Abstract
Summary
Genetics impact the propensity of different strains of mice to display hyperalgesia after opioid administration. In these studies we demonstrate that variants of the β2 adrenergic receptor gene are linked to these differences in murine opioid-induced hyperalgesia.
Background
Opioid-induced hyperalgesia (OIH) is a syndrome of increased sensitivity to noxious stimuli seen after both the acute and chronic administration of opioids which has been observed in humans and rodent models. This syndrome may reduce the clinical utility of opioids in treating acute and chronic pain.
Methods
In these studies we measured the propensity of 15 strains of inbred mice to develop mechanical manifestations of OIH. These data were subjected to in silico genetic analysis which resulted in the association of haplotypic blocks within or near several known genes. Both pharmacological agents and transgenic mice were used to confirm the functional association of the most strongly linked gene with OIH.
Results
Both baseline mechanical nociceptive thresholds as well as the percentage changes in these thresholds after 4 days of morphine treatment were found to be highly strain dependent. The haplotypic block most strongly associated with the mechanical OIH data was located within the β2 adrenergic receptor gene (β2-AR). Using the selective β2-AR antagonist butoxamine, we observed a dose-dependent reversal of OIH. Furthermore, deletion of the β2-AR gene sharply reduced the mechanical allodynia present after morphine treatment in the wild type mouse strain. Analysis of the associated β2-AR haplotypic block identified single nucleotide polymorphisms potentially explaining in part the strain specific differences in OIH.
Conclusions
Genetic variants of the β2-AR gene appear to explain some part of the differences between various strains of mice to develop OIH. The association of this gene with OIH suggests specific pharmacological strategies for reducing the impact of OIH on patients consuming opioids.
Keywords: Genetics, Hyperalgesia, Pain, Morphine, Beta Adrenergic
Introduction
Accumulating evidence suggests that the administration of opioid analgesics leads not only to analgesia, but to a paradoxical sensitization to noxious stimuli which is particularly evident after the abrupt cessation of opioid administration or at the time of serum opioid nadir between regular doses. This phenomenon is referred to as opioid-induced hyperalgesia (OIH). Among the more important human studies documenting this effect are those demonstrating hyperalgesia in former opioid addicts maintained on methadone when compared with matched controls not receiving methadone or other opioids 1–3. This hyperalgesia was most pronounced immediately prior to daily methadone administration, but was measurable even at time points closer to peak methadone serum concentrations 4. A recent prospective trial in which sustained-acting morphine was given to patients with chronic low back pain demonstrated measurable hyperalgesia within one month of beginning therapy 5. Other human data suggest that the short term infusion of opioids like the μ-opioid receptor agonist remifentanil followed by abrupt cessation exacerbates pre-existing hyperalgesia 6–8. Some evidence suggests this phenomena is due to the activation of N-methyl-D-aspartate receptors 7.
More recently rodent models have been used to study OIH. Many laboratories have reported mechanical allodynia and/or thermal hyperalgesia after the acute administration of opioids like heroin and fentanyl 9,10, the chronic (days) administration of intrathecal morphine 11,12, the local peripheral administration of morphine 13 or the chronic administration of systemic opioids of several types 14–16. Many mechanisms have been proposed to explain this type of sensitization with some of the more commonly discussed possibilities including activation of N-methyl-D-aspartate receptors 14,17,18, activation of facilitative descending pathways from the rostral ventromedial medulla (RVM) 16, the decreased reuptake of neurotransmitters from primary afferent fibers 19 and the enhanced responsiveness of spinal neurons to nociceptive neurotransmitters like substance P and glutamate 20,21. Though not previously linked to OIH, the enhanced expression of beta-2 adrenergic receptors (β2-AR) have been identified as adaptive changes occurring during chronic exposure to opioids 22,23. Likewise, the functional enhancement of β2-AR signaling has been demonstrated after chronic morphine exposure in various nervous system tissues 24,25. These observations will become relevant to the present studies. While traditional pharmacological, electrophysiological, biochemical and molecular techniques have been useful in the exploration of OIH, we are now in position to use murine genetics to identify genomic loci linked to this phenomenon.
A substantial and growing body of literature supports the conclusion that genetics influence pain sensitivity and analgesic responses. With respect to the consequences of chronic morphine administration, several reports explore the genetic basis of tolerance and dependence specifically 26–28. The genetics of OIH are not well explored in part due to the barriers posed by genetic studies. Traditional murine mapping experiments have advanced our understanding of the genetic basis of pain, but the techniques generally used are quite time consuming and accessible only to laboratories with substantial expertise in genetics and molecular biology. More recently in silico techniques have been introduced which allow mapping to be done in much expedited fashion. These techniques rely on the availability of high resolution single nucleotide polymorphism (SNP) databases. The computational algorithms then compare phenotypic trait data for a series of inbred mouse strains with the SNP alleles for those strains as organized into either genomic segments of arbitrary size 29, or more recently as organized into haplotypic blocks 30,31. These now well-described techniques have proven useful in identifying chromosomal regions and even specific genes involved in many traits including bone metabolism, alcohol withdrawal, immune system function, susceptibility to pulmonary injury, the expression of specific genes and several other traits 29,30,32. In these studies we used in silico mapping to identify haplotypic blocks associated with OIH and went on to confirm a functional association for one haplotypic block corresponding to the β2-AR using independent techniques.
Methods
Animals
All animal experiments were done after approval of protocols by our Institutional Animal Care and Use Committee and complied with the Guide for the Care and Use of Laboratory Animals available through the National Academy of Sciences.
Inbred mouse strains
Inbred mouse strains were obtained from Jackson Labs (Bar Harbor, MD) at 7–8 weeks of age. Mice were kept a further 7–10 days from the date of arrival in our animal care facility prior to use to allow for acclimation. Mice were kept under pathogen-free conditions and were provided food and water ad libitum with a 12:12h light:dark cycle. The strains used were: 129/SvlmJ, A/HeJ, A/J, AKR/J, B10.D2-H2/oSNJ, BALB/cByJ, BALB/cJ, C3H/HeJ, C57BL/6J, DBA/2J, LP/J, LG/J, MRL/MpJ, NZB/BinJ, and NZW/LaCJ (15 strains).
Transgenic mice
FVB and FVB β2-AR congenic null mutants were obtained from a local breeding colony. The generation of these mice is described by Chruscinski et al. 33. These mice were individually genotyped and used in our experiments at 7–8 weeks of age. Animal husbandry was otherwise identical to that used for the inbred strains.
Drug administration
Morphine administration
After baseline nociceptive testing morphine (Sigma Chemical, St. Louis, MO) was administered to mice subcutaneously 20mg/kg twice per day on days 1–3. On day 4 the dose was raised to 40mg/kg twice per day in 50–100μl volumes of 0.9% NaCl similar to our previous protocols for generating opioid-induced hyperalgesia 14,34. For OIH determinations, mice were assessed 16 hours after the final dose of morphine.
