Abstract
Experimental studies have demonstrated that the GABAergic system modulates acetylcholine release and, through GABAA receptors, tonically inhibits cholinergic activity. Little is known about the effects of GABA on the cholinergic activity in the human central nervous system. In vivo evaluation of some cholinergic circuits of the human brain has recently been introduced using a transcranial magnetic stimulation (TMS) protocol based on coupling peripheral nerve stimulation with TMS of the motor cortex. Peripheral nerve inputs have an inhibitory effect on motor cortex excitability at short intervals (short latency afferent inhibition, SAI). We investigated whether GABAA activity enhancement by lorazepam modifies SAI. We also evaluated the effects produced by lorazepam on a different TMS protocol of cortical inhibition, the short interval intracortical inhibition (SICI), which is believed to be directly related to GABAA activity. In 10 healthy volunteers, the effects of lorazepam were compared with those produced by quetiapine, a psychotropic drug with sedative effects with no appreciable affinity at cholinergic muscarinic and benzodiazepine receptors, and with those of a placebo using a randomized double-blind study design. Administration of lorazepam produced a significant increase in SICI (F3,9 = 3.19, P = 0.039). In contrast to SICI, SAI was significantly reduced by lorazepam (F3,9 = 9.39, P = 0.0002). Our findings demonstrate that GABAA activity enhancement determines a suppression of SAI and an increase of SICI.
Muscle responses recorded in hand muscles after transcranial magnetic stimulation (TMS) of the motor cortex can be suppressed by electrical stimulation of the median nerve if the time interval between stimulation of median nerve and motor cortex is 2–8 ms longer than the time needed by the peripheral nerve afferent input to reach the cortex (Tokimura et al. 2000). This effect, named short latency afferent inhibition (SAI) of the motor cortex, is produced by interactions within the cerebral cortex (Tokimura et al. 2000; Di Lazzaro et al. 2004c). Since this inhibitory phenomenon is reduced or abolished by intravenous injection of the muscarinic antagonist scopolamine (Di Lazzaro et al. 2000a), we suggested that it might be a non-invasive way of testing cholinergic activity in the cerebral cortex. Indeed, SAI is abolished or reduced in pathological conditions characterized by a significant cholinergic dysfunction, like Alzheimer's disease (Di Lazzaro et al. 2002; Di Lazzaro et al. 2004a; Di Lazzaro et al. 2005), while it seems normal (Sailer et al. 2003) or even enhanced (Di Lazzaro et al. 2004b) in different neurological disorders such as Parkinson's disease.
GABA, the most widespread inhibitory neurotransmitter in the brain, affects cholinergic neurotransmission at multiple central nervous system levels (Giorgetti et al. 2000). Experimental studies have shown that GABA inputs modulate the activity of the nucleus basalis magnocellularis, the major source of cholinergic innervation to the cortex (Decker & McGaugh, 1991). GABAA receptors are localized on forebrain cholinergic neurones projecting to the cortex (Zaborszky et al. 1986) and their activation results in inhibition (Khateb et al. 1998). GABAA antagonists enhance ACh release at basal forebrain level (Vazquez & Baghdoyan, 2003). At the level of the cerebral cortex, the administration of a GABAA antagonist enhances spontaneous release of ACh (Giorgetti et al. 2000; Diez-Ariza et al. 2002). Previous experimental studies suggest that GABA, through GABAA receptors, tonically inhibits cholinergic activity both at cortical and subcortical level.
The aforementioned evidence provided the rationale for the present investigation, aiming to evaluate whether a modulation in GABAA activity results in excitability changes in those cholinergic cortical networks that are involved in SAI. To do this we evaluated the effects on SAI produced by the administration of the benzodiazepine lorazepam, which binds to specific sites on the GABAA receptor enhancing chloride channel-mediated hyperpolarization of the cell membrane. An effect of lorazepam on a different intracortical inhibitory protocol, the so called short latency intracortical inhibition (SICI) (Kujirai et al. 1993), which is believed to be related to GABAA activity, has already been demonstrated in previous studies (Ziemann et al. 1996a, b; Di Lazzaro et al. 2000b; Ilic et al. 2002). The effects of lorazepam were compared with those produced by quetiapine, a psychotropic drug with sedative effects and with no appreciable affinity at cholinergic muscarinic and benzodiazepine receptors, and with those of a placebo using a randomized double-blind study design.
Methods
Subjects
Ten healthy volunteers (mean age 29 ± 5.2 (s.d.) years) participated in the experiments. All gave their written informed consent. The study was performed according to the Declaration of Helsinki and approved by the ethics committee of the Medical Faculty of the Catholic University of Rome.
