Abstract
Mechanisms of pain-related plasticity in the amygdala, a key player in emotionality, were studied at the cellular and molecular levels in a model of arthritic pain. The influence of the arthritis pain state induced in vivo on synaptic transmission and N-methyl-d-aspartate (NMDA) receptor function was examined in vitro using whole-cell voltage-clamp recordings of neurones in the latero-capsular part of the central nucleus of the amygdala (CeA), which is now defined as the ‘nociceptive amygdala’. Synaptic transmission was evoked by electrical stimulation of afferents from the pontine parabrachial area (part of the spino-parabrachio-amygdaloid pain pathway) in brain slices from control rats and from arthritic rats. This study shows that pain-related synaptic plasticity is accompanied by protein kinase A (PKA)-mediated enhanced NMDA-receptor function and increased phosphorylation of NMDA-receptor 1 (NR1) subunits. Synaptic plasticity in the arthritis pain model, but not normal synaptic transmission in control neurones, was inhibited by a selective NMDA receptor antagonist. Accordingly, an NMDA receptor-mediated synaptic component was recorded in neurones from arthritic animals, but not in control neurones, and was blocked by inhibition of PKA but not protein kinase C (PKC). Exogenous NMDA evoked a larger inward current in neurones from arthritic animals than in control neurones, indicating a postsynaptic effect. Paired-pulse facilitation, a measure of presynaptic mechanisms, was not affected by an NMDA-receptor antagonist. Increased levels of phosphorylated NR1 protein, but not of total NR1, were measured in the CeA of arthritic rats compared to controls. Our results suggest that pain-related synaptic plasticity in the amygdala involves a critical switch of postsynaptic NMDA receptor function through PKA-dependent NR1 phosphorylation.
The amygdala exhibits a high degree of plasticity in models of tetanic, pharmacologically induced and behavioural long-term modification of synaptic transmission (Davis, 1998; Maren, 1999; LeDoux, 2000; Martin et al. 2000; Neugebauer et al. 2000; Lin et al. 2001; Ressler et al. 2002; Royer & Pare, 2002; Zinebi et al. 2003). Such neuroplasticity is believed to be involved in associative learning and in certain neurological and psychiatric disorders. The central nucleus of the amygdala (CeA) provides the output pathway for major functions of the amygdala and regulates emotional responses (Davis, 1998; Ledoux, 2000; Cardinal et al. 2002). CeA neurones show synaptic plasticity in the kindling model of epilepsy and the chronic cocaine model of drug addiction (Neugebauer et al. 2000).
The latero-capsular part of the CeA is now defined as the ‘nociceptive amygdala’ (Gauriau & Bernard, 2002; Neugebauer et al. 2004). CeA neurones develop synaptic and nociceptive plasticity in persistent pain (Neugebauer et al. 2003; Neugebauer & Li, 2003; Li & Neugebauer, 2004b). Accumulating evidence suggests an important role for the amygdala in the emotional-affective component of pain (Rhudy & Meagher, 2001; Gauriau & Bernard, 2002; McGaraughty & Heinricher, 2002; Neugebauer et al. 2004). Neuroimaging pain studies have repeatedly detected a correlation between amygdala responses and pain behaviour in animals and pain experienced in humans (Becerra et al. 1999; Schneider et al. 2001; Bingel et al. 2002; Bornhovd et al. 2002; Paulson et al. 2002).
Mechanisms of pain-related plasticity in the amygdala are largely unknown. We showed recently that a switch in presynaptic metabotropic glutamate receptor (mGluR)1 function plays a critical role in nociceptive plasticity in the CeA (Neugebauer et al. 2003). The present study is the first to analyse the role of ionotropic glutamate receptors of the N-methyl-d-aspartate (NMDA) type in synaptic plasticity in the CeA. NMDA receptors are important for various forms of neuroplasticity as well as neurological and psychiatric disorders (Hollmann & Heinemann, 1994; Dingledine et al. 1999; Malenka & Nicoll, 1999). An important role of NMDA receptors in pain mechanisms in the spinal cord is well established and peripheral NMDA receptors are involved in nociception as well (Fisher et al. 2000; Fundytus, 2001; Ji & Woolf, 2001; Willis, 2001; Neugebauer & Carlton, 2002; Schaible et al. 2002). Recent evidence suggests that NMDA receptors in the forebrain modulate pain (Wei et al. 2001; Zhuo, 2002).
In the lateral and basolateral nuclei of the amygdala, NMDA receptors have been implicated in long-term potentiation of synaptic transmission (Gean et al. 1993; Huang & Kandel, 1998; Maren, 1999), fear conditioning (Maren et al. 1996; Lee & Kim, 1998; Malkani & Rosen, 2001; Walker et al. 2002) and behavioural sensitization to cocaine (Kalivas & Alesdatter, 1993). However, other studies suggest that NMDA receptors in the lateral amygdala do not play a major role in plasticity related to fear-memory (McKernan & Shinnick-Gallagher, 1997; Zinebi et al. 2003; Schroeder & Shinnick-Gallagher, 2004). Thus, the analysis of NMDA receptor function in the amygdala is not trivial. The role of NMDA receptors in synaptic plasticity in the CeA is not yet known.
The present study combines electrophysiological, pharmacological and biochemical approaches to test the hypothesis that NMDA receptors contribute to pain-related synaptic plasticity in the amygdala and that protein kinase A and NMDA receptor phosphorylation are involved in enhanced NMDA receptor function.
Methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Texas Medical Branch and conform to guidelines of the International Association for the Study of Pain (IASP) and of the National Institutes of Health (NIH). Electrophysiological and biochemical data were obtained from normal rats and rats with mono-arthritis (6–8 h postinduction). Male Sprague-Dawley rats (120–200 g) were individually housed in standard plastic boxes (40 cm × 20 cm) in a temperature-controlled room and maintained on a 12 h day and night cycle. Standard laboratory chow and tap water were continuously available. On the day of the experiment, rats were transferred from the animal facility and allowed to acclimate to the laboratory for at least 1 h.
Arthritis pain model
A localized mono-arthritis was induced in the left knee joint 6 h before brain slices were obtained as described in detail previously (Neugebauer et al. 2003; Neugebauer & Li, 2003; Li & Neugebauer, 2004b). Under brief (30 min) anaesthesia with the short-acting barbiturate sodium methohexital (50 mg kg−1, i.p.), a kaolin suspension (4%, 80–100 μl) was slowly injected into the joint cavity through the patellar ligament with the use of a syringe and needle (1 ml, 25G5/8). After repetitive flexions and extensions of the knee for 15 min, a carrageenan solution (2%, 80–100 μl) was injected into the knee joint cavity and the leg was flexed and extended for another 5 min. This treatment protocol reliably leads to inflammation and swelling of the injected knee and pain behaviour within 1–3 h and persists for days (Neugebauer et al. 2003; Neugebauer & Li, 2003; Han et al. 2005). Animals fully recovered from anaesthesia within 1 h and did not show any signs of distress as evidenced by the monitoring of core temperature, heart rate, breathing patterns, grooming behaviour, locomotion around the cage, water and food consumption, spontaneous vocalizations and interactions with the investigator.
Electrophysiology
Whole-cell voltage-clamp recordings were made from CeA neurones in brain slices from normal rats and arthritic rats (6–8 h postinduction of arthritis). Monosynaptic excitatory postsynaptic currents (EPSCs) were evoked at the parabrachial (PB)-CeA synapse, which provides nociceptive input to the CeA from the spino-parabrachio-amygdaloid pathway that connects the spinal cord with the CeA through the pontine parabrachial area (Jasmin et al. 1997; Bourgeais et al. 2001; Gauriau & Bernard, 2002). These afferent fibres can be easily identified under the microscope as previously described (Neugebauer et al. 2003). Drugs were applied by gravity-driven superfusion in artificial cerebrospinal fluid (ACSF) for at least 10 min. ACSF contained (mm): NaCl 117, KCl 4.7, NaH2PO4 1.2, CaCl2 2.5, MgCl2 1.2, NaHCO3 25 and glucose 11.
Amygdala slice preparation
Brain slices containing the CeA were obtained as previously described (Neugebauer et al. 2000, 2003). Rats were decapitated, the brains quickly dissected out and blocked in cold (4°C) ACSF (see above). ACSF was oxygenated and equilibrated to pH 7.4 with a mixture of 95% O2–5% CO2. Coronal brain slices (500 μm) were prepared using a Vibroslice (Camden Instruments, London, UK). After incubation in ACSF at room temperature (21°C) for at least 1 h, a single brain slice was transferred to the recording chamber and submerged between two nylon nets in ACSF (31 ± 1°C), which perfused the slice at 3–4 ml min−1.
Up to three brain slices (two on average) per animal were used; one neurone was recorded in each slice; and a fresh slice was used for each new experimental protocol. Numbers herein refer to the number of neurones tested for each parameter.
Whole-cell patch-clamp recording
Whole-cell recordings were obtained from CeA neurones using patch electrodes made from 1.5 mm borosilicate glass capillaries (o.d., 1.5 mm; i.d., 1.12 mm; Drummond, Broomall, PA, USA) pulled on a Flaming-Brown micropipette puller (P-80/PC, Sutter Instrument Co., Novato, CA, USA). Recording electrodes were positioned in the latero-capsular division of the CeA under visual control. The boundaries of the CeA were discerned under light microscopy; each slice was matched with the corresponding level according to Paxinos & Watson (1998). The internal solution of the recording electrodes (tip resistance, 4–5 MΩ) contained (mm): potassium gluconate 122, NaCl 5, CaCl2 0.3, MgCl2 2, EGTA 1, Hepes 10, Na2-ATP 5 and Na3-GTP 0.4; pH 7.2–7.3; 280 mOsm kg−1.
