Abstract
We investigated the hypothesis that the pulmonary oxygen uptake  slow component is related to a progressive increase in muscle lactate concentration and that prior heavy exercise (PHE) with pronounced acidosis alters
slow component is related to a progressive increase in muscle lactate concentration and that prior heavy exercise (PHE) with pronounced acidosis alters 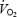 kinetics and reduces work efficiency. Subjects (n = 9) cycled at 75% of the peak
kinetics and reduces work efficiency. Subjects (n = 9) cycled at 75% of the peak 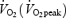 for 10 min before (CON) and after (AC) PHE.
for 10 min before (CON) and after (AC) PHE. 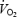 was measured continuously (breath-by-breath) and muscle biopsies were obtained prior to and after 3 and 10 min of exercise. Muscle lactate concentration was stable between 3 and 10 min of exercise but was 2- to 3-fold higher during AC (P < 0.05 versus CON). Acetylcarnitine (ACn) concentration was 6-fold higher prior to AC and remained higher during exercise. Phosphocreatine (PCr) concentration was similar prior to exercise but the decrease was 2-fold greater during AC than during CON. The time constant for the initial
was measured continuously (breath-by-breath) and muscle biopsies were obtained prior to and after 3 and 10 min of exercise. Muscle lactate concentration was stable between 3 and 10 min of exercise but was 2- to 3-fold higher during AC (P < 0.05 versus CON). Acetylcarnitine (ACn) concentration was 6-fold higher prior to AC and remained higher during exercise. Phosphocreatine (PCr) concentration was similar prior to exercise but the decrease was 2-fold greater during AC than during CON. The time constant for the initial 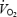 kinetics (phase II) was similar but the
kinetics (phase II) was similar but the 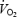 asymptote was 14% higher during AC. The slow increase in
asymptote was 14% higher during AC. The slow increase in 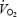 between 3 and 10 min of exercise during CON (+7.9 ± 0.2%) was not correlated with muscle or blood lactate levels. PHE eliminated the slow increase in
between 3 and 10 min of exercise during CON (+7.9 ± 0.2%) was not correlated with muscle or blood lactate levels. PHE eliminated the slow increase in 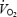 and reduced gross exercise efficiency during AC. It is concluded that the
and reduced gross exercise efficiency during AC. It is concluded that the 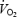 slow component cannot be explained by a progressive acidosis because both muscle and blood lactate levels remained stable during CON. We suggest that both the
slow component cannot be explained by a progressive acidosis because both muscle and blood lactate levels remained stable during CON. We suggest that both the 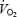 slow component during CON and the reduced gross efficiency during AC are related to impaired contractility of the working fibres and the necessity to recruit additional motor units. Despite a pronounced stockpiling of ACn during AC, initial
slow component during CON and the reduced gross efficiency during AC are related to impaired contractility of the working fibres and the necessity to recruit additional motor units. Despite a pronounced stockpiling of ACn during AC, initial 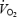 kinetics were not affected by PHE and PCr concentration decreased to a lower plateau. The discrepancy with previous studies, where initial oxidative ATP generation appears to be limited by acetyl group availability, might relate to remaining fatiguing effects of PHE.
kinetics were not affected by PHE and PCr concentration decreased to a lower plateau. The discrepancy with previous studies, where initial oxidative ATP generation appears to be limited by acetyl group availability, might relate to remaining fatiguing effects of PHE.
It is well established that pulmonary oxygen uptake 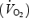 reaches a steady state only during mild to moderate intensity exercise (Whipp, 1994). At intensities above lactate threshold,
reaches a steady state only during mild to moderate intensity exercise (Whipp, 1994). At intensities above lactate threshold, 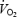 either attains a delayed steady state or continues to increase slowly until the end of exercise (Whipp, 1994). This phenomenon has been termed the ‘
either attains a delayed steady state or continues to increase slowly until the end of exercise (Whipp, 1994). This phenomenon has been termed the ‘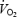 slow component’ or ‘excess
slow component’ or ‘excess 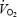 ’ and the extra
’ and the extra 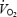 supplements the underlying initial mono-exponential response kinetics (the fundamental phase or phase II). The physiological determinants of the kinetics of both the phase II
supplements the underlying initial mono-exponential response kinetics (the fundamental phase or phase II). The physiological determinants of the kinetics of both the phase II 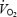 response, and the ensuing
response, and the ensuing 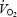 slow component remain poorly understood. The
slow component remain poorly understood. The 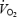 slow component will reduce exercise tolerance by increasing the metabolic rate (bringing the subject to, or closer to, their peak
slow component will reduce exercise tolerance by increasing the metabolic rate (bringing the subject to, or closer to, their peak 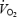 ) and therefore, revelation of the underlying mechanism(s) of the slow component is of significance to our understanding of exercise energetics and the limitations to human performance.
) and therefore, revelation of the underlying mechanism(s) of the slow component is of significance to our understanding of exercise energetics and the limitations to human performance.
Although systemic factors such as increased cardio-respiratory work and hormonal changes may contribute to the 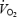 slow component, there is convincing evidence that the major contribution originates from the exercising leg muscles (Poole et al. 1991). The excess
slow component, there is convincing evidence that the major contribution originates from the exercising leg muscles (Poole et al. 1991). The excess 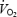 , due to the slow component, corresponds to a reduced efficiency (work accomplished/energy expended) and suggested intramuscular causes are as diverse as altered substrate utilization, altered fibre-type recruitment, increased muscle temperature, and/or lactic acidosis. The reduced efficiency could in turn relate to a reduced efficiency of the contractile machinery (increased ATP turnover rate) and/or a reduced mitochondrial efficiency (decreased ATP/O2 ratio). Estimated muscle ATP turnover rate increased after the initial period of exercise during both one-legged knee extension (Bangsbo et al. 2001; Krustrup et al. 2003) and two-legged cycling at 80% maximal
, due to the slow component, corresponds to a reduced efficiency (work accomplished/energy expended) and suggested intramuscular causes are as diverse as altered substrate utilization, altered fibre-type recruitment, increased muscle temperature, and/or lactic acidosis. The reduced efficiency could in turn relate to a reduced efficiency of the contractile machinery (increased ATP turnover rate) and/or a reduced mitochondrial efficiency (decreased ATP/O2 ratio). Estimated muscle ATP turnover rate increased after the initial period of exercise during both one-legged knee extension (Bangsbo et al. 2001; Krustrup et al. 2003) and two-legged cycling at 80% maximal 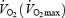 (Krustrup et al. 2004a). Furthermore, the work efficiency was less at high than at low intensity knee extension exercise (Krustrup et al. 2003). Altogether these results suggest that the energy cost of the contraction process increases with time at work intensities above the lactate threshold, and is greater at higher intensities. However, the interpretation of the results is complicated by the non-steady-state conditions. Further studies of ATP turnover rate and work efficiency during steady-state conditions at high and low tissue lactate levels are required.
(Krustrup et al. 2004a). Furthermore, the work efficiency was less at high than at low intensity knee extension exercise (Krustrup et al. 2003). Altogether these results suggest that the energy cost of the contraction process increases with time at work intensities above the lactate threshold, and is greater at higher intensities. However, the interpretation of the results is complicated by the non-steady-state conditions. Further studies of ATP turnover rate and work efficiency during steady-state conditions at high and low tissue lactate levels are required.
