Abstract
Apoptosis has been implicated in the regulation of denervation-induced muscle atrophy. However, the activation of apoptotic signal transduction during muscle denervation has not been fully elucidated. The present study examined the apoptotic responses to denervation in rat gastrocnemius muscle. Following 14 days of denervation, the extent of apoptotic DNA fragmentation as determined by a cytosolic nucleosome ELISA was increased by 100% in the gastrocnemius muscle. RT-PCR and immunoblot analyses indicated that Bax was dramatically upregulated while Bcl-2 was modestly increased; however, the Bax/Bcl-2 ratio was significantly increased in denervated muscles relative to control muscles. Analyses of ELISA and immunoblots from mitochondria-free cytosol extracts showed a significant increase in mitochondria-associated apoptotic factors, including cytochrome c, Smac/DIABLO and apoptosis-inducing factor (AIF). In addition to the upregulation of caspase-3 and -9 mRNA, pro-/cleaved caspase protein and proteolytic activity levels, the X-linked inhibitor of apoptosis (XIAP) protein level was downregulated. The cleaved product of poly(ADP-ribose) polymerase (PARP) was detected in muscle samples following denervation. Although we did not find a difference in the inhibitor of DNA binding/ differentiation-2 (Id2) and c-Myc protein contents between the denervated and control muscles, the protein content of tumour suppressor p53 was significantly increased in both the nuclear and the cytosolic fractions with denervation. Moreover, denervation increased the protein content of HSP70, whereas the MnSOD (a mitochondrial isoform of superoxide dismutase) protein content was diminished, which indicated that denervation might have induced cellular and/or oxidative stress. Our data show that mitochondria-associated apoptotic signalling is upregulated during muscle denervation. We interpret these findings to indicate that apoptosis has a physiologically important role in regulating denervation-induced muscle atrophy.
Apoptosis is an active, internal-encoded biological process that requires regulation of specific genes to tightly coordinate the corresponding apoptotic events (Steller, 1995; Yuan, 1995; Danial & Korsmeyer, 2004). It has been widely accepted that apoptotic cell death is crucial in monitoring the balance between cell survival and death in mitotic cell lineages, and it has been recognized to be an active regulatory process to degenerate or remove abnormal or damaged tissues (Ellis et al. 1991; Thompson, 1995). The role of apoptosis in health and disease has been established by demonstrating that the pathogenesis of several severe diseases, including various cancers, acquired immune deficiency syndrome, autoimmune diseases, viral infections and neurodegenerative diseases, is attributed to the aberrant regulation of apoptosis (Williams, 1991; Thompson, 1995; Duke et al. 1996; Yuan & Yankner, 2000). Recently, apoptosis has also been reported in postmitotic skeletal muscle under certain physiological and pathophysiological conditions (e.g. muscle denervation, muscle dystrophy, neuromuscular disorders, hindlimb unweighting, muscle unloading, strenuous physical exercise and ageing-associated sarcopenia) (Sandri et al. 1995, 1997, 1998; Allen et al. 1997; Tews, 2002; Dirks & Leeuwenburgh, 2002; Pollack et al. 2002; Alway et al. 2003; Leeuwenburgh, 2003; Dirks & Leeuwenburgh, 2004), and these consistent observations of activated apoptotic machinery under muscle atrophic conditions call attention to the existence of a physiological role of apoptosis in regulating the atrophic process of muscle remodelling during disuse or inactivity.
Among various experimental models of muscle atrophy, skeletal muscle denervation has been actively adopted to investigate the regulation of disuse-induced atrophy, including denervation resulting from the degeneration of motor neurones, which is closely associated with the pathogenesis of severe neurological diseases such as amyotrophic lateral sclerosis (Brown, 1997). Moreover, it has been suggested that muscle denervation may be involved in significant ageing-associated loss of muscle (i.e. sarcopenia). This is based on the histochemical evidence in aged muscles, including increased fibre number per motor unit, fibre-type grouping, disproportionate atrophy of type IIa muscle fibres, and increased coexpression of myosin heavy chain isoforms in the same fibre showing that a progressive denervation and reinnervation process continuously occurs in the aged skeletal muscle (Brown, 1972; Essen-Gustavsson & Borges, 1986; Doherty et al. 1993; Larsson, 1995). Providing that nerve innervation is essential to the growth and maintenance of skeletal myofibres (Hughes, 1998; Trachtenberg, 1998; Pette, 2001), it is certain that loss and/or dysfunction of motoneurones with neurological diseases and ageing leads to muscle atrophy and myocyte death.
There is evidence that apoptosis may regulate, at least in part, denervation-induced muscle atrophy (Migheli et al. 1997; Tews et al. 1997; Yoshimura & Harii, 1999; Borisov & Carlson, 2000; Olive & Ferrer, 2000; Tang et al. 2000; Jin et al. 2001; Jejurikar et al. 2002; Alway et al. 2003). Nevertheless, the underlying mechanism(s) accounting for the activation of apoptosis and its physiological role during denervation remain largely unknown. Therefore, the purpose of the present study was to examine apoptotic signalling and the apoptosis-associated cellular responses during denervation in skeletal muscle. We hypothesized that apoptosis is activated as a result of the promotion of mitochondria-associated pro-apoptotic factors, whereas the anti-apoptotic factors are suppressed in denervated muscle.
Methods
Animals
Experiments were conducted on eight 6-month-old adult Fischer344× Brown Norway rats that were obtained from the NIA colony. The rats were housed in pathogen-free conditions at ∼20°C. They were exposed to a reverse light condition of 12:12 h light:darkness each day, and they were fed rat chow and water ad libitum throughout the study period.
Denervation procedure
The animals were placed under a general anaesthesia using 2% isoflurane. After reflex activity had disappeared, an incision was made from the calcaneous to just proximal to the popliteal fossa. The tibial nerve was then dissected proximal to the cranial border of the gastrocnemius muscle. Care was taken to avoid any damage to the nerves, blood vessels and connective tissues. The medial and lateral branches of the tibial nerve that innervate the plantar flexor muscles (i.e. gastrocnemius and soleus) were transected close to their neuromuscular junction (Degens et al. 1995). The cut nerve ends were sutured into the biceps femoris muscle to ensure that the nerve stumps did not reinnervate the gastrocnemius muscle. Innervation to the plantaris and the deep toe flexor muscles were left intact so that the animals ambulated normally around the cage after the surgical denervation. Following the surgery, the hamstring muscle layers were closed with reabsorbable suture, and the skin incisions were closed with wound clips. The incision sites were covered with an antibacterial cream to prevent infection. The contralateral limb served as the intra-animal control. We have observed that the animals recovered quickly and were able to walk around within ∼45 min postsurgery (Alway et al. 2002, 2003).
Fourteen days after the surgical denervation, animals were anaesthetized (ketamine hydrochloride, 9 mg (100 g body weight)−1 and xylazine hydrochloride 1 mg (100 g body weight)−1, i.p.) and the gastrocnemius muscles from each limb were dissected from the surrounding connective tissues, removed, and stored at −80°C. Animals were subsequently killed with an overdose of pentobarbital sodium (5 mg (g body weight)−1, i.p.). The institutional animal use and care committee from West Virginia University School of Medicine approved all experiments. The animal care standards were followed by adhering to the recommendations for the care of laboratory animals, as advocated by the American Association for Accreditation of Laboratory Animal Care, and they fully conformed with the Animal Welfare Act of the US Department of Health and Human Services.
RT-PCR
Total RNA was extracted from the gastrocnemius muscle of both denervated and control muscles with TriReagent (Molecular Research Center, Cincinnati, OH, USA), which is based on the guanidine thiocyanate method. Frozen muscle was mechanically homogenized on ice in 1 ml of ice-cold TriReagent. Total RNA was solubilized in RNase-free H2O, and quantified in duplicate by measuring the optical density (OD) at 260 nm. Purity of RNA was assured by examining the OD260/OD280 ratio. Five micrograms of RNA was reverse transcribed with decamer primers and Superscript II reverse transcriptase (RT) in a total volume of 20 μl, according to standard methods (Invitrogen Life Technologies, Bethesda, MD, USA). Control RT reaction was done in which the RT enzyme was omitted. The control RT reaction was PCR amplified to ensure that DNA did not contaminate the RNA. One microlitre of complementary DNA (cDNA) was then amplified by PCR using 100 ng of forward and reverse primers, ribosomal 18S primer pairs (Ambion, TX, USA), 250 μm deoxyribonucleotide triphosphates (dNTPs), 1 × PCR buffer and 2.5 units Taq DNA polymerase (USB Corp., Cleveland, OH, USA) in a final volume of 50 μl. PCR was performed using a programmed thermocycler (Biometra, Göttingen, Germany). The primer pairs were designed from sequences published in GenBank (Table 1), and PCR products were verified by restriction digestions. Preliminary experiments were conducted with each gene to assure that the number of cycles represented a linear portion for the PCR OD curve for the muscle samples. The cDNAs from all muscle samples were amplified simultaneously using aliquots from the same PCR mixture. After the PCR amplification, 30 μl of each reaction was electrophoresed on 1.5% agarose gels, and stained with ethidium bromide. Images were captured, and the signals were quantified in arbitrary units as OD × band area using Kodak image analysis system (Eastman Kodak, Rochester, NY, USA). The size (number of base pairs) of each of the bands corresponded to the size of the processed mRNA. Ribosomal 18S primers were used as internal controls, while all RT-PCR signals were normalized to the 18S signal of the corresponding RT product to eliminate the measurement error from uneven sample loading, and provide a semiquantitative measure of the relative changes in gene expression.
Table 1.
