Abstract
Coding of gustatory information is complex and unique among sensory systems; information is received by multiple receptor populations located throughout the oral cavity and carried to a single central relay by four separate nerves. The geniculate ganglion is the location of the somata of two of these nerves, the greater superficial petrosal (GSP) and the chorda tympani (CT). The GSP innervates taste buds on the palate and the CT innervates taste buds on the anterior tongue. To obtain requisite taste response profiles of GSP neurones, we recorded neurophysiological responses to taste stimuli of individual geniculate ganglion neurones in vivo in the rat and compared them to those from the CT. GSP neurones had a distinct pattern of responding compared to CT neurones. For example, a small subset of GSP neurones had high response frequencies to sucrose stimulation, whereas no CT neurones had high response frequencies to sucrose. In contrast, NaCl elicited high response frequencies in a small subset of CT neurones and elicited moderate response frequencies in a relatively large proportion of GSP neurones. The robust whole-nerve response to sucrose in the GSP may be attributable to relatively few, narrowly tuned neurones, whereas the response to NaCl in the GSP may relate to proportionately more, widely tuned neurones. These results demonstrate the diversity in the initial stages of sensory coding for two separate gustatory nerves involved in the ingestion or rejection of taste solutions, and may have implications for central coding of gustatory quality and concentration as well as coding of information used in controlling energy, fluid and electrolyte homeostasis.
Integration of afferent information presents a formidable challenge to the gustatory system. Coding of taste information involves combined neural inputs from four nerves that have spatially distinct receptive fields. The chorda tympani (CT) nerve and glossopharyngeal nerve innervate separate populations of taste receptors on the tongue, the greater superficial petrosal (GSP) nerve innervates taste receptors located in the palate and the superior laryngeal nerve innervates taste receptors along the posterior oral–pharyngeal cavity, such as the epiglottis. Each nerve has its own functional profile that reflects different transduction pathways and potentially different neural coding strategies and different roles in mediating taste-related behaviours (Travers et al. 1986; Travers & Norgren, 1995).
Through the use of whole-nerve and single-fibre electrophysiology, taste response properties of CT neurones are relatively well characterized. Responses from the CT have been studied in a variety of species and experimental conditions (Beidler, 1953; Frank, 1973; Contreras & Frank, 1979; Hill et al. 1982; Boudreau et al. 1983; Frank et al. 1983; Hill & Phillips, 1994; Contreras & Lundy, 2000; Shimatani et al. 2002). Additionally, both the glossopharyngeal nerve and the superior laryngeal nerve have been studied using whole-nerve and single-fibre electrophysiology (Frank, 1973; Shingai & Beidler, 1985; Dickman & Smith, 1988; Hanamori et al. 1988; Smith & Hanamori, 1991; Danilova et al. 2002). For each of these nerves, single-fibre recordings provide different but complementary information to data derived from whole-nerve recordings. For example, whole-nerve responses provide information about how an entire receptor population responds to a taste stimulus, whereas single-fibre responses specify how the neural information is represented (or coded) in the respective nerve.
In contrast to all other gustatory nerves, few studies have described whole-nerve responses from the GSP (Nejad, 1986; Harada & Smith, 1992; Sollars & Hill, 1998; Harada & Kasahara, 2000; Sollars & Hill, 2000), and no published studies have characterized taste response properties of individual GSP neurones. Behavioural studies with rats suggest that the GSP may contribute to salt, quinine and sucrose discrimination (Krimm et al. 1987; Spector et al. 1997; St John & Spector, 1998; Roitman & Bernstein, 1999; Geran et al. 2002). Recent neurobiological advances are also providing details about gustatory function of palatal taste receptors (Boughter et al. 1997; El-Sharaby et al. 2001a, b; Gilbertson et al. 2001). Therefore, a significant missing component in an overall understanding of the neural representation of taste stimuli (especially sugars) is the lack of neurophysiological data from single GSP neurones.
Although these four gustatory nerves primarily convey taste information, they are likely to have a much wider impact centrally. Specifically, these multiple inputs all project centrally to the nucleus of the solitary tract (NTS). In fact, many of the postsynaptic neurones in the NTS receive convergent input from more than one gustatory nerve (Travers et al. 1986; Travers & Norgren, 1995). It is in the NTS where combined afferent inputs from the four gustatory nerves interact functionally to signal qualitative and quantitative (i.e. concentration) taste characteristics as well as influence metabolic, hormonal and behavioural systems (Norgren, 1984). Therefore, gustatory inputs not only convey taste information, but they are also involved in controlling energy, fluid and electrolyte homeostasis. The GSP may be a major contributor to these functions because it transmits robust salt and sugar responses (Nejad, 1986; Harada & Smith, 1992; Sollars & Hill, 1998; Harada & Kasahara, 2000).
The present study provides the first analysis of response properties of individual GSP neurones recorded in vivo in the geniculate ganglion, thereby providing requisite data in regard to the absolute response properties of individual neurones of the GSP. These taste response properties are compared directly with responses from geniculate ganglion neurones that comprise the CT, neurones from which there is a significant amount of existing data and from which responses are distinct from the GSP. Recordings from geniculate ganglion neurones were done because both CT and GSP neurones are located within the ganglion, and importantly, responses could be obtained from intact neurones. Single axon recordings from gustatory nerves involve sectioning the nerve; therefore, the potential exists for injury-induced processes to affect responses. Collectively, the results provide new insights into the strategies and complexity of sensory coding used by different gustatory nerves needed for feature extraction of gustatory stimuli by central neurones for sensory and homeostatic functions.
Methods
Animals
Adult female rats were obtained from Charles River Laboratories (Wilmington, MA, USA) and neurophysiological recordings were made when they were 40–70 days of age. All rats were maintained on standard chow and water and group housed on a 12 h light–dark cycle. Seventeen rats were used with a range of one to seven neurones recorded per animal for a total of 46 neurones (23 each of CT and GSP). All procedures were carried out under the approval of the Institutional Animal Care and Use Committee and in full accordance with NIH guidelines.
Surgery
Rats were anaesthetized with Nembutal (sodium pentobarbital, 50 mg kg−1, i.p.) with additional doses given throughout the procedure to maintain a surgical level of anaesthesia. An airway tube was placed in the trachea and a stimulus delivery tube was placed through the oral cavity and into the oesophagus. The stimulus delivery tube was made of PE 190 tubing with a flange at one end and a 2 cm piece of perforated tubing extending from the flange. The tubing exited the oesophagus in the neck through a small incision. Muscle around the tube was sutured tightly to prevent back-flow of solution into the oesophagus. The flange was placed to cover the opening to the oesophagus in the oral cavity and the perforated tubing extended the length of the palate. In this way, taste solutions could be applied through the tubing exiting the oesophagus to bathe the tongue and all three regions of the palate: the posterior palatine field, and the geschmacksstreifen and nasoincisor ducts (Miller, 1977; Miller & Spangler, 1982). Prior to establishing the stimulation procedure, we determined that both GSP and CT receptive fields were stimulated by placing dilute methylene blue through the device. In addition, this stimulation technique has been used successfully in the past to record GSP whole-nerve responses (Sollars & Hill, 1998). All recordings were obtained from the left ganglion; a suture was placed through the ventral aspect of the right side of the tongue to allow for the tongue to be retracted from the oral cavity after recording the stimulus series (see below).
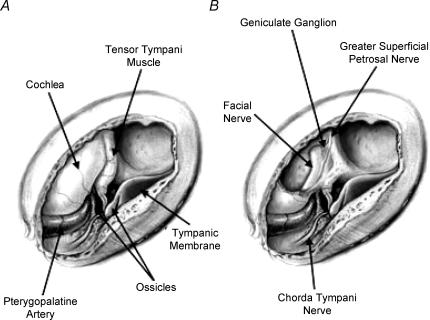
Rats were placed in a supine position in a stereotaxic device with a modified headholder that anchored the mouth just rostral to the nasoincisor duct, but did not obscure the duct. Body temperature was maintained with a water-circulating heating pad. The juncture between the anterior and posterior digastricus muscle was sectioned and the posterior muscle retracted away from the tympanic bulla. Muscles surrounding the surgical region were retracted to expose the ventral surface of the tympanic bulla. A hole was made into the tympanic bulla, and the cochlea, tensor tympani muscle, and part of the temporal bone overlying the geniculate ganglion was removed. The geniculate ganglion lies within the petrous portion of the temporal bone at the genu of the facial nerve. See Fig. 1 for an illustration of the surgical site. Care was taken to avoid injury to the chorda tympani as it courses through the tympanic bulla. The oral cavity was propped open slightly to facilitate the determination of which receptor field was innervated after a neurone was successfully recorded.
Figure 1. Illustration of the surgical approach to the geniculate ganglion.
A, a hole was cut in the ventral surface of the tympanic bulla, showing the ossicles and other internal structures within the bulla. B, the tensor tympani muscle and cochlea were removed to show the geniculate ganglion and greater superficial petrosal nerve. Illustrated by Anita Hylton.
