Abstract
Autoregulation is a vital protective mechanism that maintains stable cerebral blood flow as cerebral perfusion pressure changes. We contrasted cerebral autoregulation across sleep–wake states, as little is known about its effectiveness during sleep. Newborn lambs (n = 9) were instrumented to measure cerebral blood flow (flow probe on the superior sagittal sinus) and cerebral perfusion pressure, then studied during active sleep (AS), quiet sleep (QS) and quiet wakefulness (QW). We generated cerebral autoregulation curves by inflating an occluder cuff around the brachiocephalic artery thereby lowering cerebral perfusion pressure. Baseline cerebral blood flow was higher (P < 0.05) and cerebral vascular resistance lower (P < 0.05) in AS than in QW (76 ± 8% and 133 ± 15%, respectively, of the AS value, mean ± s.d.) and in QS (66 ± 11% and 158 ± 30%). The autoregulation curve in AS differed from that in QS and QW in three key respects: firstly, the plateau was elevated relative to QS and QW (P < 0.05); secondly, the lower limit of the curve (breakpoint) was higher (P < 0.05) in AS (50 mmHg) than QS (45 mmHg); and thirdly, the slope of the descending limb below the breakpoint was greater (P < 0.05) in AS than QS (56% of AS) or QW (56% of AS). Although autoregulation functions in AS, the higher breakpoint and greater slope of the descending limb may place the brain at risk for vascular compromise should hypotension occur.
Autoregulation of the cerebral circulation maintains cerebral blood flow (CBF) stable in the face of changes in cerebral perfusion pressure (CPP) by adjusting cerebral vascular resistance (CVR) (Paulson et al. 1990; Chillon & Baumbach, 2002). As a vital protective mechanism, autoregulation functions over a range of arterial pressures from approximately 60–150 mmHg, and helps to protect the brain from ischaemic hypoxia during hypotension and from haemorrhage and oedema during hypertension (Paulson et al. 1990; Szymonowicz et al. 1990; Chillon & Baumbach, 2002). Although the interaction between behavioural state and CBF has been studied extensively (Franzini, 1992, 2000), little is known about autoregulation of the cerebral circulation during sleep. Changes in behavioural state are accompanied by dramatic changes in CBF. Moreover, there is substantial variation of arterial pressure between and within sleep–wake states. For example, the transition into REM sleep, or active sleep (AS) as it is known in infancy, is accompanied by a large increase in CBF (Cianci et al. 1991; Franzini, 1992; Madsen, 1993; Grant et al. 1995b; Zoccoli et al. 1994; Grant et al. 1998). This increase is due to vasodilatation (Grant et al. 1995b, 1998) as arterial pressure is maintained or decreases slightly (Franzini, 1992, 2000). Although autoregulation continues to function in each sleep state, the vasodilatation of AS limits reserves for further autoregulatory vasodilatation (Grant et al. 1998). The time course of autoregulation is also slower in AS than in quiet sleep (QS) or quiet wakefulness (QW) (Grant et al. 1998). Such reduced vasodilatory reserves coupled with a slower vasodilatory response to hypotension suggest that the cerebral circulation may be particularly vulnerable to hypotension during AS. As a consequence, AS is a specific state of risk (Parmeggiani, 1991). Moreover, between 10 and 40% of strokes occur during the night (Marshall, 1977; van der Windt & van Gijn, 1988; Chamorro et al. 1998; Basetti & Aldrich, 1999), and a low blood pressure may contribute to night-time stroke (Basetti & Aldrich, 1999).
This study was designed to assess the cerebral circulation's ability to respond to hypotension during sleep. By contrasting the plateau and lower breakpoint of the autoregulation curve in sleep and awake states, we assessed the effectiveness of the cerebral circulation in maintaining blood flow during brief periods of induced hypotension.
Methods
Nine newborn lambs (Merino/Border–Leicester cross) were separated from their ewes within 24–48 h of birth and taught to feed independently from a nipple connected to a lamb milk replacer (Veanavite Pty. Ltd, Shepparton, Australia), each gaining weight at a normal rate (0.26 ± 0.04 kg day−1, mean ± s.d.). Each lamb was prepared for a series of sleep studies. All surgical and experimental procedures were performed in accordance with the guidelines of the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes established by the National Health and Medical Research Council of Australia, and were approved by the Monash Medical Centre's Committee on Ethics in Animal Experimentation.
Surgical preparation and instrumentation
Each lamb was anaesthetized (1.5% halothane, 50% O2, balance N2O), intubated, ventilated and instrumented to record CBF (Grant et al. 1995b; Grant et al. 1998). In brief, a section of the skull overlying the intersection of the lambdoid and sagittal sutures was removed and a transit-time ultrasonic flow probe (2 mm diameter, Transonic Systems Inc., Ithaca, NY, USA) was positioned to surround the superior sagittal sinus. Dental acrylic was used to stabilize the probe and to replace the section of skull that had been removed. This technique provides a quantitative and beat-by-beat measurement of CBF as validated for use on the sagittal sinus of the lamb (Grant et al. 1995b).