During submaximal exercise the phase II of 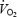 kinetics is thought to be largely determined by metabolic regulators (Grassi, 2001) including factors related to feedback control of oxidative phosphorylation (oxphos) via intramuscular phosphates (Chance & Williams, 1955; Wilson, 1994; Meyer & Foley, 1996; Hughson et al. 2001) and the redox drive (i.e. NADH/NAD+ in the mitochondrial matrix; Wilson, 1994; Hughson et al. 2001). The redox drive is influenced by the availability of tricarboxylic acid (TCA) cycle substrates (i.e. acetyl-CoA and acetylcarnitine (Acn)) and feedforward activation of TCA cycle enzymes by Ca2+. At the onset of exercise the availability of acetyl-CoA is limited by the activity of the pyruvate dehydrogenase complex (PDC) (Timmons et al. 1996). When the acetyl group availability is increased by pharmacological activation of PDC (Timmons et al. 1996, 1998; Rossiter et al. 2003) or by prior warm-up exercise (Campbell-O'Sullivan et al. 2002) phosphocreatine (PCr) degradation and lactic acidosis are reduced. The availability of acetyl groups was therefore considered to be a site of mitochondrial lag in oxidative metabolism. Although prior warm-up exercise appears to attenuate the metabolic perturbations during subsequent exercise this may not be the case if the intensity of the prior exercise is high enough to cause fatigue. The effect of fatiguing prior heavy exercise on the muscle metabolic response during subsequent exercise has not been investigated.
kinetics is thought to be largely determined by metabolic regulators (Grassi, 2001) including factors related to feedback control of oxidative phosphorylation (oxphos) via intramuscular phosphates (Chance & Williams, 1955; Wilson, 1994; Meyer & Foley, 1996; Hughson et al. 2001) and the redox drive (i.e. NADH/NAD+ in the mitochondrial matrix; Wilson, 1994; Hughson et al. 2001). The redox drive is influenced by the availability of tricarboxylic acid (TCA) cycle substrates (i.e. acetyl-CoA and acetylcarnitine (Acn)) and feedforward activation of TCA cycle enzymes by Ca2+. At the onset of exercise the availability of acetyl-CoA is limited by the activity of the pyruvate dehydrogenase complex (PDC) (Timmons et al. 1996). When the acetyl group availability is increased by pharmacological activation of PDC (Timmons et al. 1996, 1998; Rossiter et al. 2003) or by prior warm-up exercise (Campbell-O'Sullivan et al. 2002) phosphocreatine (PCr) degradation and lactic acidosis are reduced. The availability of acetyl groups was therefore considered to be a site of mitochondrial lag in oxidative metabolism. Although prior warm-up exercise appears to attenuate the metabolic perturbations during subsequent exercise this may not be the case if the intensity of the prior exercise is high enough to cause fatigue. The effect of fatiguing prior heavy exercise on the muscle metabolic response during subsequent exercise has not been investigated.
Lactic acidosis has remained a prime candidate for the cause of the slow component. A number of studies demonstrate a close relationship between blood lactate accumulation and both the temporal profile and the magnitude of the 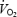 slow component (Whipp & Wasserman, 1986; Zoladz & Korzeniewski, 2001). Several studies have used prior exercise as an experimental tool to alter blood and muscle metabolic status and to assess the resulting effects on
slow component (Whipp & Wasserman, 1986; Zoladz & Korzeniewski, 2001). Several studies have used prior exercise as an experimental tool to alter blood and muscle metabolic status and to assess the resulting effects on 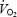 kinetics (Gerbino et al. 1996; Rossiter et al. 2001; Burnley et al. 2002a, b; Campbell-O'Sullivan et al. 2002; Tordi et al. 2003; Wilkerson et al. 2004). Prior exercise has, in a minority of cases, engendered a faster phase II
kinetics (Gerbino et al. 1996; Rossiter et al. 2001; Burnley et al. 2002a, b; Campbell-O'Sullivan et al. 2002; Tordi et al. 2003; Wilkerson et al. 2004). Prior exercise has, in a minority of cases, engendered a faster phase II 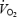 time constant (Rossiter et al. 2001; Tordi et al. 2003), but in almost all cases an increased phase II amplitude and a reduced
time constant (Rossiter et al. 2001; Tordi et al. 2003), but in almost all cases an increased phase II amplitude and a reduced 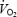 slow component during subsequent exercise were reported (Hughson et al. 2001; Burnley et al. 2002a, b; Wilkerson et al. 2004). The differences among the various studies have often been ascribed to variations in the degree of lactic acidosis induced by the prior exercise. Despite the fact that the metabolic status of both blood and muscle are so frequently implicated in the mediation of the slow component, the effects of prior heavy exercise on muscle lactate concentration and pH have not been assessed previously.
slow component during subsequent exercise were reported (Hughson et al. 2001; Burnley et al. 2002a, b; Wilkerson et al. 2004). The differences among the various studies have often been ascribed to variations in the degree of lactic acidosis induced by the prior exercise. Despite the fact that the metabolic status of both blood and muscle are so frequently implicated in the mediation of the slow component, the effects of prior heavy exercise on muscle lactate concentration and pH have not been assessed previously.
In the present study we hypothesized that the 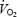 slow component is related to a progressive increase in muscle lactate concentration and that prior heavy exercise (PHE) with pronounced acidosis alters
slow component is related to a progressive increase in muscle lactate concentration and that prior heavy exercise (PHE) with pronounced acidosis alters 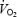 kinetics (reduces the time constant, τ), reduces work efficiency and alters the metabolic response during steady-state exercise. Therefore, the aims of the present study were to: (1) assess
kinetics (reduces the time constant, τ), reduces work efficiency and alters the metabolic response during steady-state exercise. Therefore, the aims of the present study were to: (1) assess 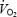 kinetics, including the magnitude of the slow component during two conditions with different muscle lactate levels; (2) assess the effect of acidosis on work efficiency during steady-state conditions; and (3) investigate the effect of prior heavy exercise on the metabolic response, including muscle acetyl group availability, during steady-state exercise.
kinetics, including the magnitude of the slow component during two conditions with different muscle lactate levels; (2) assess the effect of acidosis on work efficiency during steady-state conditions; and (3) investigate the effect of prior heavy exercise on the metabolic response, including muscle acetyl group availability, during steady-state exercise.
Methods
Nine healthy male subjects aged between 21 and 27 years (mean weight ± s.e.m., 81.4 ± 2.7 kg; height, 182.1 ± 2.9 cm) volunteered for the study. Subjects agreed to participate in the experiment after having been informed of the purpose and potential risks involved. The project was approved by the local ethics committee at the Odense University Hospital (VF 20020201). Subjects were physically active but did not participate in sports at the elite level.
Exercise protocol
The experiments were carried out on a Monark-Cardionics cycle ergometer (Varberg, Sweden) at 80 r.p.m. The work rates were calculated from pedal rate and added weights. The actual work rates were also measured with a computerized crank recording system (Schoberer Rad Messtechnik, Jülich, Germany). The difference between the calculated and measured power output was negligible and there were no systematic differences in power output between the experimental conditions.
Subjects performed an incremental cycle test (40-W increments with 4 min at each stage) until blood lactate concentration increased to > 4 mmol l−1. The linear relation between heart rate and work rate was used to estimate a target work rate corresponding to the estimated maximal heart rate. After 5–10 min rest, subjects continued the exercise at the target work rate for 3 min, after which the work rate was increased by 40 W every 1.5–2 min until exhaustion. The highest 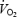 measured during a 30-s period (breath-by-breath analysis) was defined as
measured during a 30-s period (breath-by-breath analysis) was defined as 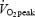 .
.
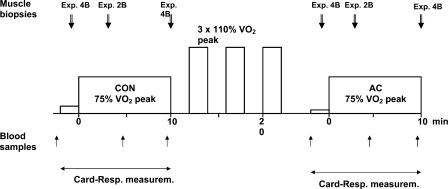
After habituation to the protocol, subjects performed the experimental exercise test on three occasions (without muscle biopsies (NB), with two muscle biopsies (2B), and with four muscle biopsies (4B)). The three exercise tests were normally performed within 1–3 weeks but due to irregularities in the test protocol, NB experiments were performed later in two subjects. The exercise test is schematically described in Fig. 1. Subjects began with 2 min pre-exercise at 80 W and cycled for 10 min at 241 ± 9 W estimated to elicit 75% of 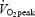 (control, CON). After 2 min rest, subjects performed three bouts of supramaximal exercise at 110% of
(control, CON). After 2 min rest, subjects performed three bouts of supramaximal exercise at 110% of 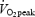 (two bouts for a 2 min duration and the last to exhaustion) separated by 2 min rest. After 3 min rest, subjects cycled for 2 min at 40 W and then repeated the 10 min submaximal cycling (acidosis, AC) at an identical work rate as during CON.