Magnetic stimulation
Magnetic stimulation was performed with a high powered Magstim 200 (Magstim Co., Whitland, Dyfed, UK). A figure-of-eight coil with external loop diameters of 9 cm, was held over the right motor cortex at the optimum scalp position to elicit electromyographic (EMG) responses in the contralateral FDI. The induced current flowed in a postero-anterior direction. Surface muscle responses were obtained via two 9 mm diameter Ag–AgCl electrodes with the active electrode over the motor point of the muscle and the reference on the metacarpophalangeal joint of the index finger. EMG responses were amplified and filtered (bandwidth 3 Hz to 3 kHz) by D360 amplifiers (Digitimer, Welwyn Garden City, UK). Data were collected on a computer with a sampling rate of 10 kHz per channel and stored for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK). Resting motor threshold (RMT) was defined as the minimum stimulus intensity that produced a liminal EMG response (about 50 µV in 50% of 10 trials) at rest. Active motor threshold (AMT) was defined as the minimum stimulus intensity that produced a liminal EMG response (about 200 µV in 50% of 10 trials) during isometric contraction of the tested muscle at about 20% maximum. A constant level of voluntary contraction was maintained with reference to an oscilloscope display of EMG in front of the subject. Auditory feedback of the EMG activity was also provided.
In order to minimize the recording time for each protocol, both single and paired stimulation of the motor cortex were performed with the stimulator(s) connected to the BiStim Module.
Short latency afferent inhibition by somatosensory input from the hand
SAI was studied using the technique that we have recently described (Tokimura et al. 2000). Conditioning stimuli were single pulses (200 µs) of electrical stimulation applied through bipolar electrodes to the median nerve at the wrist (cathode proximal). The intensity of the conditioning stimulus was set at just over motor threshold for evoking a visible twitch of the thenar muscles. The intensity of the test cortical magnetic shock was adjusted to evoke an EMG response in relaxed FDI with an amplitude of approximately 1 mV peak-to-peak.
Conditioning stimulus to the peripheral nerve preceded the test magnetic cortical stimulus. Interstimulus intervals (ISIs) were determined relative to the latency of the N20 component of the somatosensory evoked potential evoked by stimulation of the left median nerve. To record somatosensory evoked potentials, the active electrode was attached 3 cm posterior to C4 (10–20 system) and the reference was 3 cm posterior to C3. Five hundred responses were averaged to identify the latency of the N20 peak.
ISIs from the latency of the N20 plus 2 ms to the latency of the N20 plus 8 ms were investigated in steps of 1 ms. Five stimuli were delivered at each ISI. The subject was given audio-visual feedback at high gain to assist in maintaining complete relaxation. Amplitude of the conditioned EMG responses was expressed as a percentage of the amplitude of the test EMG responses. The amplitude of the conditioned responses at the seven ISIs studied was averaged obtaining a grand mean amplitude.
After drugs the intensity of the test stimulus was adjusted to ensure that the test EMG responses were matched in amplitude to the test EMG response recorded in baseline conditions.
Short interval intracortical inhibition
SICI was studied using the technique of Kujirai et al. (1993). Two magnetic stimuli were given through the same stimulating coil, using a Bistim module, over the motor cortex and the effect of the first (conditioning) stimulus on the second (test) stimulus was investigated. The conditioning stimulus was set at an intensity of 5% (of stimulator output) below AMT. The test stimulus intensity was adjusted to evoke an EMG response in relaxed FDI with an amplitude of approximately 1 mV peak-to-peak. The timing of the conditioning shock was altered in relation to the test shock. Interstimulus intervals (ISIs) of 2 and 3 ms were investigated. Five stimuli were delivered at each ISI. For these recordings muscle relaxation is very important and the subject was given audio-visual feedback at high gain to assist in maintaining complete relaxation.
Amplitude of the conditioned EMG responses was expressed as a percentage of the amplitude of the test EMG responses. The amplitude of the conditioned responses at the two ISIs studied was averaged, obtaining a grand mean amplitude.
After drugs the intensity of the test stimulus was adjusted to ensure that the test EMG responses were matched in amplitude to the test EMG responses recorded in baseline conditions.
Experimental design and data analysis
Measurements were done before (baseline) and 2 h after the administration of an oral dose of 2.5 mg lorazepam, an oral dose of 25 mg of quetiapine, or an oral placebo. Measurements were repeated 6 and 24 h after drug administration for all the three drugs. All drugs were administered double-blind. The drug sequence was determined by a random order procedure. At least 1 week passed between the administration of different drugs in each subject.
Effects of drugs on motor cortex excitability parameters (RMT, AMT, SAI and SICI) were tested separately using a repeated measures ANOVA incorporating, where necessary, a Greenhouse-Geisser correction for non-sphericity, with time as the main within-subject effect (4 levels) and with subsequent t tests. Conditional on significance of the F-value, the effects of drugs on individual ISIs of SAI and SICI were tested separately in an ANOVA model for repeated measures.