Butoxamine administration
The selective β2-AR antagonist butoxamine was obtained from (Sigma Chemical, St. Louis, MO) and diluted in 0.9% NaCl prior to use. After the measurement of baseline mechanical thresholds in C57BL/6J mice, butoxamine was injected subcutaneously. Behavioral testing was repeated in 30 minutes, and the next higher dose of butoxamine in the series was then injected into the same mice to obtain cumulative dose-response information. Control mice received saline injections at the same time points. Pilot data confirmed 30 minutes to be a time of maximal drug effect.
Local hind paw injections were performed by lightly restraining the mice and injecting 5 μl of drug containing 0.9% NaCl subcutaneously into the central plantar area of the hind paw. For these injections a 30 gauge needle and a microsyringe were employed. Mice recovered for 10 minutes in their testing enclosures which was observed to be a time of maximal drug effect.
Behavioral assays
Mechanical allodynia
Mechanical nociceptive thresholds were assayed using nylon von Frey filaments according to the “up-down” algorithm described by Chaplan et al. 35 as we have used previously to detect allodynia after chronic opioid administration 14,34. In these experiments mice were placed on wire mesh platforms in clear cylindrical plastic enclosures of 10cm diameter and 30cm in height. After 20 minutes of acclimation, fibers of sequentially increasing stiffness (0.2–2 grams, 7 fibers) were applied to the center of the plantar surface of a hind paw just distal to the first set of foot pads and left in place 5 sec with enough force to slightly bend the fiber. Withdrawal of the hind paw from the fiber was scored as a response. When no response was obtained the next stiffest fiber in the series was applied to the same paw; if a response was obtained a less stiff fiber was next applied. Testing proceeded in this manner until 4 fibers had been applied after the first one causing a withdrawal response allowing the estimation of the mechanical withdrawal threshold using curve fitting of the response data 36. Our index of mechanical OIH was calculated as the percentage decrease in baseline mechanical nociceptive threshold resulting from chronic morphine administration.
Thermal withdrawal latency
Response latencies to noxious thermal stimulation were measured using the method of Hargreaves 37 as we have modified for use with mice 14. In this assay mice were placed on a temperature controlled glass platform (29° C) in a plastic enclosure as described above. After 20 minutes of acclimation, a beam of focused light was directed towards the same area of the hind paw as described for the von Frey assay. The time to purposeful withdrawal of the foot from the beam of light was measured to 0.1sec. A 20-second cutoff was used to prevent tissue damage. In these experiments, the light beam intensity was adjusted to provide an approximate 10 second baseline latency for the C57BL/6 index strain prior to morphine treatment and the same light intensity was used for all subsequent experiments. Two measurements were made per animal per test session.
In silico mapping
Association studies using haplotypic mapping
Using “HapMapper” software developed by Roche Bioscience (Palo Alto, CA, USA) and a 158,000 SNP database organized into haplotypic blocks for all strains tested (mousesnp.roche.com) 30,31, we attempted to determine associations between our OIH trait data and individual blocks. Briefly, this approach identifies haplotypic blocks whose pattern of genetic variation correlates with the distribution of trait values among the inbred strains. The actual correlation between trait values (mechanical OIH in this case) and the strain groupings for each haplotype block is determined using analysis of variance (ANOVA) based modeling. The resulting P values are used to rank the strengths of correlation for each block in the database. This technique has been used recently to identify genes associated with a number of different murine phenotypic traits 30. At the time of analysis, this haplotyic map contained blocks corresponding to 2171 genes.
Statistical analysis
All data are displayed as the means +/− SEM unless otherwise noted. Dose-response data was fitted using a sigmoidal function with variable slope (Prism 4, GraphPad Software, San Diego, CA). Where repeated measures were employed, ANOVA analysis was applied with post-hoc t-testing.
Results
Strain differences for OIH
Figure 1 displays the mechanical baseline nociceptive responses and those observed after 4 days of morphine treatment. The differences in baseline nociceptive responses for the various strains were large with the most mechanically sensitive strain having a baseline nociceptive threshold 16% of the least sensitive strain. As can also be seen in Figure 1, the degree of mechanical allodynia acquired during morphine administration varied for the different strains. Table 1 lists the mechanical allodynia caused by chronic morphine administration as a percentage change in baseline thresholds. While some strains displayed only small mechanical changes, 6 strains had a greater than 80% reduction in mechanical nociceptive threshold. There was no correlation between baseline mechanical nociceptive thresholds and the degree of OIH developed by the individual strains.
Figure 1.
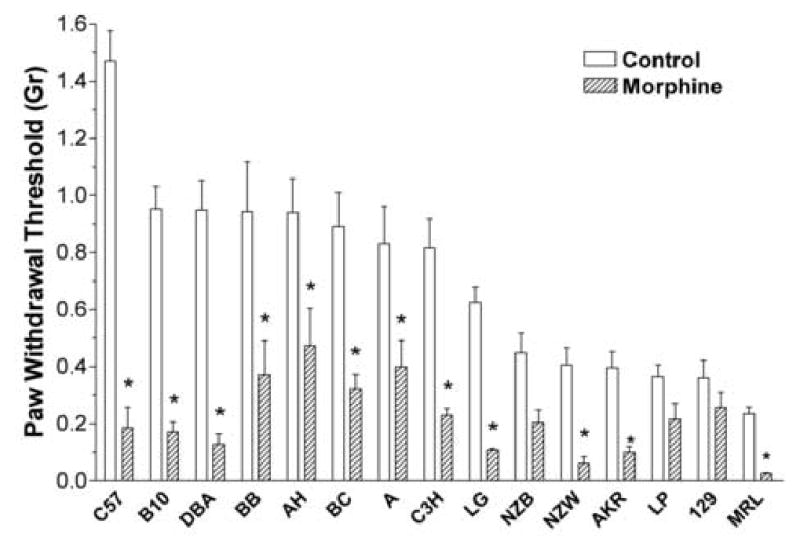
Mechanical nociceptive thresholds for the 15 strains of mice used in these studies. Mechanical thresholds were determined at baseline and after having administered morphine for 4 consecutive days. Data are presented as the means +/− SEM, *P<0.05. Eight mice were used in each group.
Table 1.
Strain-specific mechanical sensitization.
| Strain | OIH-Mechanical |
|---|---|
| 129/SvlmJ | 28.5 |
| A/HeJ | 49.5 |
| A/J | 52.0 |
| AKR/J | 74.8 |
| B10.D2-H2/oSNJ | 82.0 |
| BALB/cByJ | 60.6 |
| BALB/cJ | 63.8 |
| C3H/HeJ | 74.2 |
| C57BL/6J | 87.3 |
| DBA/2J | 86.5 |
| LP/J | 40.7 |
| LG/J | 82.6 |
| MRL/MpJ | 89.0 |
| NZB/BinJ | 54.6 |
| NZW/LaCJ | 85.0 |
Listed are the specific strains of mice used in these experiments along with the degree of opioid-induced hyperalgesia observed after chronic morphine administration. These data were calculated as the percent reduction in mechanical nociceptive thresholds obtained using von Frey fibers after 4 days of exposure to morphine.