(two bouts for a 2 min duration and the last to exhaustion) separated by 2 min rest. After 3 min rest, subjects cycled for 2 min at 40 W and then repeated the 10 min submaximal cycling (acidosis, AC) at an identical work rate as during CON.
Figure 1. Experimental protocol.
The exercise protocol was repeated three times; with no biopsies (NB), two biopsies (2B) and four biopsies (4B). Blood samples (capillary blood) and cardio-respiratory measurements were performed during all experiments. The 10-min work period at 75%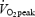 was preceded by 2 min of pre-exercise at 80 W (CON) and 40 W (AC). For further details see Methods.
was preceded by 2 min of pre-exercise at 80 W (CON) and 40 W (AC). For further details see Methods.
Biopsies were taken from vastus lateralis muscle with a modified Bergström needle with suction while the subject sat on the cycle. After local anaesthesia (2–3 ml 20 mg ml−1 Carbocain, AstraZeneca AB, Sweden) insertions (one-third (± 2 cm) of the distance between patella and anterior superior iliac spine) were made for all subsequent muscle biopsies while the subject rested in the supine position. Each insertion was used for two biopsies of which the first was directed distally and the second proximally. In experiment 4B (CON and AC), biopsies were taken during the pre-exercise period (30–60 s prior to the exercise bouts) and after 10 min of cycling. In experiment 2B, biopsies were taken after 3 min of exercise (CON and AC). During experiment 4B, the first two biopsies (CON) were taken from one leg and the other two (AC) from the other leg (order between left and right leg randomized). During experiment 2B both biopsies were taken from the same leg (randomized between left and right leg). Muscle biopsies were quick-frozen and used for analysis of metabolites and fibre-type composition. The delay between terminating exercise and plunging the needle into liquid N2 averaged 14 s (11–18 s). Capillary blood was taken from a prewarmed finger prior to pre-exercise, and after 4.5 (4–5 min) and 9 (8.5–9.5 min) min of CON and AC exercise (Fig. 1). Cardio-respiratory parameters (heart rate, 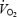 ,
, 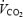 and ventilation) were measured continuously during the pre-exercise and the following 10-min exercise periods.
and ventilation) were measured continuously during the pre-exercise and the following 10-min exercise periods.
Analytical methods
Pulmonary gas exchange was measured continuously during the 2 min pre-exercise period and the subsequent 10 min cycling at 75%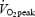 using breath-by-breath analysis (OxyCon Pro, Jaeger, Germany). Heart rate (HR) was measured with Polar Vantage NV analyser (Polar Electro KY, Kempele, Finland). Rating of perceived exertion (RPE) was estimated with Borg's 6–20 scale (Borg, 1970) after 4 and 9.5 min of exercise.
using breath-by-breath analysis (OxyCon Pro, Jaeger, Germany). Heart rate (HR) was measured with Polar Vantage NV analyser (Polar Electro KY, Kempele, Finland). Rating of perceived exertion (RPE) was estimated with Borg's 6–20 scale (Borg, 1970) after 4 and 9.5 min of exercise.
Muscle samples were freeze-dried, freed from connective tissue and blood, extracted with HClO4 (0.5 m) and neutralized with KHCO3 (2.2 m) as previously described (Harris et al. 1974). The neutralized extract was analysed with enzymatic techniques for ATP, PCr, creatine (Cr) and lactate (Harris et al. 1974) and for Acn and carnitine (Harris et al. 1987) content. Total Cr content ([TCr]=[PCr]+[Cr]) was unchanged during the experiment. To reduce the variability, due to non-muscle constituents in the samples, metabolites (except for lactate) have been adjusted to the peak value of TCr for each subject. Muscle pH was calculated from muscle lactate concentration using the linear relation between muscle pH and muscle content of lactate and pyruvate previously established during dynamic exercise (Sahlin et al. 1976). Muscle pyruvate concentration during intense dynamic exercise is < 1% of lactate concentration and was ignored in the calculations. Part of the freeze-dried muscle (one muscle sample from each subject) was used for determination of myosin heavy chain (MHC) composition by gel electrophoresis essentially as previously described (Andersen & Aagaard, 2000).
Blood lactate concentration was analysed immediately after sampling using an enzymatic lactate analyser (YSI 1500 Sport Lactate analyser, Yellow Springs Instrument, Yellow Springs, USA). Blood gases (PCO2, PO2), pH and derived acid–base parameters were analysed with an automated system (Gem Premier 3000, Instrumentation Laboratory, Lexington, MA, USA).
Calculation of 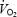 kinetics
kinetics
The data were analysed by an investigator fully blinded to the experimental condition (CON versus AC). The breath-by-breath responses of 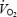 for all three trials were edited to remove outliers and then averaged every 5 s to provide the highest possible confidence for parameter estimation during the phase II region of the response (Whipp et al. 1982; Rossiter et al. 2003). The phase II time-constant (τ), asymptote
for all three trials were edited to remove outliers and then averaged every 5 s to provide the highest possible confidence for parameter estimation during the phase II region of the response (Whipp et al. 1982; Rossiter et al. 2003). The phase II time-constant (τ), asymptote 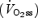 and amplitude (A), were estimated from non-linear least-squares fitting (Microcal, Origin) of the
and amplitude (A), were estimated from non-linear least-squares fitting (Microcal, Origin) of the 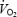 response (averaged from all three trials) from 20 s after the onset of exercise (i.e. excluding ‘phase I’) until the response deviated from exponential (discerned from the χ2 value and the residuals) using the equation:
response (averaged from all three trials) from 20 s after the onset of exercise (i.e. excluding ‘phase I’) until the response deviated from exponential (discerned from the χ2 value and the residuals) using the equation:
where 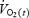 is the
is the 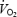 at any time, t;
at any time, t; 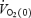 is the baseline value of
is the baseline value of 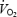 (at work rate of either 80 W in CON or 40 W in AC); e is the base of natural logarithms; and TD is the time delay after which the modelled exponential begins to increase. Phase II was in all cases terminated before the biopsy at 3 min. Mean response time (MRT) was obtained by fitting the entire
(at work rate of either 80 W in CON or 40 W in AC); e is the base of natural logarithms; and TD is the time delay after which the modelled exponential begins to increase. Phase II was in all cases terminated before the biopsy at 3 min. Mean response time (MRT) was obtained by fitting the entire 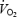 response (from exercise onset to end of phase II) to a single exponential curve; MRT corresponds to the time to reach 63% of the phase II asymptote. Oxygen deficit (O2def) was calculated from the equation:
response (from exercise onset to end of phase II) to a single exponential curve; MRT corresponds to the time to reach 63% of the phase II asymptote. Oxygen deficit (O2def) was calculated from the equation:
The magnitude of the slow component was calculated from the difference between 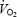 at 10 and 3 min averaged over the last 30-s interval at both times (values from experiment 2B was excluded due to the interruption for biopsy at 3 min). Gross efficiency was represented as the ratio between the work rate (adjusted for the measured r.p.m.) and
at 10 and 3 min averaged over the last 30-s interval at both times (values from experiment 2B was excluded due to the interruption for biopsy at 3 min). Gross efficiency was represented as the ratio between the work rate (adjusted for the measured r.p.m.) and 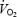 .
.
Statistics
Values are presented as mean ± s.e.m. Statistical significance of differences was tested with one-way ANOVA with repeated measures using Newman–Keuls test for post hoc analysis. The difference in 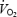 kinetic parameters between acidosis and control was tested with Student's two-tailed paired sample t test. Statistical significance was defined as P < 0.05.
kinetic parameters between acidosis and control was tested with Student's two-tailed paired sample t test. Statistical significance was defined as P < 0.05.
