Abstract
The adaptation of renal medullary cells to their hyperosmotic environment involves the accumulation of compatible organic osmolytes and the enhanced synthesis of heat shock proteins (HSP) 27 and 70. While the mechanisms leading to osmolyte accumulation are similar in papillary collecting duct (PCD) and papillary interstitial (PI) cells, the present data demonstrate that HSP27 and HSP70 are expressed differentially in these cells both in vivo and in vitro. HSP70 is abundant in PCD, but not expressed in PI cells in the papilla in situ, while HSP27 is expressed in both PCD and PI cells. These observations could be reproduced by non-permeant solutes in cultured cells. Osmotic stress strongly induced HSP70 in MDCK cells (as a model for PCD cells), but not in PI cells, while HSP27 was constitutively expressed in MDCK cells and was up-regulated in PI cells. Since prior hypertonic stress (NaCl addition) protects MDCK against subsequent exposure to high urea concentrations, this effect was also assessed in PI cells. In both cell lines, hypertonic pretreatment prior to urea exposure (400 mm) strongly attenuated caspase-3 activation. Inhibition of HSP27 expression by antisense transfection diminished the protective effect of hypertonic preconditioning in PI cells, while attenuation of HSP70 expression in MDCK cells diminished the protective effect of hypertonic preconditioning in these cells. These observations indicate that PCD and PI cells employ cell-specific mechanisms for protection against high urea concentrations as present in the renal papilla during antidiuresis.
In antidiuresis, the cells of the renal papilla are faced with a uniquely harsh environment, characterized by extreme interstitial sodium chloride and urea concentrations, built up and maintained by the renal countercurrent mechanism and urea recycling. It is well established that papillary cells are protected against the stress of hypertonicity by the intracellular accumulation of compatible organic osmolytes, which replace intracellular ions, thus allowing the cells to maintain their cell volume and intracellular ionic strength at normal levels, even when the extracellular tonicity is severalfold higher than under isotonic conditions (Woo et al. 2002).
In the past few years it has become increasingly clear that HSPs may contribute decisively to survival of papillary cells by conferring protection against the extremely high interstitial solute concentrations as present in this kidney region during antidiuresis (Neuhofer & Beck, 2005). In particular, the expression of HSP27 and HSP70 is much higher in the inner medulla than in the isoosmotic cortex and changes appropriately with the diuretic state, suggesting functions essential for inner medullary cells (Medina et al. 1996; Müller et al. 1998). However, the cellular localization of these HSPs in the renal papilla has received less attention, since the expression of HSPs has been studied primarily by Western or Northern blot analysis on papillary homogenates that do not allow discrimination between tubule and interstitial cells. Moreover, in in vitro studies regarding cellular osmoadaptation, primarily cells originating from papillary collecting duct (PCD) cells have been employed, while the adaptation of papillary interstitial (PI) cells has received comparable less attention.
Thus, it may be possible that PCD and PI cells employ cell-specific mechanisms that promote survival in the harsh environment of the renal papilla. The present data demonstrate, based on immunohistological methods, that HSP27 and HSP70 are expressed differentially in PCD and PI cells. Furthermore, this distinct expression pattern of HSP27 and HSP70 in response to osmotic stress could be reproduced in cultured cells, suggesting alternative pathways for protection against high urea concentrations in PCD and PI cells by HSP70 and HSP27, respectively. Finally, the physiological relevance of this findings was assessed, indicating that PCD and PI cells indeed require distinct HSPs for protection against high urea concentrations.
Methods
Immunohistochemistry
Immunohistochemistry for HSP27 and HSP70 was performed on kidneys obtained from Male Wistar rats (200–250 g; Charles River, Sulzfeld, Germany) as previously described (Müller et al. 1996). Briefly, after anaesthesia with sodium pentobarbitone (60 mg kg−1i.p.), the kidneys were fixed in situ via the abdominal aorta with periodate–lysine–paraformaldehyde fixative (McLean & Nakane, 1974). HSP27 and HSP70 were immunolocalized on 5-μm cryosections using an antiserum specific for HSP27 (SPA 801; Biomol, Hamburg, Germany) or monoclonal anti-HSP70 antibody (SPA 810; Biomol) and appropriate secondary antibodies as described (Müller et al. 1996). For negative controls, the sections were simultaneously incubated with recombinant HSP27 or HSP70 (Biomol). All experiments were conducted in accordance with German federal laws relating to animal experimentation.
