32 ± 4.3 | 8.94 ± 1.4* | 17.05 ± 3.4 | 10.32 ± 2.1* | 22.42 ± 3.5 | 13.13 ± 2.9* |
| UAR (cmH2O l−1 s−1) | ||||||
| Average | 1.58 ± 0.4† | 1.57 ± 0.09 | 3.13 ± 0.4 | 1.55 ± 0.3* | 3.99 ± 0.5 | 1.34 ± 0.3* |
| 0.2 l s−1 | 1.01 ± 0.1† | 0.98 ± 0.1 | 2.00 ± 0.3 | 1.21 ± 0.2* | 2.85 ± 0.6 | 0.79 ± 0.1* |
|
|
0.12 ± 0.01 | 0.09 ± 0.01 | 0.10 ± 0.03 | 0.09 ± 0.03 | 0.12 ± 0.03 | 0.10 ± 0.02 |
P < 0.05, versus OSA;
P < 0.05, control versus CPAP.
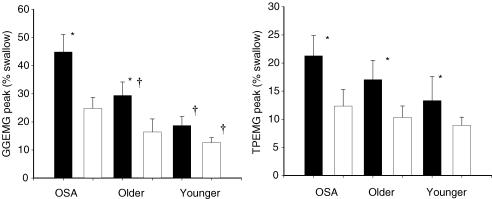
Figure 2. The effect of CPAP on GGEMG and TPEMG during wakefulness.
Bar graphs showing the mean (± s.e.m.) peak GGEMG (left) and TPEMG (right) during stable wakefulness (α breaths −5 to −2) during B (filled bar) and after the application of nasal CPAP (open bar) in all three groups. GGEMG was greater during wakefulness in the OSA patients and was greater in the middle-aged compared with younger men. Nasal CPAP led to a significant reduction in GGEMG and TPEMG and for GGEMG this effect was greatest for the OSA patients. *P < 0.05 BB vs. CPAP; †P < 0.05 vs. OSA.
Effects of CPAP during wakefulness
CPAP led to a significant reduction in GGEMG (peak GGEMG, F= 35.3, P < 0.001) and TPEMG (F= 14.4. P = 0.001) in all groups (Table 1 and Fig. 2). However, the effects of CPAP varied by group with a significant CPAP by group interaction for tonic (F= 6.48, P = 0.04) and peak GGEMG (F= 4.21, P = 0.02), as well as for UAR (UAR at 0.2 l s−1, F= 9.085, P = 0.001). Thus, CPAP caused a greater reduction in both GGEMG as well as UAR in the patients with OSA than either control group. No significant interaction between group and CPAP was seen for the TPEMG. With the application of nasal CPAP, tonic GGEMG was no longer different between the three groups (F= 0.79, P = 0.46) but both peak (F= 3.2, P = 0.05) and phasic GGEMG (F= 4.4, P = 0.02) remained greater in the OSA patients on CPAP. Post hoc tests, showed that for both peak and phasic GGEMG, significant differences in the CPAP condition were seen only between the OSA and younger control groups (Table 1). The previously noted relationship between UAR and GGEMG was no longer present during nasal CPAP.
Effect of sleep onset
Sleep onset during basal breathing
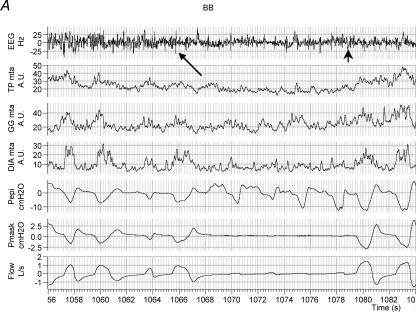
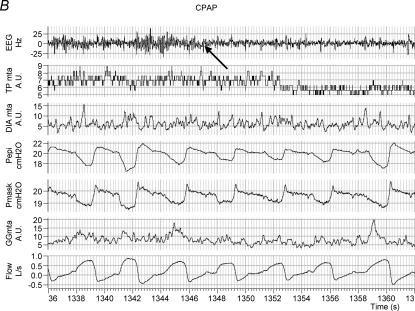
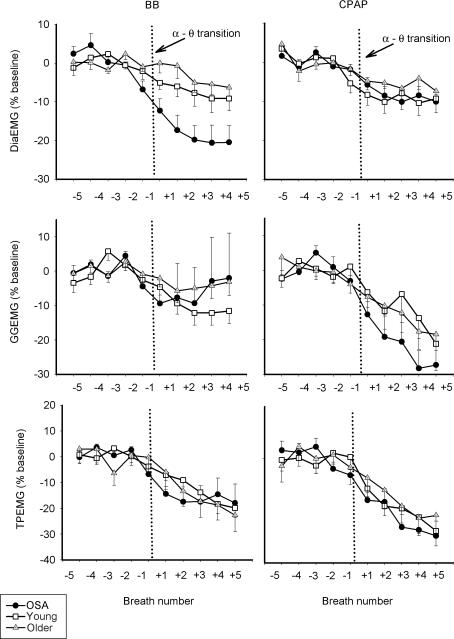
Figure 3 shows an example of raw data in one individual with OSA during BB and CPAP conditions across the α–θ transition. As can be seen, at sleep onset under basal breathing conditions (arrow, Fig. 3A), upper airway obstruction occurs in conjunction with an initial fall in upper airway and pump muscle EMG activity. However, towards the end of the apnoea, GGEMG and TPEMG begin to increase (recruit) although airway patency is not restored until arousal from sleep occurs.