Results
Subjects were moderately well trained with an average 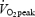 of 4.58 ± 0.19 l min−1 corresponding to 56.3 ± 1.5 ml kg−1 min−1 (range, 52–64 ml kg−1 min−1). The
of 4.58 ± 0.19 l min−1 corresponding to 56.3 ± 1.5 ml kg−1 min−1 (range, 52–64 ml kg−1 min−1). The 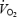 after 5 min of exercise during CON was 74.1 ± 1.1% of
after 5 min of exercise during CON was 74.1 ± 1.1% of 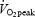 , which was close to the target intensity (75%
, which was close to the target intensity (75%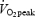 ). Muscle fibre-type composition was: 60.7 ± 2.4% slow twitch (ST) fibres with a rather narrow range among subjects (47.3–69.7).
). Muscle fibre-type composition was: 60.7 ± 2.4% slow twitch (ST) fibres with a rather narrow range among subjects (47.3–69.7).
The parameters related to phase II 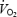 kinetics are presented in Table 1 and illustrated in Fig. 2. Despite a lower work rate during the 2 min pre-exercise period during AC (40 W versus 80 W during CON), baseline
kinetics are presented in Table 1 and illustrated in Fig. 2. Despite a lower work rate during the 2 min pre-exercise period during AC (40 W versus 80 W during CON), baseline 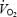 was 229 ml min−1 higher prior to AC (P < 0.05 versus CON). The phase II component of
was 229 ml min−1 higher prior to AC (P < 0.05 versus CON). The phase II component of 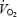 kinetics also had a greater amplitude in AC (+220 ml min−1; P < 0.05 versus CON), and as a result the
kinetics also had a greater amplitude in AC (+220 ml min−1; P < 0.05 versus CON), and as a result the 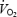 asymptote was 449 ml min−1 greater than during CON (P < 0.05). The phase II time delay and time constant (τ) were similar during CON and AC. While O2def (calculated from the onset of exercise until the end of phase II) had a tendency to be greater during AC compared to CON (due to the significantly greater amplitude in AC), this did not reach statistical significance (Table 1).
asymptote was 449 ml min−1 greater than during CON (P < 0.05). The phase II time delay and time constant (τ) were similar during CON and AC. While O2def (calculated from the onset of exercise until the end of phase II) had a tendency to be greater during AC compared to CON (due to the significantly greater amplitude in AC), this did not reach statistical significance (Table 1).
Table 1.
Parameters related to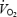 kinetics during submaximal exercise with (AC) and without (CON) prior heavy exercise
kinetics during submaximal exercise with (AC) and without (CON) prior heavy exercise
| CON | AC | |
|---|---|---|
Baseline 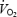 (ml min−1) (ml min−1) |
1345 ± 67 | 1574 ± 70* |
Phase II: 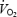 asymptote (ml min−1) asymptote (ml min−1) |
3112 ± 100 | 3561 ± 103* |
Phase II: 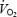 amplitude (ml min−1) amplitude (ml min−1) |
1767 ± 113 | 1987 ± 71* |
| Phase II: time delay (s) | 9.3 ± 1.3 | 9.6 ± 1.1 |
| Phase II: time constant, τ (s) | 24.9 ± 2.5 | 22.3 ± 1.3 |
| MRT (s) | 37.1 ± 1.4 | 35.3 ± 1.6 |
| Oxygen deficit (l) | 1.14 ± 0.07 | 1.23 ± 0.07 |
Values are means ± s.e.m. (n = 8) calculated from the means of all three experiments (NB, 2B and 4B). MRT, mean response time calculated from the onset of exercise until the end of phase II assuming a mono-exponential curve.
P < 0.05 AC versus CON.
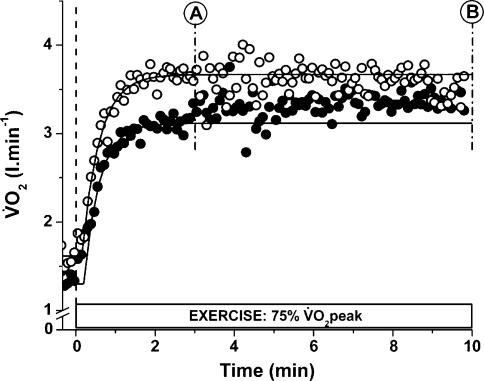
Figure 2. The 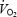 response to constant work rate submaximal cycle ergometer exercise with (AC) and without (CON) prior heavy exercise.
response to constant work rate submaximal cycle ergometer exercise with (AC) and without (CON) prior heavy exercise.
The Figure shows the results from a typical subject, time aligned to the start of either CON (o) or AC (•) exercise. The data are the average of three trials up to point A (3 min) after which the data represent the average of two trials (as during the 2B trial the exercise was briefly interrupted for the biopsy procedure at 3 min). The exponential fits are modelled from the phase II exponential region or to 3 min (whichever was the sooner) and the slow component was measured between points A and B (i.e. 3 and 10 min).
There was a slow increase in 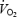 between 3 and 10 min of exercise during CON (P < 0.05, Table 2) corresponding to an increase in
between 3 and 10 min of exercise during CON (P < 0.05, Table 2) corresponding to an increase in 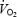 of 7.9 ± 0.2%; see also Fig. 2. The magnitude of the
of 7.9 ± 0.2%; see also Fig. 2. The magnitude of the 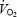 slow component was not related to fibre-type composition (r = −0.30 versus% type I fibres), blood lactate (r = 0.48) or muscle lactate concentrations (r = 0.33), or muscle pH (r = 0.12). In contrast to CON,
slow component was not related to fibre-type composition (r = −0.30 versus% type I fibres), blood lactate (r = 0.48) or muscle lactate concentrations (r = 0.33), or muscle pH (r = 0.12). In contrast to CON, 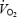 was stable between 3 (3.66 ± 0.10 l min−1) and 10 min (3.65 ± 0.10 l min−1) in AC.
was stable between 3 (3.66 ± 0.10 l min−1) and 10 min (3.65 ± 0.10 l min−1) in AC. 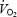 was higher during AC throughout the exercise period (P < 0.05 versus CON) but the difference was greater at 3 min (439 ± 46 ml min−1) than at 10 min (176 ± 45 ml min−1). The higher
was higher during AC throughout the exercise period (P < 0.05 versus CON) but the difference was greater at 3 min (439 ± 46 ml min−1) than at 10 min (176 ± 45 ml min−1). The higher 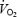 during AC corresponds to reductions in gross efficiency of 12% (3 min) and 5% (10 min) (P < 0.05) as compared to CON. HR and pulmonary ventilation
during AC corresponds to reductions in gross efficiency of 12% (3 min) and 5% (10 min) (P < 0.05) as compared to CON. HR and pulmonary ventilation 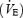 were significantly higher at baseline and throughout the 10 min of AC exercise. There were also significant increases in HR and VE from 3 to 10 min in both CON and AC (Table 2). RPE increased between 4 and 9.5 min during both AC and CON but was significantly higher during AC than during CON (Table 2).
were significantly higher at baseline and throughout the 10 min of AC exercise. There were also significant increases in HR and VE from 3 to 10 min in both CON and AC (Table 2). RPE increased between 4 and 9.5 min during both AC and CON but was significantly higher during AC than during CON (Table 2).
Table 2.