Neurophysiology and stimulation procedure
Aluminosilicate glass pipettes were pulled with an inside tip diameter of approximately 0.1 μm. The tip and tapered portion of the barrel of the pipette was filled with 0.154 m NaCl and 1.0 m potassium chloride was used to fill the remainder of the pipette to produce an electrode with resistances ranging from 75 to 150 MΩ (Renehan et al. 1994). The electrode was placed into a headstage attached to a microdriver (Exfo Burleigh, Victor, NY) that is designed to step-advance at a rate as small as 0.2 μm per step. Neural responses were amplified by an A-M Systems high-input impedance preamplifier (model 1600) and displayed and stored with PowerLab equipment (ADInstruments) attached to a Macintosh computer.
A stimulus mixture consisting of NaCl, NH4Cl, sucrose and quinine hydrochloride was applied to the oral cavity through the esophageal fistula. The electrode was advanced into the ganglion using the microdriver until activity from a cell was encountered. The stimulus mixture was rinsed off and re-applied to determine if the cell was taste-responsive. If taste-responsive, the mouth was rinsed well and neural responses to taste stimuli (see below) were recorded. Recordings were initiated with the electrode in an extracellular position. Neurones were maintained within recording range of the electrode for at least 20 min and occasionally up to 2 h or more.
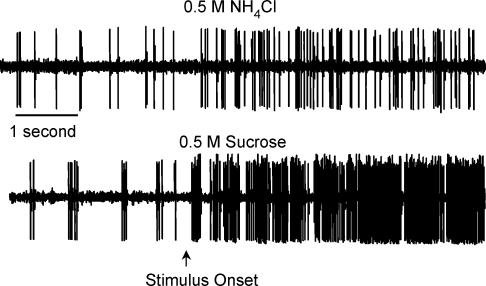
Responses were recorded to 0.1 and 0.5 m NaCl, 0.1 and 0.5 m NH4Cl, 0.5 m sucrose, 0.01 m quinine hydrochloride and 0.01 n hydrochloric acid (HCl). These stimuli focus the experiments on salt elicited responses because both the chorda tympani nerve and the greater superficial nerve respond well to salts (Hill et al. 1982; Sollars & Hill, 1998). Importantly, the number of stimuli was limited to allow completion of the majority of recordings attempted. Chemicals were reagent grade and mixed in distilled water. Stimuli flowed over the palate and tongue through the esophageal tubing. Approximately 10 ml of each solution was applied at a rate of 0.5 ml per second through the use of a computerized stimulus delivery system. A stimulus marker recorded the onset and cessation of the fluid application on a separate data channel. Stimuli remained on the tongue for approximately 20 s followed by a 40 s rinse with distilled water. Additional 40 s rinse periods were applied if the activity of the cell remained above initial spontaneous activity. After completing the stimulus series, the tongue was retracted and the tongue and palate were stimulated separately to determine the neurone's gross receptive field (i.e. palate versus tongue). Only data were analysed in which the neurone's receptive field was determined. See Fig. 2 for an example of the raw data obtained from a single GSP neurone stimulated with 0.5 m NH4Cl and 0.5 m sucrose. Signal-to-noise ratios of the magnitude illustrated were common for this procedure.
Figure 2. Raw neurophysiological data of the response to 0.5 m NH4Cl and 0.5 m sucrose recorded from a geniculate ganglion neurone that innervated palatal taste receptors.
Spontaneous activity precedes the stimulus onset.
Difficulty in obtaining large numbers of GSP neurones was encountered because of the diffuse topographical placement of this population of soma. A large grouping of chorda tympani neurones is compacted within the thickest portion of the ganglion (Gomez, 1978). However, there does not appear to be a similarly well-delineated area for GSP somata; they are dispersed throughout the ganglion and along the GSP itself (the thinnest portion of the ganglion which is difficult to penetrate with the electrode). Thus, many more CT neurones were available than GSP neurones. Since the present study was focused on analysis of GSP neurones, ‘extra’ CT neurones were bypassed throughout the experiment in an effort to maximize the number of GSP recordings. Upon completion of recording from the last unit from an animal, rats were given a lethal dose of sodium pentobarbital (100 mg kg−1, i.p.)
Data analysis
Recordings were analysed using PowerLab software programs. Measurements were taken during the time that the stimulus was flowing into the oral cavity. Spontaneous activity was defined as the 10 s of neural activity that occurred immediately prior to the onset of each respective stimulus. Spontaneous activity was subtracted from the frequency of response obtained during the 10 s of stimulation used in the analysis. The first 0.5 s after stimulus onset was eliminated from the analysis to avoid electrical noise and rapidly adapting tactile responses generated from the initial stimulus contact with the oral cavity (Hill et al. 1982). Although noise was not detected in all neurones (see Fig. 2), elimination of the initial 0.5 s provided consistency of the measured stimulus period across all neurones. The subsequent 10 s of activity was used to characterize response profiles. This produced a consistent and large period for data analysis, while minimizing variations in response adaptation that may occur after stimulus application.
Means analysis of spike frequency
Repeated measures ANOVA was performed on mean response frequencies for CT and for GSP neurones. Post hoc analyses with Least Significant Difference (LSD) tests (P < 0.05) were used to test differences in the mean activity across stimuli within the CT and GSP populations. LSD tests were also performed to determine differences in responses of CT neurones as compared to GSP neurones.
Response criteria
The standard deviation (s.d.) of the defined spontaneous activity for each individual neurone was calculated and neurones were classified according to their responses relative to the s.d. of spontaneous activity. The resultant categories are used throughout the manuscript: ‘No response’= 0 ≤ 1 s.d. higher or lower than spontaneous activity; 1 s.d. < ‘+Low’≤ 2 s.d. higher than spontaneous; 2 s.d. < ‘+Medium’≤ 5 s.d. higher than spontaneous; >5 s.d.=‘+High’. Inhibitory responses were classified similarly, but opposite in direction and are denoted by a negative sign before the label (e.g. ‘−Low’). Rather than merely categorizing all neurones above a certain threshold as ‘responsive’, the categorizations used here provide a detailed method to compare responses of individual neurones both within and across neuronal populations. Response categories of this type also provide a method for discussing the commonalities in response rates across neurones.
Across-neurone correlations
Correlation coefficients (Pearson r) were calculated for responses across stimulus pairs for geniculate ganglion neurones that innervated the tongue separately from those that innervated the palate. In this way, the similarity of response profiles between each pair of stimuli across neurones was examined.
Breadth of tuning
The degree to which a neuronal response profile was specifically or broadly tuned across stimuli was measured by calculating entropy. Entropy is based on the formula:
where H is the breadth of tuning (entropy), K (a scaling constant) = 1.66 for four stimuli and 1.18 for five stimuli, pi is the proportion that each stimulus contributes to the total frequency of response for each neurone (Smith & Travers, 1979). Entropy was calculated separately for GSP and CT neurones. Student's t test was used to analyse breadth of tuning differences between the two samples of neurones.
Breadth of tuning measurements in the gustatory system are usually confined to responses to four ‘basic’ stimuli: NaCl, sucrose, quinine and HCl (Smith & Travers, 1979; Lundy & Contreras, 1999; Di Lorenzo & Lemon, 2000; Gilbertson et al. 2001). In order to be consistent with the measures from CT neurones from other studies and to examine potential differences in tuning between CT and GSP neurones, the stimuli included in the present analysis were 0.1 m NaCl, 0.5 m sucrose, 0.01 m quinine and 0.01 n HCl.
Cluster analysis
In order to analyse groupings of neurones based on individual neural response profiles, hierarchical cluster analyses (Pearson's correlation measure, between groups linkage; SPSS) were performed on the separate neuronal populations to determine consistencies across the stimulus response profiles. Cluster analyses were performed using response data from 0.1 m NaCl, 0.5 m sucrose, 0.01 m quinine, 0.01 n HCl and 0.1 m NH4Cl. Selection of this group of stimuli provided consistency between the current report and previous research for better comparison across studies (Lundy & Contreras, 1999). NH4Cl at 0.1 m was included in the cluster analysis because it was a particularly effective stimulus in CT neurones, but less so in GSP neurones, thus providing a greater opportunity to examine functional differences between the neuronal populations.
Categorizations were determined by an agglomeration schedule that clustered the data into groups. The cluster sets were determined first by scree analyses of proximity coefficients produced in the agglomeration schedule (not shown) that were performed on each data set. Based on the response profiles of neurones within a scree-determined cluster, cluster sets were labelled as categories determined to be theoretically meaningful (e.g. ‘sucrose-best’, ‘HCl-generalists’). Individual cluster categories were further analysed using the breadth of tuning calculations mentioned above. In addition, statistical analyses using ANOVA were performed on each category formed by the cluster groupings. Each ANOVA included the responses to all seven stimuli in order to elucidate the response characteristics of the neurones in greater detail. Post hoc analyses with LSD tests (P < 0.05) were conducted to differentiate the relative effectiveness of the stimuli within the cluster categories. Entropy was calculated independently for each cluster category using all five stimuli included in the cluster analysis so that breadth of tuning measures could be compared across cluster groups.
