Abstract
The rectum receives specialized extrinsic afferent innervation by stretch-sensitive, low threshold, slowly adapting mechanoreceptors, with transduction sites shown to correspond to rectal intraganglionic laminar endings (IGLEs). Rectal IGLEs are located in myenteric ganglia, surrounded by the longitudinal and circular smooth muscle layers; in this study we investigated the mechanical stimuli to which they respond. Mechanoreceptors had graded responses to highly focal transmural compression with von Frey hairs. They were activated when stretched circumferentially by imposed increases of both length and load. Under both conditions, firing typically occurred in bursts associated with phasic muscle contractions. However, many contractions did not evoke firing. Longitudinal stretch also evoked firing, again associated with contractile activity. Thus, mechanoreceptors did not show directional sensitivity. Two agonists that excited smooth muscle directly (0.1 μm[β-Ala8]-neurokinin A (4–10) and 1 μm carbachol) activated rectal mechanoreceptors, but not in the presence of Ca2+-free solution or when preparations were kept entirely slack. We measured the dimensions, in both longitudinal and circumferential axes, of receptive fields during smooth muscle contractile activity, using video micrography. Contractile activity within the receptive field often differed significantly from the behaviour of the preparation as a whole, providing an explanation for many of the discrepancies between gross contractility and firing. Simultaneous contraction of both muscle layers within the receptive field was the strongest predictor of mechanoreceptor activation. Our results suggest that rectal mechanoreceptors do not act simply as tension receptors: rather they appear to detect mechanical deformation of myenteric ganglia – especially flattening – associated with stretch of the receptive field, or contractions of smooth muscle within the receptive field.
Distension activates a number of motor reflexes within the gastrointestinal tract. Over relatively short distances, these are mediated via the enteric nervous system, which contains intrinsic primary afferent neurones, interneurones and motor neurones arranged in functional circuits (Furness & Costa, 1987; Wood, 1994; Gershon, 1999). However, many distension-evoked reflexes involve neural pathways outside the gut. Thus, gastric accommodation, receptive relaxation, entero-gastric and entero-enteric reflexes all involve sympathetic and parasympathetic efferent pathways to the gut (Szurszewski & King, 1989; Mulak & Bonaz, 2004). These are activated either by enteric neurones that project to prevertebral ganglia (‘viscerofugal neurones’), or by extrinsic afferent neurones projecting to the central nervous system. Rectal distension activates extrinsic excitatory defaecatory reflexes mediated via pelvic nerve pathways (Yamanouchi et al. 2002), and in humans, damage to pelvic pathways plays a key role in many cases of obstructed defaecation (Denny-Brown & Robertson, 1935). Reduced sensitivity to rectal distension is associated with disordered defaecation in children and adults with spinal damage (Greving et al. 1998). Extrinsic afferent neurones also give rise to conscious sensations from the gut, such as feelings of fullness, bloating and pain, and in the rectum, urgency. Vagal afferent mechanoreceptors, with cell bodies in the nodose ganglion, are believed to mediate predominantly non-nociceptive sensations from the gut wall, especially the upper gut. Spinal afferent neurones are responsible for mediating predominantly mechano-nociceptive sensations: detecting noxious levels of distension, and activating central pain pathways (Sengupta & Gebhart, 1994).
Studies in patients with spinal lesions have indicated that although thoraco-lumbar spinal pathways are involved in mechano-nociception, sacral spinal pathways may mediate non-noxious sensations (Lembo et al. 1994). Recent studies have provided a cellular basis for these observations. Lynn et al. (2003) demonstrated the presence of low threshold, slowly adapting mechanoreceptors in the guinea pig rectum, which are very rare – or absent – more proximally in the colon. Dye filling studies have shown that the transduction sites of these low threshold mechanoreceptors correspond to specialized flattened endings within myenteric ganglia in the rectum called rectal IGLEs, or rIGLEs (Lynn et al. 2003; Olsson et al. 2004). These anatomically defined endings are rare or absent more proximally in the colon. Thus, in the rectum, but not the colon, a specialized class of low threshold afferent fibres exists which signals a wide range of distension.
Vagal mechanoreceptors to the upper gut bear many similarities to the specialized rectal mechanoreceptors described above. Both types of afferents have low mechanical thresholds, respond over a wide range of distension, and have a slowly adapting response to maintained stretch. Vagal mechanoreceptors have been shown to transduce mechanical deformation at specialized, flattened, branching structures (IGLEs) located within the myenteric ganglia that are structurally similar to rIGLEs (Zagorodnyuk & Brookes, 2000; Zagorodnyuk et al. 2001; Lynn et al. 2003). Both IGLEs and rIGLEs have been described to act like in-series tension receptors, their firing determined primarily by wall tension rather than length (Iggo, 1955; Blackshaw & Grundy, 1990; Page & Blackshaw, 1998; Zagorodnyuk et al. 2001; Lynn et al. 2003). This is despite the fact that anatomically these endings lie parallel to the muscle whose tension they reflect. In this study, we aimed to determine the adequate mechanical stimulus for specialized rectal mechanoreceptors, in terms of longitudinal and circular smooth muscle length and tension, within identified receptive fields.
Methods
Adult male and female guinea pigs (250–350 g) were killed by a blow to the occipital region and exsanguinated as approved by the Animal Welfare Committee of Flinders University. Specimens of distal bowel with attached rectal nerve trunks were excised, flushed of contents, and opened up into a flat sheet. The preparation was superfused at 3 ml min−1 with Krebs solution (mm; NaCl, 118; KCl, 4.75; NaH2PO4, 1.0; NaHCO3, 25; MgSO4, 1.2; CaCl2, 2.5; glucose, 11), chilled to approximately 10°C, and bubbled with 95% O2–5% CO2. The mucosa was removed by sharp dissection and three to seven small extrinsic nerve trunks (20–40 μm in diameter) were dissected free of connective tissue for a length of 2–6 mm. We defined the rectum as the region innervated by rectal nerve trunks emerging from the pelvic ganglia. The preparation was then cut down to size and pinned – serosal side up, with 50 μm tungsten pins – along one side.
Afferent dissection and recordings
The extrinsic nerve trunks and a strand of connective tissue were led under a coverslip partition into a recording chamber and sealed with silicon grease (Ajax, Australia). The recording chamber was then filled with paraffin oil; platinum wire electrodes were touched on a nerve trunk and on the connective tissue strand. Recorded signals were amplified and filtered through an isolated bio-amplifier (ISO-80, World Precision Instruments), then digitized with either a micro1401 (Cambridge Electronic Design) run with an IBM-compatible personal computer using Spike2 software (v.4.12, Cambridge Electronic Design), or a Powerlab/16SP (ADInstruments) and recorded on a Macintosh personal computer using Chart software (ADInstruments). The main chamber containing the preparation was superfused at 3 ml min−1 with oxygenated Krebs solution, at 34°C.
