Abstract
Magnocellular neurones of the hypothalamus release vasopressin and oxytocin from their dendrites and soma. Using a combination of electrophysiology, microdialysis, in vitro explants, and radioimmunoassay we assessed the involvement of intracellular Ca2+ stores in the regulation of dendritic vasopressin release. Thapsigargin and cyclopiazonic acid, which mobilize Ca2+ from intracellular stores of the endoplasmic reticulum, evoked vasopressin release from dendrites and somata of magnocellular neurones in the supraoptic nucleus. Thapsigargin also produced a dramatic potentiation of dendritic vasopressin release evoked by osmotic or high potassium stimulation. This effect is long lasting, time dependent, and specific to thapsigargin as caffeine and ryanodine had no effect. Furthermore, antidromic activation of electrical activity in the cell bodies released vasopressin from dendrites only after thapsigargin pretreatment. Thus, exposure to Ca2+ mobilizers such as thapsigargin or cyclopiazonic acid primes the releasable pool of vasopressin in the dendrites, so that release can subsequently be evoked by electrical and depolarization-dependent activation. Vasopressin itself is effective in inducing dendritic vasopressin release, but it is ineffective in producing priming.
The dendrites of many neurones transmit information to presynaptic nerve endings by the release of neuroactive substances (Hausser et al. 1995; Isaacson & Strowbridge, 1998; Zilberter et al. 1999; Isaacson, 2001; Ludwig & Pittman, 2003). Vasopressin- or oxytocin-containing neurosecretory granules have been visualized in dendrites and soma (with a far greater vesicle density in the dendrites) of magnocellular neurones in the hypothalamic supraoptic (SON) and paraventricular nuclei (Pow & Morris, 1989). After local release, these neuropeptides autoregulate the activity of the magnocellular neurones by both direct actions (Gouzenes et al. 1998), and indirect actions via modulation of neurotransmitter release (Kombian et al. 1997; Hirasawa et al. 2004). Dendritic release of these neuropeptides can be induced by a range of physiological and pharmacological stimuli, and is regulated by a number of brain areas (see Landgraf, 1995; Ludwig, 1998; Morris et al. 2000).
Depending upon the stimulus, neuropeptides are released from the dendrites and axonal terminals of magnocellular neurones in either a coordinated or an independent manner. For example, suckling evokes oxytocin release in the SON before significant peripheral secretion (Moos et al. 1989), whereas after osmotic stimulation, oxytocin and vasopressin release from the SON lags behind peripheral secretion (Ludwig et al. 1994). In oxytocin cells, electrical activity in the cell bodies can release neuropeptides from nerve terminals in the posterior pituitary with little or no measurable release from the dendrites. Furthermore, some stimuli induce neuropeptide release from dendrites without increasing the electrical activity of the cell body, and without inducing secretion from oxytocin cell nerve terminals (Ludwig et al. 2002; Sabatier et al. 2003).
Oxytocin and vasopressin produce a cell-type specific rise in intracellular [Ca2+] in acutely dissociated neurones of the SON; oxytocin mobilizes Ca2+ from thapsigargin-sensitive intracellular Ca2+ stores (Lambert et al. 1994), whereas the response induced by vasopressin requires an influx of external Ca2+ through identified Ca2+ channels as well as mobilization of thapsigargin-sensitive intracellular Ca2+ stores (Sabatier et al. 1997). Thapsigargin induces Ca2+ mobilization by inhibiting the endoplasmic reticulum Ca2+-ATPase, and thapsigargin-releasable stores include the IP3-sensitive stores (Thastrup et al. 1990). We have recently shown that activation by oxytocin of thapsigargin-sensitive stores in oxytocin cells induces a long-lasting potentiation (priming) of activity- or depolarization-dependent dendritic release (Ludwig et al. 2002).
Although the electrical activity of both vasopressin cells and oxytocin cells is autoregulated by somato-dendritic release of their secretory products, the cellular actions of vasopressin and oxytocin are different. Here we examine whether vasopressin regulates its dendritic release in a way similar to the regulation of dendritic oxytocin release by oxytocin.
Methods
Animals
Female Sprague-Dawley or Wistar rats (150–250 g) were housed under controlled conditions with free access to food and water. On the day of the in vivo experiments the rats were anaesthetized with urethane (ethyl carbamate, intraperitoneally 1.2 g kg−1, Sigma Chemical Co., UK). At the end of each experiment the rats were killed with pentobarbitone (Sagatal; 60 mg kg−1, intravenously). All surgical procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986, and only the minimum number of animals necessary to produce reliable scientific data was used.
Microdialysis osmotic stimulation experiment
In-house designed microdialysis probes were stereotaxically implanted in rats with the U-shaped tip (molecular weight cut-off of 6 kDa, Fleaker® hollow fibre, Spectrum Medical Inc., Los Angeles, CA, USA) located within or adjacent to the left SON (1.0 mm posterior to bregma; 1.7 mm lateral to midline; 9.3 mm below the surface of the skull; Paxinos & Watson, 1986) as previously described (Ludwig et al. 1994). After implantation, there was an equilibration period of at least 1 h with no sample collection before consecutive 30-min dialysis samples were collected at a flow rate of 3 μl min−1. The samples were frozen and stored at −20°C until assay for vasopressin. After two 30-min baseline periods, the dialysis fluid was switched to artificial cerebrospinal fluid (aCSF) containing thapsigargin (2 μm, Sigma Chemical Co., controls received aCSF) for 30 min. Rats were stimulated osmotically by intraperitoneal (i.p.) injection of 2 m NaCl (600 μl (100 g body wt)−1) after the fourth dialysis period, and a further six consecutive dialysate samples were collected.