Cell culture
Madin-Darby canine kidney (MDCK) cells were obtained from the American Type Culture Collection (ATCC; Rockville, MD, USA). Primary cultures of rat papillary interstitial (PI) cells were kindly provided by Dr C. Maric (Department of Anatomy, University of Melbourne, Parkville, Victoria, Australia) and used for experiments at passage 8–15. These cells show cytoplasmic lipid staining (Oil Red O staining, not shown), vacuolated cytoplasm, and elongated cellular outline, features that are characteristic of papillary interstitial cells (Maric et al. 1996). Both MDCK and PI cells were grown in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (Biochrom, Berlin, Germany), 50 U ml−1 penicillin (Biochrom) and 50 mg ml−1 streptomycin (Biochrom) in a humidified atmosphere (5% CO2–95% air; 37°C) on 60-mm plastic dishes (Costar, Cambridge, MA, USA). Media were made hyperosmotic by adding the indicated solutes to a final osmolality of 500 mosmol (kg H2O)−1. For experiments in hypotonic medium, Na+ -and K+ -depleted starting medium (custom prepared by Life Technologies, Karlsruhe, Germany) was supplemented with KCl (5 mm) and NaCl to a final osmolality of 200 mosmol (kg H2O)−1.
For evaluating the effect of hypertonic pretreatment on resistance against subsequent urea exposure (Neuhofer et al. 1998), the respective cells were subjected to hypertonic medium (500 mosmol (kg H2O)−1 by NaCl addition) for 24 h prior to exposure to an additional 400 mm urea for 24 h. Subsequently, the cells were processed for determination of caspase-3, 8, and 9 activity as described below.
Northern blot analysis
After the respective treatments, the cells were lysed by addition of TRIReagent (Peqlab, Erlangen, Germany) and the RNA recovered according to the manufacturer's instructions. Aliquots (20 μg) of total RNA were electrophoresed through 1% agarose/2.2 m formaldehyde gels, transferred onto positively charged nylon membranes (Hybond N+, Amersham, Freiburg, Germany) and immobilized by UV cross-linking. The mRNA abundance of the individual genes was monitored by cDNAs for HSP27 (Larsen et al. 1996), HSP70 (Wu et al. 1985) and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Neuhofer et al. 2001). Probe generation, hybridization and washing conditions were performed as previously reported (Neuhofer et al. 2001).
SDS-PAGE and Western blot analysis
After the respective treatments, the cells were washed with chilled phosphate-buffered saline (PBS) and scraped into 8 m urea/PBS (75 μl/60 mm dish) and lysed by pipetting. After centrifugation at 12 000 g for 15 min at 4°C, the protein concentration in the supernatant was determined according to Bradford (1976) using a commercially available kit (Bio-Rad, Munich, Germany), and aliquots of 40 μg of protein were subjected to SDS-PAGE and blotted onto nitrocellulose membranes (Amersham-Pharmacia, Freiburg, Germany). Immunodetection of HSP27 and HSP70 was as previously described (Müller et al. 1996).
Determination of caspase-3, -8 and -9 activity
After the respective treatments, the cells were scraped into 100 μl chilled lysis buffer containing 50 mm Hepes, pH 7.4, 5 mm CHAPS and 5 mm DTT and subjected to three cycles of snap freezing–thawing on ice. The lysates were cleared by centrifugation at 12 000 g at 4°C for 15 min and relative caspase activity in the supernatants was determined by colourimetric assays using para-nitroanilin (pNA)-conjugated, specific caspase substrates (caspase-3, Asp-Glu-Val-Asp, Promega, Madison, WI, USA; caspase-8, Ile-Glu-Thr-Asp, Sigma, Deisenhofen, Germany; caspase-9, Leu-Glu-His-Asp, Biocat, Heidelberg, Germany) according to the recommendations of the manufacturers. Caspase activity was monitored spectrophotometrically by increase in A405 and normalized to the protein concentration in the lysates and expressed as nmol pNA released per mg of protein in 1 h.
