Abstract
Oleoylethanolamide (OEA) is an endogenous lipid that regulates feeding and body weight. Although the effects of OEA are believed to depend on activation of vagal sensory afferent neurones, the mechanisms involved in exciting these neurones are unclear. Here we show that OEA directly excited nodose ganglion neurones, the cell bodies of vagal afferents. OEA depolarized these neurones and evoked inward currents that were restricted to capsaicin-sensitive cells. These currents were fully blocked by the TRPV1 inhibitor, capsazepine, and no responses to OEA were observed in neurones cultured from TRPV1-null mice. Similarly, OEA induced a rise in Ca+ concentration in wild-type but not TRPV1-deficient neurones, and responses to OEA were greater at 37°C compared to room temperature. Significantly, OEA administration in mice induced visceral pain-related behaviours that were inhibited by capsazepine and absent in TRPV1-null animals. Further, OEA reduced 30-min food intake in wild-type but not in TRPV1-null mice. Thus, the acute behavioural effects of OEA may result from visceral malaise via the activation of TRPV1.
Oleoylethanolamide (OEA) is an endogenous fatty acid ethanolamide that may play a role in feeding behaviour. The biosynthesis and release of OEA from the intestine are stimulated by feeding and reduced during fasting (Rodriguez de Fonseca et al. 2001). Furthermore, exogenous OEA reduces both food intake (for 12 h) and long-term body weight gain in rats and mice (Rodriguez de Fonseca et al. 2001; Fu et al. 2003; Gaetani et al. 2003; Nielsen et al. 2004). These anorexigenic actions of OEA are believed to occur through the activation of vagal sensory neurones. Several pieces of evidence support this hypothesis (Rodriguez de Fonseca et al. 2001). First, OEA is effective when administered peripherally but is ineffective when injected into the brain. Second, the effects of OEA are abolished by chemical destruction of vagal sensory afferents with capsaicin. Third, OEA treatment leads to enhanced c-fos expression in brain regions recruited by vagal afferents during feeding, including the nucleus of the solitary tract (NST) and the paraventricular and supraoptic nuclei in the hypothalamus. Although these data support involvement of the vagal sensory neurones, the signalling mechanisms and molecular targets of OEA are unclear. Moreover, it is not known whether OEA excites vagal neurones directly or indirectly.
One identified target of OEA is the nuclear receptor peroxisome proliferator-activated receptor-α (PPARα) (Fu et al. 2003). OEA binds PPARα, which in turn regulates expression of several genes in the small intestine including inducible nitric oxide synthase (iNOS). Accordingly, it has been proposed that a reduction in nitric oxide (NO) levels may transduce the effects of OEA on vagal nerve excitability (Fu et al. 2003). Another possibility is that OEA itself directly excites vagal afferents. However, despite its important role in feeding, no study to date has addressed the effects of OEA on vagal excitability. Recently, we have shown in an oocyte expression system that OEA can activate the capsaicin receptor (TRPV1) in a protein kinase C (PKC)-dependent fashion (Ahern, 2003). Here we sought to test whether OEA directly stimulates vagal sensory neurones, and whether this is mediated by TRPV1 and/or other receptors. Our results show that OEA can directly excite nodose sensory neurones from wild-type but not TRPV1-null mice by activating a capsazepine-sensitive TRPV1 current. Further, intraperitoneal administration of OEA in mice induces visceral pain-related behaviours and reduces food intake in a TRPV1-dependent manner. Thus, OEA can act at TRPV1 in vivo and this may contribute to the short-term behavioural effects of this lipid.
Methods
Nodose electrophysiology
Tissues were harvested using protocols approved by the Georgetown University Animal Care and Use Committee. Nodose ganglia were obtained from wild-type adult mice (C57Bl6/J) or TRPV1-null mice (B6.129S4Trpv1tm1Jul/J, Jackson Laboratories), killed by CO2 narcosis/decapitation. Ganglia were cut, digested with collagenase, and cultured in Neurobasal/B-27 medium (Gibco) on poly-d-lysine-coated glass coverslips. Neurones were used within 24–36 h of culture.