Because lorazepam may impair attention and because it has been shown that attention has an effect on different TMS-related techniques such as paired associative stimulation (Stefan et al. 2004) and cortical silent period (Mathis et al. 1998), in a subgroup of four subjects (mean age 31.2 ± 5.4 (s.d.) years) we evaluated the effects of attention on SAI. To do this we compared the baseline SAI with SAI obtained during attention manipulation. We used a simplified version of the protocol of Stefan et al. (2004). The manipulation of attention was performed using two different protocols carrying a different grade of attention directed to the left hand during SAI. In the attention-diverted condition, subjects were asked to solve continuously concatenated arithmetic tasks presented on a computer screen in front of them. In the second condition, the subjects were asked to look at their left hand during SAI and to focus attention on the left hand. During the second condition a few weak (2 times perceptual threshold) electric pulses (200 µs duration) were randomly delivered to the distal phalanx of the left index via ring electrodes. After SAI, subjects were asked to report the count of the stimuli they had identified. Effects of attention on SAI were tested using Student's two-tailed t test for paired samples.
Results
Results are summarized in Table 1 and in Figs 1 and 2.
Table 1.
Effects of lorazepam, quetiapine and placebo on motor thresholds, short latency afferent inhibition (SAI) and short latency intracortical inhibition (SICI) 2, 6 and 24 h after their administration
| Lorazepam | Quetiapine | Placebo | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Baseline | 2 h | 6 h | 24 h | F | P | Baseline | 2 h | 6 h | 24 h | F | P | Baseline | 2 h | 6 h | 24 h | F | P |
| Motor threshold (% of maximal output) | ||||||||||||||||||
| Resting | 41.7 ± 9.3 | 41.8 ± 9.4 | 41.4 ± 10 | 41.6 ± 8.2 | 0.11 | 0.95 | 41.4 ± 7.8 | 42.3 ± 8 | 42.1 ± 8 | 40.9 ± 8.4 | 2.5 | 0.08 | 41.5 ± 8.3 | 40.5 ± 6.2 | 41.7 ± 8.8 | 42.8 ± 9.5 | 1.1 | 0.3 |
| Active | 30.9 ± 7.9 | 30.6 ± 7.9 | 30.4 ± 8.1 | 30.6 ± 7.8 | 0.82 | 0.5 | 30.5 ± 7.2 | 30.7 ± 7.1 | 30.6 ± 8.3 | 29.5 ± 6.7 | 0.95 | 0.43 | 29.6 ± 7.9 | 29.2 ± 6.2 | 30 ± 7.6 | 30.9 ± 7.6 | 1.64 | 0.2 |
| Short latency afferent inhibition | ||||||||||||||||||
| Test stimulation intensity (%) | 52.4 ± 13.7 | 53.4 ± 13.7 | 54.5 ± 15 | 54.5 ± 14 | 52.8 ± 11.8 | 54.6 ± 14 | 53.7 ± 12.5 | 52.6 ± 13.1 | 56.6 ± 15.5 | 56.6 ± 15.4 | 54.3 ± 13 | 54.6 ± 13.1 | ||||||
| Test response (mV) | 1.2 ± 0.4 | 1.2 ± 0.5 | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.1 ± 0.5 | 1.2 ± 0.4 | 1.2 ± 0.4 | 1.1 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.3 | 1.0 ± 0.3 | 1.1 ± 0.3 | ||||||
| Grand mean amplitude of conditioned response (%) | 40.8 ± 19 | 68.6 ± 24.7 | 70.8 ± 26.2 | 50.5 ± 17 | 9.39 | 0.0002 | 42.3 ± 19.1 | 50.9 ± 21.5 | 50.8 ± 14.4 | 50.3 ± 15.4 | 1.83 | 0.17 | 49.1 ± 14.3 | 47.7 ± 17.1 | 47.8 ± 14.6 | 48 ± 20.1 | 0.14 | 0.9 |
| Conditioned response 2 ms interstimulus interval (%) | 24.2 ± 19.2 | 38.8 ± 25.2 | 36.8 ± 18.1 | 29.4 ± 18.7 | 1.36 | 0.28 | 27.3 ± 19.3 | 37.3 ± 33.3 | 43.2 ± 34 | 34.2 ± 22.4 | 1.2 | 0.33 | 25.5 ± 13.6 | 28.5 ± 21 | 24.7 ± 19.5 | 30.0 ± 22.4 | 0.41 | 0.74 |