We also implanted a saline-filled catheter non-occlusively (0.86 mm i.d., 1.52 mm o.d.) in the carotid artery from which we recorded blood pressure (carotid artery pressure (CAP)) and sampled blood. A saline-filled catheter (1.57 mm i.d., 2.41 mm o.d.) was positioned under the dura to record intracranial pressure (ICP). Behavioural states (QW, QS, and AS), were determined using a video image, and upon recordings from electrodes implanted on the parietal cortex (electro-corticogram, (ECOG)), at the inner and outer canthus of the left eye (electrooculogram, (EOG)), and in the dorsal musculature of the neck (nuchal electromyogram, (EMGn)) (Grant et al. 1995a).
Finally, we positioned an inflatable silicon-rubber occluder cuff around the common brachiocephalic artery through a left thoracotomy at the second intercostal space. By inflating this cuff we reduced distal arterial pressure and cerebral perfusion pressure (CPP, CPP = CAP − ICP). By adjusting the inflation of the cuff we induced a range of stable arterial pressures.
Conditions of study
Each lamb was allowed a minimum of 72 h to recover from surgery and then studied over 2–3 days (average age 16 ± 5 days, 7.8 ± 1.1 kg). During the studies the cage was partitioned to prevent the lamb from turning, while allowing it freedom to move forward and backward and to stand up and lie down. Food was available throughout the study and room temperature was maintained at 23–25°C.
We connected the flow probe to a meter (Model T101 Ultrasonic Blood Flow Meter, Transonic Systems Inc., Ithaca, NY) that, along with the electrodes, was connected to a signal conditioner (Cyberamp 380, Axon Instruments, Inc., Union City, CA, USA). Electro-physiological signals were filtered with the signal conditioner (0.3–80 Hz, 0.3–80 Hz, 30–80 Hz, for ECOG, EOG, EMGn, respectively). Vascular and intracranial catheters were connected to calibrated strain-gauge manometers (Transpac IV, Disposable Transducer, Abbott Critical Care Systems, Abbott Ireland, Sligo, Republic of Ireland) and referenced to the mid-thoracic level. The pressure and flow signals were low-pass filtered at 100 Hz prior to being recorded on a thermal chart recorder (Model 7758 A, Hewlett Packard, Waltham, MA, USA) and stored on computer at a sampling rate of 200 Hz (CVSOFT Data Acquisition and Analysis Software, Odessa Computer Systems, Ltd, Calgary, Canada).
Protocol
Data were recorded during uninterrupted, well-defined epochs of QW, QS, and AS. QW was defined as periods where the lamb was lying down, when the ECOG displayed a pattern of low-voltage and high-frequency activity, and eye movements and EMGn tone were present. In QS the ECOG displayed high-voltage and low-frequency activity and eye movements were absent (Fig. 1). During AS the ECOG displayed low-voltage and high-frequency activity, rapid eye movements were present while EMGn tone was absent. Control data were recorded for a 30-s period (control) immediately prior to lowering CPP for 60 s (hypotension) by inflating the silicon-rubber cuff around the brachiocephalic artery. Inflations were repeated over 2–3 days in order to induce a wide range of CPP in each behavioural state. A total of 255 inflations were recorded (80, 92, and 83 in AS, QS and QW, respectively), with all states usually being evaluated on any given day. Inflations (number day−1, mean ± s.d.), were 4 ± 2 in AS, 5 ± 3 in QS, and 4 ± 2 in QW. Inflations per animal were (mean ± s.d.) 9 ± 3 in AS, 10 ± 3 in QS and 9 ± 3 in QW. The lambs tolerated hypotension without distress, showed no lasting effects, and grew and behaved normally throughout the study. All lambs were killed at the end of the experiments using a lethal dose of sodium pentobarbitone (150 mg kg−1 intravenously).
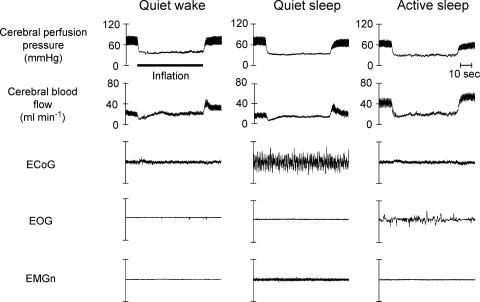
Figure 1. Physiological recordings during induced hypotension in sleep.