Figure 3. An example of raw data in one OSA patient during sleep onset.
Shown are EEG, inspiratory airflow, moving time averaged diaphragm, tensor palatini and genioglossus EMGs, mask and epiglottic pressure signals. The α–θ transition is marked with an arrow. As can be seen (A), after an initial fall in GGEMG at the α–θ transition, GGEMG activity then increases in response to upper airway obstruction during BB although airflow does not resume until arousal (arrowhead). On nasal CPAP (B), GGEMG continues to decline after the transition.
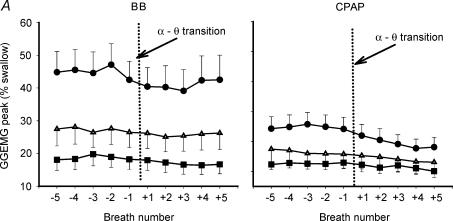
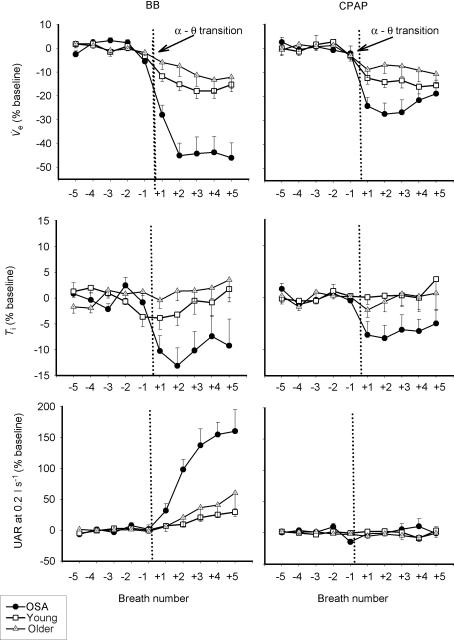
As can be seen in Table 2 and Fig. 4A, GGEMG was higher in the OSA patients than in either control group, and remained so after the α–θ transition (Fig. 4, Table 2, peak GGEMG, F= 7.09, P = 0.003). The initial fall in GGEMG over the first two breaths of the transition was greater in the OSA patients on the BB night, than either the middle-aged or young controls (5.16 versus 2.18 versus 1.08% swallow units, P = 0.015). After the initial fall in GGEMG, recruitment of muscle activity was seen on the BB night (breaths +3 to +5). Figures 5 and 6 show the effect of the α–θ transition on ventilatory and muscle parameters, respectively, in graph form as a percentage of the stable α baseline, in order to highlight the effects of sleep onset. On the basal breathing night, as expected, minute ventilation fell to a much larger degree in the OSA patients and pharyngeal resistance rose further in this group (Fig. 5; P < 0.01 versus both controls). In addition inspiratory duration (Ti) at sleep onset, also fell further in the OSA patients (F= 7.05, P = 0.003 for main effect of age) than in either control group (both P < 0.05).
Table 2.
CPAP effect on muscle activation and UAR at sleep onset (breaths +1 and +2)
| Young | Old | OSA | ||||
|---|---|---|---|---|---|---|
| BB | CPAP | BB | CPAP | BB | CPAP | |
| GGEMG (% swallow) | ||||||
| Tonic | 9.46 ± 1.7† | 6.25 ± 1.1* | 13.29 ± 1.8† | 9.51 ± 2.7* | 24.35 ± 5.4 | 10.52 ± 2.59* |
| Phasic | 11.64 ± 2.6† | 6.85 ± 2.0*† | 20.21 ± 4.9 | 8.84 ± 3.1* | 29.71 ± 5.1 | 14.43 ± 2.7* |
| Peak | 17.60 ± 3.1† | 11.76 ± 2.2*† | 25.92 ± 3.4† | 15.06 ± 4.4* | 40.34 ± 5.9 | 21.22 ± 3.8* |
| TPEMG (% swallow) | 12.27 ± 4.0 | 7.50 ± 1.2* | 15.85 ± 3.6 | 9.32 ± 1.9* | 19.23 ± 3.3 | 11.43 ± 2.8* |
| UAR (cmH2O l−1 s−1) | ||||||
| Average | 1.55 ± 0.3† | 1.38 ± 0.1 | 3.73 ± 0.5 | 1.44 ± 0.4* | 12.43 ± 1.0 | 1.19 ± 0.3* |
| 0.2 l s−1 | 1.10 ± 0.1† | 1.01 ± 0.2 | 2.36 ± 0.5 | 1.18 ± 0.2* | 4.95 ± 1.2 | 0.77 ± 0.1* |
P < 0.05, versus OSA;
P < 0.05, control versus CPAP.