Activation of stretch-sensitive units
The edge of the preparation was attached to an array of hooks, resembling a miniature garden rake. This was attached to a ‘tissue stretcher’ consisting of a microprocessor-controlled stepper motor with an in-series isometric force transducer (DSC 46-1001-01, Kistler Morse). The preparation was maintained at approximately 1 mN basal force for the duration of experiments. Using this apparatus, the tissue could be stretched at rates of 10–5000 μm s−1 over distances of 1–5 mm, while recording the tension developed by the tissue (stretching by ‘imposed length’– see Fig. 1A). In other experiments, stretch was applied by applying an ‘imposed load’ to the tissue. In these cases, the rake was attached to the arm of an isotonic transducer via a pulley, and the counterweight was increased as either a step change by hanging weights of 10–50 mN or by infusing fluid into a reservoir to increase the counterweight in a graded fashion (Fig. 1B). Using this set-up, stretch was applied by ‘imposed load’, while recording the contractions of the tissue with the isotonic transducer. In all cases, a resting load of 5 mN was applied. Using these methods, stretch could be applied in either the longitudinal and/or circumferential axis of the preparation. It should be pointed out that fully isometric recordings from smooth muscle preparations are not achievable because serial elastic elements in the tissue allow some shortening of smooth muscle cells during contraction. Likewise, true isotonic recording conditions cannot be achieved because finite loads applied to the preparation stretch passive elastic components of the tissue, and force per cross-sectional area necessarily changes as the muscle shortens. The terms ‘near-isotonic’ and ‘near-isometric’ are used throughout this study for clarity to highlight the practical limitations of our recording techniques.
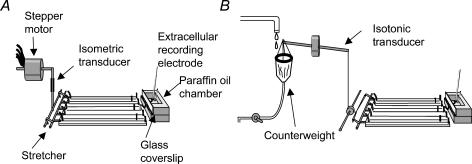
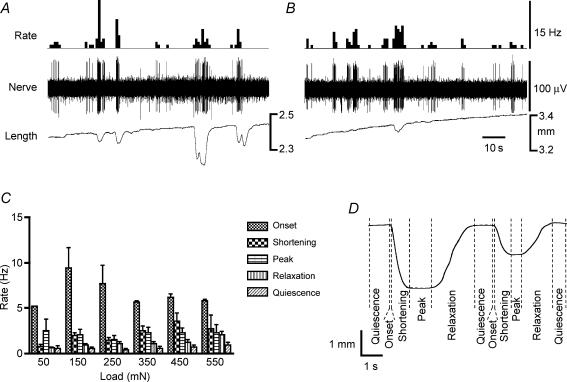
Figure 1. Preparations used to study effects of stretch on mechanoreceptor activity.
A, the preparation was pinned along one edge, serosa uppermost, and connected via a metal rake on the other edge to a microprocessor-controlled stepper motor. Changes in length of the preparation were imposed and the tension developed by the tissue was recorded via the isometric force transducer. B, the preparation was similarly pinned down one edge, but the rake on the other edge was attached to a thread that ran, via a pulley, to an isotonic length transducer. Stretch was applied by increasing the counterweight on the transducer while recording the resulting changes in length of the preparation, which was free to shorten. By aligning the preparation appropriately, it was possible to stimulate and record from longitudinal and/or circular muscle with either preparation.
Hotspot identification
Local mechanical distortion of the tissue was applied with von Frey hairs with tip diameters < 50 μm (0.1–1.10 mN). ‘Hotspots’ were then identified, and their positions marked on the tissue using fine carbon particles attached to the tip of the hair. These hotspots have previously been shown to correspond to rectal IGLEs in myenteric ganglia (Lynn et al. 2003). For this reason we refer to these sites in the text as ‘putative rIGLEs’. Stretch-sensitive units (n = 8, n = 4) were investigated for their sensitivity to von Frey hairs (0.1, 0.2, 0.3, 0.5, 0.8, 1.1 mN) under imposed length conditions.
Video imaging and spatiotemporal mapping
The location of one unit's receptive field was marked with four small opaque markers (area less than 1 mm2) placed on the preparation, after identifying hotspots with von Frey hairs. The preparation was then cut down to an L-shape, and a 1 mm wide hook was used to record longitudinal muscle tension aligned with the identified receptive field. A 5–6 mm wide hook was attached to record circular muscle force. Movements of the opaque markers were recorded at high magnification on a digital video camera recorder (DCR-TRV11E, Sony) fitted to the dissecting microscope. Video images were synchronized with electrophysiological and mechanical recordings by placing a small flashing LED in the corner of the video image, and recording the driving pulses in Chart (ADInstruments) together with electrical and mechanical activity. The area defined by the four markers, and the length of the receptive field in longitudinal and circular axes, was calculated from each frame of digital video using purpose-designed in-house software.
Drugs
Rhythmic muscle contractions were induced by application of [β-Ala8]-neurokinin A fragment 4–10 ([β-Ala8]-NKA(4–10); 0.1 μm, Sigma) or carbachol (0.06 μm, Sigma) to the bath. Tetrodotoxin was from Alomone Laboratories.
Analysis
Single units were identified and discriminated using Spike2 software or Chart Spike Histogram software; when single units could not be discriminated, recordings were discarded. Single unit activity was presented and analysed using impulses per second except where instantaneous frequency was used. Data are presented as mean ± s.e.m. unless otherwise stated; n refers to the number of single units and N to the number of animals. Statistical comparison was carried out using Student's t test and one- or two-way analysis of variance as appropriate; P < 0.05 was considered significant.
Results
Von Frey hairs
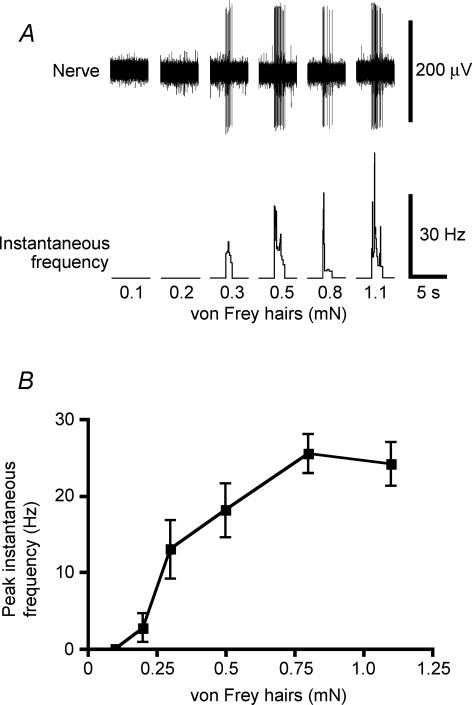
Rectal mechanoreceptors have previously been shown to have multiple small sites that are sensitive to mechanical distortion with a von Frey hair (Lynn et al. 2003). To characterize responses to vertical compression in more detail, a single transduction site was identified for each mechanoreceptor and marked on the surface of the preparation using fine carbon particles (Lynn et al. 2003). Hand-held von Frey hairs, with a range of stiffness, were then applied to the transduction site, and the peak instantaneous frequency calculated. Typically, mechanoreceptors responded with a burst of firing that stopped abruptly on removal of the stimulus (Fig. 2). Peak instantaneous frequency was positively related to stiffness of the hair (measured as a force equivalent) with a threshold between 0.1 and 0.3 mN (n = 8, n = 4), and reaching a maximal rate at 0.8–1.1 mN. Following application of 0.8 and 1.1 mN stimuli, the unit often required a recovery period of several minutes to regain its excitability. Stiffer hairs (> 2 mN) often irreversibly damaged the unit.