In silico mapping based on haplotypic analysis
HapMapper software and an expanded SNP database organized into haplotypic blocks was then used to analyze the data. In this analysis the distribution of the propensity of the strains to develop mechanical hyperalgesia after morphine administration was compared with the partitioning which would be predicted for the strains given the allelic possibilities for each available haplotypic block. Higher correlation resulted from the strain-specific trait data more closely matching the pattern predicted by the haplotypic alleles for any given block. Figure 2A displays the results of the haplotypic mapping as a Cartesian plot with the inverse of the P value plotted against the chromosomal location of the blocks. Figure 2B displays the tabular results of the HapMapper program. Note that each of the color coded blocks under “Haplotype” represents the haplotype for one strain of mice with the leftmost strain being the one with the lowest degree of mechanical OIH (129/SvJ) and the rightmost strain being the one displaying the greatest degree of mechanical OIH (MRL/MpJ). Our most highly correlated block overall, which corresponded to the β2-AR gene (Adrb2), possessed 3 possible haplotypic alleles represented as either a red, green or blue block in Figure 2B. One of the alleles was possessed by only one strain (NZW/LaCJ). The 7 strains having the least tendency to develop mechanical sensitization were of one haplotype while the 7 more extensively sensitized strains had the other haplotypic allele. This particular haplotypic block was defined by a group of 8 SNPs.
Figure 2.
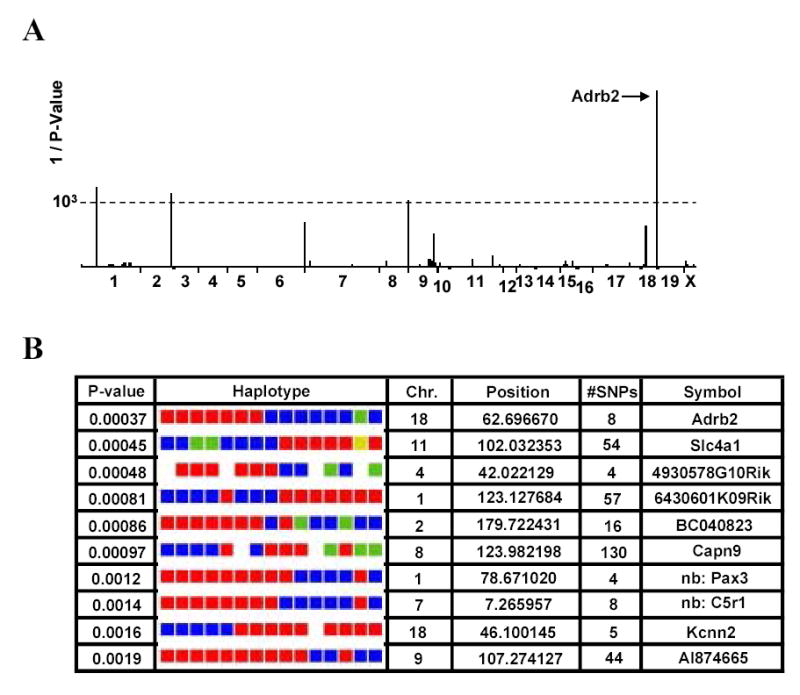
Haplotypic mapping results using data from the 15 strains of mice. For these calculations, the percent decrease in mechanical nociceptive threshold was used as the trait to be mapped. Panel A provides a plot of the significance (1/P) of correlation for each haplotypic block in the database versus the genome as displayed as tandem chromosomes. The block with the highest degree of significance (Adrb2) was part of the gene for the β2 adrenergic receptor. Panel B provides the mapping output in tabular form for the top 10 most strongly linked haplotypic blocks. The P value represents the strength of association. The actual haplotype is color coded such that all strains of mice sharing a haplotype for a specific block also share a color code. For example, the most strongly linked block (Adrb2) has 7 strains sharing the red haplotype, 7 sharing the blue haplotype and one strain uniquely possessing the green type. In this figure the colored blocks are arranged such that the leftmost block represents the strain which displayed the lowest degree of OIH, and the rightmost block is the one which displayed the highest degree of OIH. Also provided is the chromosome on which the haplotypic block is located, the starting position of the block (megabases), and the number of SNPs in the associated block.
Effects of selective β2-AR antagonists on OIH
We next hypothesized that if mechanical allodynia after chronic morphine administration was supported by β2-AR, that the administration of a β2-AR antagonist should reduce that allodynia. Control mice and mice rendered allodynic by the administration of morphine for 4 days were injected with various doses of the selective β2-AR antagonist butoxamine. The C57BL/6J strain was chosen for these experiments as this strain is very commonly used in nociceptive testing, and mechanical OIH is prominent in this strain. The data presented in Figure 3A show the dose-dependent reversal of the opioid-induced allodynia without effects of the drug on mechanical withdrawal thresholds in the control mice. The ED50 for butoxamine reversal of the allodynia was 0.25mg/kg (95% CI 0.08–0.75). Figure 3B demonstrates that butoxamine was capable of reversing the thermal manifestations of OIH as well. Experiments using the selective β2-AR antagonist ICI 118,551 provided similar results (data not shown).
Figure 3.