After tight (> 2 GΩ) seals were formed and the whole-cell configuration was obtained, neurones were included in the sample if the resting membrane potential was more negative than −50 mV and action potentials overshooting 0 mV were evoked by direct cathodal stimulation. Voltage and current signals were low-pass filtered at 1 kHz with a dual 4-pole Bessel filter (Warner Instrument Corp., Hamden, CT, USA), digitized at 5 kHz (Digidata 1200, Axon Instruments, Union City, CA, USA), and stored on a Pentium PC. Data were also continuously recorded on a pen chart recorder (Gould 3600, Gould Instruments, Valley View, OH, USA). Evoked potential and current data were acquired and analysed using pClamp8 software (Axon Instruments). Discontinuous single-electrode voltage-clamp (d-SEVC) recordings were made using an Axoclamp-2B amplifier (Axon Instruments) with a switching frequency of 5–6 kHz (30% duty cycle), gain of 3–8 nA mV−1 and time constant of 20 ms. Phase shift and anti-alias filter were optimized. The headstage voltage was monitored continuously on a digital oscilloscope (Gould 400, Gould Instruments) to ensure precise performance of the amplifier. Neurones were voltage-clamped at −60 mV. NMDA-evoked currents were also recorded over a range of holding potentials (−110 to 30 mV, using step-wise increments of 10 mV) for measurements of voltage dependency (see Fig. 3).
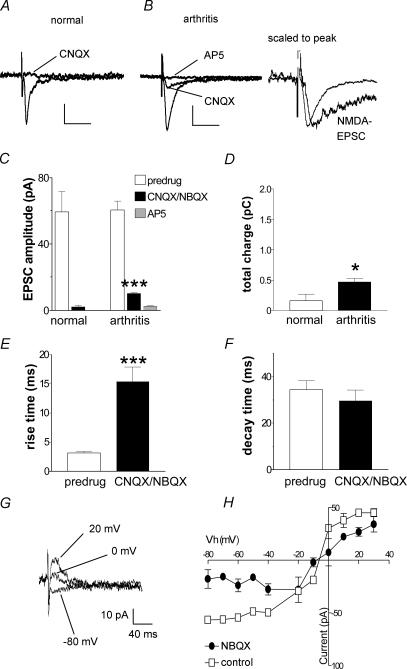
Figure 3. Pharmacological isolation of an NMDA receptor-mediated EPSC in synaptic plasticity in the arthritis pain model.
A and B, monosynaptic EPSCs recorded in a CeA neurone from a normal rat (A) and in another neurone from an arthritic rat (B). CNQX (30 μm) blocked synaptic transmission in the control neurone from a normal animal, but a resistant component remained in arthritis. Addition of AP5 (50 μm) blocked the CNQX-resistant component in arthritis. Traces to the right of B are scaled to the peak to illustrate the slow decay time of the NMDA EPSC. Scale bars in A and B are 20 pA and 40 ms, respectively. C, averaged raw data show a significant CNQX/NBQX-resistant EPSC component (measured as peak amplitude) in arthritis (n = 31; P < 0.001, paired t test), but not in control neurones from normal rats (n = 9). D, averaged raw data show the total charge of the NMDA receptor-mediated EPSC component is significantly increased in arthritis; recordings were made in the presence of CNQX/NBQX (P < 0.05, unpaired t test). E and F, averaged data show that the 10–90% rise time of the CNQX/NBQX-resistant EPSC component is significantly increased (E; P < 0.001, paired t test) compared to the predrug compound EPSC, but decay time is not affected (F). G and H, voltage-dependence of the NMDA-mediated EPSC. G, monosynaptic EPSCs recorded in the presence of NBQX (10 μm) at different holding potentials in an individual CeA neurone in a slice from an arthritic rat. H, current–voltage relationship of the NMDA receptor-mediated EPSC recorded in the presence of NBQX (10 μm) compared to the compound EPSC (predrug control) in CeA neurones (n = 3) from arthritic animals. The change in slope is consistent with the removal of a non-NMDA receptor-mediated EPSC component. *P < 0.05, ***P < 0.001.
Synaptic stimulation
The CeA is the major output nucleus of the amygdala and receives highly integrated cortical and subcortical inputs through other amygdala nuclei as well as direct inputs from brainstem areas. Our studies focused on the pontine parabrachial area (PB)-CeA synapse which provides direct nociceptive input to the latero-capsular CeA from the spinal cord and brainstem through the spino-parabrachio-amygdaloid pathway (Jasmin et al. 1997; Bourgeais et al. 2001; Gauriau & Bernard, 2002; Neugebauer et al. 2004). Using a concentric bipolar stimulating electrode (Kopf Instruments) of 22 kΩ resistance, monosynaptic excitatory postsynaptic currents (EPSCs) were evoked in CeA neurones by electrical stimulation (using a Grass S88 stimulator; Grass Instruments) of afferents at the PB-CeA synapse. The stimulation electrode was positioned under microscopic control on the fibres dorsomedial to the CeA and ventral to but outside the caudate-putamen as previously described (Neugebauer et al. 2003). Electrical stimuli (150 μs square-wave pulses) were delivered at frequencies below 0.25 Hz.
EPSC threshold was defined as the intensity that evoked a response in at least five of 10 trials. Input–output relationships were obtained by increasing the stimulus intensity in 50-μA steps. For the evaluation of drug effects on synaptically evoked responses, the stimulus intensity was adjusted to 75–80% of the intensity required for orthodromic spike generation. Drug effects were also tested on complete input–output curves (see Fig. 1) as a control for any changes of the effect of afferent fibre stimulation in the arthritis pain model. EPSC traces displayed in the figures represent the average of eight to 12 trials, which results in significantly reduced noise levels. All EPSC data are based on averaged traces from eight to 12 EPSCs at a given voltage to minimize variability. We measured both peak amplitude (single value, averaged by taking the mean of eight to 12 traces) and area (time-integral; reflecting the total charge and synaptic strength) of the EPSC (see Figs 2, 3 and 5). Rise time (10–90%) and decay time constant (t) were also analysed using pClamp8 software (Axon Instruments).
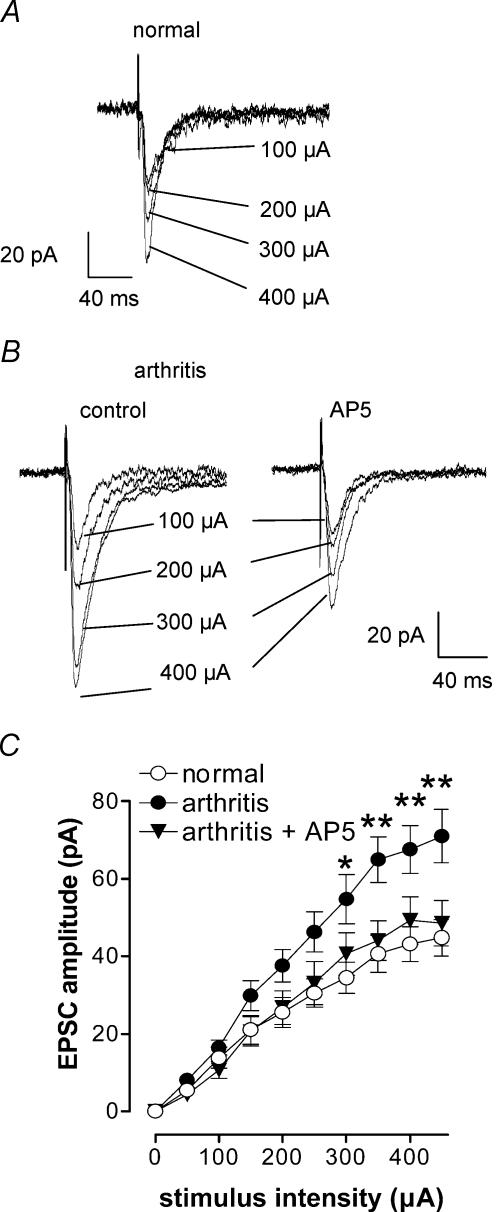
Figure 1. Synaptic plasticity in CeA neurones in the arthritis pain model.
Whole-cell voltage-clamp recordings were made from neurones in the latero-capsular part of the CeA in brain slices from normal rats and from arthritic rats (6–8 h postinduction). Monosynaptic excitatory postsynaptic currents (EPSCs) were evoked at the nociceptive parabrachial (PB)-CeA synapse (Bourgeais et al. 2001; Gauriau & Bernard, 2002; Neugebauer et al. 2004). A and B, individual traces recorded in one CeA neurone from a normal rat (A) and another CeA neurone from an arthritic rat (B) in control and in the presence of an NMDA receptor antagonist (AP5, 50 μm). Traces represent the mean of eight to 12 trials and stimulus artifacts have been truncated. C, input–output curves were generated by plotting peak EPSC amplitude as a function of increasing stimulus intensity (see Methods). Input–output relationships of neurones from arthritic animals (n = 51) were significantly different (P < 0.0001, F1,780= 40.18, two-way ANOVA) from those of control neurones (n = 29), suggesting enhanced synaptic transmission in the CeA in the arthritis pain model. In arthritis, the presence of AP5 significantly changed the input–output relationship (P < 0.0001, F9,590= 16.19, two-way ANOVA, n = 10). Neurones were voltage-clamped at −60 mV *P < 0.05, **P < 0.01, Bonferroni post hoc test.