Figure 2. Rectal mechanoreceptors responded to focal transmural compression by von Frey hairs.
After identifying stretch-sensitive units, their distortion-sensitive sites were located with von Frey hairs and marked on the tissue. Repeated responses to hand-held von Frey hairs of a range of stiffnesses up to 1.1 mN were then recorded. Stiffer von Frey hairs often caused irreversible decreases in excitability. A, typical recording shows graded responses to increasing force. B, mean data from 8 units in 4 animals.
Mechanoreceptor firing under near-isometric and near-isotonic conditions
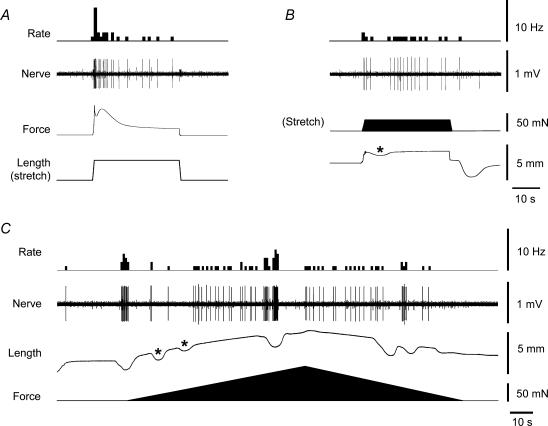
Under near-isometric conditions, imposed changes in circumference of the preparation using a ‘tissue stretcher’ (Brookes et al. 1999) evoked a brief burst of mechanoreceptor firing associated with a short-lived contraction at the onset of the stretch. Typically, this was followed by a marked adaptation of firing as the muscle tension declined after the contraction, in the presence of maintained stretch (Fig. 3A). In most cases, few additional contractions occurred (no matter how long the stretch was applied for), and correspondingly, there were few subsequent bursts of mechanoreceptor firing. Thus, firing rate was associated with force more closely than length; on this basis we previously suggested that rectal mechanoreceptors behaved primarily as tension receptors (Lynn et al. 2003). When stretched by a constant, imposed load, afferent firing in the same mechanoreceptor units usually showed less adaptation following the onset of the stimulus. The firing usually occurred in bursts, each associated with a contraction (shortening) of the muscle (Fig. 4B). Thus, under conditions of constant load, variations in mechanoreceptor firing rate occurred, which were still associated with active muscle contractions, but which could not be explained by changes in force. However, in each preparation a very different pattern of response was occasionally seen. In these cases, mechanoreceptor firing actually reduced or disappeared during contraction (shortening) of the preparation in near-isotonic conditions (asterisks in Fig. 3B and C).
Figure 3. Responses of a single unit to stretch by imposed length and imposed load.
A, the preparation was stretched by ‘imposed length’ by 3 mm at 5 mm s−1, held for 30 s, evoking an active contraction just after the onset of stretch that declined over the next 5–10 s. Rectal mechanoreceptor firing more closely followed force rather than length. B, the same preparation was stretched by ‘imposed load’ in which the counterweight on the isotonic transducer was increased by 30 mN, for approximately 30 s. Note that increasing the load evoked firing. In this particular unit, an active contraction reduced firing in the unit. C, the same preparation was stretched by graded increase in ‘imposed load’ (100 μm s−1), evoking initially bursts of firing that were associated with contraction, then constant firing. Note that at some points active contractions of the preparation appeared to be associated with a decrease in firing (asterisks), possibly reflecting ‘offloading’ of the receptor (see Discussion).
Figure 4. Responses of rectal mechanoreceptor units to a range of imposed lengths and imposed loads.
A, stretches of 1, 2, 3 and 4 mm were applied at 5 mm s−1 and held for 20 s. In each case the mechanical stimulus evoked bursts of firing that were associated with active contraction of the preparation both during the stimulus and following its removal. B, stretches by imposed loads of 10, 20, 30, 40 and 50 mN for 30 s also evoked burst of firing associated with contractions. Note, however, that in some cases, active contractions appeared to correlate with reduced firing (asterisks).
These patterns of firing were confirmed over a range of imposed length changes (near-isometric conditions, Fig. 4A), and over a range of imposed loads (near-isotonic conditions, Fig. 4B). Under imposed load conditions, most preparations showed occasional spontaneous muscle activity (mostly in the circular muscle) consisting of irregular contractions (shortenings), followed by periods of quiescence. To determine which state of the muscle was most closely associated with mechanoreceptor firing, the different phases of muscle activity – (i) quiescence, (ii) onset of contraction (< the first 200 ms), (iii) shortening (presumably active contraction), (iv) peak of contraction, and (v) the recovery (relaxation) phase – were identified from circular muscle recordings made from a 3 mm-long tissue edge. The total number of action potentials in each phase was summed, and divided by the total duration of the phase (Fig. 5). It was clear that under near-isotonic conditions, mechanoreceptor firing was significantly associated with the ‘onset’ phase of contractions, with much lower probabilities of firing during other phases. These differences were most apparent with low loads (50–150 mN) where the muscle could shorten freely. With higher loads, where the muscle was less able to generate shortening, mechanoreceptor firing was more evenly distributed between the phases. It should be noted, however, that many contractions occurred which were not associated with any mechanoreceptor discharge (Fig. 5).
Figure 5. Firing of mechanoreceptors under maintained stretch, and analysis of how mechanoreceptor firing was related to contractile activity of the preparations during maintained stretch.