Electrical stimulation of the neural stalk
The pituitary stalk and right SON were exposed by the transpharyngeal approach in urethane-anaesthetized rats as previously described (Leng & Dyball, 1991). A U-shaped dialysis probe was bent to position the membrane flat onto the exposed ventral surface of the SON after removing the meninges. A stimulating electrode was placed on the neural stalk of the pituitary to activate SON neurones (Ludwig & Leng, 1997). The SON was dialysed with aCSF at 3 μl min−1 throughout the experiments. The femoral artery was cannulated for blood sampling. After an equilibration period of at least 60 min, the stimulation electrode was set to deliver a repeated stimulus (50 Hz for 3 s 10 s−1; mean frequency 15 Hz) over 30 min. In another experiment, thapsigargin was retrodialysed onto the SON over 30 min starting 1 h before antidromic activation of SON (50 Hz for 3 s 10 s−1; mean frequency 15 Hz). Blood samples were taken from the femoral artery (0.6 ml, replaced with isotonic NaCl) for measurement of vasopressin. These samples were collected 5 min before and at the end of the stalk stimulation, and were frozen and stored at −20°C until radioimmunoassays.
Vasopressin release from SON and neurohypophysis
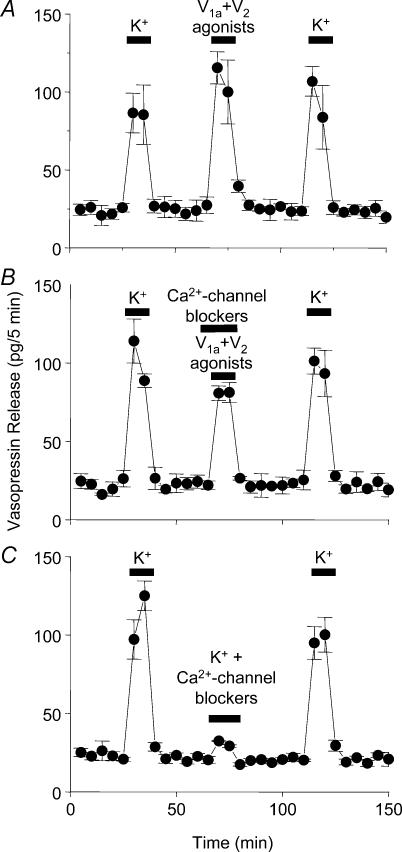
For in vitro release experiments, female rats (150–200 g) were killed by decapitation with a guillotine, and the neurohypophysis devoid of the pars intermedia, and both SON, were dissected quickly. Release was measured from individual neurohypophyses, or from either two or four SON incubated in a single dish. The tissues were preincubated with Locke buffer at 37°C for about 30–45 min during which they were rinsed every 5 min before collecting any samples. Five-minute samples were collected; high potassium (50 mm KCl) was given before and after thapsigargin (200 nm), cyclopiazonic acid (CPA, 10 μm), ryanodine (2 μm), caffeine (20 mm), or a mixture of vasopressin agonists (1 μm, Fig. 4). The SON were also exposed to a mixture of Ca2+-channel blockers (nicardipine 1 μm, ω-CgTx 800 nm, ω-Aga IVA 300 nm, SNX-482 20 nm, Ni2+ 50 μm; Sigma, UK, see Wang et al. 1999) with and without the vasopressin agonists. The V1a (F-180; Hmp-Phe-Ile-Hgn-Asn-Cys-Pro-Dab(Abu)-Gly-NH2) and V2 receptor agonists (dDAVP; 1-deamino-8-D-AVP) were generously given by Dr J. L. Junien (Ferring SA, Paris, France) and by Dr M. Manning (Medical College of Ohio, Toledo, USA), respectively. All drugs were applied for 10 min. The evoked vasopressin release during each period was calculated by subtracting the release under basal conditions (mean of five fractions before stimulus) from that observed during, and directly after, the stimulus (five fractions).
Figure 4. Effects of vasopressin agonists on vasopressin release in vitro.
A, application of a mixture of V1a and V2 (1 μm) agonists significantly increased vasopressin release from the isolated SON in vitro, but did not potentiate subsequent K+-induced release. B, this response was only partially blocked by preapplication of a mixture of Ca2+-channel blockers. C, however, pretreatment with Ca2+-channel blockers blocked the high-K+ evoked response in vasopressin release. Data are means ± s.e.m., t tests, n = 4 per group for each experiment.
