Abstract
Withdrawal from the GABA-modulatory steroid 3α-OH-5α-pregnan-20-one (3α,5α-THP) following exposure of female rats to the parent compound progesterone (P) produces a syndrome characterized by behavioural excitability in association with up-regulation of the α4 subunit of the GABAA receptor (GABAR) in the hippocampus. Similar changes are seen after 48 h exposure to its stereoisomer, 3α,5β-THP. Here, we further characterize the effects of P withdrawal on GABAR kinetics, using brief (1 ms) application of 5–10 mm GABA to outside-out patches from acutely isolated CA1 hippocampal pyramidal cells. Under control conditions, GABA-gated current deactivated biexponentially, with τfast = 12–19 ms (45–60% of the current), and τslow = 80–140 ms. P withdrawal resulted in marked acceleration of deactivation (τfast = 3–7 ms and τslow = 30–100 ms), as did 48 h exposure to 3α,5β-THP (τfast = 5–8 ms; τslow = 40–120 ms). When recombinant receptors were tested in HEK-293 cells, a similar acceleration in τfast was observed for α4β2δ and α4β2γ2 GABARs, compared to α1β2γ2 and α5β2γ2 receptors. In addition, τslow was also accelerated for α4β2δ receptors, which are increased following steroid withdrawal. As predicted by the Jones-Westbrook model, this change was accompanied by reduced receptor desensitization as well as an acceleration of the rate of recovery from rapid desensitization. A theoretical analysis of the data suggested that steroid treatment leads to receptors with a greater stability of the bound, activatable state. This was achieved by altering multiple parameters, including desensitization and gating rates, within the model. These results suggest that fluctuations in endogenous steroids result in altered GABAR kinetics which may regulate neuronal excitability.
The GABAA receptor (GABAR), as the primary mediator of fast inhibitory input in the CNS, is modulated by a wide array of exogenous compounds, including benzodiazepines (BDZs), barbiturates, alcohol (Hevers & Luddens, 1998), as well as endogenous steroids such as 3α-OH-5α-pregnan-20-one (or allopregnanolone; 3α,5α-THP) (Majewska et al. 1986) and its active isomer, pregnanolone (3α,5β-THP). Acutely applied, these steroids increase the duration of GABA-gated single channel openings (Twyman & Macdonald, 1992) leading to anxiolytic (Bitran et al. 1993) and anticonvulsant (Belelli et al. 1989; Frye, 1995) effects. Fluctuations in 3α,5α-THP during the human menstrual cycle, however, result in prolonged exposure times (Endicott et al. 1999) followed by abrupt declines (‘withdrawal’) (Rapkin et al. 1997).
In testing the consequences of these exposure conditions, animal studies from this laboratory have demonstrated a bimodal change in GABAR subunit expression in CA1 hippocampus in response to prolonged administration of 3α,5α[β]-THP or its parent compound, progesterone. An initial increase in α4 subunit expression is seen after 48–72 h of exposure, followed by recovery to control levels. A secondary increase in α4 expression then occurs 24 h after termination of steroid exposure (‘withdrawal’) (Smith et al. 1998a; Gulinello et al. 2001). Increases in hippocampal excitability as well as behavioural excitability (i.e. anxiety and seizure susceptibility) are tightly correlated with these increases in α4 expression (Smith et al. 1998a, b; Gulinello et al. 2001; Hsu & Smith, 2003). A similar bimodal pattern has been reported (Backstrom, 1976; Herzog et al. 1997; Endicott et al. 1999; Rapkin et al. 1997) for adverse mood and exacerbation of seizures across the menstrual cycle for women with premenstrual syndrome and catamenial epilepsy, respectively, when exacerbation of symptoms occurs both early and late in the luteal phase, suggesting a clinical correlation. Increased expression of the α4 subunit is also observed during the increased excitability observed after chronic exposure to or withdrawal from other GABA-modulatory drugs such as the BDZs (Holt et al. 1996; Follesa et al. 2001) and alcohol (Devaud et al. 1997; Mahmoudi et al. 1997).
Increased α4 expression following steroid exposure or withdrawal is also associated with a decrease in the decay time constant (τ) for GABA-gated current (Smith et al. 1998a; Smith & Gong, 2004). This change in kinetics, however, was determined using whole cell patch clamp recording, low agonist (EC20) concentrations, as well as relatively slow agonist application and exposure times of 40–100 ms. This approach results in overlapping times for agonist binding, channel opening, deactivation and desensitization. Agonist binding and channel opening occur on a submillisecond time scale only when saturating concentrations of GABA are rapidly (100–300 μs) applied (Maconochie et al. 1994; Burkat et al. 2001). Similarly, τ for rapid desensitization is ∼ 5–10 ms (Celentano & Wong, 1994; Jones & Westbrook, 1995; Haas & Macdonald, 1999; McClellan & Twyman, 1999; Celentano & Hawkes, 2004), which necessitates the use of brief (1–2 ms) exposure times to more accurately determine the time course of deactivation.
In order to distinguish between deactivation and desensitization, here we directly measure these parameters using brief (∼ 1 ms) or prolonged (400 ms to 5 s) application of saturating concentrations of agonist rapidly applied to outside-out patches of membrane from acutely isolated hippocampal pyramidal cells. We also compare findings from native GABAR tested across these steroid administration protocols with results from recombinant receptors with known subunit composition expressed in HEK-293 cells. Our previous findings suggest that both α4βδ (Sundstrom-Poromaa et al. 2002) and α4βγ2 (Hsu et al. 2003) GABARs are increased following 48 h steroid administration as well as following withdrawal from chronic steroid exposure. Several studies suggest that receptors composed of these subunit combinations display shorter mean open times, consistent with a faster deactivation, than GABARs normally expressed in CA1 hippocampus under control conditions (Saxena & Macdonald, 1994; Gingrich et al. 1995; Burgard et al. 1996; Fisher & Macdonald, 1997; Lavoie et al. 1997; Haas & Macdonald, 1999; Bianchi et al. 2001; Akk et al. 2004). Thus, these findings suggest that altered subunit composition may play a role in shaping receptor kinetics after steroid treatment/withdrawal. The results from the present study may be relevant to the alterations in CNS excitability and mood which have been reported across the menstrual cycle (Herzog et al. 1997; Endicott et al. 1999).
Methods
Experimental animals
Adult female Long-Evans rats (Charles River, 120–140 g) were housed in groups of three under a constant light: dark cycle (14: 10 light: dark) and room temperature (21°C). Food and water were available for ad libitum consumption. Animals were killed by decapitation during the light phase of the cycle (approx. 11.00 h). Control rats were tested only on the day of dioestrus-1, a low hormone stage, as verified by microscopic evaluation of the vaginal lavage. In all cases, conditions of animal maintenance and use were in agreement with the SUNY Downstate animal welfare committee.
Steroid administration
Two distinct steroid administration protocols were studied.
Protocol I. P withdrawal
Animals were implanted subcutaneously in the dorsal flank with silastic implants containing crystalline progesterone (P) for a 3-week period (Moran & Smith, 1998). Effects of steroid withdrawal on GABAR function were studied by killing animals 24 h following removal of the implant (‘P withdrawal’). Both placement and removal of the P implant were accomplished under halothane anaesthesia (2-bromo-2-chloro-1,1,1-trifluoro-ethane, 2% in oxygen).
Protocol II. Forty-eight hours of 3α,5β-THP
Other animals were injected with the GABA-modulatory steroid 3α,5β-THP (10 mg kg−1, i.p.) for 3 days, beginning on dioestrus-1.
Both steroid administration protocols result in physiological concentrations of 3α,5α-THP or 3α,5β-THP in the hippocampus (Moran & Smith, 1998). Control animals were injected with vehicle and tested on dioestrus-1. At the conclusion of these steroid treatment protocols, animals were killed by decapitation, hippocampi were removed, and pyramidal cells acutely isolated from the CA1 region.
Recombinant receptors: transfection
Plasmids obtained from Drs S. Vicini (Georgetown University, Washington, DC, USA; rat α1, α5, β2, γ2) and P. Whiting (Merck, Sharp & Dohme, Essex, UK; human α4, α5, δ) were prepared using Qiagen Maxi- or Midi-prep kits. HEK-293 cells were maintained in medium (Dulbecco's modified Eagle's medium (DMEM): Ham's F-12 1: 1) supplemented with 10% fetal calf serum at 37°C in a humid 5% CO2 atmosphere. Cells were transfected with various subunit combinations using Lipofectamine (Invitrogen) with the following ratios: α1β2γ2, 1: 1: 5 (based on findings from Boileau et al. 2002); α4β2γ2, 10: 1: 1, α4β2δ, 10: 1: 10, and α5β2γ2, 5: 1: 1. Currents were recorded from lifted cells 1–3 days later. Cells were also cotransfected with enhanced green fluorescent protein for visualization. Expression of α1β2γ2 was distinguished from α1β2 by a robust response to the benzodiazepine lorazepam and little or no response to zinc. Expression of α4β2 yielded little or no GABA-gated current, with no response to lanthanum or RO15-4513, compared to α4β2δ and α4β2γ2 receptors, respectively.