Whole-cell and single-channel patch-clamp recordings were performed using an EPC8 amplifier (HEKA). For whole-cell and excised patch recordings the bath solution contained (mm): NaCl 140, KCl 4, MgCl2 1, EGTA 1, Hepes 10 and glucose 10; pH 7.3 with no added Ca2+ (free [Ca2+], < 1–10 μm). The standard pipette solution contained (mm): KCl 140, NaCl 10, MgCl2 1, EGTA 5, Hepes 10, MgATP 2 and Na2GTP 0.03; pH 7.3. For some single-channel experiments KCl was replaced by CsCl in order to block K+ currents. The current signal was low-pass filtered at 1–3 kHz and sampled at 4 kHz. Currents were further filtered for display purposes. Criteria for sensitivity to OEA were > 5 mV for current clamp and > 10 pA for voltage clamp.
Ca2+ imaging
Neurones were loaded with 1 μm acetoxymethyl ester (AM) form of Fluo4 (Molecular Probes, Eugene, OR, USA) for 20 min and washed for a further 10–20 min prior to recording. The dye was excited at 488 ± 15 nm. Emitted fluorescence was filtered with a 535 ± 25-nm bandpass filter, captured by a SPOT RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA) and read into a computer. Analysis was performed off-line using Simple PCI software (Compix Inc., Cranberry Township, PA, USA). Drugs were applied via a micropipette (∼ 100 μm diameter) positioned at a distance of ∼ 0.5 mm from the neurone of interest via either a gravity feed or a pressure-ejection system.
Behavioural analysis
Behavioural procedures were approved by the Georgetown University Animal Care and Use Committee and conformed to NIH guidelines. Animals were bred from the following strains obtained from the Jackson Laboratory (Bar Harbour, ME, USA): C57Bl6/J and mice lacking the TRPV1 receptor (B6.129S4-Trpv1tm1Jul/J).
Visceral pain
Male mice (20–30 g) were allowed to habituate to the testing room for 1 h and to the observation chamber for 10 min prior to testing. Mice were injected intraperitoneally with 1 ml kg−1 of either oleoylethanolamide (OEA, 25 mg kg−1) or the same concentration of OEA plus capsazepine (20 mg kg−1) in 100% DMSO. Animals were monitored for 10 min post injection and the amount of time spent recumbent (either lying on the stomach or sitting in a hunched position with head down, not moving) was recorded.
Short-term feeding
Male mice (18–28 g) were fasted for 24 h with free access to water. Animals were allowed to habituate to the testing room for approximately 1 h. Food pellets (LabDiet 5015) were ground into a powder and mixed 2 parts food to 1 part warm tap water (w/v). A small dish of food was prepared for each mouse and weighed immediately before and after testing. Wild-type and TRPV1-null mice were given an injection of OEA (12.5 mg kg−1) or control (100% DMSO 0.5 ml kg−1i.p.) and immediately re-introduced to food. Food intake for 30 min was measured as grams of food consumed per 100 g body weight.
Chemicals
OEA and capsaicin were obtained from Tocris Cookson (Ellisville, MO, USA). All other drugs were obtained from Sigma. Drugs were prepared as stock solutions in ethanol (10 mm for 4-phorbol 12,13-dibutyrate (PDBu) and 100 mm for all other drugs) and diluted into physiological solution prior to experiments. OEA solutions were prepared fresh every 30 min.
Data are given as means ± s.e.m. and statistical significance was evaluated using Student's unpaired t test.