A, typical recording of contractions, measured as changes of length, during maintained circumferential stretch by a 20 mN load. Afferent activity was associated with contractile activity. B, during maintained circumferential stretch by a 50 mN load, contractions were no longer detectable and most bursts of afferent activity could not be associated with measurable contractions. C, probability of firing shown as rate (number of action potentials divided by total duration of each phase) during each phase of contraction. Note that the highest probability of firing was associated with the onset of contraction (defined as < 200 ms from initial deflection). This association was less marked with higher loads, reflecting the relative inability of the preparation to shorten against heavy counterweights. D, typical recording of muscular contractions divided into the phase definitions. Onset, shortening of the tissue that is presumably contractile activity, peak of contraction when the tissue is neither accelerating nor decelerating, relaxation as the tissue lengthens and quiescence when the tissue is not active.
Effect of pharmacological activation of smooth muscle
The NK2 receptor agonist [β-Ala8]-NKA(4–10) directly excites gut smooth muscle, in a tetrodotoxin-resistant manner, to produce rhythmic contractions (Zagorodnyuk et al. 1995; Zagorodnyuk & Maggi 1997). Under near-isometric conditions (20 s after onset of a 3 mm stretch, of 120 s duration), [β-Ala8]-NKA(4–10) (0.1 μm) evoked phasic contractions which were recorded as regular transient increases in circular muscle tension. These contractions were associated with significantly increased mechanoreceptor firing compared to control stretches (n = 6, n = 4, one-way ANOVA, P < 0.001, Fig. 6B). Similarly, the muscarinic cholinergic agonist carbachol (0.06 μm, n = 6, n = 4) evoked contractions associated with significantly greater firing of rectal mechanoreceptors than control stretches (n = 6, n = 4, one-way ANOVA, P < 0.001, Fig. 6C). This indicates that increased tension of the preparation evoked greater firing of mechanoreceptors. When preparations were kept slack (so that the same application of [β-Ala8]-NKA(4–10) and carbachol evoked negligible tension), the drugs were significantly less effective in increasing firing rate (n = 6, n = 4, one-way ANOVA P < 0.001, Fig. 6B and C). This suggests that rectal mechanoreceptors are not directly activated by these agonists. In the presence of Ca2+-free Krebs (containing 1 mm EDTA and 6 mm Mg2+), to prevent all muscle activity, the same application of both [β-Ala8]-NKA(4–10) and carbachol (0.06 μm) evoked neither firing nor changes in tension (n = 3, n = 3). Again this indicates that rectal mechanoreceptors are not chemosensitive to either of these agonists. These findings also support the close association between rectal mechanoreceptor firing and smooth muscle contraction, under near-isometric conditions (Fig. 6).
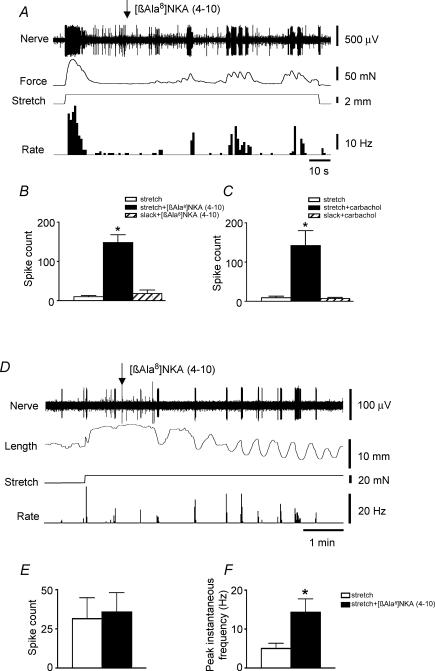
Figure 6. Pharmacologically induced smooth muscle contractions evoked mechanoreceptor firing under both near-isometric and near-isotonic conditions.
A, preparation was stretched to 3 mm and held for 2 min. After 20 s, when the muscle response to stretch had accommodated, 0.1 μm[β-Ala8]-NKA(4–10) was applied for a 100 s period of stretch (maintained by ‘imposed length’) and evoked rhythmic contractions of the circular muscle (recorded as increases in force on the isometric force transducer) which were closely associated with bursts of firing. B, summarized data from 6 units in 4 preparations show that [β-Ala8]-NKA(4–10) evoked increased firing of a mechanoreceptor (total number of action potentials) against a background of maintained stretch, but not when the tissue was kept slack, demonstrating that the NK2 receptor agonist was not acting directly on the nerve ending. C, similar findings were made using the cholinergic agonist carbachol (0.06 μm), which increased firing (n = 6, n = 4) when the tissue developed increased tension, but not when the preparation was kept slack. This again confirms that the agonist was not acting directly on the mechanoreceptor nerve endings, but indirectly, via increases in intramuscular tension. D, under conditions of stretch by an imposed load of 20 mN for 120 s, 0.1 μm[β-Ala8]-NKA(4–10) also caused rhythmic contractile activity associated with mechanoreceptor firing. E, summarized data from 9 units in 3 preparations show that [β-Ala8]-NKA(4–10) did not evoke an overall change in total number of action potentials. F, [β-Ala8]-NKA(4–10) did increase the peak instantaneous frequency in bursts of firing (n = 6, n = 3).
When [β-Ala8]-NKA(4–10) was applied under near-isotonic conditions (during stretch by an imposed load of 20 mN), it typically evoked an increase in the amplitude of spontaneous contractions, although there was an apparent slight reduction in their frequency. Mechanoreceptor units continued to fire in regular bursts – associated with the contractions – but mean firing rate was not altered (n = 9, n = 3, Student's paired t test) (Fig. 6D and E). However, the intensity of firing within bursts was increased (as measured by peak instantaneous frequency), indicating that under near-isotonic conditions, firing frequency was positively associated with contraction amplitude (n = 6, n = 3, Student's paired t test, P < 0.05, Fig. 6F).
Stretch in circumferential and longitudinal directions
To determine whether rectal mechanoreceptors show directional sensitivity to mechanical stimuli, the effects of circumferential and longitudinal stretch of preparations were compared for both near-isotonic and near-isometric conditions. Under near-isometric conditions, imposed length changes (1–3 mm for 10 s at 5 mm s−1), in both circumferential (n = 8) and longitudinal axes (n = 4), evoked significant increases in tension that were associated with mechanoreceptor firing. This was confirmed for three units in three preparations that were activated by both circumferential (Fig. 7A) and longitudinal stretch (Fig. 7B). It should be noted that under these conditions, there were clear reflex interactions between the smooth muscle layers that contributed to wall tension. When circumferential stretch was applied, the onset of the active contraction of circular muscle occurred 0.20 ± 0.05 s after the longitudinal muscle contraction (n = 8, n = 8). In addition, tetrodotoxin (1 μm for 10 min) slightly but significantly reduced the contractions of circular muscle (n = 6, n = 6, two-way ANOVA, P < 0.01) (Fig. 7C), but nearly abolished contractions of longitudinal muscle (n = 6, n = 6, two-way ANOVA, P < 0.0001) (Fig. 7D).