Results
Spontaneous activity
The average spontaneous activity was significantly greater for GSP cells than for CT cells (t(44) = 2.78, P < 0.01). The mean spontaneous activity for all GSP cells was 14.13 ± 2.4 (10 s)−1 and the average of all CT cells was 6.67 ± 1.3 (10 s)−1. Although the spontaneous activity differed between CT and GSP neurones, there was no difference in spontaneous activity rates among functional groups for each nerve (see cluster analysis categories below (P > 0.10)).
Overall response properties
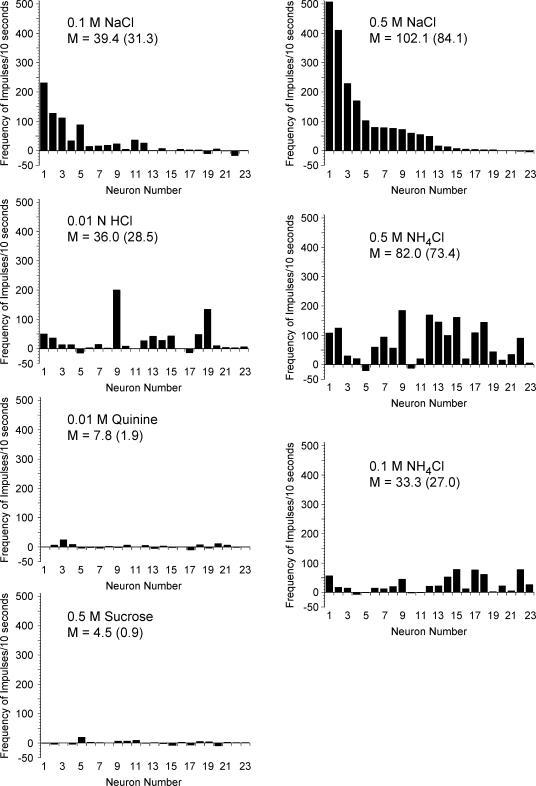
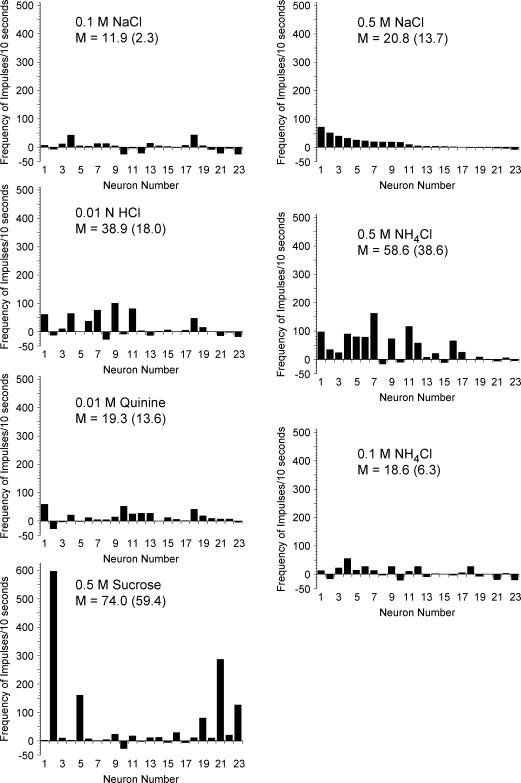
Figure 3 illustrates the response frequencies of CT neurones that innervated the tongue and Fig. 4 shows responses from GSP neurones that innervated the palate. Mean values based on all responses greater than zero are presented on the figures to provide a direct comparison with previous studies (Hill et al. 1982; Frank et al. 1983; Lundy & Contreras, 1999). The respective mean response frequencies for all neurones within a stimulus set (excitatory and inhibitory) are shown in parentheses.
Figure 3. Response frequencies above spontaneous activity for individual geniculate ganglion neurones that innervated taste receptors on the tongue.
Each neurone was assigned a number based on its frequency of response to 0.5 m NaCl and ordered with the same neurone number for each stimulus. Therefore, the pattern of activity can be tracked for each individual neurone across all stimuli. The mean (M) of all frequencies greater than 0 is presented. The mean across all neurones is included in parentheses for comparison.
Figure 4. Response frequencies above baseline for individual geniculate ganglion neurones that innervated taste receptors on the palate.
Each neurone was assigned a number based on its frequency of response to 0.5 m NaCl and ordered with the same neurone number for each stimulus. Therefore, the pattern of activity can be tracked for each individual neurone across all stimuli. Note that the y-axis value for sucrose is higher than the y-axis in other graphs. The mean (M) of all frequencies greater than 0 is presented. The mean across all neurones is included in parentheses for comparison.
Neurones that innervated the tongue were significantly different in their responses across stimuli (F6,132= 690, P < 0.0001). The mean response frequency of CT neurones to 0.5 m NaCl and to 0.5 m NH4Cl were significantly greater (P < 0.05) than responses to all other stimuli. Responses to 0.5 m NH4Cl were similar to responses to 0.5 m NaCl (P > 0.10). No other significant differences in mean responses were observed across neurones innervating the tongue.
Significant differences were also noted in mean responses for GSP neurones (F6,132= 2.82, P < 0.05). For GSP neurones, sucrose responses were significantly greater (P < 0.05) than responses to all stimuli except 0.5 m NH4Cl. Responses to sucrose and to 0.5 m NH4Cl were similar. In addition, 0.5 m NH4Cl responses were significantly higher than 0.1 m NaCl responses. No other significant differences were observed.
A comparison between CT and GSP responses across all stimuli revealed significant differences between neuronal populations (F13,321= 4.52, P < 0.0001). When mean responses were compared to the same stimulus between the CT and the GSP, responses to sucrose by GSP neurones were significantly higher (P < 0.05) than sucrose responses of the CT. In addition, CT responses to 0.5 m NaCl were significantly greater than GSP responses to 0.5 m NaCl. No other significant differences were observed across the neural populations in responses to identical stimuli.
Salt stimuli
Neurones innervating the tongue.
Neurones that innervated the tongue showed responses (Fig. 3) similar to previous reports from recordings of the chorda tympani (Hill et al. 1982; Frank et al. 1983; Lundy & Contreras, 1999). Specifically, of the 23 neurones, five had large responses to 0.5 m NaCl exceeding 100 impulses (10 s)−1. Of those, two of the neurones exceeded 400 impulses (10 s)−1. Another stimulus that elicited a high response rate in CT neurones was NH4Cl, with eight of the neurones responding to 0.5 m NH4Cl at a frequency greater than 100 impulses (10 s)−1.
Neurones innervating the palate.
Palatal units also responded strongly to salts (Fig. 4), but none showed the unusually large responses to 0.5 m NaCl as demonstrated by a few of the CT neurones. Similar to CT responses, there was not a consistent pattern of responses across salt stimuli. Many GSP neurones responded well to 0.5 m NH4Cl, with two of the cells responding at a rate exceeding 100 impulses (10 s)−1 and seven other cells exceeding 50 impulses (10 s)−1.
Neurones innervating the tongue versus neurones innervating the palate.
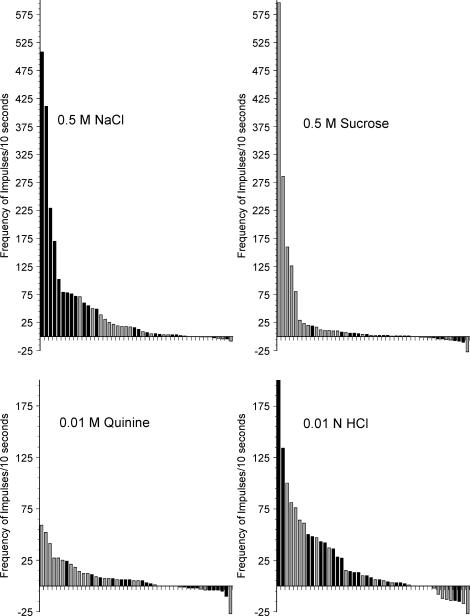
To facilitate comparisons between CT and GSP neurones, Fig. 5 shows the distribution of response frequencies for 0.5 m NaCl in both GSP and CT neurones. Response frequencies of singe neurones that innervate the tongue are shown as black bars and those that innervate the palate are shown as grey bars. Neurones are shown in descending order for each stimulus. When stimulated with 0.5 m NaCl, CT neurones dominated the highest range of responses, while GSP neurones were most common in the mid-range of response frequencies. The lowest responses were distributed across both receptor populations. The high spontaneous activity noted in a subset of neurones contributed to the occasional appearance of inhibitory responses to stimuli (i.e. bars below the x axis in Figs 3 – 5). Overall, the higher spontaneous activity of GSP neurones was reflected in greater numbers of inhibitory responses of GSP neurones as compared to CT neurones.