Physiological recording from a single lamb collected before, during (inflation) and after decreasing cerebral perfusion pressure by inflating the brachiocephalic cuff in each of the three behavioural states studied. During the control periods, cerebral perfusion pressure is equal in each state yet cerebral blood flow is higher in active sleep than it is in quiet wakefulness, which in turn is higher than it is in quiet sleep. During the inflation of the brachiocephalic occluder cuff, cerebral perfusion pressure was lowered rapidly to approximately the same level (40 mmHg) in each state. In both QW and QS, the effects of autoregulation are rapidly evident as cerebral blood flow begins to recover towards the control level, a recovery that is underpinned by cerebral vasodilatation. The recovery towards control levels of cerebral blood flow is more rapid in QS than it is in QW. Moreover, both the magnitude and the speed of this recovery are substantially less in AS than in either of the other states. EMGn = electromyogram of the nuchal (neck) muscles, EOG = electrooculogram, ECoG = electrocorticogram.
Data analysis/statistics
Data were averaged over the last 5 s of the control period that preceded cuff inflation as control values. As the time required to attain maximal cerebral vasodilatation in response to hypotension is longer in AS (35 s) that in QS (20 s) or QW (27 s) (Grant et al. 1998), hypotension data were determined as a 5 s average of data recorded after a minimum of 50 s, i.e. at the end of the inflation period. CVR was calculated from the 5 s averages of cerebral perfusion pressure and cerebral blood flow (CVR = CPP/CBF). For each lamb, CBF was normalized as a percentage of the average recorded during the control period for that state. Data from each lamb were pooled for each behavioural state (Fig. 3) and then averaged in 1 mmHg increments of CPP. The averaged data for CBF were plotted as a function of CPP for each behavioural state. A bilinear regression was fitted to these data (Wilkinson et al. 1995). Two regression lines were fitted to the data on either side of a nominated ‘breakpoint value’ (CPPbr) of CPP. CPPbr was adjusted iteratively and the regression calculation repeated. CPPbr was determined as the CPP that corresponded to the minimal residual sum of squared deviations from the bilinear prediction (Orr et al. 1982; Szymonowicz et al. 1990; Wilkinson et al. 1995; Grant et al. 2001). Finally, to determine how the relative position of the plateau and descending limb of the autoregulation curve were altered by sleep–wake state, average autoregulation curves were generated when CBF was normalized as a percentage of the control values derived in QS.
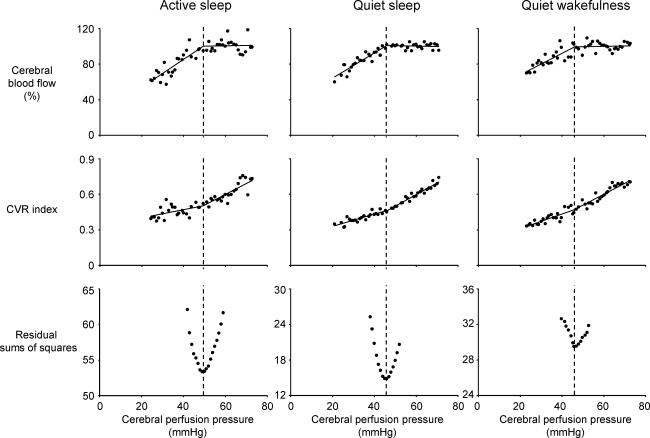
Figure 3. Average autoregulation curves derived for each behavioural state.
Bilinear regression analysis was used to determine the breakpoint position of each autoregulation curve. The breakpoint pressure was identified as the point on the pressure axis at which the combined sum of the residuals from the two least square regressions (residual sums of squares) was minimal (lower panel). Note that a plateau phase in each autoregulation curve (upper panels) was underpinned by cerebral vasodilatation (middle panel) in response to lowering of cerebral perfusion pressure. Also note that the breakpoint in cerebral blood flow coincides with a breakpoint in the cerebral vascular resistance (CVR) values.
We used analysis of variance for repeated measures to compare the average CBF, CPP, and CVR recorded from each lamb in the control periods. A Student–Newman–Keuls test was used to isolate differences that were detected by the analysis of variance. Comparison between sleep–wake states of breakpoint and slope of the descending limb of autoregulation curves was performed using a procedure for comparison of slope, intercept and identity of regression lines (Glantz, 2002). A probability (P) of ≤0.05 was considered significant. Data are presented as mean ± s.d.