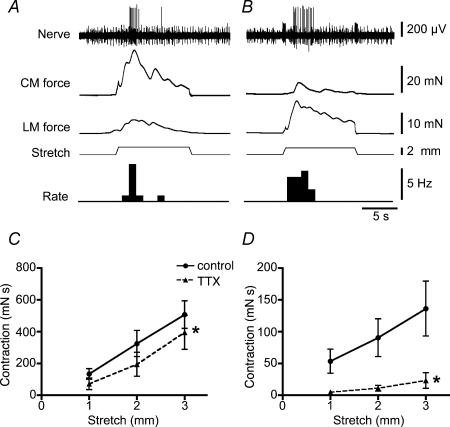
Figure 7. Response to longitudinal and circumferential stretch by the same mechanoreceptor unit and effect of tetrodotoxin on stretch-induced contractions.
Circumferential stretch by imposed change in length (A) evoked a burst of mechanoreceptor firing and an active reflex contraction of the longitudinal muscle (LM). Longitudinal stretch (B) similarly evoked a burst of mechanoreceptor firing as well as a small active reflex contraction in the circular muscle (CM). Both rakes (3 mm circumferentially and 10 mm longitudinally) were connected to tissue stretchers used to deliver imposed changes in length. During fast circumferential stretch, TTX (1 μm) slightly but significantly reduced the contractions of circular muscle (C; calculated as area under curve (mN s)) and nearly abolished contractions of longitudinal muscle (D, n = 6, n = 6).
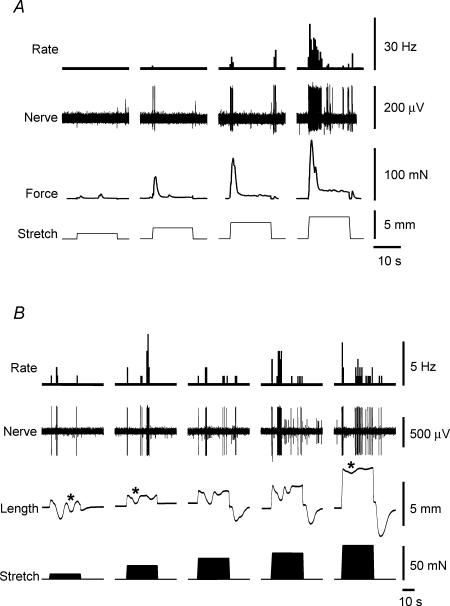
Under near-isotonic conditions, increasing the load on the preparation by 10 mN or more in both longitudinal and circumferential axes evoked comparable increases in mechanoreceptor firing (n = 8, n = 5, P < 0.05, two-way ANOVA, Fig. 8B). During maintained stretch, rhythmic contractions developed in the circular muscle, but rarely in the longitudinal muscle. Firing generally occurred in bursts (Fig. 8A) occurring near the onset of individual phasic circular muscle contractions, as described above.
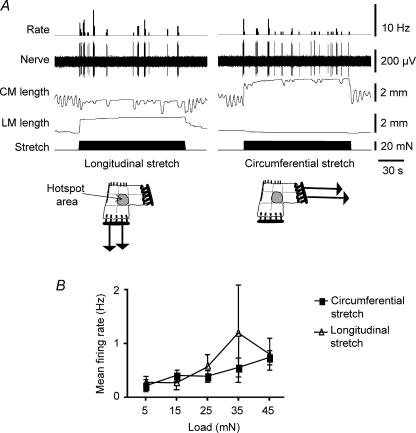
Figure 8. Both longitudinal and circumferential stretch, by imposed load, activated rectal mechanoreceptors.
A, longitudinal stretch applied by a load of 20 mN evoked bursts of firing of a rectal mechanoreceptor (left traces), as did circumferential stretch by the same load (right traces). During longitudinal stretch afferent bursts were usually associated with contractile activity within the circular muscle in the absence of longitudinal muscle activity. During circumferential stretch afferent activity also occurred in bursts that were associated with some, but not all circular muscle contractions. B, mean data from 8 units from 5 preparations shows graded responses to both longitudinal and circumferential stretch applied to the same preparations.
High-resolution video imaging and spatiotemporal mapping
Throughout the study, near-isotonic or isometric transducers were used to measure muscle activity using ‘rakes’ (3–10 mm wide) aligned with the receptive fields of the mechanoreceptive units. Despite the relatively fine resolution of these recording techniques compared to previous published studies, many muscle contractions occurred without associated bursts of afferent firing. To record the muscle activity within the exact receptive field of the units, we used high-resolution video recordings and spatiotemporal mapping techniques. Four opaque plastic markers were placed at the corners of a rectangle (about 1 mm × 1 mm) around the receptive field, which had been identified using von Frey hairs. The movements of the markers were used to track shortening in the longitudinal axis, circular axis and reductions in the receptive field area; longitudinal and circular muscle tension were concurrently recorded from the preparation using tension transducers (see Fig. 9).
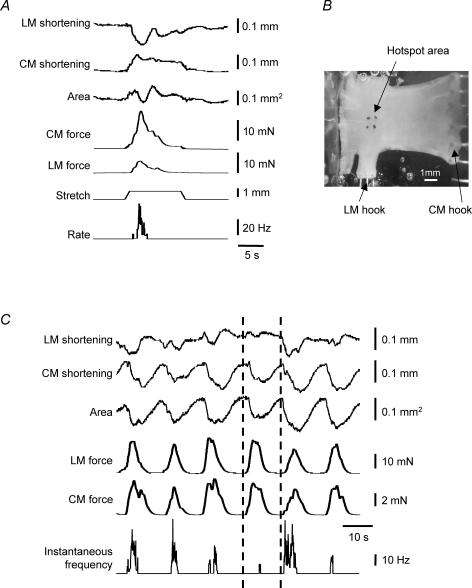
Figure 9. Circumferential stretch by an imposed change in length evoked mechanoreceptor firing.
A, video mapping of movements of markers around the receptive field of the unit were used to generate the upper three traces. Stretch evoked an increase in circular muscle tension of the preparation and a simultaneous increase in tension in the longitudinal axis. At the same time, complex changes were occurring within the receptive field, including an increase in its circumferential dimension, a decrease in its longitudinal dimension, and a reduction in its area, as the tissue was stretched and then actively contracted. B, micrograph of the preparation with a 6 mm wide rake attached to one edge to record circular muscle tension and a 1 mm wide rake arranged perpendicular to it, to record longitudinal muscle tension. C, pharmacological (0.1 μm[β-Ala8]-NKA(4–10)) activation of muscle contractions, only some of which were associated with mechanoreceptor firing. Note that of the two contractions adjacent to the vertical dashed lines, one failed to evoke significant mechanoreceptor activation. The two contractions were of approximately equal magnitude when measured by force transducers attached to orthogonal edges of the preparation. However, the first was not associated with shortening of the longitudinal muscle within the receptive field, whereas the second was. Contraction of both muscle layers within the receptive field was most strongly associated with mechanoreceptor activation.