Acute neuronal isolation
Pyramidal neurones were acutely dissociated as previously described (Smith et al. 1998a). Briefly, tissue was digested at 32°C for 50–60 min under 100% O2 in Pipes-buffered saline containing (mm): NaCl 120, KCl 5, CaCl2 1, MgCl2 1, d-glucose 25, Pipes 20 and trypsin (type XI) or pronase (0.8 mg ml−1), pH 7.0. Following a 1 h enzyme-free incubation at room temperature, tissue was dissociated by trituration in 1 ml of 20 mm Hepes-buffered DMEM which was replaced by recording medium following transfer to the recording chamber.
Electrophysiology
GABA-gated current was recorded at room temperature (20–25°C) at a holding potential of −50 mV in a bath containing (mm): NaCl 120, CsCl 5, CaCl2 2, MgCl2 1, Hepes 10 and glucose 25, pH 7.4, 320 mosmol (kg H2O)−1. Outside-out patches were pulled after a gigaseal was achieved using suction applied to 5–7 MΩ micropipettes (Sutter Instruments, filament-capillary tubes). The pipette solution contained (mm): N-methyl-d-glucamine chloride 120, Cs4BAPTA 5 and Mg-ATP 5. The ATP regeneration system consisting of Tris phosphocreatine (20 mm) and creatine kinase was added to minimize GABA rundown.
Currents were recorded using an Axopatch-1D amplifier (Axon Instruments, Union City, CA, USA) filtered at 2 kHz (four-pole Bessel filter) and acquired at an 8–10 kHz sampling frequency (pCLAMP 5.1, Axon Instruments). Ensemble averages of 6–10 responses per cell were used for determination of decay time constants (τ).
Agonist application
The kinetics of GABA-gated current were tested using a brief application protocol to administer saturating concentrations of GABA (5–10 mm) to whole cells or excised outside-out patches for ∼ 1 ms (Lavoie et al. 1997). To this end, a double-barrelled theta tube (Sutter Instruments, 80–100 μm diameter tip) containing GABA and bath solution was positioned within 50–100 μm of the patch, such that the stream of control solution contacted the patch for 1–2 s periods which were interrupted by periodic brief (< 100–300 μs) transitions to the GABA stream (maintained at 2.5 ml h−1 to yield a forward flow velocity of 125 μm ms−1). A computer generated pulse (pCLAMP 5.1, Axon Instruments) triggered the GABA application with a piezoelectric translator (Burleigh Instruments, LSS-3100). Following the recording, the patch was blown out, and the open tip potential recorded using solutions with a 5% difference in NaCl osmolarity to verify the approximate solution exchange time (see inset to Fig. 1A). Data from patches were analysed only for exposure times of < 2 ms duration and where the onset of agonist application times was < 300 μs.
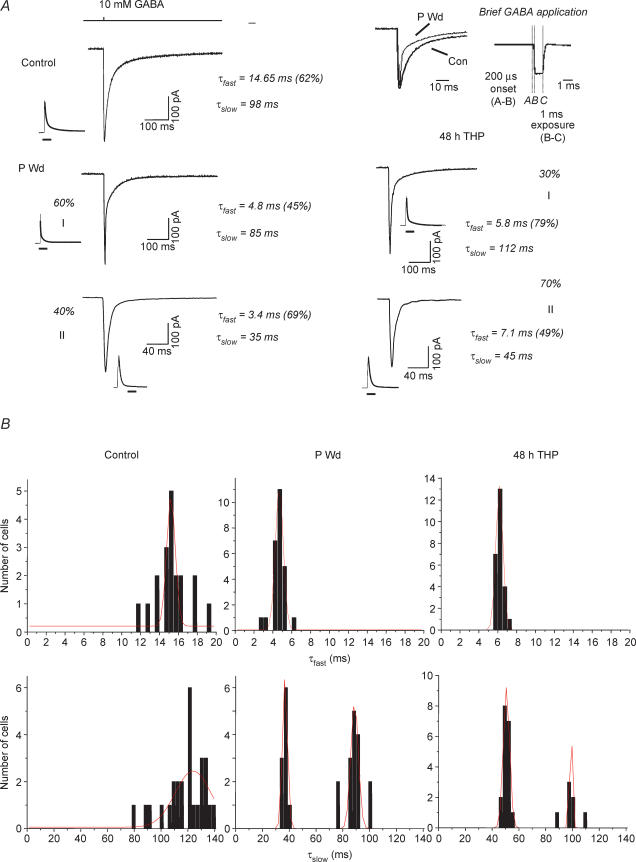
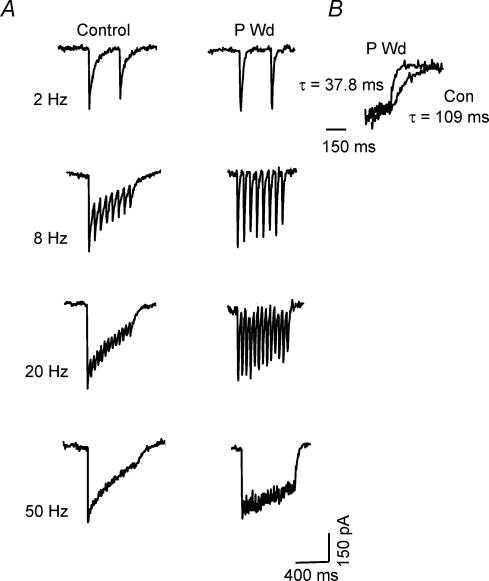
Figure 1. Progesterone withdrawal accelerates the deactivation of GABA-gated current.
A, representative traces showing responses to brief (∼ 1 ms) pulses of GABA (10 mm) recorded from outside-out patches of CA1 hippocampal pyramidal cells following progesterone withdrawal (P Wd), 48 h 3α,5β-THP (48 h THP) or sham conditions (Control). Each trace represents the average of 6–10 individual traces. (Fits are shown next to full traces.) The deactivation rate is best described as a biexponential decay, with a τfast in the range of 10–22 ms and a τslow of 80–145 ms for the control recordings. Following P withdrawal (P Wd), in Group I 60% of the current deactivated with a τfast of 3–6 ms (mean = 4.88 ± 0.61 ms), and a τslow of 80–120 ms (mean = 87.0 ± 12.0 ms). In Group II, 40% of the current recorded deactivated with a τfast of 3–7 ms, and a τslow of 30–40 ms. Forty-eight hours of treatment with 3α,5β-THP produced similar acceleration in deactivation times. Note that in both populations, τfast is significantly faster than control values, while in Group II τslow is also significantly faster than control. Average peak amplitude was unaffected by prior steroid treatment. The top trace indicates the open tip junctional current. (These results are representative of those recorded from 20 to 30 patches/group.) Inset: amplified traces illustrate an accelerated τfast following P Wd compared to control. Inset, representative open tip junction potential for a control recording. B, distribution of values for τfast and τslow for control (Con, left panels), progesterone withdrawal (P Wd, middle panels), and 48 h treatment with 3α,5β-THP (48 h THP, right panels). Values for τslow display a bimodal distribution for P Wd and 48 h THP conditions. All other distributions display a single mode.
Deactivation time constants were approximated as biexponential functions using nonlinear curve fitting routines with either Levenburg-Marquardt algorithms or the Simplex Minimization method depending on the level of background noise (Origin software, OriginLab Corp., Northampton, MA, USA). The formula I = Ife(−t/τfast)+Ise(−t/τslow) was used, where If and Is are the amplitudes of the fast and slow decay components, and τfast and τslow are their respective decay time constants. Goodness of fit was determined by minimizing the sum of the squares of deviations of the theoretical curve from the experimental points. Best fit was determined when this value was no longer improved by > 5%, with the sum of squared errors < 0.95. Averaged weighted values of τ were also determined for each case with the equation: τw =τfast (fractional amount of current) +τslow(fractional amount of current), in order to compare values of τ across steroid state. In some cases, total charge transfer was calculated by integrating the area under the curve (Origin software).
Desensitization
Desensitization in response to prolonged GABA exposure was studied by applying 5 mm GABA continuously for 400 ms or 5 s to excised outside-out patches using the piezoelectric-controlled theta tube to allow for rapid onset and offset of agonist. Desensitization was also studied using repetitive 1 ms applications of 5–10 mm GABA at different interpulse intervals to isolated pyramidal cells. As above, the open tip potential was used to verify times of agonist application and duration of exposure. The degree of desensitization following these various exposure periods was expressed as a percentage decrease from the initial GABA response. The time constant for desensitization (τdesensitization) was determined using similar non-linear curve fitting techniques as described above with two or three exponents. In this case, distinguishing between two- or three-exponential decay was accomplished using the F test (Prism statistical program, GraphPad Software Inc., San Diego, CA, USA), and best fit determined when P < 0.05 (Celentano & Wong, 1994). Deactivation following agonist removal was also evaluated as described above. As in the previous study, weighted averages of τ (τw) were used for the purposes of statistical comparison between steroid-treatment groups.
The rate of recovery from fast desensitization was determined using paired 1 ms pulses of 10 mm GABA applied at interpulse intervals of 20, 70, 120, 240, 360, 500, 1000 and 2000 ms to outside-out patches (Jones & Westbrook, 1995; Bai et al. 1999). The amplitude of the second response was compared to that of the first (see Fig. 5) and adjusted for the baseline offset. Recovery was a biexponential function and time constants were calculated as described above.
