Abstract
Because the placenta is the organ that transports nutrients, respiratory gases and wastes between the maternal and fetal systems, development of its vascular beds is essential to normal placental function, and thus in supporting normal fetal growth. Compromised fetal growth and development have adverse health consequences during the neonatal period and throughout adult life. To establish the role of placental angiogenesis in compromised pregnancies, we first evaluated the pattern of placental angiogenesis and expression of angiogenic factors throughout normal pregnancy. In addition, we and others have established a variety of sheep models to evaluate the effects on fetal growth of various factors including maternal nutrient excess or deprivation and specific nutrients, maternal age, maternal and fetal genotype, increased numbers of fetuses, environmental thermal stress, and high altitude (hypobaric) conditions. Although placental angiogenesis is altered in each of these models in which fetal growth is adversely affected, the specific effect on placental angiogenesis depends on the type of ‘stress’ to which the pregnancy is subjected, and also differs between the fetal and maternal systems and between genotypes. We believe that the models of compromised pregnancy and the methods described in this review will enable us to develop a much better understanding of the mechanisms responsible for alterations in placental vascular development.
‘The [umbilical] vessels join on the uterus like the roots of plants and through them the embryo receives its nourishment’.
Aristotle, On the Generation of Animals (ca 340 bc).
The importance of the placental circulation to fetal growth has been recognized since ancient times, as the above quotation from Aristotle makes abundantly clear (see Reynolds et al. (2005b) for a brief review of this history). More recently, Lawrence Longo (1972) put a modern spin on this concept when he stated:
‘The fetal “lifeline” thus includes an adequate maternal placental circulation and supply of blood nutrients, a placenta that transports and metabolizes various substances properly and a functional fetal placental circulation.’
Thus, adequate blood flow to the placenta is critical for normal fetal growth, and it is not surprising that conditions that affect fetal growth, such as maternal and fetal genotype, increased numbers of fetuses, maternal nutrient excess or deprivation, environmental thermal stress, and high altitude (hypobaric) conditions, typically have similar effects on placental size, and also are associated with reduced rates of fetal oxygen and nutrient uptake, as well as reduced placental angiogenesis and blood flow (Walton, 1938; Ebbs et al. 1942; Mckeown & Record, 1953; Eckstein et al. 1955; Hunter, 1956; Joubert & Hammond, 1958; Alexander, 1964; Alexander & Williams, 1971; Turman et al. 1971; Rattray et al. 1974; Corah et al. 1975; Sreenan & Beehan, 1976; Knight et al. 1977; Thompson et al. 1982; Reynolds et al. 1985; Ferrell, 1991; Ferrell & Reynolds, 1992; Reynolds & Redmer,1995; Reynolds et al. 2002; Vonnahme et al. 2001, 2002; Anthony et al. 2003). The critical role of the placental blood supply is confirmed by the observation that intrauterine growth restriction (IUGR) in third-trimester human pregnancies is characterized by impaired uterine (maternal placental) and umbilical (fetal placental) blood flows, leading to reduced fetal nutrient uptake as well as fetal hypoxia, hypoglycaemia and asymmetric organ growth (Pardi et al. 1993; Marconi et al. 1996; Ferrazzi et al. 2000; Konje et al. 2003). In addition, increased uterine vascular resistance and reduced uterine blood flow during early pregnancy can be used as predictors of high-risk pregnancies and are associated with fetal growth retardation (Trudinger et al. 1985; North et al. 1994).
Because the placenta is the organ through which respiratory gases, nutrients and wastes are exchanged between the maternal and fetal systems, placental vascular development is thought to play a central role in ensuring adequate transplacental exchange, and thus in determining, ultimately, the weight of the fetus at birth (Meschia, 1983; Reynolds & Redmer, 1995; Wulff et al. 2003; Kaufmann et al. 2004; Reynolds et al. 2005b). The full impact of birth weight or, more correctly, fetal and neonatal development, on adult health and well-being has only recently been recognized, as summarized in several recent reviews (Armitage et al. 2004; Barker, 2004; Gluckman & Hanson, 2004). This concept, which has been termed ‘developmental origins of adult health and disease’, was initially based primarily on epidemiological evidence, but has recently been confirmed by a spate of experimental work in animals (Trahair et al. 1997; Greenwood et al. 1998, 2000; Barker, 2004). In essence, this concept states that compromised fetal or neonatal growth and development, resulting from a variety of insults including maternal dietary caloric restriction, protein restriction, iron restriction, overfeeding, etc., leads to physiological ‘programming’, resulting in serious health consequences later in life, including cardiovascular disease, Type II (adult onset) diabetes, dyslipidaemia, and obesity (Breier et al. 2001; Oken & Gillman, 2003; Armitage et al. 2004; Barker, 2004; Gluckman & Hanson, 2004).
