Abstract
The cardioprotective effects of short-term exercise against myocardial ischaemia–reperfusion injury in male and female rats were examined. We subjected male and female rats to 0 (Sed; n = 8 males and 8 females), 1 (1 day; n = 10 males and 8 females), or 5 (5 day; n = 6 males and 6 females) days of treadmill running. Langendorff-perfused hearts underwent 1 h of regional ischaemia and 2 h of reperfusion, and infarct size (expressed as a percentage of the zone at risk; ZAR), left ventricular pressure development, and coronary flow were measured for each heart. Preischaemic pressure development and coronary flow did not differ between the sexes nor were they influenced by exercise. Sed females had significantly smaller infarct sizes (25 ± 3%) than Sed male hearts (37 ± 3%; P < 0.001). Short-term running significantly reduced infarct size following 1 day (27 ± 3%; P < 0.05) and 5 days (30 ± 4%; P < 0.10) of exercise in males. One day of running did not reduce infarct size in females (19 ± 3%; P = NS), but 5 day females did show a significant reduction in infarct size (13 ± 2%; P < 0.05). There was no relationship between postischaemic coronary vascular hyperaemia and infarct size across sexes or exercise training groups. Hearts from Sed females exhibited significantly higher manganese superoxide dismutase (MnSOD) protein expression than hearts from Sed males, but short-term exercise (neither 1 nor 5 days) did not alter MnSOD protein in either sex. Increased sarcolemmal ATP-sensitive K+ (KATP) channel subunit protein expression (SUR2A and/or Kir6.2) correlated closely with sex-dependent and exercise-acquired protection against myocardial infarction. These data indicate that: (1) sex-dependent and exercise-induced differences in the susceptibility of the heart to ischaemia–reperfusion injury are not associated with improved coronary flow or postischaemic hyperaemia; (2) increased MnSOD protein expression is not necessary for exercise-induced protection from infarction; and (3) one possible mechanism for sex-dependent and exercise-mediated reductions in infarct size involves an increased protein expression of cardiac sarcolemmal KATP channels.
Ischaemic heart disease claimed more lives worldwide in 2002 than any other single disease (World Health Organization, 2003). Myocardial infarction is commonly the initial manifestation of ischaemic heart disease (Manfroi et al. 2002), and exercise training has been shown to decrease a number of the risk factors for myocardial infarction including hypertension, hyperlipidaemia, obesity, and insulin resistance, and has also been reported to improve chance of survival in humans after an ischaemic event (Morris et al. 1980).
Sex (gender) also appears to have an influence on the occurrence of myocardial infarction, with the incidence of ischaemic heart disease being much lower in premenopausal women than age-matched men. Previous investigations using animal models in rat (Li & Kloner, 1995) and dog (Przyklenk et al. 1995; Lee et al. 2000) have examined the effect of sex on myocardial infarct size using in situ preparations, but the results have been equivocal, with males having either larger infarct sizes than females (Lee et al. 2000) or no difference (Li & Kloner, 1995; Przyklenk et al. 1995). While exercise-induced enhancement of postischaemic myocardial function has been shown to differ between rat sexes (Paroo et al. 2002), no study to date has examined the effect of exercise training on infarct size between males and females. Evidence from the literature demonstrating exercise-mediated infarct-sparing has either been performed in male (Yamashita et al. 1999; Yamashita et al. 2001) or female (Brown et al. 2003; Hamilton et al. 2003) rats, and the duration of the exercise regimen has varied among these studies. Since a discrepancy appears to exist regarding the protective effects of exercise between the sexes, a primary objective of the present study was to test the hypothesis that short-term exercise alters the susceptibility of rat heart to ischaemia–reperfusion tissue injury in a sex-dependent manner.
Our previous work (Brown et al. 2003) showed that chronic exercise training reduced infarct size in female rats. We attributed the exercise-induced cardioprotection to increases in manganese superoxide dismutase (MnSOD) protein expression and better preservation of coronary flow in the trained animals (Brown et al. 2003). While exercise-induced cardioprotection in rat heart has commonly been associated with increases in MnSOD (Yamashita et al. 1999; Brown et al. 2003; Hamilton et al. 2003), few studies have examined infarct size in an experimental setting where coronary flow can be measured. Since both sex and short-term exercise have been shown to influence porcine vascular reactivity (Laughlin et al. 2003a, b), we assessed coronary flow in a setting of myocardial infarction to determine if sex- and exercise-dependent differences in infarct size could be ascribed to differences in coronary flow in the rat.
Finally, we examined the myocardial protein content of the sarcolemmal ATP-sensitive potassium (KATP) channel. The opening of these channels with pharmacological agents has been shown to reduce infarct size in dogs (Gross & Auchampach, 1992)and channel blockade can ameliorate the beneficial effects of ischaemic preconditioning (Gross & Auchampach, 1992). While the role of these channels has received much attention in the context of ischaemic preconditioning across mammalian species (O'Rourke, 2000; Gross & Peart, 2003), whether or not these channels play a role in exercise-induced protection from infarction is unknown. Therefore, a final objective of this study was to examine the effects of exercise and sex on myocardial protein content of both the inwardly rectifying pore-forming subunit of the channel, Kir6.2, and the sulphonylurea receptor (SUR), the regulatory subunit of the sarcolemmal KATP channel (Inagaki et al. 1995).
Methods
Animal model
We used male and female Sprague-Dawley rats (age 5–8 months) in all experiments. Acquired cardioprotection induced by a number of stimuli has been observed across mammalian species (for reviews see Bolli, 2000; Yellon & Downey, 2003), and the cardioprotective effects of exercise training have been extensively documented in rat heart (Bowles et al. 1992; Bowles & Starnes, 1994; Powers et al. 1998; Yamashita et al. 1999, 2001; Hamilton et al. 2001, 2003; Brown et al. 2003; Starnes et al. 2003). Animals were housed in the same facility with a 12: 12-h dark–light cycle and had food and water provided ad libitum. Rats were randomly placed into one of three experimental groups: sedentary control (Sed), 1 day running (1 day) and 5 days running (5 day). All experiments received prior approval from the Institutional Animal Care and Use Committee at the University of Colorado at Boulder and were conducted in accordance with the guidelines accepted by the American Physiological Society.
Exercise protocol
Animals in the trained groups were placed on a motorized treadmill (0% grade) and familiarized for a total of 5 days by running at 15 m min−1 per day for 5 min (1st day) to 20 min (5th day) per day. For the short-term exercise, rats ran for 1 or 5 days at 15/30/15 m min−1 for 10/40/10 min, respectively. Animals in the Sed group were placed on the non-moving treadmill for the same amount of time as the trained rats to serve as handling controls. All animals were killed at least 24 h after the last training (or handling control) session.
Removal of hearts
At the time of death, animals were deeply anaesthetized with sodium pentobarbital (35 mg kg−1i.p. injection), and (after the elimination of animal sensation reflexes including eye blink, pedal and tail pressure reflexes) hearts were removed by midline thoracotomy, briefly placed in ice-cold saline, and either homogenized for biochemical analyses or rapidly hung and perfused on the cannula of a modified Langendorff apparatus for the ex vivo ischaemia–reperfusion studies.