First, small circumferential stretches were applied as imposed length changes (0.5–1 mm, n = 23, n = 9) using a ‘tissue stretcher’. Mechanoreceptor firing was associated with shortenings of the receptive field in the longitudinal axis (18 of 23 stretches, n = 9), and to a lesser degree with circular shortenings (11 of 23 stretches n = 9; in 10/11 of these latter cases, there was simultaneous shortening of longitudinal muscle, see Fig. 9). However, it should be noted that the entire preparation had been forcibly lengthened in the circumferential dimension by the tissue stretcher, interfering with circular shortening within the receptive field. We were concerned that this may have led to an underestimate of the degree of circular muscle activity. To avoid this, we studied the shortening of the two axes of the receptive field using spatiotemporal mapping, under conditions where the preparation was not activated by stretching. The NK2 agonist, [β-Ala8]-NKA(4–10) (0.1 μm) was used to stimulate circular and longitudinal muscle phasic contractions in L-shaped preparations, with force transducers attached via a 1–2 mm rake and a 5–6 mm rake to record longitudinal and circular muscle tension, respectively (Fig. 9A).
In the presence of [β-Ala8]-NKA(4–10), the dimensions of the receptive field varied considerably, in a way that was not predictable from the gross muscle contractile activity recorded with force transducers. In addition, the amplitude of longitudinal and circular muscle tension was a poor predictor of afferent firing. Indeed, contractions of near-identical tension could evoke powerful bursts, or no firing at all (Fig. 9C). Using regression analysis, gross circular muscle tension during contraction was a weak predictor of firing (mean slope = 0.15, not significant in 8 trials, n = 5). Gross longitudinal muscle tension was highly variable, with a positive correlation in 3 of 8 trials (mean slope =−1.05 ± 1.43, 8 trials, n = 5). However, when we examined shortening within the receptive field, we found more significant associations. Shortening in the longitudinal axis was correlated more strongly with firing (mean slope =−0.45 ± 0.14, significant in 5/8 trials, n = 5). Shortening in the circular axis was weakly correlated (slope =−0.05, significant in 2 out of 8 trials, n = 5), similar to changes in area of the receptive field (mean slope =−0.50 ± 0.10, significant in 2 out of 8 applications, n = 5). These results indicate that the local mechanical activity within the receptive field of the mechanoreceptor (particularly in the longitudinal axis) varied significantly, and was a better predictor of the firing activity of mechanoreceptors than the gross mechanical activity of the specimen.
Discussion
Rectal intraganglionic laminar endings (rIGLEs) have previously been shown to correspond to the transduction sites of low threshold, slowly adapting mechanoreceptors that are present in the guinea pig rectum, but not distal colon (Lynn et al. 2003). In the present study, punctate mechanoreceptor sites were shown to give highly sensitive, graded responses to focal transmural compression with von Frey hairs. In one way, rectal mechanoreceptors responded as if they were true tension receptors: they increased firing when muscle spontaneously contracted against a fixed length (i.e. under near-isometric conditions). However, under near-isotonic conditions – where the muscle could shorten against a fixed load – rectal mechanoreceptors did not behave as tension receptors. Typically, they showed variable bursts of firing, which usually coincided with the onset of contractions that subsequently led to shortening of the preparation. By definition, true tension receptors should fire at a nearly constant rate under constant load conditions (subject only to changes in cross-sectional area, according to the law of Laplace). Muscle contraction evoked by an NK2 receptor agonist increased mechanoreceptor excitability under both near-isotonic, and near-isometric conditions. Rectal mechanoreceptors were also activated by stretch in both circumferential and longitudinal axes of the gut, irrespective of whether this was evoked by imposed load or by imposed length. It should be noted that such stretches often triggered reflex activity in the orthogonal muscle layers, complicating the interpretation of their effects on mechanoreceptors. However, neither length changes nor tension changes measured from the longitudinal or circular muscle layers of the whole preparation fully predicted firing of mechanoreceptors. High-resolution video microscopy indicated that much of the variability in firing was due to highly localized contractions around the receptive field which were not necessarily detected by transducers attached to the preparation as a whole.
Directional sensitivity of rectal mechanoreceptors
Stretch in the longitudinal and circumferential axes of the gut, together with vertical compression of transduction sites, all evoked firing. In addition, contractions of the preparation under both near-isometric and near-isotonic conditions also evoked firing. The complex nature of mechanoreceptor responses is perhaps not surprising given that their transduction sites correspond to branching flattened nerve endings (rIGLEs) that occupy a substantial part of myenteric ganglia (Lynn et al. 2003). These endings are, by location, both parallel to smooth muscle and also in-series to it. It seems reasonable to assume that rIGLEs detect distortion of myenteric ganglia. The fact that putative rIGLEs investigated here respond to both longitudinal and circular muscle stretch, and contraction, suggests that they have little directional sensitivity.
Mechanoreceptor firing and contractility
In previous studies of extrinsic afferents to the large bowel, muscle activity of the gut has typically been recorded either in vivo, using a large balloon to measure intraluminal pressure (Janig & Koltzenburg 1990,1991; Sengupta & Gebhart, 1994; Thewissen et al. 2000), or in flat sheet preparations, ex vivo, where stretch has been applied without recording mechanical responses (Page & Blackshaw, 1998; Lynn & Blackshaw, 1999). In the present study, we applied mechanical stimuli and recorded muscle responses in the longitudinal, and circular axes, with transducers coupled to the tissue with stiff metal ‘rakes’ which were from 2 to 10 mm in length. This provided higher resolution mechanical recordings than previous studies. Nevertheless, the mechanical state of the muscle was poorly predictive of afferent firing. The results of the spatiotemporal mapping studies provided a simple explanation for this finding. By monitoring both longitudinal and circular dimensions within the receptive field of mechanoreceptors, it was found that contractile activity within the receptive field could not be predicted accurately from transducer recordings from the preparation as a whole, even when the receptive field was in a preparation only 2–5 mm wide. This is readily explained by the observation that electrical activity in segments of gut propagates rapidly and over long distances along bundles of smooth muscle fibres, but over shorter distances propagates perpendicularly to bundles. Thus, in our study, a receptive field that was less than 1 mm wide could have quite different mechanical activity (‘micro-contractions’) than that recorded from the full width of the preparation. There also seemed to be some variability between individual mechanoreceptors.
The firing of most units was more closely related to longitudinal rather than circular muscle microcontractions. This seems to be at variance with our observation that rectal mechanoreceptors responded equally to circular and longitudinal stretch. We can speculate on possible causes of this apparent discrepancy. In most cases, preparations were pinned along an edge close to the receptive field, which may have restricted circular muscle contractions more than longitudinal muscle contractions measured with the video mapping system. It is also possible that reflex interactions between the muscle layers may have contributed differently to stretch-evoked responses and responses to agonist-induced contractions.