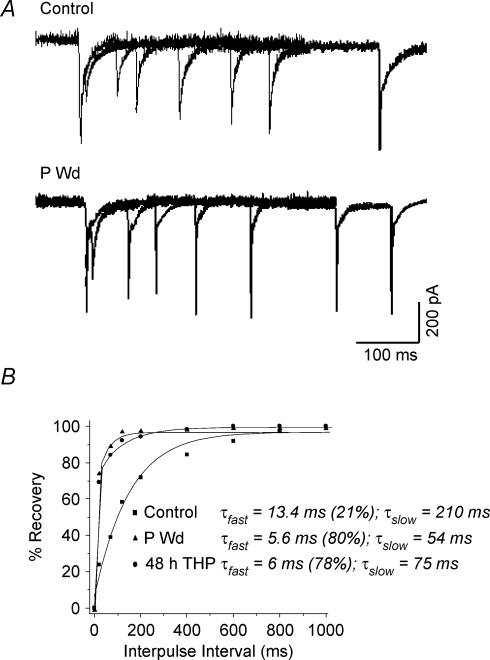
Figure 5. Recovery from fast desensitization is accelerated following progesterone withdrawal.
A, superimposed currents gated by paired 1 ms pulses of 10 mm GABA, at varying interpulse intervals (20– 2000 ms) are depicted for control, progesterone withdrawal (P Wd) or 48 h treatment with 3α,5β-THP (48 h THP). Following both steroid protocols, the extent of fast desensitization was reduced, and the rate of recovery from this desensitized state was accelerated compared to control as determined by the amplitude of the second GABA response relative to the first. B, the percentage recovery of current amplitude for the second GABA response relative to the first represents recovery from the fast desensitized state (Dfast), and is plotted as a function of the interpulse interval for averaged datapoints from both groups. Percentage recovery was calculated as ((Amptest− Onsettest)/(Ampinit− Onsettest)) × 100, where Ampinit is the amplitude of the initial GABA response, Amptest is the amplitude of the second (test) GABA response, and Onsettest is the value of the current at the onset of the second response. In all cases, the amplitude of the initial response was normalized to its maximal value during the experiment to account for variability in current. Each point represents the average from 8 to 10 different patches from 5 to 6 animals. The rate of recovery was best fitted by a biexponential equation, which was markedly faster following the steroid treatment protocols compared to control (P < 0.05). (n = 8–10 samples per point).
Kinetic modelling
In order to investigate which microscopic parameters might produce the differences in macroscopic current observed experimentally, rate constants for agonist binding, desensitization and gating were estimated using a simplified version of the Jones-Westbrook model, which contained a single open state and two desensitized states (Fig. 6A). Biliganded binding was simplified as a single step to reduce the number of free parameters. Starting values for the rate constants for kon, koff, α and β were initially based on values derived from single channel studies published by other groups, which were used for models designed to simulate control (Model I, Bai et al. 1999; Jones & Westbrook, 1995; Mozrzymas et al. 2003; Shen et al. 2000), or steroid withdrawal conditions (Models III and IV, Akk et al. 2004; Fisher & Macdonald, 1997; Haas & Macdonald, 1999). Initial estimates of the forward (df, ds) and reverse (rf, rs) rate constants (fast (f) and slow (s)) for desensitization were derived from values (time constants and extents of desensitization) obtained in the present study by solving the simultaneous equations:
as described in Celentano & Wong (1994). Forward and reverse rate constants for slow desensitization were determined by first estimating the fraction of current in the slow desensitized state under steady state conditions. Then, forward and reverse rate constants were derived using the equations:
where FDS is the fraction of receptors in the slow desensitized state, FC is the fraction of receptors in the closed state under steady state conditions. FC* is the fraction of current in the closed state at equilibrium with Dfast.
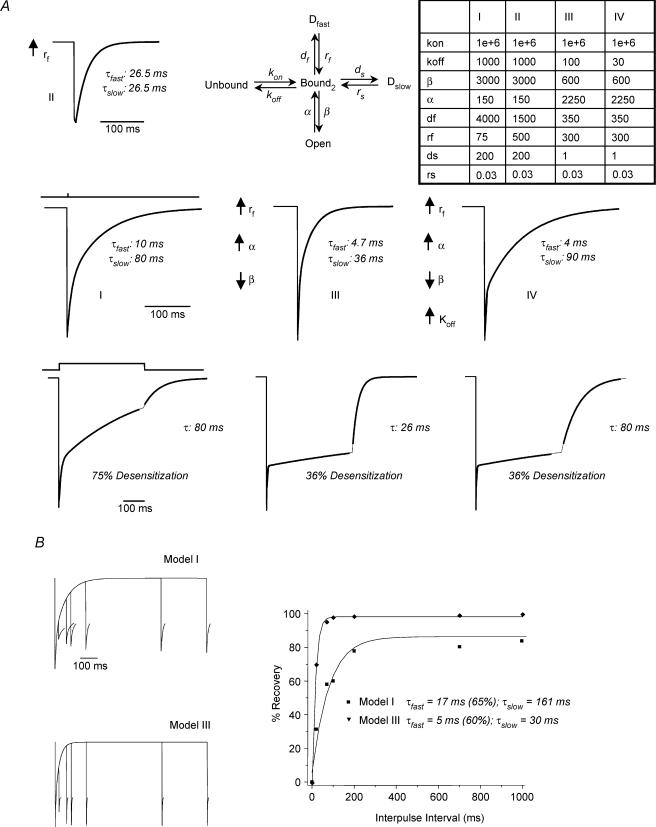
Figure 6. Computer simulation of GABAR gating following steroid treatment.
A simplified biliganded model (A, upper part, centre), based on Jones & Westbrook (1995) and Celentano & Wong (1994) was modified to simulate the data from this study. It includes one open state and two desensitized states, with rate constants (table, upper right) derived from single channel data, modified from other models and approximated from desensitization data from the present study. A, Model I results in simulated current with deactivation and desensitization kinetics similar to that from control hippocampal pyramidal cell patches. Incorporation of a more rapid rate of recovery from the fast desensitized state (↑rf) markedly accelerated τslow (Model II, inset), consistent with the Jones-Westbrook model, but failed to modify τfast. Additionally, incorporation of ↑α and ↓β to replicate single channel properties of δ-containing GABAR (Model III) replicated one subpopulation of currents following steroid treatment, with acceleration in both τfast and τslow. The second population of currents recorded following steroid treatment (faster τfast only) was simulated by additionally incorporating a ↓koff (Model IV). Bottom panel, simulations resulted in markedly different rates and extent of desensitization, comparable to those obtained from control (75% desensitization) and steroid-treated animals (36% desensitization). B, rate of recovery from fast desensitization using a paired pulse protocol. Models I and III simulate the relative differences between control and P Wd data, respectively. Left, simulated traces. (The current response to the second agonist application is truncated.) Right, percentage recovery from the fast desensitized state, estimated as a biexponential decay.
These values were used as starting estimates for the rate constants which were adjusted manually to best simulate the experimentally observed currents. Simulated current responses to 1 or 400 ms application of 10 mm GABA were generated using QUB software (Dr A. Auerbach, SUNY, Buffalo; Qin et al. 1997). Once optimal rate constants were obtained, the rate of recovery from fast desensitization was also simulated using a paired pulse protocol with locally written Q-matrix software (Celentano & Hawkes, 2004).
Statistical analysis
Differences between groups were assessed using Student's t test or ANOVA followed by the Tukey's post hoc analysis, for two or multiple groups, respectively. Differences were judged to be significant when P < 0.05. The Gaussian distribution of values for each group was determined using a Chi-square analysis (Origin).
Results
GABAR deactivation rate increases following steroid withdrawal
The deactivation of GABA-gated current was determined using brief application of GABA to outside-out patches. Agonist exposure times for the analysed currents varied between 1 and 1.4 ms (1.3 ± 0.28 ms, Control; 1.4 ± 0.3 ms, P Wd; 1.32 ± 0.2 ms, 48 h 3α,5β-THP, see inset to Fig. 1A for representative open tip potential). Agonist exposure times did not differ significantly between experimental and control groups.
Under control conditions, deactivation was best fitted as a biexponential equation, with an average τfast = 15.6 ± 3.8 ms (mean ± s.e.m.), which represented 55% of the current, and a τslow = 120.3 ± 12.6 ms (Fig. 1A, Table 1). Following withdrawal from P (Fig. 1A, Table 1, P Wd), the fast component of τ for GABA-gated current was significantly accelerated (τfast = 3–7 ms, P Wd, P < 0.05) compared to corresponding control values, and a Gaussian fit of the data revealed a single peak (Fig. 1B). However, values for the slow component of deactivation were distributed bimodally (r2 = 0.90, P < 0.05, Fig. 1B) following P withdrawal, which we have designated as separate groups. For one population, τslow was not significantly changed from control (τslow = 87.0 ± 12.0 ms, Group I, 60% of the population). However, for the second population, τslow was significantly accelerated compared to control values (τslow = 36.87 ± 2.5 ms, Group II, Fig. 1A and B). For these two populations, the distribution of current carried by the fast component was either unchanged (P Wd-I) or increased (P Wd-II) compared to control values (Fig. 1A).
Table 1.