Results
OEA directly excites nodose ganglion neurones
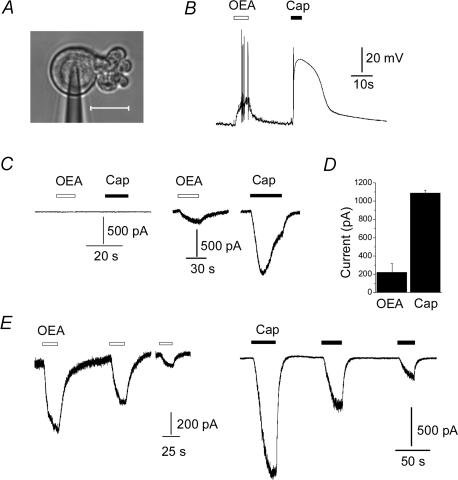
The cell bodies of vagal sensory afferents innervating the gastrointestinal tract reside in nodose ganglia. Like dorsal root ganglia, the expression of ion channels in these cell bodies is believed to reflect their distribution in nerve endings (Gold et al. 1996). Thus, electrophysiological studies made from these cell bodies can reveal properties of vagal ion channels that are otherwise inaccessible to study (Li et al. 1998). Accordingly, we used cultured nodose ganglion neurones to examine the effects of OEA on vagal sensory excitability. Whole-cell patch clamp was performed on neurones after 1 day in culture (Fig. 1A). We first explored the effect of OEA on nodose excitability under current clamp. Figure 1B shows that 10 μm OEA depolarized a neurone and evoked a burst of action potentials. The same cell subsequently responded to 250 nm capsaicin with a larger depolarization. In summary, OEA induced a depolarization of 21 ± 4 mV in 11 of 15 capsaicin-sensitive cells (53 ± 4 mV).
Figure 1. OEA excites capsaicin-sensitive vagal sensory neurones.
A, example of nodose ganglion neurone under patch clamp; scale, 20 μm. B, response of a current-clamped neurone to 10 μm OEA and 250 nm capsaicin. C, responses of capsaicin-insensitive (left) and capsaicin-sensitive (right) neurones to 10 μm OEA and 250 nm capsaicin. Cells were voltage clamped at −60 mV. D, mean current evoked by 10 μm OEA and 250 nm capsaicin in six neurones. E, capsaicin- and OEA-evoked currents exhibit similar tachyphylaxis.
To understand the nature of the current underlying this depolarization, we performed voltage-clamp experiments. OEA evoked inward currents in capsaicin-sensitive cells, but not in capsaicin-insensitive cells (Fig. 1C); a summary of these experiments is shown in Table 1. Figure 1D shows that the mean OEA-evoked current was ∼ 20% of the response evoked by 250 nm capsaicin, and this is consistent with the depolarization responses seen in current-clamp experiments. Figure 1E shows that repetitive application of OEA evoked smaller currents, despite the low concentration of extracellular calcium. This phenomenon, termed ‘tachyphylaxis’, is a hallmark of TRPV1-mediated currents (Szallasi & Blumberg, 1999; Caterina & Julius, 2001).
Table 1.
Summary of OEA-evoked responses in nodose ganglion neurones assayed by voltage clamp and Ca2+ imaging
| Wild-type | TRPV1-null | |||||||
|---|---|---|---|---|---|---|---|---|
| n | Cap+ | OEA+ | Cap+/OEA+ | n | ATP+ | KCl+ | OEA+ | |
| Voltage clamp | 98 | 40 (41%) | 22 (22%) | 22 (22%) | 20 | 14 (70%) | — | 0 (0%) |
| Ca2+ imaging | ||||||||
| 22°C | 76 | 49 (64%) | 28 (37%) | 28 (37%) | 48 | — | 39 (81%) | 0 (0%) |
| 37°C | 29 | 23 (79%) | 15 (52%) | 15 (52%) | 44 | — | 40 (91%) | 0 (0%) |
Cells were treated with OEA (10 μm) followed by capsaicin (250 nm and 3 μm for voltage clamp and Ca2+ imaging, respectively), + indicates a positive response (> 10 pA or 0.1 change in fluorescence ratio (ΔF/F0)).