Primers used for PCR amplification of cDNA
| Product | Accession no. | Sequence | Position | TA (°C) | Cycles | Product length (bp) | Restriction enzyme | Restriction products |
|---|---|---|---|---|---|---|---|---|
| Bcl-2 | NM_016993 | F: 5′-CCGGGACGCGAAGTGCTATTGGTAC-3′ | 187–211 | 55 | 39 | 950 | BamHI | 602, 348 |
| R:5′-AGCTGATTTGACCATTTGCCTGAA-3′ | 1113–1136 | Bg1I | 686,264 | |||||
| BstXI | 572, 438 | |||||||
| DraI | 865, 85 | |||||||
| Bax | AF235993 | F:5′-GCACCCCTTTCCTCCTCTCTCCACCAG-3′ | 462–488 | 55 | 39 | 654 | BamHI | 445, 209 |
| R:5′-TGCCTTTCCCCGTTCCCCATTCATC-3′ | 1091–1115 | PstI | 431, 223 | |||||
| BstXI | 521, 133 | |||||||
| AIF | AF375656 | F:5′-CCGGCTTCCAGGCAACTTGTTCC-3′ | 93–115 | 58.1 | 33 | 359 | KpnI | 290, 69 |
| R:5′-CCCGGATGGATCTAGCTGCTGCA-3′ | 429–451 | PstI | 259, 100 | |||||
| Apaf-1 | AF218388 | F:5′-CGGCCCTGCGCATCTGATTCAT-3′ | 1623–1644 | 57.8 | 36 | 288 | AluI | 193, 95 |
| R:5′-GGGCGAACGACTAAGCGGGACAG-3′ | 1888 –1910 | PstI | 198, 90 | |||||
| Caspase-3 | NM_012922 | F:5′-TGGCCCTGAAATACGAAGTC-3′ | 262–281 | 53.3 | 36 | 339 | RsaI | 260, 79 |
| R:5′-CGGCCTCCACTGGTATCT-3′ | 583–600 | SpeI | 183, 156 | |||||
| AluI | 266, 73 | |||||||
| Caspase-9 | NM_031632 | F:5′-GGCCGGTGGACATTGGTTCTGG-3′ | 543–564 | 55 | 36 | 222 | BamHI | 130, 92 |
| R:5′-CCATGAAGCGCAGCCAGCAGAA-3′ | 743–764 | HindIII | 186, 36 |
TA, annealing temperature; Accession no., GenBank accession number; F, forward primer; R, reverse primer.
Subcellular protein fractionation
The fractionation method described by Rothermel et al. (2000) was adopted with minor modification to extract the cytosolic and nuclear protein fractions from the gastrocnemius muscle. We have previously obtained the fractionated cytosolic and nuclear proteins from skeletal and heart muscles using this modified protocol (Siu & Alway, 2004; Siu et al. 2004a, 2005). Briefly, after removal of connective tissues, muscle was homogenized on ice in ice-cold lysis buffer (10 mm NaCl, 1.5 mm MgCl2, 20 mm Hepes, pH 7.4 20% glycerol, 0.1% Triton X-100, 1 mm DTT). Following centrifugation at 880 g for 3 min at 4°C to pellet the nuclei and cell debris, the supernatants were collected and subjected to further centrifugation three times at 3 500 g for 5 min at 4°C to remove residual nuclei, and stored as nuclei-free total cytosolic protein fractions. A portion of the cytosolic extract (without addition of protease inhibitors) was stored and used for fluorometric caspase protease activity assay, while a protease inhibitor cocktail containing 104 mm 4-[2-aminoethyl]-benzenesulfonylflouride hydrochloride (AEBSF), 0.08 mm aprotinin, 2 mm leupeptin, 4 mm bestatin, 1.5 mm pepstatin A and 1.4 mm E-64 (Sigma-Aldrich, St Louis, MO, USA) was added to the remaining portion. The cytosolic protein fraction with the addition of protease inhibitors was later used for cell-death ELISA and Western immunoblots. The remaining nuclear pellets were washed three times with ice-cold lysis buffer, resuspended in 300 μl of lysis buffer in the presence of 41.5 μl of 5 m NaCl and protease inhibitor cocktail, and rotated for 1 h at 4°C to lyse the nuclei. Following a spin at 21 900 g for 15 min at 4°C, the supernatants were collected and stored as cytosol-free nuclear protein fractions.
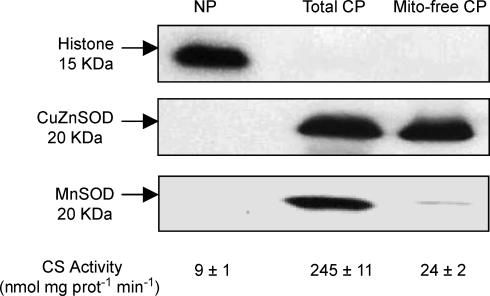
Furthermore, with the intention of estimating the release of mitochondria-residing apoptotic factors, including cytochrome c, apoptosis-inducing factor (AIF), and Smac/DIABLO, into the cytosol, a nuclei-free, mitochondria-free cytosolic protein fraction was prepared as described by Rokhlin et al. (2002), and the protein contents of these mitochondrial apoptotic factors were then measured in this mitochondria-free cytosolic fraction as described in later sections. For the extraction, muscle was dissected from the connective tissues, and minced in ice-cold extraction buffer (250 mm sucrose, 20 mm Hepes, 10 mm KCl, 1.5 mm MgCl2, 1 mm EDTA, 1 mm EGTA, 1 mm DTT and 0.1 mm phenylmethylsulphonyl fluoride, pH 7.4) in the presence of the protease inhibitor cocktail. Following a gentle homogenization with a Teflon pestle motorized with an electronic stirrer, homogenates were centrifuged at 800 g for 10 min at 4°C to pellet the nuclei and cell debris. The supernatants were then spun twice at 16 000 g for 20 min at 4°C to pellet the mitochondria, and the final supernatants were collected as nuclei-free, mitochondria-free cytosolic protein fractions. The purity of the extracted fractions was examined by immunoblotting the extracted fractions with an anti-histone H2B (a nuclear protein) rabbit polyclonal antibody (1:2000 dilution, 07371; Upstate, Lake Placid, NY, USA), an anti-CuZnSOD (a cytosolic isoform of superoxide dismutase) polyclonal rabbit antibody (1:500 dilution, sc-11407; Santa Cruz Biotechnology, Santa Cruz, CA, USA), and an anti-MnSOD (a mitochondrial isoform of superoxide dismutase) goat antibody (1:2000 dilution, A300449A; Bethyl Laboratory, Montgomery, TX, USA) (Fig. 1). In order to further verify the purity, especially that of the mitochondria-free cytosolic fraction, a kinetic enzyme activity measurement of citrate synthase (a mitochondrial oxidative enzyme) was performed in the extracted fractions as described (Fig. 1) (Siu et al. 2003, 2004b).
Figure 1. Verification of purity of protein extraction.
The purity of extracted protein fractions was verified by immunoblotting the extracted fractions with an anti-histone H2B, an anti-CuZnSOD and an anti-MnSOD antibody, and assessing the citrate synthase activity. NP, nuclear protein fraction; Total CP, total cytosolic protein fraction; Mito-free CP, mitochondria-free cytosolic protein fraction.
The protein contents of the muscle extracts were quantified in duplicate by the BCA Protein Assay (Pierce, Rockford, IL, USA) based on the biuret reaction and the bicinchoninic acid detection of cuprous cation (Smith et al. 1985). As a further means to confirm the protein contents, all the protein samples were measured in duplicate on a different occasion by DC Protein Assay (Bio-Rad, Hercules, CA, USA) based on the reaction of protein with an alkaline copper tartrate solution and Folin reagent, which was similar to Lowry assay (Lowry et al. 1951).
Apoptotic cell death ELISA
Cell death detection ELISA kit (Roche Applied Science, Indianapolis, IN, USA) was used to quantitatively determine the apoptotic DNA fragmentation by measuring the cytosolic histone-associated mono- and oligonucleosomes. Briefly, the nuclei-free cytosolic fraction of gastrocnemius muscle was used as an antigen source in a sandwich ELISA with a primary anti-histone mouse monoclonal antibody coated to the microtitre plate, and a second anti-DNA mouse monoclonal antibody coupled to peroxidase. The amount of peroxidase retained in the immunocomplex was determined photometrically by incubating with 2,2′-azino-di-[3-ethylbenzthiazoline sulphonate] (ABTS) as a substrate for 10 min at 20°C. The change in colour was measured at a wavelength of 405 nm by using a Dynex MRX plate reader controlled through PC software (Revelation; Dynatech Laboratories, CA, USA). Measurements were performed in duplicate, with denervated and contralateral control samples analysed on the same microtitre plate in the same setting. The OD405 reading was then normalized to the milligrams of protein used in the assay.
Fluorometric caspase activity assay
Previously, caspase-3, -6, -7 and caspase-8 protease activities in the denervated muscle have previously been assessed by a colourimetric method (Alway et al. 2003). In the present study, we used a more sensitive fluorometric assay to examine the protease activities of caspase-3 and -9 in the denervated gastrocnemius muscle. In brief, 50 μl of the total cytosolic protein fraction of the muscles without protease inhibitor was incubated in 50 μl of assay buffer (50 mm PIPES, 0.1 mm EDTA, 10% glycerol, 10 mm DTT, pH 7.2) with 100 μm of the fluorogenic 7-amino-4-trifluoromethyl coumarin (AFC)-conjugated substrate (Ac-DEVD-AFC for caspase-3, Ac-LEHD-AFC for caspase-9; Alexis Corp., San Diego, CA, USA) at 37°C for 2 h. Caspase specific inhibitor, Z-VAD-FMK (Calbiochem, La Jolla, CA, USA) was used as a control to validate the specificity of caspase. The change in fluorescence was measured on a spectrofluorometer with an excitation wavelength of 390/20 nm, and an emission wavelength of 530/25 nm (CytoFluor; Applied Biosystems, Foster City, CA, USA) before and after the 2 h incubation. Caspase activity was estimated as the change in arbitrary fluorescence units normalized to milligrams of protein. Measurements were performed in duplicate while denervated and intra-animal control samples were run on the same microplate in the same setting.
Western immunoblot analyses
The protein expression of Bcl-2, Bax, Bcl-2-related ovarian killer (Bok), apoptosis protease activating factor-1 (Apaf-1), X-linked inhibitor of apoptosis (XIAP), caspase-3 and -9, heat shock protein-70 (HSP70), and superoxide dismutases (CuZn-SOD and Mn-SOD) was determined in the total cytosolic protein fraction, while AIF and c-Myc were measured in the nuclear fraction. Both the total cytosolic and nuclear fractions were used to measure the protein content of cleaved poly(ADP-ribose) polymerase (PARP), p53 and inhibitor of DNA binding/differentiation-2 (Id2).