Deactivation time constants following brief GABA application
| Group | Percentage of population | τfast | Percentage of total current (τfast) | τslow | Weighted τ | n |
|---|---|---|---|---|---|---|
| Control | 100 | 15.6 ± 3.8 | 55 ± 8 | 120.3 ± 12.6 | 70.1 ± 8.5 | 52 |
| P Wd | 60 | 4.88 ± 0.61* | 50 ± 10 | 87.0 ± 12.0 | 32.5 ± 1.35* | 30 |
| 40 | 4.51 ± 0.52* | 71 ± 9 | 36.87 ± 2.5* | 13.8 ± 1.09* | 20 | |
| Average | 24.8 ± 1.25* | 50 | ||||
| 48 h THP | 30 | 6.10 ± 0.78* | 76 ± 8 | 98.2 ± 10.2 | 28.2 ± 2.10* | 15 |
| 70 | 6.50 ± 0.66* | 66 ± 10 | 50.8 ± 3.2* | 21.6 ± 1.52* | 35 | |
| Average | 23.6 ± 1.76* | 50 |
Average decay time constants (in milliseconds) for the fast (τfast) and slow (τslow) components of deactivation (means ± s.e.m.), as well as the weighted values of τ. Values were assessed using brief application (∼ 1 ms) of saturating concentrations of agonist (10 mm) to outside out patches of membrane from CA1 hippocampal pyramidal cells. Hippocampal tissue from female rats was isolated following withdrawal from 21 day progesterone treatment (P Wd) or 48 h treatment with the GABA-modulatory steroid 3α,5β-THP (48 h THP, 10 mg kg−1, i.p., for 3 days). Significant decreases in τfast and the weighted τ were observed following both steroid protocols compared to control. Values for τslow exhibited a bimodal distribution following the steroid protocols (% of population indicated). (n = number of patches/group,* P < 0.05 versus control).
Forty-eight hours of exposure to 3α,5β-THP resulted in altered kinetics similar to those observed following P withdrawal: τfast was consistently accelerated compared to control values (τfast = 5–8 ms, 66–76% of the current, Fig. 1A, Table 1). As observed following P withdrawal, values for τslow displayed a bimodal distribution (r2 = 0.87, P < 0.05, Fig. 1B): Group I, τslow = 98.2 ± 10.2 ms (30% of the population) and Group II, τslow = 50.8 ± 3.2 ms (Fig. 1A and B, and Table 1). The range of deactivation time constants obtained is within the range reported for native and recombinant receptor isoforms (Banks & Pearce, 2000; Jones & Westbrook, 1995; McClellan & Twyman, 1999), including the 30–50 ms values for τslow observed after steroid treatment and withdrawal. In addition, the 10–90% rise time was slightly accelerated when GABA responses were tested following either steroid exposure protocols (0.75–0.90 ms) compared to control (1.1–1.3 ms).
When the values of τ were converted to weighted values, both P withdrawal and 48 h 3α,5β-THP exposure resulted in a similar threefold faster rate of deactivation for GABA-gated current (Table 1) compared to the control value. Thus, these results demonstrate that both steroid treatment conditions significantly decrease τ for deactivation of GABA-gated current.
GABAR subunit composition alters deactivation kinetics
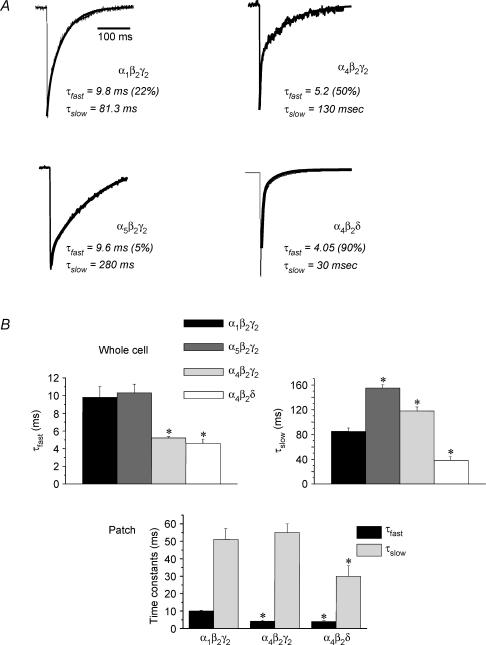
Because our previous findings have shown that a common outcome of the two steroid treatment protocols is to increase hippocampal expression of α4- and δ-containing GABARs (Smith et al. 1998a; Gulinello et al. 2001; Sundstrom-Poromaa et al. 2002), recombinant receptors were expressed in HEK-293 cells in order to compare deactivation rate as a function of subunit composition using whole cell mode and outside-out patches. As observed for native GABAR, all recombinant isoforms deactivated with a biexponential decay (Fig. 2A and B). Both α4-containing GABARs deactivated with an accelerated τfast, which was approximately 50% decreased compared to the α1β2γ2 and α5β2γ2 isoforms (P < 0.05). In addition, α4β2δ GABAR deactivated with an accelerated τslow compared to the other isoforms (P < 0.05), while α4β2γ2 deactivated with a τslow which was marginally slower than α1β2γ2. In contrast, α5β2γ2 GABAR deactivated with the slowest rate constants, displaying a τslow which was twofold greater than α4β2γ2 receptors (P < 0.05). These relative differences were similar in recordings from patches and whole cells, suggesting that the internal milieu is not required for the observed variations in kinetics associated with subunit composition. Values of τfast for all three subunit combinations were nearly identical in patches and whole cell recordings. However, values of τslow for α4β2γ2 GABAR were more prolonged in whole cell recordings compared to patches. This may reflect different states of phosphorylation or other post-translational mechanisms (Jones & Westbrook, 1997), which have been shown to alter the slow component of deactivation.
Figure 2. Kinetics of recombinant GABAA receptor isoforms.
Representative current traces (A) and averaged values (B) illustrate the different kinetics exhibited by recombinant α1β2γ2, α4β2γ2, α5β2γ2 or α4β2δ GABAR recorded from HEK-293 cells using whole cell or outside-out patch recording techniques. Both α4-containing GABAR isoforms deactivate with a faster τfast than α1 or α5-containing GABAR. (These results are averaged from 6–8 cells/group, *P < 0.05 versusα1β2γ2.)
When the area under the curve was integrated to produce a value for the total charge transfer, this value varied as predicted by the variations in deactivation τ. Total charge transfer was approximately twofold greater for α5β2γ2 GABAR compared to α1β2γ2 (1.25 ± 0.14, α5β2γ2 versus 0.7 ± 0.08, α1β2γ2, P < 0.05; all values × 106 per 500 pA). In contrast, both α4β2γ2 and α4β2δ GABARs resulted in values for total charge transfer which were significantly (P < 0.05) less than α1β2γ2 (0.46 ± 0.04, α4β2γ2; 0.265 ± 0.06, α4β2δ, P < 0.05).
GABAR desensitization – varying pulse frequency
Repetitive agonist application was used to study the effects of steroid treatment on GABAR desensitization. To this end, 1 ms pulses of GABA were applied at frequencies of 2, 8, 20 and 50 Hz (Fig. 3A). Under control conditions, a 15% desensitization was observed at frequencies as low as 2 Hz. As predicted, the degree of desensitization increased with increasing frequency of applied GABA pulses to a maximum level of 82.3 ± 15.0% desensitization at a 50 Hz GABA pulse frequency. Approximate values of τ for desensitization using this protocol were estimated as τD1 = 12–20 ms; τD2 = 180 ms. As seen for continuous agonist exposure (Table 2), desensitization in response to GABA application was markedly attenuated (P < 0.05) following P withdrawal for all frequencies, with significant desensitization beginning at 20 Hz frequencies of GABA application (Fig. 3A). Maximal desensitization (50 Hz) was 28.5 ± 5.2%, a value similar to that seen after 400 ms continuous agonist exposure (Table 2). The approximate τ for this desensitization process was 2200 ms, also similar to the τ for desensitization calculated after continuous agonist exposure (Table 2).
Figure 3. Desensitization in response to episodic agonist application is attenuated following progesterone withdrawal.
A, representative traces illustrate responses of pyramidal cells to trains of 1 ms GABA (10 mm) pulses applied at frequencies of 2, 8, 20 or 50 Hz. Following progesterone withdrawal (P Wd), desensitization developed at higher agonist application frequencies than seen for control, first apparent at 8 Hz and reaching a maximum of 18% at 50 Hz application frequencies. In contrast, under control conditions, desensitization was apparent with 2 Hz GABA pulses (200 ms interpulse interval) and reached an 84% maximum desensitization at a 50 Hz application. B, deactivation following 50 Hz GABA application was (P < 0.001) faster following P withdrawal (38.2 ± 4.3 ms) compared to control (110.2 ± 5.6 ms). (n = 12–16 cells/group).
Table 2.