Figure 5. Frequency of response (minus spontaneous activity) of individual neurones that innervated tongue taste receptors (black bars) or palatal taste receptors (grey bars).
Neurones are ordered within each stimulus based on the degree of response to that stimulus.
Sucrose
Neurones innervating the tongue.
CT neurones were largely unresponsive or were inhibited to sucrose; the highest response rate was 19 impulses (10 s)−1 (Fig. 3). This finding is consistent with previous reports in which most neurones had low response frequencies to sucrose (Frank et al. 1983; Lundy & Contreras, 1999).
Neurones innervating the palate.
Sucrose elicited a large response in a subset of GSP neurones (see Fig. 4). Four cells had a response larger than 100 impulses (10 s)−1. Of that group, one neurone had a response to 0.5 m sucrose of 596 impulses (10 s)−1. This response was the highest observed from any cell (whether CT or GSP) with any stimulus throughout this experiment. Considering that there is a high GSP whole-nerve response to sucrose (Nejad, 1986; Harada & Smith, 1992; Sollars & Hill, 1998, 2000), it might be expected that most GSP neurones would be strongly responsive to sucrose. This was not the case; only five GSP neurones (22%) responded with a frequency rate greater than 50 impulses (10 s)−1 (Fig. 4). Indeed, eight GSP neurones (35%) were either non-responsive or inhibited by sucrose.
Figure 5 shows the frequency of impulses to sucrose for both CT and GSP neurones, presented in order of responsiveness. As expected, the neurones that responded with high frequencies were from the GSP.
HCl
Neurones innervating the tongue.
Responses to 0.01 n HCl were variable across CT neurones (see Fig. 3). Two of the neurones had responses exceeding 100 impulses (10 s)−1 and 11 of the neurones had responses in the range of 10–50 impulses (10 s)−1.
Neurones innervating the palate.
Similar to CT neurones, responses of GSP neurones to 0.01 n HCl varied widely. Five neurones responded at a rate exceeding 50 impulses (10 s)−1, while the remaining cells (n = 15; 65%) responded to HCl at a rate less than or equal to 10 impulses (10 s)−1 and seven GSP neurones were unresponsive (see Fig. 4).
Figure 5 shows that CT and GSP neurones did not segregate on the basis of HCl responses. GSP and CT neurones were interspersed along the range of absolute response frequencies to HCl.
Quinine
Both CT and GSP neurones had relatively low response frequencies to quinine (Figs 3 and 4). Two GSP neurones responded to 0.01 m quinine at a rate above 50 impulses (10 s)−1, whereas nine GSP neurones failed to respond to quinine (Fig. 4). Overall, CT neurones had lower response frequencies to quinine than GSP neurones (Fig. 5).
Across-neurone correlations
Neurones innervating the tongue.
Across-neurone correlations (Pearson r) indicated significant relationships between three stimulus pairs for CT neurones (Table 1; numbers in standard type). The pairs showing similar patterns of eliciting responses were 0.5 m NaCl–0.1 m NaCl, 0.5 m NH4Cl–0.1 m NH4Cl and 0.5 m NH4Cl–0.01 n HCl. Examining the relationships further, 39% of CT neurones that responded to 0.5 m NaCl at a +High rate (see Table 2) responded at a +High rate to 0.1 m NaCl and 35% of the neurones were not responsive to either stimulus. Additionally, 56% of CT neurones that responded at a +High rate to 0.5 m NH4Cl responded at a similar rate to 0.1 m NH4Cl. Although response rates were high toward both concentrations of salts, absolute responses were generally lower to the lower concentration of salts as compared to the higher concentration. As can be observed in Fig. 3 and Table 2, 39% of neurones that responded at a +High rate to 0.5 m NaCl responded at a similar rate to 0.5 m NH4Cl. However, the overall pattern of responses across all CT units was not similar for the two stimuli and produced a non-significant correlation between the groups (Table 1). A significant correlation was observed between 0.5 m NH4Cl and 0.01 n HCl with 39% of neurones responding at a +High rate to both these stimuli.
Table 1.
Correlation matrices for stimulus pairs recorded from geniculate ganglion neurones
| 0.1 m NaCl | 0.5 m NaCl | 0.1 m NH4Cl | 0.5 m NH4Cl | 0.5 m sucrose | 0.01 m quinine | 0.01 n HCl | |
|---|---|---|---|---|---|---|---|
| 0.1 m NaCl | — | 0.945* | −0.039 | 0.021 | 0.066 | 0.244 | 0.012 |
| 0.5 m NaCl | 0.205 | — | −0.067 | 0.082 | −0.075 | 0.260 | 0.058 |
| 0.1 m NH4Cl | 0.651* | 0.285 | — | 0.638* | −0.399 | −0.264 | 0.143 |
| 0.5 m NH4Cl | 0.224 | 0.444* | 0.539* | — | −0.316 | −0.238 | 0.503* |
| 0.5 m sucrose | −0.273 | 0.241 | −0.398 | 0.099 | — | −0.182 | 0.085 |
| 0.01 m quinine | 0.147 | 0.127 | 0.201 | 0.082 | −0.550* | — | −0.132 |
| 0.01 n HCl | 0.442 | 0.308 | 0.662* | 0.742* | −297 | 0.351 | — |
Numbers in standard type are from neurones innervating the tongue. Numbers in italics are from neurones innervating the palate.
Correlation is significant at the 0.05 level (2-tailed).
Table 2.
Number of geniculate ganglion neurones within response categories
| 0.1 m NH4Cl | 0.1 m NaCl | 0.5 m NH4Cl | 0.5 m NaCl | 0.5 m sucrose | 0.01 m quinine | 0.01 n HCl | |
|---|---|---|---|---|---|---|---|
| Tongue | |||||||
| No response | 5 | 8 | 0 | 6 | 10 | 12 | 4 |
| +Low | 0 | 1 | 1 | 1 | 6 | 3 | 4 |
| +Medium | 3 | 4 | 3 | 3 | 1 | 3 | 3 |
| +High | 15 | 9 | 17 | 12 | 1 | 2 | 10 |
| −Low | 0 | 1 | 0 | 1 | 3 | 3 | 1 |
| −Medium | 0 | 0 | 2 | 0 | 2 | 0 | 1 |
| Palate | |||||||
| No response | 5 | 7 | 5 | 10 | 5 | 9 | 7 |
| +Low | 2 | 4 | 2 | 3 | 3 | 3 | 3 |
| +Medium | 6 | 4 | 1 | 3 | 5 | 8 | 2 |
| +High | 3 | 2 | 12 | 6 | 7 | 2 | 6 |
| −Low | 4 | 3 | 2 | 1 | 2 | 0 | 2 |
| −Medium | 3 | 3 | 1 | 0 | 1 | 1 | 3 |
No response = 0 ≤ 1 s.d. higher or lower than spontaneous activity; 1 s.d. < + Low ≤ 2 s.d. higher than spontaneous; 2 s.d. < +Medium ≤ 5 s.d. higher than spontaneous; > 5 s.d.=+High. Inhibitory responses (−Low and −Medium) were classified similarly, but opposite in direction. Standard deviations (s.d.) are based on the spontaneous activity of individual neurones.
Neurones innervating the palate.
Significant positive correlations were also observed across stimulus pairs for GSP neurones (Table 1; numbers in italics). The pairs showing similar patterns of eliciting responses were 0.1 m NH4Cl–0.5 m NH4Cl, 0.1 m NaCl–0.1 m NH4Cl, 0.5 m NaCl–0.5 m NH4Cl, 0.1 m NH4Cl–0.01 n HCl and 0.5 m NH4Cl–0.01 n HCl. Unlike CT neurones, a significant negative correlation was observed between 0.5 m sucrose and 0.01 m quinine in GSP neurones. The similarities in response magnitudes occurred across all response rate levels for the 0.1 m NaCl–0.1 m NH4Cl and 0.1 m NH4Cl–0.01 n HCl pairs. However, the 0.5 m NaCl–0.5 m NH4Cl and 0.5 m NH4Cl–0.01 n HCl pairs had clusters of rate pairings at the +High response magnitude (5 neurones each; 22%) and clusters (26% and 17%, respectively) of neurones that responded similarly to both stimuli at the +Low/no response range. The 0.1 m NH4Cl–0.5 m NH4Cl pairing consisted of three (13%) neurones in the +High range and five (22%) in the +Low/no response range to both stimuli.
The 0.5 m sucrose−0.01 m quinine pairing generated only two neurones in agreement at +Medium response rate and four neurones that were in agreement within the +Low/no response range. Interestingly, 6 of the 12 neurones that were in the moderate-to-high response range to sucrose were not responsive or were inhibited by quinine. Similarly, of the 10 neurones that were in the moderate-to-high range of response to quinine, four were not responsive or were inhibited by sucrose.