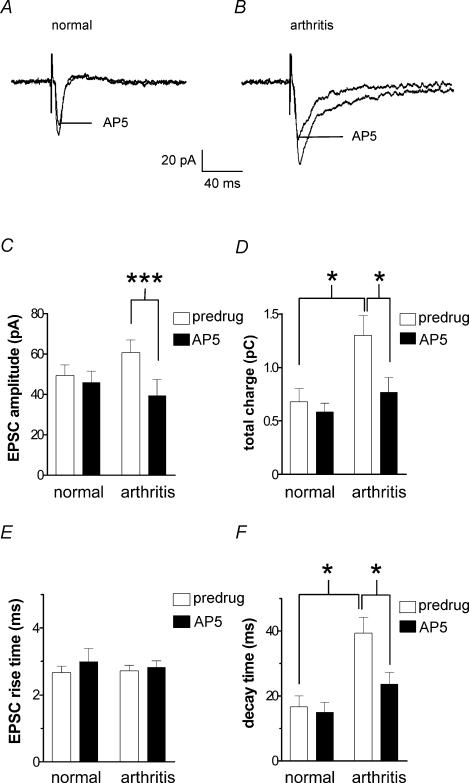
Figure 2. Inhibition of synaptic transmission by an NMDA receptor antagonist (AP5) is enhanced in the arthritis pain model.
A and B, monosynaptic EPSCs recorded in a CeA neurone from a normal rat (A) and in another neurone from an arthritic rat (B). AP5 (50 μm) inhibited synaptic transmission in arthritis but not under normal conditions. C and D, averaged raw data show that AP5 inhibited peak EPSC amplitude (C) and total charge (area under the curve, D) of monosynaptic EPSCs in arthritis (n = 11 neurones) but not in CeA neurones from normal animals (n = 12). Increase of the total charge of EPSC in the arthritis pain model suggests increased synaptic strength. E and F, differential effects of AP5 (50 μm) on the 10–90% rise time (E) and decay time constant (t, F) in neurones from arthritic rats (n = 11 neurones) and from normal animals (n = 10 neurones). AP5 shortened the increased decay time of monosynaptic EPSCs in the arthritis pain model. Monosynaptic EPSCs were evoked at the PB-CeA synapse using a stimulus intensity that generated 80% of the maximum EPSC amplitude. Neurones were voltage-clamped at −60 mV. *P < 0.05, ***P < 0.001, paired and unpaired t tests.
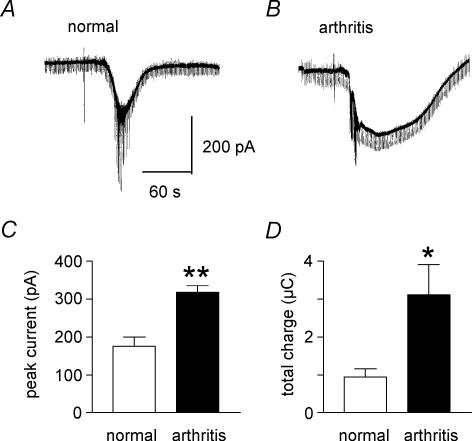
Figure 5. Direct membrane effect of NMDA is increased in CeA neurones in the arthritis pain model.
A and B, current traces show that NMDA (2 mm) evokes a larger inward current in a neurone recorded in a brain slice from an arthritic animal (B) than in another neurone from a normal animal (A). In each experiment one microdrop (10 μl) of NMDA was applied to the recording chamber using a pipette (see artifact) as described in the Methods. Hyperpolarizing voltage steps producing inward currents (see downward deflections) were used for the continuous monitoring of membrane conductance changes. Sodium spike currents appeared around the peak of the NMDA-evoked membrane current. C and D, averaged data show that significantly larger inward currents were evoked by NMDA in arthritis (n = 7 neurones) than under normal conditions (n = 4 neurones) both in terms of peak current amplitude (C) and total charge (area under the curve, D). Neurones were voltage-clamped at −60 mV. *P < 0.05, **P < 0.01, unpaired t test.
Paired-pulse facilitation
Paired-pulse facilitation (PPF) allows for the definition of pre- versus postsynaptic mechanisms in the CNS (see McKernan & Shinnick-Gallagher, 1997) and has been applied successfully to the study of drug effects in the CeA (Neugebauer et al. 2003). Two orthodromic synaptic stimuli of equal intensity were applied at varying intervals and the resulting EPSCs were recorded. PPF refers to a form of short-term synaptic plasticity in which the amplitude of the second EPSC (EPSC2) undergoes facilitation compared to the initial EPSC (EPSC1) if the interstimulus interval is sufficiently small. In whole-cell voltage-clamp, peak amplitudes were measured as the difference between the current level before the stimulus artifact and the peak of the EPSC. PPF was calculated as the ratio of EPSC2 to EPSC1 and expressed as a percentage. Any alterations in PPF suggest a presynaptic site of action (see McKernan & Shinnick-Gallagher, 1997; Neugebauer et al. 2003). PPF was tested before and during drug application.
Drugs and drug application
The following agents were tested: dl-2-amino-5-phosphonopentanoic acid (AP5, NMDA receptor anta-gonist); 6-cyano-7-nitroquinoxaline-2,3-dione disodium salt (CNQX, non-NMDA receptor antagonist); 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulphonamide disodium salt (NBQX, non-NMDA receptor antagonist); N-methyl-d-aspartic acid (NMDA); 2-[1-(3-dimethylaminopropyl)indol-3-yl]-3-(indol-3-yl) maleimide (GF109203X; potent and selective protein kinase C (PKC) inhibitor; Toullec et al. 1991); (9R, 10S, 12S)-2, 3, 9, 10, 11, 12-hexahydro-10-hydroxy-9-methyl-1-oxo-9, 12-epoxy-1H-diindolo[1, 2, 3-fg:3′, 2′, 1′-kl]pyrrolo[3, 4-i][1, 6]benzodiazocine-10-carboxylic acid, hexyl ester (KT5720; potent and selective PKA inhibitor; Cabell & Audesirk, 1993); all drugs were purchased from Tocris Cookson Inc., Ellisville, MO, USA.
Drugs were applied by gravity-driven superfusion in the ACSF at the rate of 3–4 ml min−1. Solution flow into the recording chamber was controlled with a three-way stopcock. For brief application of NMDA a pipette was used to apply a single microdrop to the inlet of the chamber as previously described (Keele et al. 2000). By the latter method, final drug concentration in the chamber was calculated (1: 100 dilution factor) on the basis of the microdrop volume (10 μl) added into the chamber (1 ml total capacity).
Western blotting
Amygdala slices (500 μm thick; three slices per animal) were obtained from normal rats and from arthritic rats (6–8 h postinduction). The CeA was cut out quickly, homogenized and processed as previously described (Zou et al. 2002, 2004; Neugebauer et al. 2003). The following phosphatase inhibitors were present in the homogenization and solubilization buffer: 50 mm NaF, 50 mm sodium pyrophosphate, 20 mm 2-glycerolphosphate, 1 mm p-nitrophosphate and 2 μm microcystin LR. The homogenate was centrifuged twice at 13 000 g for 10 min at 4°C. The supernatant was used for all Western blot analyses. The concentration of protein in the homogenate was measured using a bicinchoninic acid (BCA) kit. Equal amounts of protein (40 μg) were fractionated by 10% (w/v) sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE) and transferred onto a polyvinylidene difluoride (PVDF) membrane. The PVDF membrane was blocked in freshly prepared Tris-buffered saline containing 3% bovine serum albumin (BSA) and 0.1% Triton X-100 and 400 U ml−1γ-phosphatase then incubated with immunoaffinity purified antibody selective for phospho-NR1 or total NR1 on serine 897 (1: 1000) (Upstate, Lake Placid, NY, USA) overnight at 4°C. The blots were washed three times for 0.5 h each with washing buffer and then incubated with horseradish peroxidase conjugated with IgG (Santa Cruz, San Francisco, CA, USA) diluted in 2.5% (w/v) non-fat milk in washing buffer. The membranes were washed with buffer three times for 0.5 h and enhanced with a chemiluminescence reagent (ECL kit, Amersham, Arlington Height, IL, USA). The blots were exposed to autoradiographic film (Kodak, Rochester, NY, USA) and the intensity of immuno-reactive bands of interest was quantified using densitometric-scanning analyses.
Data analysis
Arthritis pain-related changes of synaptic transmission, drug effects and densitometry of NMDA-receptor immuno-reactivity were measured in an unpaired protocol. Statistical significance of the effects of arthritis or drugs on input–output relationships of EPSCs was determined using a two-way ANOVA with Bonferroni post hoc tests as appropriate (GraphPad Prism 3.0). Differences of drug effects in arthritis compared to normal conditions were analysed using unpaired t tests (GraphPad Prism 3.0). Significance of drug effects in each neurone was evaluated using a paired protocol and paired t tests (GraphPad Prism 3.0). The relative densities of immuno-reactive bands in tissues from normal and arthritic rats were compared statistically using post hoct tests after one-way ANOVA (GraphPad Prism 3.0). All averaged values are given as the means ± s.e.m. Statistical significance was accepted at the level P < 0.05.