Results
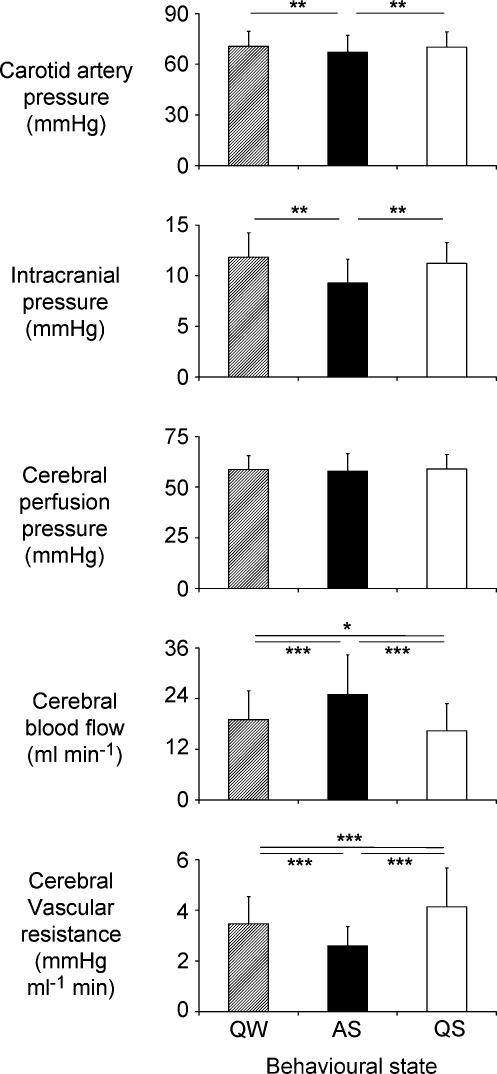
No significant differences were observed in blood gas and pH data among behavioural states (Table 1). During the control period, CPP was equal in all states. Nevertheless, CBF and CVR differed according to behavioural state under control conditions (Figs 1 and 2, Table 1). In AS, control values of CBF were greater (P≤ 0.001) and CVR (P≤ 0.001) less than in both QS and QW. Control values of CBF were also higher (P≤ 0.05) and CVR lower (P≤ 0.05) in QW than in QS.
Table 1. Behavioural state effects upon carotid artery pressure (CAP), intracranial pressure (ICP), cerebral blood flow (CBF), cerebral perfusion pressure (CPP), and cerebral vascular resistance (CVR) during control states.
| Active sleep | Quiet sleep | Quiet wakefulness | ||||
|---|---|---|---|---|---|---|
| CAP (mmHg) | 67 ± 10 | 70 ± 9 | 71 ± 9 | |||
| ICP (mmHg) | 9 ± 2 | 11 ± 2 | 12 ± 2 | |||
| CBF (ml min−1) | 25.0 ± 9.4 | 16.4 ± 6.4* | 18.9 ± 7.0*† | |||
| CPP (mmHg) | 59 ± 9 | 59 ± 7 | 59 ± 6 | |||
| CVR (mmHg ml−1.min) | 2.6 ± 0.8 | 4.1 ± 1.4* | 3.5 ± 1.0*† | |||
| SaO2 (%) | 95 ± 2 | 96 ± 2 | 95 ± 2 | |||
| Hb (g dl−1) | 7.7 ± 0.7 | 7.7 ± 0.8 | 7.9 ± 0.6 | |||
| PaO2 (mmHg) | 96 ± 10 | 101 ± 7 | 102 ± 7 | |||
| PaCO2 (mmHg) | 40 ± 2 | 41 ± 2 | 41 ± 2 | |||
| pH | 7.435 ± 0.023 | 7.432 ± 0.034 | 7.426 ± 0.027 | |||
| HCO3− (mmol l−1) | 26 ± 2 | 26 ± 1 | 26 ± 1 | |||
| BE (mmol l−1) | 3 ± 2 | 3 ± 2 | 2 ± 2 | |||
Values represent mean ± s.d. CPP = CAP − ICP; CVR = CPP/CBF; BE, base excess (n = 7 for blood gas data, n = 9 for all other data).
P < 0.001 versus active sleep
P < 0.05 versus quiet sleep.
Figure 2. Average data recorded during the control period in quiet wakefulness (QW), active sleep (AS) and quiet sleep (QS).
Although carotid artery pressure and intracranial pressure were lower in AS than in QW and QS, cerebral perfusion pressure did not differ between the three behavioural states. Nevertheless, cerebral blood flow was greater in AS than in either QS or QW, and greater in QW than in QS. Also, cerebral vascular resistance was lower in AS than in either QS or QW and lower in QW than in QS. Values = mean ± s.d., n = 9. Bars indicate significant differences: *P < 0.05; **P < 0.01; ***P < 0.001.
Brachiocephalic occlusions induced a rapid decrease in CPP that remained at a constant level throughout the inflation period (Fig. 1). CBF initially fell rapidly with the onset of hypotension, then began to return towards control values as a result of the underlying autoregulation process. The ability of autoregulation to limit the decrease in CBF in response to lowering CPP, was limited in AS, more complete in QS and complete in QW.