Forty micrograms of protein was boiled for 5 min at 95°C in Laemmli buffer, loaded on each lane of a 12% polyacrylamide gel, and separated by SDS-PAGE at room temperature. The gels were blotted to nitrocellulose membranes (VWR, West Chester, PA, USA) and stained with Ponceau S red (Sigma-Aldrich) to verify equal loading and transferring of proteins to the membrane in each lane. As another approach to validate similar loading between the lanes, gels were loaded in duplicate with one gel stained with Coomassie blue. The membranes were then blocked in 5% nonfat milk in phosphate-buffered saline with 0.05% Tween 20 (PBS-T) at room temperature for 1 h, and probed with the following primary antibodies diluted in PBS-T with 2% BSA: anti-Bcl-2 mouse monoclonal antibody (1:100 dilution, sc-7382), anti-Bcl-2 mouse monoclonal antibody (1:100 dilution, 610538), anti-Bax rabbit polyclonal antibody (1:200 dilution, sc-493), anti-Bok rabbit polyclonal antibody (1:100 dilution, ab2304; Abcam, Cambridge, MA, USA), anti-Apaf-1 rabbit polyclonal antibody (1:200 dilution, 3018; BioVision, Mountain View, CA, USA), anti-hILP/XIAP mouse monoclonal antibody (1:250 dilution, 610762), antipro-/cleaved caspase-3 rabbit polyclonal antibody (1:2000 dilution, 552037), anti-cleaved caspase-9 rabbit polyclonal antibody (1:100 dilution, sc-22182), anti-HSP70 rabbit polyclonal antibody (1:2000 dilution, SPA812; StressGen, Victoria, BC, Canada), anti-SOD-1 rabbit polyclonal antibody (1:500 dilution, sc-11407), anti-MnSOD goat antibody (1:2000 dilution, A300449A), anti-AIF mouse monoclonal antibody (1:800 dilution, sc-13116 HP), anticleaved PARP rabbit polyclonal antibody (1:1000 dilution, G734; Promega, Madison, WI, USA), anti-c-Myc mouse monoclonal antibody (1:200 dilution, 3800-1), anti-Id2 rabbit polyclonal antibody (1:100 dilution, sc-489), anti-p53 mouse monoclonal antibody (1:100 dilution, sc-99). Bcl-2 (sc-7382), Bax, cleaved caspase-9, SOD-1, AIF, Id2 and p53 antibodies were purchased from Santa Cruz Biotechnology, while Bcl-2 (610538), XIAP, caspase-3, and c-Myc antibodies were purchased from BD Biosciences (San Jose, CA, USA). With the intention of examining the protein content of pro-caspase-9, immunoblots were performed with anti-pro-caspase-9 rabbit polyclonal antibodies purchased from different sources (sc-8355, Santa Cruz Biotechnology; 3016, BioVision, Mountain View, CA, USA), but we failed to detect an ∼46 kDa immunoreactive corresponding to the predicted molecular mass of pro-caspase-9. All primary antibody incubations were performed overnight at 4°C. Secondary antibodies were conjugated to horseradish peroxidase (Chemicon International, Temecula, CA, USA), and signals were developed by West Pico chemiluminescent substrate (Pierce). The signals were then visualized by exposing the membranes to X-ray films (BioMax MS-1; Eastman Kodak, Rochester, NY, USA), and digital records of the films were captured with a Kodak 290 camera. Resulting bands were quantified as OD × band area by a one-dimensional image analysis system (Eastman Kodak), and recorded in arbitrary units. The molecular sizes of the immunodetected proteins were verified by using prestained standard (LC5925; Invitrogen Life Technologies, Bethesda, MD, USA).
Estimation of mitochondrial cytochrome c, Smac/DIABLO and AIF release
Cytochrome c, AIF and Smac/DIABLO (second mitochondria-derived activator of caspase) are apoptotic factors normally confined to mitochondria, and their release into the cytosol has been demonstrated during the activation of apoptosis (Susin et al. 1999). In the present study, the release of Smac/DIABLO and AIF into the cytosol was estimated by measuring their protein contents in the extracted mitochondria-free cytosolic protein fraction by immunoblotting with an anti-Smac/DIABLO mouse monoclonal antibody (1:500 dilution, 612244; BD Biosciences) and an anti-AIF monoclonal mouse antibody. Moreover, a cytochrome c ELISA kit (MBL International, Woburn, MA, USA) was used to assess the protein content of cytochrome c in the mitochondria-free cytosol fraction to evaluate the release of the mitochondrial cytochrome c into the cytosol. By following the manufacturer's protocol, 60 μl the extracted mitochondria-free cytosolic fraction was used as an antigen source in a sandwich ELISA with a horseradish peroxidase-conjugated anti-cytochrome c polyclonal antibody in microwell strips coated with an anti-cytochrome c antibody. After washing, the peroxidase retained in the immunocomplex was detected by incubating with a chromogenic substrate, tetramethylbenzidine/hydrogen peroxide (TMB/H2O2), followed by adding an acid solution to terminate the enzyme reaction and to stabilize the developed colour. The change in colour was monitored at a wavelength of 450 nm using a Dynex MRX plate reader. Measurements were performed in duplicate with the denervated and contralateral control samples analysed on the same microplate, and the cytochrome c content was expressed as OD450 per milligram of protein.
Statistical analyses
Statistical analyses were performed using the SPSS 10.0 software package. Student's t test for paired data was used to examine differences between the denervated and contralateral control muscles. Statistical significance was accepted at P < 0.05. Data are expressed as the means of the percentages relative to the contralateral control muscle (i.e. control refers to 100%).
Results
Apoptotic DNA fragmentation
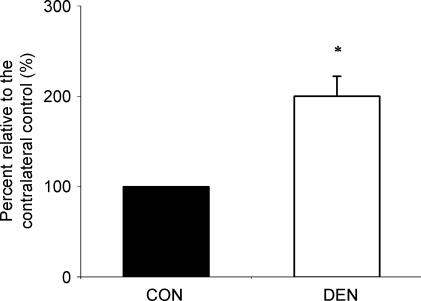
The cell-death ELISA analysis indicated that the extent of the apoptotic DNA fragmentation in the denervated muscle was 100% (P < 0.05, Fig. 2) higher than that in the control muscle.
Figure 2. Apoptotic DNA fragmentation.
The extent of apoptotic DNA fragmentation was determined by measuring the cytosolic mono- and oligo-nucleosomes. The data are presented as means ± standard error of mean (s.e.m.) of percentages relative to CON (i.e. CON refers to 100%). *P < 0.05, data are significantly different from CON. CON, control muscle; DEN, denervated muscle.
BCL-2 family: Bcl-2, Bax and Bok
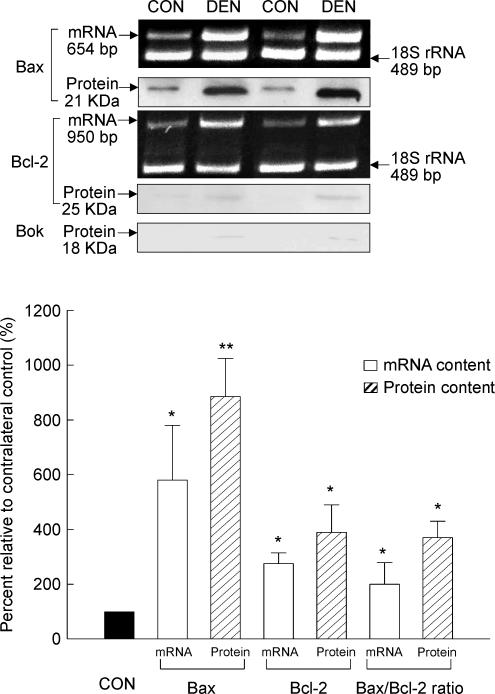
As estimated by RT-PCR, denervated muscle had a 177% increase in Bcl-2 mRNA, and a 482% increase in Bax mRNA when compared to the control muscle (P < 0.05, Fig. 3). The ratio of Bax/Bcl-2 mRNA content was further evaluated, and was found to be increased by 131% in the denervated muscle relative to the control muscle (P < 0.05, Fig. 3).
Figure 3. Bcl-2, Bax and Bok.
The content of mRNA and protein was examined by RT-PCR and Western immunoblot, respectively, in the CON and DEN muscles. The insets show representative results for the mRNA and protein. The data are presented as means ± s.e.m. of percentages relative to CON (i.e. CON refers to 100%). *P < 0.05, **P < 0.01, data are significantly different from CON.
In our Western blot analyses, we detected immunoreactive bands corresponding to the Bcl-2 and Bax proteins. Consistent with the mRNA data, both Bcl-2 and Bax protein content increased in the muscle following denervation relative to the control muscle. We found that the Bcl-2 and Bax protein content in denervated muscle was 295% (P < 0.05) and 785% (P < 0.01) higher than that of control muscle, respectively (Fig. 3). It is noted that the increased Bcl-2 protein content was verified by using anti-Bcl-2 mouse monoclonal antibodies obtained from two different sources (sc-7382 from Santa Cruz Biotechnology, and 610538 from BD Biosciences). As a result of a relatively greater increase in the Bax protein content compared with Bcl-2, the ratio of Bax/Bcl-2 was increased by 263% in the denervated muscle when compared with the control muscle (P < 0.05, Fig. 3). In our immunoblots, although an ∼18 kDa immunoreactive band corresponding to Bok protein (a pro-apoptotic member of BCL-2 family) was not detected in most of the muscle samples, we observed that it was present weakly in two muscle samples following denervation (Fig. 3).