Figure 4. Breath-by-breath mean (± s.e.m.) peak GGEMG (top panel,% of swallow) and TPEMG (bottom panel,% of swallow) in OSA patients, younger and older controls during basal breathing and nasal CPAP across the α–θ transition.
Data are shown from breath −5 to +5 with the transition marked by the vertical dotted line. GGEMG was highest in the OSA patients and remained higher after the transition. Muscle activity was reduced more by the application of nasal CPAP in the OSA patients. The recruitment of GGEMG seen in the BB condition did not occur while on nasal CPAP. • OSA; ▪, young; ▵, older.
Figure 5. Breath-by-breath mean (± s.e.m.) changes in inspiratory minute ventilation (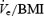 top), inspiratory duration (Ti, middle) and upper airway resistance (UAR at 0.2 l s−1, bottom), as a percentage of the stable α baseline during basal breathing and CPAP.
top), inspiratory duration (Ti, middle) and upper airway resistance (UAR at 0.2 l s−1, bottom), as a percentage of the stable α baseline during basal breathing and CPAP.
Even with application of nasal CPAP and no rise in resistance at the α–θ transition, ventilation fell to a greater degree in the OSA patients, primarily due to a larger reduction in inspiratory duration. •, OSA; □, young; ▵, older.
Figure 6. Breath-by-breath mean (± s.e.m.) values of GGEMG, TPEMG and DiaEMG (as % of the stable α baseline) across the transition during basal breathing and CPAP.
During basal breathing, there is an initial fall in GGEMG after the α–θ transition, followed by a recruitment of muscle activity in the OSA patients and older controls. This recruitment is not seen while on CPAP and GGEMG continues to decline until breath +5. While on CPAP the change in muscle activity is similar between the three groups. •, OSA; □, young; ▵ older.
Sleep onset during CPAP and comparison with BB
In comparison to the BB night, on the CPAP night (Fig. 3B) a fall in ventilation is seen in conjunction with a decrease in muscle activation. However, in the absence of any increase in upper airway resistance, no GGEMG or TPEMG increase is noted as sleep deepens. Although the initial fall in GGEMG in the first two breaths after the α–θ transition (Fig. 4, Table 2) was not different between conditions for each group, it was still greater in the OSA patients in the CPAP condition (6.03 versus 2.34 versus 1.31% swallow units, P = 0.006). Furthermore, repeated measures ANOVA revealed a significant interaction between breath number and breathing condition (CPAP versus BB) as CPAP prevented the recruitment in GGEMG after sleep onset seen during BB (peak GGEMG, F= 2.90, P = 0.018, Fig. 4). Post hoc testing showed that this significant CPAP by breath interaction was seen only in the OSA patients and middleaged controls, but not in the younger controls. No interaction between CPAP and breath number was seen for TPEMG as the magnitude and pattern of EMG reduction was similar for TP under both breathing conditions.
On the CPAP night, there was no increase in UAR in any of the three groups at the α–θ transition (Table 2, Fig. 5) and there was no significant difference in UAR between the three groups on CPAP before or after the α–θ transition. Significant interactions were seen between subject type and breath number (Fig. 5, F= 6.63, P < 0.001), as well as for CPAP and subject type (F= 11.07, P < 0.001) as CPAP eliminated the rise in resistance seen in the older men and OSA patients at sleep onset. Despite no difference in UAR, 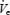 fell further in the OSA patients than either control group on the CPAP night as well (Fig. 5, F= 10.38, P < 0.001, main effect of age) and this fall in
fell further in the OSA patients than either control group on the CPAP night as well (Fig. 5, F= 10.38, P < 0.001, main effect of age) and this fall in 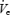 appeared to be primarily due to a greater reduction in inspiratory duration in the apnoea patients (Fig. 5, F= 5.47, P = 0.009). Consistent with this (Fig. 6) was the fact that the change in pump and upper airway muscle activation during the sleep onset period did not differ between the three groups on the CPAP night (although the fall in GGEMG as percentage baseline tended toward being greater in the OSA patients, P = 0.13). Also consistent with this is that the fall in
appeared to be primarily due to a greater reduction in inspiratory duration in the apnoea patients (Fig. 5, F= 5.47, P = 0.009). Consistent with this (Fig. 6) was the fact that the change in pump and upper airway muscle activation during the sleep onset period did not differ between the three groups on the CPAP night (although the fall in GGEMG as percentage baseline tended toward being greater in the OSA patients, P = 0.13). Also consistent with this is that the fall in 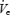 across the transition on the CPAP night was correlated with the change in Ti (r= 0.37, P = 0.03), for the group as a whole, and even more strongly for the OSA patients (r= 0.53, P = 0.04), than for the control subjects (r= 0.35, P = 0.09).
across the transition on the CPAP night was correlated with the change in Ti (r= 0.37, P = 0.03), for the group as a whole, and even more strongly for the OSA patients (r= 0.53, P = 0.04), than for the control subjects (r= 0.35, P = 0.09).
