Abstract
The biophysical properties of native cardiac erg1 and recombinant HERG1 channels have been shown to be influenced by the extracellular K+ concentration ([K+]o). The erg1 conductance, for example, increases dramatically with a rise in [K+]o. In the brain, where local [K+]o can change considerably with the extent of physiological and pathophysiological neuronal activity, all three erg channel subunits are expressed. We have now investigated and compared the effects of an increase in [K+]o from 2 to 10 mm on the three rat erg channels heterologously expressed in CHO cells. Upon increasing [K+]o, the voltage dependence of activation was shifted to more negative potentials for erg1 (ΔV0.5 =−4.0 ± 1.1 mV, n = 28) and erg3 (ΔV0.5 =−8.4 ± 1.2 mV, n = 25), and was almost unchanged for erg2 (ΔV0.5 =−2.0 ± 1.3 mV, n = 6). For all three erg channels, activation kinetics were independent of [K+]o, but the slowing of inactivation by increased [K+]o was even more pronounced for erg2 and erg3 than for erg1. In addition, with increased [K+]o, all three erg channels exhibited significantly slower time courses of recovery from inactivation and of deactivation. Whole-cell erg-mediated conductance was determined at the end of 4 s depolarizing pulses as well as with 1 s voltage ramps starting from the fully activated state. The rise in [K+]o resulted in increased conductance values for all three erg channels which were more pronounced for erg2 (factor 3–4) than for erg1 (factor 2.5–3) and erg3 (factor 2–2.5). The data demonstrate that most [K+]o-dependent changes in the biophysical properties are well conserved within the erg K+ channel family, despite gradual differences in the magnitude of the effects.
Ether-à-go-go-related gene (erg) K+ channels belong to the superfamily of voltage-gated K+ channels. A number of different physiological functions of erg channels have been demonstrated (reviewed in Bauer & Schwarz, 2001) including the repolarization of the cardiac action potential (Sanguinetti et al. 1995), the frequency adaptation of action potentials in neuroblastoma cells (Chiesa et al. 1997) and the maintenance of the resting membrane potential in lactotroph cells (Barros et al. 1997; Bauer, 1998; Bauer et al. 1999). Three different rat erg channels are known which differ in their voltage dependence of activation as well as in the extent of inward rectification. Erg2 is the strongest inward rectifier activating at the most positive potential, and erg3 is the weakest inward rectifier activating at the most negative potential (Shi et al. 1997; Schledermann et al. 2001; Wimmers et al. 2001, 2002).
A huge body of information is available about the biophysical properties of the first cloned member of the erg channel family, HERG (the human erg1; Warmke & Ganetzky, 1994), and their alterations by different external ion concentrations. The sensitivity of erg1 channels to changes in the extracellular K+ concentration ([K+]o) has been investigated in detail, and in particular the paradoxical increase in erg1 current amplitude in elevated [K+]o is well documented (Sanguinetti et al. 1995; Trudeau et al. 1995; Kiehn et al. 1996; Wang et al. 1996; Zou et al. 1998) and can be clinically exploited to treat abnormally prolonged QT intervals (Compton et al. 1996; Choy et al. 1997).
Up to now, there exists no information about [K+]o-dependent changes in the biophysical properties of the two other members of the erg subfamily, erg2 and erg3. Whereas erg1 channels are expressed in various tissues, including the heart, endocrine and nervous tissue as well as smooth muscle cells (reviewed in Bauer & Schwarz, 2001), erg2 and erg3 are described as ‘nervous system-specific’ channels (Shi et al. 1997). The three erg channels are differentially expressed in the brain (Shi et al. 1997; Saganich et al. 2001; Papa et al. 2003) where they can be exposed to considerable changes in [K+]o. Abnormally high electrical activity during epileptic seizures, for example, can increase the [K+]o to values of about 10 mm (Heinemann & Dietzel, 1984; Xiong & Stringer, 1999), and prolonged anoxia can result in [K+]o of several tens of millimolar (Croning & Haddad, 1998; Stiefel & Marmarou, 2002). Therefore, we were interested in the effects of changes in [K+]o within the pathophysiological range on the biophysical properties of the three erg channels. Using the rat homologues of the erg channels, we compared the influence of a rise in [K+]o from 2 mm to 10 mm on the whole-cell erg channel conductance, and the potential dependence and time course of erg channel gating.
Recently, the unusual dependence of HERG1 channels on [K+]o has partially been explained by a blocking effect of external Na+ which is counteracted by external K+ (Numaguchi et al. 2000; Mullins et al. 2002, 2004). A comparable observation had first been made in cardiac Purkinje cells where the decrease in delayed rectifier current in low [K+]o depended on the presence of external Na+ (Scamps & Carmeliet, 1989). However, the aim of the present study was not to further analyse the molecular mechanisms underlying the paradoxical effects of [K+]o on erg channels, but to make some data on erg2 and erg3 channels available for reflections on the possible physiological role of these channels in the nervous system. Our experiments show that the unusual K+ dependence of erg1 is preserved in erg2 and erg3 channels. We demonstrate that even moderate changes in [K+]o are able to exert significant changes in the gating behaviour of all three erg channels.
Part of the results have been published in abstract form (Sturm et al. 2004).
Methods
Cell culture
Chinese hamster ovary (CHO) cells were grown in MEM medium (Gibco) containing 1% penicillin–streptomycin–glutamine (Gibco) and 10% fetal calf serum (Biother). Cells were maintained at 37°C in a water-saturated atmosphere of 95% air and 5% CO2. The medium was changed every 2–3 days and cells were passaged when they reached confluence, usually every 2 or 3 days. For microinjection and electrophysiological recordings cells were plated onto poly d-lysine-coated CELLocate grids (Eppendorf) in 35 mm plastic culture dishes (Nunc).
Molecular biology
All three rat erg subunits (erg1, Acc. No. Z96106; erg2, Acc. No. AF016192; erg3, Acc. No. AF016191) were subcloned into the pcDNA3 vector (Invitrogen) for microinjection as reported previously (Wimmers et al. 2001). The clones were transferred into CHO cells by microinjection using an InjectMan (Eppendorf). Coinjection of EGFP-N1pcDNA3, encoding a green fluorescent protein (Clontech), was used to detect cells with successful expression. The erg cDNA was injected in a concentration between 10 (erg1) and 850 ng μl−1 (erg2). After 3–8 h of incubation injected cells began to show fluorescence.
Electrophysiology
Whole-cell recordings were made within 8–20 h after injection of the clones using the nystatin-perforated-patch method. An EPC9 patch-clamp amplifier was used in combination with PULSE stimulation and data acquisition software (HEKA Elektronik). The pipette resistance ranged from 2 to 5 MΩ when filled with intracellular solution. Data were low-pass filtered at 3 kHz and compensated for both fast and slow capacity transients. Series resistance errors were compensated to at least 75%. All experiments were done at room temperature (22–25°C).
Solutions
The extracellular 2 mm K+ solution contained (mm): 143 NaCl, 2 KCl, 2 CaCl2, 2 MgCl2, 10 Hepes, 5 glucose, pH = 7.3 adjusted with NaOH. The 10 mm K+ solution had the same composition except that NaCl was replaced by equimolar concentrations of KCl. The pipette solution contained (mm): 140 KCl, 2 MgCl2, 1 CaCl2, 2.5 EGTA, 10 Hepes (pH = 7.3 adjusted with KOH). Nystatin was dissolved in DMSO (60 mg ml−1), its final concentration in the pipette solution was 0.24 mg ml−1. With respect to the standard pipette solution, the liquid junction potential was estimated to be 4.4 mV for the 2 mm K+ solution, and 4.1 mV for the 10 mm K+ solution. Data are given without correction for liquid junction potential errors because an additional undefined potential error corresponding to the Donnan potential was present in the perforated-patch whole-cell experiments.
Data analysis
The voltage dependence of activation and erg current availability was fitted with a Boltzmann equation: y= 1/[1 + exp(−(V−V0.5)/k)], where k is the slope factor and V0.5 is the potential of half-maximal erg current amplitude. The time course of activation was determined by an envelope-of-tail protocol. For erg1 and erg2, the peak current amplitudes elicited with a hyperpolarizing pulse after different durations of a depolarizing prepulse were normalized and plotted against prepulse duration. For erg3, the mean instead of the maximal tail current amplitudes were used which represent a measure of the total charge flowing through open erg channels, thus eliminating the strong effect of the large instantaneous current amplitudes induced by the hyperpolarizations starting during the initial outward current transient. Apart from the first data points, the normalized current amplitudes could be well fitted with a single exponential function. Extrapolation of the fit to zero current yielded a delay probably produced by closed–closed transitions of the erg channels (see Wang et al. 1997; Gomez-Varela et al. 2002). The time course of inactivation was fitted with a single exponential function. The time constants of recovery from inactivation were determined by fitting current traces elicited by voltage steps to potentials between +40 and −120 mV which followed a prepulse to +80 mV with the sum of two exponential functions describing recovery from inactivation as well as subsequent deactivation. The time constants of fast and slow deactivation were obtained by fitting the decay phase of current traces elicited with hyperpolarizations to potentials between −60 and −120 mV with the sum of two exponential functions. In addition to the experiments with CHO cells injected with erg channel cDNA, all sets of experiments were performed with uninjected CHO cells to determine the contribution of unspecific currents to the recorded membrane currents. Where appropriate and noted, data of current amplitudes were corrected for the contribution of the unspecific currents. Statistical significance was tested either with Student's two-tailed paired or unpaired (Fig. 6) t test and errors indicate s.e.m.