Cardio-respiratory parameters during submaximal exercise with (AC) and without (CON) prior heavy exercise
| CON | AC | |||||
|---|---|---|---|---|---|---|
| Pre-exercise | 3 min | 10 min | Pre-exercise | 3 min | 10 min | |
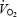 (l min−1) (l min−1) |
1.35 ± 0.05 | 3.23 ± 0.10a | 3.48 ± 0.10a# | 1.57 ± 0.04* | 3.66 ± 0.10a* | 3.65 ± 0.11a* |
Δ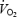 (ml min−1) (ml min−1) |
— | — | 252 ± 8 | — | — | −11 ± 28* |
| Gross efficiency (W (l O2)−1 min−1) | — | 74.6 ± 1.3 | 69.2 ± 1.3# | — | 65.7 ± 1.5* | 65.9 ± 1.5* |
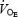 (l min−1) (l min−1) |
32.6 ± 1.3 | 73.0 ± 3.2a | 83.2 ± 4.2a# | 57.8 ± 1.6* | 98.0 ± 3.9a* | 106.4 ± 3.3a* |
| RER | 0.86 ± 0.02 | 0.97 ± 0.02a | 0.97 ± 0.01a | 0.98 ± 0.02* | (0.84 ± 0.012a*) | (0.92 ± 0.01a#*) |
| HR (beats min−1) | 104.7 ± 5.4 | 149.8 ± 3.7a | 163.3 ± 4.1a# | 133.7 ± 3.6* | 170.5 ± 4.4a* | 180.8 ± 2.8a#* |
| RPE | — | 12.1 ± 0.1 | 13.6 ± 0.3# | — | 14.5 ± 0.4* | 16.0 ± 0.4#* |
Values are means ± s.e.m. (n = 9) and have been averaged over experiment NB and 4B. RPE, rate of perceived exertion was measured at 4 and 9.5 min. Δ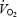 is the difference between
is the difference between 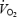 at 10 and 3 min. Respiratory exchange ratio (RER) does not reflect substrate utilization during AC (values in brackets) due to the non-stable acid–base conditions.
at 10 and 3 min. Respiratory exchange ratio (RER) does not reflect substrate utilization during AC (values in brackets) due to the non-stable acid–base conditions.
P < 0.05 versus pre-exercise;
P < 0.05 versus 3–4 min;
P < 0.05 AC versus CON.
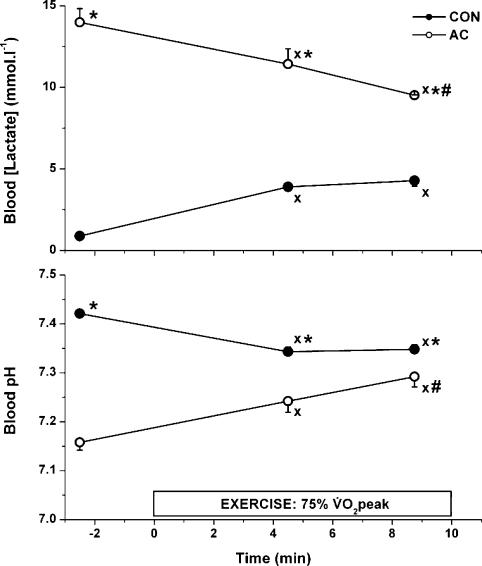
Blood lactate concentration was much higher prior to AC than prior to CON (14.0 ± 0.83 versus 0.88 ± 0.08 mmol l−1) and remained higher during exercise both at 4.5 (change in lactate concentration (Δ lactate), 8.73 ± 0.98 mmol l−1) and 9 min (Δ lactate, 5.25 ± 0.67 mmol l−1; Fig. 3). In contrast to CON, where lactate concentration increased and remained stable between 4.5 and 9 min, it decreased throughout AC. Blood pH reflected the lactate concentration values and was significantly lower during AC than during CON both prior to and during exercise. Blood pH was stable during CON (7.34 ± 0.01 and 7.35 ± 0.01 after 4.5 and 9 min, respectively) but increased slightly during AC from 7.24 ± 0.02 at 4.5 min to 7.29 ± 0.02 at 9 min (P < 0.05).
Figure 3. Blood lactate and pH during submaximal exercise.
Values are means ± s.e.m. (n = 9) from two separate experiments (NB and 4B). XP < 0.05 versus pre-exercise; #P < 0.05, 9 versus 4.5 min; *P < 0.05 AC (o) versus CON (•).
Muscle lactate concentration (Fig. 4) increased 4-fold above rest after 3 min of CON exercise, but remained stable between 3 and 10 min. Muscle lactate concentration was highly elevated prior to AC (68.5 ± 2.3 mmol (kg dry wt)−1), but decreased by 38% after 3 min and was stable during the remainder of the exercise period. Muscle lactate concentration was 2- to 3-fold higher during AC than during CON (P < 0.05). Estimated muscle pH (Fig. 4) was stable between 3 and 10 min of exercise (both during AC and CON) and was about 0.1 pH units lower during AC than during CON (P < 0.05).
Figure 4. Muscle lactate and muscle pH during submaximal exercise.
Values are means ± s.e.m. (n = 9) from two separate experiments (2B and 4B). Muscle pH was calculated from muscle lactate concentration (see Methods). XP < 0.05 versus pre-exercise; *P < 0.05 AC (o) versus CON (•).
ATP concentration did not change during CON or AC exercise but was lower during AC after 3 min of exercise (P < 0.05 AC versus CON Table 3). PCr concentration was similar prior to CON and AC but decreased during exercise to different levels (Table 3). PCr concentration was about 20% lower during AC than during CON. The change in PCr concentration between 3 and 10 min varied considerably between subjects with some showing an increase and some a decrease. The individual response in PCr concentration during CON exercise was not related to the magnitude of 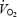 slow component. Muscle Cr concentration increased during exercise and the change was inversely related to the change in PCr concentration. The sum of Cr and PCr concentrations was similar prior to CON (120.2 ± 3.1 mmol (kg dry wt)−1) and AC (123.6 ± 2.9 mmol (kg dry wt)−1) and did not change during exercise.
slow component. Muscle Cr concentration increased during exercise and the change was inversely related to the change in PCr concentration. The sum of Cr and PCr concentrations was similar prior to CON (120.2 ± 3.1 mmol (kg dry wt)−1) and AC (123.6 ± 2.9 mmol (kg dry wt)−1) and did not change during exercise.
Table 3.
Muscle metabolites during submaximal exercise with (AC) and without (CON) prior heavy exercise
| CON | AC | |||||
|---|---|---|---|---|---|---|
| Pre-exercise | 3 min | 10 min | Pre-exercise | 3 min | 10 min | |
| ATP | 24.8 ± 0.4 | 26.1 ± 0.5 | 25.2 ± 0.8 | 23.3 ± 0.4 | 24.2 ± 0.7* | 24.3 ± 0.4 |
| PCr | 80.2 ± 3.1 | 63.6 ± 3.0a | 65.5 ± 4.2a | 84.1 ± 2.2 | 52.0 ± 4.1a* | 51.8 ± 3.8a* |
| Cr | 49.9 ± 2.3 | 66.0 ± 3.4a | 64.1 ± 3.9a | 45.6 ± 1.6 | 77.7 ± 3.4a* | 77.9 ± 3.1a* |
| PCr/Cr | 1.65 ± 0.13 | 1.00 ± 0.10a | 1.09 ± 0.14a | 1.87 ± 0.11 | 0.70 ± 0.09a* | 0.69 ± 0.08a* |
| Acetylcarnitine | 2.1 ± 0.3 | 9.3 ± 0.7a | 7.2 ± 1.0a | 12.6 ± 1.1* | 13.4 ± 1.5* | 14.9 ± 0.9* |
| Carnitine | 20.7 ± 0.5 | 13.4 ± 1.0 | 15.3 ± 1.5 | 9.3 ± 0.7 | 9.3 ± 0.8 | 7.9 ± 0.7 |
Values are means ± s.e.m. and expressed as mmol (kg dry wt)−1; n = 9, except for acetylcarnitine (n = 7) and carnitine (n = 6–7) and pre-exercise during CON (n = 8). Carnitine values were not analysed statistically due to too many missing values.
P < 0.05 versus pre-exercise;
P < 0.05 AC versus CON.