Thus, in comparing the effects of sleep onset during basal breathing and CPAP conditions, we see that under both conditions there is an initial fall in 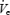 , upper airway and pump muscle activity that is similar during the early portion of this transition (first two breaths) and that this fall is greater in OSA patients than controls. A recruitment of GGEMG occurs during BB in those subjects in whom UAR increases (OSA and older subjects) and this recruitment is not seen during CPAP.
, upper airway and pump muscle activity that is similar during the early portion of this transition (first two breaths) and that this fall is greater in OSA patients than controls. A recruitment of GGEMG occurs during BB in those subjects in whom UAR increases (OSA and older subjects) and this recruitment is not seen during CPAP.
Discussion
In the present study, we have both confirmed prior data and have demonstrated a number of novel findings regarding control of the upper airway and ventilation in both normal subjects and men with obstructive sleep apnoea. First, we confirmed our prior findings that GGEMG is higher during wakefulness in OSA patients versus controls (Mezzanotte et al. 1992; Fogel et al. 2003b). The origin of this increased GGEMG in OSA patients is probably a product of several mechanisms. We believe that the great majority of this increased upper airway muscle activity is a neuromuscular response to a more collapsible pharyngeal airway, probably due to greater activation of the negative pressure reflex. We have previously shown in both normal individuals and in those with sleep apnoea, that increasing the negative pressure generated in the pharynx either with the addition of an inspiratory resistive load (Malhotra et al. 2000b) or using a negative pressure ventilator (Fogel et al. 2001), leads to an increase in GGEMG that is proportional to both the increasingly negative epiglottic pressure and UAR developed. Several of our current findings are also consistent with this hypothesis. First, all measures of GGEMG (tonic, peak and phasic) were systematically related to UAR during BB, but not after the application of nasal CPAP (which minimized epiglottic pressure changes during inspiration and UAR). Second, while nasal CPAP led to a decrement in upper airway EMG in all subjects, this effect was greater in the OSA patients (45% reduction in peak GGEMG in the OSA patients versus 37% in the controls) and was associated with a greater reduction in UAR. However, even with the application of nasal CPAP and elimination of UAR differences between the three groups, both phasic and peak GGEMG remained higher in the OSA patients during wakefulness. If all of the increased GGEMG was in compensation for the increased collapsibility of the upper airway, this difference should have been eliminated by the application of nasal CPAP. Thus other factors must contribute to the increased upper airway muscle tone in OSA as well. This may be due to chronic changes in the sensitivity of muscle activation (a plasticity or learning effect within the CNS) or perhaps due to other drives to the genioglossus such as heightened input from the central pattern generating neurones in the brainstem (Peever et al. 2001a, b, 2002). Another possibility would be an independent effect of wakefulness on this muscle. While the tensor palatini showed similar differences between groups, these were not statistically significant.
An important observation from the present study was that there was a gradation of upper airway muscle activity as well as UAR between young men, middle-aged men and middle-aged men with OSA. If, as we and others have hypothesized, resting upper airway muscle tone is a reflection of the intrinsic anatomy and collapsibility of the pharyngeal airway, these data would suggest that with normal ageing the human upper airway becomes smaller or more collapsible, thus approaching the characteristics seen in patients with OSA. The aetiology of these changes are unclear, but may be secondary to alterations in either the anatomical structure or tissue characteristics of the pharyngeal airway associated with ageing. While several prior upper airway imaging studies have not demonstrated consistent anatomic changes in the pharyngeal lumen or soft tissue structures associated with ageing (Burger et al. 1992; Martin et al. 1997), no studies, to our knowledge, have systematically compared histological tissue characteristics of the pharyngeal airway in healthy humans of different age groups. Our data would suggest, however, that these healthy middle-aged men without snoring or evidence of upper airway obstruction during sleep, had airway characteristics moving on a continuum toward those of the OSA patient. Such changes may thus be responsible for the increasing prevalence of OSA in middle-aged males.