Figure 6. An increase in [K+]o slows recovery from inactivation and deactivation kinetics of erg1, erg2 and erg3 channels.
Time constants of recovery from inactivation (τrec) and fast (τfast) and slow (τslow) deactivation for erg1 (a), erg2 (b) and erg3 (c) currents determined from experiments as shown in Fig. 5. τrec (A), τfast (B) and τslow (C) as functions of the hyperpolarizing test pulse potential determined for the three erg channels in 2 mm[K+]o (filled symbols) and 10 mm[K+]o (open symbols). The number of evaluated current traces varied, mainly due to the small current amplitudes close to the reversal potentials. *P≤ 0.05 and **P≤ 0.01, significant differences with two-tailed unpaired t test.
Results
All three rat erg channels were heterologously expressed in CHO cells and exposed to an increase in [K+]o during perforated-patch whole-cell experiments which allowed long-lasting reproducible recordings of erg currents as has been found previously (Schledermann et al. 2001).
Effect of [K+]o on erg current activation
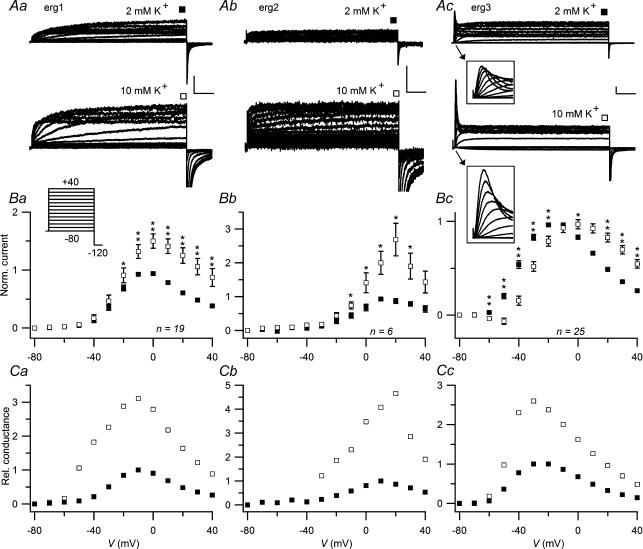
The voltage dependence of erg channel activation was studied with a double-pulse protocol. Starting from a holding potential of −80 mV at which the three erg channels are completely deactivated, 4 s variable depolarizing test pulses were applied followed by a hyperpolarization to −120 mV to determine the proportion of erg channels activated during the preceding test pulses. Figure 1 shows the effect of a rise in [K+]o from 2 to 10 mm on the outward currents of CHO cells previously injected with erg1, erg2 or erg3 cDNA. The erg current amplitudes determined at the end of the test pulses exhibited a bell-shaped voltage dependence typical for the functional inward rectification of erg channels due to increased steady-state inactivation upon stronger depolarizations and faster inactivation than activation. In the higher [K+]o, the current amplitudes were clearly increased for erg1 and erg2 at potentials equal or positive to −30 mV. For erg3, there was no clearly recognizable increase in the maximum of sustained outward current, but the potential at which the maximal erg current occurred shifted from about −20 to 0 mV. In contrast to the sustained current, the transient erg3 currents at the beginning of the test pulses increased continuously with stronger depolarizations. The rise in [K+]o induced the same effects on transient and sustained erg3 current amplitudes: they were decreased below −10 mV, and increased at potentials positive to −10 mV (see Fig. 1Ac, insets).
Figure 1. Influence of [K+]o on erg1, erg2 and erg3 currents.
Using a holding potential of −80 mV, erg current activation was measured with 4 s depolarizing pulses to potentials between −80 and +40 mV. A, comparison of erg1 (a), erg2 (b) and erg3 (c) currents recorded with 2 mm[K+]o and after a change to 10 mm[K+]o. Vertical and horizontal scale bars denote 200 pA and 500 ms, respectively. The insets in Ac show the first 100 ms on an expanded time scale. B, mean normalized current amplitudes at the end of the 4 s test pulses as a function of the test pulse potential for the three erg channels. For each experiment, current amplitudes were normalized with respect to the maximal outward current obtained in 2 mm[K+]o. C, relative conductance values, which were normalized to the maximal erg conductance obtained in 2 mm[K+]o, are plotted as a function of the test pulse potential. Absolute conductance values (G) were calculated according to the equation G = I/(V−Vrev) using the mean current amplitudes (I) shown in B and reversal potential values (Vrev) determined by voltage ramps (see Fig. 7). ▪, 2 mm[K+]o; □, 10 mm[K+]o. The number of experiments (n) is indicated; error bars denote s.e.m.*P≤ 0.05; **P≤ 0.01, significant differences with two-tailed paired t test.
To allow a comparison of the effect of increased [K+]o and to compensate for the differences in the voltage dependence of the three erg channels, the erg whole-cell chord conductances were calculated using the mean normalized steady-state erg current amplitudes (Fig. 1B) and reversal potential values observed in protocols with voltage ramps (see below). The potential at which the erg channels exhibited their maximal conductance in 2 mm[K+]o was about −10 mV for erg1, +10 mV for erg2, and around −25 mV for erg3. Upon increasing the [K+]o to 10 mm, the potential at which the maximal erg conductance occurred changed only slightly. In contrast, the amplitude of the relative erg conductance at the end of the 4 s test pulses multiplied in 10 mm[K+]o compared with 2 mm[K+]o, with a factor of about 3 for erg1, about 4.5 for erg2 and about 2.5 for erg3 (Fig. 1Ca–c).
The voltage dependence of erg channel activation in the differing [K+]o was determined from the maximal amplitude of the transient erg inward current elicited upon the constant hyperpolarization following the variable test pulses (Fig. 2A). Current increase mirrors the process of recovery from inactivation and the subsequent decay is due to erg channel deactivation. In Fig. 2B, the mean normalized erg current amplitudes were plotted versus the potential of the preceding test pulses, and the resulting data points were fitted with a Boltzmann equation yielding the potential for half-maximal isochronal (4 s) activation (V0.5) of the three erg channels. As previously described (Shi et al. 1997; Schledermann et al. 2001), the voltage dependence differed considerably for the three erg channels. In 2 mm K+ solution, the V0.5 values were for erg1, −19.4 ± 1.1 mV (n = 28); for erg2, 5.2 ± 2.1 mV (n = 6); and for erg3, −36.5 ± 1.3 mV (n = 25). After increasing [K+]o to 10 mm, the V0.5 values for the voltage dependence of activation were slightly more negative. This shift in V0.5 (ΔV0.5) amounted to −8.4 ± 1.2 mV for erg3 (n = 25, P≤ 0.0001), to −4.0 ± 1.1 mV for erg1 (n = 28, P≤ 0.001) and to −2.0 ± 1.3 mV for erg2 (n = 6, not significant). Significant differences in the steepness of the activation curves were only found for erg3 where the slope factor k decreased from 8.5 ± 0.4 mV to 6.2 ± 0.3 mV (n = 25, P≤ 0.0001).
Figure 2. A rise in [K+]o shifts the voltage dependence of erg1 and erg3 channel activation to more negative potentials.
Data from the same set of experiments as shown in Fig. 1. The maximal current amplitudes at repolarization to −120 mV served as a measure of erg channel activation during the preceding 4 s depolarizing test pulse. A, representative examples of erg1 (a), erg2 (b) and erg3 (c) currents recorded in 2 mm and 10 mm[K+]o. Vertical and horizontal scale bars denote 500 pA and 20 ms. B, mean normalized maximal current amplitudes as a function of the preceding test pulse potential for the three erg channels in 2 mm and in 10 mm[K+]o solutions. Data points were fitted with a Boltzmann equation (continuous lines) yielding the indicated potentials of half-maximal activation (V0.5). Number of experiments (n) is indicated; error bars denote s.e.m.•, 2 mm[K+]o; ○: 10 mm[K+]o. **P≤ 0.01, significant difference with two-tailed paired t test as described in the text.