Results
Synaptic plasticity in CeA neurones from arthritic rats
Compared to control animals, rats with the kaolin/carrageenan-induced mono-arthritis had a significantly increased joint diameter in the arthritic, but not the contralateral, knee and showed stronger spontaneous and mechanically evoked pain behaviour as described before (Neugebauer et al. 2003; Han et al. 2005). Whole-cell voltage-clamp recordings of CeA neurones were made in brain slices from untreated normal rats (n = 43 neurones) and from rats in which the arthritis had been induced 6–8 h beforehand (n = 97 neurones). In agreement with our previous study (Neugebauer et al. 2003), CeA neurones from arthritic rats showed changes in membrane properties compared to control neurones from normal rats. In slices from arthritic rats, neurones had a significantly more depolarized resting membrane potential (RMP: arthritis, −55.51 ± 0.52 mV, n = 72; normal, −58.94 ± 0.91 mV, n = 39; P < 0.001, unpaired t test). The slope conductance calculated from the linear portion of the current–voltage (I–V) relationship was significantly increased in arthritis (6.02 ± 0.21 nS, n = 63) compared to control neurones from normal animals (5.04 ± 0.26 nS, n = 31; P < 0.01, unpaired t test). These data suggest increased membrane excitability of CeA neurones in the arthritis pain state.
CeA neurones recorded in slices from arthritic rats showed significantly enhanced synaptic transmission compared to control neurones from normal rats (Fig. 1). Monosynaptic EPSCs with progressively larger amplitudes were evoked at the nociceptive PB-CeA synapse (see Methods) by electrical stimulation with increasing intensities (see individual examples in Fig. 1A and B). Compared to a control neurone (Fig. 1A), synaptic transmission was enhanced in a CeA neurone recorded in a brain slice from an arthritic rat (6–8 h postinduction, Fig. 1B). In arthritis, monosynaptic EPSCs had larger amplitudes. Input–output relationships were obtained by measuring EPSC peak amplitude (pA) as a function of afferent fibre stimulus intensity (μA) for each neurone (Fig. 1C). The comparison of input–output relationships between neurones from arthritic rats (n = 51) and control neurones from normal rats (n = 29) showed enhanced synaptic transmission in the arthritis pain model. Changes in synaptic transmission preserved in the brain slice are referred to as ‘synaptic plasticity’ (see Neugebauer et al. 2003). The arthritis led to a steeper slope of the input–output relationship (Fig. 1C) resulting in an upward shift at higher stimulus intensities compared to normal conditions and a statistically significant difference between the two curves (P < 0.0001, F1,780= 40.18, two-way ANOVA, column factor). Post hoc analysis showed significant differences at stimulus intensities ≥ 300 μA (P < 0.05, Bonferroni post hoc test).
In addition to the increased peak synaptic current (Fig. 1), two other parameters of synaptic transmission were altered in the arthritis pain model. Total charge (area under the curve) of monosynaptic EPSCs increased as a measure of synaptic strength (Fig. 2D, open bars; see below for details) and was accompanied by an increased decay time constant (compare open bars in Fig. 2F; see below for details). These data suggest not only a quantitative but also qualitative change of synaptic transmission in the CeA in the arthritis pain model. Next we addressed mechanisms underlying pain-related synaptic plasticity.
PKA-dependent enhanced NMDA-receptor function in synaptic plasticity
NMDA receptor antagonist (AP5) effects
To analyse the contribution of NMDA receptors to normal synaptic transmission and pain-related synaptic plasticity, we tested whether the effect of a selective NMDA receptor antagonist (AP5) was altered in CeA neurones in slices from arthritic animals. Input–output relationships in arthritis were changed significantly in the presence of AP5 (50 μm, n = 10; P < 0.0001, F9,590= 16.19, two-way ANOVA, column factor; Fig. 1C). Figure 1B shows monosynaptic EPSCs before (control) and during application of AP5 in an individual CeA neurone. Data summarized in Fig. 1C show that AP5 changed the input–output relationships in arthritis to the level of those recorded under normal conditions (no arthritis).
AP5 (50 μm) had no effect on synaptic transmission in a neurone recorded in a slice from a normal rat (Fig. 2A) but inhibited EPSCs in a neurone from an arthritic rat (Fig. 2B). Pooled data (Fig. 2C) show that in neurones recorded in slices from arthritic animals (n = 11), AP5 significantly reduced the peak amplitude of monosynaptic EPSCs (P < 0.001, paired t test), but had no significant effect on normal synaptic transmission (n = 12 neurones). The total charge (time-integral of the area under the curve) of monosynaptic EPSCs was significantly enhanced in arthritis compared to normal (no arthritis) conditions (P < 0.05, unpaired t test; Fig. 2D, open bars), suggesting increased synaptic strength in the arthritis pain model. AP5 reduced the increased total charge (area under the curve) of EPSCs in arthritis (P < 0.05, paired t test; Fig. 2D, filled bars) but not in normal neurones.
Analysis of the kinetics of monosynaptic EPSCs (Fig. 2E and F) showed that the rise time (10–90%; Fig. 2E) did not change in the arthritis pain model (n = 11 neurones) compared to normal conditions (n = 10 neurones) and was not affected by AP5 (50 μm). In contrast, the decay time constant (τ; Fig. 2F) was significantly increased in arthritis (P < 0.05, unpaired t test; open bars). AP5 significantly shortened the decay time in arthritis (P < 0.05, paired t test; Fig. 2F, filled bars) but had no effect on normal synaptic transmission.
NMDA receptor-mediated EPSC in synaptic plasticity: CNQX/NBQX effects
Next we analysed in more detail the contribution of NMDA receptors to synaptic transmission under normal conditions and to synaptic plasticity in the arthritis pain model. Using non-NMDA receptor antagonists (CNQX, 30 μm, and NBQX, 10 μm), we attempted to isolate the NMDA receptor-mediated component of the increased EPSC in the arthritis pain model. Figure 3A and B shows individual examples of monosynaptic EPSCs recorded in one CeA neurone from a normal rat (Fig. 3A) and another CeA neurone from an arthritic rat (Fig. 3B). Under normal conditions, CNQX blocked synaptic transmission completely, but in arthritis a CNQX-resistant EPSC remained, which was blocked by AP5 (50 μm). The slow decay time of this NMDA receptor-mediated EPSC is illustrated in Fig. 3B (scaled traces on the right).
In the whole sample of neurones recorded in slices from arthritic rats, a significant residual EPSC component was recorded in the presence of CNQX/NBQX (P < 0.001, paired t test; n = 31; Fig. 3C, bars on the right) but not in control neurones from normal rats (n = 9 neurones; Fig. 3C, bars on the left). The addition of AP5 (n = 9) abolished the CNQX/NBQX-resistant synaptic response in arthritis, confirming that the residual EPSC was NMDA receptor-mediated. The NMDA component was revealed by measuring the peak EPSC amplitude (Fig. 3C) as well as the total charge (area under the curve; Fig. 3D). The total charge of the NMDA receptor-mediated EPSC in the presence of CNQX/NBQX increased significantly in arthritis compared to normal conditions (Fig. 3D, P < 0.05, unpaired t test), suggesting increased synaptic strength due to the appearance of the NMDA receptor-mediated synaptic component. Analysis of EPSC kinetics (Fig. 3E and F) in arthritis showed a highly significant increase of rise time in the presence CNQX/NBQX (P < 0.001, paired t test; Fig. 3E) whereas the decay time constant was not affected (Fig. 3F) compared to the predrug compound EPSC.
The analysis of the voltage-dependence (Fig. 3G and H) of the CNQX/NBQX-resistant EPSC further suggested that this EPSC is NMDA receptor-mediated. Monosynaptic EPSCs were recorded in the presence of NBQX (10 μm) at different holding potentials (Fig. 3G, individual CeA neurone; Fig. 3H, averaged data from n = 3 neurones) in slices from arthritic rats. The nearly linear current–voltage relationship of the compound EPSC (control) changed in the presence of NBQX to reveal a slope at negative holding potentials. The change in slope is consistent with the removal of a non-NMDA receptor-mediated EPSC component by NBQX and the unmasking of the NMDA receptor-mediated EPSC.
Inhibition of PKA, but not of PKC, blocks the NMDA receptor-mediated EPSC
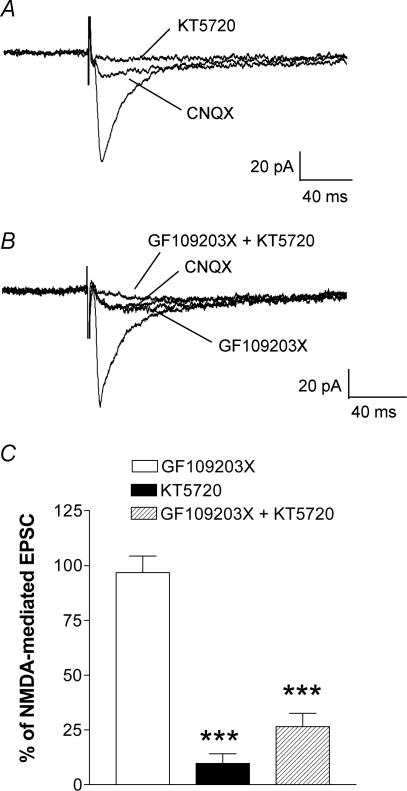
NMDA mediated, CNQX/NBQX-resistant EPSCs were blocked by a selective PKA inhibitor (KT5720, 1 μm; see Methods), but not by a selective PKC inhibitor (GF109203X, 1 μm; see Methods). Individual examples are shown in Fig. 4A and B. The monosynaptic EPSC recorded in one CeA neurone in the arthritis pain model was partially inhibited by CNQX. KT5720 completely blocked this NMDA receptor-mediated EPSC (Fig. 4A). In another CeA neurone, GF109203X had no effect on the NMDA receptor-mediated EPSC recorded in the presence of CNQX in the arthritis pain model (Fig. 4B). The subsequent addition of KT5720 largely blocked the NMDA receptor-mediated EPSC. In the sample of neurones (n = 7) a selective PKA inhibitor (KT5720) significantly inhibited the NMDA component (P < 0.001, unpaired t test; Fig. 4C). In contrast, a selective PKC inhibitor (GF109203X) had no significant effect on the CNQX/NBQX-resistant EPSC (n = 8), but the subsequent addition of KT5720 (n = 7) significantly reduced the EPSC amplitude (P < 0.001, unpaired t test). These data suggest that PKA rather than PKC is required for enhanced NMDA receptor-mediated function in the CeA in pain-related synaptic plasticity.