Data derived from the control and inflation epochs were utilized to generate autoregulation curves (Fig. 3). An improved fit of the data was attained using bilinear regression versus a single regression (F1,43= 26.1 P < 0.01, F1,45= 80.0 P < 0.01, F1,46= 27.6 P < 0.01, for AS, QS and QW, respectively). The breakpoint of the autoregulation curve was higher in AS (50 mmHg) than in QS (45 mmHg, P < 0.05). The breakpoint in QW (46 mmHg) was similar to that in QS.
The average autoregulation curves (normalized to the control values in QS, the most stable sleep–wake state) differed in AS, QS and QW (Fig. 4). The sleep state-dependent difference in CBF observed during the control period was maintained over the entire range of CPP, i.e. during both the plateau phase and the descending limb. In the plateau phase of the autoregulation curves, CBF was greater in AS than in QW and in QS. In addition, the slope of the descending limb (ml min−1 mmHg−1) was greater (P < 0.01) in AS (y= 2.5x+ 34, R2= 0.74) than in QS (y= 1.4x+ 36, R2= 0.84) or QW (y= 1.4x+ 52, R2= 0.66).
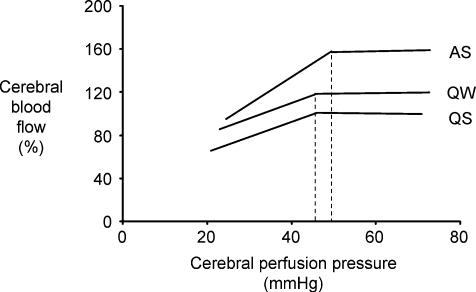
Figure 4. Average autoregulation curves generated from the nine lambs studied in each of the behavioural states.
Data were normalized to the control value recorded for each lamb in quiet sleep and replotted to reveal the relationship that exists between sleep state and autoregulation. Note that the sleep state-dependent differences in cerebral blood flow are maintained across all levels of cerebral perfusion pressure. Note also that cerebral blood flow in active sleep (AS) exceeds that in quiet wakefulness (QW) which in turn exceeds that in quiet sleep (QS). Moreover, the position of the breakpoint of the autoregulation curve is shifted to the right in AS (50 mmHg) relative to that in QW (46 mmHg) and QS (45 mmHg). As a result of the higher cerebral blood flow in AS, the slope of the descending limb of the autoregulation curve in AS (y= 2.5x+ 34) is also steeper than in QS (y= 1.4x+ 36) or QW (y= 1.4x+ 52). Both these characteristics (higher breakpoint and greater slope) may place the brain in danger of ischaemia and hypoxia should hypotension occur during AS.
Discussion
We identified major differences in the form of the cerebral autoregulation curve during active sleep (AS) that may place the brain at greater risk of ischaemia than in other sleep–wake states. Both the speed and the magnitude of the vasoactive response to hypotension are diminished in AS compared with quiet sleep (QS) and quiet wakefulness (QW, Fig. 1) (Grant et al. 1998). Our new observations indicate that the plateau and descending limbs of the autoregulation curves of AS, QS and QW also differ in three important ways. First, the autoregulation curve of AS is shifted upward relative to that in QS and QW, and the autoregulation curve of QW is shifted upward relative to that in QS (i.e. CBF at any given CPP is higher in AS than in QW and higher in QW than in QS). Thus, the behavioural state-specific differences that exist in CBF are maintained throughout the plateau phase (Franzini, 1992; Grant et al. 1995b, 1998). Moreover, these differences are maintained in the descending limb of the curve. The second behavioural state-related difference is that the lower breakpoint of the autoregulation curve appears first in AS. This earlier breakpoint in AS, in conjunction with the limitations to the magnitude and rate of autoregulation (Grant et al. 1998) places the brain in a position of vulnerability to hypotension in this state. Finally, the autoregulation curves also differ in the descending limb, with the slope being greatest in AS. Should hypotension occur during AS, a situation that has been described to occur in specific subpopulations of patients with obstructive sleep apnoea (McGinty et al. 1988), not only will autoregulation fail at a higher CPP but once it fails CBF will subsequently fall at a greater rate than in QS or QW.
Autoregulation in the face of decreasing CPP is dependent upon the ability of the cerebral circulation to vasodilate (Fig. 3 and Chillon & Baumbach, 2002). The high level of neural activity in AS requires a high basal level of CBF (Lenzi et al. 1999) underpinned by a substantial vasodilatation (Grant et al. 1995b, 1998). The present results indicate that in AS, although widely vasodilated, the cerebral circulation maintains further autoregulatory reserves as demonstrated by the plateau phase in the autoregulation curve. The mechanisms that function to determine sleep state-dependent CBF differ from those involved in the autoregulatory response to hypotension. This is suggested as the sleep state-dependent differences in CBF are maintained at CPP below the breakpoint of QS, and thus well below the breakpoint in AS. At these low pressures CBF remained higher in AS than in QS, indicating that there are vasodilatory reserves available in AS that cannot be accessed in QS.