Effect of [K+]o on the time course of activation
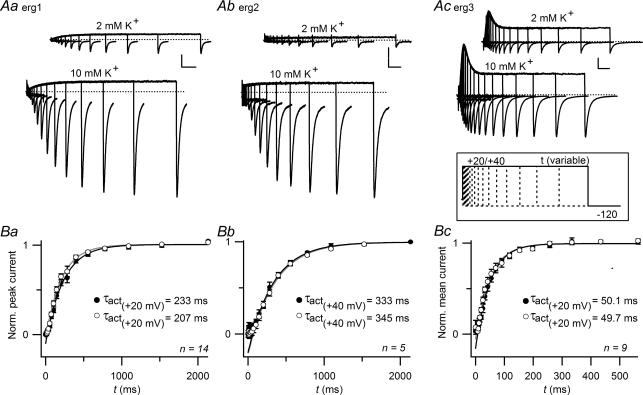
The time course of erg current activation was assessed with an envelope-of-tail protocol (Fig. 3). Due to the considerably slower activation of erg2 (Wimmers et al. 2002), a more depolarized test pulse potential of 40 mV instead of 20 mV was used for the depolarizing pulse with variable duration. The amplitudes of the inward erg currents elicited by a subsequent hyperpolarization to −120 mV increased with prepulse duration. Following a delay phase, the normalized peak amplitudes of the erg1 and erg2 tail currents could be well fitted with single exponential functions yielding the time constants of activation, τact. In contrast, the peak amplitudes of the erg3 tail currents were less well fitted with a single exponential function due to considerable differences in the percentage of inactivated channels during the course of the depolarization-induced current transient (see also Wimmers et al. 2002). Assuming that all inactivated erg channels have to pass the open state before deactivation can take place (linear gating model, Wang et al. 1997), we used the mean amplitude of the whole erg3 tail currents recorded during the 145 ms hyperpolarizations as a measure of the fraction of erg channels activated during the preceding depolarization. For all three erg channels, the rise in [K+]o produced no significant changes in the time constants of activation. For erg1 and erg3, there was a tendency to shorter delay phases in 10 mm[K+]o, but the differences were not significant.
Figure 3. Activation kinetics of erg channels do not depend on [K+]o.
A, erg1, erg2 and erg3 current traces evoked by depolarizing pulses to +20 mV (erg1 and erg3) or +40 mV (erg2) of increasing duration followed by a hyperpolarization to −120 mV. The holding potential was −80 mV. Vertical and horizontal scale bars denote 500 pA and 200 ms in Aa and Ab, and 500 pA and 50 ms in Ac. Peak amplitudes (Ba, Bb) or mean amplitudes (Bc) of erg currents elicited upon the hyperpolarizations were normalized, averaged and plotted against the duration of the preceding depolarization to 20 or 40 mV. After a delay, the erg current amplitudes could be well fitted with a single exponential function yielding the indicated time constants. Number of experiments (n) is indicated; note the different time scale for erg3. •, 2 mm[K+]o; ○, 10 mm[K+]o.
Effect of [K+]o on the inactivation of erg currents
Erg current inactivation was studied with a triple-pulse protocol according to Wang et al. (1997) and Schledermann et al. (2001). Starting from the fully activated state, a short 25 ms hyperpolarization inducing recovery from inactivation was followed by variable test pulses. For erg3, a less negative hyperpolarization to −60 mV instead of −100 mV was used taking into account the faster deactivation kinetics. During the depolarizing test pulses the erg currents decayed in a voltage-dependent manner (Fig. 4A). The decay phase was fitted by single exponential functions to obtain the time constants of inactivation (τinact; Fig. 4B). To be able to discriminate between erg channel inactivation and deactivation as the cause of the current decay, the variable test pulses were followed by an additional 25 ms hyperpolarization and a depolarization to 60 mV (data not shown). If no deactivation occurs during the variable test pulses, the instantaneous current elicited with the second depolarization to 60 mV should always be maximal. Channel deactivation was regarded as exhibiting no substantial contribution to the current decay during the 400 ms test pulses if the instantaneous current elicited with the constant depolarization to 60 mV amounted to at least 80% of the maximal current. More pronounced current deactivation occurred at test pulse potentials below −40 mV for erg1 and erg2, and below −30 mV (2 mm[K+]o) or −40 mV (10 mm[K+]o) for erg3. Nevertheless, erg3 current traces elicited with test pulses more negative than −10 mV were not fitted because they exhibited only a small inactivating component (see Fig. 4Cc). Figure 4B shows that the inactivation kinetics of all three erg channels were markedly slowed after the rise in [K+]o. This effect was more pronounced for erg2 and erg3 than for erg1.
Figure 4. A rise in [K+]o slows the inactivation of erg channels.
A, representative current traces evoked by test pulses to potentials between −80 and +60 mV following a 2 s depolarizing pulse to +20 mV with a subsequent 25 ms hyperpolarization to −100 mV (for erg1 and erg2) or −60 mV (for erg3) in cells expressing erg1, erg2 or erg3 channels. The holding potential was −20 mV. Vertical and horizontal scale bars denote 1 nA and 100 ms. B, mean time constants of inactivation (τinact) as a function of the test pulse potential for the three erg channels determined in 2 mm K+ and after a change to 10 mm K+ solution. C, voltage dependence of the ratio of the steady-state current to the maximal erg current at the onset of inactivation. *P≤ 0.05 and **P≤ 0.01, significant differences with two-tailed paired t test. The insets show the voltage dependence of the extrapolated instantaneous current (Imax) in the two different [K+]o concentrations. Filled symbols, 2 mm[K+]o; open symbols, 10 mm[K+]o.
As a measure of steady-state inactivation, the ratio of the sustained erg current to the maximal current at the onset of inactivation was determined using the values derived from the exponential fits. In accordance with previous results (Shi et al. 1997; Schledermann et al. 2001) erg3 exhibited considerably less steady-state inactivation in the voltage range studied compared to erg1 and erg2 (Fig. 4C). Upon the rise in [K+]o, steady-state inactivation was slightly reduced for all three erg channels. The increased steepness of the voltage dependence of the instantaneous current amplitudes (insets in Fig. 4C) in 10 mm[K+]o compared to 2 mm[K+]o again reflects the increased erg conductance.
Effect of [K+]o on the availability of erg currents
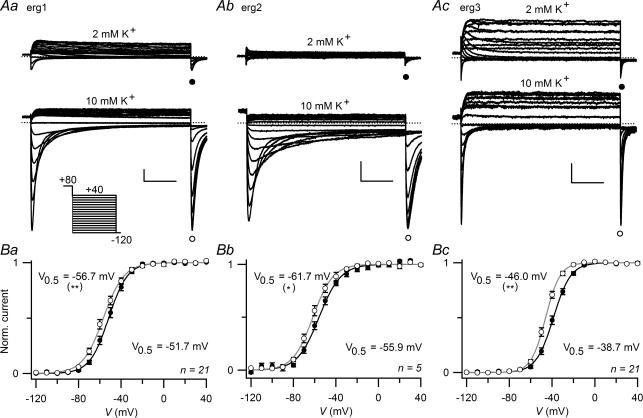
‘Fully activated’ erg currents were measured with 1 s variable hyperpolarizing test pulses following a depolarizing 400 ms prepulse to +80 mV from a holding potential of −20 mV. During the more negative test pulses, erg currents first increased due to recovery from inactivation and – at increasingly more negative test potentials – subsequently decreased as a consequence of channel deactivation. The amplitude of the transient inward currents dramatically increased with the rise in [K+]o (Fig. 5A). At the end of the test pulses, the availability of erg channels was determined with a subsequent hyperpolarizing pulse to −120 mV. Figure 5B shows the voltage dependence of current availability for the three erg channels with 2 and 10 mm K+ in the bath solution. The increase in [K+]o caused significant shifts of the availability curves to the left (erg1: ΔV0.5 =−4.9 ± 1.1 mV, n = 21, P≤ 0.001; erg2: ΔV0.5 =−5.4 ± 1.5 mV, n = 6, P≤ 0.05; erg3: ΔV0.5 =−7.2 ± 1.3 mV, n = 21, P≤ 0.001). As for the activation curves, the shift was strongest for erg3. The voltage dependence of the erg 3 availability curves was similar to the corresponding activation curves due to the considerably faster activation and deactivation kinetics of erg3 compared with the other two erg channels. For erg1 and erg2, much longer test pulses would be necessary to bring the activation and the availability curves into line as described in more detail by Schönherr et al. (1999).
Figure 5. Voltage dependence of erg current availability is slightly shifted by increased [K+]o.