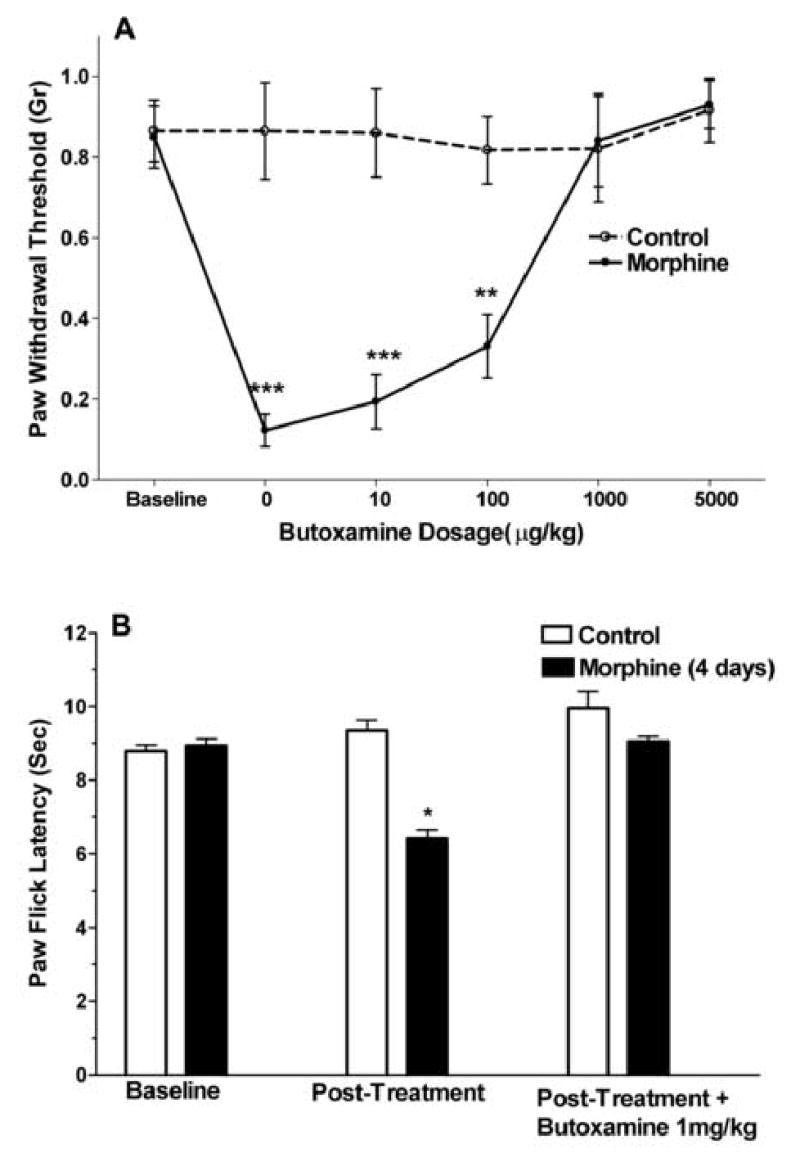
The pharmacological reversal of the mechanical and thermal manifestations of OIH using the selective β2-AR antagonist butoxamine. Mice of the C57BL/6J strain were used after 4 days of morphine treatment to induce OIH or after 4 days of saline administration as a control. In panel A, data representing the measurement of mechanical withdrawal thresholds after the subcutaneous administration of butoxamine are presented. In panel B, control mice or mice treated with morphine were administered a just-maximal dose of butoxamine to determine the effects on the thermal manifestations of OIH. Data are presented as the means +/− SEM, *P<0.05, **P<0.01, ***P<0.001. Eight mice were used in each group.
OIH in β2-AR null mutant mice
We next sought a pharmacologically independent approach to confirming or refuting a role for β2-AR in supporting the mechanical and thermal manifestations of OIH. In these experiments β2-AR null mutants and littermate FVB wild type mice were used. Figure 4 presents data demonstrating that the wild type and null mutant strains had similar baseline mechanical and thermal withdrawal thresholds, but that after morphine treatment, the null mutant mice developed no discernable mechanical allodynia or thermal hyperalgesia. The wild type mice, on the other hand, displayed statistically significant reductions in both the mechanical and thermal indices of OIH. Not all morphine induced changes were different in the β2-AR null mutants, however. The weight loss which characterizes the chronic treatment of mice with morphine was similar for null mutants and wild-type mice (7.5% versus 7.8%, no statistical difference).
Figure 4.
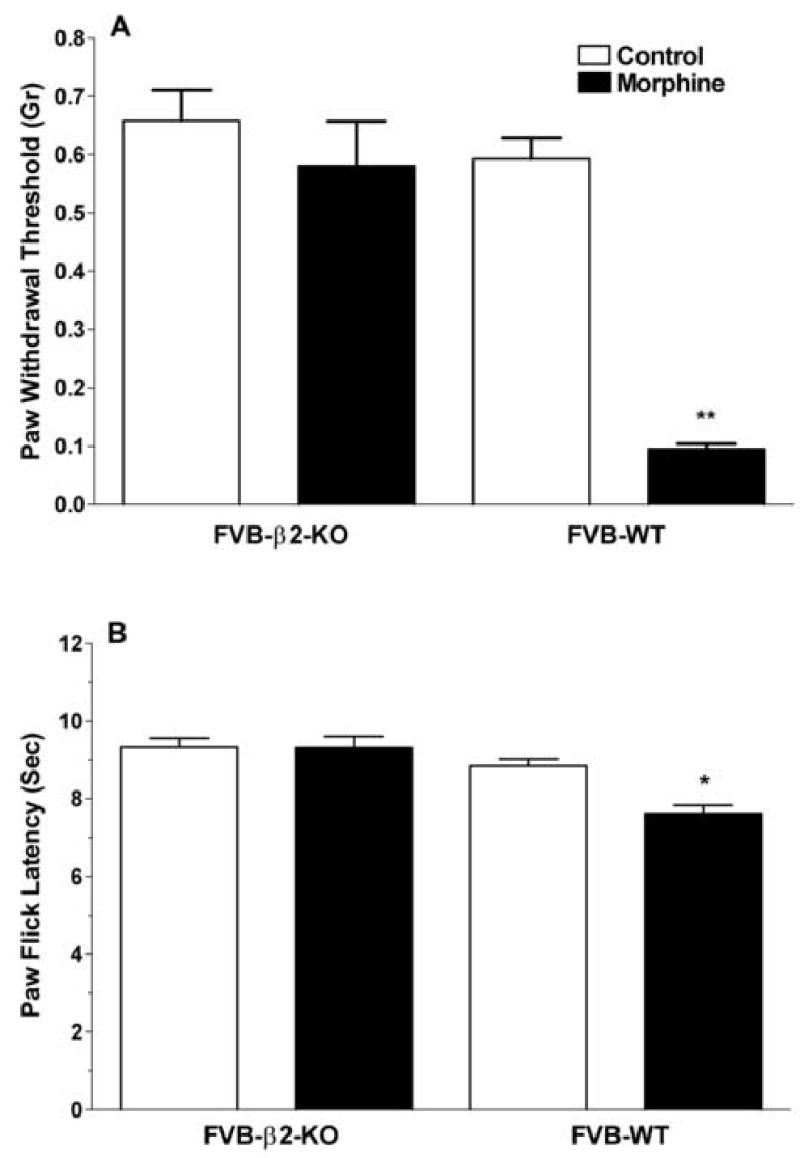
The assessment of mechanical and thermal manifestations of OIH in β2-AR null mutant (knockout) mice versus FVB wild type littermates. Panel A displays data demonstrating mechanical manifestations of OIH in control FVB wild type mice, but no significant sensitization in the β2-AR knockouts after 4 days of morphine administration. Panel B provides similar data using thermal nociceptive measurements. Data are presented as the means +/− SEM, *P<0.05, *P<0.01. Ten mice were used in each group.
Effects of local injections of β2-AR ligands
Because reports in the literature suggest that peripherally expressed β2-ARs support states of enhanced mechanical nociceptive sensitivity 38,39, we undertook studies directed at determining whether the peripheral administration of butoxamine could reduce mechanical-OIH, and conversely whether a selective β2-AR agonist could cause mechanical allodynia if injected locally. Figure 5A demonstrates that the injection of a butoxamine into the plantar surface of the mouse hind paw fully reversed the mechanical sensitization in that paw. The 1μg butoxamine dose chosen had minimal impact when administered systemically (Figure 3). As shown in Figure 5, neither saline nor butoxamine had effects on baseline nociceptive thresholds. Figure 5B shows that the selective β2-AR agonist terbutaline dose-dependently caused mechanical allodynia after local injection supporting the hypothesis that this receptor is an important regulator of nociceptive sensitivity.