Figure 4. A PKA inhibitor (KT5720), but not a PKC inhibitor (GF109203X), blocks the NMDA receptor-mediated synaptic plasticity.
A, monosynaptic EPSCs recorded in a CeA neurone from an arthritic rat are only partially inhibited by CNQX (10 μm). This NMDA receptor-mediated component is completely blocked by KT5720 (1 μm). B, in another CeA neurone, application of GF109203X (1 μm) had no effect on the CNQX/NBQX-resistant NMDA receptor-mediated EPSC, but addition of KT5720 (1 μm) completely blocked the response. C, averaged normalized data show a significant inhibition of the CNQX/NBQX-resistant NMDA receptor-mediated synaptic component by KT5720 (n = 7) and by co-application of GF109203X and KT5720 (n = 7), but not by GF109203X alone (n = 8). Monosynaptic EPSCs were evoked at the PB-CeA synapse using a stimulus intensity that generated 80% of the maximum EPSC amplitude. Each trace in A and B represents the average of 10–12 EPSCs. Neurones were voltage-clamped at −60 mV. ***P < 0.001, unpaired t test (compared to predrug controls in the presence of CNQX).
Evidence for a postsynaptic rather than presynaptic mechanism
Direct membrane effects of NMDA receptor activation were measured as inward currents generated by exogenous NMDA (2 mm), using the microdrop (10 μl) application technique as described in the Methods (see Keele et al. 2000). Brief application of NMDA into the recording chamber evoked a larger inward current in a CeA neurone recorded in a brain slice from an arthritic rat (Fig. 5B) than in a CeA neurone from a normal rat (Fig. 5A). Averaged data show that peak amplitude (Fig. 5C) and total charge (area under the curve, Fig. 5D) of the NMDA-evoked membrane current were significantly larger in CeA neurones from arthritic animals (n = 7 neurones) than in control neurones from normal animals (n = 4; Fig. 5C, P < 0.01; Fig. 5D, P < 0.05, unpaired t test).
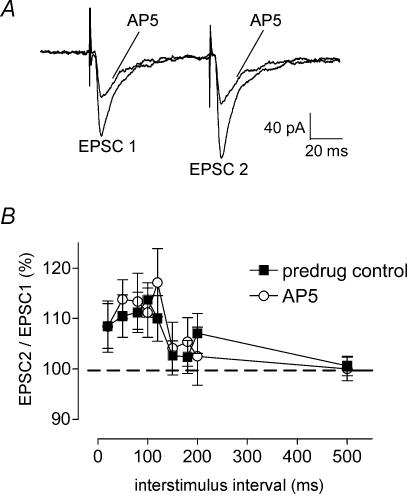
To determine whether a presynaptic site was involved in the increased endogenous NMDA-receptor activation in synaptic plasticity, we measured the effects of AP5 on paired-pulse facilitation (PPF, see Methods) as described before (Neugebauer et al. 2003). PPF refers to the phenomenon that the amplitude of the second of two consecutive EPSCs evoked by electrical synaptic stimulation is larger than the initial EPSC if the interstimulus interval is sufficiently small. If a drug (e.g. AP5) decreases transmitter release, PPF is enhanced. Any changes in PPF suggest a presynaptic site of action (see Neugebauer et al. 2003). Pairs of monosynaptic EPSCs were evoked by electrical stimulation of the PB-CeA synapse at progressively increasing interstimulus intervals (20–500 ms; see Fig. 6A for individual example). Consistent with our previous study (Neugebauer et al. 2003) PPF was recorded at the PB-CeA synapse in CeA neurones from arthritic rats (n = 14 neurones; Fig. 6B). In the presence of AP5 (n = 12 neurones) the PPF ratio did not change across the range of interstimulus intervals (Fig. 6A, individual example; Fig. 6B, averaged data). The lack of changes of PPF during block of endogenous NMDA-receptors and the direct membrane effects of exogenous NMDA strongly suggest that NMDA receptors contribute to pain-related synaptic plasticity through post- rather than presynaptic mechanisms.
Figure 6. Paired-pulse facilitation (PPF) is not altered by block of NMDA receptors.
The effect of AP5 on PPF was studied in CeA neurones in brain slices from arthritic rats. PPF, a measure of presynaptic transmitter release mechanisms, was evoked by electrical stimulation of the PB-CeA synapse at progressively increasing interstimulus intervals. A, pairs of monosynaptic EPSCs were recorded in one CeA neurone before and during application of AP5 (50 μm). Each trace is the average of 10–12 EPSCs. B, averaged normalized data show that PPF in the presence of AP5 (n = 12 neurones) was not significantly different than PPF before drug application (predrug control; n = 14 neurones). Peak amplitudes were measured as the difference between the current level before the stimulus artifact (see A) and the peak of the EPSC. PPF was calculated as EPSC2/EPSC1 × 100. Neurones were voltage-clamped at −60 mV. The lack of changes of PPF during block of endogenous NMDA-receptors argues against the involvement of any presynaptic NMDA receptors.
Increased phosphorylation, but not up-regulation, of NR1 protein in the CeA in the arthritis pain model
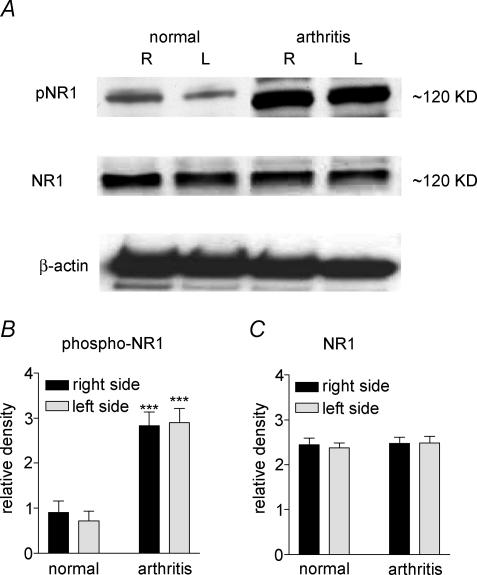
Increased postsynaptic NMDA receptor function could be due to receptor phosphorylation, receptor up-regulation or a combination of both. As our electrophysiological data suggested the involvement of PKA in enhanced NMDA receptor function, we measured the levels of phosphorylated NMDA receptor 1 subunit (pNR1) and total NR1 protein expression in the CeA from normal and from arthritic animals (6 h postinduction). Using antibodies with selectivity for total NR1 or for the PKA phosphorylation site of NR1, the relative density of immunoblots of NR1 and pNR1 protein was measured in tissues of the left (ipsilateral to the arthritis) and right (contralateral) CeA. Figure 7 shows that the relative density of pNR1 (Fig. 7B), but not total NR1 (Fig. 7C), immunoreactivity was significantly increased in the ipsi- and contralateral CeA obtained from arthritic rats (n = 6) compared with normal rats (n = 6) (P < 0.001, unpaired t test). There was no significant difference between the two brain hemispheres, which is consistent with the large receptive fields of CeA neurones and the bilateral input the CeA receives from the body (Neugebauer & Li, 2002). These data suggest that increased receptor phosphorylation rather than receptor up-regulation contributes to the changes of NMDA receptor function in pain-related synaptic plasticity.
Figure 7. Increased phospho-NR1, but not total NR1, expression in the CeA in the arthritis pain model.
A, immunoblots of phosphorylated NR1 (pNR1) and total NR1 in the CeA of the right (R, contralateral to the arthritis) and left (L, ipsilateral) brain hemispheres of normal and arthritic rats (6–8 h postinduction) are shown compared to a β-actin standard. B and C, averaged data of immunoblot densitometry show that the relative density of phospho-NR1 protein (B) is significantly increased in the right and left CeA of arthritic rats (n = 6) compared to normal rats (n = 6; P < 0.001, unpaired t test). There was no significant difference of total NR1 protein expression (C) in normal (n = 6) and arthritic (n = 6) rats.
Discussion
Previous studies from our laboratory have demonstrated enhanced synaptic plasticity in the central nucleus of the amygdala (CeA) in vitro in a model of arthritic pain induced in vivo (Neugebauer et al. 2003). Here we show that NMDA receptor function is enhanced in CeA neurones recorded in brain slices from arthritic rats and that this may well reflect a mechanism of pain-related plasticity in the CeA. The NMDA receptor change in arthritis is manifested as an increased effect of NMDA receptor block on synaptic transmission, the appearance of an NMDA receptor-mediated synaptic component and enhanced membrane currents evoked by exogenous NMDA. Enhanced NMDA receptor function depends on PKA but not PKC activation and results from increased phosphorylation rather than up-regulation of the NMDA receptor NR1 subunit in the arthritis pain model.