Plasmid construction and transfection
For inhibition of tonicity-induced up-regulation of HSP27 and HSP70 in PI and MDCK cells, pcDNA3 (Invitrogen, Karlsruhe, Germany) based antisense constructs were prepared. Briefly, for down-regulation of HSP27, a full-length canine HSP27 fragment was excised from pBluescript SK+ (kindly provided by Dr E. Hickey, University of Nevada, NV, USA; Larsen et al. 1995) by EcoRI and inserted into the respective sites of pcDNA3. Constructs with antisense orientation were identified and used for transfection. Tonicty-induced up-regulation of HSP70 was attenuated using a construct containing 322 bp of 5′ end of the inducible HSP70 gene in antisense orientation as previously described (Neuhofer et al. 1998). PI and MDCK were transfected by the calcium-phosphate procedure (20 μg plasmid DNA per 100-mm dish) with subsequent isolation of stable clones by geneticin (G418; Invitrogen; 600 μg ml−1 for MDCK cells, 400 μg ml−1 for PI cells). At least two healthy clones with significant inhibition of NaCl-induced HSP27 or HSP70 expression, as assessed by Western blot analysis, were pooled, expanded and used for further experiments. Mock-transfected cells, i.e. cells transfected with empty vector, were established by transfection with pcDNA3 lacking the respective inserts.
Presentation of data and statistical analysis
Quantification of Northern and Western blots was performed using Image J software (NIH, Bethesda, MD, USA). Data are shown as means ± s.e.m. Significance of differences between means was assessed using Student's t test for unpaired samples. P < 0.05 was regarded as significant.
Results
Immunolocalization of HSP27 and HSP70 in rat papilla
Although the abundance of both HSP27 and HSP70 is much higher in homogenates obtained from the renal papilla than from cortex, it is conceivable that these chaperones are differently expressed in different papillary cells, here interstitial and collecting duct cells. To address this question, the intrapapillary expression of HSP27 and HSP70 was assessed by immunohistochemistry in normal rat kidney. As shown in Fig. 1, HSP27 was detectable in all cell types in the papilla. In contrast, HSP70 was detectable primarily in collecting duct cells, while there was only marginal interstitial immunoreactivity. Of note, the epithelium lining the papillary tip was positive for both HSP27 and HSP70 (not shown).
Figure 1. Expression of HSP27 and HSP70 in cross-sections in the inner medulla of rats with free access to water.
A, HSP70; B, negative control for HSP70; C, HSP27; D, negative control for HSP27. Bar represents 100 μm.
Expression of HSP27 and HSP70 in MDCK and PI cells by osmotic stress
To confirm the expression pattern observed by immunohistochemistry and to address the question whether HSP27 and HSP70 are regulated differentially in PCD and PI cells, the effect of osmotic stress on the expression of these HSPs was evaluated in MDCK and PI cells. MDCK cells display many characteristics of PCD cells and express osmosensitive genes in a fashion similar to that of primary cultures of PCD cells in response to changes in tonicity (Neuhofer et al. 2002a, b). PI cells are derived from primary cultures of papillary interstitial cells and are very similar to papillary interstitial cells in situ (Maric et al. 1996; Zhuo et al. 1998).
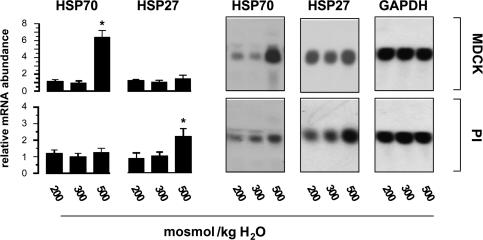
As shown in Fig. 2, osmotic stress induced distinct expression patterns of HSP27 and HSP70 in MDCK and PI cells. HSP70 mRNA was strongly induced in MDCK cells exposed to hypertonic stress (medium osmolality 500 mosmol (kg H2O)−1 by NaCl addition) for 24 h, whilst the expression of HSP27 was not affected substantially. Hypotonicity (medium osmolality 200 mosmol (kg H2O)−1) had no effect on HSP27 and HSP70 mRNA expression. In contrast to MDCK cells, hypertonic stress significantly enhanced HSP27 mRNA expression in PI cells, while HSP70 mRNA abundance was only elevated marginally.