Figure 5.
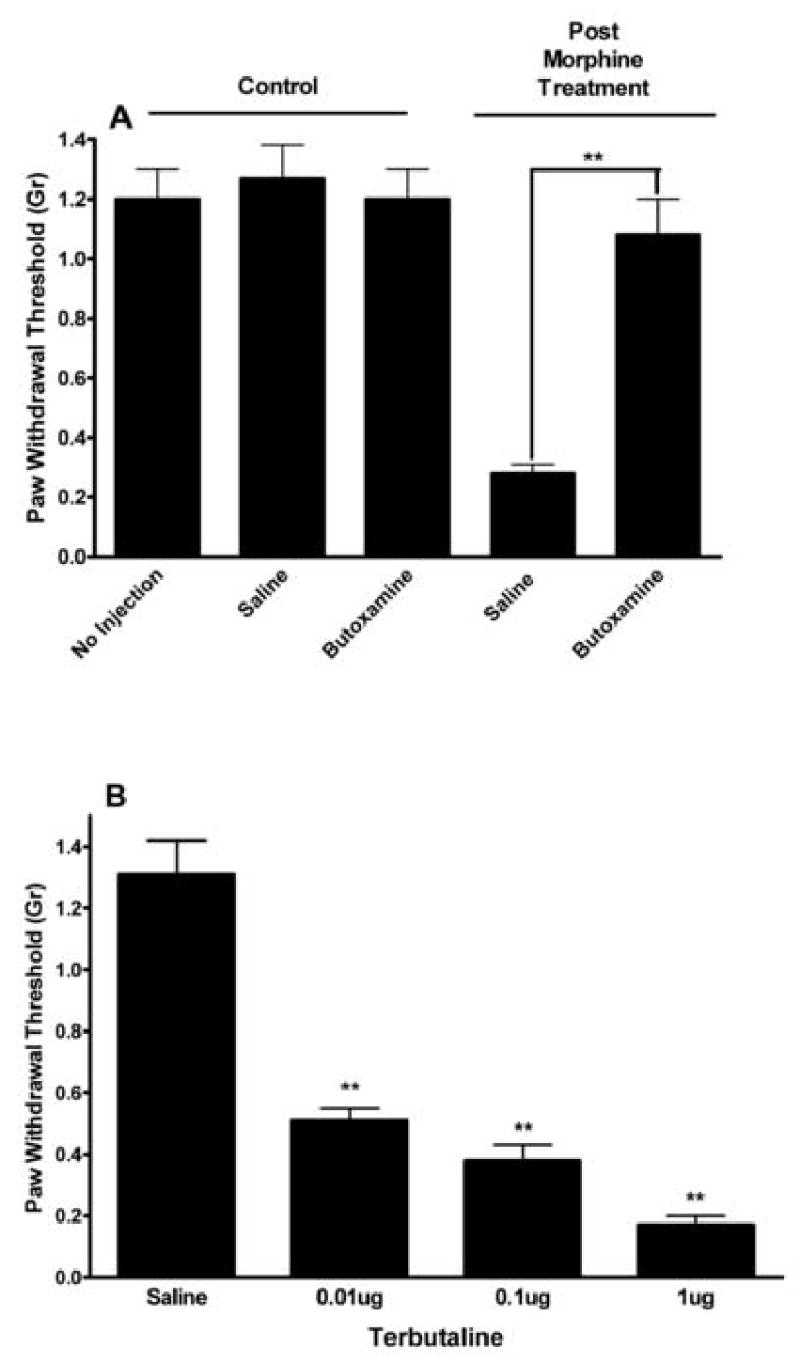
The effects of locally injected β2-AR ligands. In panel A, local injection of saline or butoxamine (1 μg ) had no effect on baseline mechanical nociceptive thresholds, while the injection of 1 μg butoxamine but not saline significantly reversed the allodynia displayed by mice after 4 days of morphine treatment (N=8/group). In panel B, separate groups of 5 mice received plantar injections of saline or each of the doses of terbutaline indicated. Data are presented as the means +/− SEM, **P<0.01.
Structure of the β2-AR haplotype block associated with morphine induced allodynia
Figure 6 provides a diagram of the haplotype block associated with morphine induced mechanical allodynia. This block begins on the 5’ side of the β2-AR gene on chromosome 18 and extends through the coding region to the 3’ side of the gene. The block contains 8 SNP’s. The single SNP located within the coding region of the gene is associated with a silent mutation and thus does not change the receptor’s amino acid sequence.
Figure 6.
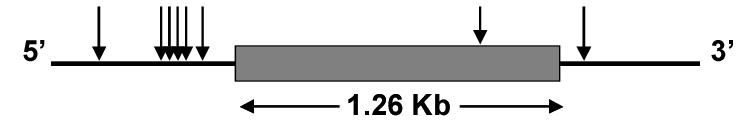
Diagram of the β2-AR haplotypic block associated with OIH. Arrows indicate the relative positions of the 8 SNPs defining the block.
Discussion
The goals of this project were, 1) to use in silico techniques to derive predictions as to what specific genes are associated with the propensity of mice to develop OIH, and 2) to pursue one of these predictions to confirm or refute a functional role in supporting OIH. The data presented in Figure 1 as well as Table 1 demonstrate strong inter-strain differences in the propensity to develop mechanical sensitization. Subsequent in silico genetic mapping identified the β2-AR gene as a candidate gene involved in modulating mechanical OIH in mice (Figure 2). Pharmacological and β2-AR null mutant mouse experiments provided results consistent with the genetic association (Figures 3–5). Ours were not the first efforts to us genetics to investigate pain-related traits. Though specific genes were not identified, previous studies demonstrated influences of genetics on nociceptive measurements of many types 40–42, and other consequences of chronic morphine administration like tolerance and dependence 26–28,43.
The in silico mapping approach used a set of SNPs, (158,000 at the time of analysis) organized into haplotypic blocks using an algorithm described by Wang et al. 31. The significance of naturally occurring haplotypic blocks with respect to genomics and genomic mapping strategies has been reviewed in recent publications 44,45. This technique took advantage of the naturally occurring linkage disequilibrium which exists for the SNPs corresponding to the 2171 genes within the approximately 75Mb of the murine genome for which SNP discovery had been undertaken. As these blocks tend to be small in genomic terms, 39Kb on average, linkage of phenotypic traits to these haplotypic blocks often results in the identification of specific genes associated with the traits of interest. Figure 2 displays our results from these studies. From the group of haplotypic blocks present in the HapMapper database, the one corresponding to the β2-AR was most strongly associated. Though three haplotypes were represented in the 15 strains of mice, 14 strains had one of the two predominant haplotypes. One of the reasons for the high degree of association of this block with mechanical-OIH was the partitioning of the strains into non-overlapping groups by the two predominant haplotypes.