| Conditioned response 3 ms interstimulus interval (%) | 32.4 ± 14.4 | 59.4 ± 30.3 | 38.9 ± 22.9 | 31.0 ± 20.8 | 3.28 | 0.036 | 24.7 ± 19.1 | 38.3 ± 26.9 | 30.6 ± 20 | 24.0 ± 12.8 | 1.73 | 0.18 | 28.5 ± 20.1 | 32.9 ± 25.2 | 20.2 ± 19.4 | 25.5 ± 17.7 | 1.48 | 0.25 |
| Conditioned response 4 ms interstimulus interval (%) | 35.3 ± 21.5 | 66.3 ± 47.8 | 65.7 ± 44.2 | 40.5 ± 19.8 | 2.81 | 0.06 | 29.4 ± 23.6 | 43.3 ± 20.7 | 47.3 ± 28.9 | 45.2 ± 12.2 | 2.49 | 0.08 | 42.0 ± 24.4 | 40.7 ± 28.9 | 27.0 ± 19 | 37.3 ± 18.4 | 1.96 | 0.14 |
| Conditioned response 5 ms interstimulus interval (%) | 38.4 ± 26.9 | 79.6 ± 37.7 | 69.1 ± 38.4 | 51.7 ± 36.8 | 6.51 | 0.002 | 42.8 ± 25.9 | 53.1 ± 25.9 | 58.8 ± 31.7 | 55.6 ± 41.3 | 0.74 | 0.54 | 51.1 ± 17.6 | 45.4 ± 24.2 | 51.2 ± 27.6 | 52.5 ± 31.9 | 0.3 | 0.83 |
| Conditioned response 6 ms interstimulus interval (%) | 39.0 ± 14.7 | 76.2 ± 29.9 | 87.5 ± 30.6 | 65.9 ± 31.1 | 7.86 | 0.0006 | 51.2 ± 35.0 | 52.5 ± 25.7 | 56.2 ± 24.6 | 58.3 ± 22.3 | 0.21 | 0.89 | 51.7 ± 24.8 | 54.5 ± 25.9 | 60.7 ± 26.1 | 58.4 ± 32.3 | 0.42 | 0.74 |
| Conditioned response 7 ms interstimulus interval (%) | 47.1 ± 26.4 | 75.6 ± 36 | 92.4 ± 44 | 67.5 ± 22.1 | 3.56 | 0.029 | 61.2 ± 24.8 | 62.6 ± 27.9 | 65.6 ± 20.4 | 65.2 ± 19.3 | 0.09 | 0.96 | 70.2 ± 28.4 | 62.5 ± 27.6 | 71.0 ± 23.6 | 51.6 ± 31.8 | 2.02 | 0.13 |
| Conditioned response 8 ms interstimulus interval (%) | 76.5 ± 46.8 | 95.5 ± 39.6 | 113.6 ± 73.3 | 80.7 ± 24.9 | 1.39 | 0.27 | 59.1 ± 30.6 | 69.5 ± 31.8 | 83.9 ± 29.3 | 69.4 ± 30.8 | 3.18 | 0.04 | 75.0 ± 25.7 | 61.6 ± 23.1 | 80.9 ± 37.5 | 90.6 ± 36.4 | 1.84 | 0.16 |
| Short latency intracortical inhibition | ||||||||||||||||||
| Test stimulation intensity (%) | 52.4 ± 13.7 | 53.7 ± 15.0 | 55.2 ± 14.2 | 54.5 ± 14 | 53 ± 11.9 | 54.2 ± 13.9 | 53.4 ± 12.7 | 53 ± 13.8 | 54.2 ± 18.5 | 50.9 ± 18.8 | 54.5 ± 13 | 54 ± 13 | ||||||
| Test response (mV) | 1.3 ± 0.4 | 1.1 ± 0.5 | 1.3 ± 0.7 | 1.6 ± 1.0 | 1.2 ± 0.5 | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.5 | 1.4 ± 0.7 | 1.1 ± 0.4 | 1.2 ± 0.4 | 1.4 ± 0.6 | ||||||
| Grand mean amplitude of conditioned response (%) | 44.4 ± 18.6 | 28.5 ± 13.6 | 34.8 ± 15 | 38.1 ± 18.4 | 3.19 | 0.039 | 37.3 ± 19 | 39.8 ± 16 | 31 ± 20.5 | 35.2 ± 13.7 | 2.6 | 0.07 | 39.7 ± 25.4 | 41.8 ± 20.2 | 42.6 ± 29.6 | 36.9 ± 18.5 | 0.38 | 0.7 |
| Conditioned response 2 ms interstimulus interval (%) | 36.3 ± 18.9 | 30.6 ± 14.8 | 29.0 ± 16 | 26.4 ± 14.6 | 0.89 | 0.46 | 32.8 ± 20.0 | 37.8 ± 19.8 | 28.6 ± 18.4 | 30.4 ± 16.8 | 1.4 | 0.26 | 36.5 ± 30.9 | 36.6 ± 24.1 | 40.3 ± 24.5 | 29.0 ± 17.6 | 1.32 | 0.29 |
| Conditioned response 3 ms interstimulus interval (%) | 52.5 ± 23.9 | 26.4 ± 15.7 | 40.7 ± 19.7 | 49.7 ± 24.8 | 6.33 | 0.0022 | 41.9 ± 26.7 | 41.8 ± 17.7 | 33.1 ± 26 | 40.1 ± 15 | 0.71 | 0.55 | 42.9 ± 25.4 | 46.9 ± 22.5 | 44.9 ± 39.6 | 44.8 ± 24.8 | 0.062 | 0.98 |
All values are expressed as mean ±s.d. F and P-values reflect repeated-measure ANOVA with the main within-subject effect of time; P-values below mean data indicate significant post hoc comparisons of measurements after drug intake with measurements at baseline (paired t test with Bonferroni correction for multiple comparisons. Significant effects are indicated in bold).