Electrophysiology
The SON and neural stalk were exposed transpharyngeally and a U-shaped microdialysis probe was placed flat on the surface of the SON as described above. Magnocellular neurosecretory neurones were identified by antidromic stimulation from the neural stalk. Oxytocin neurones were distinguished from vasopressin neurones by their firing pattern and by their response to i.v. cholecystokinin (CCK, 20 μg kg−1, Bachem Ltd, Saffron Walden, Essex, UK), i.e. transient excitation of oxytocin neurones, and no effect or short-term inhibition of vasopressin neurones (Sabatier et al. 2004). After a period of continuous dialysis with aCSF, the dialysis medium was changed to aCSF containing thapsigargin (2 μm) for between 20 and 60 min. The firing rates of cells were recorded using Spike2 software and CED 1401 interface (Cambridge Electronic Design, Cambridge, UK) on a personal computer. The mean firing rate (spikes s−1) in the 5 min before retrodialysis was compared with the rate in the 25 min after retrodialysis. When appropriate, the mean intraburst firing rate, burst duration, interburst interval, and activity quotient (proportion of time active relative to total time) of each neurone were calculated before and during drug administration. Phasic bursts were characterized using the ‘bursts’ script in Spike2; bursts were defined as activity lasting at least 5 s, containing 20 or more action potentials and with more than 5 s interval between bursts; using these parameters > 95% of spikes were categorized as occurring within bursts for each phasic cell (Brown et al. 1998).
Radioimmunoassay
Microdialysis samples and samples from the in vitro release studies were measured in different assays as previously described. Briefly, vasopressin in microdialysates was measured after lyophilization by a highly sensitive and selective radioimmunoassay (RIA; detection limit: 0.1 pg sample−1; cross-reactivity less than 0.7%). Plasma vasopressin was measured after extraction (Landgraf et al. 1995). Vasopressin released from the explants was assayed as described before (Cazalis et al. 1985), with antibody kindly supplied by Dr R. J. Bicknell (Babraham Institute, Cambridge, UK). The final antibody dilution was 1 : 100 000 or 1 : 140 000 and the cross-reactivity of the vasopressin antiserum with oxytocin was 0.001%. The sensitivity of the assay was 0.5 pg. All in vitro experiments were assayed in Montpellier as previously described (Cazalis et al. 1985) except for the CPA experiment, which was performed and assayed in Edinburgh using the same protocol, but using four SON per dish instead of two as we measured a lower level of basal release than expected in this series of experiments. The inter- and intra-assay coefficients of variation for the assays as performed in Edinburgh were 5–7%, similar to those reported for the assay performed in Montpellier.
Statistical analysis
Statistical analyses were performed using SigmaStat software package (Systat Software Inc., Richmond, Ca, USA). Data were analysed by t tests (paired or unpaired as appropriate after passed normality tests) or, when appropriate, by ANOVA followed by Student-Newman-Keuls post hoc tests. All values are expressed as means ± s.e.m., and differences were considered significant at P≤ 0.05.
Results
Dendritic release in response to osmotic stimulation
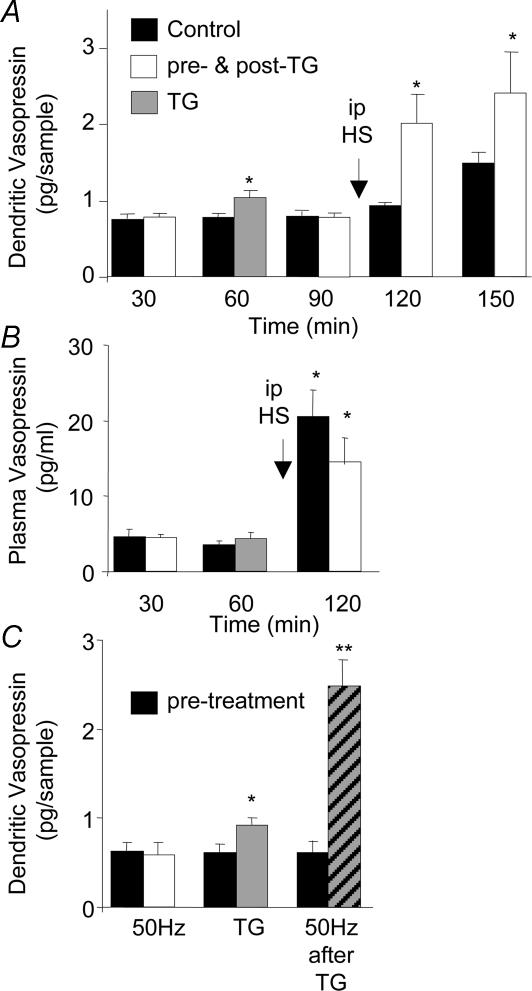
In anaesthetized rats, vasopressin release was measured in response to systemic osmotic stimulation with and without prior application of thapsigargin, which was administered to the SON via the dialysis probe. Thapsigargin caused a slight but significant increase in SON vasopressin release that returned to control levels after washout (P≤ 0.05). Subsequent systemic osmotic stimulation resulted in a gradual and long-lasting increase in dendritic vasopressin release as previously described (Ludwig et al. 1994). Thapsigargin pretreatment, however, caused a more rapid onset in vasopressin release, reaching significance only 30 min following osmotic stimulation (P≤ 0.001, compared between groups, Fig. 1A). Vasopressin secretion into the circulation in response to exposure of one SON to thapsigargin was unaffected. Intraperitoneal injection of hypertonic saline resulted in significantly increased plasma vasopressin concentrations (P≤ 0.05) with no significant differences between the groups (Fig. 1B).