Desensitization time constants
| Group | Control | P Wd | Control | 48 h THP |
|---|---|---|---|---|
| Duration of GABA application | 400 ms | 400 ms | 5 s | 5 s |
| τfast | 31.1 ± 4.05 | 97.6 ± 17.1* | 18.5 ± 4.2 | 63.2 ± 8.5* |
| % of total desensitization | 80 ± 11 | 5 ± 2 | 34 ± 5 | 18 ± 4 |
| τslow,1 | 308.6 ± 44.1 | 2888 ± 274* | 205.1 ± 31.2 | 3100 ± 280* |
| % of total desensitization | 18 ± 2 | 95 ± 1 | 14 ± 2 | 82 ± 11 |
| τslow,2 | 2156 ± 236 | |||
| % of total desensitization | 52 ± 7 | |||
| Weighted τ | 87.3 ± 21.1 | 2546 ± 433* | 1148 ± 272 | 2550 ± 265* |
| % Desensitization | 86.1 ± 7.8 | 28.5 ± 3.4* | 92.6 ± 5.6 | 36.2 ± 2.3* |
Average values of τ (means ± s.e.m. in milliseconds) calculated for multiexponential desensitization kinetics and the combined weighted τ. Values were assessed following prolonged application of 5 mm GABA (400 ms, left or 5 s, right) to outside-out patches using a theta tube. Progesterone withdrawal (P Wd) and 48 h treatment with 3α,5β-THP (48 h THP) both resulted in a slower rate of desensitization best fitted as a biexponential function compared to control, where a three-exponential fit was optimal. In addition, the extent of desensitization compared to the peak current (% desensitization) was attenuated following steroid treatment. (n = 20–25 excised patches/group, *P < 0.05 versus control values).
Using the 50 Hz GABA pulse application protocol, the rate of deactivation was also assessed following agonist washout (Fig. 3B). Deactivation following pulse agonist application was faster in ∼ 50% of the cases tested (τw = 40.2 ± 8.2 ms, P Wd versusτw = 116 ± 15.3 ms, Control, P < 0.05).
Desensitization – prolonged agonist exposure
For this study, saturating concentrations of GABA were applied continuously for either 400 ms or 5 s with rapid onset and washout of agonist provided by the piezo-electric delivery system. Both desensitization and deactivation rate constants were determined.
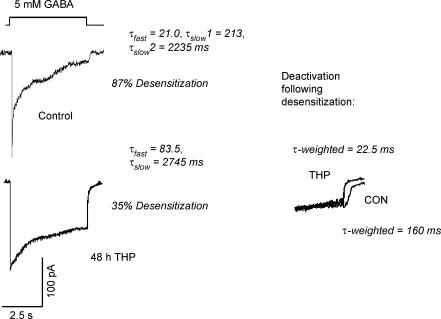
Following 48 h 3α,5β-THP exposure, the extent of desensitization in patches from CA1 hippocampal pyramidal cells exposed to 5 mm GABA for 5 s was only 36% compared with 93% in control patches (P < 0.001, Fig. 4, Table 2). In addition, the rate of desensitization was also significantly slower after steroid treatment compared to control (Fig. 4, Table 2). This comparison is more easily made with the weighted time constants: τw = 2550 ± 265 ms, 48 h 3α,5β-THP versus 1148 ± 272 ms, Control (P < 0.01). When the individual exponential components were evaluated, desensitization was best fitted as a three-exponential decay for control patches, as reported by others (Celentano & Wong, 1994; Jones & Westbrook, 1995; Haas & Macdonald, 1999; Celentano & Hawkes, 2004), while 48 h 3α,5β-THP treatment resulted in desensitization kinetics best fitted as a two-exponential decay. Both τfast and τslow,1 were significantly faster for control versus steroid treatment conditions (Table 2), with the faster values of τ representing a greater fraction of the desensitizing current for control traces. However, a smaller percentage of the cells recorded (15%) following steroid treatment exhibited a faster rate of desensitization (not shown), τw = 620 ms, than the observed control traces. Deactivation, following washout of agonist after a 5 s application, also reflected a bimodal distribution following steroid treatment, with values either faster (20–27 ms) or not significantly different (150–200 ms) from control (80–170 ms), similar to the bimodal distribution of τslow.
Figure 4. Desensitization in response to prolonged agonist exposure is attenuated following steroid treatment.
Representative traces illustrate significant attenuation in both the rate and degree of desensitization of GABA response following 48 h treatment with 3α,5β-THP (48 h THP). Desensitization kinetics (τfast, τslow) were determined for 5 s exposure to GABA (5 mm) using outside-out patches of membrane from acutely isolated CA1 hippocampal pyramidal cells following steroid treatment. Inset: deactivation following this prolonged exposure period was also significantly (P < 0.01) faster following steroid pretreatment (THP, average weighted τ= 20.45 ± 9.2 ms) compared to control (CON, average weighted τ= 148.11 ± 18.8 ms). (n = 20–25 patches/group).
Desensitization in response to continuous application of GABA for 400 ms was also attenuated following P withdrawal (Table 2): τw = 2545.6 ± 433 ms, compared to control (τw = 87.3 ± 21.1 ms), with the desensitization τ calculated independently of the slowest component (τslow,2). This shorter exposure time is sufficient to compare the fastest two components of desensitization across control and experimental groups. In this case, the difference in decay time constants observed across steroid treatment groups is highly significant (P < 0.001), suggesting that the greatest difference in decay times for desensitization is observed during the initial period of desensitization for P withdrawal versus control. After P withdrawal, the extent of desensitization was markedly attenuated to about 29% of the maximal GABA-gated current, compared to an 86% desensitization observed under control conditions (Table 2), similar to results obtained with repetitive agonist application (Fig. 3A). Deactivation following removal of agonist after sustained administration was also faster after P withdrawal (τw = 27 versus 79 ms, control, P < 0.05), as predicted by deactivation kinetics following brief exposure (Fig. 1A, Table 1). Thus, both steroid administration protocols decreased the rate and extent of desensitization compared to control conditions.
Recovery from fast desensitization
According to the Jones-Westbrook GABAR model (1995) an observed change in the τslow of deactivation could be a result of a change in the rate of recovery from fast desensitization. Therefore, we assessed this parameter in outside-out patches by measuring the current response to the second of two paired 1 ms pulses of 5–10 mm GABA delivered at varying interpulse intervals (Fig. 5A).
Using this protocol, the rate of recovery from fast desensitization was significantly accelerated following P withdrawal or 48 h 3α,5β-THP exposure (Fig. 5A and B) compared to control. The time course of recovery was best described by a biexponential function, as has been reported previously (Jones & Westbrook, 1995). Values of both τfast and τslow were significantly reduced following the steroid treatment protocols compared to control (τfast = 13.4 ± 2.0 ms, control, versusτfast = 5.6 ± 0.67 ms, P Wd and τfast = 6.0 ± 0.41 ms, 48 h 3α,5β-THP, P < 0.05; τslow = 210 ± 31.3 ms, control, versus τslow = 54 ± 4.8 ms, P Wd and τslow = 75 ± 6.2 ms, 48 h 3α,5β-THP, P < 0.05). In addition, a greater fraction of the current was carried by the fast component following the steroid protocols (78–80%, P Wd and 48 h 3α,5β-THP, versus 21%, control).
Kinetic modelling
In order to explore the microscopic parameters which might predict the observed change in macroscopic kinetics obtained following steroid treatment, a biliganded model of the GABAR was employed, based on Jones & Westbrook (1995) and Celentano & Wong (1994), but simplified to reduce the number of free parameters. Briefly, the model included the predominant biliganded state with a single open state as well as fast and slow desensitized states. In order to reduce the number of free parameters, the binding of two molecules of GABA was reduced to a single binding step. Rate constants were approximated from values reported in a number of studies (Jones & Westbrook, 1995; Fisher & Macdonald, 1997; Bai et al. 1999; Haas & Macdonald, 1999; Shen et al. 2000; Mozrzymas et al. 2003) and combined with those approximated here from the desensitization studies. Computer simulations of macroscopic current in response to a 1 ms pulse of agonist (Fig. 6A) resulted in a deactivation similar to the control traces (Fig. 1A) with a biexponential decay: τfast = 10 ms; τslow = 80 ms (Model 1, Fig. 6A). Desensitization in response to a 400 ms pulse of agonist resulted in a 75% desensitization, with a τfast = 9 ms; τslow = 379 ms, approximating the control values reported in Fig. 4. Deactivation following desensitization was identical to τslow, 80 ms.
A number of alterations in the rate constants were tested to determine which would predict a deactivation and desensitization time course similar to that observed following steroid exposure. The Jones-Westbrook model predicts that a faster recovery from the fast desensitized state, as observed following steroid treatment, would accelerate the slow component of deactivation. Amending the control model to incorporate an increased rate of recovery from Dfast, rf (Model II, Fig. 6A) indeed yielded a faster τslow of deactivation, but was insufficient to model the steroid data. This current appeared to exhibit a monoexponential decay, because τfast was equal to τslow under these conditions. In contrast, for the biological data, τfast was consistently faster than τslow. Because, we have shown increased expression of α4βδ GABAR following P withdrawal (Sundstrom-Poromaa et al. 2002), we also incorporated a decrease in α and an increase in β, to approximate values derived from single channel recordings of δ-containing GABAR (Fisher & Macdonald, 1997; Haas & Macdonald, 1999; Akk et al. 2004). This combination of changes to the model yielded a current response to a 1 ms application of agonist which closely replicated current deactivation following steroid treatment, with acceleration in both τfast and τslow (4.7 ms and 36 ms, respectively, Fig. 6A). Use of such a model also effectively reflected current desensitization where the extent (36%versus 75%, respectively) and rate of desensitization were reduced compared to control (Fig. 6A). In the absence of a significant fast desensitized state, prolongation of τslow could be accomplished with incorporation of a slower koff (Model IV, Fig. 6A), consistent with the findings of Chang & Weiss (1999), and reflecting the second population of currents recorded following steroid treatment (τfast = 4 ms, τslow = 90 ms). Values for the 10–90% rise time using these simulations closely corresponded to those obtained with patch recordings (0.8–0.9 ms, Models III and IV versus 1.2 ms, Model I).