Homogenization for biochemical analysis
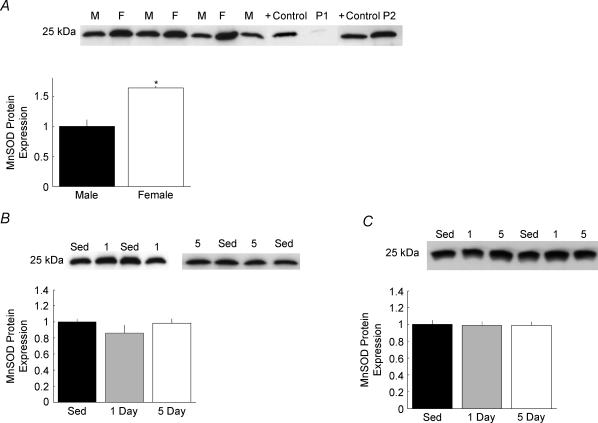
Left ventricular (LV) tissue was dissected from each heart, quickly rinsed in saline, and rapidly frozen with liquid nitrogen. Frozen samples were pulverized via a nitrogen-cooled mortar and pestle apparatus and LV powder was stored under liquid nitrogen until the time of homogenization. At the time of homogenization, 30–50 mg of heart powder was homogenized in cold (2–4°C) buffer containing (mm) 100 KCl, 50 Mops, 5 MgCl2, 1 ATP, and 1 EGTA, and stored at −80°C. When ready for use, homogenates were diluted 1: 4 in lysis buffer containing (mm): 20 Hepes, 150 NaCl, 1 EDTA, 1% NP-40 and 0.1% SDS. After equilibrating in lysis buffer (25°C) for 10 min, samples were clarified by low-speed centrifugation (3000 g for 5 min). Supernatants and pellets were separated and protein concentrations determined using the BCA assay (Pierce Biotechnology, Inc., Rockford, IL, USA). For all biochemical assays, the supernatant was used to probe for the protein of interest. However, the presence of the protein of interest was also examined in the pellets from each sample to ensure accurate separation. No measurable amounts of the proteins of interest were found in any of the pellet fractions when 1% NP-40 was used in the homogenizing buffer (Fig. 3A).
Figure 3. Manganese superoxide dismutase (MnSOD) protein expression in rat heart.
Representative Western blots for MnSOD protein expression and band density quantification are presented in 3 panels. A, MnSOD expression from Sed male (M) and Sed female (F) hearts. Band density for all hearts (n = 4 males and 4 females) is quantified and expressed as a function of Sed male density (*P < 0.05 versus Sed male). To ensure accurate data collection, pellet fractions from the clarifying spin were tested for the presence of MnSOD. Pellets were obtained from homogenizing buffer containing either 1% NP-40 (P1) or no NP-40 (P2) and each band is expressed alongside a positive control (+ control) for MnSOD protein. B, MnSOD expression from male hearts exposed to 0 (Sed, n = 4), 1 (n = 5), or 5 (n = 5) days of treadmill running. Band density is presented as a function of Sed males. C, MnSOD expression from female hearts exposed to 0 (Sed, n = 4), 1 (n = 5), or 5 (n = 5) days of treadmill running. Band density is expressed as a function of Sed females.
Western blotting
Western blots were performed using SDS-PAGE with 7.5% (SUR) or 15% (MnSOD, Kir6.2) polyacrylamide mini-gels (Bio-Rad), and 3 μg (MnSOD) or 30 μg (SUR, Kir6.2) of homogenate protein loaded in each lane. Following transfer to PVDF membrane primary antibody was added (1: 100 000 MnSOD (Stressgen Biotechnologies, Inc., San Diego, CA, USA); 1: 100 SUR1/2 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA); 1: 1000 Kir6.2 (Santa Cruz)). The addition of a horseradish peroxidase-conjugated secondary antibody followed by chemiluminescent reaction yielded blots when exposed to film. Bands were scanned into a computer and band density quantified using ImageJ software (http://rsb.info.nih.gov/nih-image/Default.htm). Data are normalized to either sedentary bands (for training comparisons) or male bands (for sex comparisons).
Whole-heart perfusion protocol
Following excision of the heart and placement on a modified Langendorff apparatus, hearts were perfused with buffer containing (mm) 117.4 NaCl, 4.7 KCl, 1.9 CaCl2, 1.2 MgSO4, 1.2 KH2PO4, 5 Pyruvate, 11 glucose, 0.5 EDTA, 25 NaHCO3, and 1200 U l−1 heparin, and equilibrated with 95% O2–5% CO2 at 37°C. A 3-F pressure-transducing catheter was inserted through the aortic valve into the left-ventricular chamber of each heart to monitor pressure and heart rate. Coronary flow was measured via one-minute collection of the coronary effluent. After a 5 min stabilization period and baseline recordings, ischaemia was initiated.
Ischaemia–reperfusion protocol
Regional ischaemia was induced by threading a suture around the left anterior coronary artery 3–5 mm distal to the aorta and tightening the suture to occlude the artery with the use of a removable snare. Ischaemia lasted for 1 h. At the end of ischaemia, the snare was loosened and reperfusion ensued for 2 h. Recordings of left ventricular pressure development and coronary flow were collected every 15 min, with additional recordings every other minute for the first 5 min of reperfusion.
Measurement of infarct size
At the end of reperfusion, the snare was re-tightened and 100 μl of 0.5% Evans blue dye was injected into the Langendorff apparatus by way of a valve attached to the cannula. Following administration of Evans blue, hearts were cut down from the cannula, the right ventricle and atria removed, and the heart sliced into four equal slices from apex to base. Each slice was placed in a triphenyltetrazolium chloride solution to stain the zone at risk and incubated at 37°C for 10 min in a shaking water-bath. Following this brief incubation, both sides of each slice were photographed with a digital camera attached to a microscope. Images were transferred to a personal computer and areas were quantified in a single-blinded manner using ImageJ software. Total area (TA), zone at risk (ZAR), and infarct area (IA) were measured for each side of each slice. ZAR was determined by the exclusion of Evans blue dye, and IA was determined by white-appearing tissue within the ZAR. The mean TA, ZAR and IA for each slice were determined using the average of both sides of a slice. Areas were converted to weights by multiplying the TA, ZAR and IA of each slice by the slice weight. Summation of the TA weight, ZAR weight and IA weight for each slice yielded the TA, ZAR and IA weights for the entire left ventricle. Finally, ZAR weight was divided by TA weight, and IA weight was divided by ZAR weight to obtain the percentage of the heart at risk for ischaemia and the percentage of the ZAR that was infarcted, respectively. Infarct size is uniformly presented as a function of the ZAR.