Central to the function of the NMDA receptor is the voltage-sensitive channel blockade by Mg2+, the removal of which increases ligand-gated permeability to cations including Ca2+. Influx of this ion in particular has been linked to neuroplasticity. The regulation of NMDA receptor activity occurs at a number of different levels. Functional NMDA receptors are composed of a heteromeric assembly of NR1 and NR2 subunits (Hollmann & Heinemann, 1994; Laube et al. 1998; Dingledine et al. 1999). The essential subunit for channel formation is NR1 while selective addition of NR2 subunits (NR2A–D) into the assembly allows for modulation of channel kinetics (Hollmann & Heinemann, 1994). A single amino acid residue in the NR1 subunit, asparagine 598 (N598), serves as a critical determinant for key properties of the NMDA receptor, such as high Ca2+ permeability and voltage-dependent Mg2+ block (Burnashev et al. 1992). NMDA receptors in the CeA are composed of NR1 and NR2B subunits (Zhuo, 2002; Sah & Lopez De Armentia, 2003). NMDA receptor channel modulation occurs through subunit (NR1 and NR2) phosphorylation by various intracellular protein kinases, including PKA and PKC, as well as through dephosphorylation via the Ca2+/calmodulin-dependent phosphatase calcineurin (Chen & Huang, 1992; Lieberman & Mody, 1994; Moon et al. 1994; Tong & Jahr, 1994; Omkumar et al. 1996; Tingley et al. 1997).
The central finding of our present study is that synaptic plasticity in the arthritis pain model depends on enhanced NMDA receptor function at the PB-CeA synapse, which transmits nociceptive information from the spino-parabrachial-amygdaloid pain pathway to the CeA (see Neugebauer et al. 2004). The present study shows increased effects of an NMDA receptor antagonist (AP5) and appearance of a CNQX/NBQX-resistant synaptic component. The peak synaptic current and total charge of the synaptic response as a measure of synaptic strength increased in the arthritis pain model. Both parameters were sensitive to AP5. Analysis of the kinetics of the AP5-sensitive CNQX/NBQX-resistant EPSC showed a slower decay time consistent with the involvement of NMDA receptors. The altered EPSC kinetics suggest not only a quantitative but also a qualitative change of synaptic transmission in the CeA in the arthritis pain model. Further, these data allow the conclusion that pain-related plasticity in the CeA requires an NMDA-mediated rather than (or in addition to) an NMDA-modulated synaptic response.
We analysed whether the change in NMDA receptor function involved a pre- or postsynaptic mechanism. Our data suggest a postsynaptic site of action, because the addition of exogenous NMDA produced a larger membrane current in the arthritis pain model than under normal conditions. Further, the lack of effect of AP5 on PPF, a measure of presynaptic transmitter release, argues against presynaptic mechanisms. NR1 subunit phosphorylation by PKA is known to occur at serine 890 and 897 and by PKC on serine 896, and our Western blot analysis using an antibody selective for the PKA phosphorylation site confirmed increased PKA-dependent phosphorylation of the NR1 subunit in arthritis. There was no increase in total NR1, suggesting an important role for enhanced receptor phosphorylation rather than receptor up-regulation in pain-related synaptic plasticity. Of importance, our electrophysiological data demonstrate that enhanced NMDA receptor-mediated synaptic transmission can be blocked by selective inhibition of PKA but not of PKC.
The results of the present work in the amygdala show similarities to in vitro studies in spinal cord dorsal horn neurones where enhanced NR1 subunit phosphorylation by PKA or PKC increased agonist effects (Chen & Huang, 1991; Cerne et al. 1992, 1993; Rusin et al. 1993). PKC-mediated phosphorylation has been suggested to result in a reduction of the Mg2+-dependent blockade of NMDA receptors (Chen & Huang, 1992) via direct and indirect mechanisms (Zheng et al. 1999; Liao et al. 2001). Of importance, phosphorylation-mediated removal of the Mg2+-dependent blockade has not previously been demonstrated in the amygdala. Our finding that increased NMDA receptor function in arthritis can be reduced by a selective PKA inhibitor, whereas a selective PKC inhibitor has no effect, indicates that enhanced NMDA receptor function in the CeA in pain-related plasticity depends upon the activity of PKA rather than PKC.
In the amygdala, neuroplasticity related to behavioural modifications and disorders has been mainly studied in the lateral and basolateral nuclei in models of epilepsy, drug addiction, conditioned fear and associative learning (McKernan & Shinnick-Gallagher, 1997; Neugebauer et al. 1997; Post et al. 1998; Davis, 1998; Huang & Kandel, 1998; Maren, 1999; Ledoux, 2000; Rolls, 2000; Schroeder & Shinnick-Gallagher, 2004). However it is the latero-capsular part of the CeA with its high content of nociceptive neurones that has been designated as the ‘nociceptive amygdala’ (see Neugebauer et al. 2004).
We recently demonstrated that pain-related synaptic plasticity in the latero-capsular CeA involves an increased contribution of presynaptic group I mGluR1 subtype receptors (Neugebauer et al. 2003). In these studies presynaptic mGluR1, but not mGluR5, were up-regulated in the CeA in the arthritis pain model. Block of mGluR1 reversed pain-related synaptic plasticity in the CeA (Neugebauer et al. 2003). The significance of these data was confirmed in our electrophysiological analysis of individual CeA neurones recorded in anaesthetized animals in vivo (Li & Neugebauer, 2004b) and in behavioural studies of higher integrated pain responses in awake animals (Han & Neugebauer, 2005). The consequence of enhanced presynaptic mGluR function would be enhanced transmitter release and, subsequently, increased activation of postsynaptic receptors.
Of particular importance to the present study is the now well-established interaction between group I mGluRs and NMDA receptors to influence plasticity. In spinal dorsal horn neurones, group I mGluR activation promotes the relief of the Mg2+-dependent blockade of NMDA receptors and results in increased neuronal responses to exogenous NMDA and enhanced nociceptive transmission (Neugebauer et al. 1994; Gerber et al. 2000; Fundytus et al. 2001). However, a direct interaction between group I mGluRs and NMDA receptors is unlikely to play a role in pain-related plasticity in the CeA that was addressed in the present study. Group I mGluRs act presynaptically to regulate transmitter release in the CeA (Neugebauer et al. 2003) whereas NMDA receptors regulate postsynaptic membrane processes (present study). The involvement of PKA rather than PKC in enhanced NMDA receptor function would also argue for an indirect rather than direct interaction between NMDA receptors and group I mGluRs, because the latter do not directly couple to PKA activation (see references in Neugebauer, 2002). Thus, a serial arrangement would explain why antagonists at mGluR1 and NMDA receptors produce seemingly similar results, as removing either step will interrupt the signalling cascade.
The analysis of NMDA-receptor function in pain-related synaptic plasticity in the amygdala is novel and not trivial. Although NMDA receptors have been shown to play important roles in various forms of neuroplasticity and nervous system disorders (Hollmann & Heinemann, 1994; Dingledine et al. 1999; Malenka & Nicoll, 1999), NMDA-independent forms of neuroplasticity are well documented (Malenka & Nicoll, 1999) and recent studies suggest that NMDA receptors in the lateral amygdala do not play a major role in plasticity related to fear-memory processes (McKernan & Shinnick-Gallagher, 1997; Zinebi et al. 2003; Schroeder & Shinnick-Gallagher, 2004).
This study is the first to show the contribution of postsynaptic NMDA receptors to pain-related synaptic plasticity in the CeA. Our work is consistent with the literature in that NMDA receptors do not contribute to normal synaptic transmission in CeA neurones (Sah & Lopez De Armentia, 2003). However, NMDA receptors play a crucial role in nociceptive transmission and neuronal plasticity at several sites of the pain neuraxis, including peripheral (Lawand et al. 1997; Du et al. 2003) and central (spinal) sensitization (Woolf & Thompson, 1991; Coderre & Melzack, 1992; Dougherty et al. 1992; Neugebauer et al. 1993; Guo & Huang, 2001; Zou et al. 2002, 2004). In a recent electrophysiological study from our group (Li & Neugebauer, 2004a), NMDA receptor activation was shown to contribute to the enhanced responses of CeA neurones recorded extracellularly in anaesthetized animals in the arthritis pain model. The patch-clamp analysis in the present study does not simply confirm these findings but provides novel information about mechanisms and sites of enhanced NMDA receptor function in synaptic plasticity in the CeA. Our data show that PKA-dependent increased phosphorylation of postsynaptic NMDA receptors alters synaptic transmission qualitatively and quantitatively and produces pain-related synaptic plasticity in the CeA.
Acknowledgments
We would like to thank Vicki Wilson for secretarial assistance. This work was supported by John Sealy Memorial Endowment Fund for Biomedical Research grants 2528–99 (V.N.) and 2521–04 (V.N.) and by NIH grants NS38261 (V.N.) and NS11255 (W.D.W.).