ACn concentration increased 3- to 4-fold (versus pre-exercise) during CON (Table 3) but remained unchanged between 3 and 10 min. ACn concentration was 6-fold higher prior to AC (versus CON P < 0.05) and remained higher during exercise (44% and 107% higher after 3 and 10 min exercise, respectively). The sum of ACn and carnitine concentrations was 22.7 ± 1.1 and 21.8 ± 1.4 mmol (kg dry wt)−1 (n = 4) prior to CON and AC exercise, and remained unchanged during exercise. As a consequence, the change in Cn concentration showed the reverse pattern in comparison to ACn concentration.
Discussion
The present study is unique in that we were able to create two very different muscle metabolic conditions which remained stable between 3 and 10 min of an identical bout of submaximal exercise. In one condition muscle and blood lactate concentrations were only slightly increased above baseline conditions (CON) whereas in the other condition (AC), lactate concentrations were highly elevated in muscle and blood. The main findings in this study were that: (i) despite stable muscle pH and muscle lactate concentration there was an evident 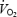 slow component during CON; (ii)
slow component during CON; (ii) 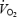 slow component was eliminated and gross exercise efficiency reduced at high muscle lactate concentration; and (iii) the combination of acidosis and stockpiling of ACn resulted in lower levels of PCr during exercise but did not affect initial
slow component was eliminated and gross exercise efficiency reduced at high muscle lactate concentration; and (iii) the combination of acidosis and stockpiling of ACn resulted in lower levels of PCr during exercise but did not affect initial 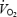 .
.
The slow increase of 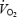 between 3 and 10 min of CON exercise (252 ± 8 ml min−1 or 7.9 ± 0.2% of
between 3 and 10 min of CON exercise (252 ± 8 ml min−1 or 7.9 ± 0.2% of 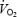 ) was associated with elevated but constant levels of blood lactate (∼4 mmol l−1). This is in accordance with previous studies where excess
) was associated with elevated but constant levels of blood lactate (∼4 mmol l−1). This is in accordance with previous studies where excess 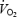 occurs when the exercise intensity exceeds the lactate threshold (Whipp, 1994; Zoladz & Korzeniewski, 2001). The lactate anion per se is unlikely to mediate the slow component as direct lactate infusion into a blood-perfused muscle model caused no change in the
occurs when the exercise intensity exceeds the lactate threshold (Whipp, 1994; Zoladz & Korzeniewski, 2001). The lactate anion per se is unlikely to mediate the slow component as direct lactate infusion into a blood-perfused muscle model caused no change in the 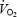 /developed force ratio during contractions (Poole et al. 1994). Although acidosis has been implicated as a cause of
/developed force ratio during contractions (Poole et al. 1994). Although acidosis has been implicated as a cause of 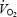 slow component, the present study is one of the few studies of
slow component, the present study is one of the few studies of 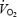 kinetics where both muscle lactate concentration and muscle pH have been assessed. A new finding was that muscle lactate concentration and muscle pH remained stable between 3 and 10 min during which time there was a considerable
kinetics where both muscle lactate concentration and muscle pH have been assessed. A new finding was that muscle lactate concentration and muscle pH remained stable between 3 and 10 min during which time there was a considerable 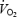 slow component (7.9% increase in
slow component (7.9% increase in 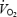 ). The individual amplitude of the
). The individual amplitude of the 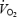 slow component in CON was neither related to lactate concentration nor to muscle pH. The link between lactic acid and the
slow component in CON was neither related to lactate concentration nor to muscle pH. The link between lactic acid and the 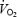 slow component appears therefore to be coincidental rather than causal. During AC,
slow component appears therefore to be coincidental rather than causal. During AC, 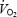 slow component was eliminated despite signs of remaining fatigue (RPE). However the asymptote of phase II
slow component was eliminated despite signs of remaining fatigue (RPE). However the asymptote of phase II 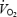 kinetics was higher during AC and the possibility cannot be excluded that
kinetics was higher during AC and the possibility cannot be excluded that 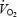 slow component merged into a higher
slow component merged into a higher 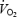 asymptote and that the two phenomena are interrelated.
asymptote and that the two phenomena are interrelated.
Contrary to previous findings where prior exercise was submaximal (Rossiter et al. 2001; Campbell-O'Sullivan et al. 2002) and the increase in muscle lactate concentration was small (Campbell-O'Sullivan et al. 2002), the present data show that a very high muscle lactate concentration and low pH resulted in a higher oxygen and PCr cost for AC exercise. Although non-working tissues may contribute to the increased 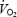 (see below), the increased
(see below), the increased 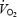 amplitude and the lower PCr concentration during AC suggest that at least part of the increased
amplitude and the lower PCr concentration during AC suggest that at least part of the increased 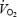 is derived from the working muscle.
is derived from the working muscle.
Contractile efficiency, estimated from heat production and power output in isolated mouse muscle, has been shown to decrease at fatigue (Barclay, 1996). RPE was augmented during AC and the reduced gross exercise efficiency during AC may therefore relate to an impaired work capacity possibly caused by a greater accumulation of metabolic end products such as H+ or inorganic phosphate. In order to maintain the work rate when power output of recruited motor units is reduced, additional (possibly less efficient) motor units or muscles will be recruited and the 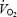 will increase. In the present study, muscle metabolites were measured in mixed fibre samples from vastus lateralis. The pattern of fibre recruitment and recruitment of other muscles (e.g. vastus medialis, rectus femoris) can therefore not be evaluated from the present data. However, measurements of PCr concentration in single fibres demonstrate an enhanced recruitment of fast-twitch fibres after prior glycogen depletion of slow-twitch fibres (Krustrup et al. 2004b). Furthermore, the integrated EMG amplitude appeared to be larger (especially in vastus medialis) after prior exercise (Burnley et al. 2002a; Tordi et al. 2003). Recruitment of a larger muscle mass would increase total ATP turnover rate and
will increase. In the present study, muscle metabolites were measured in mixed fibre samples from vastus lateralis. The pattern of fibre recruitment and recruitment of other muscles (e.g. vastus medialis, rectus femoris) can therefore not be evaluated from the present data. However, measurements of PCr concentration in single fibres demonstrate an enhanced recruitment of fast-twitch fibres after prior glycogen depletion of slow-twitch fibres (Krustrup et al. 2004b). Furthermore, the integrated EMG amplitude appeared to be larger (especially in vastus medialis) after prior exercise (Burnley et al. 2002a; Tordi et al. 2003). Recruitment of a larger muscle mass would increase total ATP turnover rate and 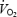 and may thus be the major explanation for the observed reduction in gross efficiency. Although acidosis per se is unlikely to be the cause of
and may thus be the major explanation for the observed reduction in gross efficiency. Although acidosis per se is unlikely to be the cause of 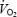 slow component during CON it remains a possible mediator for impaired work capacity and thus the higher oxygen demand during AC.
slow component during CON it remains a possible mediator for impaired work capacity and thus the higher oxygen demand during AC.
Several (Coyle et al. 1992; Jones et al. 2004) but not all (Pedersen et al. 2002) studies have shown a correlation between mechanical efficiency and the proportion of ST fibres. In the present study we could not find any relation between fibre-type composition and gross exercise efficiency or the amplitude of 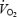 slow component. However, the fibre-type composition was rather similar between subjects (range, 47–70% ST fibres) and the hypothesis can therefore not be refuted.
slow component. However, the fibre-type composition was rather similar between subjects (range, 47–70% ST fibres) and the hypothesis can therefore not be refuted.