The wide vasodilatation of cerebral vessels in AS (evidenced by the low CVR) appears to limit the vasodilatory reserve of this state, and might also shorten the autoregulatory plateau. Although increases in CO2 are also known to increase CBF and to narrow the plateau phase of the autoregulation curve (Harper, 1966; Raichle & Stone, 1971; Paulson et al. 1990; Panerai et al. 1999), arterial blood gas and pH analysis failed to reveal any state-dependent differences. Nevertheless, it remains possible that local increases in tissue CO2 or decreases in tissue pH might have contributed to the higher breakpoint observed in the autoregulation curve during AS.
The lamb has active and quiet sleep phases comparable to those of the human infant (Baker & Fewell, 1988; Horne et al. 1991; Grant et al. 1995a; Johnston et al. 1998) and has proven of value in the study of the cerebral circulation (Purves & James, 1969; Papile et al. 1985; Szymonowicz et al. 1990) and the cerebral circulation in sleep (Grant et al. 1998; Zoccoli et al. 2001). Like humans and most other species (Greenberg, 1980) the lamb exhibits a stereotyped increase of blood flow during AS and a decrease in QS (Madsen & Vorstrup, 1991; Franzini, 1992). In addition, the presence of a common brachiocephalic artery allowed us to induce cerebral hypotension while avoiding the complications of decreased haemoglobin (Hb) concentrations and decreased O2 content associated with previous studies that have relied upon haemorrhagic hypotension (Szymonowicz et al. 1990).
Our approach has potential limitations as data were pooled across animals and experimental days in order to collect the wide range of data points needed to generate autoregulation curves, and to identify breakpoints, in each of the states of sleep and wakefulness. When pooling data it is important that sampling be uniform to avoid bias from day-to-day variations and ensure valid comparisons between experimental conditions. These potential problems were avoided by collecting data over a time period that was sufficiently short (2–3 days) to ensure that no major developmental changes in cardiovascular or sleep physiology occurred (Horne et al. 1991; Grant et al. 1995a; Johnston et al. 1998). We also ensured that the number of tests was similar between animals and sleep–wake states so as to avoid bias from highly sampled animals.
No previous study has examined the autoregulation curve in sleep, possibly because of the technical difficulties of recording CBF. By continuously recording the blood flow in the superior sagittal sinus, our technique provides a quantitative measurement of cerebral blood flow that is linearly related to arterial inflow (Grant et al. 1995b). Although this measurement is continuous and responds rapidly (within one heart beat) to variations in cerebral perfusion pressure, the processes that underlie autoregulation are less rapid (Florence & Seylaz, 1992; Grant et al. 1998). To ensure that the maximal vasodilatory response induced by the hypotension had been attained in each sleep–wake state, we only collected data at the end of the 60 s hypotensive periods.
Previous studies in newborn lambs indicate that the lower breakpoint of the autoregulation curve exists somewhere over the range of pressures between 40 and 50 mmHg (Purves & James, 1969; Laptook et al. 1983; Papile et al. 1985; Szymonowicz et al. 1990). In the newborn lamb, the resting level of mean arterial blood pressure consistently sits 30–40 mmHg above the breakpoint (Papile et al. 1985), and thus provides a substantial buffer that preserves CBF during periods of hypotension. Papile et al. (1985) also demonstrated that the lower breakpoint of the autoregulation curve in preterm fetal lambs resides over the same range that has been described for newborn lambs, indicating that the lower breakpoint does not vary with gestational age. However, in the preterm lamb the resting level of mean arterial blood pressure lies only 10 mmHg above the autoregulation breakpoint, suggesting that the preterm brain is in a position of greater vulnerability to hypotension that that of the newborn lamb (Szymonowicz et al. 1990). Although conducted in chronically instrumented fetal lamb models, these early studies did not control for behavioural state. Our observation of a breakpoint in QW of 46 mmHg is in accord with the values of previous studies. However, our observations differ in that we have identified an elevated breakpoint in AS. This, coupled with the tendency for mean arterial pressure to decrease in AS, places the newborn lamb as close as, or closer than, the preterm fetus is to the lower breakpoint of the autoregulation curve. Moreover, the steeper slope of the descending limb of the autoregulation curve in AS compared to QS and QW may further compromise the brain's ability to autoregulate its blood supply in the face of hypotension. Thus, the newborn brain in AS, may not be as well protected from fluctuations in blood pressure as previously thought from studies of awake lambs.