Figure 2. Effect of osmotic stress on the abundance of HSP27 and HSP70 mRNA.
MDCK and PI cells were incubated for 24 h in hypotonic (200 mosmol (kg H2O)−1) or hypertonic medium (500 mosmol (kg H2O)−1 by NaCl addition), or remained in isotonic medium (300 mosmol (kg H2O)−1). Subsequently, the mRNA abundance of HSP27 and HSP70 was determined by Northern Blot analysis. To demonstrate equal RNA loading, the blots were also probed for GAPDH abundance. Data are means ± s.e.m. of 3 experiments. *P < 0.05 versus respective isotonic control.
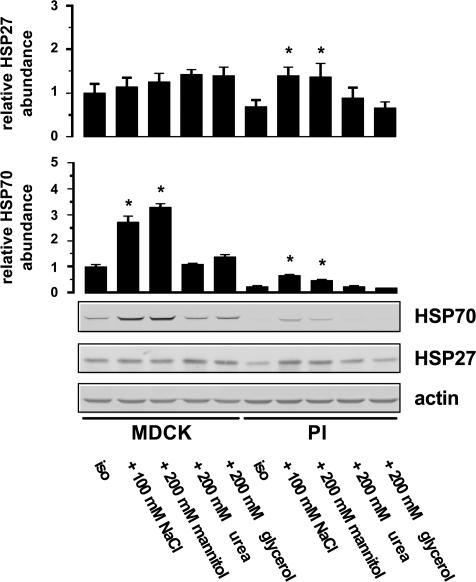
The results obtained at the mRNA level were confirmed and extended at the protein level. As demonstrated in Fig. 3, non-permeant solutes such as NaCl and mannitol, added to a final medium osmolality of 500 mosmol (kg H2O)−1, significantly induced HSP70 to comparable levels in MDCK cells. In contrast, permeant solutes like urea or glycerol, added in equiosmolar concentrations, failed to enhance HSP70 expression. In PI cells, HSP70 was close to the detection limit under isotonic conditions and was slightly, but significantly, induced in cells exposed to hypertonic stress. HSP27 was readily detectable in MDCK cells incubated in isotonic medium, but no further induction was evident after hypertonic stress. In contrast, in PI cells, HSP27 was detectable at low abundance under basal conditions and increased significantly by incubation in medium supplemented with impermeant solutes like NaCl and mannitol while the addition of urea or glycerol failed to enhance HSP27 expression.
Figure 3. Effect of osmotic stress on the abundance of HSP27 and HSP70.
MDCK and PI cells were incubated for 24 h with the indicated solutes, or maintained in isotonic medium. Subsequently, the expression of HSP27 and HSP70 was determined by Western blot analysis. To demonstrate equal protein loading, the blots were also probed with β-actin antiserum. Data are means ± s.e.m. for 3–4 separate experiments; *P < 0.05 versus respective isotonic control (iso).
Effect of hypertonic preconditioning on urea-induced caspase activation in MDCK and PI cells
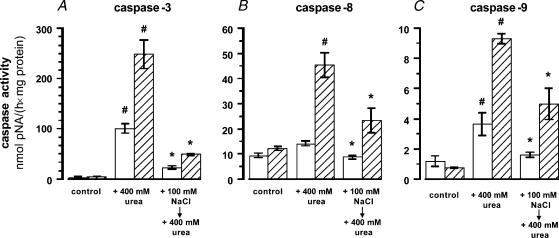
Since pretreatment with hypertonic NaCl protects against subsequent exposure to high urea concentrations (Neuhofer et al. 1998), the effect of hypertonic preconditioning on activation of caspases-3, -8, and -9 after urea exposure was assessed in MDCK and PI cells. As shown in Fig. 4, incubation in medium containing 400 mm urea for 24 h dramatically increased caspase-3 activity in both cell types (Fig. 4A), whilst the activity of the upstream caspases-8 and -9 were affected differently: no significant increase in caspase-8 activity was evident in MDCK cells, but a 4-fold increase was observed in PI cells (Fig. 4B). Caspase-9 activity was elevated 3-fold in MDCK cells and 12-fold in PI cells after urea exposure (Fig. 4C).
Figure 4. Effect of hypertonic pretreatment on urea-induced caspase activation.