The effect of sleep onset on these upper airway muscles is of interest as well. As we have previously shown (Fogel et al. 2003b), there was an initial reduction in both GGEMG and TPEMG, followed by an increase in the activity of the genioglossus on the BB night, as UAR resistance began to rise. While the initial fall in the first two breaths was somewhat greater in the OSA patients, these patients were not different from the controls in that the genioglossus subsequently responded to the occlusion of the upper airway and increased activity after sleep onset. In fact by breath +5, peak GGEMG in the OSA patient was the same as it was prior to the transition (breath −5 versus breath +5, 44.82 versus 45.50% swallow). Thus, there was no evidence that the GGEMG response to sleep onset was in any way defective in these patients, but rather that it was ineffective in re-opening the upper airway once occlusion had occurred. This may be due to loss of activity in other upper airway muscles that did not respond as well to airway occlusion (such as the TPEMG), or due to the fact that greater muscle activity would be required to re-open the occluded pharyngeal airway (due to surface tension forces, loss of lung volume (Begle et al. 1990), etc.) than could be generated before arousal. Whether the greater fall in GGEMG seen at the α–θ transition in the OSA patients is due to a specific effect of sleep onset on the upper airway muscles or a greater decrement in ventilatory drive at sleep onset (as evidence by the greater fall in DiaEMG) cannot be distinguished from our data. The rise in GGEMG in the face of increasingly more negative epiglottic pressure after sleep onset suggests that the negative pressure reflex is at least partially active after sleep onset, consistent with our prior data using either pulses of negative pressure or negative pressure ventilation (Shea et al. 1999; Fogel et al. 2003a). While this increase in GGEMG could be due to other inputs as well (such as central pattern generating neurones) the fact that it persisted during passive negative pressure ventilation (in the absence of phasic diaphragm EMG) suggests that the response is locally mediated (Fogel et al. 2003a). That the GGEMG is responding to the rise in UAR (or more negative epiglottic pressure) is confirmed by the fact that on the nasal CPAP night (when UAR and epiglottic pressure changes across inspiration are similar before and after sleep onset), GGEMG behaves in a manner similar to the TPEMG, continuing to fall after the α–θ transition. Of note, the changes that occur at sleep onset may not be reflective of the physiological conditions that exist during established stable NREM sleep. However, the importance of the discrete α–θ transition is demonstrated by the fact that upper airway obstruction frequently occurs in this time period, while such obstruction is less commonly seen during established NREM (especially slow-wave) sleep.
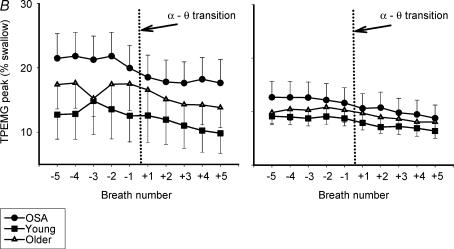
In this study, we also found suggestions that the tensor palatini may be able to respond to local upper airway conditions as well. First, TPEMG fell in all subjects with the addition of nasal CPAP and a reduction in UAR, and this effect was larger in the middle-aged and OSA men than in the younger ones. Furthermore, while the tensor palatini is classically described as having a tonic pattern of activity (not modulated across the respiratory cycle) we found evidence of a clear inspiratory phasic pattern of activity in 5 of our 12 patients with OSA (see Fig. 3A). This pattern of phasic activation was only seen on the BB night when UAR was elevated and not after the application of nasal CPAP (Fig. 3B). Finally, while not statistically significant, TPEMG showed a similar trend to GGEMG among our three groups, with the highest activity found in the OSA patients, followed by the middle-aged men and then the younger men. Taken together, these findings suggest that the tensor palatini can be recruited in a similar manner (although with considerably less sensitivity) as can the genioglossus.
Lastly, our findings regarding the effects of sleep onset on inspiratory duration and minute ventilation in our three groups were somewhat surprising. The greater fall in weight-corrected 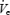 at sleep onset in OSA patients despite nasal CPAP could result from a greater wakeful drive to the respiratory system, which is lost at sleep onset. Alternatively they could start from a similar level of respiratory drive and fall to a lower level during sleep. To distinguish these possibilities an absolute measure of respiratory drive would be required. Our measure of DiaEMG is imperfect in this regard as the use of surface electrodes produces a signal that is contaminated by abdominal/chest wall musculature, and any maximal manoeuvre used to create a scale would be influenced by effort.
at sleep onset in OSA patients despite nasal CPAP could result from a greater wakeful drive to the respiratory system, which is lost at sleep onset. Alternatively they could start from a similar level of respiratory drive and fall to a lower level during sleep. To distinguish these possibilities an absolute measure of respiratory drive would be required. Our measure of DiaEMG is imperfect in this regard as the use of surface electrodes produces a signal that is contaminated by abdominal/chest wall musculature, and any maximal manoeuvre used to create a scale would be influenced by effort.