Models I and III were also tested for their ability to simulate the rate of recovery from fast desensitization for control and steroid withdrawal conditions, respectively (Fig. 6B). These simulations approximated the actual data in their relative rates of recovery, estimated as biexponential decays: Model I produced values of τfast and τslow for recovery which were markedly slower than calculated for Model III. Values for the 80% recovery time estimated for Models I and III (approximately 220 and 30 ms, respectively) were close to values estimated from the data for control and P withdrawal conditions (approximately 270 and 30 ms, respectively). Thus, these models were successful in approximating deactivation, desensitization and recovery from fast desensitization under control and steroid withdrawal conditions. Therefore, the models most successful in simulating steroid withdrawal kinetics incorporated both an accelerated rate of recovery from fast desensitization, as well as shorter mean open times consistent with increased expression of δ-containing GABAR.
Discussion
The results from this study indicate that steroid withdrawal alters the kinetics of GABA-gated current in pyramidal cells of CA1 hippocampus, producing faster deactivation and slower desensitization. Similar changes in kinetics were observed following 48 h exposure to 3α,5β-THP. We suggest that the decrease in deactivation τ, which would decrease the total charge transfer for inhibitory current, may contribute to the neuronal excitability which characterizes steroid withdrawal (Smith et al. 1998a; Hsu & Smith, 2003), and is similar to the withdrawal hyperexcitability of other GABA-modulatory compounds (Xie & Tietz, 1991; Kang et al. 1996).
One possible mechanism for the decrease in τfast produced by steroid administration and withdrawal is via α4-containing GABARs, which we have shown are increased by these steroid protocols (Smith et al. 1998a; Gulinello et al. 2001). Results from the present study demonstrate that recombinant α4β2δ and α4β2γ2 GABARs deactivate with an accelerated τfast, compared to α1β2γ2 and α5β2γ2, which are normally highly expressed in CA1 hippocampal pyramidal cells (Wisden et al. 1992; Nusser et al. 1996; Crestani et al. 2002; Liang et al. 2004). We have previously shown that 48 h treatment with 3α,5β-THP also results in miniature inhibitory postsynaptic currents (mIPSCs) which deactivate with an accelerated τfast (Hsu et al. 2003). In this study, τfast was prolonged when α4 expression was suppressed with in vivo antisense treatment, suggesting that increased expression of α4-containing GABAR resulted in this change in kinetics (Hsu et al. 2003). Other conditions, such as ethanol withdrawal, which increase hippocampal α4 expression also produce acceleration in the τfast of mIPSCs (Cagetti et al. 2003). In a separate study, suppression of α4 expression prevented the faster decay of GABA-gated current recorded following P withdrawal, which was assessed using slower agonist exposure times (Smith et al. 1998a). Taken together, these findings support the idea that increases in α4 expression underlie the faster deactivation τ observed in the present study after steroid exposure and withdrawal.
A number of reports have demonstrated that α2βγ2 (Lavoie et al. 1997), α3βγ2 (Gingrich et al. 1995) and α6βγ2 (Tia et al. 1996) GABARs deactivate more slowly than α1β2γ2. Taken together with the present data, these findings suggest that α4-containing GABARs may exhibit the fastest deactivation rates of the diverse population of GABAR subunit combinations which have been evaluated to date.
Unlike τfast, values for τslow, assessed in the present study, displayed a bimodal distribution following steroid treatment. This bimodal pattern could be a result of two different GABAR isoforms with altered kinetics or result from state-dependent changes in a single isoform. The first possibility is more likely, as the α4βδ and α4βγ2 receptors, which are increased by these steroid administration protocols (Smith et al. 1998a; Sundstrom-Poromaa et al. 2002), were shown in the present study to deactivate with slow components similar to the two modes distinguished after steroid treatment. Alternatively, recent reports have identified modal gating patterns in ligand-gated receptors such as NMDA and acetylcholine receptors (Naranjo & Brehm, 1993; Popescu & Auerbach, 2003), which result from transitions of the fully liganded receptor to open and closed states. In these studies, modal gating at the single channel level produced alterations in decay of synaptic current comparable to the bimodal distribution reported here. Modal gating cannot be ruled out in the present study, where it could result from spontaneous thermodynamic changes in a single GABAR isoform or post-translational mechanisms.
The GABAR kinetics recorded after steroid treatment and withdrawal are similar to those reported for δ-containing GABARs which deactivate more quickly and exhibit much less desensitization (Haas & Macdonald, 1999; Bianchi et al. 2001; Brown et al. 2002) than most commonly expressed GABAR subtypes in CA1 hippocampus (Gingrich et al. 1995; Burgard et al. 1996; Lavoie et al. 1997; Haas & Macdonald, 1999; Bianchi et al. 2001), although variations in αβγ2 kinetics have been reported (Boileau et al. 2003). δ-Containing GABARs also desensitize with a two-exponential decay (Haas & Macdonald, 1999; Bianchi et al. 2001) similar to the desensitization kinetics we report here following steroid treatment. This contrasts with the three-exponential decay (Celentano & Wong, 1994; Haas & Macdonald, 1999; Celentano & Hawkes, 2004) reported for desensitization of native hippocampal GABA-gated currents and recombinant α1βγ2 GABAR. These similarities between our kinetic findings following steroid treatment/withdrawal and those exhibited by α4βδ and α4βγ2 GABARs suggest that these receptors may mediate the faster deactivation and slower desensitization observed following steroid exposure and withdrawal.
In determining the microscopic rate constants which might change in order to produce the macroscopic changes observed after steroid treatment, we implemented a receptor model. Although multiple models for GABAR binding and gating have been proposed (Jones & Westbrook, 1995; Lavoie et al. 1997; Bianchi et al. 2001; Burkat et al. 2001; Weiss & Magleby, 2001; Chang et al. 2002; Mozrzymas et al. 2003; Celentano & Hawkes, 2004), the model of Jones & Westbrook (1995) is useful in relating deactivation and desensitization rates. Increases in the fast desensitized state of the receptor prolong deactivation, by delaying unbinding and subsequent relaxation of the channel. Both steroids and anaesthetics can prolong deactivation by delaying recovery from this fast desensitized state (Zhu & Vicini, 1997; Bai et al. 1999; Banks & Pearce, 1999; Shen et al. 2000). In the present study, the accelerated rate of recovery from Dfast after the steroid protocols reduced τslow in the model, but required additional reductions in β, to reflect the decreased mean open time and reduced open probability for δ-containing GABAR, to accurately simulate the data. In contrast to τfast, the bimodal population of values for τslow may be reflected by differences in koff, in agreement with recent studies (Chang & Weiss, 1999). These changes would stabilize the bound, activatable state of the receptor, which may then yield a receptor which is highly modifiable, as demonstrated for δ-containing GABAR, which have increased sensitivity to ethanol (Sundstrom-Poromaa et al. 2002; Wallner et al. 2003) and neurosteroids (Bianchi et al. 2002; Stell et al. 2003).
Consistent with the modelling results, single channel recording experiments have established a mean open time for α4β2γ2 GABAR (Akk et al. 2004) which is approximately one-half of that reported for α1βγ2 receptors (Fisher & Macdonald, 1997). δ-Containing GABARs, including α4β2δ, have a yet lower open probability than α4β2γ2 (Akk et al. 2004) and lack the longest open channel time that is reported for α1βγ2, resulting in a mean open time only one-third that established for α1βγ2 (Fisher & Macdonald, 1997; Haas & Macdonald, 1999; Bianchi et al. 2001; Bianchi et al. 2002).
The currents recorded in the present study may largely represent currents gated by extrasynaptic receptors. In fact, α4βδ GABARs, which are increased by steroid treatment/withdrawal, are extrasynaptic or perisynaptic (Wei et al. 2003). Under conditions of increased activity from GABAergic afferents, spillover (Wei et al. 2003) from adjacent synapses may then access the peri-synaptic receptor population, to act as a resistive shunt to decrease excitability (Brickley et al. 2001; Bai et al. 2001; Hamann et al. 2002; Nusser & Mody, 2002; Wei et al. 2003; Yeung et al. 2003). In the absence of a change in the total GABAR population, as suggested by similar peak current amplitudes following steroid treatment/withdrawal (Smith et al. 1998a), steroid-induced increases in these extrasynaptic GABARs which deactivate quickly, would result in less inhibition during transient spill-over events (Wei et al. 2003) than would slower deactivating GABARs (such as α5βγ2) found under control conditions (Crestani et al. 2002; Caraiscos et al. 2004). However, following steroid withdrawal, more prolonged activation of GABARs at sites distant from the synapse would increase inhibition due to their relative lack of desensitization. Thus, the effect of altered GABAR kinetics observed after steroid withdrawal may depend upon the ambient activity of inhibitory afferents to individual pyramidal cells which would determine the time course of transmitter exposure.
Conclusions
In conclusion, the results from the present study suggest that 48 h exposure to and withdrawal from the GABA-modulatory steroid 3α,5α/β-THP produces GABARs which deactivate quickly, due at least in part to a decrease in the fast desensitized state. The resulting decrease in inhibition may serve as a mechanism for alterations in mood, susceptibility to seizures and CNS activation associated with fluctuations in endogenous steroids across the menstrual cycle.