Mitochondria-mediated apoptotic factors: mitochondrial cytochrome c release and Apaf-1 content
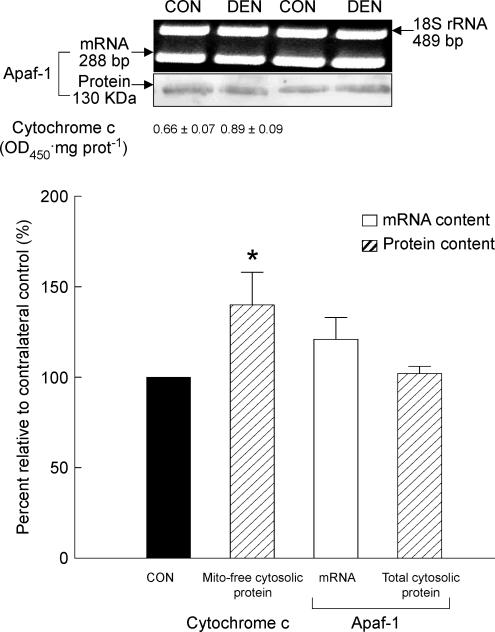
The ELISA analyses on the extracted mitochondria-free cytosolic fraction showed that the protein content of cytosolic cytochrome c in the denervated muscle increased by 40% when compared with the control muscle (P < 0.05, Fig. 4). Given that cytochrome c is primarily housed in the mitochondria under normal conditions, this indicated that cytochrome c was relocated to the cytosol during denervation. In contrast, we did not find any difference in the mRNA and the cytosolic protein content of Apaf-1 between the denervated and control samples as measured by RT-PCR and Western immunoblotting, respectively (P > 0.05, Fig. 4).
Figure 4. Cytochrome c and Apaf-1.
The release of mitochondrial cytochrome c was estimated by measuring the protein content of cytochrome c in the extracted mitochondria-free cytosolic fraction using an ELISA. The mRNA and total cytosolic protein content of Apaf-1 was determined by RT-PCR and Western immunoblot, respectively. The insets show representative results for the mRNA and protein of Apaf-1. The data are presented as means ± s.e.m. of percentages relative to CON (i.e. CON refers to 100%). *P < 0.05, data are significantly different from CON. Mito-free cytosolic protein, mitochondria-free cytosolic protein.
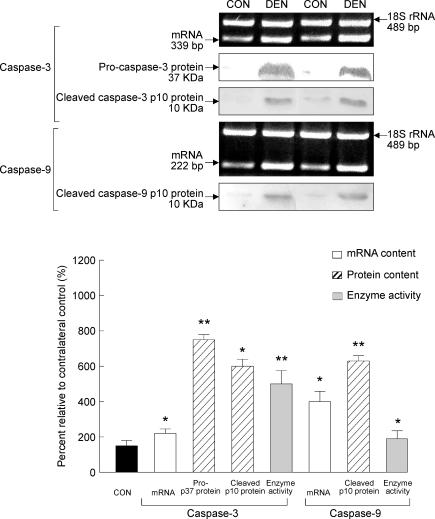
Caspase-3 and -9 mRNA, protein content and protease activity
The responses of caspase-3 and -9 to muscle denervation were evaluated by examining caspase mRNA, protein and protease activity. As estimated by RT-PCR, the mRNA content of caspase-3 and -9 in the denervated muscle increased by 125 and 300% relative to the control muscle, respectively (P < 0.05, Fig. 5). In our immunoblots, we detected immunoreactive bands corresponding to the predicted positions for pro-caspase-3 and cleaved caspase-3. There was a 649% increase (P < 0.01) in the protein content of pro-caspase-3, and the cleaved caspase-3 protein content increased by 500% (P < 0.05) in the muscle following denervation when compared to the control muscle (Fig. 5). In addition, an immunoreactive band corresponding to predicted molecular weight of the cleaved caspase-9 was detected, and there was a 519% increase in the denervated muscle compared with the control muscle (P < 0.01, Fig. 5). The activation of caspase-3 and -9 was further confirmed by a fluorometric caspase enzymatic activity analysis showing that there was a 394% (P < 0.01) and 69% (P < 0.05) increase in the protease activity of caspase-3 and -9, respectively (Fig. 5).
Figure 5. Caspase-3 and -9.
The mRNA and protein content of caspase-3 and -9 were determined by RT-PCR and Western immunoblot, respectively. The enzyme activity was assessed by a fluorometric caspase activity assay. The insets show representative results for the mRNA and protein. The data are presented as means ± s.e.m. of percentages relative to CON (i.e. CON refers to 100%). *P < 0.05, **P < 0.01, data are significantly different from CON.
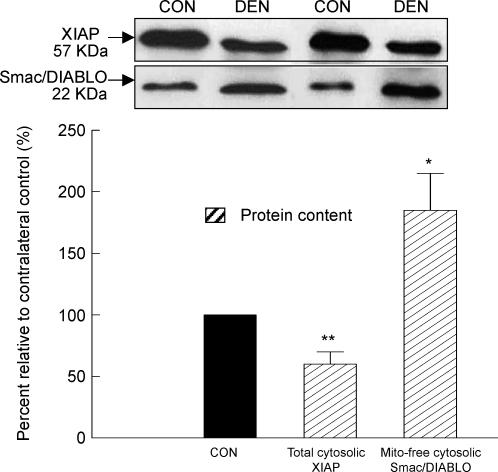
Modulators of caspases: XIAP protein content and mitochondrial Smac/DIABLO release
An immunoreactive band that corresponded to the predicted position for XIAP protein, an inhibitor of caspase-3 and -9, was detected in the immunoblots of the total cytosolic fractions. In the muscle, following denervation, the protein content of XIAP was 39% lower than that in the control muscle (P < 0.01, Fig. 6). For the immunoblots of the extracted mitochondria-free cytosolic fractions, we detected an immunoreactive band that corresponded to the predicted position of the Smac/DIABLO protein, an upstream inhibitor of XIAP. We found that the Smac/DIABLO protein content increased by 81% (P < 0.05, Fig. 6) in the cytosolic fraction (in the absence of mitochondria) of the denervated muscle relative to the control muscle, signifying that mitochondrial Smac/DIABLO was released into the cytosol during denervation.
Figure 6. XIAP and Smac/DIABLO protein.
The protein content of XIAP and Smac/DIABLO was measured by Western immunoblot in the total cytosolic and mitochondria-free cytosolic fraction, respectively. The insets show representative blots. The data are presented as means ± s.e.m. of percentages relative to CON (i.e. CON refers to 100%). *P < 0.05, **P < 0.01, data are significantly different from CON.
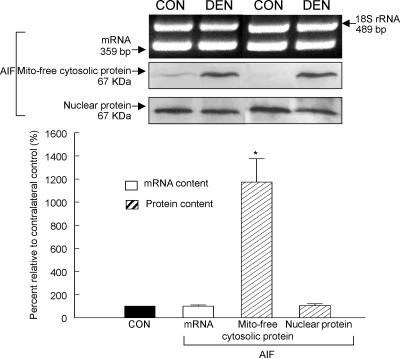
Caspase-independent mitochondrial AIF release and nuclear translocation
Translocation of mitochondrial AIF to the nuclei has been suggested to be a caspase-independent apoptogenic event (Joza et al. 2001; Cande et al. 2002). In the present study, we evaluated the mRNA, nuclear and mitochondria-free cytosolic protein contents of AIF in the denervated and control muscles. As assessed by RT-PCR and immunoblots of the nuclear protein fraction, we found that the mRNA and the nuclear AIF were not different between the denervated and control muscles (P > 0.05, Fig. 7). However, as an estimate of the mitochondrial AIF release to the cytosol, the AIF protein content measured in the mitochondria-free cytosolic fraction of the denervated muscle was 1029% higher than the control muscle (P < 0.05, Fig. 7), indicating that AIF was significantly released from mitochondria into the cytosol during denervation.
Figure 7. Apoptosis-inducing factor (AIF).
The mRNA and protein content of AIF was evaluated by RT-PCR and Western immunoblot, respectively. The mitochondria-free cytosolic and nuclear AIF protein content indicated the extent of mitochondrial release and nuclear translocation of AIF, correspondingly. The insets show representative results for the mRNA and protein. The data are presented as means ± s.e.m. of percentages relative to CON (i.e. CON refers to 100%). *P < 0.05, data are significantly different from CON.
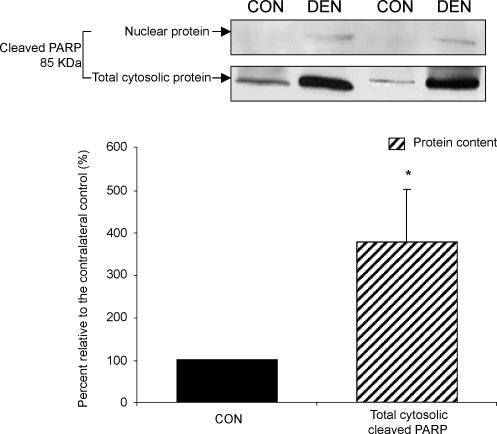
Cleaved PARP protein content
PARP plays an important role in the process of DNA repairing, and it has been demonstrated to be a cleavage target for the effector caspases (e.g. caspase-3) during the execution of apoptosis (Lazebnik et al. 1994). Although PARP is a nuclear protein, there have been reports illustrating the presence of cleaved PARP protein in the cytosol (Rosenthal et al. 1997; Cookson et al. 1999). Thus, by using immunoblotting, we examined the protein content of cleaved PARP in both the nuclear and total cytosolic fractions. For the nuclear fraction, an immunoreactive band corresponding to the cleaved PARP protein was not detected in any of the control muscles, but it was found in the denervated muscle samples of five animals (Fig. 8). We found that the cleaved PARP protein content was increased by 275% in the cytosolic fraction of denervated muscle when compared with the control muscle (P < 0.05, Fig. 8).
Figure 8. Cleaved poly(ADP-ribose) polymerase (PARP).
The cleaved PARP protein content was determined by Western immunoblot in the CON and DEN muscles. The insets show representative blots for the cleaved PARP protein. The data are presented as means ± s.e.m. of percent relative to CON (i.e. CON refers to 100%). *P < 0.05, data are significantly different from CON.