Exclusion criteria
Hearts were excluded from analysis if coronary flow (CF) did not decrease when the coronary snare was tightened (indicative of ineffective snare placement), if CF increased during ischaemia (indicative of snare loosening), or if there were problems with the injection of dyes and/or with the clarity of the digital images of the heart slices that precluded clear assessment of ZAR or IA. If any of these exclusion criteria were met, all data from the affected heart were omitted from analysis; a total of 12 hearts were excluded from analysis in this study. We only included hearts from which both infarct size and flow/pressure data were successfully obtained.
Statistical analysis
Infarct size, zone at risk, and morphological data were analysed using a 2 (sex) × 3 (training group) ANOVA. Coronary flow and LV pressure data were analysed using a repeated measures (time) ANOVA. When appropriate, simple effects of training (within sex) and sex (within Sed group) were evaluated with Student's t test. Comparisons of Western blot band density were made with a two-tailed t test. In the exercise studies, a one-tailed t test was used to evaluate infarct size using the a priori hypothesis that exercise training would result in a decrease in infarct size. There is strong literature precedent for this a priori hypothesis (Yamashita et al. 1999, 2001; Brown et al. 2003; Hamilton et al. 2003). All data are presented as means ± standard error of the mean (s.e.m.).
Results
Morphology
Body weights for male and female rats used in the study were 448 ± 6 g and 268 ± 4 g, respectively. Body weight was significantly lower in female rats compared to male rats (P < 0.001), and neither 1 nor 5 days of exercise training altered body weight in either sex. Left ventricle (LV) weight in Sed, 1 day and 5 day males was 0.985 ± 0.028 g, 0.954 ± 0.026 g and 0.956 ± 0.034 g, respectively. LV weight in female rats was 0.609 ± 0.021 g, 0.673 ± 0.024 g and 0.670 ± 0.028 g in Sed, 1 day and 5 day animals, respectively. Male rats had significantly greater LV weights than females (P < 0.001), and exercise training did not influence LV weight in either sex (ANOVA).
Infarct Size
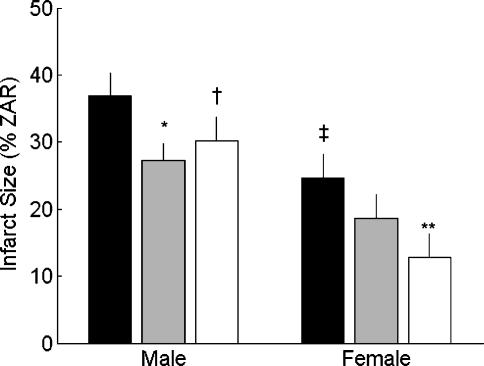
There were no significant differences in zone-at-risk (ZAR) between any of the experimental groups. Importantly, within each experimental group and across all experimental groups combined, there was no relationship between infarct size and ZAR (r= 0.01). This provides assurance that between-group differences in infarct size were due to experimental conditions rather than to differences in ZAR. Infarct size data are presented in Fig. 1. Main effects of the 2 × 3 ANOVA revealed significant sex (P < 0.01) and exercise group (P < 0.05) differences in infarct size. Sed female hearts had significantly smaller infarct sizes (expressed as a percentage of the ZAR) than Sed males (25 ± 3%versus 37 ± 3%, respectively; P < 0.001). One day of exercise did not significantly reduce infarct size in females (19 ± 3%), but female hearts from the 5 day group had smaller infarct sizes when compared to Sed females (13 ± 2%versus 25 ± 3%, respectively; P < 0.05). Contrary to the females, hearts from the 1 day males had smaller infarcts when compared to Sed males (27 ± 3%versus 37 ± 3%, respectively; P < 0.05), and infarct sparing appeared to be sustained in the 5 day males compared to the Sed males (30 ± 4%versus 37 ± 3%, P = 0.095).
Figure 1. Left ventricular infarct size following ischaemia–reperfusion.
Infarct size is expressed as a percentage of the zone at risk (ZAR). Bars represent means ± s.e.m. for sedentary (n = 8 males and 8 females, black bars), 1 day (n = 10 males and 8 females, grey bars), and 5 day (n = 6 males and 6 females, white bars) groups (*P < 0.05 versus Sed male; †P < 0.1 versus Sed male; ‡P < 0.01 versus Sed male; **P < 0.05 versus Sed female).
Coronary Flow
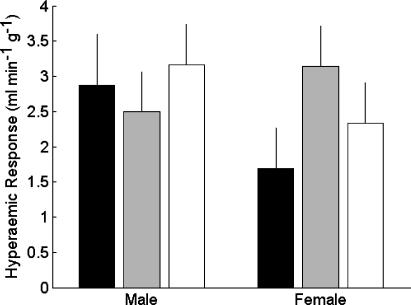
In the interest of clarity, only the flow measurements from the baseline, 1 h of ischaemia, and 1 h of reperfusion recordings are presented (Table 1). There were no differences in coronary flow at any time points between Sed male and female or within a sex as a function of training group. The decline in coronary flow following the onset of ischaemia was not different between experimental groups, consistent with our observation that all hearts had similar ZAR. The increase in flow from the last minute of ischaemia to the first minute of reperfusion was used as an index of hyperaemic response (Fig. 2), and no statistically significant differences in the hyperaemic response were observed between sexes or training groups.
Table 1.
Coronary flow and left ventricular developed pressure (LVDP) at baseline, 1 h following the onset of ischaemia, and 1 h following the onset of reperfusion
| Flow baseline (ml min−1 g−1) | Flow 60 min post-onset Ischaemia (ml min−1 g−1) | Flow 60 min post-onset reperfusion (ml min−1 g−1) | LVDP baseline (mmHg) | LVDP 60 min post-onset ischaemia (mmHg) | LVDP 60 min post-onset reperfusion (mmHg) | |
|---|---|---|---|---|---|---|
| Sed male | 17.4 ± 0.6 | 12.5 ± 0.9 | 10.8 ± 0.9 | 96 ± 1 | 88 ± 4 | 51 ± 11 |
| 1 day male | 17.2 ± 1.1 | 12.2 ± 1.2 | 10.6 ± 1.5 | 94 ± 1 | 90 ± 3 | 64 ± 11 |
| 5 day male | 15.1 ± 0.9 | 12.1 ± 0.6 | 11.2 ± 1.6 | 94 ± 2 | 93 ± 2 | 65 ± 10 |
| Sed female | 18.8 ± 0.9 | 10.7 ± 1.0 | 9.8 ± 0.6 | 92 ± 1 | 67 ± 8 | 43 ± 3 |
| 1 day female | 18.6 ± 0.7 | 11.4 ± 1.1 | 13.0 ± 1.4 | 97 ± 2 | 76 ± 11 | 72 ± 13 |
| 5 day female | 20.2 ± 2.8 | 13.3 ± 2.0 | 12.8 ± 2.1 | 89 ± 2 | 72 ± 10 | 48 ± 11 |
Figure 2. Hyperaemic response for male and female hearts at the onset of reperfusion.