The results from spatiotemporal mapping of microcontractions also explain why, under near-isotonic conditions, some contractions of the circular muscle were associated with decreased firing of rectal mechanoreceptors (Figs 3 and 4). This may have occurred when circular muscle shortening outside the receptive field effectively ‘unloaded’ mechanoreceptor endings in an area that did not contract. In these ways, microcontractile activity around the receptive field may decrease the correlation between length and force recorded with transducers, and afferent firing.
Ganglionic distortion as an adequate stimulus
In the light of our findings, it is worth considering the true nature of the adequate stimulus for rectal mechanoreceptors. Recent studies have demonstrated that similar endings of vagal afferents in the upper gut probably detect distortion via the opening of stretch-activated ion channels (Zagorodnyuk et al. 2003). Preliminary data suggest that this is also likely to be the case for rectal mechanoreceptors (authors' unpublished observations). For this to be the case, there must be physical movement of part, but not all, of the mechanotransducing molecular complex located in the membrane of rIGLEs. Rectal IGLEs are branching flattened structures that are not aligned with either longitudinal or circular muscle, but rather appear to course in all directions through myenteric ganglia, often close to the ensheathing connective tissue (Lynn et al. 2003; Olsson et al. 2004). Mechanosensitive ion channel complexes are unlikely to show preferential alignment with either the longitudinal or circumferential axis of the gut. Thus, distortion of ganglia in any direction would necessarily activate a subset of channels and excite endings. Any movement of tissue that either compresses or stretches ganglia could activate rIGLEs. We suggest that contraction of smooth muscle fibres above or below a ganglion would increase their rigidity and thus compress the interposed ganglion. Simultaneous contraction of both muscle layers should thus be particularly effective in activating mechanosensitive endings, as was observed. Several studies have shown that simultaneous contractions of longitudinal and circular muscle commonly occur during neurogenic propulsive activity (Brookes et al. 1999; Hennig et al. 1999; Spencer et al. 1999; Spencer & Smith, 2001) and during myogenic activity evoked by pacemaker cells, which are common to both muscle layers (Dickens et al. 1999). In addition, contractions of distant smooth muscle that stretch the receptive field should cause flattening of the ganglion (to preserve volume), and thus also activate mechanoreceptors.
Significance of multiple transduction sites
Individual rectal mechanoreceptors have previously been shown to have multiple hotspots spread over a few square millimetres of tissue, each capable of responding to localized transmural compression with a von Frey hair (Lynn et al. 2003). We have previously shown, using simple arithmetic modelling, that the multiple transduction sites of similar mechanoreceptors in the oesophagus do not function independently (Zagorodnyuk & Brookes, 2000). Rather, action potentials from the most strongly activated site ‘reset’ other transduction sites, and thus determine the overall firing frequency of the unit. A similar mechanism has recently been proposed for rabbit pulmonary receptors (Yu & Zhang, 2004). If, as seems likely, the same mechanism operates for rectal mechanoreceptors, the presence of multiple mechanosensitive sites spread over several square millimetres of the gut wall would tend to smooth out variations in firing frequency caused by the localized nature of contractile activity. Thus, rectal mechanoreceptors should encode the state of contractile activity of the most active region of muscle anywhere within the receptive field, at any particular moment.
Physiological significance of transduction
The present study has shown that specialized rectal mechanoreceptors are typically activated during muscle contraction, whether this occurs under near-isotonic conditions or under near-isometric conditions. It is known that faecal matter moving in the gut of the guinea pig causes deformation of myenteric ganglia in which these endings reside (Gabella, 1990). It also triggers neurogenic motor activity involving both longitudinal and circular muscle (Costa & Furness, 1976). In the present study, stretch of the gut wall not only evoked activity of rectal mechanoreceptors directly, but also triggered neurogenic contractions in the stretched muscle layer and in the orthogonal layer, via a combination of neurogenic and myogenic mechanisms (see Fig. 7). There is extensive evidence that activation of rectal mechanoreceptors, presumably by both distension and by contractile activity of the rectal wall, activate extrinsic and intrinsic reflexes that play a key role in defaecatory motor behaviour (Yamanouchi et al. 2002).
References
- Blackshaw LA, Grundy D. Effects of cholecystokinin (cck-8) on two classes of gastroduodenal vagal afferent fibre. J Auton Nerv Syst. 1990;31:191–201. doi: 10.1016/0165-1838(90)90185-l. [DOI] [PubMed] [Google Scholar]
- Brookes SJ, Chen BN, Costa M, Humphreys CM. Initiation of peristalsis by circumferential stretch of flat sheets of guinea-pig ileum. J Physiol. 1999;516:525–538. doi: 10.1111/j.1469-7793.1999.0525v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M, Furness JB. The peristaltic reflex: An analysis of the nerve pathways and their pharmacology. Naunyn Schmiedebergs Arch Pharmacol. 1976;294:47–60. doi: 10.1007/BF00692784. [DOI] [PubMed] [Google Scholar]
- Denny-Brown D, Robertson EG. An investigation of the nervous control of defaecation. Brain. 1935;58:256–310. doi: 10.1111/j.1463-1318.2004.00636.x. [DOI] [PubMed] [Google Scholar]
- Dickens EJ, Hirst GD, Tomita T. Identification of rhythmically active cells in guinea-pig stomach. J Physiol. 1999;514:515–531. doi: 10.1111/j.1469-7793.1999.515ae.x. 10.1111/j.1469-7793.1999.515ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB, Costa M. The Enteric Nervous System. Edinburgh: Churchill Livingstone; 1987. [Google Scholar]
- Gabella G. On the plasticity of form and structure of enteric ganglia. J Auton Nerv Syst. 1990;30:S59–S66. doi: 10.1016/0165-1838(90)90103-p. 10.1016/0165-1838(90)90103-P. [DOI] [PubMed] [Google Scholar]