References
- Becerra LR, Breiter HC, Stojanovic M, Fishman S, Edwards A, Comite AR, Gonzalez RG. Human brain activation under controlled thermal stimulation and habituation of noxious heat: an fMRI study. Magn Reson Med. 1999;41:1044–1057. doi: 10.1002/(sici)1522-2594(199905)41:5<1044::aid-mrm25>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bingel U, Quante M, Knab R, Bromm B, Weiller C, Buchel C. Subcortical structures involved in pain processing: evidence from single-trial fMRI. Pain. 2002;99:313–321. doi: 10.1016/s0304-3959(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Bornhovd K, Quante M, Glauche V, Bromm B, Weiller C, Buchel C. Painful stimuli evoke different stimulus-response functions in the amygdala, prefrontal, insula and somatosensory cortex: a single-trial fMRI study. Brain. 2002;125:1326–1336. doi: 10.1093/brain/awf137. [DOI] [PubMed] [Google Scholar]
- Bourgeais L, Gauriau C, Bernard J-F. Projections from the nociceptive area of the central nucleus of the amygdala to the forebrain: a PHA-L study in the rat. Eur J Neurosci. 2001;14:229–255. doi: 10.1046/j.0953-816x.2001.01640.x. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Schoepfer R, Monyer H, Ruppersberg JP, Gunther W, Seeburg PH, Sakmann B. Control by asparagine residues of calcium permeability and magnesium blockade in the NMDA receptor. Science. 1992;257:1415–1419. doi: 10.1126/science.1382314. [DOI] [PubMed] [Google Scholar]
- Cabell L, Audesirk G. Effects of selective inhibition of protein kinase C, cyclic AMP-dependent protein kinase, and Ca2+-calmodulin-dependent protein kinase on neurite development in cultured rat hippocampal neurons. Int J Dev Neurosci. 1993;11:357–368. doi: 10.1016/0736-5748(93)90007-z. [DOI] [PubMed] [Google Scholar]
- Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- Cerne R, Jiang M, Randic M. Cyclic adenosine 3′5′-monophosphate potentiates excitatory amino acid and synaptic responses of rat spinal dorsal horn neurons. Brain Res. 1992;596:111–123. doi: 10.1016/0006-8993(92)91538-p. [DOI] [PubMed] [Google Scholar]
- Cerne R, Rusin KI, Randic M. Enhancement of the N-methyl-D-aspartate response in spinal dorsal horn neurons by cAMP-dependent protein kinase. Neurosci Lett. 1993;161:124–128. doi: 10.1016/0304-3940(93)90275-p. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LY. Sustained potentiation of NMDA receptor-mediated glutamate responses through activation of protein kinase C by a mu opioid. Neuron. 1991;7:319–326. doi: 10.1016/0896-6273(91)90270-a. [DOI] [PubMed] [Google Scholar]
- Chen L, Huang LY. Protein kinase C reduces Mg2+ block of NMDA-receptor channels as a mechanism of modulation. Nature. 1992;356:521–523. doi: 10.1038/356521a0. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci. 1992;12:3671–3675. doi: 10.1523/JNEUROSCI.12-09-03671.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. Anatomic and physiologic substrates of emotion in an animal model. J Clin Neurophysiol. 1998;15:378–387. doi: 10.1097/00004691-199809000-00002. 10.1097/00004691-199809000-00002. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Dougherty PM, Palecek J, Paleckova V, Sorkin LS, Willis WD. The role of NMDA and non-NMDA excitatory amino acid receptors in the excitation of primate spinothalamic tract neurons by mechanical, chemical, thermal, and electrical stimuli. J Neurosci. 1992;12:3025–3041. doi: 10.1523/JNEUROSCI.12-08-03025.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. 10.1016/S0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ, Hagen NA. Targeting the N-methyl-D-aspartate receptor for chronic pain management. Preclinical animal studies, recent clinical experience and future research directions. J Pain Symptom Manage. 2000;20:358–373. doi: 10.1016/s0885-3924(00)00213-x. 10.1016/S0885-3924(00)00213-X. [DOI] [PubMed] [Google Scholar]
- Fundytus ME. Glutamate receptors and nociception. Implications for the drug treatment of pain. CNS Drugs. 2001;15:29–58. doi: 10.2165/00023210-200115010-00004. [DOI] [PubMed] [Google Scholar]
- Fundytus ME, Yashpal K, Cabot JG, Osborne MG, Lefebvre CD, Dray A, Henry JL, Coderre TJ. Knockdown of spinal metabotropic glutamate receptor 1 (mGluR (1)) alleviates pain and restores opioid efficacy after nerve injury in rats. Br J Pharmacol. 2001;132:354–367. doi: 10.1038/sj.bjp.0703810. 10.1038/sj.bjp.0703810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauriau C, Bernard J-F. Pain pathways and parabrachial circuits in the rat. Exp Physiol. 2002;87:251–258. doi: 10.1113/eph8702357. 10.1113/eph8702357. [DOI] [PubMed] [Google Scholar]
- Gean PW, Chang FC, Huang CC, Lin JH, Way LJ. Long-term enhancement of EPSP and NMDA receptor-mediated synaptic transmission in the amygdala. Brain Res Bull. 1993;31:7–11. doi: 10.1016/0361-9230(93)90003-t. 10.1016/0361-9230(93)90003-T. [DOI] [PubMed] [Google Scholar]
- Gerber G, Youn DH, Hsu CH, Isaev D, Randic M. Spinal dorsal horn synaptic plasticity: involvement of group I metabotropic glutamate receptors. Prog Brain Res. 2000;129:115–134. doi: 10.1016/S0079-6123(00)29009-2. [DOI] [PubMed] [Google Scholar]
- Guo H, Huang LY. Alteration in the voltage dependence of NMDA receptor channels in rat dorsal horn neurones following peripheral inflammation. J Physiol. 2001;537:115–123. doi: 10.1111/j.1469-7793.2001.0115k.x. 10.1111/j.1469-7793.2001.0115k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Bird GC, Li W, Jones J, Neugebauer V. Computerized analysis of audible and ultrasonic vocalizations of rats as a standardized measure of pain-related behavior. J Neurosci Methods. 2005;141:261–269. doi: 10.1016/j.jneumeth.2004.07.005. 10.1016/j.jneumeth.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Han JS, Neugebauer V. mGluR1 and mGluR5 antagonists in the amygdala inhibit different components of audible and ultrasonic vocalizations in a model of arthritic pain. Pain. 2005;113:211–222. doi: 10.1016/j.pain.2004.10.022. 10.1016/j.pain.2004.10.022. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huang YY, Kandel ER. Postsynaptic induction and PKA-dependent expression of LTP in the lateral amygdala. Neuron. 1998;21:169–178. doi: 10.1016/s0896-6273(00)80524-3. 10.1016/S0896-6273(00)80524-3. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Burkey AR, Card JP, Basbaum AI. Transneuronal labeling of a nociceptive pathway, the spino-(trigemino-) parabrachio-amygdaloid, in the rat. J Neurosci. 1997;17:3751–3765. doi: 10.1523/JNEUROSCI.17-10-03751.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Alesdatter JE. Involvement of N-methyl-D-aspartate receptor stimulation in the ventral tegmental area and amygdala in behavioral sensitization to cocaine. J Pharmacol Exp Ther. 1993;267:486–495. [PubMed] [Google Scholar]
- Keele NB, Zinebi F, Neugebauer V, Shinnick-Gallagher P. Epileptogenesis up-regulates metabotropic glutamate receptor activation of sodium-calcium exchange current in the amygdala. J Neurophysiol. 2000;83:2458–2462. doi: 10.1152/jn.2000.83.4.2458. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawand NB, Willis WD, Westlund KN. Excitatory amino acid receptor involvement in peripheral nociceptive transmission in rats. Eur J Pharmacol. 1997;324:169–177. doi: 10.1016/s0014-2999(97)00072-1. 10.1016/S0014-2999(97)00072-1. [DOI] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lee H, Kim JJ. Amygdalar NMDA receptors are critical for new fear learning in previously fear-conditioned rats. J Neurosci. 1998;18:8444–8454. doi: 10.1523/JNEUROSCI.18-20-08444.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Block of NMDA and non-NMDA receptor activation results in reduced background and evoked activity of central amygdala neurons in a model of arthritic pain. Pain. 2004a;110:112–122. doi: 10.1016/j.pain.2004.03.015. 10.1016/j.pain.2004.03.015. [DOI] [PubMed] [Google Scholar]
- Li W, Neugebauer V. Differential roles of mGluR1 and mGluR5 in brief and prolonged nociceptive processing in central amygdala neurons. J Neurophysiol. 2004b;91:13–24. doi: 10.1152/jn.00485.2003. 10.1152/jn.00485.2003. [DOI] [PubMed] [Google Scholar]
- Liao GY, Wagner DA, Hsu MH, Leonard JP. Evidence for direct protein kinase-C mediated modulation of N-methyl-D-aspartate receptor current. Mol Pharmacol. 2001;59:960–964. doi: 10.1124/mol.59.5.960. [DOI] [PubMed] [Google Scholar]
- Lieberman DN, Mody I. Regulation of NMDA channel function by endogenous Ca 2+-dependent phosphatase. Nature. 1994;369:235–239. doi: 10.1038/369235a0. 10.1038/369235a0. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. 10.1016/S0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- McGaraughty S, Heinricher MM. Microinjection of morphine into various amygdaloid nuclei differentially affects nociceptive responsiveness and RVM neuronal activity. Pain. 2002;96:153–162. doi: 10.1016/s0304-3959(01)00440-7. 10.1016/S0304-3959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- McKernan MG, Shinnick-Gallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation – a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malkani S, Rosen JB. N-Methyl-D-aspartate receptor antagonism blocks contextual fear conditioning and differentially regulates early growth response-1 messenger RNA expression in the amygdala: implications for a functional amygdaloid circuit of fear. Neuroscience. 2001;102:853–861. doi: 10.1016/s0306-4522(00)00531-5. 10.1016/S0306-4522(00)00531-5. [DOI] [PubMed] [Google Scholar]