Acknowledgments
This work was supported by NIH grants DA09618 and AA12958 to S.S.S. The authors are grateful to Y. Ruderman for expert technical assistance and to A. Auerbach for the QUB program. We thank J. J. Celentano and R. A. Pearce for a critical reading of the manuscript, and we also thank J. J. Celentano for assistance in simulating the paired pulse protocol.
References
- Akk G, Bracamontes J, Steinbach JH. Activation of GABAA receptors containing the α4 subunit by GABA and pentobarbital. J Physiol. 2004;556:387–399. doi: 10.1113/jphysiol.2003.058230. 10.1113/jphysiol.2003.058230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backstrom T. Epileptic seizures in women related to plasma estrogen and progesterone during the menstrual cycle. Acta Neurol Scand. 1976;54:321–347. doi: 10.1111/j.1600-0404.1976.tb04363.x. [DOI] [PubMed] [Google Scholar]
- Bai D, Pennefather P, MacDonald J, Orser BA. The general anesthetic propofol slows deactivation and desensitization of GABAA receptors. J Neurosci. 1999;19:10635–10646. doi: 10.1523/JNEUROSCI.19-24-10635.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai D, Zhu G, Pennefather P, Jackson MF, Macdonald JF, Orser BA. Distinct functional and pharmacological properties of tonic and quantal inhibitory postsynaptic currents mediated by γ-aminobutyric acidA receptors in hippocampal neurons. Mol Pharmacol. 2001;59:814–824. doi: 10.1124/mol.59.4.814. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Dual actions of volatile anesthetics on GABAA IPSCs: dissociation of blocking and prolonging effects. J Neurosci. 1999;90:120–134. doi: 10.1097/00000542-199901000-00018. [DOI] [PubMed] [Google Scholar]
- Banks MI, Pearce RA. Kinetic differences between synaptic and extrasynaptic GABAA receptors in CA1 pyramidal cells. J Neurosci. 2000;20:937–948. doi: 10.1523/JNEUROSCI.20-03-00937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belelli D, Bolger MB, Gee KW. Anticonvulsant profile of the progesterone metabolite 5 alpha-pregnan-3 alpha-ol-20-one. Eur J Pharmacol. 1989;166:325–329. doi: 10.1016/0014-2999(89)90077-0. [DOI] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Structural determinants of fast desensitization and desensitization-deactivation coupling in GABAa receptors. J Neurosci. 2001;21:1127–1136. doi: 10.1523/JNEUROSCI.21-04-01127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi MT, Haas KF, Macdonald RL. Alpha1 and alpha6 subunits specify distinct desensitization, deactivation and neurosteroid modulation of GABAA receptors containing the delta subunit. Neuropharm. 2002;43:492–502. doi: 10.1016/s0028-3908(02)00163-6. [DOI] [PubMed] [Google Scholar]
- Bitran D, Hilvers RJ, Kellog CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: Endogenous metabolites of progesterone that are active at the GABAA receptor function. Brain Res. 1993;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Baur R, Sharkey LM, Sigel E, Czajkowski C. The relative amount of cRNA coding for gamma2 subunits affects stimulation by benzodiazepines in GABAA receptors expressd in Xenopus oocytes. Neuropharm. 2002;43:695–700. doi: 10.1016/s0028-3908(02)00036-9. [DOI] [PubMed] [Google Scholar]
- Boileau AJ, Benkwitz C, Czajkowski C, Pearce RA. Effects of gamma2S subunit incorporation on GABA-A receptor macroscopic kinetics. Neuropharm. 2003;44:1003–1012. doi: 10.1016/s0028-3908(03)00114-x. [DOI] [PubMed] [Google Scholar]
- Brickley SG, Revilla V, Cull-Candy SG, Wisden W, Farrant M. Adaptive regulation of neuronal excitability by a voltage-independent potassium conductance. Nature. 2001;409:88–92. doi: 10.1038/35051086. [DOI] [PubMed] [Google Scholar]
- Brown N, Kerby J, Bonnert TP, Whiting PJ, Wafford KA. Pharmacological characterization of a novel cell line expressing human alpha4 beta3 delta GABAA receptors. Br J Pharmacol. 2002;136:965–974. doi: 10.1038/sj.bjp.0704795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgard EC, Tietz EI, Neelands TR, Macdonald RL. Properties of recombinant gamma-aminobutyric acid A receptor isoforms containing the alpha 5 subunit subtype. Mol Pharmacol. 1996;50:119–127. [PubMed] [Google Scholar]
- Burkat P, Yang J, Gingrich K. Dominant gating governing transient GABAA receptor activity: a first latency and Po/o analysis. J Neurosci. 2001;21:7026–7036. doi: 10.1523/JNEUROSCI.21-18-07026.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cagetti E, Liang J, Spigelman I, Olsen RW. Withdrawal from chronic intermittent ethanol treatment changes subunit composition, reduces synaptic function, and decreases behavioral responses to positive allosteric modulators of GABA-A receptors. Mol Pharmacol. 2003;63:53–64. doi: 10.1124/mol.63.1.53. [DOI] [PubMed] [Google Scholar]
- Caraiscos VB, Elliott EM, You-Ten KE, Cheng VY, Belelli D, Newell JG, Jackson MF, Lambert JJ, Rosahl TW, Wafford K, MacDonald JF, Orser BA. Tonic inhibition in mouse hippocampal CA1 pyramidal neurons is mediated by alpha 5 subunit-containing gamma-aminobutyric acid type A receptors. Proc Natl Acad Sci U S A. 2004;101:3662–3667. doi: 10.1073/pnas.0307231101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano JJ, Hawkes AG. Use of the covariance matrix in directly fitting kinetic parameters: Application to GABA-A receptors. Biophys J. 2004;87:276–294. doi: 10.1529/biophysj.103.036632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celentano JJ, Wong RKS. Multiphasic desensitization of the GABAA receptor in outside-out patches. Biophys J. 1994;66:1039–1050. doi: 10.1016/S0006-3495(94)80885-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Ghanasah E, Chen Y, Ye J, Weiss DS. Desensitization mechanism of GABA receptors revealed by single oocyte binding and receptor function. J Neurosci. 2002;22:7982–7990. doi: 10.1523/JNEUROSCI.22-18-07982.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Weiss DS. Channel opening locks agonist onto the GABAC receptor. Nat Neurosci. 1999;2:219–225. doi: 10.1038/6313. 10.1038/6313. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy J-M, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha-5 GABAA receptors. Proc Natl Acad Sci U S A. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Sieghart W, Morrow AL. Bidirectional alterations of GABAA receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem. 1997;69:126–130. doi: 10.1046/j.1471-4159.1997.69010126.x. [DOI] [PubMed] [Google Scholar]
- Endicott J, Amsterdam J, Eriksson E, Frank E, Freeman E, Hirschfeld R, Ling F, Parry B, Pearlstein T, Rosenbaum J, Rubinow D, Schmidt P, Severino S, Steiner M, Stewaart D, Thys-Jacobs S. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med. 1999;8:663–679. doi: 10.1089/jwh.1.1999.8.663. [DOI] [PubMed] [Google Scholar]
- Fisher JL, Macdonald RL. Single channel properties of recombinant GABAA receptors containing gamma 2 or delta subtypes expressed with alpha 1 and beta 3 subtypes in mouse L929 cells. J Physiol. 1997;505:283–297. doi: 10.1111/j.1469-7793.1997.283bb.x. 10.1111/j.1469-7793.1997.283bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follesa P, Cagetti E, Mancuso L, Biggio F, Manca A, Maciocco E, Massa F, Desole M, Carta M, Busonero F, Sanna E, Biggio G. Increase in expression of the GABAA receptor alpha4 subunit gene induced by withdrawal of, but not by long-term treatment with, benzodiazepine full or partial agonists. Brain Res Mol Brain Res. 2001;92:138–148. doi: 10.1016/s0169-328x(01)00164-4. 10.1016/S0169-328X(01)00164-4. [DOI] [PubMed] [Google Scholar]
- Frye CA. The neurosteroid 3α,5α-THP has antiseizure and possible neuroprotective effects in an animal model of epilepsy. Brain Res. 1995;696:113–120. doi: 10.1016/0006-8993(95)00793-p. 10.1016/0006-8993(95)00793-P. [DOI] [PubMed] [Google Scholar]
- Gingrich KL, Roberts WA, Kass RS. Dependence of the GABAA receptor gating kinetics on the alpha subunit isoform: implications for structure-function relations and synaptic transmission. J Physiol. 1995;489:529–543. doi: 10.1113/jphysiol.1995.sp021070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulinello M, Gong QH, Li X, Smith SS. Short-term exposure to a neuroactive steroid increases α4 GABAA receptor subunit levels in association with increased anxiety. Brain Res. 2001;910:55–66. doi: 10.1016/s0006-8993(01)02565-3. 10.1016/S0006-8993(01)02565-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas KF, Macdonald RL. GABAA receptor subunit gamma2 and delta subtypes confer unique kinetic properties on recombinant GABAA receptor currents in mouse fibroblasts. J Physiol. 1999;514:27–45. doi: 10.1111/j.1469-7793.1999.027af.x. 10.1111/j.1469-7793.1999.027af.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann M, Rossi DJ, Attwell D. Tonic and spillover inhibition of granule cells control information flow through cerebellar cortex. Neuron. 2002;33:625–633. doi: 10.1016/s0896-6273(02)00593-7. 10.1016/S0896-6273(02)00593-7. [DOI] [PubMed] [Google Scholar]