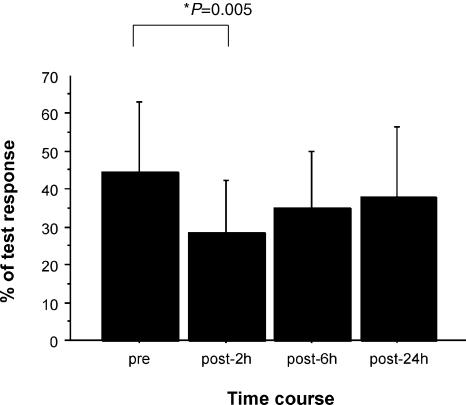
Figure 1. Effects of lorazepam on short latency intracortical inhibition (SICI).
Bar graph showing mean values in baseline conditions and 2, 6 and 24 h after lorazepam administration; error bars are standard deviations. Amplitude of the conditioned EMG response is reported as a percentage of control EMG response. The amount of inhibition is increased after lorazepam (F3,9 = 3.19, P = 0.039). The decrease in SICI is significant 2 h after drug intake (P = 0.005).
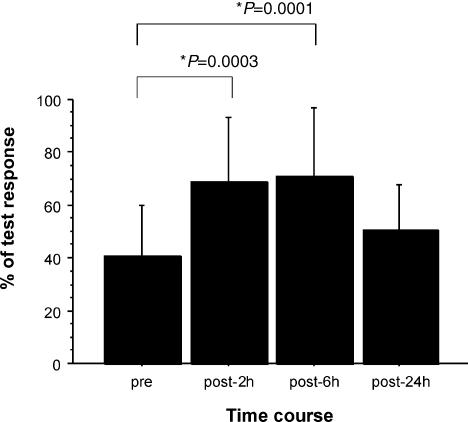
Figure 2. Effects of lorazepam on short latency afferent inhibition (SAI).
Bar graph showing mean values in baseline conditions and 2, 6 and 24 h after lorazepam administration; error bars are standard deviations. Amplitude of the conditioned EMG response is reported as a percentage of control EMG response. The amount of inhibition is reduced after lorazepam (F3,9 = 9.39, P = 0.0002). The decrease in SAI is significant 2 and 6 h after drug intake (P = 0.0003 and P = 0.0001, respectively).
The RMT was not significantly modified by lorazepam (F3,9 = 0.11, P > 0.05), quetiapine (F3,9 = 2.51, P > 0.05), or placebo (F3,9 = 1.16, P > 0.05).
SICI
The amount of inhibition was increased after lorazepam (F3,9 = 3.19, P = 0.039; Figs 1 and 3). In the post hoc comparisons, the increase in SICI was significant 2 h after drug intake (P = 0.005). An analysis of individual interstimulus interval showed that lorazepam had a significant effect at 3 ms ISI (Table 1). SICI was not significantly modified by quetiapine (F3,9 = 2.6, P > 0.05) or placebo (F3,9 = 0.38, P > 0.05).
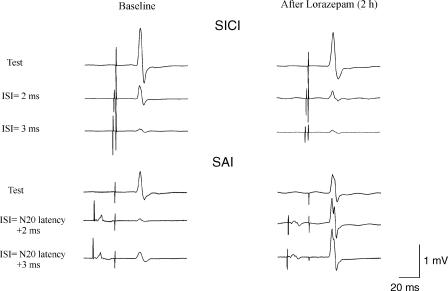
Figure 3. Short latency intracortical inhibition (SICI) at 2 and 3 ms interstimulus intervals and short latency afferent inhibition (SAI).
The top traces on the left show the average (of 5 trials each) of EMG responses evoked in the FDI by cortical stimulation alone and cortical stimulation conditioned by a cortical stimulus subthreshold for motor responses (5% of maximum magnetic stimulator output below active motor threshold) given 2–3 ms earlier. The lower traces on the left show the average (of 5 trials each) of EMG responses evoked in the FDI by cortical stimulation alone and cortical stimulation conditioned by a median nerve stimulus with an interstimulus interval corresponding to the N20 latency plus 2 and 3 ms. Traces on the right show SICI and SAI after lorazepam. After lorazepam SICI is increased while, in contrast, SAI is suppressed.
SAI
The amount of inhibition was reduced after lorazepam (F3,9 = 9.39, P = 0.0002; Figs 2 and 3). In the post hoc comparisons, the decrease in SAI was significant 2 and 6 h after drug intake (P = 0.0003 and P = 0.0001, respectively). An analysis of individual interstimulus interval showed that lorazepam had a significant effect on 3, 5, 6 and 7 ms ISIs (Table 1).
SAI was not significantly modified by quetiapine (F3,9 = 1.83, P > 0.05), or placebo (F3,9 = 0.14, P > 0.05).
Effects of attention
SAI was not significantly modified by attention manipulation. The mean amplitude of the conditioned EMG response in baseline condition was 51.6 ± 15.2% of control size. The amount of inhibition was not significantly modified in the attention-diverted condition (the mean amplitude of the conditioned EMG response was 51.3 ± 10.5% of control size; P = 0.94, paired t test) and in the attention focused condition (the mean amplitude of the conditioned EMG response was 52.8 ± 16.8% of control size; P = 0.61, paired t test).