It is not clear why Ti should have been affected by sleep onset to a greater degree in the OSA patients than in the control subjects. The fall in Ti at the α–θ transition did not differ between the BB and CPAP night in any of the three groups suggesting the group difference is independent of upper airway resistance-mediated output (the NPR). It may represent a consistent effect of the loss of a greater wakefulness drive to the respiratory system in OSA or a heightened wakefulness-dependent reflex input to the respiratory pump from lung or chest wall afferents in OSA subjects. One caveat, however, would be that while Ti was always calculated based on the flow signal in the control groups, by necessity, it had to be calculated using the Pepi signal in some of the OSA patients on the BB night, when flow approached 0 during apnoeas. However, on the CPAP night, the finding of a greater reduction in Ti in the OSA patients was still seen, and this fall in Ti was correlated with the fall in 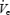 at sleep onset. Along with the greater reduction in GGEMG at sleep onset in the OSA patients on CPAP, these data suggest that there may be a greater pure ‘wakefulness’ drive to the respiratory system in these patients and this loss of wakefulness drive could potentially contribute to the development of upper airway obstruction. The origin of the wakefulness drive to the respiratory system is not completely understood, but may include descending inputs from the cortex, basal forebrain or thalamus to the ventrolateral medulla respiratory centres (Orem, 1990).
at sleep onset. Along with the greater reduction in GGEMG at sleep onset in the OSA patients on CPAP, these data suggest that there may be a greater pure ‘wakefulness’ drive to the respiratory system in these patients and this loss of wakefulness drive could potentially contribute to the development of upper airway obstruction. The origin of the wakefulness drive to the respiratory system is not completely understood, but may include descending inputs from the cortex, basal forebrain or thalamus to the ventrolateral medulla respiratory centres (Orem, 1990).
Our study had a number of limitations, which must be recognized in order to adequately interpret the findings presented. First, while the patients with OSA were well matched to the middle-aged men in terms of age, they were not matched for BMI. Thus, it is theoretically possible that some of the findings presented may be due to the effects of obesity on mechanisms other than the pharyngeal airway. It is clear that obesity leads to a complex series of changes in both the human upper airway and the ventilatory apparatus. Obesity leads to a reduction in chest wall compliance with an increased requirement of pump muscle activity for a given level of ventilation. Central respiratory drive increases as well. Whether the effects of sleep onset on the ventilatory system differ in obese individuals in unknown, however. Thus, we cannot state with certainty that all of our findings were due to OSA rather than to a non-specific effect of obesity. However, to study obese individuals without sleep apnoea would probably introduce other confounders as obesity is such an important determinant of apnoea severity.
Second, our measure of UAR is not accurate when flow limitation develops (as was seen in the OSA patients after sleep onset on the BB night). Thus we acknowledge that the exact numbers provided may not be completely correct. However, it is clear that UAR rises to a greater degree in the OSA patients at this time. Third, while we used nasal CPAP to eliminate upper airway resistance differences among our three groups, we acknowledge that CPAP has multiple effects. These include an increase in resting lung volume, as well as potential shape change in the upper airway. Multiple investigations, including several from our group, have shown that increasing lung volume will lead to a decrease in UAR and a decrease in GGEMG (Stanchina et al. 2003). However, we believe the likely stimulus for the reduction in GGEMG in these cases is not the change in lung volume per se but rather a reduction in UAR with increasing lung volume which leads to a decrease in GGEMG acting through reflex mechanisms. Thus, even if CPAP led to alterations in lung volume in these subjects, it would not invalidate our interpretation of the results. In addition, by necessity, the level of CPAP applied to the upper airway was greater in the OSA patients, as they required a higher pressure to minimize upper airway resistance and this could potentially confound interpretation of these results. Fourth, our method of quantifying GGEMG and TPEMG between nights and between subjects (% of activity during a swallow) could be challenged. As we did in our prior study, we chose this method because it led to a highly reproducible and effort-independent value for both of these muscles (Fogel et al. 2003b). We also believe the use of multiple swallows to develop an average value gave us a more reliable scale to use for these individuals. For this methodology to produce artificial results, CPAP would have to led to a differential effect on swallow activity among our three groups, making it appear that basal activity was being altered more in one group than another. We know of no reason why this should be true. Fifth, while we did not perform formal polysomnography to exclude OSA in our control subjects, we believe it unlikely that any had substantial sleep-disordered breathing. All were studied on the control night in the supine posture, with a nasal mask in place and a pressure catheter in the airway, all factors likely to increase the load on the upper airway. Yet none demonstrated any sleep apnoea during this study. The only caveat to this would be that we cannot exclude OSA isolated to REM sleep in these individuals as we did not study these subjects during REM. However, we doubt that mild REM-associated apnoea would change our conclusions. Finally, as all of these studies were conducted in men, whether these results can be generalized to females is not clear.
In summary, we found that there was a gradation of upper airway muscle activity during normal respiration between normal young men, normal middle-aged men and middle-aged men with OSA. This, in combination with the increased reduction in GGEMG seen with the application of nasal CPAP in the OSA patients, suggests that anatomy is the major difference between these groups and that upper airway muscle activity directly reflects these changes. Thus ageing moves the upper airway from that of a young norma1 male toward that of a patient with sleep apnoea. Furthermore, the recruitment of GGEMG seen after the α–θ transition occurred in OSA patients as well, proof that their muscles also retain the ability to respond to stimuli at this time. In the setting of increased airway collapsibility along with the other changes that occur at sleep onset (loss of tonic muscles, change in lung volume and a decreased central respiratory drive), however, this muscle activity is not adequate to maintain airway patency. Finally, changes in ventilation and ventilatory timing at sleep onset were greater in the patients with OSA, suggesting that wakefulness per se may have a greater effect on ventilatory drive in these subjects.