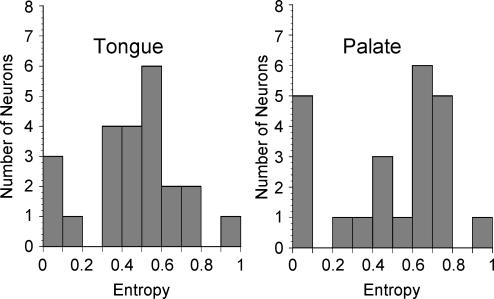
Breadth of responsiveness
The average entropy measures were similar between GSP and CT neurones (Fig. 6; mean ± s.e.m., GSP = 0.488 ± 0.06; CT = 0.454 ± 0.05; t(44) = 0.434 P > 0.10). The entropy value of the majority of neurones ranged between 0.3 and 0.8, indicating they were responsive to more than one, but not to all four of the stimuli included in the analysis (0.1 m NaCl, 0.5 m sucrose, 0.01 m quinine and 0.01 n HCl).
Figure 6. Histograms of entropy distributions for geniculate ganglion neurones responsive to tongue stimulation and those responsive to palatal stimulation.
Entropy of ‘0’ indicates neurones that were highly responsive to a single stimulus or narrowly tuned. Entropy of ‘1’ indicates neurones that were broadly responsive across multiple stimuli. Analysis included responses to 0.1 m NaCl, 0.5 m sucrose, 0.01 m quinine and 0.01 n HCl.
Cluster analysis
In order to examine potential functional neuronal groups of CT and GSP neurones, cluster analyses were performed using response data from 0.1 m NaCl, 0.1 m NH4Cl, 0.5 m sucrose, 0.01 m quinine and 0.01 n HCl.
CT neurones
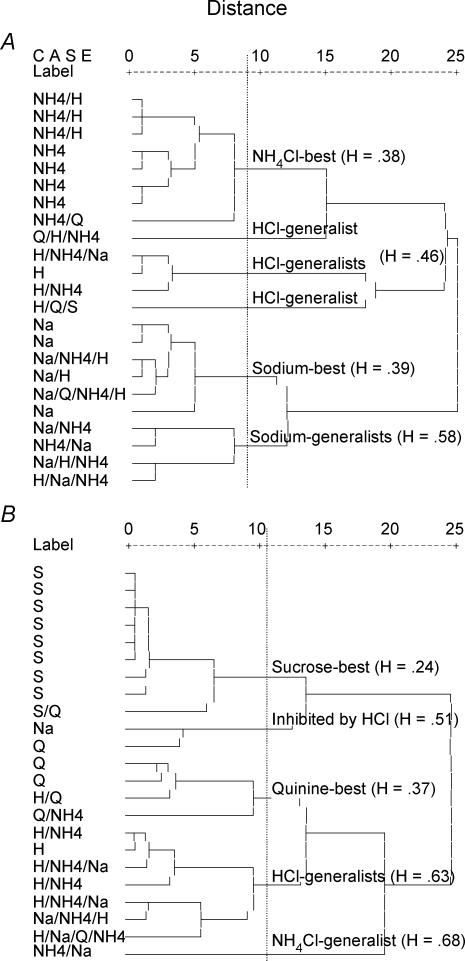
Cluster groupings.
The analysis for CT neurones resulted in six groups based on the scree analysis (Fig. 7A). Initial designations were ‘NH4Cl-best’, ‘sodium-best’, ‘sodium-generalists’ and three small groups that were later combined into one cluster termed ‘HCl-generalists’ (Fig. 7A). All designations except the ‘NH4Cl-best’ category are typical of other studies of CT neurones (Frank et al. 1983; Lundy & Contreras, 1999).
Figure 7. Hierarchical cluster analysis based on individual neurone response from geniculate ganglion neurones that innervated the tongue (A) and those that innervated the palate (B).
Analyses included neural responses to 0.1 m NaCl, 0.1 m NH4Cl, 0.5 m sucrose, 0.01 m quinine and 0.01 n HCl. Each cell (left side of each graph) is labelled according to the stimuli that it responded to the best (Na = NaCl; NH4 = NH4Cl, Q = quinine; S = sucrose; H = HCl) followed by stimuli that were in the +High category (see Table 2) or were ≥ 50% of the response with the highest frequency. The vertical dashed line indicates the demarcation in groupings indicated by a scree analysis. ‘H’ indicates the entropy values of each cluster classification.
‘Sodium-best’.
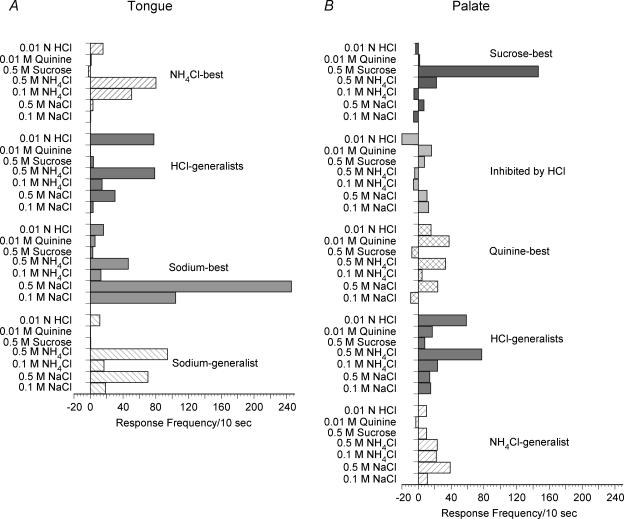
Entropy measures indicated that the ‘sodium-best’ neurones as a group were relatively specific in their responses (H= 0.39), reflecting the especially strong responses to 0.1 m NaCl. Figure 8A shows that the mean responses to 0.1 m and 0.5 m NaCl were high in this group (0.1 m NaCl, mean M= 104.3 ± 29.8; 0.5 m, M= 245.8 ± 72.8). The mean response to 0.5 m NH4Cl was also large (M= 46.3 ± 23.1). An ANOVA indicated a significant difference in response when analysed across all stimuli (F6,41= 7.90, P < 0.0001). LSD tests indicated that the response of the ‘sodium-best’ group to 0.5 m NaCl was significantly greater than responses to all other stimuli. In addition, responses to 0.1 m NaCl were significantly greater than responses to 0.1 m NH4Cl, sucrose and quinine.
Figure 8. Mean responses to all seven stimuli recorded from geniculate ganglion neurones that innervated the tongue (A) or the palate (B).
Data are grouped according to the classifications generated from the cluster analyses that used five stimuli (0.1 m NaCl, 0.1 m NH4Cl, 0.5 m sucrose, 0.01 m quinine and 0.01 n HCl) to determine categories.
‘Sodium-generalist’.
The ‘sodium-generalist’ group had a broader breadth of tuning (H= 0.58) than the sodium-specific group, demonstrating the high degree of similarity in response to 0.1 m NaCl, 0.1 m NH4Cl and 0.01 n HCl. However, when all seven stimuli were analysed using ANOVA, there was a significant effect of stimulus (F6,27= 12.02, P < 0.0001). LSD tests indicated that responses to 0.5 m NaCl and 0.5 m NH4Cl were significantly higher than responses to the other five stimuli, but 0.5 m NaCl and NH4Cl responses were not different from each other (see Fig. 8A). No other significant differences in responses were noted within this cluster.
‘NH4Cl-best’.
This response cluster has not been described in previous reports of CT single fibres or neurones. The cluster was specific in breadth of tuning (H= 0.38) indicating the specificity in response to 0.1 m NH4Cl. When responses to all seven stimuli were examined, there was a significant ANOVA (F6,55= 11.33, P < 0.0001) with LSD tests showing that responses to 0.5 m NH4Cl were significantly greater than responses to all other stimuli. Additionally, responses to 0.1 m NH4Cl were significantly greater than responses to 0.1 m and 0.5 m NaCl, sucrose, quinine and HCl. Mean responses to all stimuli are presented in Fig. 8A.
In two cases of neurones in this cluster, had NH4Cl not been included as a stimulus the neurones might not have been considered as ‘taste responsive’ and thus excluded from the overall analysis. However, their responses to NH4Cl were high (0.1 m, 76 and 77 impulses; 0.5 m, 90 and 108 impulses). It is unlikely that the acidic pH of NH4Cl was responsible for the high frequency of response in these neurones because quinine and HCl were not effective stimuli for either of the two cells.
‘HCl-generalist’.
The breadth of tuning analysis indicated that the ‘HCl-generalists’ were neither broadly responsive nor specifically tuned (H= 0.46) across the five stimuli. There was a significant effect of stimulus in the responses across all seven stimuli (F6,34= 3.18, P < 0.05). Responses to 0.01 n HCl and 0.5 m NH4Cl were significantly higher than responses to 0.1 m NaCl, 0.1 m NH4Cl, sucrose and quinine (Fig. 8A).