- Herzog AG, Klein P, Ransil BJ. Three patterns of catamenial epilepsy. Epilepsia. 1997;38:1082–1088. doi: 10.1111/j.1528-1157.1997.tb01197.x. [DOI] [PubMed] [Google Scholar]
- Hevers W, Luddens H. The diversity of GABAA receptors. Pharmacological and electrophysiological properties of GABAA channel subtypes. Mol Neurobiol. 1998;18:35–86. doi: 10.1007/BF02741459. [DOI] [PubMed] [Google Scholar]
- Holt RA, Bateson AN, Martin IL. Chronic treatment with diazepam or abecarnil differently affects the expression of GABAA receptor subunit mRNAs in the rat cortex. Neuropharm. 1996;35:1457–1463. doi: 10.1016/s0028-3908(96)00064-0. 10.1016/S0028-3908(96)00064-0. [DOI] [PubMed] [Google Scholar]
- Hsu F-C, Smith SS. Progesterone withdrawal reduces paired-pulse inhibition in rat hippocampus: Dependence on GABA-A receptor alpha-4 upregulation. J Neurophysiol. 2003;89:186–198. doi: 10.1152/jn.00195.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu F-C, Waldeck R, Faber DS, Smith SS. Neurosteroid effects on GABAergic synaptic plasticity in hippocampus. J Neurophysiol. 2003;89:1929–1940. doi: 10.1152/jn.00780.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Shaping of IPSCs by endogenous calcineurin activity. J Neurosci. 1997;17:7626–7633. doi: 10.1523/JNEUROSCI.17-20-07626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Spigelman I, Sapp DW, Olsen RW. Persistent reduction of GABAA receptor-mediated inhibition in rat hippocampus after chronic intermittent ethanol treatment. Brain Res. 1996;709:221–228. doi: 10.1016/0006-8993(95)01274-5. 10.1016/0006-8993(95)01274-5. [DOI] [PubMed] [Google Scholar]
- Lavoie AM, Tingey JJ, Harrison NL, Pritchett DB, Twyman RE. Activation and deactivation rates of recombinant GABAA receptor channels are dependent on α-subunit isoform. Biophys J. 1997;73:1–9. doi: 10.1016/S0006-3495(97)78280-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. Altered pharmacology of synaptic and extrasynaptic GABA-A receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther. 2004;310:1234–1245. doi: 10.1124/jpet.104.067983. 10.1124/jpet.104.067983. [DOI] [PubMed] [Google Scholar]
- Maconochie DJ, Zempel JM, Steinbach JH. How quickly can GABAA receptors open? Neuron. 1994;12:61–71. doi: 10.1016/0896-6273(94)90152-x. 10.1016/0896-6273(94)90152-X. [DOI] [PubMed] [Google Scholar]
- Mahmoudi M, Kang MH, Tillakaratne N, Tobin AJ, Olsen RW. Chronic intermittent ethanol treatment in rats increases GABAA receptor α4 subunit expression – possible relevance to alcohol dependence. J Neurochem. 1997;68:2485–2492. doi: 10.1046/j.1471-4159.1997.68062485.x. [DOI] [PubMed] [Google Scholar]
- Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- McClellan AM, Twyman RE. Receptor system response kinetics reveal functional subtypes of native murine and recombinant human GABAA receptors. J Physiol. 1999;515:711–727. doi: 10.1111/j.1469-7793.1999.711ab.x. 10.1111/j.1469-7793.1999.711ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran MH, Smith SS. Progesterone withdrawal I: Pro-convulsant effects. Brain Res. 1998;807:91–100. doi: 10.1016/s0006-8993(98)00782-3. 10.1016/S0006-8993(98)00781-1. [DOI] [PubMed] [Google Scholar]
- Mozrzymas JW, Barberis A, Mercik K, Zarnowska ED. Binding sites, singly bound states, and conformation coupling shape GABA-evoked currents. J Neurophysiol. 2003;89:871–883. doi: 10.1152/jn.00951.2002. [DOI] [PubMed] [Google Scholar]
- Naranjo D, Brehm P. Modal shifts in acetylcholine receptor channel gating confer subunit-dependent desensitization. Science. 1993;260:1811–1814. doi: 10.1126/science.8511590. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Mody I. Selective modulation of tonic and phasic inhibitions in dentate gyrus granule cells. J Neurophysiol. 2002;87:2624–2628. doi: 10.1152/jn.2002.87.5.2624. [DOI] [PubMed] [Google Scholar]
- Nusser Z, Sieghart W, Benke D, Fritschy J-M, Somogyi P. Differential synaptic localization of two major γ-aminobutyric acid type A receptor α subunits on hippocampal pyramidal cells. Proc Natl Acad Sci U S A. 1996;93:11939–11944. doi: 10.1073/pnas.93.21.11939. 10.1073/pnas.93.21.11939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G, Auerbach A. Modal gating of NMDA receptors and the shape of their synaptic response. Nat Neurosci. 2003;6:476–483. doi: 10.1038/nn1044. [DOI] [PubMed] [Google Scholar]
- Qin R, Auerbach A, Sachs R. Maximum likelihood estimation of aggregated Markov processes. Proc Roy Soc Lond B Biol Sci. 1997;264:375–383. doi: 10.1098/rspb.1997.0054. 10.1098/rspb.1997.0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapkin A, Morgan M, Goldman L, Brann D, Simone D, Mahesh V. Progesterone metabolite allopregnanolone in women with premenstrual syndrome. Obstet Gynecol. 1997;90:709–714. doi: 10.1016/S0029-7844(97)00417-1. 10.1016/S0029-7844(97)00417-1. [DOI] [PubMed] [Google Scholar]
- Saxena NC, Macdonald RL. Assembly of GABAA receptor subunits: role of the delta subunit. J Neurosci. 1994;14:7077–7086. doi: 10.1523/JNEUROSCI.14-11-07077.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen W, Mennerick S, Covey DF, Zorumski CF. Pregnenolone sulfate modulates inhibitory synaptic transmission by enhancing GABAA receptor desensitization. J Neurosci. 2000;20:3571–3579. doi: 10.1523/JNEUROSCI.20-10-03571.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SS, Gong QH. Ethanol administration rapidly reverses alpha-4 GABA-A receptor subunit upregulation following steroid exposure. Neuropharm. 2004;47:9–16. doi: 10.1016/j.neuropharm.2004.03.010. 10.1016/j.neuropharm.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Hsu F-C, Markowitz RS, ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–929. doi: 10.1038/31948. 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu F-C. Withdrawal from 3α-OH-5α-pregnan-20-one withdrawal using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stell BM, Brickley SG, Tang CY, Farrant M, Mody I. Neuroactive steroids reduce neuronal excitability by selectively enhancing tonic inhibition mediated by delta subunit-containing GABA-A receptors. Proc Natl Acad Sci U S A. 2003;100:14439–14444. doi: 10.1073/pnas.2435457100. 10.1073/pnas.2435457100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith S. Hormonally regulated α4β2δ GABAA receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tia S, Wang JF, Kotchabhakdi N, Vicini S. Distinct deactivation and desensitzation kinetics of recombinant GABA-A receptors. Neuropharmacology. 1996;35:1375–1382. doi: 10.1016/s0028-3908(96)00018-4. 10.1016/S0028-3908(96)00018-4. [DOI] [PubMed] [Google Scholar]
- Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properities of mouse spinal cord neurons in culture. J Physiol. 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. Ethanol enhances alpha 4 beta 3 delta and alpha 6 beta 3 delta gamma-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci U S A. 2003;100:15218–15223. doi: 10.1073/pnas.2435171100. 10.1073/pnas.2435171100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhang N, Peng Z, Houser CR, Mody I. Perisynaptic localization of delta subunit-containing GABAA receptors and their activation by GABA spillover in the mouse dentate gyrus. J Neurosci. 2003;23:10650–10661. doi: 10.1523/JNEUROSCI.23-33-10650.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss DS, Magleby K. Gating scheme for single GABA-activated Cl− channels determined from stability plots, dwell-time distributions, and adjacent-interval durations. J Neurosci. 2001;9:1314–1324. doi: 10.1523/JNEUROSCI.09-04-01314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisden W, Laurie DJ, Monyer H, Seeburg P. The distribution of 13 GABA-A receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X-H, Tietz EI. Chronic benzodiazepine treatment of rats induces reduction of paired-pulse inhibition in CA1 region of in vitro hippocampus. Brain Res. 1991;561:69–76. doi: 10.1016/0006-8993(91)90750-p. 10.1016/0006-8993(91)90750-P. [DOI] [PubMed] [Google Scholar]
- Yeung JY, Canning KJ, Zhu G, Pennefather P, MacDonald JF, Orser BA. Tonically activated GABA-A receptors in hippocampal neurons are high-affinity, low-conductance sensors for extracellular GABA. Mol Pharmacol. 2003;63:2–8. doi: 10.1124/mol.63.1.2. 10.1124/mol.63.1.2. [DOI] [PubMed] [Google Scholar]
- Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]