Mean increases in coronary flow from the end of ischaemia (minute 60) through the first minute of reperfusion (minute 61) are plotted as means ± s.e.m. Black bars represent Sed (n = 8 males and 8 females), grey bars represent 1 day (n = 10 males and 8 females), and white bars represent 5 day (n = 6 males and 6 females) hearts. No statistically significant differences were observed in the hyperaemic response from hearts in the study.
Mechanical performance
Left ventricular developed pressure (LVDP) recordings from the baseline, 1 h of ischaemia, and 1 h of reperfusion time points are also presented in Table 1. There was a strong positive correlation between LVDP and coronary flow for all data points in the study (n = 653; r= 0.64, P < 0.001). Using a repeated measures analysis of variance, we found no differences in LVDP between any of the groups at any time. There were no differences in the maximal rate of contraction or relaxation (± dp/dt) in either sex or between training groups at any point during the I/R protocol (repeated measures ANOVA).
Superoxide dismutase protein expression
Sed female (n = 4) hearts had significantly greater MnSOD (Fig. 3A) and CuZnSOD protein expression than Sed male (n = 4) hearts (P < 0.05, Student's t test). Neither 1 day (n = 5) nor 5 days (n = 5) of exercise training altered the expression of either isoform of SOD protein in males (Fig. 3B) or females (Fig. 3C).
ATP-sensitive potassium channel expression
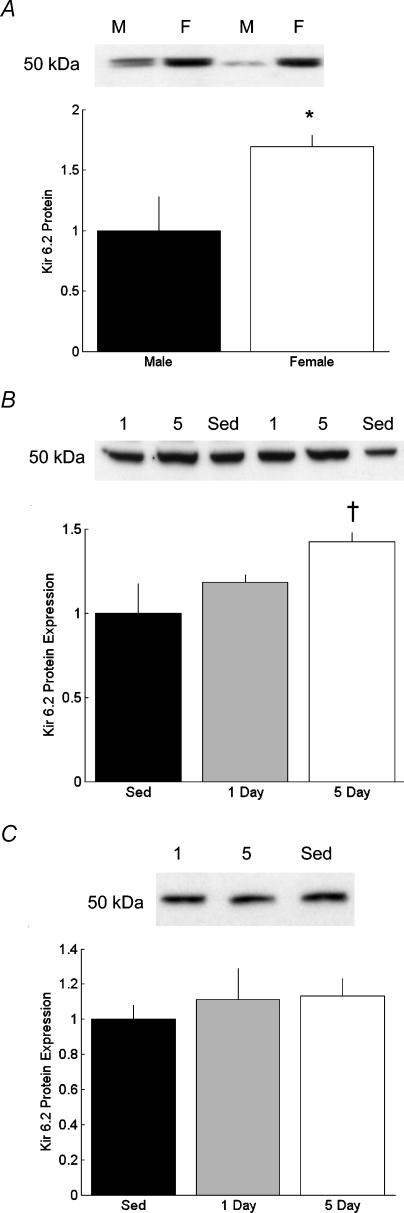
Sed female (n = 4) had significantly greater (P < 0.05, Student's t test) Kir6.2 protein density than Sed males (n = 4; Fig. 4A). Five day males had significantly greater Kir6.2 protein density than Sed males (P < 0.1; Fig. 4B), while there were no differences in Kir6.2 following training in the females (Fig. 4C). A sex difference was also observed in the expression of SUR protein. Female hearts (n = 4) had significantly higher SUR protein than male hearts (n = 4) (Fig. 5A). Exercise training led to a significant increase in SUR protein in the 1 day male group (n = 4, P < 0.05), and a statistically trendy increase in SUR in the 5 day males (Fig. 5B; n = 5, P = 0.09). In female hearts, SUR did not increase significantly in the 1 day group (n = 5, P = 0.185), but did increase significantly in the 5 day group (Fig. 5C; n = 5, P < 0.05).
Figure 4. Myocardial protein expression of the pore-forming subunit of the sarcolemmal ATP-sensitive K+ channel (Kir6.2).
Representative Western blots and band density for Kir6.2 from male and female hearts. A, Kir6.2 protein from Sed male (M, n = 4) and Sed female (F, n = 4) hearts. Data are expressed as a function of Sed male (*P < 0.05 versus Sed male) band density. B, myocardial Kir6.2 protein from male rats exposed to 0 (Sed, n = 4), 1 (n = 5) or 5 (n = 5) days of exercise (†P < 0.1 versus Sed male). C, myocardial Kir6.2 protein from female rats exposed to 0 (Sed, n = 4), 1 (n = 5) or 5 (n = 5) days of exercise.
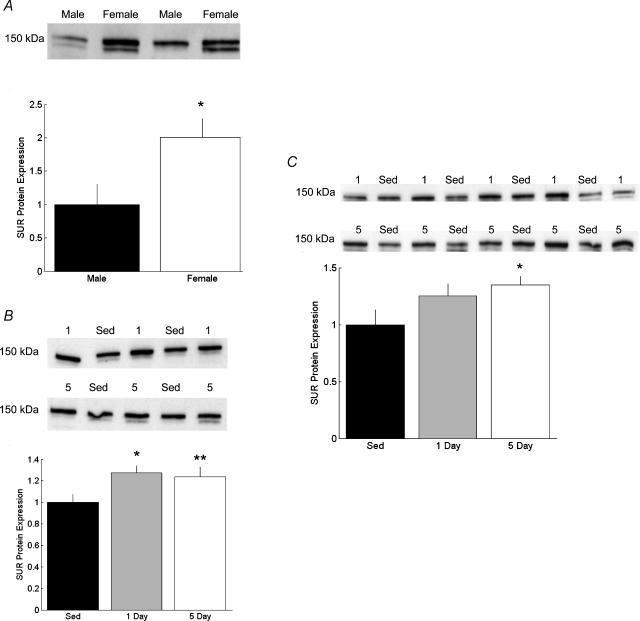
Figure 5. Myocardial protein expression of the sulphonylurea receptor (SUR).
Representative Western blots and band density for SUR from male and female hearts. A, SUR protein from Sed male (n = 4) and Sed female (n = 4) hearts. Band density is presented as a function of Sed male (*P < 0.05 versus Sed male). B, myocardial SUR protein expression from male rats exercised for 0 (Sed, n = 4), 1 (n = 4), or 5 (n = 5) days (*P < 0.05 versus Sed male; **P < 0.1 versus Sed male). C, myocardial SUR protein expression from female rats exercised for 0 (Sed, n = 4), 1 (n = 5), or 5 (n = 5) days (*P < 0.05 versus Sed female).