GSP neurones
Cluster groupings.
The cluster analyses of GSP neurones indicated markedly different groupings from those reported for CT neurones. The scree analysis used on the cluster based on five stimuli formed five groupings we termed ‘sucrose-best’, ‘inhibited by HCl’, ‘quinine-best’, ‘HCl generalists’ and ‘NH4Cl-generalist’.
‘Sucrose-best’.
The largest and most specific grouping (H= 0.24) was composed of sucrose-best neurones, although one of these neurones was also responsive to quinine. The mean response frequency to sucrose was high in this group (Fig. 8B), and there was a high degree of response frequency variability (146.6 ± 63.8). The ANOVA indicated a significant effect of stimulus type (F6,62= 5.00, P < 0.0001). Sucrose responses were significantly greater than the responses to each of the other six stimuli and there were no other statistically significant differences in responses between other stimuli in the ‘sucrose-best’ group. It is important to note, that although responses to all other stimuli were statistically smaller than the sucrose response of these neurones, four neurones were +High responsive to stimuli (most often 0.5 m NH4Cl and/or 0.5 m NaCl) not included in the cluster analysis. In addition, six of the nine ‘sucrose-best’ neurones showed inhibitory responses to at least one of the other six stimuli.
‘Quinine-best’.
The ‘quinine-best’ group showed specificity in tuning across the four stimuli (H= 0.37), but the ANOVA results did not indicate a significant effect of stimulus type within this classification. Although quinine was the ‘best’ stimulus when examined using the four ‘basic’ stimuli, the neurones in this group were also quite responsive to 0.5 m NaCl and NH4Cl (Fig. 8B).
‘HCl-generalist’.
Similar to that observed for CT neurones, an ‘HCl-generalist’ classification was noted for GSP neurones. This group was broadly tuned across the four stimuli (H= 0.63). Responses to 0.5 m NH4Cl were also high (Fig. 8B) and comparable in the frequency of response of CT neurones to this stimulus. A significant effect was observed (F6,48= 6.94, P < 0.0001) across all seven stimuli; LSD tests showed that responses to 0.01 n HCl and 0.5 m NH4Cl were significantly greater than responses to all other stimuli, with the exception that they were comparable to each other. This finding is identical to that noted for the ‘HCl-generalist’ class within the population of CT neurones with the exception that GSP responses to 0.5 m NaCl were significantly less than 0.5 m NH4Cl and HCl responses, whereas 0.5 m NaCl responses were not statistically different from responses to those stimuli within CT neurones of the ‘HCl-generalist’ class.
‘NH4Cl-generalist’.
One GSP neurone was classified as a ‘NH4Cl-generalist’ because it was broadly tuned (H= 0.68) and clearly did not cluster with any other grouping. This neurone showed the highest response to 0.5 m NaCl, but had nearly equivalent responses to 0.1 and 0.5 m NH4Cl.
‘Inhibited by HCl’.
In addition, there was an unusual cluster of two neurones; one neurone was most responsive to NaCl and the other most responsive to quinine (Fig. 8B). The likely reason that they clustered as a group was that they both exhibited inhibitory responses to HCl (M= −20.0 ± 7.0).
Discussion
Geniculate ganglion neurones innervating taste receptors on the palate had response patterns that distinguished them from neurones innervating taste receptors on the tongue. This is the first report to describe details of the neurophysiological response properties of individual GSP neurones to taste stimuli, thereby providing the remaining sample of single taste neurone responses from all four gustatory nerves. By recording from both GSP and CT neurones, we were able to make direct response profile comparisons between the two neuronal populations. Qualities that differentiated GSP from CT neurones were (1) a relatively small subset of GSP neurones were highly responsive to sucrose, whereas no CT neurones had responses to sucrose that were close in magnitude to the robust GSP responses, (2) a substantial subset of CT neurones were highly responsive to NaCl, whereas no such group was seen in GSP neurones, (3) based on the cluster analysis, 0.1 m NH4Cl was the best stimulus for a number of CT neurones, but was the best stimulus for only one GSP neurone, (4) the average spontaneous activity of GSP neurones was higher than that observed in CT neurones, and (5) inhibitory responses were observed for each class of neurone, but more inhibitory responses occurred in GSP neurones than in CT neurones.
Comparison with whole-nerve responses
The current results from ganglion neurones that supply the GSP are not necessarily what would be predicted from whole-nerve responses. Results of whole-nerve electrophysiological studies indicate that both the GSP and CT are strongly responsive to sodium salt taste stimulation when expressed as responses relative to a 0.5 m NH4Cl standard (Sollars & Hill, 1998; Hendricks et al. 2002). However, relative response measures are not necessarily indicative of the absolute contribution of the afferent signal from the CT as compared to the GSP. For CT neurones, a subset of neurones especially responsive to NaCl may account for the high whole-nerve response of the CT to NaCl. However, in the GSP, no neurones were found that responded as strongly to NaCl. Thus, the robust whole-nerve response of the GSP to NaCl (Sollars & Hill, 1998) may be due to a considerable proportion of neurones responding at +Medium and +Low response frequencies instead of relatively few +High responsive fibres contributing disproportionately to the whole-nerve response, as may be the case for the CT (see Fig. 5 and Table 2). Therefore, the sum of responses from a large number of GSP neurones results in a robust response to NaCl (Sollars & Hill, 1998). Interestingly, there was not a significant correlation between 0.1 m and 0.5 m NaCl for GSP neurones (Table 1). Therefore, unlike the CT where the correlation was very high, such a result suggests that different taste transduction processes for NaCl are involved in the palate compared to the tongue and/or neural coding is distinctly different. We must also acknowledge that, due to the relatively small sample size, it is possible that GSP neurones highly responsive to NaCl exist but are not included in the present sample.
In contrast to salt responses, sucrose was the most potent stimulus in a subset of GSP neurones. Given the large whole-nerve response of the GSP to sucrose and the pattern of single neurone responses to NaCl (Nejad, 1986; Harada & Smith, 1992; Sollars & Hill, 1998), it might be expected that most GSP neurones would be sucrose responsive. However, 48% of GSP neurones had small response frequencies, no response, or were inhibited by sucrose. The implication of this finding is that the large whole-nerve response of the GSP to sucrose is likely to be the result of a subset of neurones that respond with high frequencies to sucrose and that many GSP neurones do not substantially contribute to the sucrose response.
Specificity of gustatory information
The relatively small proportion of single neurones in the CT and GSP that respond with very high frequencies to NaCl and to sucrose, respectively, suggests that they are critical in transmitting limited and specific taste information to neurones in the NTS. This would be consistent with the specificity of ‘X’ and ‘Y’ cells identified in retinal ganglia (Enroth-Cugell & Robson, 1984).
Not only does it appear that CT neurones are distinct from GSP neurones, it is evident that GSP neurones and/or their receptor populations are distinct from one another. One intriguing, but untested, possibility is that the various receptor populations innervated by the GSP (e.g. nasoincisor duct, geschmacksstreifen, soft palate; Miller, 1977; Miller & Spangler, 1982) have distinct regional distributions of membrane receptors that transduce sucrose. That is, the three palatal receptive fields may have unique functional sensitivities to taste stimuli that are reflected in the response properties of their respective innervating neurones. Such a functional distinction may lead to similar distinctions in central processing and behavioural outcomes.
Functional categories and breadth of tuning
A study by Lundy & Contreras (1999) of CT ganglion neurones recorded in vivo in the rat showed functional categories of CT neurones that were similar to those found for CT neurones in the present study (‘NaCl-specialist’, ‘NaCl-generalist’ and ‘HCl-generalist’ groups). They were the first to characterize the NaCl-generalist group that was also observed in our study. One important difference between the current study and that of Lundy & Contreras (1999) was uncovered by the addition of 0.1 m NH4Cl in our cluster analyses. A clear and specific category we termed ‘NH4Cl-best’ neurones was differentiated as a group separate from the ‘HCl-generalists’. Some of these neurones were also responsive to HCl, though not all of them were strongly responsive to it. Interestingly, two of the neurones were specifically tuned to NH4Cl such that they would not have been considered ‘taste-responsive’ had NH4Cl not been included as a stimulus. It is unclear if NH4Cl serves as a separate taste stimulus with a functional role to the adult rat. However, preweanling rats will ingest large quantities of NH4Cl (Sollars & Bernstein, 1994) and NH4Cl is a potent stimulus in eliciting electrophysiological responses from the CT prior to the development of NaCl responses (Hill et al. 1982). Therefore, this functional group of neurones may be critically involved in taste-mediated behaviours during early development.