OEA excites nodose neurones via TRPV1
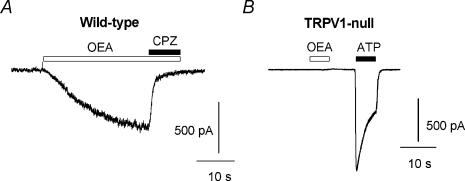
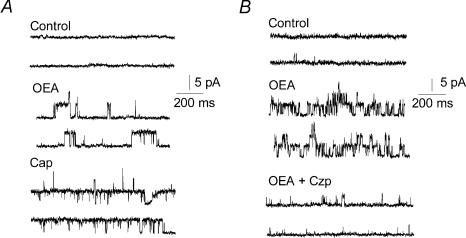
To examine the involvement of TRPV1, we tested whether responses to OEA were sensitive to the TRPV1 inhibitor, capsazepine. Capsazepine (1 μm) produced an almost complete block of OEA-induced currents (Fig. 2A and 94 ± 3%, n = 7, P < 5 × 10−12). Consistent with this result, OEA failed to evoke any current in sensory neurones cultured from TRPV1-null mice (Fig. 2B and Table 1, n = 20). The majority of these neurones responded to ATP with desensitizing inward currents, reflecting expression of purinergic (P2X) ion channels and confirming their status as viable nociceptors. Further, in cell-free patches OEA evoked single-channel activity that was enhanced by capsaicin (Fig. 3A) and blocked by capsazepine (Fig. 3B). These OEA and capsaicin-activated channels had a conductance of ∼ 90 pS (at +50 mV). Taken together, these data suggest that TRPV1 is necessary for the direct excitatory effects of OEA on vagal sensory neurones.
Figure 2. OEA evokes vanilloid-sensitive currents in wild-type but not in TRPV1-null sensory neurones.
A, the current evoked by 10 μm OEA is blocked by the TRPV1 inhibitor, capsazepine (1 μm). B, response to 10 μm OEA and 100 μm ATP in a neurone cultured from a TRPV1-null mouse.
Figure 3. OEA activates TRPV1 single channel activity.
A, representative channel activity (opening upwards) in an outside-out patch (+50 mV) showing that 10 μm OEA and 250 nm capsaicin both activate the same TRPV1 channel protein. B, TRPV1 channels activated by OEA (10 μm) and inhibition by capsazepine (1 μm).
OEA evokes a temperature-sensitive Ca2+ rise in wild-type but not TRPV1-null neurones
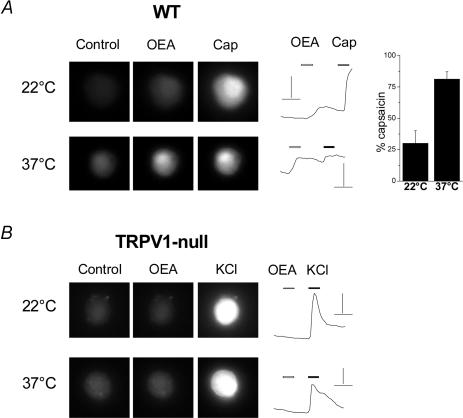
To confirm that OEA activated TRPV1 under more physiological conditions, we performed calcium-imaging experiments at both room temperature (22°C) and 37°C. OEA (10 μm) induced a calcium rise in 37% (28/76) of neurones from wild-type mice; all of these OEA sensitive-neurones responded to a saturating concentration of capsaicin (3 μm, see Fig. 4A and Table 1). At 22°C, the OEA-induced Ca2+ rise was ∼ 30% of the capsaicin-induced response; however, this value increased to 80% at 37°C (Fig. 4A, right panel). This is consistent with the known temperature sensitivity of TRPV1 (Tominaga et al. 1998) whereby increases in temperature (below the heat threshold of 43°C) synergistically enhance agonist-evoked activation. In contrast, OEA failed to evoke any Ca2+ increase in neurones from TRPV1-null mice, either at 22°C or 37°C (Fig. 4B, Table 1). The majority of these cells responded to a high-K+ solution confirming that they were viable.