Discussion
Sex differences in infarct size
A significant finding of our research was that infarcts produced by a standardized protocol of ischaemia–reperfusion (I/R) were smaller in hearts from female than from male rats (Fig. 1). Previous studies using different species and an in situ model of I/R have yielded equivocal results with investigators finding no sex difference (Li & Kloner, 1995; Przyklenk et al. 1995; McNulty et al. 2000) or a decrease in infarct size in female hearts (Lee et al. 2000) following I/R. A major difference between our work and these previous studies is that we used an in vitro model of I/R to induce myocardial infarction. We have previously commented in detail on the advantages and disadvantages of the in situ versus in vitro model of I/R (Brown et al. 2003), but will briefly reiterate that the in vitro preparation permits observation of intrinsic myocardial differences as well as changes in coronary flow in response to I/R. To the best of our knowledge, this is the first demonstration that hearts from Sed females have smaller infarcts following in vitro ischaemia–reperfusion than do hearts from males.
There could be several mechanisms underlying our finding that hearts from females are intrinsically more resistant to I/R tissue damage than hearts from males (results are summarized in Table 2). The absence of sex-related differences in coronary flow and postischaemic hyperaemia (see Fig. 2) rules out a coronary vascular explanation. One possible explanation for the sex difference in infarct size is a significant increase in MnSOD protein expression in female versus male rats (Fig. 3). In several rat models of acquired cardioprotection, increased MnSOD activity and/or protein expression have repeatedly been correlated with infarct-sparing (Yamashita et al. 1999; Brown et al. 2003; Hamilton et al. 2003). Furthermore, prevention of the increase in MnSOD expression with the use of antisense oligonucleotides has consequently abolished the cardioprotection afforded by heat stress (Yamashita et al. 2000), adenosine A1 receptor agonist treatment (Dana et al. 2000), and an acute bout of exercise (Yamashita et al. 1999). In this context, the inverse relationship between MnSOD protein expression and I/R-induced infarct size across males and females that we observed is not surprising. Previous investigations of sex differences in total SOD activity have found either no differences in rabbit (Furuya & Chaudhuri, 1993) or greater total SOD activity in female rats (Barp et al. 2002), but these studies did not examine MnSOD specifically. Sex differences in the generation of superoxide anion have been observed in rat vasculature (Brandes & Mugge, 1997), and sex hormones have been shown to influence the activity of SOD (Barp et al. 2002). The data presented herein provide the first demonstration of a sex-specific difference in myocardial MnSOD protein expression.
Table 2.
Summary of sedentary (Sed) male versus Sed female differences in myocardial susceptibility to post-I/R tissue injury, coronary flow, post-I/R hyperaemia, and sarcolemmal ATP-sensitive potassium channel subunit protein expression
| Sed female (relative to Sed male) | |
|---|---|
| Infarct Size | ↓ |
| Coronary Flow | NoΔ |
| Post-I/R reactive hyperaemia | NoΔ |
| MnSOD protein expression | ↑ |
| Kir6.2 protein expression | ↑ |
| SUR(2 A) protein expression | ↑ |
‘NoΔ’, ‘↓’, and ‘↑’ are, respectively, indicative of no change, a decrease, or an increase compared to the sedentary (Sed) male group.
A novel finding from our study, and one which may help explain the observed sex-dependent susceptibility of the heart to I/R-induced tissue injury, is that the protein expression of the sarcolemmal ATP-sensitive potassium (KATP) channel was highest in hearts from female rats. In the heart, the KATP channel exists as an octomer consisting of four pore-forming subunits, Kir6.2, and four accessory proteins characterized as sulphonylurea receptors (SUR); the cardiac isoform is SUR2A (Inagaki et al. 1995). We found that both Kir6.2 and SUR protein expression were highest in hearts from females (Figs 4A and 5A). Previous studies have reported a greater protein expression of SUR, but not Kir6.2 protein in hearts from female rats (Ranki et al. 2001). These prior studies also concluded that increased expression of SUR alone was sufficient to increase the assembly of functional sarcolemmal KATP channels on the basis of the observation that pinacidil-activated KATP current density was greatest in cardiocytes isolated from female rats (Ranki et al. 2001).
The precise role of sarcolemmal KATP channels in cardioprotection is unknown. However, opening of sarcolemmal KATP channels with pharmacological agents has been shown to reduce infarct size in dogs (Gross & Auchampach, 1992), and blockade or genetic knockout of these sarcolemmal channels abolishes the protective effects of ischaemic preconditioning (Gross & Auchampach, 1992; Gumina et al. 2003). It has been proposed that the sarcolemmal KATP channel may act as an important cellular energy sensor that is involved in a mechanism for sustaining cellular ATP levels under conditions of metabolic stress and in conferring tissue resistance to infarction. Improved communication between the membrane sensors of ATP concentration (i.e. sarcolemmal KATP channels) and myocardial producers of ATP (i.e. glycolytic pathways and, to a lesser extent in ischaemia, the mitochondria) through phosphotransfer networks (Dzeja & Terzic, 1998) may be associated with the protection from myocardial infarction that we observed in hearts from sedentary females.
Exercise-induced infarct sparing
While sex differences in postischaemic ventricular performance have been observed in the rat following a brief exercise regimen (Paroo et al. 2002), no study to date has examined sex differences in infarct size following short-term exercise. Several studies have been conducted using a rat model in which the infarct sparing effects of short-term exercise have shown reductions in infarct size in males following 1 day (Yamashita et al. 1999; Yamashita et al. 2001) and in females following 5 days (Hamilton et al. 2003) of exercise. Our data corroborate these earlier findings (see Fig. 1), and also extend them by showing that while short-term exercise training leads to infarct sparing in both sexes, the acquisition of cardioprotection against infarction occurs more rapidly in males than females (see Table 3). The finding that males acquire protection more rapidly than females after an exercise bout has been previously described in a setting of myocardial stunning (Paroo et al. 2002) and will be discussed in more detail below.
Table 3.
Summary of training-induced differences in myocardial susceptibility to post-I/R tissue injury, coronary flow, post-I/R hyperaemia, and sarcolemmal ATP-sensitive potassium channel subunit protein expression
| Female | Male | |||||
|---|---|---|---|---|---|---|
| Sed | 1 d | 5 d | Sed | 1 d | 5 d | |
| Infarct Size | — | noΔ | ↓ | — | ↓ | ↓ |
| Coronary Flow | — | noΔ | noΔ | — | noΔ | noΔ |
| Post-I/R reactive hyperaemia | — | noΔ | noΔ | — | noΔ | noΔ |
| MnSOD protein expression | — | noΔ | noΔ | — | noΔ | noΔ |
| Kir6.2 protein expression | — | noΔ | noΔ | — | noΔ | ↑ |
| SUR(2 A) protein expression | — | noΔ | ↑ | — | ↑ | ↑ |
‘NoΔ’, ‘↓’, and ‘↑’ are, respectively, indicative of no change, a decrease, or an increase compared to the corresponding sedentary (Sed) control group.
From a methodological standpoint it is notable that the aforementioned investigations demonstrating exercise-induced infarct sparing have been performed using both in vitro (Yamashita et al. 1999; this study) and in situ perfusion protocols (Yamashita et al. 2001; Hamilton et al. 2003). While a potential limitation to the in vitro experiments could be the use of a crystalloid perfusate, our results demonstrating exercise-induced infarct sparing (Fig. 1) are consistent with studies using an in situ preparation in both males (Yamashita et al. 1999) and females (Hamilton et al. 2003). Moreover, our finding that one bout of exercise reduced infarct size in males by approximately 30% is consistent in magnitude with published data using the in situ preparation (Yamashita et al. 1999).