MDCK (open bars) and PI (hatched bars) cells were left untreated (control), exposed to medium containing 400 mm urea for 24 h (+ 400 mm urea) or incubated in hypertonic medium (500 mosmol (kg H2O)−1 by NaCl addition) for 24 h prior to exposure to medium containing an additional 400 mm urea for 24 h. Means ± s.e.m. for 4–8 experiments; #P < 0.05 versus respective control; *P < 0.05 versus respective +400 mm urea.
Preexposure to medium made hypertonic by NaCl addition (+ 100 mm NaCl, final osmolality 500 mosmol (kg H2O)−1) for 24 h prior to adding an additional 400 mm urea for 24 h strongly attenuated urea-induced caspase-3 activation in both cell types (Fig. 4A), although the final medium osmolality was even higher under this condition (900 mosmol (kg H2O)−1). Urea-induced activation of caspase-8 was attenuated by 50% in NaCl-pretreated PI cells (Fig. 4B). Activation of caspase-9 by 400 mm urea was reduced by 50% in both MDCK and PI cells (Fig. 4C).
Effect of impaired HSP27 and HSP70 expression on urea-induced caspase-3 activation
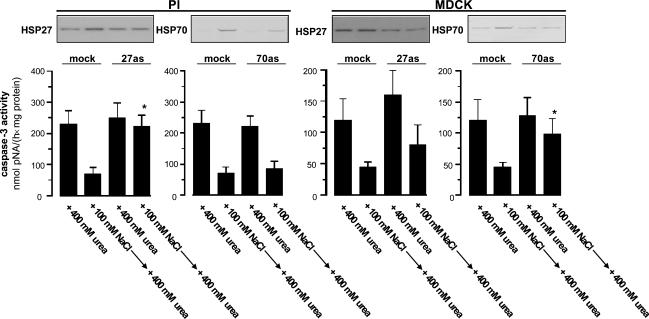
To address the physiological relevance of HSP27 and HSP70 in PI and MDCK cells, expression of these HSPs was attenuated in PI and MDCK cells by antisense transfection. Since HSP27 induction by hypertonicity in PI cells is associated with reduced caspase-3 activation after exposure to an additional 400 mm urea, tonicity-induced up-regulation of HSP27 was diminished by antisense transfection. In these cells, NaCl-induced HSP27 expression was reduced by at least 50% compared to mock-transfected cells (Fig. 5). If these cells were subsequently exposed to an additional 400 mm urea for 24 h, the protective effect of hypertonic pretreatment on caspase-3 activity was diminished. Since hypertonic pretreatment lead to a minor, but detectable up-regulation of HSP70, the contribution of this HSP to urea resistance was also assessed in PI cells transfected with a HSP70 antisense construct. Although HSP70 induction was substantially repressed in these cells, the protective effect of hypertonic pretreatment on caspase-3 activity after urea exposure was still evident (Fig. 5).
Figure 5. Effect of HSP27 and HSP70 antisense transfection on caspase-3 activation.
PI and MDCK cells were transfected with either HSP27 or HSP70 antisense constructs or empty vector (mock). Subsequently, the respective cells were exposed to medium containing 400 mm urea for 24 h (+400 mm urea) or pretreated with hypertonic medium (500 mosmol (kg H2O)−1 by NaCl addition) for 24 h prior to exposure to medium containing an additional 400 mm urea for 24 h. Subsequently, caspase-3 activity was assessed as described in Methods. Immunoblots demonstrating the expression of HSP27 and HSP70 prior to urea exposure are shown. Data are means ± s.e.m. for 4 experiments; *P < 0.05 versus respective mock transfected cells.
Despite the lack of induction by tonicity, HSP27 is abundant in MDCK cells (Fig. 3) and in PCD cells in situ (Fig. 1). Thus, this HSP may also be involved in urea resistance in MDCK cells. As demonstrated in Fig. 5, MDCK cells transfected with a HSP27 antisense construct had at least 50% reduced HSP27 levels and tended to show higher caspase-3 activity after urea exposure, either with or without hypertonic pretreatment compared to the respective mock transfected cells. This effect however, did not reach statistical significance. In MDCK cells transfected with a HSP70 antisense vector, urea-induced caspase-3 activity was significantly higher than in the respective mock-transfected cells and was associated with > 50% reduced HSP70 abundance.