Discussion
The present results provide the first evidence in humans that the enhancement of GABAA activity produced by lorazepam is associated with a reduction of short latency afferent inhibition, a form of inhibition that is believed to involve muscarinic cholinergic activity (Di Lazzaro et al. 2000a). SAI was not significantly affected by quetiapine and placebo.
Excitability of the motor cortex to single magnetic stimuli
As reported in previous studies (Ziemann et al. 1996a, b) lorazepam did not significantly modify RMT.
Effects of lorazepam on SAI and SICI
SAI was significantly decreased by lorazepam. This inhibitory phenomenon is related to muscarinic cholinergic activity (Di Lazzaro et al. 2000a). Several studies have demonstrated a GABAA-mediated inhibition of acetylcholine release both at cortical (Giorgetti et al. 2000) and subcortical levels (Vazquez & Baghdoyan, 2003). Therefore, the reduction in SAI produced by lorazepam might be explained by an inhibition in acetylcholine release due to the enhancement of GABAA activity. However, other more complex interactions are also possible. It has been demonstrated that acetylcholine has a rapid excitatory effect on the fast spiking (presumably GABAergic) cortical neurones (McCormick & Prince, 1985, 1986), and that cholinergic activity differentially modulates subsets of cortical GABA neurones (Xiang et al. 1998). Xiang et al. (1998) have shown that cortical cholinergic activation has a differential effect on different forms of cortical GABAergic inhibition, reducing intralaminar inhibition and promoting intracolumnar inhibition. Therefore, it can be hypothesized that the reduction in SAI after lorazepam reflects the imbalance between these two different forms of GABAergic inhibition differentially modulated by cholinergic activation. Another possibility is that the cholinergic and GABAergic systems are independently affected by lorazepam and have an independent influence on a third system.
In contrast with SAI and as shown in previous studies (Ziemann et al. 1996a, b; Di Lazzaro et al. 2000b; Ilic et al. 2002), SICI was significantly increased by lorazepam.
Is the change in SAI directly related to the enhanced activity of those GABAergic connections that determine SICI? This possibility should be taken into consideration because there are interactions between cortical inhibitory circuits (Sanger et al. 2001; Chen, 2004). It could be hypothesized that SAI is produced through the exci tatory effect of acetylcholine on the inhibitory GABAergic networks that are also responsible for SICI and, when the excitability of this latter system is shifted close to the maximum by a GABAergic drug, the inhibition produced indirectly by the afferent inputs is attenuated. However, this hypothesis seems unlikely because our previous study on the effects of scopolamine, a muscarinic antagonist, on SAI and SICI (Di Lazzaro et al. 2000a) strongly suggests that different populations of inhibitory neurones are involved in SICI and SAI in that they respond differentially to muscarinic blockade. After the administration of scopolamine SICI remained unchanged while SAI was suppressed (Di Lazzaro et al. 2000a). If the effect on SAI was due to an excitatory action on mechanisms generating SICI, a reduction of SICI could be expected after muscarinic blockade, but this was not the case. It could also be hypothesized that cortical connections responsible for SICI may determine a presynaptic inhibition of structures determining SAI. Therefore, an enhancement in SICI could result in a decrease of SAI.
It has been reported that lorazepam and the anticholinergic drug scopolamine produce similar impairment in memory function (Mintzer & Griffiths, 2003). The findings of the present work, demonstrating a depression of central muscarinic activity, as evaluated with SAI testing, after GABAA enhancement, might contribute to explain the impairment in memory function reported by Mintzer & Griffiths (2003). Indeed, ACh has a central role in memory function (Hasselmo & Bower, 1993) and the depression of ACh activity produced either directly through anticholinergic drugs or indirectly through a GABAA agonist could well result in a memory impairment.
Effects of quetiapine on SAI
We also evaluated the effects of quetiapine, a psychotropic drug with sedative effects, on SAI. Quetiapine is an antagonist at multiple neurotransmitter receptors in the brain: serotonin 5HT1A and 5HT2, dopamine D1 and D2, histamine, and adrenergic α1 and α2 receptors, with no appreciable affinity at cholinergic muscarinic and benzodiazepine receptors, which produces somnolence and sedation. Quetiapine showed no significant effect on SAI and on SICI. The lack of effects of atypical antipsychotic drugs on SICI has been demonstrated in a previous study in which the effects of olanzapine were evaluated (Daskalakis et al. 2003). However, the effects of quetiapine on SICI could have been masked by its concomitant antagonist action at multiple receptors. Quetiapine is an antagonist of dopamine D1 and D2 and adrenergic α1 and α2 receptors. Because it has been shown that the antagonists of dopamine receptors may reduce SICI (Ziemann et al. 1997) while adrenergic α1 and α2 receptor agonists may also decrease SICI (Ilic et al. 2003; Korchounov et al. 2003; Ziemann, 2004), it could be that the antagonist action of quetiapine at both levels results in no measurable effect on SICI. Because we used a dose of queitapine much lower than that used for treatment of schizophrenia, we cannot exclude that higher doses of quetiapine could result in changes in TMS parameters.