Acknowledgments
This work was supported by National Institutes of Health–National Center for Research Resources GCR MO1 RR02635, 1 P50 HL60292 and K23 HL04400.
References
- Akahoshi T, White DP, Edwards JK, Beauregard J, Shea SA. Phasic mechanoreceptor stimuli can induce phasic activation of upper airway muscles in humans. J Physiol. 2001;531:677–691. doi: 10.1111/j.1469-7793.2001.0677h.x. 10.1111/j.1469-7793.2001.0677h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlett D, Jr, St John WM. Influence of lung volume on phrenic, hypoglossal and mylohyoid nerve activities. Respir Physiol. 1988;73:97–109. doi: 10.1016/0034-5687(88)90130-2. [DOI] [PubMed] [Google Scholar]
- Begle RL, Badr S, Skatrud JB, Dempsey JA. Effect of lung inflation on pulmonary resistance during NREM sleep. Am Rev Respir Dis. 1990;141:854–860. doi: 10.1164/ajrccm/141.4_Pt_1.854. [DOI] [PubMed] [Google Scholar]
- Bianchi A, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–31. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Burger CD, Stanson AW, Sheedy PFD, Daniels BK, Shepard JW., Jr Fast-computed tomography evaluation of agerelated changes in upper airway structure and function in normal men. Am Rev Respir Dis. 1992;145:846–852. doi: 10.1164/ajrccm/145.4_Pt_1.846. [DOI] [PubMed] [Google Scholar]
- Flemons WW, Tsai W. Quality of life consequences of sleep-disordered breathing. J Allergy Clin Immunol. 1997;99:S750–756. doi: 10.1016/s0091-6749(97)70123-4. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, Pillar G, Edwards JK, Beauregard J, Shea SA, White DP. Genioglossal activation in patients with obstructive sleep apnea versus control subjects. Mechanisms of muscle control. Am J Respir Crit Care Med. 2001;164:2025–2030. doi: 10.1164/ajrccm.164.11.2102048. [DOI] [PubMed] [Google Scholar]
- Fogel RB, Malhotra A, White DP. Sleep. 2: pathophysiology of obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:159–163. doi: 10.1136/thorax.2003.015859. 10.1136/thorax.2003.015859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel RB, Trinder J, Malhotra A, Stanchina M, Edwards JK, Schory KE, White DP. Within-breath control of genioglossal muscle activation in humans: effect of sleep–wake state. J Physiol. 2003a;550:899–910. doi: 10.1113/jphysiol.2003.038810. 10.1113/jphysiol.2003.038810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogel RB, White DP, Pierce RJ, Malhotra A, Edwards JK, Dunai J, Kleverlaan D, Trinder J. Control of upper airway muscle activity in younger versus older men during sleep onset. J Physiol. 2003b;553:533–544. doi: 10.1113/jphysiol.2003.045708. 10.1113/jphysiol.2003.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haponik E, Smith P, Bohlman M, Allan R, Goldman S, Bleecker E. Computerized tomography in obstructive sleep apnea: correlation of airway size with physiology during sleep and wakefulness. Am Rev Respir Dis. 1983;127:221–226. doi: 10.1164/arrd.1983.127.2.221. [DOI] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Holden HB, Guz A. Afferent pathway(s) for pharyngeal dilator reflex to negative pressure in man: a study using upper airway anaesthesia. J Physiol. 1991a;436:31–44. doi: 10.1113/jphysiol.1991.sp018537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horner RL, Innes JA, Murphy K, Guz A. Evidence for reflex upper airway dilator muscle activation by sudden negative airway pressure in man. J Physiol. 1991b;436:15–29. doi: 10.1113/jphysiol.1991.sp018536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra A, Fogel RB, Edwards JK, Shea SA, White DP. Local mechanisms drive genioglossus activation in obstructive sleep apnea. Am J Respir Crit Care Med. 2000a;161:1746–1749. doi: 10.1164/ajrccm.161.5.9907109. [DOI] [PubMed] [Google Scholar]
- Malhotra A, Pillar G, Fogel RB, Beauregard J, Edwards JK, Slamowitz DI, Shea SA, White DP. Genioglossal but not palatal muscle activity relates closely to pharyngeal pressure. Am J Respir Crit Care Med. 2000b;162:1058–1062. doi: 10.1164/ajrccm.162.3.9912067. [DOI] [PubMed] [Google Scholar]
- Martin SE, Mathur R, Marshall I, Douglas NJ. The effect of age, sex, obesity and posture on upper airway size. Eur Respir J. 1997;10:2087–2090. doi: 10.1183/09031936.97.10092087. 10.1183/09031936.97.10092087. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Genioglossus muscle response to upper airway pressure changes: afferent pathways. J Appl Physiol. 1982a;52:445. doi: 10.1152/jappl.1982.52.2.445. [DOI] [PubMed] [Google Scholar]