Figure 4. OEA evokes a temperature-dependent [Ca2+] increase in nodose neurones from wild-type but not TRPV1-null mice.
A, Fluo-4 fluorescence from a neurone cultured from a wild-type mouse, before and after OEA (10 μm) and capsaicin (3 μm) application, at 22 and 37°C. Scale bars, 2 ΔF/F0 and 20 s. The right panel shows the mean response in OEA-sensitive cells expressed as a percentage of capsaicin at 22 and 37°C (n = 7 for both). B, Fluo-4 fluorescence from a TRPV1-deficient neurone under the same conditions as in A. Scale bars, 1 ΔF/F0 and 20 s.
OEA-evoked currents require phosphorylation
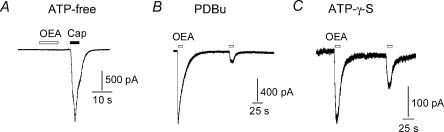
The fact that OEA sensitivity was not observed in all capsaicin-sensitive neurones (Table 1) may reflect different levels of TRPV1 expression and phosphorylation among these cells. Previously, we have demonstrated that OEA activates TRPV1 in Xenopus oocytes in a PKC-dependent fashion and that TRPV1-mutants lacking critical PKC phosphorylation sites are unresponsive to OEA (Ahern, 2003). We therefore investigated the requirement of phosphorylation for OEA activation of TRPV1 in nodose ganglia. To disrupt phosphorylation, we performed whole-cell recordings using ATP-free patch pipette solutions or by replacing ATP by 5′-adenylylimidodiphosphate (AMP-PNP, a non-hydrolysable ATP analogue). Under these conditions, OEA evoked little to no current in capsaicin-sensitive neurones (Fig. 5A and Table 2). However, robust responses to OEA were restored when neurones were pretreated with PDBu to stimulate PKC prior to break-in (Fig. 5B). Similarly, responses to OEA were observed when the pipette solution was supplemented with ATPγS (an ATP analogue that promotes irreversible thio-phospshorylation) (Fig. 5C) or simply with ATP. A summary of these experiments is shown in Table 2. Note that these experiments were performed in a low-Ca2+ extracellular medium and tonic phosphorylation of TRPV1 may occur under these conditions (Vellani et al. 2001) leading to greater sensitivity to OEA under control conditions. Taken together, these data confirm a requirement of phosphorylation (presumably of TRPV1) for OEA excitation of vagal neurones.
Figure 5. OEA activates TRPV1 in a phosphorylation-dependent manner.
A, a voltage clamped nodose neurone responds to capsaicin but not to OEA when ATP is omitted from the patch pipette solution. B, OEA evokes currents under the same conditions as in A when the cell is pretreated with PDBu (0.4 μm) to stimulate PKC prior to break-in. C, responses to OEA are observed when ATPγS (1 mm) is included in the patch pipette solution (see text and Table 2).
Table 2.
Summary of OEA-evoked responses under different phosphorylating conditions
| n | OEA+ | Current (pA) | |
|---|---|---|---|
| ATP (2 mm) | 123 | 30 (24%) | 239 ± 58** |
| ATP-γ-S (1 mm) | 33 | 15 (45%) | 298 ± 33 |
| AMP-PNP (1 mm) | 6* | 0 (0%) | 0 |
| ATP-free | 7 | 0 (0%) | 0 |
| PDBu pretreated (ATP-free) | 13 | 6 (46%) | 582 ± 213 |
Patch pipettes contained concentrations of ATP and analogues as indicated. PDBu (400 nm) was applied in the bathing solution.
denotes neurones were also capsaicin sensitive,
from 15 neurones in voltage clamp.