Effects of attention on SAI
At variance with different TMS-related techniques, such as paired associative stimulation (Stefan et al. 2004) and cortical silent period (Mathis et al. 1998), changes in attention level seem to have no effect on SAI.
Conclusion
The present study on conscious human motor cortex is the first demonstration of the effect of GABAergic drugs on short latency afferent inhibition, a form of inhibition related to the cholinergic activity in the cerebral cortex.
References
- Chen R. Interactions between inhibitory and excitatory circuits in the human motor cortex. Exp Brain Res. 2004;154:1–10. doi: 10.1007/s00221-003-1684-1. [DOI] [PubMed] [Google Scholar]
- Daskalakis ZJ, Christensen BK, Chen R, Fitzgerald PB, Zipursky RB, Kapur S. Effect of antipsychotics on cortical inhibition using transcranial magnetic stimulation. Psychopharmacology (Berl) 2003;170:255–262. doi: 10.1007/s00213-003-1548-1. 10.1007/s00213-003-1548-1. [DOI] [PubMed] [Google Scholar]
- Decker MW, McGaugh JL. The role of interactions between the cholinergic system and other neuromodulatory systems in learning and memory. Synapse. 1991;7:151–168. doi: 10.1002/syn.890070209. 10.1002/syn.890070209. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Meglio M, Cioni B, Tamburrini G, Tonali P, Rothwell JC. Direct demonstration of the effect of lorazepam on the excitability of the human motor cortex. Clin Neurophysiol. 2000b;111:794–799. doi: 10.1016/s1388-2457(99)00314-4. 10.1016/S1388-2457(99)00314-4. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Bentivoglio AR, Tonali PA. Normal or enhanced short latency afferent inhibition in Parkinson's disease? Brain. 2004b;127:E8. doi: 10.1093/brain/awh089. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Daniele A, Ghirlanda S, Gainotti G, Tonali PA. Motor cortex hyperexcitability to transcranial magnetic stimulation in Alzheimer's disease. J Neurol Neurosurg Psychiat. 2004b;75:555–559. doi: 10.1136/jnnp.2003.018127. 10.1136/jnnp.2003.018127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Marra C, Ghirlanda S, Ranieri F, Gainotti G, Tonali P. Neurophysiological predictors of long-term response to AChE inhibitors in AD patients. J Neurol Neurosurg Psychiat. 2005 doi: 10.1136/jnnp.2004.051334. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004c;115:255–266. doi: 10.1016/j.clinph.2003.10.009. 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Pennisi MA, Di Giovanni S, Zito G, Tonali P, Rothwell JC. Muscarinic receptor blockade has differential effects on the excitability of intracortical circuits in the human motor cortex. Exp Brain Res. 2000a;135:455–461. doi: 10.1007/s002210000543. 10.1007/s002210000543. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Tonali PA, Marra C, Daniele A, Profice P, Saturno E, Pilato F, Masullo C, Rothwell JC. Noninvasive in vivo assessment of cholinergic cortical circuits in AD using transcranial magnetic stimulation. Neurology. 2002;59:392–397. doi: 10.1212/wnl.59.3.392. [DOI] [PubMed] [Google Scholar]
- Diez-Ariza M, Garcia-Alloza M, Lasheras B, Del Rio J, Ramirez MJ. GABAA receptor antagonists enhance cortical acetylcholine release induced by 5-HT3 receptor blockade in freely moving rats. Brain Res. 2002;956:81–85. doi: 10.1016/s0006-8993(02)03483-2. 10.1016/S0006-8993(02)03483-2. [DOI] [PubMed] [Google Scholar]
- Giorgetti M, Bacciottini L, Giovannini MG, Colivicchi MA, Goldfarb J, Blandina P. Local GABAergic modulation of acetylcholine release from the cortex of freely moving rats. Eur J Neurosci. 2000;12:1941–1948. doi: 10.1046/j.1460-9568.2000.00079.x. 10.1046/j.1460-9568.2000.00079.x. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Bower JM. Acetylcholine and memory. Trends Neurosci. 1993;16:218–222. doi: 10.1016/0166-2236(93)90159-j. 10.1016/0166-2236(93)90159-J. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Korchounov A, Ziemann U. Methylphenidate facilitates and disinhibits the motor cortex in intact humans. Neuroreport. 2003;14:773–776. doi: 10.1097/00001756-200304150-00023. [DOI] [PubMed] [Google Scholar]
- Ilic TV, Meintzschel F, Cleff U, Ruge D, Kessler KR, Ziemann U. Short-interval paired-pulse inhibition and facilitation of human motor cortex: the dimension of stimulus intensity. J Physiol. 2002;545:153–167. doi: 10.1113/jphysiol.2002.030122. 10.1113/jphysiol.2002.030122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khateb A, Fort P, Williams S, Serafin M, Muhlethaler M, Jones BE. GABAergic input to cholinergic nucleus basalis neurons. Neuroscience. 1998;86:937–947. doi: 10.1016/s0306-4522(98)00094-3. 10.1016/S0306-4522(98)00094-3. [DOI] [PubMed] [Google Scholar]