Animal models have been central to the study of the placenta and the placental circulation since the earliest times, and much of our knowledge of placental vascular development continues to be derived from comparative studies in animals (Kaufmann et al. 2004; Reynolds et al. 2005b). Fortunately, the power of the comparative approach in solving complex biological problems is widely recognized. Nevertheless, almost 50 years ago Donald Barron (1959) opined, ‘Obstetricians, by and large, deprive themselves to a surprising degree of sources of information which could be available to them from animal experiments … animal experiments are carried out to obtain vistas and to get ideas of the mechanisms of biological operation’.
In this review, we will focus on sheep models that have been used to study placental angiogenesis, first in normal then in compromised pregnancies. However, when appropriate we will discuss how results using sheep models compare with those from other animals, including primates. For a discussion of angiogenesis in the primate placenta we refer the reader to several excellent recent reviews (Wulff et al. 2003; Charnock-Jones et al. 2004; Kaufmann et al. 2004; Mayhew et al. 2004).
The sheep as a model for studying placental angiogenesis
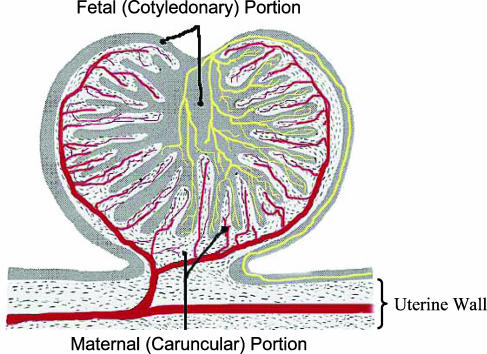
Macroscopically, the sheep placenta comprises 60–100 discreet structures, or units, known as placentomes, which are spaced relatively evenly over the surface of the fetal membranes (Ramsey, 1982; Mossman, 1987). Each of these placentomes consists of maternal caruncular and fetal cotyledonary portions, which interdigitate and thus are in close apposition (Fig. 1; Ramsey, 1982). Interestingly, maternal caruncles are present on the endometrial surface from shortly after birth, and their number and location remain constant throughout the female's life, although obviously they will grow during each pregnancy and then regress back to their non-pregnant size during each postpartum interval (Ramsey, 1982). The caruncles serve as specialized points of contact between the fetal membranes and the endometrium, and their location therefore dictates the ultimate location of the placentomes.
Figure 1. Schematic representation of the sheep placentome.
The maternal, or caruncular, portion is represented by the stippled areas, and the fetal, or cotyledonary, portion is represented by the greyish areas. The vascular supply for each portion of the placentome is represented by the red (maternal, caruncular) or yellow (fetal, cotyledonary) vessels. Figure adapted from The Placenta: Human and Animal, Ramsey, Elizabeth Mapelsden. Copyright © (1982) by Praeger Publishers. Reproduced with Permission of Greenwood Publishing Group, Inc., Westfoot, CT.