- Mathew OP, Abu-Osba YK, Thach BT. Influence of upper airway pressure changes on genioglossus and muscle respiratory activity. J Appl Physiol. 1982b;52:438. doi: 10.1152/jappl.1982.52.2.438. [DOI] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal EMG in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanisms) J Clin Inves. 1992;89:1571–1579. doi: 10.1172/JCI115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mezzanotte WS, Tangel DJ, White DP. Influence of sleep onset on upper-airway muscle activity in apnea patients versus normal controls. Am J Respir Crit Care Med. 1996;153:1880–1887. doi: 10.1164/ajrccm.153.6.8665050. [DOI] [PubMed] [Google Scholar]
- Onal E, Lopata M, O'Connor TD. Diaphragmatic and genioglossal electromyogram responses to CO2 rebreathing in humans. J Appl Physiol. 1981a;50:1052–1055. doi: 10.1152/jappl.1981.50.5.1052. [DOI] [PubMed] [Google Scholar]
- Onal E, Lopata M, O'Connor T. Diaphragmatic and genioglossal electromyogram responses to isocapnic hypoxia in humans. Am Rev Respir Dis. 1981b;124:215–217. doi: 10.1164/arrd.1981.124.3.215. [DOI] [PubMed] [Google Scholar]
- Orem J. The nature of the wakefulness stimulus for breathing. Prog Clin Biol Res. 1990;345:23–30. discussion 31. [PubMed] [Google Scholar]
- Peever JH, Mateika JH, Duffin J. Respiratory control of hypoglossal motoneurones in the rat. Pflugers Arch. 2001a;442:78–86. doi: 10.1007/s004240000502. 10.1007/s004240000502. [DOI] [PubMed] [Google Scholar]
- Peever JH, Necakov A, Duffin J. Nucleus raphe obscurus modulates hypoglossal output of neonatal rat in vitro transverse brain stem slices. J Appl Physiol. 2001b;90:269–279. doi: 10.1152/jappl.2001.90.1.269. [DOI] [PubMed] [Google Scholar]
- Peever JH, Shen L, Duffin J. Respiratory pre-motor control of hypoglossal motoneurons in the rat. Neuroscience. 2002;110:711–722. doi: 10.1016/s0306-4522(01)00594-2. 10.1016/S0306-4522(01)00594-2. [DOI] [PubMed] [Google Scholar]
- Peppard P, Young T, Palta M, Skatrud J. Prospective study of the association between sleep disordered breathing and hypertension. N Engl J Med. 2000;342:1378–1384. doi: 10.1056/NEJM200005113421901. 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Degroot WJ, Saureland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Schwab RJ, Gupta KB, Gefter WB, Metzger LJ, Hoffman EA, Pack AI. Upper airway and soft tissue anatomy in normal subjects and patients with sleep-disordered breathing. Significance of the lateral pharyngeal walls. Am J Respir Crit Care Med. 1995;152:1673–1689. doi: 10.1164/ajrccm.152.5.7582313. [DOI] [PubMed] [Google Scholar]
- Shea SA, Edwards JK, White DP. Effect of wake–sleep transitions and rapid eye movement sleep on pharyngeal muscle response to negative pressure in humans. J Physiol. 1999;520:897–908. doi: 10.1111/j.1469-7793.1999.00897.x. 10.1111/j.1469-7793.1999.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanchina ML, Malhotra A, Fogel RB, Trinder J, Edwards JK, Schory K, White DP. The influence of lung volume on pharyngeal mechanics, collapsibility, and genioglossus muscle activation during sleep. Sleep. 2003;26:851–856. doi: 10.1093/sleep/26.7.851. [DOI] [PubMed] [Google Scholar]
- Teran-Santos J, Jimenez-Gomez A, Cordero-Guevara J. The association between sleep apnea and the risk of traffic accidents. Cooperative Group Burgos-Santander. N Engl J Med. 1999;340:847–851. doi: 10.1056/NEJM199903183401104. 10.1056/NEJM199903183401104. [DOI] [PubMed] [Google Scholar]
- Wheatley J, Mezzanotte W, Tangel D, White D. Influence of sleep on genioglossus muscle activation by negative pressure in normal men. Am Rev Respir Dis. 1993;148:597–605. doi: 10.1164/ajrccm/148.3.597. [DOI] [PubMed] [Google Scholar]
- Worsnop C, Kay A, Kim Y, Trinder J, Pierce R. Effect of age on sleep onset-related changes in respiratory pump and upper airway muscle function. J Appl Physiol. 2000;88:1831–1839. doi: 10.1152/jappl.2000.88.5.1831. 10.1063/1.1305832. [DOI] [PubMed] [Google Scholar]
- Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. New Engl J Med. 1993;32:1230–1235. doi: 10.1056/NEJM199304293281704. 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]