In summary, determination of the form of the cerebral autoregulation curve during hypotension has revealed major differences during AS compared with QS and QW. Notably, the point at which cerebral blood flow begins to fall as cerebral perfusion pressure is reduced (breakpoint) appears at a higher cerebral perfusion pressure in AS than QS. Moreover, the fall of cerebral blood flow below the breakpoint is steepest in AS. These relative deficiencies of autoregulation during AS, along with its reduced vasodilatory reserves and slower vasodilatation, place the brain at greater risk of ischaemia should hypotension occur in this sleep state.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia (D.A.G.) and (A.M.W.), Monash Research Foundation for Mothers and Babies (D.A.G.), and the Windermere Foundation (D.A.G., A.M.W., and C.F). We express our gratitude to Mr V. Brodecky for his technical assistance.
References
- Baker SB, Fewell JE. Heart rate response to arousal and lung inflation following upper airway obstruction in lambs. Sleep. 1988;11:233–241. doi: 10.1093/sleep/11.3.233. [DOI] [PubMed] [Google Scholar]
- Basetti C, Aldrich M. Night time versus day time transient ischaemic attack and ischaemic stroke: a prospective study of 110 patients. J Neurol Neurosurg Psychiatry. 1999;67:463–467. doi: 10.1136/jnnp.67.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chamorro A, Vila N, Ascaso C, Elices E, Schonewille W, Blanc R. Blood pressure and functional recovery in acute ischemic stroke. Stroke. 1998;29:1850–1853. doi: 10.1161/01.str.29.9.1850. [DOI] [PubMed] [Google Scholar]
- Chillon J-M, Baumbach GL. Autoregulation: arterial and intracranial pressure. In: Edvinsson L, Krause DN, editors. Cerebral Blood Flow and Metabolism. Philadelphia: Lippincott. Williams & Wilkins; 2002. pp. 395–412. [Google Scholar]
- Cianci T, Zoccoli G, Lenzi P, Franzini C. Loss of integrative control of peripheral circulation during desynchronized sleep. Am J Physiol Regul Integr Comp Physiol. 1991;261:R373–R377. doi: 10.1152/ajpregu.1991.261.2.R373. [DOI] [PubMed] [Google Scholar]
- Florence G, Seylaz J. Rapid autoregulation of cerebral blood flow: a laser-doppler flowmetry study. J Cereb Blood Flow Metab. 1992;12:674–680. doi: 10.1038/jcbfm.1992.92. [DOI] [PubMed] [Google Scholar]
- Franzini C. Brain metabolism and blood flow during sleep. J Sleep Res. 1992;1:3–16. [Google Scholar]
- Franzini C. Cardiovascular physiology: the peripheral circulation. In: Kryger M, Roth T, Dement WC, editors. Principles and Practice of Sleep Medicine. Philadelphia: Saunders; 2000. pp. 193–203. [Google Scholar]
- Glantz SA. Primer of Biostatics. San Francisco: McGraw-Hill; 2002. pp. 254–256. [Google Scholar]
- Grant DA, Davidson TL, Fewell JE. Automated scoring of sleep in the neonatal lamb. Sleep. 1995a;18:439–445. doi: 10.1093/sleep/18.6.439. [DOI] [PubMed] [Google Scholar]
- Grant DA, Fauchère J-C, Eede KJ, Tyberg JV, Walker AM. Left ventricular stroke volume in the fetal sheep is limited by extracardiac constraint and arterial pressure. J Physiol. 2001;535:231–239. doi: 10.1111/j.1469-7793.2001.t01-1-00231.x. 10.1111/j.1469-7793.2001.t01-1-00231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DA, Franzini C, Wild J, Walker AM. Continuous measurement of blood flow in the superior sagittal sinus of the lamb. Am J Physiol Regul Comp Integr Physiol. 1995b;269:R274–R279. doi: 10.1152/ajpregu.1995.269.2.R274. [DOI] [PubMed] [Google Scholar]
- Grant DA, Franzini C, Wild J, Walker AM. Cerebral circulation in sleep: vasodilatory response to cerebral hypotension. J Cereb Blood Flow Metab. 1998;18:639–645. doi: 10.1097/00004647-199806000-00006. 10.1097/00004647-199806000-00006. [DOI] [PubMed] [Google Scholar]
- Greenberg JH. Sleep and the cerebral circulation. In: Orem J, Barnes CD, editors. Physiology in Sleep. New York: Academic Press; 1980. pp. 57–95. [Google Scholar]