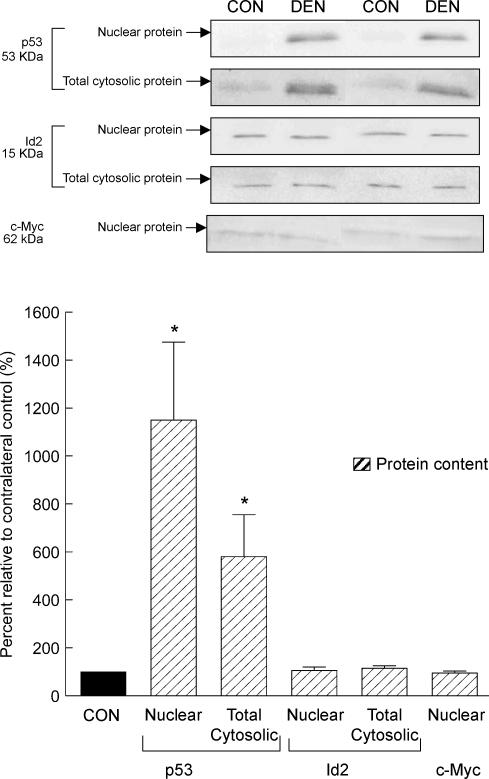
Cellular regulatory factors: p53, Id2, and c-Myc protein contents
In our immunoblot analyses, an immunoreactive band corresponding to the predicted position of the p53 protein was barely detected in the nuclear and the cytosolic protein fraction of all the control muscles, while the p53 protein content was markedly elevated in both the nuclear (∼10-fold increase, P < 0.05) and the cytosolic fraction (∼fivefold increase, P < 0.05, Fig. 9) of the muscle following denervation. In contrast, we found that the nuclear and cytosolic Id2 and nuclear c-Myc protein content was not different between the denervated and control muscles (P > 0.05, Fig. 9).
Figure 9. p53, Id2 and c-Myc protein.
The protein content of p53, Id2 and c-Myc was determined by Western immunoblot. The protein content of p53 and Id2 was measured in both the nuclear and total cytosolic fraction, while that for c-Myc was determined in the nuclear fraction. The insets show representative blots. The data are presented as means ± s.e.m. of percentages relative to CON (i.e. CON refers to 100%). *P < 0.05, data are significantly different from CON.
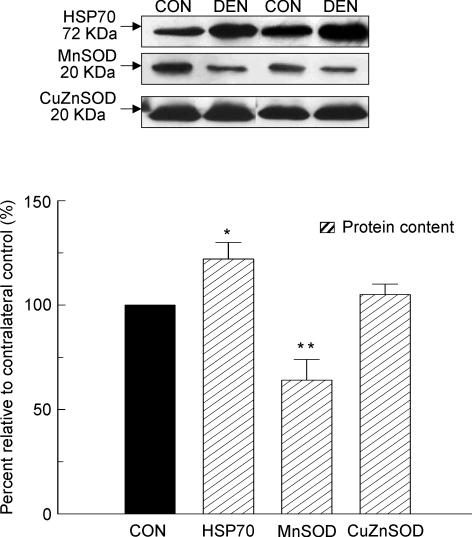
Stress protein and antioxidant enzyme: HSP70, MnSOD and CuZnSOD protein contents
According to our immunoblot analyses, the protein content of CuZnSOD in the denervated muscle was similar to the control muscle (P > 0.05, Fig. 10). However, we found that the HSP70 protein content was elevated by 22% (P < 0.05), while the protein content of MnSOD was diminished by 36% (P < 0.01, Fig. 10) in the muscle after denervation relative to the control muscle. These denoted that the denervated muscle examined in the current study endured some degree of cellular and/or oxidative stress.
Figure 10. HSP70, MnSOD and CuZnSOD protein.
The protein content of HSP70, MnSOD and CuZnSOD was determined in the total cytosolic fraction by Western immunoblot. The insets show representative blots. The data are presented as means ± s.e.m. of percentages relative to CON (i.e. CON refers to 100%). *P < 0.05, **P < 0.01, data are significantly different from CON.
Discussion
Apoptosis is a cellular process that is conserved from worms to humans. It has been extensively studied, but primarily in mitotic cells/tissues. Apoptosis plays a crucial role in a variety of biological events, including embryonic development, tissue turnover and immunological defence (Thompson, 1995). Recently, there have been several studies suggesting that apoptosis may be involved in the loss of postmitotic skeletal muscle during denervation (Migheli et al. 1997; Tews et al. 1997; Yoshimura & Harii, 1999; Borisov & Carlson, 2000; Olive & Ferrer, 2000; Tang et al. 2000; Jin et al. 2001; Jejurikar et al. 2002; Alway et al. 2003). These studies have demonstrated increases in TUNEL-positive cells and BCL-2 family expression to denervation. In the present study, we have provided novel data that show activation of mitochondria-associated apoptotic signalling in post-mitotic skeletal muscle tissue. We found apoptotic DNA fragmentation, an increase in Bax/Bcl-2 relative ratio, mitochondrial release of cytochrome c, Smac/DIABLO and AIF, an increase in caspase-3 and -9 mRNA, active protein and proteolytic activity, a decrease in XIAP, an elevation of cleaved PARP protein, and upregulation of p53 in the 14 day denervated gastrocnemius muscle. Our data extend previous indications of apoptosis during denervation by clearly showing that mitochondria-associated apoptosis pathways are activated by skeletal muscle denervation.
BCL-2 family and mitochondria-mediated apoptosis
The mitochondrion, an essential organelle, has been generally implicated to have an important apoptotic role in acting as a central modulator for the activation of the apoptotic machinery in response to a variety of apoptogenic stimuli (Desagher & Martinou, 2000; Kuwana & Newmeyer, 2003). Among the known apoptotic regulators in the mitochondria-mediated apoptosis, the BCL-2 protein family has been suggested to be a pivotal upstream regulator and constitutes a key intracellular checkpoint in the corresponding apoptotic signal transduction (Burlacu, 2003; Danial & Korsmeyer, 2004; Sharpe et al. 2004). In particular, among the entire BCL-2 family, Bax (a pro-apoptotic member) and Bcl-2 (an antiapoptotic member) have been relatively well examined and have been designated to be the main protagonists in the regulation of apoptotic machinery. This designation is principally originated from the existing evidence showing that Bax can translocate to the mitochondria and expose its N-terminus via a conformational change upon induction of apoptosis (Wolter et al. 1997; Desagher & Martinou, 2000). This conformational change allows the Bax/Bax-homo-oligomerization and therefore insertion of Bax into the outer mitochondrial membrane (Zha et al. 1996). This is followed rapidly by the formation of a channel, and subsequent release of the mitochondria-resided apoptogenic factors (e.g. cytochrome c, AIF and Smac/DIABLO) into the cytosol. Conversely, the anti-apoptotic role of Bcl-2 has been established by showing that Bcl-2 is capable of forming Bcl-2/Bax-heterodimers which oppose the pro-apoptotic activity of Bax by preventing the process of Bax/Bax-homo-oligomerization (Antonsson et al. 2000; Burlacu, 2003; Danial & Korsmeyer, 2004; Sharpe et al. 2004). Although the precise mechanism of Bax/Bcl-2-mediated apoptogenic factor release from the mitochondrial intermembrane space is still under active investigation, it is generally acknowledged that the relative content of pro-/anti-apoptotic BCL-2 family members provides a tight control in promoting the execution of apoptotic cascades (Desagher & Martinou, 2000; Burlacu, 2003; Kuwana & Newmeyer, 2003; Danial & Korsmeyer, 2004; Sharpe et al. 2004). In accordance with this idea, we have demonstrated that following denervation, the relative Bax/Bcl-2 ratios (both the mRNA and protein) are significantly elevated concomitant with the increased release of mitochondrial apoptogenic factors, including cytochrome c, AIF and Smac/DIABLO. Notably, we found that the antiapoptotic Bcl-2 mRNA and protein content increased, rather than decreased as anticipated, in the denervated muscle, and this observation appeared to be in contradiction to the overall pro-apoptotic changes that we have demonstrated in the denervated muscle, as well as our previously reported Bcl-2 response to unloading in the quail hypertrophied muscle (Siu et al. 2005). However, we interpret these observations of increase in Bcl-2 as an adaptive response of the skeletal muscle as an attempt to offset the pro-apoptotic stimulus provoked from denervation. In support of this interpretation, there have been studies reporting that the level of Bcl-2 in skeletal muscle increased concurrent with the elevation of Bax in response to denervation (Tews et al. 1997; Jejurikar et al. 2002). It is unclear whether our observed differences in the response of Bcl-2 between the denervated rat muscle and the unloaded quail hypertrophied muscle are attributed to the different natures of the experimental models (e.g. differences in innervation or the starting muscle level prior to the onset of the atrophy process), and it warrants further investigation. Nonetheless, our findings suggested that the relative content of the pro-/anti-apoptotic members (i.e. the ratio of Bax/Bcl-2) is a better predictor of apoptotic events than solely assessing the expression of individual levels of BCL-2 family members in denervated muscles.
Tumour suppressor p53 has been implicated as one of the regulators in apoptotic signal transduction, although it is unclear if this is by a transcription-dependent and/or -independent mechanism because both of these mechanisms are related to Bax (Schuler & Green, 2001; Chipuk et al. 2004). Recently, we have shown that upregulation of p53 possibly contributes to part of the apoptotic signalling during unloading-induced muscle atrophy in quail hypertrophied muscle (Siu & Alway, 2004; Siu et al. 2005). Here, we found that both the nuclear and cytosolic p53 protein contents are markedly elevated in parallel with the upregulation of Bax in the denervated muscle. These findings suggest that p53 may also be involved in the denervation-induced apoptotic signalling transduction in skeletal muscle. Nevertheless, more research is needed to reveal the possible regulatory role of p53 in the apoptosis-associated muscle atrophy during denervation. Interestingly, several regulatory factors shown to have roles in apoptosis in other models, including Id2, c-Myc, and CuZnSOD, were not altered by denervation. These results are in accordance with our previous findings indicating that Id2 and c-Myc may not be directly involved in regulating apoptosis during denervation and unloading following hypertrophy, respectively (Alway et al. 2003; Siu & Alway, 2004). Furthermore, we observed that variations in the response of MnSOD protein expression exist between the denervated rat muscle studied in here, and the quail unloaded muscle following hypertrophy studied previously (Siu & Alway, 2004). Since that oxidative stress has been shown to be elevated during muscle disuse (Lawler et al. 2003), and it may have an important role in regulating muscle atrophy (Powers et al. 2005), it is reasonable to interpret our findings as due at least in part to a disturbance of redox balance in both the denervated rat muscle and the unloaded quail hypertrophied muscle (Siu & Alway, 2004).