The current results also complement functional data obtained from the taste receptor cells that they innervate. Gilbertson et al. (2001) examined gustatory sensitivity of taste receptor cells on the tongue and soft palate and demonstrated that the majority of receptor cells within each population were responsive to more than one stimulus. The breadth of tuning measures obtained by Gilbertson and colleagues for tongue receptor cells (H= 0.43) and soft palate receptor cells (H= 0.55) were similar to the breadth of tuning for CT neurones (H= 0.45) and GSP neurones (H= 0.49) found in the present study. Thus, although each neurone contacts several receptor cells, the convergent signal appears to maintain the breadth of responsiveness of individual receptor cells. This consistency at the periphery may provide a key to coding of basic tastes as they reach the first gustatory relay in the NTS.
Comparisons of single neurone responses among gustatory nerves
Further insight into the functional dynamics of gustatory processing is possible through an examination of previous studies of the glossopharyngeal nerve and the superior laryngeal nerve that innervate taste receptors on the posterior tongue and epiglottis, respectively. The superior laryngeal nerve has a functional profile that makes it unique among gustatory nerves. In contrast to other gustatory populations, rat superior laryngeal fibres respond robustly to water (Shingai, 1980; Hanamori, 2001). Responses of superior laryngeal fibres to other stimuli vary depending on the species under investigation, but include significant responses to KCl and acids (Shingai, 1980; Stedman et al. 1980; Shingai & Beidler, 1985; Smith & Hanamori, 1991; see also Bradley, 2000). Therefore, there are clear taste response differences between the superior laryngeal nerve and the CT and GSP nerves that may reflect the differences in functions of the nerves; the superior laryngeal nerve may mediate reflexive rejection and airway protection responses compared to more taste quality- and concentration-related behaviours attributed to other taste nerves.
Interesting similarities exist between recordings of glossopharyngeal single fibres of the rat (Frank, 1991) and the recordings of GSP neurones in the present report. Parallels may be drawn because total numbers of neurones analysed in the cluster analyses of the two studies are comparable (glossopharyngeal, n = 18; GSP, n = 23), and NH4Cl was used in both analyses. Similar stimulus categories were formed from the glossopharyngeal fibre recordings and during the GSP analysis. The ‘acid-best’ group (46%) of the glossopharyngeal nerve matches with the ‘HCl-generalist’ and ‘NH4Cl-best’ groups (35% combined) of the GSP; in addition to being highly responsive to HCl, the ‘acid-best/HCl-generalist’ groups were strongly responsive to NH4Cl and less responsive to NaCl, sucrose and quinine. The overall characteristics of the ‘quinine-best’ cluster (glossopharyngeal, 31%; GSP, 17%) and ‘sucrose-best’ group (glossopharyngeal, 23%; GSP, 39%) also match closely. However, the absolute response rate of glossopharyngeal fibres to quinine was higher than that recorded in GSP neurones and the quinine-best neurones appear more narrowly tuned in the glossopharyngeal nerve than in the GSP. A noteworthy similarity between the two populations occurred in the slight inhibition toward sucrose and 0.1 m NaCl in ‘quinine-best’ units. In addition, the ‘sucrose-best’ units in the Frank report appeared to be more specifically tuned to sucrose than the neurones of the GSP; ‘side-band’ responses to the high concentrations of NH4Cl and NaCl were apparent in some GSP ‘sucrose-best’ neurones. Thus, the functional overlap between glossopharyngeal and GSP neurones appears to be high, although functional differences are apparent. One important caveat to the similarities between the glossopharyngeal nerve and GSP is the effectiveness of amiloride to suppress whole nerve sodium responses of the GSP (Sollars & Hill, 1998), whereas amiloride is ineffective in suppressing responses of the glossopharyngeal nerve (Formaker & Hill, 1991; Kitada et al. 1998).
Summary
In summary, we show here that the GSP transmits fundamentally different types of afferent information from the CT. Many CT and GSP neurones exhibit similar response frequencies to individual stimuli even though their overall response profiles differ. Within the group of neurones that innervated the palate, not all neurones were sucrose responsive, suggesting that the high whole-nerve response of the GSP may be primarily due to the activity of a subset of neurones. In contrast, the neurones that innervated the tongue included a subset of neurones that were highly responsive to NaCl. A number of GSP neurones were also NaCl responsive, but no GSP neurones were found in the present study that showed a frequency of responding to NaCl that was comparable to the subset of high-response CT neurones. When compared with other gustatory receptor populations, there appear to be many common features between the glossopharyngeal nerve and the GSP, while the superior laryngeal nerve is distinctly different from the other nerves.
Gustatory coding involves complex processing of diverse inputs. Single neurones of the CT and GSP appear to retain the breadth of tuning of taste receptors (Gilbertson et al. 2001) that then converge onto postsynaptic neurones in the NTS (Travers et al. 1986; Travers & Norgren, 1995) resulting in an integration of inputs from tongue and palatal taste receptors. Given the diversity of response profiles, this convergent information may serve to amplify and/or attenuate the relative contribution of spatially distinct receptors. As an ingestive stimulus moves from the anterior to the posterior oral cavity, temporal features may be extracted in concert with the movement of the stimulus. Temporal disparities may also be related to distinct receptor profiles across the tongue and palate. Across the tongue, there are fungiform receptors near the tip and mid regions and foliate and circumvallate receptors in the back. Across the palate, there are the nasoincisor receptors at the tip, the geschmacksstreifen in the centre and the soft palate receptors at the back. Thus, extraction of the character of a taste stimulus is likely to be related to the diversity and spatial distribution of gustatory neurones and their respective receptor populations. This complex afferent signal representing different coding strategies must then be used by central neurones to guide ingestion or rejection of food and solutions.
Acknowledgments
The authors thank Dr William E. Renehan for support and instruction with the in vivo neural recording technique. This work was supported by the National Institutes of Health, grant numbers: DC04648 (S.I.S.) and DC00407 (D.L.H.).
References
- Beidler LM. Properties of chemoreceptors of tongue of rat. J Neurophysiol. 1953;16:595–607. doi: 10.1152/jn.1953.16.6.595. [DOI] [PubMed] [Google Scholar]
- Boudreau JC, Hoang NK, Oravec J, Do LT. Rat neurophysiological taste responses to salt solutions. Chem Senses. 1983;8:131–150. [Google Scholar]
- Boughter JD, Jr, Pumplin DW, Yu C, Christy RC, Smith DV. Differential expression of alpha-gustducin in taste bud populations of the rat and hamster. J Neurosci. 1997;17:2852–2858. doi: 10.1523/JNEUROSCI.17-08-02852.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RM. Sensory receptors of the larynx. Am J Med. 2000;108:47S–50S. doi: 10.1016/s0002-9343(99)00339-3. [DOI] [PubMed] [Google Scholar]
- Contreras RJ, Frank ME. Sodium deprivation alters neural responses to gustatory stimuli. J General Physiol. 1979;73:569–594. doi: 10.1085/jgp.73.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras RJ, Lundy RF. Gustatory neuron types in the periphery: a functional perspective. Physiol Behav. 2000;69:41–52. doi: 10.1016/s0031-9384(00)00187-6. [DOI] [PubMed] [Google Scholar]
- Danilova V, Danilov Y, Roberts T, Tinti JM, Nofre C, Hellekant G. Sense of taste in a new world monkey, the common marmoset: recordings from the chorda tympani and glossopharyngeal nerves. J Neurophysiol. 2002;88:579–594. doi: 10.1152/jn.2002.88.2.579. [DOI] [PubMed] [Google Scholar]
- Di Lorenzo PM, Lemon CH. The neural code for taste in the nucleus of the solitary tract of the rat: effects of adaptation. Brain Res. 2000;852:383–397. doi: 10.1016/s0006-8993(99)02187-3. 10.1016/S0006-8993(99)02187-3. [DOI] [PubMed] [Google Scholar]