Figure 1. Vasopressin release in the SON.
A, release after intraperitoneal (i.p.) injection of hypertonic saline (HS) after dialysis of one SON with thapsigargin (TG, 200 nm, given during the second 30-min dialysis interval) or control solution. n = 6, *P < 0.05, analysis of variance (ANOVA). B, vasopressin measured in the plasma in the same experiments as A. *P < 0.05, compared to basal. C, vasopressin release in the SON before (black columns) and in response to electrical stimulation of the neural stalk (white columns) or thapsigargin (grey column) and electrical stimulation after exposure of one SON to thapsigargin (hatched column). Data are means ± s.e.m., n = 8, *P < 0.05, **P < 0.01, compared to controls, t tests.
Dendritic release in response to axonal stimulation
To test whether thapsigargin potentiated spike-dependent release from the SON, we placed a microdialysis loop on the ventral surface of the SON, where the dendrites form a dense mat, and a stimulating electrode was placed on the neural stalk of the pituitary for antidromic activation of magnocellular neurones (Ludwig & Leng, 1997). Neural stalk stimulation did not significantly alter the concentration of vasopressin recovered in the SON dialysates (Fig. 1C). Electrical stimulation at 50 Hz resulted in significantly increased plasma concentrations of vasopressin (from 15.0 ± 3.0 to 117.8 ± 14.0 pg ml−1, n = 12), indicating that the stimulation was highly effective in eliciting action potentials in the axons of the magnocellular neurones. Retrodialysis of thapsigargin onto the SON significantly increased dendritic vasopressin release (P≤ 0.05, compared to controls), which returned to basal levels in the poststimulation periods. Subsequent antidromic activation now resulted in a significant increase in dendritic vasopressin release (Fig. 1C, P≤ 0.001, compared to controls). No significant effect of thapsigargin retrodialysis was observed on plasma vasopressin release in response to stalk stimulation (data not shown).
Dendritic release in response to depolarization in vitro
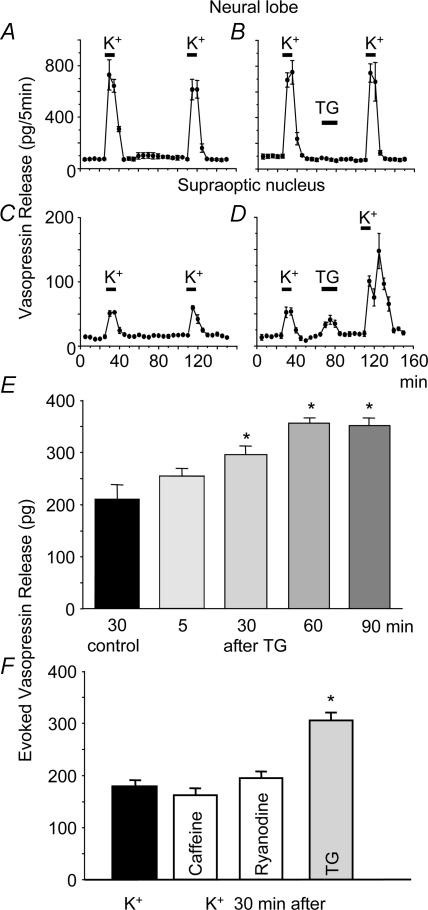
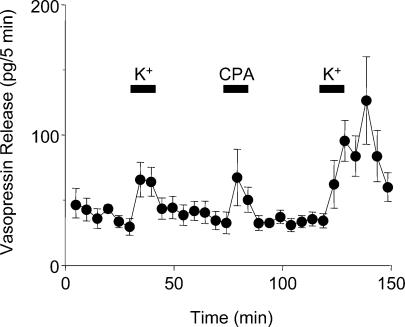
To test whether the effects of thapsigargin were specific to dendritic release of vasopressin, we studied vasopressin release from isolated neural lobes and SON in vitro. The responses of vasopressin secretion from isolated SONs and neural lobes were similar after repeated stimulation with high potassium solution (Fig. 2A and C). Vasopressin secretion from the neural lobe, evoked by depolarization with high-K+ solutions, was unaffected by thapsigargin (Fig. 2B). By contrast, thapsigargin (but not ryanodine or caffeine, Fig. 2F) caused significant vasopressin release from the SON, and produced a dramatic potentiation of subsequent K+-induced release (P < 0.01, paired t tests; Fig. 2D). No significant potentiation was seen in response to high-K+ administered 5 min after exposure to thapsigargin (Fig. 2E), but a large potentiation was observed in response to high K+ administered 30, 60 or 90 min after thapsigargin (Fig. 2E). As the thapsigargin-induced effect on the sarcoplasmic reticulum Ca2+-ATPase is irreversible (Thastrup et al. 1990), in one set of experiments we used the reversible inhibitor CPA (Plenge-Tellechea et al. 1997). Like thapsigargin, CPA caused significant vasopressin release from the SON (P < 0.05, n = 7), and produced a dramatic potentiation of subsequent K+-induced release (P < 0.001, Fig. 3), with no effect on vasopressin release from axon terminals (Sasaki et al. 2005). Thus, exposure to thapsigargin and CPA primes dendritic vasopressin release in response to subsequent depolarization, as previously described for dendritic oxytocin release (Ludwig et al. 2002).