A, erg1 (a), erg2 (b) and erg3 (c) currents recorded with 2 mm or 10 mm[K+]o in the bath solution. The pulse protocol consisted of variable 1 s test pulses to potentials between 40 and −120 mV in steps of 10 mV from a holding potential of −20 mV. A 400 ms depolarization to 80 mV preceded the test pulses to fully activate the erg channels. The variable test pulses were followed by a hyperpolarization to −120 mV. Vertical and horizontal scale bars denote 500 pA and 200 ms. B, the normalized maximal erg current amplitudes at the hyperpolarizing pulse to −120 mV were averaged and plotted versus the preceding test pulse potential. •, 2 mm[K+]o; ○, 10 mm[K+]o. Data points were fitted with a Boltzmann equation (continuous lines) yielding the indicated potentials of half-maximal erg current (V0.5). Number of experiments (n) is indicated. *P≤ 0.05 and **P≤ 0.01, significant differences with two-tailed paired t test as described in the text.
Time course of recovery from inactivation and deactivation
The effects of changes in [K+]o on the time course of recovery from inactivation and deactivation of erg channels were investigated by fitting current traces elicited by hyperpolarizing pulses as shown in Fig. 5A with exponential functions as described in the Methods. Due to the strong depolarizing prepulse to +80 mV, recovery from inactivation could be studied within the entire voltage range of the test pulses (−120 to +40 mV) yielding a bell-shaped voltage dependence of the respective time constant (τrec) for all three erg channels. The recovery from inactivation of erg3 channels recorded with 2 mm K+ bath solution at potentials more negative than −90 mV was too fast to yield reliable data. Like the inactivation process, the recovery from inactivation of all three erg channels was markedly slowed by the rise to 10 mm[K+]o (Fig. 6A). For erg2, only the bigger differences in the time constants at −70, −60 and 0 mV reached significance (Fig. 6Ab), which might be due to the relatively small number of experiments with erg2. Especially pronounced was the slowing of recovery for erg3 at more depolarized potentials.
The comparison of the different erg currents elicited with the hyperpolarizing pulses shown in Fig. 5 demonstrates that erg3 channels deactivate considerably faster than erg1 and erg2 channels. After the rise in [K+]o from 2 to 10 mm, the time constants of the fast as well as of the slowly deactivating current components of all three erg channels were slightly, but significantly increased (Fig. 6B and C). Given the higher amplitude of erg inward currents at more negative potentials in raised [K+]o, increased time constants could result from remaining uncompensated series resistance potential errors. However, this technical problem is not assumed to account for the observed increases in deactivation time constants of the three erg channels, since no positive correlations between absolute erg current amplitudes and corresponding values of the deactivation time constants were detected (data not shown).
Effect of [K+]o on erg whole-cell conductance
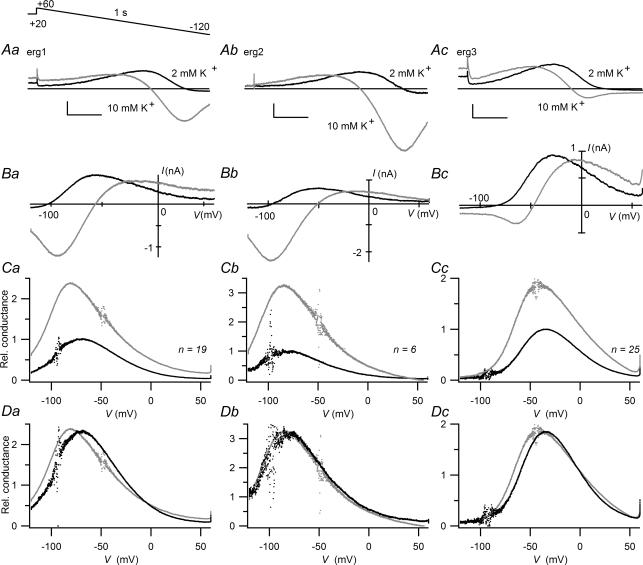
The effect of a rise in [K+]o on changes in the maximally available erg channel conductance was also investigated with 1 s voltage ramps from +60 mV to −120 mV. The holding potential was −20 mV and a depolarizing 2 s prepulse to +20 mV preceded the voltage ramp to ensure that the erg channels were in the fully activated state. During the voltage ramp, all three erg currents first increased due to recovery from inactivation and then decreased due to voltage-dependent deactivation and changes in the driving force (Fig. 7A and B). To compensate for the differences in this driving force, relative whole-cell conductance values were calculated using the observed reversal potentials. The plots of the normalized mean chord conductance values against the ramp potential demonstrate the differences in the voltage dependence of the erg channels as well as the pronounced increases in conductance produced by the rise in [K+]o (Fig. 7C). With this special pulse protocol, K+ conductance was maximal in the range of −70 to −80 mV for erg1, −80 to −85 mV for erg2, and around −40 mV for erg3. In Fig. 7D, the conductance values determined in 2 mm K+ were scaled to the corresponding data determined in 10 mm K+ using a factor of 2.3 for erg1, a factor of 3.2 for erg2 and a factor of 1.85 for erg3. After the increase in [K+]o, the voltage dependence of the erg2 conductance was almost unchanged, whereas the conductance curve for erg1 was mainly shifted to the left by about 8.5 mV, and the conductance curve for erg3 was broadened with a shift of the left part of the curve by about −7 mV.
Figure 7. A rise in [K+]o increases erg1, erg2 and erg3 whole-cell conductance.
Erg currents were elicited by a 1 s voltage ramp from 60 to −120 mV starting from the fully activated state using a holding potential of −20 mV and a 2 s prepulse to +20 mV. A, superimposition of erg1 (a), erg2 (b) and erg3 currents (c) recorded with the voltage ramp protocol in 2 mm[K+]o and after a change to 10 mm[K+]o. Vertical and horizontal scale bars denote 500 pA and 200 ms. B, erg current amplitudes as a function of the ramp voltage. Same experiments as shown in A. C, the erg whole-cell conductance was calculated using mean ramp current values which were corrected for unspecific currents measured in control CHO cells and the resulting corrected reversal potentials (Vrev in 2 mm[K+]o: −93.4, −97.5 and −92.5 mV; Vrev in 10 mm[K+]o: −49.0, −49.0 and −45.0 mV for erg1, erg2 and erg3, respectively). Conductance values were normalized with respect to the maximal mean conductance obtained in 2 mm[K+]o and plotted versus the ramp voltage. D, superimposed mean conductance curves as shown in C, with the data obtained with 2 mm[K+]o scaled to the maximal amplitude obtained with 10 mm[K+]o. Black lines or dots, 2 mm[K+]o; grey lines or dots, 10 mm[K+]o. The number of experiments (n) is indicated.
Effect of [K+]o on erg1 and erg3 currents during simulated action potential bursts
To estimate the effect of a rise in [K+]o on a possible contribution of erg1 and erg3 currents to frequency accommodation, erg1 and erg3 currents were recorded with a spike train voltage protocol (Fig. 8) which consisted of 20 voltage ramps of 5 ms duration separated by intervals of 20 ms at a plateau potential of −30 mV. Each voltage ramp was preceded by a 1 ms pulse to +30 mV to reduce capacity transients at the onset of the voltage ramps. As for all sets of experiments, uninjected CHO cells served as control. In these control cells, no significant changes in current amplitude were found during successive voltage ramps in both external solutions. To eliminate most of the contribution of capacitive transients, endogenous non-erg currents and leakage currents, data from CHO cells expressing erg channels were averaged and corrected for erg-unspecific currents recorded from control cells.
Figure 8. Erg1 and erg3 currents elicited with spike train protocols in 2 and 10 mM [K+]o.
Averages of erg1 (A,n = 7) and erg3 (B,n = 29) current traces obtained in 2 mm and 10 mm[K+]o after subtraction of unspecific currents. Membrane currents were elicited from a holding potential of −60 mV using the pulse protocol shown at the top which consisted of 20 5-ms voltage ramps from +30 to −30 mV following 1 ms pulses to +30 mV, thus simulating a burst of action potentials with a frequency of 38.5 Hz. On the right of the current traces, mean erg current amplitudes during the subsequent ramp pulses are shown as a function of the ramp voltage.
Figure 8 shows the erg1 and erg3 currents recorded during the course of the 520 ms pulse protocol. In addition, the current traces obtained during the 20 5-ms ramps are shown superimposed as a function of the ramp voltage. In both [K+]o, the erg3 current was relatively large compared to the small erg1 current amplitude (note the different current scales), which is consistent with the faster activation and the more negative activation threshold of erg3 channels compared to erg1 channels. Almost no erg1 current was elicited during the first part of the pulse protocol including the first three voltage ramps. After this delay, the erg1 current at the plateau potential of −30 mV, as well as the erg1 current elicited upon subsequent voltage ramps, increased continuously during the course of the test protocol with no indication of saturation. In contrast, erg3 exhibited a much faster increase in the current amplitude at −30 mV. Concomitantly, the erg3 ramp currents increased dramatically with the first four voltage ramps. Thereafter, the erg3 current amplitude at the plateau potential, as well as during the voltage ramps, started to saturate. The rise in [K+]o did not alter these characteristics of erg1 and erg3 currents. In spite of the decreased driving force for K+, the erg1 current amplitude at −30 mV was only slightly smaller, and the maximal amplitude during the voltage ramps was even clearly increased. For erg3, the current at the plateau potential was considerably reduced in elevated [K+]o, but the ramp currents reached essentially the same maximal amplitude.