Microscopically, the sheep placenta belongs to a classification, first used by Grosser in 1927, known as epitheliochorial, which signifies that the fetal chorion is in direct contact with the maternal uterine (endometrial or uterine mucosal) epithelium (Ramsey, 1982; Mossman, 1987). Most investigators further classify the sheep placenta as a subset of epitheliochorial, termed syndesmochorial or synepithelial, to indicate that the uterine epithelium is fused with chorionic binucleate cells to form a feto-maternal syncytium (Wooding & Flint, 1994). Chorion refers to the outer tissue layer (ectodermal epithelium plus underlying mesoderm) of the fetal chorioallantois, which is the outer fetal membrane surrounding the fetal compartment (Ramsey, 1982; Mossman, 1987). In mammals with epitheliochorial placentation (moles, manatees, whales, horses, pigs, cattle, sheep, and a few prosimians), the chorioallantois is minimally invasive and thus the uterine epithelium remains intact during pregnancy (Ramsey, 1982; Mossman, 1987). In this regard, the sheep placenta is an ideal model for studying placental development because the maternal and fetal portions of the placenta remain closely associated but completely intact throughout gestation, and thus one can evaluate each tissue separately (Ramsey, 1982; Reynolds & Redmer, 1995; Reynolds et al. 2005a, b).
Placental angiogenesis in normal pregnancy
Based on the concept that chronic increases in blood flow to any growing tissue depend on vascular growth, or angiogenesis, Meschia (1983) reasoned ‘the large increase of blood flow to the uterus during pregnancy … results primarily from the formation and growth of the placental vascular bed.’ In fact, not only does the sustained increase in gravid uterine and umbilical blood flows depend on development of the placental vascular beds, but placental growth itself depends on placental angiogenesis since tissue growth of any magnitude cannot occur in the absence of vascular growth (Bassingthwaighte & Goresky, 1984; Hudlicka, 1984). For example, Folkman and coworkers (see Folkman & Klagsbrun, 1987) demonstrated that recruitment of a blood supply is requisite for sustained growth of tumours beyond about 1 mm3 in size. This dependence on vascular development results from the high metabolic demands associated with tissue growth and the limited ability of respiratory gases, nutrients, and metabolic wastes to diffuse through the extracellular compartment (Bassingthwaighte & Goresky, 1984; Adair et al. 1990).
Numerous studies have indicated that angiogenesis is indeed a major component of the increase in placental blood flow throughout gestation. For example, by day 24 after mating, vascular density of the uterine mucosa, or endometrium, exhibits a 2-fold increase (Reynolds & Redmer, 1992). Vascular density of endometrial (maternal placental) tissues continues to increase gradually throughout gestation (Reynolds & Redmer, 2001; Reynolds et al. 2005b). In contrast, vascular density of the fetal placental tissues, or cotyledons, remains relatively constant until mid-gestation then increases dramatically thereafter. These patterns of placental angiogenesis coincide with the dramatic increases that have been reported for uterine and umbilical blood flows throughout gestation (Reynolds & Redmer, 1995). Thus, growth and development of the vascular beds are critical components of tissue growth, including that of the utero-placenta, and as already mentioned the importance of vascular development to placental function has long been recognized. However, research on placental vascular growth comprises primarily descriptive histological studies, and only a handful of quantitative studies of placental angiogenesis have been reported (see Reynolds et al. 2005b, for review).
We have recently conducted an extensive quantitative analysis of placental vascular development and have used those data to model placental angiogenesis and its relationship to placental expression of angiogenic factors throughout the last two-thirds of gestation in sheep. For these studies, we utilized fixation methodologies involving vascular perfusion and casting, and subsequently analysed vascular development using morphometric methods similar to those we have previously described (Reynolds & Redmer, 1992; Reynolds et al. 1998) but with application of computerized image analysis. Although they provide results similar to those using standard manual morphometric methods, the computerized methodologies have enabled us to rapidly generate several measures of vascular development, and thus have allowed us to model angiogenesis in the maternal and fetal portions of the placentome (Borowicz et al. 2003; Reynolds et al. 2005b).