In our previous work (Brown et al. 2003), we showed that 20 weeks of endurance training was sufficient to reduce infarct size, and that one potential mechanism underlying the training-induced cardioprotection was a training-induced improvement in coronary flow, specifically an increase in flow upon the onset of reperfusion (e.g. postischaemic hyperaemia). Therefore, another objective of the current study was to determine the influence of coronary flow, and specifically a hyperaemic response to the ZAR, on infarct-sparing following short-term exercise. Exercise training has been shown to alter vascular reactivity in many models (Laughlin et al. 1998) but few studies have examined I/R-induced infarct size and coronary flow simultaneously. One such study examining the effects of one day of exercise on infarct size (in male rats) and using a markedly shorter ischaemic bout reported modest changes in coronary flow following training, with the trained animals better preserving flow towards the end of reperfusion (Yamashita et al. 2001). However, these authors did not observe a hyperaemic response at the onset of reperfusion (Yamashita et al. 2001). Our findings in hearts from male rats (Fig. 2) corroborate these data. These data lead to the novel conclusion that the infarct-sparing effects of acute exercise training can be obtained in the complete absence of any improvements in coronary flow or postischaemic hyperaemia.
Interestingly, the infarct-sparing effects of short-term exercise that we observed were not correlated with increased MnSOD protein expression. While we did observe an inverse relationship between infarct size and MnSOD expression in Sed animals across sexes, we did not observe increases in MnSOD protein expression in any of our exercise groups. To the best of our knowledge, this is the first study to show that exercise-induced reductions in infarct size can be observed in the absence of elevations in MnSOD expression. It is likely that there are multiple pathways, both related and in parallel, that can lead to infarct-sparing that may involve sex hormones (Sbarouni et al. 1998; Lee et al. 2000; Sbarouni et al. 2003), cytokines (Yamashita et al. 1999), opioids (Cohen et al. 2001), and the KATP channel (see below) to name a few. Our observation that MnSOD protein levels were related to infarct-sparing between the sexes but not following exercise may be a reflection of the complexity of the myocardial defence system to I/R damage. Our finding that short-term exercise did not increase MnSOD protein expression differs from that of Yamashita et al. (1999). These authors found that 1 day of training reduced infarct size 36–60 h post-exercise, and that increased MnSOD content occurred 48 h post-exercise. It should be noted, however, that these authors did not measure MnSOD content at the other two corresponding time points where infarct sparing was observed. Possible explanations for the differences between our findings that MnSOD did not change (Fig. 3B and C) and that of the previous study could be methodological in nature. Yamashita et al. used an ELISA to measure MnSOD, while Western blotting was used herein. Furthermore, we used a low-speed clarifying spin and homogenizing buffer containing 1% NP-40 to purify our samples before electrophoresis (see Methods). Testing of the pellet fraction revealed that MnSOD did not sediment in the pellet following the centrifugation in solution containing NP-40 (Fig. 3A) and that our measurements of MnSOD in the supernatant were truly representative of the cellular total. Removal of NP-40 from the homogenizing buffer led to contamination of the pellet fraction with MnSOD (Fig. 3A). It is not clear from earlier work (Yamashita et al. 1999) how the samples were prepared, which may also explain the discrepancy between these two studies. Since we did not observe increased MnSOD expression following short-term exercise, it appears that other pathways, independent of increases in MnSOD protein content, are responsible for the infarct-sparing observed in this study.
In this study, the only cellular correlate of exercise-acquired infarct sparing was the increased expression of one or both of the subunits of the sarcolemmal KATP channel. In both males and females, increased SUR protein expression was always observed when infarct sparing was evident (Figs 1 and 5). In addition, we observed increases in Kir6.2 protein in 5 day males compared to Sed males (Fig. 4B). Again, early studies using guinea pigs have shown that increased expression of SUR, not Kir6.2, was the rate-limiting factor in functional sarcolemmal KATP channel formation (Ranki et al. 2001). Our observation that both sex- and exercise-dependent increases in SUR protein expression were always coincident with infarct sparing suggests that protection from infarction may be related to increased functional expression of sarcolemmal KATP channels. One potential limitation to this interpretation is that we used LV homogenate (composed of septum and LV free wall) for Western blotting, yet the major part of the zone at risk for infarction was in the LV free wall. The possibility that exercise increased KATP channel expression preferentially in the septal wall and was not responsible for the infarct-sparing observed primarily in the LV free wall cannot be completely disregarded. However, we are unaware of any data indicating regional heterogeneity of sarcolemmal KATP channel expression so this alternative explanation seems unlikely at present.
Although a paucity of data exists relating the role of these channels with the protective effects of exercise, previous experiments from our laboratory have examined KATP current characteristics in a chronic training model. Jew & Moore (2002) found that chronic training decreased the anoxia-induced expression and magnitude of the sarcolemmal KATP current in single rat cardiocytes, a finding that may be related to an improved sustaining of myocardial ATP levels in chronically trained animals following I/R that we previously observed (Jew & Moore, 2001). An increase in KATP channel protein expression and our earlier work demonstrating a training-induced reduction in the magnitude of anoxia-induced KATP current density seems counter-intuitive, but this counter-intuition may assume an oversimplification of the regulatory system. Previous work correlating sex-dependent increases in KATP current with protection from I/R-induced Ca2+ overload (Ranki et al. 2001) did not elicit KATP current with ischaemia, but rather with a pharmacological opener of the channel, pinacidil. It is likely that a pharmacological opener would elicit a near-maximal response of the channels that, while informative, may not be physiologically relevant. Cardioprotection could be obtained by increasing the number of KATP channels, which would in turn improve the cellular sensitivity of the sarcolemmal energy sensor to declining ATP levels, and thus better preserve the metabolic state of the cell during I/R. Previous studies by our laboratory and others have clearly demonstrated that exercise training ultimately improves cellular ATP levels following I/R (Bowles et al. 1992; Jew & Moore, 2001). An improved metabolic status of the cell (i.e. increased ATP levels) would in turn have a suppressant effect on the sarcolemmal KATP current in the face of metabolic stress. Whether or not this scenario is physiologically accurate warrants further investigation.