Comparable experiments have not been performed with erg2, because erg2 channels activate even slower and at still more positive potentials than erg1, resulting in negligible small erg2 currents elicited with the spike train voltage protocol.
Discussion
We have analysed the effect of a change in [K+]o from 2 to 10 mm on the biophysical properties of the three rat erg channels. This increase in [K+]o shifted the activation threshold of erg1 and erg3 channels slightly to more negative potentials. In addition, all three erg channels exhibited a reduced steady-state inactivation, a slowing of inactivation and deactivation and, most strikingly, a significantly increased chord conductance. Most of the effects exerted on rat erg1 channels are qualitatively similar to those described for HERG1 as expected from the high degree of the subunit homology (Bauer et al. 1998). It should be stated, though, that most HERG studies were performed in Xenopus oocytes and involved stronger differences in [K+]o, since mainly 2 and 98 mm K+ have been used. Some inconsistencies might arise from different endogenously expressed proteins interacting with erg channels. In Xenopus oocytes, the presence of four MinK-related peptides has recently been demonstrated (Anantharam et al. 2003). In addition, batches of oocytes from some donor frogs may express endogenous erg-like currents (Bauer et al. 1996). CHO cells which were also used in the present study are probably devoid of MinK-related peptides as suggested by negative RT-PCR results using degenerate primers (Lu et al. 2003). The present study presents data for all three rat erg channels obtained with almost identical expression and recording conditions allowing a direct comparison of the [K+]o dependence of the three erg channels. Although most effects on the biophysical properties seem to be well conserved within the erg channel family, there exist some quantitative differences in these effects between the three erg channels which might be of functional importance.
Influence of [K+]o on erg current amplitude
Our results show that the dramatic increase in conductance with elevated [K+]o which is typical for classical inward rectifiers (Hagiwara & Takahashi 1974), is also a distinctive feature of all three erg channels. The increase in conductance was even more pronounced for erg2, and less pronounced for erg3 compared to erg1. For HERG1 channels, the increase in current amplitude is the most often described effect of elevating [K+]o (Sanguinetti et al. 1995; Trudeau et al. 1995; Kiehn et al. 1996; Schönherr & Heinemann, 1996; Wang et al. 1996, 1997; Zou et al. 1998). This effect has first been observed in rabbit nodal heart cells for the delayed rectifier current IKr (Shibasaki, 1987) which is now known to be mediated by erg1 channels. Shibasaki (1987) found that the IKr single channel conductance varies almost proportionally to the square root of [K+]o. These data were derived from experiments performed in high [K+]o (50–300 mm). The method of non-stationary noise analysis applied on heterologously expressed HERG1 channels yielded the small single channel conductance value of about 2 pS at 5 mm[K+]o compared to 9 pS at 100 mm[K+]o (Kiehn et al. 1996). Our data for the K+ dependence of the maximally available whole-cell conductance of rat erg1 obtained with voltage ramps starting from the fully activated state fit well to the single channel data: the square root of 5 (as the factor of the two different [K+]o used) equals 2.236 and we used a factor of 2.3 to scale the erg1 conductance values. This correspondence suggests that the increase in the maximally available whole-cell conductance results predominantly from the increase in single channel conductance.
Recently, the mechanism of the unusual [K+]o dependence of the HERG1 conductance has partially been explained by a blocking effect of external Na+ ions which can be relieved by increases in [K+]o within the physiological range (Numaguchi et al. 2000; Mullins et al. 2002, 2004). External Na+ block of HERG1 channels was reduced by impaired inactivation of the channel suggesting that inactivated channels have a high affinity to external Na+ (Mullins et al. 2004). Given that binding of external Na+ would also be able to block erg3 channels, this mechanism could explain the smaller [K+]o-induced increase in conductance for erg3 compared to erg1 channels found in our experiments, since erg3 channels exhibit considerably less steady-state inactivation than erg1.
For all three rat erg channels, the K+-induced whole-cell conductance increase was consistently larger for the maximum ‘steady-state’ conductance determined at the end of 4 s depolarizing test pulses than for the maximally available conductance measured with the voltage ramp protocol. This difference in conductance increase is most probably due to the additional effects induced by the rise in [K+]o, such as the shift of the activation curve to the left, slower inactivation and less steady-state inactivation as discussed below.
Effects of [K+]o on erg channel activation and deactivation
With respect to the voltage dependence of activation, the three rat erg channels differed in their sensitivity to [K+]o. Upon the rise in [K+]o from 2 to 10 mm, the erg1 activation curve shifted slightly, but significantly, by about 4 mV to more negative potentials. This [K+]o dependence of channel activation was stronger for erg3 with an approximately −8 mV shift of the activation curve, whereas it was not significant for erg2. Although a shift in the voltage dependence of −4 mV sounds small, together with a steep voltage dependence as indicated by a slope factor k of about 8 mV it means an approximately 20% increase in the fraction of activated erg1 channels close to the midpoint potential V0.5.
Only for erg3 was a significant increase in the steepness of the activation curve found upon the rise in [K+]o. All erg activation curves were obtained indirectly by measuring the peak amplitudes of the tail currents following the depolarizing test pulses. In the critical voltage range for erg3 channel activation (−50 to −20 mV), little inactivation occurred resulting in large instantaneous tail currents. The rise in [K+]o decreased steady-state inactivation thereby reducing the small percentage of erg3 channels which have to recover from inactivation before they contribute to the tail current. To investigate whether differences in steady-state inactivation and the time course of deactivation could underlie the observed increase in the steepness of the activation curve, we performed an additional evaluation of the data using the averaged amplitudes of the whole erg3 tail currents in exactly the same way as in the analysis of the envelope-of-tail experiments. With this method, the differences in the steepness of the activation curves were smaller (k decreased from 8.8 ± 0.3 mV to 7.6 ± 0.4 mV; n = 25, P≤ 0.01), indicating that part of the calculated difference in the steepness was caused by the evaluation method and mirrored the changes in steady-state inactivation and deactivation kinetics. In the latter evaluation, the shift in the voltage dependence of erg3 channel activation was only slightly smaller and amounted to 7.0 ± 1.0 mV (n = 25, P≤ 0.0001).
Little information is available about [K+]o effects on the voltage dependence of HERG1 channel activation. Wang et al. (1997) showed a not significant left shift for an elevation of [K+]o from 2 to 98 mm. In contrast, an almost 30 mV shift of the HERG1 availability curve to the left by elevating [K+]o from 2 to 20 mm has been measured at 0.5 mm[Ca2+]o (Ho et al. 1998). The fact that the same group observed no [K+]o effect at 5 mm[Ca2+]o suggests an interaction of these two external ions regarding the voltage dependence of erg channel gating.
With respect to the time course of activation which differs considerably within the erg channel family (Wimmers et al. 2002 and this study), all three rat erg channels were unaffected by a change in [K+]o as previously described for HERG1 channels (Wang et al. 1997; Terai et al. 2000). Not only the time course of activation, but also the time course of deactivation of erg3 channels is much faster than that of erg1 and erg2 channels. Nevertheless, deactivation kinetics of all three erg channels were comparably slowed by the rise in [K+]o.
Most published data concerning the [K+]o dependence of HERG1 channel deactivation kinetics have been obtained in Xenopus oocytes and involve stronger changes in [K+]o than used in the present experiments. Interestingly, clearly increased time constants of HERG1 channel deactivation have been shown in 20 mm compared to 2 mm[K+]o (Terai et al. 2000), but in another study, HERG1 deactivation was not significantly slower in 98 mm[K+]o than in 2 mm[K+]o (Wang et al. 1997). Further elevations of [K+]o to values up to 300 mm used in ‘knock-off’ experiments even resulted in an acceleration of HERG1 deactivation kinetics (Wang et al. 1998; Aydar & Palmer, 2001). These data suggest that the effects of an increase in [K+]o on erg deactivation kinetics can change with the concentration range used.
The voltage dependence of the availability curves of the three erg channels depends not only on their voltage dependence of activation, but also on their deactivation kinetics. The slower the activation and deactivation kinetics, the bigger the potential differences between the isochronal activation and availability curves (Schönherr et al. 1999). The left shift in the erg2 availability curve produced by the rise in [K+]o despite a significant effect on erg2 activation can therefore be explained by the slower deactivation in elevated [K+]o. A mechanism by which a negative shift in the voltage dependence of activation induced by high external [K+]o can result from a slowing of deactivation and stabilization of the open state of the channel has recently been described for Kv4.3 channels (Wang et al. 2004).