Apoptotic cascade of caspases
A specific family of broadly conserved proteases named caspase (cysteine-dependent aspartate protease) has also been extensively investigated and advocated to be another important component in mediating the apoptotic signal transduction (Earnshaw et al. 1999; Grutter, 2000; Chang & Yang, 2000; Degterev et al. 2003). With the exception of caspase-9 (Stennicke et al. 1999), caspases are normally synthesized and present in the cell as inactive zymogens termed procaspases. Structural and biochemical analyses revealed that the activation of procaspases requires self-/auto-activation through oligomerization (for some initiator caspases), scaffold-mediated transactivation, or limited proteolytic cleavage by upstream proteases for the acquisition of the protease activity once upon triggered by an apoptotic signal (Earnshaw et al. 1999; Chang & Yang, 2000). Categorized as an initiator caspase, caspase-9 has been demonstrated to mediate the mitochondrial apoptosis by assembling an apoptosome complex through the interaction of procaspase-9 with Apaf-1, dATP/ATP, and mitochondrial released-cytochrome c, which subsequently recruits procaspase-3 to the complex and efficiently activates caspase-3, a common effector caspase, by the caspase-9-mediated proteolytic cleavage (Chang & Yang, 2000; Acehan et al. 2002; Shi, 2002a). A variety of cellular molecules, including proteins for DNA metabolism and repair (e.g. PARP and ICAD or inhibitor of caspase-activated DNase), signalling transduction (e.g. Akt/PKB), cell cycle regulation (e.g. p21Cip1/Waf1), cytoskeletal scaffold (e.g. gelsolin) and cell death (e.g. Bcl-2 and IAP), are the potential cleavage substrates for caspase-3 or other effector caspases (Chang & Yang, 2000). In the current study, we have demonstrated that cytochrome c was significantly released into the cytosol, while both caspase-3 and -9 increased based upon the levels of mRNA, active protein, as well as enzyme activity in the denervated muscle. These changes indicate that the mitochondria-mediated apoptotic caspase cascade was activated in the muscle following denervation. In agreement with this observation, we have also observed that the cleaved protein content of PARP (a cleavage substrate for caspase-3) elevated in company with the increase in the extent of apoptotic DNA fragmentation as assessed by a cytosolic nucleosome ELISA in the denervated muscle.
As a modulating mechanism for the cascade of caspases, the proteolytic activity of both initiator and effector caspases is subject to the suppression by an anti-apoptotic protein family called IAP (Deveraux et al. 1998; Earnshaw et al. 1999; Chang & Yang, 2000). This IAP-mediated inhibition of caspases is repressed by Smac/DIABLO, a mitochondria-resided apoptotic protein, which is released into the cytosol to promote apoptosis by neutralizing the inhibitory effect of multiple IAPs (Shi, 2002b). XIAP is one of the potent IAP members in inhibiting caspase-3, -7 and -9 (Deveraux et al. 1998; Earnshaw et al. 1999; Chang & Yang, 2000; Shi, 2002b). We have shown that the protein content of XIAP was downregulated, while the release of mitochondrial Smac/DIABLO increased in concomitant with the elevation of the caspase-3 and -9 protease activity in the muscle following denervation. These data demonstrate that the cascade of caspases and the XIAP-Smac/DIABLO-mediated modulating mechanism are involved in the activated apoptotic signal transduction in the denervated muscle.
While our data show that the caspase cascade indisputably plays an important role in the apoptotic signal transduction in denervated muscle, it is possible, as has been suggested, that apoptosis can be executed without the involvement of caspase in certain experimental models (Cande et al. 2002; Jaattela, 2002). Since caspase-independent apoptotic signalling is primarily driven by specific mitochondria-housed apoptotic factors (e.g. AIF and endonuclease G) that are released into the cytosol during apoptosis (Cande et al. 2002; Jaattela, 2002), the striking elevation of the AIF protein content in the mitochondria-free cytosol of our examined denervated muscle prompted a reasonable suspicion that the caspase-independent machinery may have contributed to the activated apoptotic signalling during denervation. Although we failed to acquire evidence showing nuclear translocation of AIF, it is unclear that the increase in cytosolic AIF may have functioned to the apoptotic signal transduction in the absence of nuclear translocation during muscle denervation. Nonetheless, additional research is required to inspect the suspicious role of AIF in promoting the denervation-induced apoptosis in skeletal muscle.
Inflammation caused by surgical trauma has been implicated as a confounding factor in several experimental muscle models (e.g. tenotomy and muscle ablation) (Armstrong et al. 1979; Lowe & Alway, 2002). However, the present muscle denervation model induces relatively lower levels of trauma, because there is less bleeding during surgery resulting in less post-surgery inflammation when compared with other models (Lowe & Alway, 2002; Alway et al. 2005). In addition, the inflammatory response has been shown to peak at 1–5 days post-surgery in the muscle ablation model, a model causes severe inflammation, and the response subsides to control level by 16 days after surgery (Armstrong et al. 1979). Taken together, it is unlikely that our results are masked by the post-surgery inflammation.
Some non-muscle cell populations (e.g. endothelia and fibroblasts) are included in the muscle tissue homogenates that we used to study apoptosis in this study. Nevertheless, it is not likely that the marked changes in our study are the results of major contributions by these nonmuscle cells due to their small proportion relative to the skeletal myocytes in our homogenates. Furthermore, most models of decreased neuromuscular activity and possibly denervation are known to determine a slow-to-fast fibre transformation (Hamalainen & Pette, 2001; Geiger et al. 2003). However, the short-term denervation used in this study is unlikely to determine a large change in fibre-type distribution, and therefore our results are not likely to be significantly affected by the factor of fibre transformation.
In conclusion, we have identified mitochondrial-apoptotic signalling in denervated skeletal muscle (summarized in Fig. 11). Our data demonstrate increases in BCL-2 family proteins, mitochondria-mediated apoptosis regulators, caspase family proteases, caspase modulators, caspase-independent apoptogenic factor and apoptosis-associated cellular regulatory factors to muscle denervation. We show pro-apoptotic increases in DNA fragmentation, and increases in Bax/Bcl-2 ratio, mitochondrial cytochrome c, Smac/DIABLO, AIF release, and upregulation of caspase-3 and -9 mRNA, active protein and protease activity, diminished XIAP, and increased cleaved PARP following 14 days of denervation. In addition to the signs of cellular stress, including increased HSP70 and diminished MnSOD, we found that p53 was upregulated concomitant with the pro-apoptotic signalling during denervation. These observations raise the possibility that p53 may contribute to denervation-induced muscle remodelling. These findings support the hypothesis that apoptosis has a physiological role in the regulation of the muscle loss during skeletal muscle denervation. It has been proposed that apoptosis may occur at the level of nuclei in addition to the death of whole multinucleated myofibres. This so called ‘nuclear apoptosis’ is hypothesized to have a role in the regulation of muscle remodelling based on the concept of myonuclear domain (Allen et al. 1997; Leeuwenburgh et al. 2005). In the present study, although the extent of myofibre death or the number of apoptotic nuclei was not determined, our cell-death ELISA data indicate a significant increase in apoptosis DNA fragmentation in muscle following 14 days of denervation.
Figure 11. Proposed activated mitochondrial apoptotic signal transduction in denervated skeletal muscle.
Following 14 days of denervation, apoptosis is evident based on the elevation of apoptotic DNA fragmentation and PARP cleavage. Regarding the mitochondrial apoptotic signalling, pro-apoptotic Bax dramatically increases while anti-apoptotic Bcl-2 is modestly upregulated resulting in an increase in Bax/Bcl-2 ratio. Cytosolic release of mitochondria-residing apoptotic factors including cytochrome c (Cyto c) and Smac/DIABLO are found concomitant with the activation of caspases (Casp)-9 and -3. XIAP, a potent inhibitor of caspases is downregulated, whereas p53 is markedly elevated in both nuclei and cytosol. Moreover, mitochondrial AIF is found to be released to the cytosol in the absence of nuclear translocation. ICAD, inhibitor of caspase-activated DNase; CAD, caspase-activated DNase.
It has been shown that skeletal muscle may exhibit distinct ultrastructural apoptotic characteristics following long-term denervation (Borisov & Carlson, 2000). These findings raise the possibility that multinucleated myocytes may execute the apoptotic programme differently from classical apoptosis. Although we did not use electron microscopy to examine classical apoptotic features in the denervated myocytes of the current study, our data show increased DNA laddering, and direct evidence of mitochondrial apoptotic signalling in denervated skeletal muscle. Collectively, our data indicate that ‘classical’ mitochondria-associated apoptosis is well conserved in mature denervated skeletal muscle.
Acknowledgments
We are grateful to Dr William Wonderlin for providing access to the CytoFluor spectrofluorometer in his laboratory. This study was supported by NIH: National Institute on Aging grant R01 AG021530.