Figure 2. Effects of thapsigargin (TG) on vasopressin release in vitro.
A and C, repeated stimulation with high-K+ solutions results in repeatable responses in vasopressin release from isolated neural lobes (A) and SON (C). B and D, vasopressin release from isolated SON (D), and neural lobes (B) before and after thapsigargin (TG). E, release from the SON only, evoked by depolarization with high-K+ solutions, was strongly potentiated by thapsigargin; the potentiation was long-lasting and time dependent. F, release from the SON only, evoked by depolarization with high-K+ solutions, was strongly potentiated by thapsigargin, but not by caffeine or ryanodine. Data are means ± s.e.m., n = 4 per group for each experiment, *P < 0.05, t tests.
Figure 3. Effects of cyclopiazonic acid (CPA) on vasopressin release in vitro.
Vasopressin release from isolated SON evoked by depolarization with high-K+ solutions was strongly potentiated by CPA (n = 7). The potentiation was similar to the potentiation seen with thapsigargin (Fig. 2D).
We then tested whether vasopressin itself evoked dendritic vasopressin release in our in vitro preparation, just as oxytocin evokes dendritic oxytocin release (Ludwig et al. 2002). Application of a mixture of V1a and V2 agonists significantly increased vasopressin release (P < 0.05); but exposure to the agonists did not significantly potentiate release evoked by a subsequent challenge with high K+ (Fig. 4A). Thus vasopressin, unlike oxytocin, does not prime subsequent depolarization-evoked peptide release. Vasopressin release in response to vasopressin agonists was attenuated, but not fully blocked, in the presence of a mixture of Ca2+-channel blockers (Fig. 4B), although in these conditions vasopressin release in response to 50 mm KCl was blocked (P < 0.05, Fig. 4C). This suggests that vasopressin release in response to vasopressin is partly, though not wholly accounted for by Ca2+ entry through voltage-gated channels. By contrast, oxytocin release in response to oxytocin is independent of voltage-gated Ca2+ entry, and is wholly accounted for by Ca2+ mobilization from thapsigargin-sensitive stores (Lambert et al. 1994).
Responses of vasopressin cells to thapsigargin
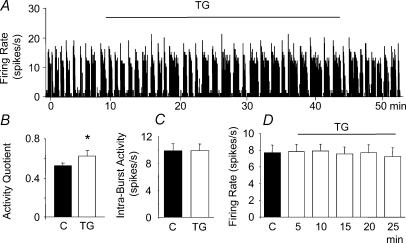
To investigate the effects of thapsigargin on the electrical activity of 14 vasopressin neurones, we recorded from single vasopressin neurones in virgin female rats while dialysing the SON with thapsigargin. Retrodialysis of thapsigargin (2 μm) for 20–60 min increased the activity quotient of phasic firing vasopressin neurones (mean change from 0.52 ± 0.03 to 0.62 ± 0.07 spikes s−1, P < 0.05, n = 6, Fig. 5A and B) without changing the intraburst activity (from 9.86 ± 1.07 to 9.91 ± 0.99 spikes s−1, Fig. 5C). However, thapsigargin treatment did not significantly alter the electrical discharge rate of continuously active vasopressin cells (n = 8, Fig. 5D).
Figure 5. Effects of thapsigargin (TG) on the electrical activity of vasopressin cells.
A, recording of the electrical activity of a phasically firing vasopressin neurone showing an increase in the activity induced by microdialysis administration (retrodialysis) of thapsigargin (TG, 200 nm) onto the SON. B and C, changes in activity quotient (B) but not intraburst activity (C) after TG (P < 0.05, n = 6). D, continuously active vasopressin neurones were not affected by thapsigargin. n = 8, Data are means ± s.e.m., *P < 0.05, t tests.
Discussion
Exposure to thapsigargin, which mobilizes Ca2+ from intracellular stores, produced a dramatic potentiation of dendritic vasopressin release in response to subsequent activation of SON neurones by osmotic stimulation, high potassium or antidromic activation, but produced no potentiation of secretion from the axon terminals. Thus exposure to thapsigargin or CPA, but not exposure to ryanodine or caffeine, appears to selectively prime activity-dependent vasopressin release from dendrites. Priming in vasopressin cells is not a consequence of long-lasting elevation of intracellular Ca2+ as priming outlasts the increase in intracellular Ca2+ induced by thapsigargin (Lambert et al. 1994; Dayanithi et al. 1996) or CPA (data not shown). No potentiation was seen in response to high K+ administered 5 min after exposure to thapsigargin, but a large potentiation was observed in response to high K+ administered 30, 60 or 90 min after thapsigargin. Prolonged dendritic release has been noted in dialysis experiments where dendritic release outlasts that from the axon terminals in the pituitary (Ludwig et al. 1994). There is a similarly persistent neurohormone secretion in Aplysia bag cells long after cytosolic [Ca2+] has returned to baseline (Michel & Wayne, 2002).