Effects of [K+]o on erg channel inactivation and recovery from inactivation
The increase in [K+]o from 2 to 10 mm significantly slowed inactivation as well as recovery from inactivation of all three rat erg channels. It should be noted that the slowing of inactivation was even more pronounced for erg2 and erg3 than for erg1. For HERG1, a slowing of the time course of inactivation and of recovery from inactivation by increased [K+]o has been consistently observed (Kiehn et al. 1996; Wang et al. 1996, 1997). The same dependence of inactivation kinetics on [K+]o has also been reported for native erg currents such as in atrial cells (Yang et al. 1997) and neuroblastoma cells which can express all three erg channel subunits (Meves, 1999; Meves et al. 1999).
In Shaker K+ channels, the slowing of inactivation by increased [K+]o has been described as a characteristic of C-type inactivation (Lopez-Barneo et al. 1993). In contrast, the slowing of HERG1 recovery kinetics by increased [K+]o differs from the behaviour of K+ channels with classical C-type inactivation. The recovery from inactivation of Kv1.4 and Kv1.3 channels, for instance, is accelerated by increased [K+]o (Pardo et al. 1992; Rasmusson et al. 1995; Levy & Deutsch, 1996).
Not only the inactivation kinetics, but also the voltage dependence of erg channel inactivation depends on [K+]o. Steady-state inactivation decreases with rises in [K+]o, and for HERG1 channels, a 30 mV shift of the steady-state inactivation curve is reported upon a change in [K+]o from 2 to 98 mm (Wang et al. 1997), and a 20 mV shift for a change in [K+]o from 2 to 20 mm (Zou et al. 1998). A significant decrease in steady-state inactivation upon raising [K+]o is also found for all three rat erg channels in the voltage range studied (−40 to 60 mV; Fig. 4C). The results for erg3 clearly show, and an extrapolation of the data for erg1 and erg2 to more negative potentials suggests, a smaller shift in the voltage dependence of steady-state inactivation. This could partially be due to the smaller differences in [K+]o used in the present study.
Physiological implications
The comparative investigation of the effects of a rise in [K+]o on the biophysical properties of the three rat erg channels yielded a huge body of information helpful in assessing the physiological function of the erg channels in the nervous system. In situ hybridization studies performed in rat brain demonstrated a differential regional expression of the three erg channels (Saganich et al. 2001; Papa et al. 2003). The relative abundance of the erg channel subunits also differed with a higher level of erg1 and erg3 expression than that of erg2 (Saganich et al. 2001). In some brain regions, an overlapping expression of erg channel subunits was found, and even coexpression at the cellular level like that in CA1 pyramidal neurones (Saganich et al. 2001; Papa et al. 2003) and brainstem serotonergic neurones (Hirdes et al. 2004) has been detected.
It has been demonstrated that the three erg subunits are able to form heteromultimeric channels (Wimmers et al. 2001). Since our data now show that most [K+]o effects on the three erg channels are qualitatively similar, comparable effects are expected also for heteromultimeric erg channels. In addition to erg1 (also denoted erg1a), the splice variant erg1b is expressed in the brain (Lees-Miller et al. 1997). These two isoforms differ only in their intracellular N-termini, suggesting a similar sensitivity of the homomultimeric as well as putative heteromultimeric erg1/1b channels to [K+]o.
The range of [K+]o used in the present study can probably be achieved in vivo in the brain because the ion composition of the extracellular fluid is very sensitive to hypoxic conditions and the activity state. Hyperactivity, e.g. during an epileptic seizure, results in an increase in [K+]o up to about 10 mm (Benninger et al. 1980; Heinemann & Dietzel, 1984). This concentration represents the so-called ‘ceiling’ level of [K+]o which is normally maintained in spite of increasing amounts of K+ entering the extracellular space (Heinemann & Lux, 1977; Xiong & Stringer, 1999). One explanation for this phenomenon is a very high buffering capacity around 10 mm[K+]o provided in part by the glial K+ uptake system. Nevertheless, even higher local [K+]o in the range of several tens of millimolar have been reported to occur during spreading depression (Nicholson et al. 1978; Krnjevic et al. 1980; Gorji et al. 2001) and prolonged anoxia (Croning & Haddad, 1998; Stiefel & Marmarou, 2002).
Increased [K+]o also plays an important experimental role in the identification and analysis of small endogenous erg currents (Bauer et al. 1990). Together with a low external [Ca2+], high [K+]o was often used to enhance the erg current amplitude and to separate the erg currents from other endogenous K+ currents. Also the first studies of neuronal erg currents used elevated [K+]o (40 mm[K+]o; Sacco et al. 2003; Hirdes et al. 2004). These neuronal erg currents described so far have been detected in cerebellar Purkinje neurones from postnatal mice (Sacco et al. 2003) and rat embryonic neurones of the raphe nuclei (Hirdes et al. 2004). Both the electrophysiological properties of these neuronal erg currents as well as the erg expression pattern suggest that the endogenous erg channels could well involve erg1 and erg3 or even all three erg channel subunits. Therefore, a detailed knowledge about the K+ dependence of all three erg channels is necessary to get an idea of the current properties in a more physiological range of [K+]o.
In addition to the neuronal expression, erg1 channels have been described in hippocampal astrocytes (Papa et al. 2003) where they were suggested to be functionally important for K+ homeostasis (Emmi et al. 2000). In this case, the paradoxical K+ dependence of erg1 channels might be crucial for the clearance of increased extracellular K+. In neurones, erg channels could contribute to diverse tasks such as the maintenance of the resting membrane potential of cells with less negative resting potential, action potential properties or frequency adaptation. Recently, the first hints of an involvement of erg channels in the generation of afterhyperpolarizations in substantia nigra compacta neurones have emerged (Nedergaard, 2004). A functional role of erg channels in frequency accommodation has first been studied in neuroblastoma cells (Chiesa et al. 1997) and has now been detected in adult cerebellar Purkinje neurones (Sacco et al. 2003). In this preparation, a block of erg channels resulted in an increased firing rate already at the onset of long depolarizing current pulses. The present data obtained with the spike train protocol suggest that this fast contribution of an erg current to frequency accommodation is more likely to be carried by erg3 than by erg1 channels. Increased [K+]o reduced the large amplitude of the erg3 current in the interspike intervals, probably resulting in a similarly reduced ability to mediate frequency accommodation. The relatively fast activation kinetics and little steady-state inactivation up to −20 mV make erg3 channels suited to efficiently contribute to action potential repolarization already during short action potential bursts. This capability is probably not affected by differences in [K+]o, since the maximal erg3 current amplitudes during the simulated action potentials were almost the same in both tested [K+]o. In contrast to erg3, the slow activation kinetics of erg1 suggest that erg1 channels mediate frequency accommodation and contribute to action potential repolarization only after prolonged bursts of action potentials. In increased [K+]o, which favours repetitive firing, the erg1 current amplitudes are preserved or even enhanced, thus enabling a neurone to bring a burst to an end.
In a model of a hippocampal CA1 pyramidal cell, bursts of action potentials during normal excitability are suggested to raise the local [K+]o around the soma to levels around 10 mm already within 50 ms (Kager et al. 2000). Every action potential produces a stepwise increase in [K+]o and concomitantly a decrease in EK. Kager et al. (2000) suggested that prolonged seizure discharges or spreading depression mainly result from a positive feedback mechanism. During a burst of action potentials or prolonged depolarizations, repolarizing K+ currents increase [K+]o which in turn reduces the amplitude of the K+ currents by decreasing the driving force. The reduction of K+ currents would then result in a further depolarization. The unusual [K+]o dependence of erg channels resulting in increased erg current amplitudes in elevated [K+]o comprises a possible neuronal negative feedback mechanism which could help to suppress pathological hyperactivity. Interestingly, in this model neurone trains of action potentials as well as prolonged depolarizations produced by spreading depression are followed by a period where the increased activity of the electrogenic Na+–K+ pump restores a membrane potential more negative than EK in the soma. In this situation, open K+ channels would produce an influx of K+ ions back into the neurone. It is tempting to speculate that the functionally inward-rectifying erg channels with their slow deactivation kinetics and their paradoxical K+ dependence could mediate a substantial K+ inward current thus helping to restore basal levels of [K+]o and the normal transmembrane K+ gradient.
To summarize the results of the present experiments, an increase in [K+]o shifts the activation threshold of erg1 and erg3 channels slightly, but significantly, to more negative potentials. In addition, all three erg channels exhibit a slowing of inactivation, a slightly reduced steady-state inactivation and a slowing of deactivation. These effects are well suited to produce an increased activity of erg channels in elevated [K+]o. This acts in concert with the drastically increased conductance to produce the phenomenon of considerable erg outward current in increased [K+]o which can counteract high frequency firing during normal excitability as well as during pathological hyperactivity such as clonic burst behaviour.