Discussion
The adaptive processes conferring protection against high interstitial NaCl and urea concentrations involve both the accumulation of compatible organic osmolytes and the enhanced synthesis of HSPs. Although most studies on osmoadaptation have been performed on cell lines displaying characteristics of the inner medullary collecting duct or on primary cultures of the PCD, experiments on PI cells demonstrate that these cells also accumulate organic osmolytes in response to hypertonicity by mechanisms similar to PCD cells (Burger-Kentischer et al. 1999; Moeckel et al. 2003), thus preventing cell shrinkage and a rise in cellular ionic strength.
During the past few years it has become increasingly clear that the accumulation of organic osmolytes is only one component of the adaptive process allowing medullary cells to survive in the harsh environment of the renal papilla. While organic osmolytes protect against high salt concentrations, HSPs appear not to be involved in this process (Alfieri et al. 2004). However, HSP27 and HSP70 are expressed at high levels in the renal papilla and their abundance changes appropriately with the diuretic state (Medina et al. 1996; Müller et al. 1998), suggesting protective functions against the adverse effects of high solute concentrations as present in this kidney region during antidiuresis. The functional significance of HSP70 for cells of the collecting duct has been demonstrated recently, since forced expression of HSP70 protects MDCK cells against the detrimental effects of high urea concentrations as present in the renal papilla of the concentrating kidney (Neuhofer et al. 2001). This effect is at least partially attributable to the chaperoning activities of HSP70, since urea is a potent protein-destabilizing agent and HSP70 counteracts the urea-mediated decrease in the activity of several enzymes (Neuhofer & Beck 2005). Urea also induces apoptosis in IMCD3 cells (Colmont et al. 2001), while HSP70 prevents the execution of the apoptotic pathway by several mechanisms, including inhibition of Apaf-1 and cytochrome c release from mitochondria (Beere et al. 2000). In agreement, targeted disruption of the tonicity-inducible HSP70 gene in mice is associated with renal papillary apoptosis after stimulation of the renal concentrating mechanism (Shim et al. 2002). These results are in agreement with the present observations that demonstrate that inhibition of NaCl-induced HSP70 expression in MDCK cells is associated with higher caspase-3 activity after urea exposure compared to mock transfected cells.
The lack of HSP70 expression in interstitial cells in the renal papilla and in cultured PI cells after exposure to osmotic stress suggests that HSPs other than HSP70 may protect these cells against high urea concentrations. The present data demonstrate that HSP27 is induced significantly by hypertonic stress in PI cells, supporting the view that tonicity-induced HSP27 expression may be of relevance for protection of PI cells from high urea concentrations. This assumption is strengthened further by the observation that PI cells exposed to hypertonic medium for 24 h prior to exposure to medium containing an additional 400 mm urea displayed much less caspase-3 activity than non-preconditioned controls. These pretreated cells showed a significant increase in HSP27 abundance (Figs 2 and 3), while no osmolyte accumulation is detectable after this period (Steffgen et al. 2002), suggesting that organic osmolytes do not contribute significantly to the suppression of caspase-3 activation in PI cells preconditioned with hypertonic NaCl prior to ureaexposure. Indeed, attenuation of NaCl-induced HSP27 expression in PI cells diminished the protective effect of hypertonic pretreatment on caspase-3 activation. Because HSP27 possesses chaperone activity and promotes refolding of enzymes after thermal- and urea-induced denaturation (Jakob et al. 1993), HSP27 may oppose the destabilizing effects of high urea concentrations on intracellular proteins in PI cells. In addition, HSP27 inhibits apoptosis by preventing apoptosome formation and by sequestering pro-caspase-3 (Concannon et al. 2001). These observations are compatible with the view that NaCl-induced up-regulation of HSP27 expression attenuates the urea-mediated induction of apoptosis in PI cells.
In summary, HSP27 and HSP70 are expressed differentially in MDCK and PI cells and in PCD and interstitial cells in the papilla in situ. While HSP27 was induced significantly by tonicity stress in PI, but not in MDCK cells, HSP70 up-regulation was much more pronounced in MDCK cells. Tonicity-induced increases in the abundance of HSP70 in MDCK cells and HSP27 in PI cells was associated with reduced caspase activation after exposure to high urea concentrations, indicating that the mechanisms conferring urea resistance may be different in these cells.