OEA induces visceral pain in mice
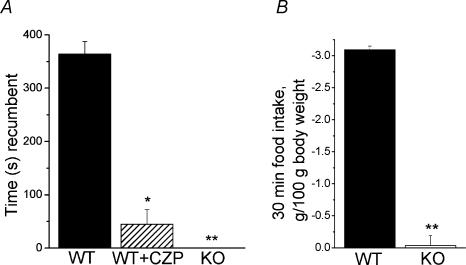
TRPV1 has a well-described role in pain signalling and is essential for the development of inflammatory thermal hyperalgesia in mice (Caterina et al. 2000; Davis et al. 2000; Caterina & Julius, 2001). Moreover, activation of TRPV1 is believed to contribute to visceral pain; TRPV1 antagonists inhibit the writhing caused by acetic acid (Urban et al. 2000; Ikeda et al. 2001; Rigoni et al. 2003). We therefore examined whether OEA could modulate visceral nociception. We found that intraperitoneal administration of 25 mg kg−1 OEA, a dosage similar to those used in satiety studies, produced pronounced nociceptive behaviours. Within ∼ 1–2 min of injection, animals assumed a hunched, recumbent posture and exhibited rapid, shallow breathing and occasional abdominal writhes. This behaviour lasted for approximately 30 min to 1 h. The vehicle (DMSO, 1 ml kg−1) alone was without effect. The mean time spent recumbent for 10 min postinjection was 360 s and this was reduced to 50 s (P < 0.001) by co-administration of capsazepine (Fig. 6A). Furthermore, TRPV1-null animals spent no time recumbent (Fig. 6A) and did not exhibit any signs of distress. Thus, OEA has a pronounced nociceptive action in mice and this is mediated by activation of TRPV1.
Figure 6. OEA induces visceral pain and decreases short-term food intake in mice via TRPV1.
A, wild-type mice (C57Bl6) were injected with 25 mg kg−1 OEA (filled bar, n = 5) or 25 mg kg−1 OEA plus 20 mg kg−1 capsazepine (striped bar, n = 5). The time spent recumbent (either lying on stomach or sitting hunched and immobile) was recorded for the first 10 min after injection. TRPV1-null mice were injected with 25 mg kg−1 OEA (n = 6) and spent no time recumbent. *P < 5.0 × 10−5**P < 5.0 × 10−8 compared with wild-type injected with OEA. B, wild-type and TRPV1-null mice were fasted for 24 h prior to experiment. Mice were injected with either 12.5 mg kg−1 OEA or control solution (100% DMSO). Mice were immediately re-introduced to food and food intake was monitored after 30 min, food intake was measured as grams of food consumed per 100 g body weight. Bars show difference in each mouse injected with OEA from the mean food intake of the control group, n = 4–5 for each group. **P < 5.0 × 10−7 compared with wild-type mice.
OEA reduces short-term food intake in wild-type but not TRPV1 null mice
To test whether OEA-induced visceral discomfort translated into changes in short-term feeding, we examined 30-min food consumption in mice fasted for 24 h. Figure 6B shows that intraperitoneal administration of OEA (12.5 mg kg−1) substantially reduced food intake in wild-type mice compared to the vehicle control. In contrast, OEA did not significantly change food intake in TRPV1-null mice. Thus, the short-term inhibition of food intake by OEA correlates with the induction of visceral pain.
Discussion
OEA directly excites the vagus and induces malaise via TRPV1
Our data indicate that OEA can directly excite a subset of vagal sensory neurones and several lines of evidence indicate that this excitation is mediated by the TRPV1 ion channel. First, responses to OEA were restricted to capsaicin-sensitive neurones. Second, OEA induced capsazepine-sensitive currents and capsaicin/capsazepine-sensitive single channel activity. Third, OEA induced a temperature-sensitive rise in Ca2+ concentration in capsaicin-sensitive cells. Finally, no responses to OEA were observed in neurones cultured from TRPV1-null mice. This activation of TRPV1 by OEA was manifest at the whole animal level. We found that OEA administration induced visceral pain-related behaviour in mice that occurred in a TRPV1-dependent manner. This is consistent with the activation of vagal and spinal C-fibres and demonstrates that OEA has in vivo agonist activity at TRPV1. Although unexpected, this pain behaviour is consistent with activation of a nociceptive ion channel. In agreement with our findings, McKay & Ritter (2004) reported in a preliminary study that OEA causes physical discomfort when administered to rats. Further, we found that OEA reduced short-term (30 min) food intake in a TRPV1-dependent manner. This result is consistent with OEA inducing visceral discomfort. Noxious agents are well known to suppress feeding in a manner distinct from satiety signalling. TRPV1 may therefore represent a candidate for transducing some forms of visceral malaise which in turn affects feeding behaviour. On this note, it is relevant that dietary capsaicin has been shown to reduce food consumption in humans (Yoshioka et al. 1999).