References
- Acehan D, Jiang X, Morgan DG, Heuser JE, Wang X, Akey CW. Three-dimensional structure of the apoptosome: implications for assembly, procaspase-9 binding, and activation. Mol Cell. 2002;9:423–432. doi: 10.1016/s1097-2765(02)00442-2. 10.1016/S1097-2765(02)00442-2. [DOI] [PubMed] [Google Scholar]
- Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, Edgerton VR. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol Cell Physiol. 1997;273:C579–587. doi: 10.1152/ajpcell.1997.273.2.C579. [DOI] [PubMed] [Google Scholar]
- Alway SE, Degens H, Krishnamurthy G, Chaudhrai A. Denervation stimulates apoptosis but not Id2 expression in hindlimb muscles of aged rats. J Gerontol A Biol Sci Med Sci. 2003;58:687–697. doi: 10.1093/gerona/58.8.b687. [DOI] [PubMed] [Google Scholar]
- Alway SE, Degens H, Krishnamurthy G, Smith CA. Potential role for Id myogenic repressors in apoptosis and attenuation of hypertrophy in muscles of aged rats. Am J Physiol Cell Physiol. 2002;283:C66–76. doi: 10.1152/ajpcell.00598.2001. [DOI] [PubMed] [Google Scholar]
- Alway SE, Siu PM, Murlasits Z, Butler DC. Muscle hypertrophy models: applications for aging research. Can J Appl Physiol. 2005 doi: 10.1139/h05-143. (in press) [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Lauper S, Eskes R, Martinou JC. Bax oligomerization is required for channel-forming activity in liposomes and to trigger cytochrome c release from mitochondria. Biochem J. 2000;345:271–278. 10.1042/0264-6021:3450271. [PMC free article] [PubMed] [Google Scholar]
- Armstrong RB, Marum P, Tullson P, Saubert CW. Acute hypertrophic response of skeletal muscle to removal of synergists. J Appl Physiol. 1979;46:835–842. doi: 10.1152/jappl.1979.46.4.835. [DOI] [PubMed] [Google Scholar]
- Borisov AB, Carlson BM. Cell death in denervated skeletal muscle is distinct from classical apoptosis. Anat Rec. 2000;258:305–318. doi: 10.1002/(SICI)1097-0185(20000301)258:3<305::AID-AR10>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Brown WF. A method for estimating the number of motor units in thenar muscles and the changes in motor unit count with ageing. J Neurol Neurosurg Psychiatry. 1972;35:845–852. doi: 10.1136/jnnp.35.6.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RH., Jr Amyotrophic lateral sclerosis. Insights from genetics. Arch Neurol. 1997;54:1246–1250. doi: 10.1001/archneur.1997.00550220050013. [DOI] [PubMed] [Google Scholar]
- Burlacu A. Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med. 2003;7:249–257. doi: 10.1111/j.1582-4934.2003.tb00225.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cande C, Cohen I, Daugas E, Ravagnan L, Larochette N, Zamzami N, Kroemer G. Apoptosis-inducing factor (AIF): a novel caspase-independent death effector released from mitochondria. Biochimie. 2002;84:215–222. doi: 10.1016/s0300-9084(02)01374-3. [DOI] [PubMed] [Google Scholar]
- Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspases. Microbiol Mol Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Cookson MR, Ince PG, Usher PA, Shaw PJ. Poly (ADP-ribose) polymerase is found in both the nucleus and cytoplasm of human CNS neurons. Brain Res. 1999;834:182–185. doi: 10.1016/s0006-8993(99)01559-0. [DOI] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ. Cell death: critical control points. Cell. 2004;116:205–219. doi: 10.1016/s0092-8674(04)00046-7. [DOI] [PubMed] [Google Scholar]
- Degens H, Meessen NE, Wirtz P, Binkhorst RA. The development of compensatory hypertrophy in the plantaris muscle of the rat. Anat Anz. 1995;177:285–289. doi: 10.1016/S0940-9602(11)80203-7. [DOI] [PubMed] [Google Scholar]
- Degterev A, Boyce M, Yuan J. A decade of caspases. Oncogene. 2003;22:8543–8567. doi: 10.1038/sj.onc.1207107. 10.1038/sj.onc.1207107. [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou JC. Mitochondria as the central control point of apoptosis. Trends Cell Biol. 2000;10:369–377. doi: 10.1016/s0962-8924(00)01803-1. 10.1016/S0962-8924(00)01803-1. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, Alnemri ES, Salvesen GS, Reed JC. IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J. 1998;17:2215–2223. doi: 10.1093/emboj/17.8.2215. 10.1093/emboj/17.8.2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirks A, Leeuwenburgh C. Apoptosis in skeletal muscle with aging. Am J Physiol Regul Integr Comp Physiol. 2002;282:R519–527. doi: 10.1152/ajpregu.00458.2001. [DOI] [PubMed] [Google Scholar]
- Dirks AJ, Leeuwenburgh C. Aging and lifelong calorie restriction result in adaptations of skeletal muscle apoptosis repressor, apoptosis-inducing factor, X-linked inhibitor of apoptosis, caspase-3, and caspase-12. Free Radic Biol Med. 2004;36:27–39. doi: 10.1016/j.freeradbiomed.2003.10.003. 10.1016/j.freeradbiomed.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Doherty TJ, Vandervoort AA, Taylor AW, Brown WF. Effects of motor unit losses on strength in older men and women. J Appl Physiol. 1993;74:868–874. doi: 10.1152/jappl.1993.74.2.868. 10.1063/1.354879. [DOI] [PubMed] [Google Scholar]
- Duke RC, Ojcius DM, Young JD. Cell suicide in health and disease. Sci Am. 1996;275:80–87. doi: 10.1038/scientificamerican1296-80. [DOI] [PubMed] [Google Scholar]
- Earnshaw WC, Martins LM, Kaufmann SH. Mammalian caspases: structure, activation, substrates, and functions during apoptosis. Annu Rev Biochem. 1999;68:383–424. doi: 10.1146/annurev.biochem.68.1.383. 10.1146/annurev.biochem.68.1.383. [DOI] [PubMed] [Google Scholar]
- Ellis RE, Yuan JY, Horvitz HR. Mechanisms and functions of cell death. Annu Rev Cell Biol. 1991;7:663–698. doi: 10.1146/annurev.cb.07.110191.003311. 10.1146/annurev.cb.07.110191.003311. [DOI] [PubMed] [Google Scholar]
- Essen-Gustavsson B, Borges O. Histochemical and metabolic characteristics of human skeletal muscle in relation to age. Acta Physiol Scand. 1986;126:107–114. doi: 10.1111/j.1748-1716.1986.tb07793.x. [DOI] [PubMed] [Google Scholar]
- Geiger PC, Bailey JP, Zhan WZ, Mantilla CB, Sieck GC. Denervation-induced changes in myosin heavy chain expression in the rat diaphragm muscle. J Appl Physiol. 2003;95:611–619. doi: 10.1152/japplphysiol.00862.2002. [DOI] [PubMed] [Google Scholar]
- Grutter MG. Caspases: key players in programmed cell death. Curr Opin Struct Biol. 2000;10:649–655. doi: 10.1016/s0959-440x(00)00146-9. 10.1016/S0959-440X(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Hamalainen N, Pette D. Myosin and SERCA isoform expression in denervated slow-twitch muscle of euthyroid and hyperthyroid rabbits. J Muscle Res Cell Motil. 2001;22:453–457. doi: 10.1023/a:1014543507149. 10.1023/A:1014543507149. [DOI] [PubMed] [Google Scholar]
- Hughes SM. Muscle development: electrical control of gene expression. Curr Biol. 1998;8:R892–894. doi: 10.1016/s0960-9822(07)00554-4. 10.1016/S0960-9822(07)00554-4. [DOI] [PubMed] [Google Scholar]
- Jaattela M. Programmed cell death: many ways for cells to die decently. Ann Med. 2002;34:480–488. doi: 10.1080/078538902321012423. 10.1080/078538902321012423. [DOI] [PubMed] [Google Scholar]
- Jejurikar SS, Marcelo CL, Kuzon WM., Jr Skeletal muscle denervation increases satellite cell susceptibility to apoptosis. Plast Reconstr Surg. 2002;110:160–168. doi: 10.1097/00006534-200207000-00027. 10.1097/00006534-200207000-00027. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu Z, Tian T, Gu Y. Apoptosis in atrophic skeletal muscle induced by brachial plexus injury in rats. J Trauma. 2001;50:31–35. doi: 10.1097/00005373-200101000-00005. [DOI] [PubMed] [Google Scholar]
- Joza N, Susin SA, Daugas E, Stanford WL, Cho SK, Li CY, Sasaki T, Elia AJ, Cheng HY, Ravagnan L, Ferri KF, Zamzami N, Wakeham A, Hakem R, Yoshida H, Kong YY, Mak TW, Zuniga-Pflucker JC, Kroemer G, Penninger JM. Essential role of the mitochondrial apoptosis-inducing factor in programmed cell death. Nature. 2001;410:549–554. doi: 10.1038/35069004. 10.1038/35069004. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Newmeyer DD. Bcl-2-family proteins and the role of mitochondria in apoptosis. Curr Opin Cell Biol. 2003;15:691–699. doi: 10.1016/j.ceb.2003.10.004. 10.1016/j.ceb.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Larsson L. Motor units: remodeling in aged animals. J Gerontol A Biol Sci Medical Sci. 1995;50:91–95. doi: 10.1093/gerona/50a.special_issue.91. [DOI] [PubMed] [Google Scholar]
- Larsson L. The age-related motor disability: underlying mechanisms in skeletal muscle at the motor unit, cellular and molecular level. Acta Physiol Scand. 1998;163:S27–29. doi: 10.1046/j.1365-201x.1998.00375.x. 10.1046/j.1365-201x.1998.00375.x. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Song W, Demaree SR. Hindlimb unloading increases oxidative stress and disrupts antioxidant capacity in skeletal muscle. Free Radic Biol Med. 2003;35:9–16. doi: 10.1016/s0891-5849(03)00186-2. 10.1016/S0891-5849(03)00186-2. [DOI] [PubMed] [Google Scholar]