Acknowledgments
We wish to thank Dr I. Wulfsen for the erg pcDNA3 constructs, Ms C. Reißmann for technical help and the Deutsche Forschungsgemeinschaft for financial support (SFB444/A3 and Ba-1436/2). P.S. was a fellow of the DFG Graduiertenkolleg GK255.
References
- Anantharam A, Lewis A, Panaghie G, Gordon E, McCrossan ZA, Lerner DJ, Abbott GW. RNA interference reveals that endogenous Xenopus MinK-related peptides govern mammalian K+ channel function in oocyte expression studies. J Biol Chem. 2003;278:11739–11745. doi: 10.1074/jbc.M212751200. 10.1074/jbc.M212751200. [DOI] [PubMed] [Google Scholar]
- Aydar E, Palmer C. Functional characterization of the C-terminus of the human ether-à-go-go-related gene K+ channel (HERG) J Physiol. 2001;534:1–14. doi: 10.1111/j.1469-7793.2001.t01-3-00001.x. 10.1111/j.1469-7793.2001.t01-3-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros F, del Camino D, Pardo LA, Palomero T, Giraldez T, de la Pena P. Demonstration of an inwardly rectifying K+ current component modulated by thyrotropin-releasing hormone and caffeine in GH3 rat anterior pituitary cells. Pflugers Arch. 1997;435:119–129. doi: 10.1007/s004240050491. 10.1007/s004240050491. [DOI] [PubMed] [Google Scholar]
- Bauer CK. The erg inwardly rectifying K+ current and its modulation by thyrotrophin-releasing hormone in giant clonal rat anterior pituitary cells. J Physiol. 1998;510:63–70. doi: 10.1111/j.1469-7793.1998.063bz.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CK, Engeland B, Wulfsen I, Ludwig J, Pongs O, Schwarz JR. RERG is a molecular correlate of the inward-rectifying K current in clonal rat pituitary cells. Receptors Channels. 1998;6:19–29. [PubMed] [Google Scholar]
- Bauer CK, Falk T, Schwarz JR. An endogenous inactivating inward-rectifying potassium current in oocytes of Xenopus laevis. Pflugers Arch. 1996;432:812–820. doi: 10.1007/s004240050203. [DOI] [PubMed] [Google Scholar]
- Bauer CK, Meyerhof W, Schwarz JR. An inward-rectifying K+ current in clonal rat pituitary cells and its modulation by thyrotrophin-releasing hormone. J Physiol. 1990;429:169–189. doi: 10.1113/jphysiol.1990.sp018250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CK, Schäfer R, Schiemann D, Reid G, Hanganu I, Schwarz JR. A functional role of the erg-like inward-rectifying K+ current in prolactin secretion from rat lactotrophs. Mol Cell Endocrinol. 1999;148:37–45. doi: 10.1016/s0303-7207(98)00241-x. [DOI] [PubMed] [Google Scholar]
- Bauer CK, Schwarz JR. Physiology of EAG K+ channels. J Membr Biol. 2001;182:1–15. doi: 10.1007/s00232-001-0031-3. [DOI] [PubMed] [Google Scholar]
- Benninger C, Kadis J, Prince DA. Extracellular calcium and potassium changes in hippocampal slices. Brain Res. 1980;187:165–182. doi: 10.1016/0006-8993(80)90502-8. [DOI] [PubMed] [Google Scholar]
- Chiesa N, Rosati B, Arcangeli A, Olivotto M, Wanke E. A novel role for HERG K+ channels: Spike-frequency adaptation. J Physiol. 1997;501:313–318. doi: 10.1111/j.1469-7793.1997.313bn.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy AM, Lang CC, Chomsky DM, Rayos GH, Wilson JR, Roden DM. Normalization of acquired QT prolongation in humans by intravenous potassium. Circulation. 1997;96:2149–2154. doi: 10.1161/01.cir.96.7.2149. [DOI] [PubMed] [Google Scholar]
- Compton SJ, Lux RL, Ramsey MR, Strelich KR, Sanguinetti MC, Green LS, Keating MT, Mason JW. Genetically defined therapy of inherited long-QT syndrome. Correction of abnormal repolarization by potassium. Circulation. 1996;94:1018–1022. doi: 10.1161/01.cir.94.5.1018. [DOI] [PubMed] [Google Scholar]
- Croning MD, Haddad GG. Comparison of brain slice chamber design for investigations of oxygen deprivation in vitro. J Neurosci Meth. 1998;81:103–111. doi: 10.1016/s0165-0270(98)00023-5. [DOI] [PubMed] [Google Scholar]
- Emmi A, Wenzel HJ, Schwartzkroin PA, Taglialatela M, Castaldo P, Bianchi L, et al. Do glia have heart? Expression and functional role for ether-a-go-go currents in hippocampal astrocytes. J Neurosci. 2000;20:3915–3925. doi: 10.1523/JNEUROSCI.20-10-03915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Varela D, de la Pena P, Garcia J, Giraldez T, Barros F. Influence of amino-terminal structures on kinetic transitions between several closed and open states in human erg K+ channels. J Membr Biol. 2002;187:117–133. doi: 10.1007/s00232-001-0156-4. 10.1007/s00232-001-0156-4. [DOI] [PubMed] [Google Scholar]
- Gorji A, Scheller D, Straub H, Tegtmeier F, Köhling R, Höhling JM, Tuxhorn I, Ebner A, Wolf P, Panneck HW, Oppel F, Speckmann EJ. Spreading depression in human neocortical slices. Brain Res. 2001;906:74–83. doi: 10.1016/s0006-8993(01)02557-4. 10.1016/S0006-8993(01)02557-4. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Takahashi K. The anomalous rectification and cation selectivity of the membrane of a starfish egg cell. J Membr Biol. 1974;18:61–80. doi: 10.1007/BF01870103. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Dietzel I. Extracellular potassium concentration in chronic alumina cream foci of cats. J Neurophysiol. 1984;52:421–434. doi: 10.1152/jn.1984.52.3.421. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Lux HD. Ceiling of stimulus-induced rises in extracellular potassium concentration in the cerebral cortex of cats. Brain Res. 1977;120:231–249. doi: 10.1016/0006-8993(77)90903-9. 10.1016/0006-8993(77)90903-9. [DOI] [PubMed] [Google Scholar]
- Hirdes W, Schweizer M, Schuricht KS, Guddat SS, Wulfsen I, Schwarz JR. Program No. 400.14 2004 Abstract Viewer/Itinerary Planner. Washington, DC, USA: Society for Neuroscience; 2004. Erg K+ currents in rat embryonic rhombencephalon neurons. [Google Scholar]
- Ho WK, Kim I, Lee CO, Earm YE. Voltage-dependent blockade of HERG channels expressed in Xenopus oocytes by external Ca2+ and Mg2+ J Physiol. 1998;507:631–638. doi: 10.1111/j.1469-7793.1998.631bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kager H, Wadman WJ, Somjen GG. Simulated seizures and spreading depression in a neuron model incorporating interstitial space and ion concentrations. J Neurophysiol. 2000;84:495–512. doi: 10.1152/jn.2000.84.1.495. [DOI] [PubMed] [Google Scholar]
- Kiehn J, Lacerda AE, Wible B, Brown AM. Molecular physiology and pharmacology of HERG: Single-channel currents and block by dofetilide. Circulation. 1996;94:2572–2579. doi: 10.1161/01.cir.94.10.2572. [DOI] [PubMed] [Google Scholar]
- Krnjevic K, Morris ME, Reiffenstein RJ. Changes in extracellular Ca2+ and K+ activity accompanying hippocampal discharges. Can J Physiol Pharmacol. 1980;58:579–582. doi: 10.1139/y80-097. [DOI] [PubMed] [Google Scholar]
- Lees-Miller JP, Kondo C, Wang L, Duff HJ. Electrophysiological characterization of an alternatively processed ERG K+ channel in mouse and human hearts. Circ Res. 1997;81:719–726. doi: 10.1161/01.res.81.5.719. [DOI] [PubMed] [Google Scholar]
- Levy DI, Deutsch C. Recovery from C-type inactivation is modulated by extracellular potassium. Biophys J. 1996;70:798–805. doi: 10.1016/S0006-3495(96)79619-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Hoshi T, Heinemann SH, Aldrich RW. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1993;1:61–71. [PubMed] [Google Scholar]
- Lu Y, Mahaut-Smith MP, Huang CL, Vandenberg JI. Mutant MiRP1 subunits modulate HERG K+ channel gating: a mechanism for pro-arrythmia in long QT syndrome type 6. J Physiol. 2003;551:253–262. doi: 10.1113/jphysiol.2003.046045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meves H. Inactivation of the ERG current in NG108-15 cells. Biochem Biophys Res Commun. 1999;263:510–515. doi: 10.1006/bbrc.1999.1378. [DOI] [PubMed] [Google Scholar]