Statistical analysis using a paired t test following a passed test for normality indicated significant effects of thapsigargin induced priming 30, 60 and 90 min after thapsigargin administration. Conclusions drawn with an n value of 4 require careful inspection, and the evidence from such experiments has been implicitly tested for coherence and compatibility with other available evidence. A group size of 4 is inadequate for rigorously demonstrating significance with conservative non-parametric tests, and though the present data passed normality tests, these only demonstrate that the data are not demonstrably inconsistent with the assumption that they are normally distributed. However, in the present study, the data showing the effects of thapsigargin are effectively replicated (with different protocols) several times (at three time points, 30, 60 and 90 min) and are also replicated using a different drug (CPA, n = 7). In all cases the effects are very large – these are not marginal changes. The effectiveness of thapsigargin in priming vasopressin release is therefore robustly established.
Priming might involve actions on translation/protein processing (e.g. local synthesis of proteins supporting exocytosis, Ma & Morris, 2002), vesicle tethering and/or active vesicle transport. We have recently shown that thapsigargin-induced priming involves translocation of neurosecretory vesicles closer to the release site at the dendritic membrane (Tobin et al. 2004).
The long-lasting priming of activity-dependent dendritic release of vasopressin is similar to that seen in oxytocin cells, but the magnitude of the priming is much lower. In oxytocin cells, oxytocin itself plays a critical role in priming; in dissociated magnocellular neurones from the SON, oxytocin-induced increases in intracellular [Ca2+] are due to mobilization from thapsigargin-sensitive stores and are independent of external Ca2+ (Lambert et al. 1994). By contrast, although vasopressin produces a rise in intracellular [Ca2+] in vasopressin cells that is similar in magnitude to that induced in oxytocin cells by oxytocin, most of the response to vasopressin reflects Ca2+ entry through voltage-gated channels. The response induced by vasopressin requires about 70% influx of external Ca2+ through L-, N-, and T-type channels and only 30% mobilization of Ca2+ from thapsigargin-sensitive stores (Sabatier et al. 1997).
Although vasopressin increases intracellular [Ca2+] (Dayanithi et al. 1996), the present data show that vasopressin does not prime dendritic release. In oxytocin cells, priming does not result from increases in intracellular [Ca2+]per se, but specifically accompanies mobilization of thapsigargin-sensitive stores; it seems that the limited activation of these stores by vasopressin is insufficient to induce priming.
Exposure to thapsigargin evokes immediate release of vasopressin from dendrites, as previously described for oxytocin cells. In oxytocin cells this occurred without an increase of electrical discharge rate and without triggering secretion from axon terminals. In vasopressin cells, thapsigargin administration resulted in an increase in electrical activity in phasic cells only. The simplest explanation for the difference in responses is that, in vasopressin cells, thapsigargin-induced increases in intracellular [Ca2+] activate Ca2+-dependent currents that underlie activation of the depolarizing afterpotential (DAP) that is exhibited by vasopressin cells but not by oxytocin cells (see Hatton & Li, 1998). The DAP underlies the genesis of the phasic firing pattern that is specific to vasopressin cells (see Armstrong, 1995). Although thapsigargin caused an increase in electrical activity and an increase in dendritic release, these might not be causally related. It seems likely that, as in oxytocin cells, a rise in intracellular [Ca2+] is a direct trigger for dendritic vasopressin release. Alternatively, the observed differences between oxytocin and vasopressin neurones might reflect differences in their calcium buffering capacity. Oxytocin neurones express high levels of calbindin and also express calretinin, whereas vasopressin cells have very low levels of calbindin and no calretinin (Miyata et al. 1998).
Physiological consequences
After priming, it seems likely that activity-dependent dendritic release of oxytocin underlies the oxytocin-dependent generation of bursting activity in response to suckling (Ludwig et al. 2002). These observations suggest that priming can change the nature of interactions between magnocellular neurones and their inputs over a long time period. However, the physiological consequence of vesicle priming in vasopressin cells has yet to be determined. Neuropeptides released from dendrites are at very high local concentrations (Ludwig & Leng, 1997) and might have long half-lives, and even degradation produces shorter, but still active, fragments (Gouzenes et al. 1999). Not only can their local actions be prolonged, but diffusion through the brain extracellular fluid might allow them to act on receptors on neurones at a distance from their sites of release (Landgraf & Neumann, 2004), including regions involved in behaviour regulation that are known to be influenced by oxytocin and vasopressin, but which have no direct anatomical connection with magnocellular neurones (see Engelmann et al. 1996; Ferguson et al. 2002). However, the precise physiological role of priming of dendritic vasopressin release remains to be shown.
Acknowledgments
The Wellcome Trust and the BBSRC supported this work.