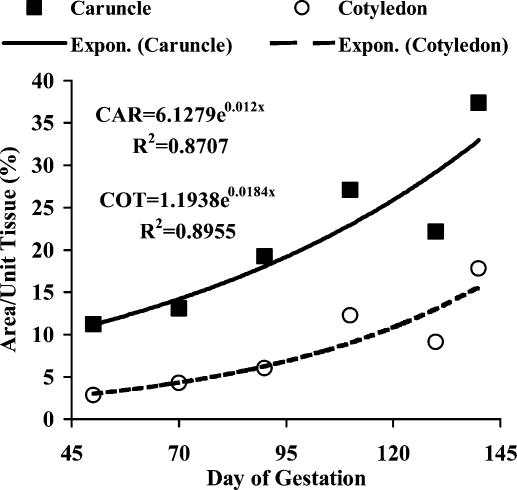
As shown in Fig. 2, capillary area density (capillary area as a proportion of total tissue area) of both maternal caruncular and fetal cotyledonary tissues increased exponentially (3.3-fold for maternal and 6.2-fold for fetal; Fig. 3) from day 50 through day 140 after mating. Capillary area density is a commonly used measure of angiogenesis, and based on stereological principles also is equivalent to capillary volume density (capillary volume as a proportion of total tissue volume; Weibel, 1972; Hudlicka, 1984). In contrast, another common measure of vascular growth, capillary number density (number of capillaries per unit of tissue; Weibel, 1972), increased exponentially and dramatically (12.3-fold) in the fetal cotyledon but only slightly (1.5-fold) in the maternal caruncle from day 50 to day 140 (Fig. 3). Similarly, the capillary surface density (perimeter or surface area of capillaries per unit of tissue) increased exponentially (6-fold) in cotyledons but only slightly (1.7-fold) in caruncles (Fig. 3). The large increase in number density of cotyledonary capillaries was associated with a 1.9-fold decrease in their size, whereas the size of caruncular capillaries increased 2.2-fold from day 50 to 140 of gestation (Fig. 3). This model also agrees with the observations of Stegeman, who reported a 4-fold increase in ‘degree of branching’ of the fetal cotyledonary villi, accompanied by a 9-fold increase in cotyledonary capillary density from day 50 to day 140 of gestation in sheep (Stegeman, 1974; Reynolds et al. 2005b).
Figure 2. Vascular development of maternal caruncle (CAR) and fetal cotyledon (COT) throughout the last two-thirds of gestation in sheep.
Capillary area density (capillary area as a proportion of total tissue area) of both maternal (caruncle) and fetal (cotyledon) placental tissues increased exponentially from day 50 through day 140 after mating. Capillary area density is a commonly used measure of angiogenesis, and based on stereological principles also is equivalent to capillary volume density (capillary volume as a proportion of total tissue volume; Weibel, 1972; Hudlicka, 1984). Figure taken from Reynolds et al. (2005b).
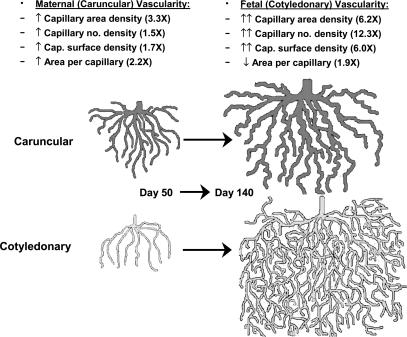
Figure 3. Empirical model of angiogenesis in the sheep placenta throughout the last two-thirds of gestation.
The maternal (caruncular) capillary beds grow primarily by increased capillary size, with only small increases in capillary number or surface densities, resulting in a modest increase in capillary area density. In contrast, the fetal (cotyledonary) capillary beds grow primarily by branching, resulting in a large increase in capillary number density accompanied by a decrease in capillary size; this branching pattern of growth also explains the relatively large increase in capillary area and surface densities. For illustration the capillary beds are depicted as open-ended, rather than as a closed loop. Taken from Reynolds et al. (2005b).
Based on these observations, we have developed an empirical model of angiogenesis in the maternal and fetal portions of the sheep placenta throughout the last two-thirds of gestation (Fig. 3). In this model, the caruncular capillary beds grow primarily by increased size of the capillaries with only small increases in capillary number or surface densities, resulting in a modest, 3.3-fold increase in capillary area density. In contrast, the cotyledonary capillary beds grow primarily by branching, resulting in a large, 12.3-fold increase in capillary number density accompanied by a decrease in capillary size. The relatively large, 6-fold increase in capillary area and surface densities of the fetal cotyledons can be explained by this branching pattern of growth.