Acknowledgments
We are indebted to Dr Christine Maric and Dr Frederick A. O. Mendelsohn (University of Melbourne, Australia) for providing the PI cells. The present work was supported by grants from the Deutsche Forschungsgemeinschaft and the German Kidney Foundation to W.N. We thank Dr J. Davis for critical reading of the manuscript.
References
- Alfieri RR, Petronini PG, Bonelli MA, Desenzani S, Cavazzoni A, Borghetti AF, Wheeler KP. Roles of compatible osmolytes and heat shock protein 70 in the induction of tolerance to stresses in porcine endothelial cells. J Physiol. 2004;555:757–767. doi: 10.1113/jphysiol.2003.058412. 10.1113/jphysiol.2003.058412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beere HM, Wolf BB, Cain K, Mosser DD, Mahboubi A, Kuwana T, Tailor P, Morimoto RI, Cohen GM, Green DR. Heat-shock protein 70 inhibits apoptosis by preventing recruitment of procaspase-9 to the Apaf-1 apoptosome. Nature Cell Biol. 2000;2:469–475. doi: 10.1038/35019501. 10.1038/35019501. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burger-Kentischer A, Müller E, März J, Fraek ML, Thurau K, Beck FX. Hypertonicity-induced accumulation of organic osmolytes in papillary interstitial cells. Kidney Int. 1999;55:1417–1425. doi: 10.1046/j.1523-1755.1999.00382.x. [DOI] [PubMed] [Google Scholar]
- Colmont C, Michelet S, Guivarc'h D, Rousselet G. Urea sensitizes mIMCD3 cells to heat shock-induced apoptosis: protection by NaCl. Am J Physiol Cell Physiol. 2001;280:C614–C620. doi: 10.1152/ajpcell.2001.280.3.C614. [DOI] [PubMed] [Google Scholar]
- Concannon CG, Orrenius S, Samali A. Hsp27 inhibits cytochrome c-mediated caspase activation by sequestering both pro-caspase-3 and cytochrome c. Gene Expression. 2001;9:195–201. doi: 10.3727/000000001783992605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J Biol Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Larsen JK, Gerthoffer WT, Hickey E, Weber LA. Cloning and sequencing of a cDNA encoding the canine HSP27 protein. Gene. 1995;19:305–606. doi: 10.1016/0378-1119(95)00290-m. 10.1016/0378-1119(95)00290-M. [DOI] [PubMed] [Google Scholar]
- Larsen JK, Gerthoffer WT, Hickey E, Weber LA. Cloning and sequencing of a cDNA encoding the canine HSP27 protein. Gene. 1996;161:305–306. doi: 10.1016/0378-1119(95)00290-m. 10.1016/0378-1119(95)00290-M. [DOI] [PubMed] [Google Scholar]
- Maric C, Aldred GP, Antoine AM, Dean RG, Eitle E, Mendelsohn FA, Williams DA, Harris PJ, Alcorn D. Effects of angiotensin II on cultured rat renomedullary interstitial cells are mediated by AT1A receptors. Am J Physiol. 1996;271:F1020–F1080. doi: 10.1152/ajprenal.1996.271.5.F1020. [DOI] [PubMed] [Google Scholar]
- McLean IW, Nakane PK. Periodate-lysine-paraformaldehyde fixative: a new fixative for immunoelectron microscopy. J Histochem Cytochem. 1974;22:1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Medina R, Cantley L, Spokes K, Epstein FH. Effect of water diuresis and water restriction on expression of HSPs-27-60 and 70 in rat kidney. Kidney Int. 1996;50:1191–1194. doi: 10.1038/ki.1996.427. [DOI] [PubMed] [Google Scholar]
- Moeckel GW, Zhang L, Fogo AB, Hao CM, Pozzi A, Breyer MD. COX2 activity promotes organic osmolyte accumulation and adaptation of renal medullary interstitial cells to hypertonic stress. J Biol Chem. 2003;278:19352–19357. doi: 10.1074/jbc.M302209200. 10.1074/jbc.M302209200. [DOI] [PubMed] [Google Scholar]