- Gershon MD. The enteric nervous system: a second brain. Hosp Pract. 1999;34:31–42. doi: 10.3810/hp.1999.07.153. [DOI] [PubMed] [Google Scholar]
- Greving I, Tegenthoff M, Nedjat S, Orth G, Botel U, Meister V, Micklefield G, May B, Enck P. Anorectal functions in patients with spinal cord injury. Neurogastroenterol Motil. 1998;10:509–515. doi: 10.1046/j.1365-2982.1998.00124.x. 10.1046/j.1365-2982.1998.00124.x. [DOI] [PubMed] [Google Scholar]
- Hennig GW, Costa M, Chen BN, Brookes SJ. Quantitative analysis of peristalsis in the guinea-pig small intestine using spatio-temporal maps. J Physiol. 1999;51:575–590. doi: 10.1111/j.1469-7793.1999.0575t.x. 10.1111/j.1469-7793.1999.0575t.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iggo A. Tension receptors in the stomach and the urinary bladder. J Physiol. 1955;128:593–607. doi: 10.1113/jphysiol.1955.sp005327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janig W, Koltzenburg M. On the function of spinal primary afferent fibres supplying colon and urinary bladder. J Auton Nerv Syst. 1990;30:S89–S96. doi: 10.1016/0165-1838(90)90108-u. 10.1016/0165-1838(90)90108-U. [DOI] [PubMed] [Google Scholar]
- Janig W, Koltzenburg M. Receptive properties of sacral primary afferent neurons supplying the colon. J Neurophysiol. 1991;65:1067–1077. doi: 10.1152/jn.1991.65.5.1067. [DOI] [PubMed] [Google Scholar]
- Lembo T, Munakata J, Mertz H, Niazi N, Kodner A, Nikas V, Mayer EA. Evidence for the hypersensitivity of lumbar splanchnic afferents in irritable bowel syndrome. Gastroenterology. 1994;107:1686–1696. doi: 10.1016/0016-5085(94)90809-5. [DOI] [PubMed] [Google Scholar]
- Lynn PA, Blackshaw LA. In vitro recordings of afferent fibres with receptive fields in the serosa, muscle and mucosa of rat colon. J Physiol. 1999;518:271–282. doi: 10.1111/j.1469-7793.1999.0271r.x. 10.1111/j.1469-7793.1999.0271r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynn PA, Olsson C, Zagorodnyuk V, Costa M, Brookes SJ. Rectal intraganglionic laminar endings are transduction sites of extrinsic mechanoreceptors in the guinea pig rectum. Gastroenterology. 2003;125:786–794. doi: 10.1016/s0016-5085(03)01050-3. 10.1016/S0016-5085(03)01050-3. [DOI] [PubMed] [Google Scholar]
- Mulak A, Bonaz B. Irritable bowel syndrome: A model of the brain–gut interactions. Med Sci Monit. 2004;10:RA55–62. [PubMed] [Google Scholar]
- Olsson C, Costa M, Brookes SJ. Neurochemical characterization of extrinsic innervation of the guinea pig rectum. J Comp Neurol. 2004;470:357–371. doi: 10.1002/cne.20000. 10.1002/cne.20000. [DOI] [PubMed] [Google Scholar]
- Page AJ, Blackshaw LA. An in vitro study of the properties of vagal afferent fibres innervating the ferret oesophagus and stomach. J Physiol. 1998;512:907–916. doi: 10.1111/j.1469-7793.1998.907bd.x. 10.1111/j.1469-7793.1998.907bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta JN, Gebhart GF. Characterization of mechanosensitive pelvic nerve afferent fibers innervating the colon of the rat. J Neurophysiol. 1994;71:2046–2060. doi: 10.1152/jn.1994.71.6.2046. [DOI] [PubMed] [Google Scholar]
- Spencer NJ, Smith TK. Simultaneous intracellular recordings from longitudinal and circular muscle during the peristaltic reflex in guinea-pig distal colon. J Physiol. 2001;533:787–799. doi: 10.1111/j.1469-7793.2001.00787.x. 10.1111/j.1469-7793.2001.00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer N, Walsh M, Smith TK. Does the guinea-pig ileum obey the ‘law of the intestine’? J Physiol. 1999;517:889–898. doi: 10.1111/j.1469-7793.1999.0889s.x. 10.1111/j.1469-7793.1999.0889s.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurszewski JH, King BF. Physiology of prevertebral ganglia in mammals wth special reference to inferior mesenteric ganglion. In: Schultz SG, Wood JD, Rauner BB, editors. Handbook of Physiology, section 6, The Gastrointestinal System. part 1. Vol. 1. Bethesda: American Physiological Society; 1989. pp. 519–592. chap. 15. [Google Scholar]
- Thewissen M, Ruhl A, Enck P. On the adequate stimulus for rectal mechanoreception and perception: a study in cat and humans. Neurogastroenterol Motil. 2000;12:43–52. doi: 10.1046/j.1365-2982.2000.00176.x. 10.1046/j.1365-2982.2000.00176.x. [DOI] [PubMed] [Google Scholar]
- Wood JD. Application of classification schemes to the enteric nervous system. J Auton Nerv Syst. 1994;48:17–29. doi: 10.1016/0165-1838(94)90156-2. 10.1016/0165-1838(94)90156-2. [DOI] [PubMed] [Google Scholar]
- Yamanouchi M, Shimatani H, Kadowaki M, Yoneda S, Nakagawa T, Fujii H, Takaki M. Integrative control of rectoanal reflex in guinea pigs through lumbar colonic nerves. Am J Physiol Gastrointest Liver Physiol. 2002;283:G148–G156. doi: 10.1152/ajpgi.00497.2001. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Brookes SJ. Transduction sites of vagal mechanoreceptors in the guinea pig esophagus. J Neurosci. 2000;20:6249–6255. doi: 10.1523/JNEUROSCI.20-16-06249.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Brookes SJ. Intraganglionic laminar endings are mechano-transduction sites of vagal tension receptors in the guinea-pig stomach. J Physiol. 2001;534:255–268. doi: 10.1111/j.1469-7793.2001.00255.x. 10.1111/j.1469-7793.2001.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk VP, Chen BN, Costa M, Brookes SJ. Mechanotransduction by intraganglionic laminar endings of vagal tension receptors in the guinea-pig oesophagus. J Physiol. 2003;553:575–587. doi: 10.1113/jphysiol.2003.051862. 10.1113/jphysiol.2003.051862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagorodnyuk V, Maggi CA. Tachykinin nk1 and nk2 receptors mediate non-adrenergic non-cholinergic excitatory neuromuscular transmission in the guinea-pig stomach. Neuroscience. 1997;80:625–634. doi: 10.1016/s0306-4522(97)00169-3. 10.1016/S0306-4522(97)00169-3. [DOI] [PubMed] [Google Scholar]
- Zagorodnyuk V, Santicioli P, Maggi CA, Giachetti A. Evidence that tachykinin nk1 and nk2 receptors mediate non-adrenergic non-cholinergic excitation and contraction in the circular muscle of guinea-pig duodenum. Br J Pharmacol. 1995;115:237–246. doi: 10.1111/j.1476-5381.1995.tb15869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Zhang J. A single pulmonary mechano-sensory unit possesses multiple encoders in rabbits. Neurosci Lett. 2004;362:171–175. doi: 10.1016/j.neulet.2004.01.047. 10.1016/j.neulet.2004.01.047. [DOI] [PubMed] [Google Scholar]