Thus, although the size of the fetal and maternal portions of the placenta is highly correlated, their capillary beds exhibit dramatic differences in their microscopic microvascular architecture and, most likely, in their physiological function as well (Reynolds & Redmer, 1995; Borowicz et al. 2003; Reynolds et al. 2005b). The microvasculature of the maternal placental compartment is composed almost exclusively of very large capillaries, which would dictate a low-velocity, ‘irrigation-flow’ or percolating type of system, designed primarily as a delivery (and, conversely, a waste-removal) system (Reynolds et al. 2005b). In contrast, the microvascular architecture of the fetal placenta is highly branched and composed primarily of abundant small capillaries, designed as a high velocity rapid transport system.
These dramatic differences in microvascular architecture agree with several physiological observations. For example, it has recently been shown that, throughout the last half of gestation in sheep, resistance across the fetal placental microvascular bed in an in vitro perfusion system is 2-fold greater than that of the maternal placental microvascular bed at physiological flow rates (Vonnahme et al. 2004a, b). In addition, because of increased branching and thus larger numbers of capillaries per unit of tissue, the surface area available for exchange is greater in fetal cotyledons compared with maternal caruncles during the last trimester (Reynolds et al. 2005a, b). Moreover, the thickness of the barrier between the fetal and maternal capillaries also may be reduced throughout gestation (Faber & Thornburg, 1983; Longo, 1987). Taken together, these observations help to explain why the proportion of the nutrients and oxygen taken up by the gravid uterus that is transported to the fetus increases by 2- to 4-fold from mid- to late gestation, essentially keeping pace with the rate of fetal growth (Reynolds & Redmer, 1995).
Thus, the placental microvascular architecture of sheep is ideally suited for both nutrient delivery, on the maternal side, and nutrient uptake and transport, on the fetal side. This arrangement, however, does not appear to be unique to sheep. For example, there are several striking similarities in placental microvascular architecture and function among the sheep, cow and pig (Assheton, 1906; King et al. 1982; Reynolds & Redmer, 1992; Reynolds et al. 2005a). These similarities may extend to other mammals as well. For example, in primates including humans the chorioallantois is so invasive that a portion of the maternal endometrium is lost, and the fetal villi are bathed in maternal blood (Ramsey, 1982). The arrangement of the primate placenta thus represents the ultimate in a low-velocity, irrigation-flow or percolating system such as that we have proposed for the sheep (Reynolds et al. 2005b). It is interesting to note that this ‘haemochorial’ type of placenta (designating the chorion or outer layer of the chorioallantois being bathed in maternal blood) is widespread among mammals, being present not only in the vast majority of primates but also in rodents, insectivores and bats, which together comprise 95% of the more than 4000 species of mammals (Ramsey, 1982; Nowak, 1991).
To begin to model the regulation of placental angiogenesis in sheep we have calculated the simple correlations among measures of vascular growth and expression of angiogenic factors (Borowicz et al. 2003, 2004a, b; Reynolds et al. 2005b). As shown in Table 1, the angiogenic factors evaluated included vascular endothelial growth factor (VEGF) and its major receptors VEGFR-1 and -2, basic fibroblast growth factor (bFGF), and angiopoietins (ANG)-1 and -2 and their major receptor TIE-2, which represent several of the major factors regulating angiogenesis (Charnock-Jones et al. 2004; Reynolds et al. 2005b).
Table 1.