- Müller E, Neuhofer W, Burger-Kentischer A, Ohno A, Thurau K, Beck FX. Effects of long-term changes in medullary osmolality on heat shock proteins HSP25, HSP60, HSP72 and HSP73 in the rat kidney. Pflugers Arch. 1998;435:705–712. doi: 10.1007/s004240050572. 10.1007/s004240050572. [DOI] [PubMed] [Google Scholar]
- Müller E, Neuhofer W, Ohno A, Rucker S, Thurau K, Beck FX. Heat shock proteins HSP25, HSP60, HSP72, HSP73 in isoosmotic cortex and hyperosmotic medulla of rat kidney. Pflugers Arch. 1996;431:608–617. doi: 10.1007/BF02191910. 10.1007/s004240050042. [DOI] [PubMed] [Google Scholar]
- Neuhofer W, Bartels H, Fraek ML, Beck FX. Relationship between intracellular ionic strength and expression of tonicity-responsive genes in rat papillary collecting duct cells. J Physiol. 2002a;543:147–153. doi: 10.1113/jphysiol.2002.021931. 10.1113/jphysiol.2002.021931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuhofer W, Beck FX. Cell survival in the hostile environment of the renal medulla. Annu Rev Physiol. 2005;67:531–555. doi: 10.1146/annurev.physiol.67.031103.154456. 10.1146/annurev.physiol.67.031103.154456. [DOI] [PubMed] [Google Scholar]
- Neuhofer W, Lugmayr K, Fraek ML, Beck FX. Regulated overexpression of heat shock protein 72 protects Madin-Darby canine kidney cells from the detrimental effects of high urea concentrations. J Am Soc Nephrol. 2001;12:2565–2571. doi: 10.1681/ASN.V12122565. [DOI] [PubMed] [Google Scholar]
- Neuhofer W, Müller E, Burger-Kentischer A, Fraek ML, Thurau K, Beck FX. Pretreatment with hypertonic NaCl protects MDCK cells against high urea concentrations. Pflugers Arch. 1998;435:407–414. doi: 10.1007/s004240050531. 10.1007/s004240050531. [DOI] [PubMed] [Google Scholar]
- Neuhofer W, Woo SK, Na KY, Grünbein R, Park WK, Nahm O, Beck FX, Kwon HM. Regulation of TonEBP transcriptional activator in MDCK cells following changes in ambient tonicity. Am J Physiol. 2002b;283:C1604–C1611. doi: 10.1152/ajpcell.00216.2002. [DOI] [PubMed] [Google Scholar]
- Shim EH, Kim JI, Bang ES, Heo JS, Lee JS, Kim EY, Lee JE, Park WY, Kim SH, Kim HS, Smithies O, Jang JJ, Jin DI, Seo JS. Targeted disruption of hsp70.1 sensitizes to osmotic stress. EMBO Reports. 2002;3:857–861. doi: 10.1093/embo-reports/kvf175. 10.1093/embo-reports/kvf175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steffgen J, Kampfer K, Grupp C, Langenberg C, Muller GA, Grunewald RW. Osmoregulation of aldose reductase and sorbitol dehydrogenase in cultivated interstitial cells of rat renal inner medulla. Nephrol Dial Transplant. 2002;18:2255–2261. doi: 10.1093/ndt/gfg397. 10.1093/ndt/gfg397. [DOI] [PubMed] [Google Scholar]
- Woo SK, Lee SD, Kwon HM. TonEBP transcriptional activator in the cellular response to increased osmolality. Pflugers Arch. 2002;444:579–585. doi: 10.1007/s00424-002-0849-2. 10.1007/s00424-002-0849-2. [DOI] [PubMed] [Google Scholar]
- Wu B, Hunt C, Morimoto R. Structure and expression of the human gene encoding major heat shock protein HSP70. Mol Cellular Biol. 1985;5:330–341. doi: 10.1128/mcb.5.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo J, Dean R, Maric C, Aldred PG, Harris P, Alcorn D, Mendelsohn FA. Localization and interactions of vasoactive peptide receptors in renomedullary interstitial cells of the kidney. Kidney Int. 1998;67:S22–S28. doi: 10.1046/j.1523-1755.1998.06705.x. 10.1046/j.1523-1755.1998.06705.x. [DOI] [PubMed] [Google Scholar]