Left ventricular pressure development
We found no differences in developed pressure between the sexes or training groups, indicating that protection from infarction that we observed was not associated with improved myocardial function between the groups. Our finding that short-term exercise training did not influence LVDP during I/R differs from those of other investigators (Demirel et al. 2001; Hamilton et al. 2001) who found that 3–5 days of exercise led to the sustaining of mechanical performance in rat during both ischaemia and reperfusion. Paroo et al. (2002) reported improved postischaemic cardiac function after a single exercise bout in male, but not female, rats. A likely explanation for this discrepancy is that our 1 h/2 h I/R protocol necessary for infarction was significantly more severe than the myocardial stunning protocols used by these investigators. While we have previously observed a training-induced preservation of LVDP with this protocol (Brown et al. 2003), the experimental animals were exercised for 20 weeks as opposed to a few days (this study). It may be that gradual intrinsic adaptations of the myocardium after exposure to short-term exercise permit observation of improved mechanical performance with shorter I/R protocols, but that preservation of LVDP after a 1 h/2 h I/R requires a longer training period. More experiments are needed to characterize the temporal onset of training-induced preservation in LVDP following I/R.
Summary and conclusion
In summary, we have demonstrated that female hearts are intrinsically more resistant to ischaemia–reperfusion injury than male hearts. Exercise training significantly reduced infarct size in both sexes, but protection against infarction was acquired more rapidly in males. Several mechanisms previously thought to be involved in exercise-mediated infarct-sparing including increased MnSOD and improved coronary flow were not associated with the cardioprotection observed in this study. A putative mechanism that may have contributed to infarct sparing was the novel finding that sulphonylurea receptor protein expression was elevated in every experimental setting where infarct size was reduced. These data indicate that both intrinsic sex differences in the susceptibility to infarct size and exercise-induced reductions in infarct size may be related to increased expression of sarcolemmal KATP channel subunits.
Acknowledgments
This work was supported by PHS grant HL40306 (R.L.M.), National Institute of Ageing Training Grant (AG 279-04; D.A.B.), Beverly Sears Graduate Student Grant Program at the University of Colorado (D.A.B.), and the National Institutes of Health/Howard Hughes Medical Institute Scholarship Program for Diversity (NIH GM066728-01; M.S.J.). The authors wish to acknowledge the contributions of Nadia Reynolds for her help with the experimental animals, and Drs Adam Chicco and Korinne Jew for editorial assistance with the manuscript.
References
- Barp J, Araujo AS, Fernandes TR, Rigatto KV, Llesuy S, Bello-Klein A, et al. Myocardial antioxidant and oxidative stress changes due to sex hormones. Braz J Med Biol Res. 2002;35:1075–1081. doi: 10.1590/s0100-879x2002000900008. [DOI] [PubMed] [Google Scholar]
- Bolli R. The late phase of preconditioning. Circ Res. 2000;87:972–983. doi: 10.1161/01.res.87.11.972. [DOI] [PubMed] [Google Scholar]
- Bowles DK, Farrar RP, Starnes JW. Exercise training improves cardiac function after ischemia in the isolated, working rat heart. Am J Physiol. 1992;263:H804–H809. doi: 10.1152/ajpheart.1992.263.3.H804. [DOI] [PubMed] [Google Scholar]
- Bowles DK, Starnes JW. Exercise training improves metabolic response after ischemia in isolated working rat heart. J Appl Physiol. 1994;76:1608–1614. doi: 10.1152/jappl.1994.76.4.1608. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Mugge A. Gender differences in the generation of superoxide anions in the rat aorta. Life Sci. 1997;60:391–396. doi: 10.1016/s0024-3205(96)00663-7. [DOI] [PubMed] [Google Scholar]
- Brown DA, Jew KN, Sparagna GC, Musch TI, Moore RL. Exercise training preserves coronary flow and reduces infarct size following ischemia-reperfusion in rat heart. J Appl Physiol. 2003;95:2510–2518. doi: 10.1152/japplphysiol.00487.2003. [DOI] [PubMed] [Google Scholar]
- Cohen MV, Yang XM, Liu GS, Heusch G, Downey JM. Acetylcholine, bradykinin, opioids, and phenylephrine, but not adenosine, trigger preconditioning by generating free radicals and opening mitochondrial KATP channels. Circ Res. 2001;89:273–278. doi: 10.1161/hh1501.094266. [DOI] [PubMed] [Google Scholar]
- Dana A, Jonassen AK, Yamashita N, Yellon DM. Adenosine A1 receptor activation induces delayed preconditioning in rats mediated by manganese superoxide dismutase. Circulation. 2000;101:2841–2848. doi: 10.1161/01.cir.101.24.2841. [DOI] [PubMed] [Google Scholar]
- Demirel HA, Powers SK, Zergeroglu MA, Shanely RA, Hamilton K, Coombes J, et al. Short-term exercise improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. J Appl Physiol. 2001;91:2205–2212. doi: 10.1152/jappl.2001.91.5.2205. [DOI] [PubMed] [Google Scholar]
- Dzeja PP, Terzic A. Phosphotransfer reactions in the regulation of ATP-sensitive K+ channels. FASEB J. 1998;12:523–529. doi: 10.1096/fasebj.12.7.523. [DOI] [PubMed] [Google Scholar]
- Furuya K, Chaudhuri G. Estradiol does not influence myocardial superoxide dismutase activity in rabbits. J Cardiovasc Pharmacol. 1993;22:65–68. doi: 10.1097/00005344-199307000-00011. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Auchampach JA. Blockade of ATP-sensitive potassium channels prevents myocardial preconditioning in dogs. Circ Res. 1992;70:223–233. doi: 10.1161/01.res.70.2.223. [DOI] [PubMed] [Google Scholar]
- Gross GJ, Peart JN. KATP channels and myocardial preconditioning: an update. Am J Physiol Heart Circ Physiol. 2003;285:H921–H930. doi: 10.1152/ajpheart.00421.2003. [DOI] [PubMed] [Google Scholar]
- Gumina RJ, Pucar D, Bast P, Hodgson DM, Kurtz CE, Dzeja PP, et al. Knockout of Kir6.2 negates ischemic preconditioning-induced protection of myocardial energetics. Am J Physiol Heart Circ Physiol. 2003;284:H2106–H2113. doi: 10.1152/ajpheart.00057.2003. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Powers SK, Sugiura T, Kim S, Lennon S, Tumer N, et al. Short-term exercise training can improve myocardial tolerance to I/R without elevation in heat shock proteins. Am J Physiol Heart Circ Physiol. 2001;281:H1346–H1352. doi: 10.1152/ajpheart.2001.281.3.H1346. [DOI] [PubMed] [Google Scholar]