References
- Armstrong WE. Morphological and electrophysiological classification of hypothalamic supraoptic neurons. Prog Neurobiol. 1995;47:291–339. 10.1016/0301-0082(95)00025-9. [PubMed] [Google Scholar]
- Brown CH, Ludwig M, Leng G. Kappa-opioid regulation of neural activity in the rat supraoptic nucleus in vivo. J Neurosci. 1998;18:9480–9488. doi: 10.1523/JNEUROSCI.18-22-09480.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazalis M, Dayanithi G, Nordmann JJ. The role of patterned burst and interburst interval on the excitation–coupling mechanism in the isolated rat neural lobe. J Physiol. 1985;369:45–60. doi: 10.1113/jphysiol.1985.sp015887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dayanithi G, Widmer H, Richard P. Vasopressin-induced intracellular Ca2+ increase in isolated rat supraoptic cells. J Physiol. 1996;490:713–727. doi: 10.1113/jphysiol.1996.sp021180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann M, Wotjak CT, Neumann I, Ludwig M, Landgraf R. Behavioral consequences of intracerebral vasopressin and oxytocin: focus on learning and memory. Neurosci Biobehav Rev. 1996;20:341–358. doi: 10.1016/0149-7634(95)00059-3. 10.1016/0149-7634(95)00059-3. [DOI] [PubMed] [Google Scholar]
- Ferguson JN, Young LJ, Insel TR. The neuroendocrine basis of social recognition. Front Neuroendocrinol. 2002;23:200–224. doi: 10.1006/frne.2002.0229. 10.1006/frne.2002.0229. [DOI] [PubMed] [Google Scholar]
- Gouzenes L, Dayanithi G, Moos FC. Vasopressin (4–9) fragment activates V-1a-type vasopressin receptor in rat supraoptic neurones. Neuroreport. 1999;10:1735–1739. doi: 10.1097/00001756-199906030-00020. [DOI] [PubMed] [Google Scholar]
- Gouzenes L, Desarmenien MG, Hussy N, Richard P, Moos FC. Vasopressin regularizes the phasic firing pattern of rat hypothalamic magnocellular neurons. J Neurosci. 1998;18:1879–1885. doi: 10.1523/JNEUROSCI.18-05-01879.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton GI, Li Z. Intrinsic controls of intracellular calcium and intercellular communication in the regulation of neuroendocrine cell activity. Cell Mol Neurobiol. 1998;18:13–28. doi: 10.1023/A:1022519008991. 10.1023/A:1022519008991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausser M, Stuart G, Racca C, Sakmann B. Axonal initiation and active dendritic propagation of action potentials in substantia nigra neurons. Neuron. 1995;15:637–647. doi: 10.1016/0896-6273(95)90152-3. 10.1016/0896-6273(95)90152-3. [DOI] [PubMed] [Google Scholar]
- Hirasawa M, Schwab Y, Natah S, Hillard CJ, Mackie K, Sharkey KA, Pittman QJ. Dendritically released transmitters cooperate via autocrine and retrograde actions to inhibit afferent excitation in rat brain. J Physiol. 2004;559:611–624. doi: 10.1113/jphysiol.2004.066159. 10.1113/jphysiol.2004.066159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS. Mechanisms governing dendritic gamma-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc Natl Acad Sci U S A. 2001;98:337–342. doi: 10.1073/pnas.021445798. 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaacson JS, Strowbridge BW. Olfactory reciprocal sysnapses – dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. 10.1016/S0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- Kombian SB, Mouginot D, Pittman QJ. Dendritic released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. 10.1016/S0896-6273(00)80971-X. [DOI] [PubMed] [Google Scholar]
- Lambert RC, Dayanithi G, Moos FC, Richard P. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol. 1994;478:275–288. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landgraf R. Intracerebrally released vasopressin and oxytocin: measurement, mechanisms and behavioural consequences. J Neuroendocrinol. 1995;7:243–253. doi: 10.1111/j.1365-2826.1995.tb00754.x. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID. Neuropeptide release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front Neuroendocrinol. 2004;25:150–176. doi: 10.1016/j.yfrne.2004.05.001. 10.1016/j.yfrne.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Holsboer F, Pittman QJ. Interleukin-1 beta stimulates both central and peripheral release of vasopressin and oxytocin in the rat. Eur J Neurosci. 1995;7:592–598. doi: 10.1111/j.1460-9568.1995.tb00663.x. [DOI] [PubMed] [Google Scholar]
- Leng G, Dyball REJ. Functional identification of magnocellular neuroendocrine neurones. In: Greenstein B, editor. Neuroendocrine Research Methods. Chur, Switzerland: Harwood Academic Publishers GmbH; 1991. pp. 769–791. In. [Google Scholar]