It is interesting that it has previously been shown that high doses of OEA (20 mg kg−1) reduce locomotor activity in rats (Rodriguez de Fonseca et al. 2001). Our data suggest that activation of TRPV1 in visceral sensory afferents and subsequent visceral pain may contribute to this reduced mobility. In contrast, Rodriguez de Fonseca et al. did not observe any effect of OEA (i.p.) in a hot-plate nociceptive assay. However, it is possible that intraperitoneal injection may specifically modulate visceral and not peripheral pain signalling.
PPARα and TRPV1: two distinct targets of OEA
OEA is thought to regulate feeding through the activation of capsaicin-sensitive vagal sensory neurones. Indeed, neonatal ablation of this population by capsaicin treatment abolishes the actions of OEA (Rodriguez de Fonseca et al. 2001). Our data therefore suggest that TRPV1 may contribute to the excitatory actions of OEA on vagal neurones and indeed in feeding regulation. However, OEA acting at TRPV1 appears to signal discomfort rather than satiety. A previous study has identified PPARα as a relevant molecular substrate for OEA. OEA can activate PPARαin vitro and disruption of the PPARα gene inhibits the long-term effects of OEA whereby a single injection reduces food intake for 12 h (Fu et al. 2003). PPARα in intestinal epithelia regulates expression of several genes including an inhibition of iNOS and it has been proposed that reduced levels of NO may mediate stimulation of vagal afferents (Fu et al. 2003). Indeed, NO is known to have an inhibitory effect on vagal excitability via depression of voltage-gated Na+ currents (Li et al. 1998). The transcriptional regulation of iNOS by PPARα occurs with a time course of several hours (Colville-Nash et al. 1998). This would lead to a slow-onset, long-lasting increase in vagal excitability and matches the 12-h suppression of feeding in OEA-treated animals (Fu et al. 2003). However, this mechanism would not account for an immediate suppression of feeding. In this respect, the direct activation of TRPV1 by OEA that we describe may represent an additional, rapid form of OEA signalling that can operate within minutes of feeding. This would be consistent with a role for the vagus in regulating short-term meal termination (Woods, 2004). Thus, OEA may transduce short- and long-term feeding behaviours by temporally distinct actions on the vagus nerve.
In summary, this study shows that OEA can directly excite vagal sensory neurones and induce visceral pain via activation of TRPV1. An OEA–TRPV1 pathway may thus contribute to some of the behavioural effects of OEA.
Acknowledgments
We thank David Julius for kindly providing us with cDNA for rat TRPV1.