- Korchounov A, Ilic TV, Ziemann U. The alpha2-adrenergic agonist guanfacine reduces excitability of human motor cortex through disfacilitation and increase of inhibition. Clin Neurophysiol. 2003;114:1834–1840. doi: 10.1016/s1388-2457(03)00192-5. 10.1016/S1388-2457(03)00192-5. [DOI] [PubMed] [Google Scholar]
- Kujirai T, Caramia MD, Rothwell JC, Day BL, Thompson PD, Ferbert A, Wroe S, Asselman P, Marsden CD. Corticocortical inhibition in human motor cortex. J Physiol. 1993;471:501–519. doi: 10.1113/jphysiol.1993.sp019912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathis J, de Quervain D, Hess CW. Dependence of the transcranially induced silent period on the ‘instruction set’ and the individual reaction time. Electroencephalogr Clin Neurophysiol. 1998;109:426–435. doi: 10.1016/s0924-980x(98)00042-3. 10.1016/S0924-980X(98)00042-3. [DOI] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Two types of muscarinic response to acetylcholine in mammalian cortical neurons. Proc Natl Acad Sci U S A. 1985;82:6344–6348. doi: 10.1073/pnas.82.18.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince DA. Mechanisms of action of acetylcholine in the guinea-pig cerebral cortex in vitro. J Physiol. 1986;375:169–194. doi: 10.1113/jphysiol.1986.sp016112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintzer MZ, Griffiths RR. Lorazepam and scopolamine: a single-dose comparison of effects on human memory and attentional processes. Exp Clin Psychopharmacol. 2003;11:56–72. doi: 10.1037//1064-1297.11.1.56. 10.1037//1064-1297.11.1.56. [DOI] [PubMed] [Google Scholar]
- Sailer A, Molnar GF, Paradiso G, Gunraj CA, Lang AE, Chen R. Short and long latency afferent inhibition in Parkinson's disease. Brain. 2003;126:1883–1894. doi: 10.1093/brain/awg183. [DOI] [PubMed] [Google Scholar]
- Sanger TD, Garg RR, Chen R. Interactions between two different inhibitory systems in the human motor cortex. J Physiol. 2001;530:307–317. doi: 10.1111/j.1469-7793.2001.0307l.x. 10.1111/j.1469-7793.2001.0307l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefan K, Wycislo M, Classen J. Modulation of associative human motor cortical plasticity by attention. J Neurophysiol. 2004;92:66–72. doi: 10.1152/jn.00383.2003. 10.1152/jn.00383.2003. [DOI] [PubMed] [Google Scholar]
- Tokimura H, Di Lazzaro V, Tokimura Y, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Short latency inhibition of human hand motor motor cortex by somatosensory input from the hand. J Physiol. 2000;523:503–513. doi: 10.1111/j.1469-7793.2000.t01-1-00503.x. 10.1111/j.1469-7793.2000.t01-1-00503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez J, Baghdoyan HA. Muscarinic and GABAA receptors modulate acetylcholine release in feline basal forebrain. Eur J Neurosci. 2003;17:249–259. doi: 10.1046/j.1460-9568.2003.02451.x. 10.1046/j.1460-9568.2003.02451.x. [DOI] [PubMed] [Google Scholar]
- Xiang Z, Huguenard JR, Prince DA. Cholinergic switching within neocortical inhibitory networks. Science. 1998;281:985–988. doi: 10.1126/science.281.5379.985. 10.1126/science.281.5379.985. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Heimer L, Eckenstein F, Leranth C. GABAergic input to cholinergic forebrain neurons: an ultrastructural study using retrograde tracing of HRP and double immunolabeling. J Comp Neurol. 1986;250:282–295. doi: 10.1002/cne.902500303. 10.1002/cne.902500303. [DOI] [PubMed] [Google Scholar]
- Ziemann U. TMS and drugs. Clin Neurophysiol. 2004;115:1717–1729. doi: 10.1016/j.clinph.2004.03.006. 10.1016/j.clinph.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhof BJ, Paulus W. The effect of lorazepam on the motor cortical excitability in man. Exp Brain Res. 1996a;109:127–135. doi: 10.1007/BF00228633. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Lonnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptics drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996b;40:367–378. doi: 10.1002/ana.410400306. 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- Ziemann U, Tergau F, Bruns D, Baudewig J, Paulus W. Changes in human motor cortex excitability induced by dopaminergic and anti-dopaminergic drugs. Electroencephalogr Clin Neurophysiol. 1997;105:430–437. doi: 10.1016/s0924-980x(97)00050-7. 10.1016/S0924-980X(97)00050-7. [DOI] [PubMed] [Google Scholar]