Part of the increased 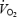 during AC may relate to increased cardio-respiratory work. The oxygen cost of pulmonary ventilation and cardiac work has been estimated as 1.8 ml per l of ventilation (Aaron et al. 1992) and 0.2 ml beat−1 (Kitamura et al. 1972). By using these data it can be calculated that increased cardio-respiratory work during AC versus CON may contribute ∼11 and 26% of the increased
during AC may relate to increased cardio-respiratory work. The oxygen cost of pulmonary ventilation and cardiac work has been estimated as 1.8 ml per l of ventilation (Aaron et al. 1992) and 0.2 ml beat−1 (Kitamura et al. 1972). By using these data it can be calculated that increased cardio-respiratory work during AC versus CON may contribute ∼11 and 26% of the increased 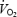 at 3 and 10 min, respectively. Similarly, increased cardio-respiratory work may contribute 8% of the
at 3 and 10 min, respectively. Similarly, increased cardio-respiratory work may contribute 8% of the 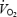 slow component during CON exercise. These estimates are consistent with actual measurements of
slow component during CON exercise. These estimates are consistent with actual measurements of 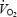 of the exercising legs during cycling, where 86% of the pulmonary
of the exercising legs during cycling, where 86% of the pulmonary 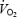 slow component was attributed to increased leg oxygen consumption (Poole et al. 1991).
slow component was attributed to increased leg oxygen consumption (Poole et al. 1991).
Mitochondrial efficiency is substrate dependent with carbohydrate (CHO) being a more efficient substrate than fat. However, a switch from CHO to fat oxidation did not occur during CON, as RER was 0.97 both after 3 and 10 min exercise, and is unlikely to occur during AC due to the high levels of muscle lactate and ACn. ATP formation in glycolysis will increase the yield of ATP during CHO oxidation but not during lactate oxidation. The yield of ATP per oxygen consumed will therefore be 10% lower during lactate oxidation than during CHO oxidation. However, major net lactate oxidation between 3 and 10 min of exercise is unlikely because muscle lactate concentration was stable during both AC and CON.
There is substantial evidence that a deficit in availability of acetyl groups may limit oxidative phosphorylation at the onset of exercise (Timmons et al. 1996, 1998; Campbell-O'Sullivan et al. 2002; Rossiter et al. 2003). Low intensity prior exercise (55%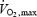 ) increased ACn concentration 2-fold (Campbell-O'Sullivan et al. 2002) and resulted in reduced PCr degradation (Rossiter et al. 2001; Campbell-O'Sullivan et al. 2002) and a faster initial
) increased ACn concentration 2-fold (Campbell-O'Sullivan et al. 2002) and resulted in reduced PCr degradation (Rossiter et al. 2001; Campbell-O'Sullivan et al. 2002) and a faster initial 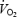 kinetics (reduced MRT and O2def) (Campbell-O'Sullivan et al. 2002). However, in the present study, despite a 6-fold higher ACn concentration prior to AC, neither τ of phase II nor MRT (calculated with exclusion of the slow component; see Campbell-O'Sullivan et al. 2002) was altered. Furthermore, instead of being attenuated, PCr degradation was higher and reached a lower plateau during AC. The differences between studies may be related to the pronounced acidosis in the present study and the associated fatigue, which might override the potential benefit of prior acetyl group accumulation.
kinetics (reduced MRT and O2def) (Campbell-O'Sullivan et al. 2002). However, in the present study, despite a 6-fold higher ACn concentration prior to AC, neither τ of phase II nor MRT (calculated with exclusion of the slow component; see Campbell-O'Sullivan et al. 2002) was altered. Furthermore, instead of being attenuated, PCr degradation was higher and reached a lower plateau during AC. The differences between studies may be related to the pronounced acidosis in the present study and the associated fatigue, which might override the potential benefit of prior acetyl group accumulation.
PCr concentration decreased by 21% after 3 min CON exercise and remained unaltered at this plateau until exercise cessation at 10 min. However, there was a large variation in the response between subjects. The variability may in part relate to the fact that muscle biopsies at 3 min, for technical reasons, were performed on a separate occasion than muscle biopsies taken before and after 10 min exercise. Previous studies of exercise with small muscle groups, showed a slow downward drift of about 5% in PCr concentration measured with the 31P magnetic resonance spectroscopy technique (Rossiter et al. 2001; Haseler et al. 2004). With the technique used in the present study we would be unable to demonstrate a 5% decline in PCr.
The present study demonstrated that 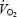 does not reach a steady state but increases slowly during CON, despite stable values of lactate concentration and pH in both muscle and blood. The hypothesis that acidosis is the sole cause of the
does not reach a steady state but increases slowly during CON, despite stable values of lactate concentration and pH in both muscle and blood. The hypothesis that acidosis is the sole cause of the 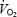 slow component is therefore rejected. Prior heavy exercise resulted in a markedly different metabolic condition (AC versus CON) with a 2- to 3-fold higher lactate concentration in blood and muscle, 20% lower PCr levels and 6-fold elevation in ACn concentration. Prior heavy exercise eliminated
slow component is therefore rejected. Prior heavy exercise resulted in a markedly different metabolic condition (AC versus CON) with a 2- to 3-fold higher lactate concentration in blood and muscle, 20% lower PCr levels and 6-fold elevation in ACn concentration. Prior heavy exercise eliminated 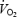 slow component but increased the
slow component but increased the 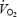 asymptote of the initial (phase II) component of
asymptote of the initial (phase II) component of 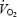 kinetics. This may suggest that the
kinetics. This may suggest that the 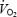 slow component merged into the initial component of
slow component merged into the initial component of 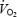 kinetics and that both phenomena are therefore related to impaired contractility of the working fibres. Despite a pronounced stockpiling of ACn during AC, initial
kinetics and that both phenomena are therefore related to impaired contractility of the working fibres. Despite a pronounced stockpiling of ACn during AC, initial 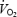 kinetics were not affected by PHE, and PCr levels decreased to a lower plateau. This is in contrast with previous studies, where initial oxidative ATP generation appears to be limited by acetyl group availability. The differences between studies may be related to the pronounced acidosis in the present study and the associated fatigue, which might override the potential benefit of prior acetyl group accumulation.
kinetics were not affected by PHE, and PCr levels decreased to a lower plateau. This is in contrast with previous studies, where initial oxidative ATP generation appears to be limited by acetyl group availability. The differences between studies may be related to the pronounced acidosis in the present study and the associated fatigue, which might override the potential benefit of prior acetyl group accumulation.
Acknowledgments
This work was supported by Team Danmark and Statens Sundhedsvidenskabelige Forskningsråd. Harry B. Rossiter is an International Travelling Fellow of the Wellcome Trust (UK) (064898).