With a spectrum of data in hand implicating β2-AR in OIH, we must ask if these observations fit with existing literature. One set of observations suggesting β2-AR might be involved in opioid-induced hypersensitivity states involves measurements of increased β2-AR density in the central nervous system after chronic exposure of rats to morphine 22–24 as well as the up-regulation of guanosine triphosphate binding proteins, the molecules utilized by β-AR to activate ion channels and second messenger systems 46. In cell culture systems, chronic exposure to morphine enhances stimulated cyclic adenosine monophosphate production by a mechanism involving an increased expression of β2-AR 25. Though the mechanism was not well described, it is interesting to note that serum levels of cyclic adenosine monophosphate were significantly increased in rats treated chronically with morphine as well 47.
Independently reported animal behavioral data supports possible roles for peripherally expressed β2-AR in states of enhanced pain sensitivity. These data lead us to test the hypothesis that the hind paw injection of β-AR antagonists could reduce mechanical OIH. For example, Khasar et al. demonstrated that both epinephrine and the more selective β-AR agonist isoproterenol caused a mechanical hyperalgesia when injected into the hindpaws of rats. This sensitization was blocked by β-AR antagonists 38. In this study the authors went on to provide evidence that the mechanical sensitization might be related to the sensitization of small diameter dorsal root ganglion neurons. In a later study, Aley et al. reproduced the earlier data and provided further evidence that β2-AR was the likely receptor subtype responsible for the sensitization. In additional studies, cultured dorsal root ganglion neurons were found to respond to β2-AR stimulation with phosphorylation of extracellular signal-related kinase (ERK) 39. This type of phosphorylation has been linked to enhanced nociception by many laboratories. Additionally, β2-AR have been shown to enhance inflammation in models of arthritis 48,49 possibly involving enhanced production of tumor necrosis factor α (TNFα)50.
Our own data using the local hind paw injection of butoxamine and terbutaline to decrease and increase mechanical nociceptive sensitivity respectively are consistent with the peripheral β2-AR effects outlined above. An additional factor perhaps amplifying the role of peripheral β2-AR in supporting OIH is the increased levels of circulating catecholamines present after the acute administration of morphine and the increases during opioid abstinence in rodents 51–53. Though we have focused on the periphery, it is possible that in the setting of OIH, β2-AR expressed in other locations participate in modulating the nociceptive sensitization as well. Ultimately our findings will need to be integrated in a model for OIH which includes the other brain and spinal cord level mechanisms which have been described 12,16,19,21.
While the results we obtained are notable for the association made with β2-AR, they are equally notable for the lack of association with genes coding for proteins well demonstrated to modulate OIH. The protein perhaps best associated with the modulation of OIH at this point is the N-methyl-D-aspartate receptor 7,9,10,12,14,17. Haplotype blocks pertaining to the genes coding for the principal subunits of this receptor are represented in the data base used by the haplotypic in silico mapping program we employed. The lack of a high strength association with any of these subunits should not be interpreted as inconsistent with the existing pharmacological data, however. It may be that the N-methyl-D-aspartate receptor is critical in the modulation of the OIH trait, but that the genetic variants of receptor subunits which exist do not lead to important functional differences in the resulting proteins and thus are not likely to be identified in this type of mapping study.
It is also notable that this dataset did not lead to the identification of factors influencing the distribution or elimination of morphine though haplotype blocks pertaining to various drug transporters and metabolic enzymes using morphine as a substrate were included. Simple morphine brain level measurements after acute administration did not predict mechanical OIH. (data not shown). It is possible circulating or brain morphine levels would influence other consequences of chronic morphine administration, however. Similarly, we have completed mapping studies using only morphine. While other opioids can cause OIH, it is not clear that β2-AR would always play as prominent a role.
A number of factors limit the strength and meaning of these mapping results. The first is that while the sets of SNPs and haplotypic blocks used here were relatively large, they were not comprehensive. SNPs are the most common type of genetic polymorphisms. Although one of the advantages of haplotypic analysis is that not all SNPs need to be known to define the common haplotypes, the map we used was far from complete. Our haplotypic mapping likely analyzed only about 1/10th of all murine genes at optimal resolution. Thus it is possible if not likely that some genes influencing OIH were not identified in these studies. Also, while the number of strains used was relatively large (15), many more strains and corresponding SNP data would be needed to have the power to identify all haplotypic blocks associated with complex traits like OIH, opioid tolerance or pain sensitivity. An analysis of the strain dependence of power in haplotypic analysis has been published recently 31. This analysis suggests that the use of 30–40 strains would very substantially enhance the power of this type of study, and will be required for whole genome analyses. The use of 15 strains of mice would result in an power of about 0.8 in detecting causal genetic loci having genetic effects in the range of 0.5, i.e. explaining 50% of the genetic variance. Thus continuing to accumulate strain specific data may allow us to identify more genes involved in this complex trait.
A list of the most useful tools and techniques available for neuroscience research would likely include pharmacological approaches, electrophysiology, molecular biology, the generation of transgenic models, immunohistochemistry and others. We are poised to add in silico murine genetic studies to this list. In this set of studies a relatively simple paradigm for measuring OIH in various strains of readily available inbred mice was used to obtain a dataset which was directly subjected to in silico genetic analysis. Having demonstrated the ability of β2-AR blockade to reduce or eliminate OIH in mice, we are now in position to translate these findings into human studies. It may be possible to determine the ability of β2-AR blockade to reduce OIH in human models 6–8. Conceivably, the addition of β2-AR to a chronic opioid regime might improve the long term efficacy of this form of treatment.