References
- Ahern GP. Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem. 2003;278:30429–30434. doi: 10.1074/jbc.M305051200. 10.1074/jbc.M305051200. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Julius D. The vanilloid receptor: a molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Leffler A, Malmberg AB, Martin WJ, Trafton J, Petersen-Zeitz KR, Koltzenburg M, Basbaum AI, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- Colville-Nash PR, Qureshi SS, Willis D, Willoughby DA. Inhibition of inducible nitric oxide synthase by peroxisome proliferator-activated receptor agonists: correlation with induction of heme oxygenase 1. J Immunol. 1998;161:978–984. [PubMed] [Google Scholar]
- Davis JB, Gray J, Gunthorpe MJ, Hatcher JP, Davey PT, Overend P, et al. Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature. 2000;405:183–187. doi: 10.1038/35012076. 10.1038/35012076. [DOI] [PubMed] [Google Scholar]
- Fu J, Gaetani S, Oveisi F, Lo Verme J, Serrano A, Rodriguez De Fonseca F, Rosengarth A, Luecke H, Di Giacomo B, Tarzia G, Piomelli D. Oleylethanolamide regulates feeding and body weight through activation of the nuclear receptor PPAR-alpha. Nature. 2003;425:90–93. doi: 10.1038/nature01921. 10.1038/nature01921. [DOI] [PubMed] [Google Scholar]
- Gaetani S, Oveisi F, Piomelli D. Modulation of meal pattern in the rat by the anorexic lipid mediator oleoylethanolamide. Neuropsychopharmacology. 2003;28:1311–1316. doi: 10.1038/sj.npp.1300166. 10.1038/sj.npp.1300166. [DOI] [PubMed] [Google Scholar]
- Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275. doi: 10.1016/0306-4522(95)00433-5. 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Ueno A, Naraba H, Oh-ishi S. Involvement of vanilloid receptor VR1 and prostanoids in the acid-induced writhing responses of mice. Life Sci. 2001;69:2911–2919. doi: 10.1016/s0024-3205(01)01374-1. 10.1016/S0024-3205(01)01374-1. [DOI] [PubMed] [Google Scholar]
- Li Z, Chapleau MW, Bates JN, Bielefeldt K, Lee HC, Abboud FM. Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. Neuron. 1998;20:1039–1049. doi: 10.1016/s0896-6273(00)80484-5. 10.1016/S0896-6273(00)80484-5. [DOI] [PubMed] [Google Scholar]
- McKay BM, Ritter RC. Oleoylethanolamide induces rapid reduction of short-term food intake in intact and vagotomized rats. Abstr Soc Neurosci. 2004:427.12. [Google Scholar]
- Nielsen MJ, Petersen G, Astrup A, Hansen HS. Food intake is inhibited by oral oleoylethanolamide. J Lipid Res. 2004;45:1027–1029. doi: 10.1194/jlr.C300008-JLR200. 10.1194/jlr.C300008-JLR200. [DOI] [PubMed] [Google Scholar]
- Rigoni M, Trevisani M, Gazzieri D, Nadaletto R, Tognetto M, Creminon C, Davis JB, Campi B, Amadesi S, Geppetti P, Harrison S. Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin. Br J Pharmacol. 2003;138:977–985. doi: 10.1038/sj.bjp.0705110. 10.1038/sj.bjp.0705110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez de Fonseca F, Navarro M, Gomez R, Escuredo L, Nava F, Fu J, Murillo-Rodriguez E, Giuffrida A, LoVerme J, Gaetani S, Kathuria S, Gall C, Piomelli D. An anorexic lipid mediator regulated by feeding. Nature. 2001;414:209–212. doi: 10.1038/35102582. 10.1038/35102582. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple painproducing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. 10.1016/S0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- Urban L, Campbell EA, Panesar M, Patel S, Chaudhry N, Kane S, Buchheit K, Sandells B, James IF. In vivo pharmacology of SDZ 249–665, a novel, non-pungent capsaicin analogue. Pain. 2000;89:65–74. doi: 10.1016/S0304-3959(00)00349-3. 10.1016/S0304-3959(00)00349-3. [DOI] [PubMed] [Google Scholar]
- Vellani V, Mapplebeck S, Moriondo A, Davis JB, McNaughton PA. Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J Physiol. 2001;534:813–825. doi: 10.1111/j.1469-7793.2001.00813.x. 10.1111/j.1469-7793.2001.00813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SC. Gastrointestinal satiety signals I. An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004;286:G7–G13. doi: 10.1152/ajpgi.00448.2003. 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- Yoshioka M, St-Pierre S, Drapeau V, Dionne I, Doucet E, Suzuki M, Tremblay A. Effects of red pepper on appetite and energy intake. Br J Nutr. 1999;82:115–123. [PubMed] [Google Scholar]