- Meves H, Schwarz JR, Wulfsen I. Separation of M-like current and ERG current in NG108-15 cells. Br J Pharmacol. 1999;127:1213–1223. doi: 10.1038/sj.bjp.0702642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins FM, Stepanovic SZ, Gillani NB, Desai RR, George AL, Balser JR. Extracellular sodium interacts with the HERG channel at an outer pore site. J Gen Physiol. 2002;120:517–537. doi: 10.1085/jgp.20028589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullins FM, Stepanovic SZ, Gillani NB, George AL, Jr, Balser JR. Functional interaction between extracellular sodium, potassium and inactivation gating in HERG channels. J Physiol. 2004;558:729–744. doi: 10.1113/jphysiol.2004.065193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Numaguchi H, Johnson JP, Jr, Petersen CI, Balser JR. A sensitive mechanism for cation modulation of potassium current. Nat Neurosci. 2000;3:429–430. doi: 10.1038/74793. [DOI] [PubMed] [Google Scholar]
- Nedergaard S. A Ca2+-independent slow afterhyperpolarization in substantia nigra compacta neurons. Neuroscience. 2004;125:841–852. doi: 10.1016/j.neuroscience.2004.02.030. [DOI] [PubMed] [Google Scholar]
- Nicholson C, ten Bruggencate G, Stockle H, Steinberg R. Calcium and potassium changes in extracellular microenvironment of cat cerebellar cortex. J Neurophysiol. 1978;41:1026–1039. doi: 10.1152/jn.1978.41.4.1026. [DOI] [PubMed] [Google Scholar]
- Papa M, Boscia F, Canitano A, Castaldo P, Sellitti S, Annunziato L, Taglialatela M. Expression pattern of the ether-a-go-go-related (ERG) K+ channel-encoding genes ERG1, ERG2, and ERG3 in the adult rat central nervous system. J Comp Neurol. 2003;466:119–135. doi: 10.1002/cne.10886. [DOI] [PubMed] [Google Scholar]
- Pardo LA, Heinemann SH, Terlau H, Ludewig U, Lorra C, Pongs O, Stühmer W. Extracellular K+ specifically modulates a rat brain K+ channel. Proc Natl Acad Sci U S A. 1992;89:2466–2470. doi: 10.1073/pnas.89.6.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmusson RL, Morales MJ, Castellino RC, Zhang Y, Campbell DL, Strauss HC. C-type inactivation controls recovery in a fast inactivating cardiac K+ channel (Kv1.4) expressed in Xenopus oocytes. J Physiol. 1995;489:709–721. doi: 10.1113/jphysiol.1995.sp021085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco T, Bruno A, Wanke E, Tempia F. Functional roles of an ERG current isolated in cerebellar Purkinje neurons. J Neurophysiol. 2003;90:1817–1828. doi: 10.1152/jn.00104.2003. [DOI] [PubMed] [Google Scholar]
- Saganich MJ, Machado E, Rudy B. Differential expression of genes encoding subthreshold-operating voltage-gated K+ channels in brain. J Neurosci. 2001;21:4609–4624. doi: 10.1523/JNEUROSCI.21-13-04609.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Scamps F, Carmeliet E. Delayed K+ current and external K+ in single cardiac Purkinje cells. Am J Physiol. 1989;257:C1086–C1092. doi: 10.1152/ajpcell.1989.257.6.C1086. [DOI] [PubMed] [Google Scholar]
- Schledermann W, Wulfsen I, Schwarz JR, Bauer CK. Modulation of rat erg1, erg2, erg3 and HERG K+ currents by thyrotropin-releasing hormone in anterior pituitary cells via the native signal cascade. J Physiol. 2001;532:143–163. doi: 10.1111/j.1469-7793.2001.0143g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr R, Heinemann SH. Molecular determinants for activation and inactivation of HERG, a human inward rectifier potassium channel. J Physiol. 1996;493:635–642. doi: 10.1113/jphysiol.1996.sp021410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schönherr R, Rosati B, Hehl S, Rao VG, Arcangeli A, Olivotto M, Heinemann SH, Wanke E. Functional role of the slow activation property of ERG K+ channels. Eur J Neurosci. 1999;11:753–760. doi: 10.1046/j.1460-9568.1999.00493.x. [DOI] [PubMed] [Google Scholar]
- Shi WM, Wymore RS, Wang HS, Pan ZM, Cohen IS, McKinnon D, Dixon JE. Identification of two nervous system-specific members of the erg potassium channel gene family. J Neurosci. 1997;17:9423–9432. doi: 10.1523/JNEUROSCI.17-24-09423.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibasaki T. Conductance and kinetics of delayed rectifier potassium channels in nodal cells of the rabbit heart. J Physiol. 1987;387:227–250. doi: 10.1113/jphysiol.1987.sp016571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiefel MF, Marmarou A. Cation dysfunction associated with cerebral ischemia followed by reperfusion: a comparison of microdialysis and ion-selective electrode methods. J Neurosurg. 2002;97:97–103. doi: 10.3171/jns.2002.97.1.0097. [DOI] [PubMed] [Google Scholar]
- Sturm P, Wimmers S, Schwarz JR, Bauer CK. Program No. 400.15 2004 Abstract Viewer/Itinerary Planner. Washington, DC, USA: Society for Neuroscience; 2004. A rise in the external K+ concentration alters the biophysical properties of erg1, erg2 and erg3 K+ channels. [Google Scholar]
- Terai T, Furukawa T, Katayama Y, Hiraoka M. Effects of external acidosis on HERG current expressed in Xenopus oocytes. J Mol Cell Cardiol. 2000;32:11–21. doi: 10.1006/jmcc.1999.1048. [DOI] [PubMed] [Google Scholar]
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- Wang S, Bondarenko VE, Qu Y, Morales MJ, Rasmusson RL, Strauss HC. Activation properties of Kv4.3 channels: time, voltage and [K+]o dependence. J Physiol. 2004;557:705–717. doi: 10.1113/jphysiol.2003.058578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Liu SG, Morales MJ, Strauss HC, Rasmusson RL. A quantitative analysis of the activation and inactivation kinetics of HERG expressed in Xenopus oocytes. J Physiol. 1997;502:45–60. doi: 10.1111/j.1469-7793.1997.045bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Morales MJ, Liu SG, Strauss HC, Rasmusson RL. Time, voltage and ionic concentration dependence of rectification of h-erg expressed in Xenopus oocytes. FEBS Lett. 1996;389:167–173. doi: 10.1016/0014-5793(96)00570-4. [DOI] [PubMed] [Google Scholar]
- Wang JL, Trudeau MC, Zappia AM, Robertson GA. Regulation of deactivation by an amino terminal domain in human ether-a-go-go-related gene potassium channels. J Gen Physiol. 1998;112:637–647. doi: 10.1085/jgp.112.5.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warmke JW, Ganetzky B. A family of potassium channel genes related to eag in Drosophila and mammals. Proc Natl Acad Sci U S A. 1994;91:3438–3442. doi: 10.1073/pnas.91.8.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmers S, Bauer CK, Schwarz JR. Biophysical properties of heteromultimeric erg K+ channels. Pflugers Arch. 2002;445:423–430. doi: 10.1007/s00424-002-0936-4. [DOI] [PubMed] [Google Scholar]
- Wimmers S, Wulfsen I, Bauer CK, Schwarz JR. Erg1, erg2 and erg3 K channel subunits are able to form heteromultimers. Pflugers Arch. 2001;441:450–455. doi: 10.1007/s004240000467. [DOI] [PubMed] [Google Scholar]
- Xiong Z-Q, Stringer JL. Astrocytic regulation of the recovery of extracellular potassium after seizures in vivo. Eur J Neurosci. 1999;11:1677–1684. doi: 10.1046/j.1460-9568.1999.00587.x. [DOI] [PubMed] [Google Scholar]
- Yang T, Snyders DJ, Roden DM. Rapid inactivation determines the rectification and [K+]o dependence of the rapid component of the delayed rectifier K+ current in cardiac cells. Circ Res. 1997;80:782–789. doi: 10.1161/01.res.80.6.782. [DOI] [PubMed] [Google Scholar]
- Zou AR, Xu QP, Sanguinetti MC. A mutation in the pore region of HERG K+ channels expressed in Xenopus oocytes reduces rectification by shifting the voltage dependence of inactivation. J Physiol. 1998;509:129–137. doi: 10.1111/j.1469-7793.1998.129bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]