- Lazebnik YA, Kaufmann SH, Desnoyers S, Poirier GG, Earnshaw WC. Cleavage of poly (ADP-ribose) polymerase by a proteinase with properties like ICE. Nature. 1994;371:346–347. doi: 10.1038/371346a0. 10.1038/371346a0. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C. Role of apoptosis in sarcopenia. J Gerontol A Biol Sci Med Sci. 2003;58:999–1001. doi: 10.1093/gerona/58.11.m999. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Gurley CM, Strotman BA, Dupont-Versteegden EE. Age-related differences in apoptosis with disuse atrophy in soleus muscle. Am J Physiol Regul Integr Comp Physiol. 2005 doi: 10.1152/ajpregu.00576.2004. (in press) [DOI] [PubMed] [Google Scholar]
- Lowe DA, Alway SE. Animal models for inducing muscle hypertrophy: are they relevant for clinical applications in humans? J Orthop Sports Phys Ther. 2002;32:36–43. doi: 10.2519/jospt.2002.32.2.36. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Migheli A, Mongini T, Doriguzzi C, Chiado-Piat L, Piva R, Ugo I, Palmucci L. Muscle apoptosis in humans occurs in normal and denervated muscle, but not in myotonic dystrophy, dystrophinopathies or inflammatory disease. Neurogenetics. 1997;1:81–87. doi: 10.1007/s100480050012. 10.1007/s100480050012. [DOI] [PubMed] [Google Scholar]
- Olive M, Ferrer I. Bcl-2 and bax immunohistochemistry in denervation-reinnervation and necrosis-regeneration of rat skeletal muscles. Muscle Nerve. 2000;23:1862–1867. doi: 10.1002/1097-4598(200012)23:12<1862::aid-mus10>3.0.co;2-q. 10.1002/1097-4598(200012)23:12<1862::AID-MUS10>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Pette D. Historical perspectives: plasticity of mammalian skeletal muscle. J Appl Physiol. 2001;90:1119–1124. doi: 10.1152/jappl.2001.90.3.1119. [DOI] [PubMed] [Google Scholar]
- Pollack M, Phaneuf S, Dirks A, Leeuwenburgh C. The role of apoptosis in the normal aging brain, skeletal muscle, and heart. Ann N Y Acad Sci. 2002;959:93–107. doi: 10.1111/j.1749-6632.2002.tb02086.x. [DOI] [PubMed] [Google Scholar]
- Powers SK, Kavazis AN, DeRuisseau KC. Mechanisms of disuse muscle atrophy: role of oxidative stress. Am J Physiol Regul Integr Comp Physiol. 2005;288:R337–344. doi: 10.1152/ajpregu.00469.2004. [DOI] [PubMed] [Google Scholar]
- Rokhlin OW, Glover RA, Taghiyev AF, Guseva NV, Seftor RE, Shyshynova I, Gudkov AV, Cohen MB. Bisindolylmaleimide IX facilitates tumor necrosis factor receptor family-mediated cell death and acts as an inhibitor of transcription. J Biol Chem. 2002;277:33213–33219. doi: 10.1074/jbc.M204612200. 10.1074/jbc.M204612200. [DOI] [PubMed] [Google Scholar]
- Rosenthal DS, Ding R, Simbulan-Rosenthal CM, Vaillancourt JP, Nicholson DW, Smulson M. Intact cell evidence for the early synthesis, and subsequent late apopain-mediated suppression, of poly (ADP-ribose) during apoptosis. Exp Cell Res. 1997;232:313–321. doi: 10.1006/excr.1997.3536. 10.1006/excr.1997.3536. [DOI] [PubMed] [Google Scholar]
- Rothermel B, Vega RB, Yang J, Wu H, Bassel-Duby R, Williams RS. A protein encoded within the Down syndrome critical region is enriched in striated muscles and inhibits calcineurin signaling. J Biol Chem. 2000;275:8719–8725. doi: 10.1074/jbc.275.12.8719. 10.1074/jbc.275.12.8719. [DOI] [PubMed] [Google Scholar]
- Sandri M, Carraro U, Podhorska-Okolov M, Rizzi C, Arslan P, Monti D, Franceschi C. Apoptosis, DNA damage and ubiquitin expression in normal and mdx muscle fibers after exercise. FEBS Lett. 1995;373:291–295. doi: 10.1016/0014-5793(95)00908-r. 10.1016/0014-5793(95)00908-R. [DOI] [PubMed] [Google Scholar]
- Sandri M, El Meslemani AH, Sandri C, Schjerling P, Vissing K, Andersen JL, Rossini K, Carraro U, Angelini C. Caspase 3 expression correlates with skeletal muscle apoptosis in Duchenne and facioscapulo human muscular dystrophy. A potential target for pharmacological treatment? J Neuropathol Exp Neurol. 2001;60:302–312. doi: 10.1093/jnen/60.3.302. [DOI] [PubMed] [Google Scholar]
- Sandri M, Minetti C, Pedemonte M, Carraro U. Apoptotic myonuclei in human Duchenne muscular dystrophy. Lab Invest. 1998;78:1005–1016. [PubMed] [Google Scholar]
- Sandri M, Podhorska-Okolow M, Geromel V, Rizzi C, Arslan P, Franceschi C, Carraro U. Exercise induces myonuclear ubiquitination and apoptosis in dystrophin-deficient muscle of mice. J Neuropathol Exp Neurol. 1997;56:45–57. doi: 10.1097/00005072-199701000-00005. [DOI] [PubMed] [Google Scholar]
- Schuler M, Green DR. Mechanisms of p53-dependent apoptosis. Biochem Soc Trans. 2001;29:684–688. doi: 10.1042/0300-5127:0290684. 10.1042/BST0290684. [DOI] [PubMed] [Google Scholar]
- Sharpe JC, Arnoult D, Youle RJ. Control of mitochondrial permeability by Bcl-2 family members. Biochim Biophys Acta. 2004;1644:107–113. doi: 10.1016/j.bbamcr.2003.10.016. 10.1016/j.bbamcr.2003.10.016. [DOI] [PubMed] [Google Scholar]
- Shi Y. Apoptosome: the cellular engine for the activation of caspase-9. Structure (Camb) 2002a;10:285–288. doi: 10.1016/s0969-2126(02)00732-3. 10.1016/S0969-2126(02)00732-3. [DOI] [PubMed] [Google Scholar]
- Shi Y. Mechanisms of caspase activation and inhibition during apoptosis. Mol Cell. 2002b;9:459–470. doi: 10.1016/s1097-2765(02)00482-3. 10.1016/S1097-2765(02)00482-3. [DOI] [PubMed] [Google Scholar]
- Siu PM, Alway SE. Id2 and p53 participate in apoptosis during unloading-induced muscle atrophy. Am J Physiol Cell Physiol. 2004 doi: 10.1152/ajpcell.00495.2004. (in press) [DOI] [PubMed] [Google Scholar]
- Siu PM, Bryner RW, Martyn JK, Alway SE. Apoptotic adaptations from exercise training in skeletal and cardiac muscles. FASEB J. 2004a;18:1150–1152. doi: 10.1096/fj.03-1291fje. [DOI] [PubMed] [Google Scholar]
- Siu PM, Donley DA, Bryner RW, Alway SE. Citrate synthase expression and enzyme activity after endurance training in cardiac and skeletal muscles. J Appl Physiol. 2003;94:555–560. doi: 10.1152/japplphysiol.00821.2002. [DOI] [PubMed] [Google Scholar]
- Siu PM, Donley DA, Bryner RW, Alway SE. Myogenin and oxidative enzyme gene expression levels are elevated in rat soleus muscles after endurance training. J Appl Physiol. 2004b;97:277–285. doi: 10.1152/japplphysiol.00534.2004. [DOI] [PubMed] [Google Scholar]
- Siu PM, Pistilli EE, Butler DC, Alway SE. Aging influences cellular and molecular responses of apoptosis to skeletal muscle unloading. Am J Physiol Cell Physiol. 2005;288:C338–349. doi: 10.1152/ajpcell.00239.2004. [DOI] [PubMed] [Google Scholar]
- Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Steller H. Mechanisms and genes of cellular suicide. Science. 1995;267:1445–1449. doi: 10.1126/science.7878463. [DOI] [PubMed] [Google Scholar]
- Stennicke HR, Deveraux QL, Humke EW, Reed JC, Dixit VM, Salvesen GS. Caspase-9 can be activated without proteolytic processing. J Biol Chem. 1999;274:8359–8362. doi: 10.1074/jbc.274.13.8359. 10.1074/jbc.274.13.8359. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, Marzo I, Snow BE, Brothers GM, Mangion J, Jacotot E, Costantini P, Loeffler M, Larochette N, Goodlett DR, Aebersold R, Siderovski DP, Penninger JM, Kroemer G. Molecular characterization of mitochondrial apoptosis-inducing factor. Nature. 1999;397:441–446. doi: 10.1038/17135. 10.1038/17135. [DOI] [PubMed] [Google Scholar]
- Tang H, Cheung WM, Ip FC, Ip NY. Identification and characterization of differentially expressed genes in denervated muscle. Mol Cell Neurosci. 2000;16:127–140. doi: 10.1006/mcne.2000.0864. 10.1006/mcne.2000.0864. [DOI] [PubMed] [Google Scholar]
- Tews DS. Apoptosis and muscle fibre loss in neuromuscular disorders. Neuromuscul Disord. 2002;12:613–622. doi: 10.1016/s0960-8966(02)00030-5. 10.1016/S0960-8966(02)00030-5. [DOI] [PubMed] [Google Scholar]
- Tews DS, Goebel HH, Schneider I, Gunkel A, Stennert E, Neiss WF. DNA-fragmentation and expression of apoptosis-related proteins in experimentally denervated and reinnervated rat facial muscle. Neuropathol Appl Neurobiol. 1997;23:141–149. 10.1046/j.1365-2990.1997.9298092.x. [PubMed] [Google Scholar]
- Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–1462. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- Trachtenberg JT. Fiber apoptosis in developing rat muscles is regulated by activity, neuregulin. Dev Biol. 1998;196:193–203. doi: 10.1006/dbio.1998.8871. 10.1006/dbio.1998.8871. [DOI] [PubMed] [Google Scholar]
- Williams GT. Programmed cell death: apoptosis and oncogenesis. Cell. 1991;65:1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura K, Harii K. A regenerative change during muscle adaptation to denervation in rats. J Surg Res. 1999;81:139–146. doi: 10.1006/jsre.1998.5504. 10.1006/jsre.1998.5504. [DOI] [PubMed] [Google Scholar]
- Yuan J. Molecular control of life and death. Curr Opin Cell Biol. 1995;7:211–214. doi: 10.1016/0955-0674(95)80030-1. 10.1016/0955-0674(95)80030-1. [DOI] [PubMed] [Google Scholar]
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407:802–809. doi: 10.1038/35037739. 10.1038/35037739. [DOI] [PubMed] [Google Scholar]
- Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]