- Harper AM. Autoregulation of cerebral blood flow: influence of the arterial pressure on the blood flow through the cortex. J Neurol Neurosurg Psychiatry. 1966;29:398–403. doi: 10.1136/jnnp.29.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horne RSC, de Preu ND, Berger PJ, Walker AM. Arousal responses to hypertension in lambs: effect of sinoaortic denervation. Am J Physiol Heart Circ Physiol. 1991;260:H1283–H1289. doi: 10.1152/ajpheart.1991.260.4.H1283. [DOI] [PubMed] [Google Scholar]
- Johnston RV, Grant DA, Wilkinson MH, Walker AM. Repetitive hypoxia rapidly depresses arousal from active sleep in newborn lambs. J Physiol. 1998;510:651–659. doi: 10.1111/j.1469-7793.1998.651bk.x. 10.1111/j.1469-7793.1998.651bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laptook AR, Stonestreet BS, Oh W. Brain blood flow and O2 delivery during hemmorhagic hypotension in the piglet. Pediatr Res. 1983;17:77–80. doi: 10.1203/00006450-198301000-00015. [DOI] [PubMed] [Google Scholar]
- Lenzi P, Zoccoli G, Walker AM, Franzini C. Cerebral blood flow regulation in sleep: a model for flow metabolism coupling. Arch Ital Biol. 1999;137:165–179. [PubMed] [Google Scholar]
- McGinty D, Beahm E, Stern N, Littner M, Sowers J, Reige W. Nocturnal hypotension in older men with sleep-related breathing disorders. Chest. 1988;94:305–311. doi: 10.1378/chest.94.2.305. [DOI] [PubMed] [Google Scholar]
- Madsen PL. Blood flow and oxygen uptake in the human brain during various states of sleep and wakefulness. Acta Neurol Scand Suppl. 1993;148:7–25. [PubMed] [Google Scholar]
- Madsen PL, Vorstrup S. Cerebral blood flow and metabolism during sleep. Cerebrovas Brain Metab Rev. 1991;3:281–296. [PubMed] [Google Scholar]
- Marshall J. Diurnal variation in occurrence of strokes. Stroke. 1977;8:230–231. doi: 10.1161/01.str.8.2.230. [DOI] [PubMed] [Google Scholar]
- Orr GW, Green HJ, Hughson RL, Bennett GW. A computer linear regression model to determine ventilatory anaerobic threshold. J Appl Physiol. 1982;52:1349–1352. doi: 10.1152/jappl.1982.52.5.1349. [DOI] [PubMed] [Google Scholar]
- Panerai RB, Deverson ST, Mahony P, Hayes P, Evans DH. Effect of CO2 on dynamic cerebral autoregulation measurement. Physiol Meas. 1999;20:265–275. doi: 10.1088/0967-3334/20/3/304. 10.1088/0967-3334/20/3/304. [DOI] [PubMed] [Google Scholar]
- Papile LA, Rudolph AM, Heymann MA. Autoregulation of cerebral blood flow in the preterm fetal lamb. Pediatr Res. 1985;19:159–161. doi: 10.1203/00006450-198502000-00001. [DOI] [PubMed] [Google Scholar]
- Parmeggiani PL. Physiological risks in sleep. In: Peter JH, Penzel T, Podszus T, von Wichert P, editors. Sleep and Health Risk. Heidelberg: Springer-Verlag; 1991. pp. 119–123. [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Purves MJ, James IM. Observations on the control of cerebral blood flow in the sheep fetus and newborn lamb. Circ Res. 1969;25:651–667. doi: 10.1161/01.res.25.6.651. [DOI] [PubMed] [Google Scholar]
- Raichle ME, Stone HL. Cerebral blood flow autoregulation and graded hypercapnia. Eur Neurol. 1971;6:5. doi: 10.1159/000114443. [DOI] [PubMed] [Google Scholar]
- Szymonowicz W, Walker AMYuVYH, Stewart ML, Cannata J, Cussen L. Regional cerebral blood flow after hemorrhagic hypotension in the preterm, near-term, and newborn lamb. Pediatr Res. 1990;28:361–366. doi: 10.1203/00006450-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Wilkinson MH, Cranage S, Berger PJ, Blanch N, Adamson TM. Changes in the temporal structure of periodic breathing with postnatal development in preterm infants. Pediatr Res. 1995;38:533–538. doi: 10.1203/00006450-199510000-00010. [DOI] [PubMed] [Google Scholar]
- van der Windt C, van Gijn J. Cerebral infarctions do not occur typically at night. J Neurol Neurosurg Psychiatry. 1988;51:109–111. doi: 10.1136/jnnp.51.1.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoccoli G, Bach V, Cianci T, Lenzi P, Franzini C. Brain blood flow and extracerebral carotid circulation during sleep in rat. Brain Res. 1994;641:46–50. doi: 10.1016/0006-8993(94)91813-9. 10.1016/0006-8993(94)91813-9. [DOI] [PubMed] [Google Scholar]
- Zoccoli G, Grant DA, Wild J, Walker AM. Nitric oxide inhibition abolishes sleep-wake differences in cerebral circulation. Am J Physiol heart Circ Physiol. 2001;280:H2598–H2606. doi: 10.1152/ajpheart.2001.280.6.H2598. [DOI] [PubMed] [Google Scholar]