References
- Aaron EA, Seow KC, Johnson BD, Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol. 1992;72:1818–1825. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- Andersen JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2000;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. 10.1002/1097-4598(200007)23:7<1095::AID-MUS13>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Bangsbo J, Krustrup P, Gonzalez-Alonso J, Saltin B. ATP production and efficiency of human skeletal muscle during intense exercise: effect of previous exercise. Am J Physiol Endocrinol Metab. 2001;280:E956–E964. doi: 10.1152/ajpendo.2001.280.6.E956. [DOI] [PubMed] [Google Scholar]
- Barclay CJ. Mechanical efficiency and fatigue of fast and slow muscles of the mouse. J Physiol. 1996;497:781–794. doi: 10.1113/jphysiol.1996.sp021809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–98. [PubMed] [Google Scholar]
- Burnley M, Doust JH, Ball D, Jones AM. Effects of prior heavy exercise on VO2 kinetics during heavy exercise are related to changes in muscle activity. J Appl Physiol. 2002a;93:167–174. doi: 10.1152/japplphysiol.01217.2001. [DOI] [PubMed] [Google Scholar]
- Burnley M, Doust JH, Jones AM. Effects of prior heavy exercise, prior sprint exercise and passive warming on oxygen uptake kinetics during heavy exercise in humans. Eur J Appl Physiol. 2002b;87:424–432. doi: 10.1007/s00421-002-0647-8. [DOI] [PubMed] [Google Scholar]
- Campbell-O'Sullivan SP, Constantin-Teodosiu D, Peirce N, Greenhaff PL. Low intensity exercise in humans accelerates mitochondrial ATP production and pulmonary oxygen kinetics during subsequent more intense exercise. J Physiol. 2002;538:931–939. doi: 10.1113/jphysiol.2001.013238. 10.1113/jphysiol.2001.013238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oygen utilisation. J Biol Chem. 1955;217:383–393. [PubMed] [Google Scholar]
- Coyle EF, Sidossis LS, Horowitz JF, Beltz JD. Cycling efficiency is related to the percentage of type I muscle fibers. Med Sci Sports Exerc. 1992;24:782–788. [PubMed] [Google Scholar]
- Gerbino A, Ward SA, Whipp BJ. Effects of prior exercise on pulmonary gas-exchange kinetics during high-intensity exercise in humans. J Appl Physiol. 1996;80:99–107. doi: 10.1152/jappl.1996.80.1.99. [DOI] [PubMed] [Google Scholar]
- Grassi B. Regulation of oxygen consumption at exercise onset: is it really controversial? Exerc Sport Sci Rev. 2001;29:134–138. doi: 10.1097/00003677-200107000-00009. 10.1097/00003677-200107000-00009. [DOI] [PubMed] [Google Scholar]
- Harris RC, Foster CV, Hultman E. Acetylcarnitine formation during intense muscular contraction in humans. J Appl Physiol. 1987;63:440–442. doi: 10.1152/jappl.1987.63.1.440. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Methods and variance of values. Scand J Clin Lab Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Haseler LJ, Kindig CA, Richardson RS, Hogan MC. The role of oxygen in determining phosphocreatine onset kinetics in exercising humans. J Physiol. 2004;558:985–992. doi: 10.1113/jphysiol.2004.062042. 10.1113/jphysiol.2004.062042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughson RL, Tschakovsky ME, Houston ME. Regulation of oxygen consumption at the onset of exercise. Exerc Sport Sci Rev. 2001;29:129–133. doi: 10.1097/00003677-200107000-00008. 10.1097/00003677-200107000-00008. [DOI] [PubMed] [Google Scholar]
- Jones AM, Campbell IT, Pringle JS. Influence of muscle fibre type and pedal rate on the VO2-work rate slope during ramp exercise. Eur J Appl Physiol. 2004;91:238–245. doi: 10.1007/s00421-003-0971-7. 10.1007/s00421-003-0971-7. [DOI] [PubMed] [Google Scholar]
- Kitamura K, Jorgensen CR, Gobel FL, Taylor HL, Wang Y. Hemodynamic correlates of myocardial oxygen consumption during upright exercise. J Appl Physiol. 1972;32:516–522. doi: 10.1152/jappl.1972.32.4.516. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Ferguson RA, Kjar M, Bangsbo J. ATP and heat production in human skeletal muscle during dynamic exercise: higher efficiency of anaerobic than aerobic ATP resynthesis. J Physiol. 2003;549:255–269. doi: 10.1113/jphysiol.2002.035089. 10.1113/jphysiol.2002.035089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krustrup P, Soderlund K, Mohr M, Bangsbo J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004a;447:855–866. doi: 10.1007/s00424-003-1203-z. 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Soderlund K, Mohr M, Bangsbo J. Slow-twitch fiber glycogen depletion elevates moderate-exercise fast-twitch fiber activity and O2 uptake. Med Sci Sports Exerc. 2004b;36:973–982. doi: 10.1249/01.mss.0000128246.20242.8b. [DOI] [PubMed] [Google Scholar]
- Meyer RA, Foley JM. Cellular processes integrating the metabolic response to exercise. In: Rowell LB, Shepherd JT, editors. Handbook of Physiology. New York: Oxford University Press; 1996. pp. 841–869. [Google Scholar]
- Pedersen PK, Sorensen JB, Jensen K, Johansen L, Levin K. Muscle fiber type distribution and nonlinear VO2-power output relationship in cycling. Med Sci Sports Exerc. 2002;34:655–661. doi: 10.1097/00005768-200204000-00015. 10.1097/00005768-200204000-00015. [DOI] [PubMed] [Google Scholar]
- Poole DC, Gladden LB, Kurdak S, Hogan MC. L-(+)-lactate infusion into working dog gastrocnemius: no evidence lactate per se mediates VO2 slow component. J Appl Physiol. 1994;76:787–792. doi: 10.1152/jappl.1994.76.2.787. [DOI] [PubMed] [Google Scholar]
- Poole DC, Schaffartzik W, Knight DR, Derion T, Kennedy B, Guy HJ, Prediletto R, Wagner PD. Contribution of excising legs to the slow component of oxygen uptake kinetics in humans. J Appl Physiol. 1991;71:1245–1260. doi: 10.1152/jappl.1991.71.4.1245. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Howe FA, Wood DM, Kowalchuk JM, Griffiths JR, Whipp BJ. Effects of dichloroacetate on VO2 and intramuscular 31P metabolite kinetics during high-intensity exercise in humans. J Appl Physiol. 2003;95:1105–1115. doi: 10.1152/japplphysiol.00964.2002. [DOI] [PubMed] [Google Scholar]
- Rossiter HB, Ward SA, Kowalchuk JM, Howe FA, Griffiths JR, Whipp BJ. Effects of prior exercise on oxygen uptake and phosphocreatine kinetics during high-intensity knee-extension exercise in humans. J Physiol. 2001;537:291–303. doi: 10.1111/j.1469-7793.2001.0291k.x. 10.1111/j.1469-7793.2001.0291k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahlin K, Harris RC, Nylind B, Hultman E. Lactate content and pH in muscle obtained after dynamic exercise. Pflugers Arch. 1976;367:143–149. doi: 10.1007/BF00585150. 10.1007/BF00585150. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Gustafsson T, Sundberg CJ, Jansson E, Greenhaff PL. Muscle acetyl group availability is a major determinant of oxygen deficit in humans during submaximal exercise. Am J Physiol. 1998;274:E377–E380. doi: 10.1152/ajpendo.1998.274.2.E377. [DOI] [PubMed] [Google Scholar]
- Timmons JA, Poucher SM, Constantin-Teodosiu D, Worrall V, Macdonald IA, Greenhaff PL. Increased acetyl group availability enhances contractile function of canine skeletal muscle during ischemia. J Clin Invest. 1996;97:879–883. doi: 10.1172/JCI118490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tordi N, Perrey S, Harvey A, Hughson RL. Oxygen uptake kinetics during two bouts of heavy cycling separated by fatiguing sprint exercise in humans. J Appl Physiol. 2003;94:533–541. doi: 10.1152/japplphysiol.00532.2002. [DOI] [PubMed] [Google Scholar]
- Whipp BJ. The slow component of O2 uptake kinetics during heavy exercise. Med Sci Sports Exerc. 1994;26:1319–1326. [PubMed] [Google Scholar]
- Whipp BJ, Ward SA, Lamarra N, Davis JA, Wasserman K. Parameters of ventilatory and gas exchange dynamics during exercise. J Appl Physiol. 1982;52:1506–1513. doi: 10.1152/jappl.1982.52.6.1506. [DOI] [PubMed] [Google Scholar]
- Whipp BJ, Wasserman K. Effect of anaerobiosis on the kinetics of O2 uptake during exercise. Fed Proc. 1986;45:2942–2947. [PubMed] [Google Scholar]
- Wilkerson DP, Koppo K, Barstow TJ, Jones AM. Effect of prior multiple-sprint exercise on pulmonary O2 uptake kinetics following the onset of perimaximal exercise. J Appl Physiol. 2004;97:1227–1236. doi: 10.1152/japplphysiol.01325.2003. 10.1152/japplphysiol.01325.2003. [DOI] [PubMed] [Google Scholar]
- Wilson DF. Factors affecting the rate and energetics of mitochondrial oxidative phosphorylation. Med Sci Sports Exerc. 1994;26:37–43. [PubMed] [Google Scholar]
- Zoladz JA, Korzeniewski B. Physiological background of the change point in VO2 and the slow component of oxygen uptake kinetics. J Physiol Pharmacol. 2001;52:167–184. [PubMed] [Google Scholar]