- Dickman JD, Smith DV. Response properties of fibers in the hamster superior laryngeal nerve. Brain Res. 1988;450:25–38. doi: 10.1016/0006-8993(88)91541-7. 10.1016/0006-8993(88)91541-7. [DOI] [PubMed] [Google Scholar]
- El-Sharaby A, Ueda K, Kurisu K, Wakisaka S. Development and maturation of taste buds of the palatal epithelium of the rat: histological and immunohistochemical study. Anat Rec. 2001a;263:260–268. doi: 10.1002/ar.1095. 10.1002/ar.1095. [DOI] [PubMed] [Google Scholar]
- El-Sharaby A, Ueda K, Wakisaka S. Differentiation of the lingual and palatal gustatory epithelium of the rat as revealed by immunohistochemistry of alpha-gustducin. Arch Histol Cytol. 2001b;64:401–409. doi: 10.1679/aohc.64.401. [DOI] [PubMed] [Google Scholar]
- Enroth-Cugell C, Robson JG. Functional characteristics and diversity of cat retinal ganglion cells. Invest Ophthal Visual Sci. 1984;25:250–257. [PubMed] [Google Scholar]
- Formaker BK, Hill DL. Lack of amiloride sensitivity in SHR and WKY glossopharyngeal taste responses to NaCl. Physiol Behav. 1991;50:765–769. doi: 10.1016/0031-9384(91)90015-g. 10.1016/0031-9384(91)90015-G. [DOI] [PubMed] [Google Scholar]
- Frank ME. An analysis of hamster afferent taste nerve response functions. J General Physiol. 1973;61:588–618. doi: 10.1085/jgp.61.5.588. 10.1085/jgp.61.5.588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank ME. Taste-responsive neurons of the glossopharyngeal nerve of the rat. J Neurophysiol. 1991;65:1452–1463. doi: 10.1152/jn.1991.65.6.1452. [DOI] [PubMed] [Google Scholar]
- Frank ME, Contreras RJ, Hettinger TP. Nerve fibers sensitive to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol. 1983;50:941–960. doi: 10.1152/jn.1983.50.4.941. [DOI] [PubMed] [Google Scholar]
- Geran LC, Garcea M, Spector AC. Transecting the gustatory branches of the facial nerve impairs NH4Cl vs. KCl discrimination in rats. Am J Physiol Regul Integr Comp Physiol. 2002;283:R739–R747. doi: 10.1152/ajpregu.00103.2002. [DOI] [PubMed] [Google Scholar]
- Gilbertson TA, Boughter JD, Jr, Zhang H, Smith DV. Distribution of gustatory sensitivities in rat taste cells: whole-cell responses to apical chemical stimulation. J Neurosci. 2001;21:4931–4941. doi: 10.1523/JNEUROSCI.21-13-04931.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez MM. Wake Forest University; 1978. The peripheral distribution of the rat geniculate ganglion. PhD Thesis. [Google Scholar]
- Hanamori T. Effects of various ion transport inhibitors on the water response in the superior laryngeal nerve in rats. Chem Senses. 2001;26:897–903. doi: 10.1093/chemse/26.7.897. 10.1093/chemse/26.7.897. [DOI] [PubMed] [Google Scholar]
- Hanamori T, Miller IJ, Jr, Smith DV. Gustatory responsiveness of fibers in the hamster glossopharyngeal nerve. J Neurophysiol. 1988;60:478–498. doi: 10.1152/jn.1988.60.2.478. [DOI] [PubMed] [Google Scholar]
- Harada S, Kasahara Y. Inhibitory effect of gurmarin on palatal taste responses to amino acids in the rat. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1513–R1517. doi: 10.1152/ajpregu.2000.278.6.R1513. [DOI] [PubMed] [Google Scholar]
- Harada S, Smith DV. Gustatory sensitivities of the hamster's soft palate. Chem Senses. 1992;17:37–51. [Google Scholar]
- Hendricks SJ, Sollars SI, Hill DL. Injury-induced functional plasticity in the peripheral gustatory system. J Neurosci. 2002;22:8607–8613. doi: 10.1523/JNEUROSCI.22-19-08607.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Mistretta CM, Bradley RM. Developmental changes in taste response characteristics of rat single chorda tympani fibers. J Neurosci. 1982;2:782–790. doi: 10.1523/JNEUROSCI.02-06-00782.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill DL, Phillips LM. Functional plasticity of regenerated and intact taste receptors in adult rats unmasked by dietary sodium restriction. J Neurosci. 1994;14:2904–2910. doi: 10.1523/JNEUROSCI.14-05-02904.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitada Y, Mitoh Y, Hill DL. Salt taste responses of the IXth nerve in Sprague-Dawley rats: lack of sensitivity to amiloride. Physiol Behav. 1998;63:945–949. doi: 10.1016/s0031-9384(98)00009-2. 10.1016/S0031-9384(98)00009-2. [DOI] [PubMed] [Google Scholar]
- Krimm RF, Nejad MS, Smith JC, Miller IJ, Jr, Beidler LM. The effect of bilateral sectioning of the chorda tympani and the greater superficial petrosal nerves on the sweet taste in the rat. Physiol Behav. 1987;41:495–501. doi: 10.1016/0031-9384(87)90086-2. 10.1016/0031-9384(87)90086-2. [DOI] [PubMed] [Google Scholar]
- Lundy RF, Jr, Contreras RJ. Gustatory neuron types in rat geniculate ganglion. J Neurophysiol. 1999;82:2970–2988. doi: 10.1152/jn.1999.82.6.2970. [DOI] [PubMed] [Google Scholar]
- Miller IJ., Jr . Gustatory receptors of the palate. In: Katsuki Y, Sato M, Tagagi S, Oomura Y, editors. Food Intake and Chemical Senses. Tokyo: University of Tokyo Press; 1977. pp. 173–185. [Google Scholar]
- Miller IJ, Jr, Spangler M. Taste bud distribution and innervation on the palate of the rat. Chem Senses. 1982;7:99–108. [Google Scholar]
- Nejad MS. The neural activities of the greater superficial petrosal nerve of the rat in response to chemical stimulation of the palate. Chem Senses. 1986;11:283–293. [Google Scholar]
- Norgren R. Central mechanisms of taste. In: Darian-Smith I, editor. Handbook of Physiology, section 1 The Nervous System, vol. III, Sensory Processes. Bethesda, MD, USA: American Physiological Society; 1984. pp. 1087–1128. [Google Scholar]
- Renehan WE, Jin Z, Zhang X, Schweitzer L. The structure and function of gustatory neurons in the nucleus of the solitary tract. I. A classification of neurons based on morphological features. J Comp Neurol. 1994;347:531–544. doi: 10.1002/cne.903470405. 10.1002/cne.903470405. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Bernstein IL. Amiloride-sensitive sodium signals and salt appetite: multiple gustatory pathways. Am J Physiol. 1999;276:R1732–R1738. doi: 10.1152/ajpregu.1999.276.6.R1732. [DOI] [PubMed] [Google Scholar]
- Shimatani Y, Grabauskiene S, Bradley RM. Long-term recording from the chorda tympani nerve in rats. Physiol Behav. 2002;76:143–149. doi: 10.1016/s0031-9384(02)00684-4. 10.1016/S0031-9384(02)00684-4. [DOI] [PubMed] [Google Scholar]
- Shingai T. Water fibers in the superior laryngeal nerve of the rat. Japan J Physiol. 1980;30:305–307. doi: 10.2170/jjphysiol.30.305. [DOI] [PubMed] [Google Scholar]
- Shingai T, Beidler LM. Response characteristics of three taste nerves in mice. Brain Res. 1985;335:245–249. doi: 10.1016/0006-8993(85)90476-7. 10.1016/0006-8993(85)90476-7. [DOI] [PubMed] [Google Scholar]
- Smith DV, Hanamori T. Organization of gustatory sensitivities in hamster superior laryngeal nerve fibers. J Neurophysiol. 1991;65:1098–1114. doi: 10.1152/jn.1991.65.5.1098. [DOI] [PubMed] [Google Scholar]
- Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses Flav. 1979;4:215–229. [Google Scholar]
- Sollars SI, Bernstein IL. Amiloride sensitivity in the neonatal rat. Behav Neurosci. 1994;108:981–987. doi: 10.1037//0735-7044.108.5.981. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. Taste responses in the greater superficial petrosal nerve: substantial sodium salt and amiloride sensitivities demonstrated in two rat strains. Behav Neurosci. 1998;112:991–1000. doi: 10.1037//0735-7044.112.4.991. 10.1037//0735-7044.112.4.991. [DOI] [PubMed] [Google Scholar]
- Sollars SI, Hill DL. Lack of functional and morphological susceptibility of the greater superficial petrosal nerve to developmental dietary sodium restriction. Chem Senses. 2000;25:719–727. doi: 10.1093/chemse/25.6.719. 10.1093/chemse/25.6.719. [DOI] [PubMed] [Google Scholar]
- Spector AC, Markison S, St John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol. 1997;272:R1210–R1208. doi: 10.1152/ajpregu.1997.272.4.R1210. [DOI] [PubMed] [Google Scholar]
- St John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 1998;18:4353–4362. doi: 10.1523/JNEUROSCI.18-11-04353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stedman HM, Bradley RM, Mistretta CM, Bradley BE. Chemosensitive responses from the cat epiglottis. Chem Senses. 1980;5:233–245. [Google Scholar]
- Travers SP, Norgren R. Organization of orosensory responses in the nucleus of the solitary tract of rat. J Neurophysiol. 1995;73:2144–2162. doi: 10.1152/jn.1995.73.6.2144. [DOI] [PubMed] [Google Scholar]
- Travers SP, Pfaffmann C, Norgren R. Convergence of lingual and palatal gustatory neural activity in the nucleus of the solitary tract. Brain Res. 1986;365:305–320. doi: 10.1016/0006-8993(86)91642-2. 10.1016/0006-8993(86)91642-2. [DOI] [PubMed] [Google Scholar]