- Maren S. Long-term potentiation in the amygdala: a mechanism for emotional learning and memory. Trends Neurosci. 1999;22:561–567. doi: 10.1016/s0166-2236(99)01465-4. 10.1016/S0166-2236(99)01465-4. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Stote DL, Fanselow MS. N-methyl-D-aspartate receptors in the basolateral amygdala are required for both acquisition and expression of conditional fear in rats. Behav Neurosci. 1996;110:1365–1374. doi: 10.1037//0735-7044.110.6.1365. 10.1037//0735-7044.110.6.1365. [DOI] [PubMed] [Google Scholar]
- Martin SJ, Grimwood PD, Morris RG. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- Moon IS, Apperson ML, Kennedy MB. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-D-aspartate receptor subunit 2B. Proc Natl Acad Sci U S A. 1994;91:3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V. Metabotropic glutamate receptors – important modulators of nociception and pain behavior. Pain. 2002;98:1–8. doi: 10.1016/s0304-3959(02)00140-9. 10.1016/S0304-3959(02)00140-9. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Carlton SM. Peripheral metabotropic glutamate receptors as drug targets for pain relief. Expert Opin Ther Targets. 2002;6:349–361. doi: 10.1517/14728222.6.3.349. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Keele NB, Shinnick-Gallagher P. Epileptogenesis in vivo enhances the sensitivity of inhibitory presynaptic metabotropic glutamate receptors in basolateral amygdala neurons in vitro. J Neurosci. 1997;17:983–995. doi: 10.1523/JNEUROSCI.17-03-00983.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Processing of nociceptive mechanical and thermal information in central amygdala neurons with knee-joint input. J Neurophysiol. 2002;87:103–112. doi: 10.1152/jn.00264.2001. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W. Differential sensitization of amygdala neurons to afferent inputs in a model of arthritic pain. J Neurophysiol. 2003;89:716–727. doi: 10.1152/jn.00799.2002. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Bhave G, Gereau RW. Synaptic plasticity in the amygdala in a model of arthritic pain: differential roles of metabotropic glutamate receptors 1 and 5. J Neurosci. 2003;23:52–63. doi: 10.1523/JNEUROSCI.23-01-00052.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer V, Li W, Bird GC, Han JS. The amygdala and persistent pain. Neuroscientist. 2004;10:221–234. doi: 10.1177/1073858403261077. 10.1177/1073858403261077. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Lucke T, Schaible HG. N-methyl-D-aspartate (NMDA) and non-NMDA receptor antagonists block the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat's knee joint. J Neurophysiol. 1993;70:1365–1377. doi: 10.1152/jn.1993.70.4.1365. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Lucke T, Schaible HG. Requirement of metabotropic glutamate receptors for the generation of inflammation-evoked hyperexcitability in rat spinal cord neurons. Eur J Neurosci. 1994;6:1179–1186. doi: 10.1111/j.1460-9568.1994.tb00616.x. [DOI] [PubMed] [Google Scholar]
- Neugebauer V, Zinebi F, Russell R, Gallagher JP, Shinnick-Gallagher P. Cocaine and kindling alter the sensitivity of group II and III metabotropic glutamate receptors in the central amygdala. J Neurophysiol. 2000;84:759–770. doi: 10.1152/jn.2000.84.2.759. [DOI] [PubMed] [Google Scholar]
- Omkumar RV, Kiely MJ, Rosenstein AJ, Min KT, Kennedy MB. Identification of a phosphorylation site for calcium/calmodulin-dependent protein kinase II in the NR2B subunit of the N-methyl-D-aspartate receptor. J Biol Chem. 1996;271:31670–31678. doi: 10.1074/jbc.271.49.31670. 10.1074/jbc.271.49.31670. [DOI] [PubMed] [Google Scholar]
- Paulson PE, Casey KL, Morrow TJ. Long-term changes in behavior and regional cerebral blood flow associated with painful peripheral mononeuropathy in the rat. Pain. 2002;95:31–40. doi: 10.1016/s0304-3959(01)00370-0. 10.1016/S0304-3959(01)00370-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. New York: Academic Press; 1998. [Google Scholar]
- Post RM, Weiss SR, Li H, Smith MA, Zhang LX, Xing G, Osuch EA, McCann UD. Neural plasticity and emotional memory. Dev Psychopathol. 1998;10:829–855. doi: 10.1017/s0954579498001898. 10.1017/S0954579498001898. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Paschall G, Zhou XI, Davis M. Regulation of synaptic plasticity genes during consolidation of fear conditioning. J Neurosci. 2002;22:7892–7902. doi: 10.1523/JNEUROSCI.22-18-07892.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. The role of emotion in pain modulation. Curr Opin Psychiatry. 2001;14:241–245. 10.1097/00001504-200105000-00012. [Google Scholar]
- Rolls ET. Memory systems in the brain. Annu Rev Psychol. 2000;51:599–630. doi: 10.1146/annurev.psych.51.1.599. 10.1146/annurev.psych.51.1.599. [DOI] [PubMed] [Google Scholar]
- Royer S, Pare D. Bidirectional synaptic plasticity in intercalated amygdala neurons and the extinction of conditioned fear responses. Neuroscience. 2002;115:455–462. doi: 10.1016/s0306-4522(02)00455-4. 10.1016/S0306-4522(02)00455-4. [DOI] [PubMed] [Google Scholar]
- Rusin KI, Bleakman D, Chard PS, Randic M, Miller RJ. Tachykinins potentiate N-methyl-D-aspartate responses in acutely isolated neurons from the dorsal horn. J Neurochem. 1993;60:952–960. doi: 10.1111/j.1471-4159.1993.tb03242.x. [DOI] [PubMed] [Google Scholar]
- Sah P, Lopez De Armentia M. Excitatory synaptic transmission in the lateral and central amygdala. Ann NY Acad Sci. 2003;985:67–77. doi: 10.1111/j.1749-6632.2003.tb07072.x. [DOI] [PubMed] [Google Scholar]
- Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann NY Acad Sci. 2002;966:343–354. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- Schneider F, Habel U, Holthusen H, Kessler C, Posse S, Muller-Gartner HW, Arndt JO. Subjective ratings of pain correlate with subcortical-limbic blood flow: an fMRI study. Neuropsychobiology. 2001;43:175–185. doi: 10.1159/000054887. 10.1159/000054887. [DOI] [PubMed] [Google Scholar]
- Schroeder BW, Shinnick-Gallagher P. Fear memories induce a switch in stimulus response and signaling mechanisms for long-term potentiation in the lateral amygdala. Eur J Neurosci. 2004;20:549–556. doi: 10.1111/j.1460-9568.2004.03517.x. 10.1111/j.1460-9568.2004.03517.x. [DOI] [PubMed] [Google Scholar]
- Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-D-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- Tong G, Jahr CE. Regulation of glycine-insensitive desensitization of the NMDA receptor in outside-out patches. J Neurophysiol. 1994;72:754–761. doi: 10.1152/jn.1994.72.2.754. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of d-cycloserine as assessed with fear- potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei F, Wang GD, Kerchner GA, Kim SJ, Xu HM, Chen ZF, Zhuo M. Genetic enhancement of inflammatory pain by forebrain NR2B overexpression. Nat Neurosci. 2001;4:164–169. doi: 10.1038/83993. 10.1038/83993. [DOI] [PubMed] [Google Scholar]
- Willis WD. Role of neurotransmitters in sensitization of pain responses. Ann NY Acad Sci. 2001;933:142–156. doi: 10.1111/j.1749-6632.2001.tb05821.x. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Thompson SW. The induction and maintenance of central sensitization is dependent on N-methyl-D-aspartic acid receptor activation; implications for the treatment of post-injury pain hypersensitivity states. Pain. 1991;44:293–299. doi: 10.1016/0304-3959(91)90100-C. 10.1016/0304-3959(91)90100-C. [DOI] [PubMed] [Google Scholar]
- Zheng X, Zhang L, Wang AP, Bennett MV. Protein kinase C potentiation of N-methyl-D-aspartate receptor activity is not mediated by phosphorylation of N-methyl-D-aspartate receptor subunits. Proc Natl Acad Sci U S A. 1999;96:15262–15267. doi: 10.1073/pnas.96.26.15262. 10.1073/pnas.96.26.15262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuo M. Glutamate receptors and persistent pain: targeting forebrain NR2B subunits. Drug Discov Today. 2002;7:259–267. doi: 10.1016/s1359-6446(01)02138-9. 10.1016/S1359-6446(01)02138-9. [DOI] [PubMed] [Google Scholar]
- Zinebi F, Xie J, Liu J, Russell RT, Gallagher JP, McKernan MG, Shinnick-Gallagher P. NMDA currents and receptor protein are downregulated in the amygdala during maintenance of fear memory. J Neurosci. 2003;23:10283–10291. doi: 10.1523/JNEUROSCI.23-32-10283.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Role of protein kinase A in phosphorylation of NMDA receptor 1 subunits in dorsal horn and spinothalamic tract neurons after intradermal injection of capsaicin in rats. Neuroscience. 2002;115:775–786. doi: 10.1016/s0306-4522(02)00490-6. 10.1016/S0306-4522(02)00490-6. [DOI] [PubMed] [Google Scholar]
- Zou X, Lin Q, Willis WD. Effect of protein kinase C blockade on phosphorylation of NR1 in dorsal horn and spinothalamic tract cells caused by intradermal capsaicin injection in rats. Brain Res. 2004;1020:95–105. doi: 10.1016/j.brainres.2004.06.017. 10.1016/j.brainres.2004.06.017. [DOI] [PubMed] [Google Scholar]