Footnotes
Financial support: This work was supported by a grant from the National Institute on Drug Abuse, Bethesda, MD, USA, #DA017129-01
References
- 1.Compton P, Charuvastra VC, Kintaudi K, Ling W. Pain responses in methadone-maintained opioid abusers. J Pain Symptom Manage. 2000;20:237–45. doi: 10.1016/s0885-3924(00)00191-3. [DOI] [PubMed] [Google Scholar]
- 2.Compton P, Charuvastra VC, Ling W. Pain intolerance in opioid-maintained former opiate addicts: effect of long-acting maintenance agent. Drug Alcohol Depend. 2001;63:139–46. doi: 10.1016/s0376-8716(00)00200-3. [DOI] [PubMed] [Google Scholar]
- 3.Doverty M, White JM, Somogyi AA, Bochner F, Ali R, Ling W. Hyperalgesic responses in methadone maintenance patients. Pain. 2001;90:91–6. doi: 10.1016/s0304-3959(00)00391-2. [DOI] [PubMed] [Google Scholar]
- 4.Doverty M, Somogyi AA, White JM, Bochner F, Beare CH, Menelaou A, Ling W. Methadone maintenance patients are cross-tolerant to the antinociceptive effects of morphine. Pain. 2001;93:155–63. doi: 10.1016/S0304-3959(01)00306-2. [DOI] [PubMed] [Google Scholar]
- 5.Chu LF, Clark DJ, Angst MS: Opioid Tolerance and Hyperalgesia in Chronic Pain Patients After One Month of Oral Morphine Therapy: A Preliminary Prospective Study. J Pain: (In Press) [DOI] [PubMed]
- 6.Angst MS, Koppert W, Pahl I, Clark DJ, Schmelz M. Short-term infusion of the mu-opioid agonist remifentanil in humans causes hyperalgesia during withdrawal. Pain. 2003;106:49–57. doi: 10.1016/s0304-3959(03)00276-8. [DOI] [PubMed] [Google Scholar]
- 7.Koppert W, Sittl R, Scheuber K, Alsheimer M, Schmelz M, Schuttler J. Differential modulation of remifentanil-induced analgesia and postinfusion hyperalgesia by S-ketamine and clonidine in humans. Anesthesiology. 2003;99:152–9. doi: 10.1097/00000542-200307000-00025. [DOI] [PubMed] [Google Scholar]
- 8.Hood DD, Curry R, Eisenach JC. Intravenous remifentanil produces withdrawal hyperalgesia in volunteers with capsaicin-induced hyperalgesia. Anesth Analg. 2003;97:810–5. doi: 10.1213/01.ANE.0000078811.80093.88. [DOI] [PubMed] [Google Scholar]
- 9.Celerier E, Laulin J, Larcher A, Le Moal M, Simonnet G. Evidence for opiate-activated NMDA processes masking opiate analgesia in rats. Brain Res. 1999;847:18–25. doi: 10.1016/s0006-8993(99)01998-8. [DOI] [PubMed] [Google Scholar]
- 10.Celerier E, Rivat C, Jun Y, Laulin JP, Larcher A, Reynier P, Simonnet G. Long-lasting hyperalgesia induced by fentanyl in rats: preventive effect of ketamine. Anesthesiology. 2000;92:465–72. doi: 10.1097/00000542-200002000-00029. [DOI] [PubMed] [Google Scholar]
- 11.Ibuki T, Dunbar SA, Yaksh TL. Effect of transient naloxone antagonism on tolerance development in rats receiving continuous spinal morphine infusion. Pain. 1997;70:125–32. doi: 10.1016/s0304-3959(96)03283-6. [DOI] [PubMed] [Google Scholar]
- 12.Mao J, Price DD, Mayer DJ. Thermal hyperalgesia in association with the development of morphine tolerance in rats: roles of excitatory amino acid receptors and protein kinase C. J Neurosci. 1994;14:2301–12. doi: 10.1523/JNEUROSCI.14-04-02301.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aley KO, Green PG, Levine JD. Opioid and adenosine peripheral antinociception are subject to tolerance and withdrawal. J Neurosci. 1995;15:8031–8. doi: 10.1523/JNEUROSCI.15-12-08031.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li X, Angst MS, Clark JD. A murine model of opioid-induced hyperalgesia. Brain Res Mol Brain Res. 2001;86:56–62. doi: 10.1016/s0169-328x(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 15.Li X, Angst MS, Clark JD. Opioid-induced hyperalgesia and incisional pain. Anesth Analg. 2001;93:204–9. doi: 10.1097/00000539-200107000-00040. [DOI] [PubMed] [Google Scholar]
- 16.Vanderah TW, Suenaga NM, Ossipov MH, Malan TP, Jr, Lai J, Porreca F. Tonic descending facilitation from the rostral ventromedial medulla mediates opioid-induced abnormal pain and antinociceptive tolerance. J Neurosci. 2001;21:279–86. doi: 10.1523/JNEUROSCI.21-01-00279.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Celerier E, Laulin JP, Corcuff JB, Le Moal M, Simonnet G. Progressive enhancement of delayed hyperalgesia induced by repeated heroin administration: a sensitization process. J Neurosci. 2001;21:4074–80. doi: 10.1523/JNEUROSCI.21-11-04074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Laulin JP, Larcher A, Celerier E, Le Moal M, Simonnet G. Long-lasting increased pain sensitivity in rat following exposure to heroin for the first time. Eur J Neurosci. 1998;10:782–5. doi: 10.1046/j.1460-9568.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- 19.Mao J, Sung B, Ji RR, Lim G. Chronic morphine induces downregulation of spinal glutamate transporters: implications in morphine tolerance and abnormal pain sensitivity. J Neurosci. 2002;22:8312–23. doi: 10.1523/JNEUROSCI.22-18-08312.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.King T, Gardell LR, Wang R, Vardanyan A, Ossipov MH, Philip Malan T, Jr, Vanderah TW, Hunt SP, Hruby VJ, Lai J, Porreca F. Role of NK-1 neurotransmission in opioid-induced hyperalgesia. Pain. 2005;116:276–88. doi: 10.1016/j.pain.2005.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X, Clark JD. Hyperalgesia during opioid abstinence: mediation by glutamate and substance p. Anesth Analg. 2002;95:979–84. doi: 10.1097/00000539-200210000-00035. table of contents. [DOI] [PubMed] [Google Scholar]
- 22.Moises HC, Smith CB. Electrophysiological responsiveness to isoproterenol in rat hippocampal slices correlates with changes in beta-adrenergic receptor density induced by chronic morphine treatment. Brain Res. 1989;485:67–78. doi: 10.1016/0006-8993(89)90667-7. [DOI] [PubMed] [Google Scholar]
- 23.Moises HC, Smith CB. Changes in cortical beta-adrenergic receptor density and neuronal sensitivity to norepinephrine accompany morphine dependence and withdrawal. Brain Res. 1987;400:110–26. doi: 10.1016/0006-8993(87)90658-5. [DOI] [PubMed] [Google Scholar]
- 24.Moises HC, Smith CB. Changes occur in central adrenoreceptor function following long-term morphine treatment and during morphine withdrawal. Neuropeptides. 1984;5:29–32. doi: 10.1016/0143-4179(84)90019-2. [DOI] [PubMed] [Google Scholar]
- 25.Ammer H, Schulz R. Chronic morphine treatment increases stimulatory beta-2 adrenoceptor signaling in A431 cells stably expressing the mu opioid receptor. J Pharmacol Exp Ther. 1997;280:512–20. [PubMed] [Google Scholar]