Simple correlations among measures maternal placental (caruncular) and fetal placental (cotyledonary) angiogenesis and expression of mRNA for angiogenic factors throughout the last two-thirds of gestation in sheep
| Maternal caruncle | Fetal cotyledon | |||||
|---|---|---|---|---|---|---|
| Angiogenic factor | Capillary area density (%) | Capillary number density (no. per unit tissue) | Capillary surface density (mm mm−2) | Capillary area density (%) | Capillary number density (no. per unit tissue) | Capillary surface density (mm mm−2) |
| VEGF | 0.24 | −0.05 | −0.14 | 0.46 | 0.52 | 0.45 |
| P = 0.19 | P = 0.80 | P = 0.45 | P < 0.01 | P < 0.01 | P < 0.01 | |
| VEGFR-1 | 0.54 | 0.11 | 0.28 | 0.36 | 0.31 | 0.42 |
| P < 0.01 | P = 0.55 | P = 0.12 | P < 0.04 | P = 0.08 | P < 0.01 | |
| VEGFR-2 | 0.24 | −0.14 | −0.01 | −0.11 | −0.19 | −0.07 |
| P = 0.18 | P = 0.44 | P = 0.97 | P = 0.52 | P = 0.29 | P = 0.71 | |
| bFGF | −0.11 | −0.19 | −0.22 | 0.37 | 0.34 | 0.52 |
| P = 0.53 | P = 0.30 | P = 0.22 | P < 0.03 | P = 0.06 | P < 0.01 | |
| ANG-1 | 0.24 | 0.05 | −0.01 | 0.33 | 0.32 | 0.37 |
| P = 0.16 | P = 0.79 | P = 0.95 | P = 0.06 | P = 0.07 | P < 0.04 | |
| ANG-2 | 0.50 | 0.02 | 0.17 | 0.54 | 0.45 | 0.60 |
| P < 0.01 | P = 0.89 | P = 0.33 | P < 0.01 | P < 0.01 | P < 0.01 | |
| TIE-2 | 0.08 | −0.30 | −0.14 | 0.07 | −0.01 | 0.09 |
| P = 0.65 | P = 0.09 | P = 0.42 | P = 0.70 | P = 0.98 | P = 0.63 | |
Taken from Borowicz et al. (2004a,b), and unpublished observations (n = 4 ewes per day). Values represent Pearson correlation coefficients and probability levels. Significant correlations are shown in bold.
Capillary area density of maternal caruncular tissues was correlated with expression of VEGFR-1 and ANG-2, but not with the other angiogenic factors (Table 1). However, neither capillary number density nor capillary surface density of caruncles was correlated with expression of any of the angiogenic factors. For cotyledons, all three measures of angiogenesis were correlated with expression of VEGF and ANG-2 (Table 1). In addition, cotyledonary capillary area density and capillary surface density were correlated with expression of VEGFR-1, bFGF and ANG-1 mRNA.
Although these are only simple correlations, they have begun to yield useful information concerning placental angiogenesis in sheep. For example, these data indicate that maternal placental vascularization is dependent on VEGFR-1 and ANG-2 expression. In contrast, fetal placental angiogenesis seems to depend on numerous angiogenic factors. The observation that cotyledonary angiogenesis is so strongly correlated with VEGF and ANG-2 expression argues for a critical interaction between these two angiogenic factors, which has been suggested for the placenta as well as for other tissues (Koh et al. 2002; Reynolds et al. 2005b).
Placental angiogenesis in sheep models of compromised pregnancy
Thus, as described in the previous section, we have evaluated the pattern of placental angiogenesis and expression of angiogenic factors throughout normal pregnancy. To determine the role of placental angiogenesis in compromised pregnancies, we and others have established a variety of sheep models to evaluate various factors that influence fetal growth, including maternal nutrient excess or deprivation and specific nutrients; maternal age; maternal and fetal genotype; increased numbers of fetuses; environmental thermal stress; and high altitude (hypobaric) conditions. We will first discuss observations made by laboratories other than our own concerning the effects on placental angiogenesis of both environmental thermal stress and high altitude conditions. We will then discuss observations that we have made very recently using several models to investigate the influence of maternal nutritional stress, maternal age, maternal genotype, and numbers of fetuses on placental angiogenesis.
Maternal environmental thermal stress and high altitude (chronic hypoxia)
In ruminants, including sheep and cattle, environmental thermal (or heat) stress; that is, pregnancy at elevated environmental temperature, results in decreased fetal and placental weights by late in pregnancy (Reynolds et al. 1985; Regnault et al. 2003). In addition, both uterine and umbilical blood flows are reduced, resulting in compromised transport capacity of the placenta (Reynolds et al. 1985; Regnault et al. 2003). In sheep, this adverse effect of heat stress is associated with a reduced expression of placental angiogenic factors by mid-gestation and continuing through late gestation (Regnault et al. 2002b, 2003). In conjunction with reduced angiogenic factor expression, Regnault et al. (2002a) also reported that the fetal placental capillaries from the heat stressed ewes were more ‘coiled and appeared in a more random orientation relative to neighbouring vessels. Additionally, sinusoidal dilations also appeared to be more numerous in … fetal vessels.’ Although they did not quantify these differences, they did suggest that the changes in fetal placental microvascular architecture were similar to those observed in ewes subjected to high altitude-induced hypoxia during pregnancy, as discussed in the next paragraph.