- Ludwig M. Dendritic release of vasopressin and oxytocin. J Neuroendocrinol. 1998;10:881–895. doi: 10.1046/j.1365-2826.1998.00279.x. 10.1046/j.1365-2826.1998.00279.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Callahan MF, Neumann I, Landgraf R, Morris M. Systemic osmotic stimulation increases vasopressin and oxytocin release within the supraoptic nucleus. J Neuroendocrinol. 1994;6:369–373. doi: 10.1111/j.1365-2826.1994.tb00595.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Leng G. Autoinhibition of supraoptic nucleus vasopressin neurons in vivo – a combined retrodialysis/electrophysiological study in rats. Eur J Neurosci. 1997;9:2532–2540. doi: 10.1111/j.1460-9568.1997.tb01682.x. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Pittman QJ. Talking back: dendritic neurotransmitter release. Trends Neurosci. 2003;26:255–261. doi: 10.1016/S0166-2236(03)00072-9. 10.1016/S0166-2236(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Sabatier N, Bull PM, Landgraf R, Dayanithi G, Leng G. Intracellular calcium stores regulate activity-dependent neuropeptide release from dendrites. Nature. 2002;418:85–89. doi: 10.1038/nature00822. 10.1038/nature00822. [DOI] [PubMed] [Google Scholar]
- Ma D, Morris JF. Protein synthetic machinery in the dendrites of the magnocellular neurosecretory neurons of wild-type Long-Evans and homozygous Brattleboro rats. J Chem Neuroanat. 2002;23:171–186. doi: 10.1016/s0891-0618(01)00158-2. 10.1016/S0891-0618(01)00158-2. [DOI] [PubMed] [Google Scholar]
- Michel S, Wayne NL. Neurohormone secretion persists after post-afterdischarge membrane depolarization and cytosolic calcium elevation in peptidergic neurons in intact nervous tissue. J Neurosci. 2002;22:9063–9069. doi: 10.1523/JNEUROSCI.22-20-09063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata S, Khan AM, Hatton GI. Colocalization of calretinin and calbindin-D28k with oxytocin and vasopressin in rat supraoptic nucleus neurons: a quantitative study. Brain Res. 1998;785:178–182. doi: 10.1016/s0006-8993(97)01375-9. 10.1016/S0006-8993(97)01375-9. [DOI] [PubMed] [Google Scholar]
- Moos F, Poulain DA, Rodriguez F, Guerne Y, Vincent JD, Richard P. Release of oxytocin within the supraoptic nucleus during the milk ejection reflex in rats. Exp Brain Res. 1989;76:593–602. doi: 10.1007/BF00248916. 10.1007/BF00248916. [DOI] [PubMed] [Google Scholar]
- Morris JF, Christian H, Ma D, Wang H. Dendritic secretion of peptides from hypothalamic magnocellular neurosecretory neurones: a local dynamic control system and its functions. Exp Physiol. 2000;85:131S–138S. doi: 10.1111/j.1469-445x.2000.tb00016.x. 10.1111/j.1469-445X.2000.tb00016.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Sydney: Academic Press; 1986. [Google Scholar]
- Plenge-Tellechea F, Soler F, Fernandez-Belda F. On the inhibition mechanism of sarcoplasmic or endoplasmic reticulum Ca2+-ATPases by cyclopiazonic acid. J Biol Chem. 1997;272:2794–2800. doi: 10.1074/jbc.272.5.2794. 10.1074/jbc.272.5.2794. [DOI] [PubMed] [Google Scholar]
- Pow DV, Morris JF. Dendrites of hypothalamic magnocellular neurons release neurohypophysial peptides by exocytosis. Neuroscience. 1989;32:435–439. doi: 10.1016/0306-4522(89)90091-2. 10.1016/0306-4522(89)90091-2. [DOI] [PubMed] [Google Scholar]
- Sabatier N, Brown CH, Ludwig M, Leng G. Phasic spike patterning in rat supraoptic neurones in vivo and in vitro. J Physiol. 2004;558:161–180. doi: 10.1113/jphysiol.2004.063982. 10.1113/jphysiol.2004.063982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Caquineau C, Dayanithi G, Bull P, Douglas AJ, Guan XM, Jiang M, Van der Ploeg L, Leng G. α-Melanocyte-stimulating hormone stimulates oxytocin release from dendrites of hypothalamic neurons while inhibiting oxytocin release from their terminals in the neurohypopysis. J Neuroci. 2003;23:10351–10358. doi: 10.1523/JNEUROSCI.23-32-10351.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatier N, Richard P, Dayanithi G. L-, N- and T- but neither P- nor Q-type Ca2+ channels control vasopressin-induced Ca2+ influx in magnocellular vasopressin neurones isolated from the rat supraoptic nucleus. J Physiol. 1997;503:253–268. doi: 10.1111/j.1469-7793.1997.253bh.x. 10.1111/j.1469-7793.1997.253bh.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Dayanithi G, Shibuya I. Ca2+ clearance mechanisms in neurohypophysial terminals of the rat. Cell Calcium. 2005;37:45–56. doi: 10.1016/j.ceca.2004.06.007. 10.1016/j.ceca.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2+-ATPase. Proc Natl Acad Sci U S A. 1990;87:2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobin VA, Hurst G, Norrie L, Dal Rio F, Bull PM, Ludwig M. Thapsigargin-induced mobilisation of dendritic dense-cored vesicles in rat supraoptic neurones. Eur J Neurosci. 2004;19:2909–2912. doi: 10.1111/j.1460-9568.2004.03388.x. 10.1111/j.1460-9568.2004.03388.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Dayanithi G, Newcomb R, Lemos JR. An R-type Ca2+ current in neurohypophysial terminals preferentially regulates oxytocin secretion. J Neurosci. 1999;19:9235–9241. doi: 10.1523/JNEUROSCI.19-21-09235.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y, Kaiser KMM, Sakmann B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron. 1999;24:979–988. doi: 10.1016/s0896-6273(00)81044-2. 10.1016/S0896-6273(00)81044-2. [DOI] [PubMed] [Google Scholar]