- 26.Kest B, Hopkins E, Palmese CA, Adler M, Mogil JS. Genetic variation in morphine analgesic tolerance: a survey of 11 inbred mouse strains. Pharmacol Biochem Behav. 2002;73:821–8. doi: 10.1016/s0091-3057(02)00908-5. [DOI] [PubMed] [Google Scholar]
- 27.Kest B, Palmese CA, Hopkins E, Adler M, Juni A, Mogil JS. Naloxone-precipitated withdrawal jumping in 11 inbred mouse strains: evidence for common genetic mechanisms in acute and chronic morphine physical dependence. Neuroscience. 2002;115:463–9. doi: 10.1016/s0306-4522(02)00458-x. [DOI] [PubMed] [Google Scholar]
- 28.Kest B, Palmese CA, Juni A, Chesler EJ, Mogil JS. Mapping of a quantitative trait locus for morphine withdrawal severity. Mamm Genome. 2004;15:610–7. doi: 10.1007/s00335-004-2367-3. [DOI] [PubMed] [Google Scholar]
- 29.Grupe A, Germer S, Usuka J, Aud D, Belknap JK, Klein RF, Ahluwalia MK, Higuchi R, Peltz G. In silico mapping of complex disease-related traits in mice. Science. 2001;292:1915–8. doi: 10.1126/science.1058889. [DOI] [PubMed] [Google Scholar]
- 30.Liao G, Wang J, Guo J, Allard J, Cheng J, Ng A, Shafer S, Puech A, McPherson JD, Foernzler D, Peltz G, Usuka J. In silico genetics: identification of a functional element regulating H2-Ealpha gene expression. Science. 2004;306:690–5. doi: 10.1126/science.1100636. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Liao G, Usuka J, Peltz G. Computational genetics: from mouse to human? Trends Genet. 2005;21:526–32. doi: 10.1016/j.tig.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 32.Savov JD, Whitehead GS, Wang J, Liao G, Usuka J, Peltz G, Foster WM, Schwartz DA. Ozone-induced acute pulmonary injury in inbred mouse strains. Am J Respir Cell Mol Biol. 2004;31:69–77. doi: 10.1165/rcmb.2003-0001OC. [DOI] [PubMed] [Google Scholar]
- 33.Chruscinski AJ, Rohrer DK, Schauble E, Desai KH, Bernstein D, Kobilka BK. Targeted disruption of the beta2 adrenergic receptor gene. J Biol Chem. 1999;274:16694–700. doi: 10.1074/jbc.274.24.16694. [DOI] [PubMed] [Google Scholar]
- 34.Liang D, Li X, Lighthall G, Clark JD. Heme oxygenase type 2 modulates behavioral and molecular changes during chronic exposure to morphine. Neuroscience. 2003;121:999–1005. doi: 10.1016/s0306-4522(03)00483-4. [DOI] [PubMed] [Google Scholar]
- 35.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 36.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg. 1998;87:941–8. doi: 10.1097/00000539-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 37.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 38.Khasar SG, McCarter G, Levine JD. Epinephrine produces a beta-adrenergic receptor-mediated mechanical hyperalgesia and in vitro sensitization of rat nociceptors. J Neurophysiol. 1999;81:1104–12. doi: 10.1152/jn.1999.81.3.1104. [DOI] [PubMed] [Google Scholar]
- 39.Aley KO, Martin A, McMahon T, Mok J, Levine JD, Messing RO. Nociceptor sensitization by extracellular signal-regulated kinases. J Neurosci. 2001;21:6933–9. doi: 10.1523/JNEUROSCI.21-17-06933.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception I: responses of 11 inbred mouse strains on 12 measures of nociception. Pain. 1999;80:67–82. doi: 10.1016/s0304-3959(98)00197-3. [DOI] [PubMed] [Google Scholar]
- 41.Mogil JS, Wilson SG, Bon K, Lee SE, Chung K, Raber P, Pieper JO, Hain HS, Belknap JK, Hubert L, Elmer GI, Chung JM, Devor M. Heritability of nociception II. 'Types' of nociception revealed by genetic correlation analysis. Pain. 1999;80:83–93. doi: 10.1016/s0304-3959(98)00196-1. [DOI] [PubMed] [Google Scholar]
- 42.Lariviere WR, Wilson SG, Laughlin TM, Kokayeff A, West EE, Adhikari SM, Wan Y, Mogil JS. Heritability of nociception. III Genetic relationships among commonly used assays of nociception and hypersensitivity. Pain. 2002;97:75–86. doi: 10.1016/s0304-3959(01)00492-4. [DOI] [PubMed] [Google Scholar]
- 43.Nitsche JF, Schuller AG, King MA, Zengh M, Pasternak GW, Pintar JE. Genetic dissociation of opiate tolerance and physical dependence in delta-opioid receptor-1 and preproenkephalin knock-out mice. J Neurosci. 2002;22:10906–13. doi: 10.1523/JNEUROSCI.22-24-10906.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.van den Oord EJ, Neale BM. Will haplotype maps be useful for finding genes? Mol Psychiatry. 2004;9:227–36. doi: 10.1038/sj.mp.4001449. [DOI] [PubMed] [Google Scholar]
- 45.Belmont JW, Gibbs RA. Genome-wide linkage disequilibrium and haplotype maps. Am J Pharmacogenomics. 2004;4:253–62. doi: 10.2165/00129785-200404040-00005. [DOI] [PubMed] [Google Scholar]
- 46.Kaewsuk S, Hutamekalin P, Ketterman AJ, Khotchabhakdi N, Govitrapong P, Casalotti SO. Morphine induces short-lived changes in G-protein gene expression in rat prefrontal cortex. Eur J Pharmacol. 2001;411:11–16. doi: 10.1016/s0014-2999(00)00768-8. [DOI] [PubMed] [Google Scholar]
- 47.Nakaki T, Muraki T, Tokunaga Y, Kato R. Plasma cyclic AMP in the morphine-tolerant rat. Biochem Pharmacol. 1981;30:2217–20. doi: 10.1016/0006-2952(81)90090-3. [DOI] [PubMed] [Google Scholar]
- 48.Levine JD, Coderre TJ, Helms C, Basbaum AI. Beta 2-adrenergic mechanisms in experimental arthritis. Proc Natl Acad Sci U S A. 1988;85:4553–6. doi: 10.1073/pnas.85.12.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Basbaum AI, Levine JD. The contribution of the nervous system to inflammation and inflammatory disease. Can J Physiol Pharmacol. 1991;69:647–51. doi: 10.1139/y91-096. [DOI] [PubMed] [Google Scholar]
- 50.Chou RC, Dong XL, Noble BK, Knight PR, Spengler RN. Adrenergic regulation of macrophage-derived tumor necrosis factor-alpha generation during a chronic polyarthritis pain model. J Neuroimmunol. 1998;82:140–8. doi: 10.1016/s0165-5728(97)00196-3. [DOI] [PubMed] [Google Scholar]
- 51.Conway EL, Brown MJ, Dollery CT. Plasma catecholamine and cardiovascular responses to morphine and D-ala2-d-leu5-enkephalin in conscious rats. Arch Int Pharmacodyn Ther. 1983;265:244–58. [PubMed] [Google Scholar]
- 52.Delle M, Thoren P. Changes in sympathetic nerve activity during morphine abstinence in the rat. Acta Physiol Scand. 1987;130:47–54. doi: 10.1111/j.1748-1716.1987.tb08110.x. [DOI] [PubMed] [Google Scholar]
- 53.Delle M, Ricksten SE, Thoren P. Renal sympathetic nerve activity during morphine abstinence in sino-aortic baroreceptor-denervated rats. Acta Physiol Scand. 1988;134:479–91. doi: 10.1111/j.1748-1716.1998.tb08522.x. [DOI] [PubMed] [Google Scholar]