- Hamilton KL, Staib JL, Phillips T, Hess A, Lennon SL, Powers SK. Exercise, antioxidants, and HSP72: protection against myocardial ischemia/reperfusion. Free Radic Biol Med. 2003;34:800–809. doi: 10.1016/s0891-5849(02)01431-4. 10.1016/S0891-5849(02)01431-4. [DOI] [PubMed] [Google Scholar]
- Inagaki N, Gonoi T, Clement J, Pt Namba N, Inazawa J, Gonzalez G, et al. Reconstitution of IKATP: an inward rectifier subunit plus the sulfonylurea receptor. Science. 1995;270:1166–1170. doi: 10.1126/science.270.5239.1166. [DOI] [PubMed] [Google Scholar]
- Jew KN, Moore RL. Glibenclamide improves postischemic recovery of myocardial contractile function in trained and sedentary rats. J Appl Physiol. 2001;91:1545–1554. doi: 10.1152/jappl.2001.91.4.1545. [DOI] [PubMed] [Google Scholar]
- Jew KN, Moore RL. Exercise training alters an anoxia-induced, glibenclamide-sensitive current in rat ventricular cardiocytes. J Appl Physiol. 2002;92:1473–1479. doi: 10.1152/japplphysiol.00513.2001. 10.1063/1.1485110. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Oltman CL, Bowles DK. Exercise training-induced adaptations in the coronary circulation. Med Sci Sports Exerc. 1998;30:352–360. doi: 10.1097/00005768-199803000-00004. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Rubin LJ, Rush JW, Price EM, Schrage WG, Woodman CR. Short-term training enhances endothelium-dependent dilation of coronary arteries, not arterioles. J Appl Physiol. 2003a;94:234–244. doi: 10.1152/japplphysiol.00246.2002. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Welshons WV, Sturek M, Rush JW, Turk JR, Taylor JA, et al. Gender, exercise training, and eNOS expression in porcine skeletal muscle arteries. J Appl Physiol. 2003b;95:250–264. doi: 10.1152/japplphysiol.00061.2003. [DOI] [PubMed] [Google Scholar]
- Lee TM, Su SF, Tsai CC, Lee YT, Tsai CH. Cardioprotective effects of 17 beta-estradiol produced by activation of mitochondrial ATP-sensitive K+ channels in canine hearts. J Mol Cell Cardiol. 2000;32:1147–1158. doi: 10.1006/jmcc.2000.1167. 10.1006/jmcc.2000.1167. [DOI] [PubMed] [Google Scholar]
- Li Y, Kloner RA. Is there a gender difference in infarct size and arrhythmias following experimental coronary occlusion and reperfusion? J Thromb Thrombolysis. 1995;2:221–225. doi: 10.1007/BF01062713. [DOI] [PubMed] [Google Scholar]
- Manfroi WC, Peukert C, Berti CB, Noer C, Gutierres Dde A, Silva FT. Acute myocardial infarction: the first manifestation of ischemic heart disease and relation to risk factors. Arq Bras Cardiol. 2002;78:392–395. doi: 10.1590/s0066-782x2002000400006. [DOI] [PubMed] [Google Scholar]
- McNulty PH, Jagasia D, Whiting JM, Caulin-Glaser T. Effect of 6-wk estrogen withdrawal or replacement on myocardial ischemic tolerance in rats. Am J Physiol Heart Circ Physiol. 2000;278:H1030–H1034. doi: 10.1152/ajpheart.2000.278.4.H1030. [DOI] [PubMed] [Google Scholar]
- Morris JN, Everitt MG, Pollard R, Chave SP, Semmence AM. Vigorous exercise in leisure-time: protection against coronary heart disease. Lancet. 1980;2:1207–1210. doi: 10.1016/s0140-6736(80)92476-9. 10.1016/S0140-6736(80)92476-9. [DOI] [PubMed] [Google Scholar]
- O'Rourke B. Myocardial KATP channels in preconditioning. Circ Res. 2000;87:845–855. doi: 10.1161/01.res.87.10.845. [DOI] [PubMed] [Google Scholar]
- Paroo Z, Haist JV, Karmazyn M, Noble EG. Exercise improves postischemic cardiac function in males but not females: consequences of a novel sex-specific heat shock protein 70 response. Circ Res. 2002;90:911–917. doi: 10.1161/01.res.0000016963.43856.b1. 10.1161/01.RES.0000016963.43856.B1. [DOI] [PubMed] [Google Scholar]
- Powers SK, Demirel HA, Vincent HK, Coombes JS, Naito H, Hamilton KL, et al. Exercise training improves myocardial tolerance to in vivo ischemia-reperfusion in the rat. Am J Physiol. 1998;275:R1468–R1477. doi: 10.1152/ajpregu.1998.275.5.R1468. [DOI] [PubMed] [Google Scholar]
- Przyklenk K, Ovize M, Bauer B, Kloner RA. Gender does not influence acute myocardial infarction in adult dogs. Am Heart J. 1995;129:1108–1113. doi: 10.1016/0002-8703(95)90390-9. 10.1016/0002-8703(95)90390-9. [DOI] [PubMed] [Google Scholar]
- Ranki HJ, Budas GR, Crawford RM, Jovanovic A. Gender-specific difference in cardiac ATP-sensitive K+ channels. J Am Coll Cardiol. 2001;38:906–915. doi: 10.1016/s0735-1097(01)01428-0. 10.1016/S0735-1097(01)01428-0. [DOI] [PubMed] [Google Scholar]
- Sbarouni E, Iliodromitis EK, Bofilis E, Kyriakides ZS, Kremastinos DT. Short-term estrogen reduces myocardial infarct size in oophorectomized female rabbits in a dose-dependent manner. Cardiovasc Drugs Ther. 1998;12:457–462. doi: 10.1023/a:1007750015372. 10.1023/A:1007750015372. [DOI] [PubMed] [Google Scholar]
- Sbarouni E, Iliodromitis EK, Bofilis E, Kyriakides ZS, Kremastinos DT. Estrogen alone or combined with medroxyprogesterone but not raloxifene reduce myocardial infarct size. Eur J Pharmacol. 2003;467:163–168. doi: 10.1016/s0014-2999(03)01627-3. 10.1016/S0014-2999(03)01627-3. [DOI] [PubMed] [Google Scholar]
- Starnes JW, Taylor RP, Park Y. Exercise improves postischemic function in aging hearts. Am J Physiol Heart Circ Physiol. 2003;285:H347–H351. doi: 10.1152/ajpheart.00952.2002. [DOI] [PubMed] [Google Scholar]
- World Health Organization. The World Health Report 2003. 2003. Facts and figures; pp. 1–6. World Health Organization. [Google Scholar]
- Yamashita N, Baxter GF, Yellon DM. Exercise directly enhances myocardial tolerance to ischaemia-reperfusion injury in the rat through a protein kinase C mediated mechanism. Heart. 2001;85:331–336. doi: 10.1136/heart.85.3.331. 10.1136/heart.85.3.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Otsu K, Asahi M, Kuzuya T, Hori M. Exercise provides direct biphasic cardioprotection via manganese superoxide dismutase activation. J Exp Med. 1999;189:1699–1706. doi: 10.1084/jem.189.11.1699. 10.1084/jem.189.11.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Hoshida S, Otsu K, Taniguchi N, Kuzuya T, Hori M. Involvement of cytokines in the mechanism of whole-body hyperthermia-induced cardioprotection. Circulation. 2000;102:452–457. doi: 10.1161/01.cir.102.4.452. [DOI] [PubMed] [Google Scholar]
- Yellon DM, Downey JM. Preconditioning the myocardium: from cellular physiology to clinical cardiology. Physiol Rev. 2003;83:1113–1151. doi: 10.1152/physrev.00009.2003. [DOI] [PubMed] [Google Scholar]