In humans, birth weight declines when the mother resides at high altitude (> 2500 m) throughout pregnancy, although the magnitude of this effect depends on whether the population is adapted to high altitude (Moore et al. 2004). There is good evidence that maternal hypoxia, including that resulting from hypobaric stress at high altitude, also results in compromised placental growth and function in humans (Mayhew et al. 2004). Nevertheless, probably as a means to attempt to compensate for maternal hypoxia, VEGF concentrations in serum are elevated in pregnant women at high altitude compared with normoxic controls (Wheeler et al. 2002).
Pregnancy at high altitude in humans also is associated with compensatory changes in fetal placental microvascular architecture, including increased capillary branching, associated with increased capillary area density and surface density (Mayhew et al. 2004). Similarly, in sheep at high altitude throughout pregnancy both maternal and fetal placental microvascular architecture is altered (Krebs et al. 1997). Although capillary number density was reduced in the fetal placenta, capillary size was increased, resulting in increased capillary area density. In maternal placenta, capillary number density was not affected by high altitude-associated maternal hypoxia, but capillary size and capillary area density were increased as in the fetal placenta. Thus, the sheep model appears to mirror the changes in placental microvascular architecture observed in humans.
Maternal nutrient excess in adolescents
Adolescent pregnancy remains a major problem, especially in the US and UK, because adolescent girls have an increased risk of delivering premature and low birth weight babies compared with mothers > 20 years of age (NVSR, 2001; MOD, 2002a; NLM, 2002a; NCPTP, 2004). In 2000, there were more than 479 000 births to adolescents in the US (NVSR, 2001). Moreover, as discussed above, both preterm delivery and fetal growth restriction are associated with a much greater risk of neonatal mortality and morbidity, and also of developing cardiovascular disease, diabetes, or other significant health consequences as adults (MOD, 2002a; NLM, 2002a; Armitage et al. 2004; Barker, 2004; Gluckman & Hanson, 2004).
Many adolescents retain the potential to grow while pregnant. For example, the study from Camden, New Jersey (one of the poorest cities in the United States) showed that almost 50% of adolescents continue to grow while pregnant (Scholl et al. 1993, 1994, 1997). This continued growth was associated with larger pregnancy weight gains, increased fat stores and greater postpartum weight retention compared with non-growing pregnant adolescents and mature pregnant women. Paradoxically, birth weights were reduced by 150–200 g in the growing adolescents despite maternal changes typically associated with increased fetal size (e.g. increased weight gain and fat stores). The reduction in fetal growth rate in adolescents has been attributed to a competition for nutrients between the maternal body and her gravid uterus (Redmer et al. 2004b).
Using a unique, robust and highly controlled experimental protocol it has been shown that overnourishing the singleton-bearing adolescent ewe results in rapid maternal growth, and most particularly of maternal adipose tissue, at the expense of the nutrient requirements of the gravid uterus (Wallace et al. 1996, 1999b, 2001; Redmer et al. 2004b). This model therefore reflects not only overfeeding to promote rapid maternal growth but also maternal obesity, and results in major placental growth restriction (30–40%), leading to the premature delivery of low birth weight lambs (25–30% reduction in birth weight) compared with moderately nourished adolescents of equivalent gynaecological age.
Although maternal obesity and excessive weight gain during pregnancy in human adults do not compromise fetal growth, and may even result in increased birth weight, they do result in fetal growth abnormalities and increased risk of fetal and neonatal morbidity and mortality (Naeye, 1990; Cnattingius et al. 1998; Ogunyemi et al. 1998; Schieve et al. 1999; Abrams et al. 2000; Stephansson et al. 2001; Castro & Avina, 2002; MOD, 2002b; Luke et al. 2003). In very young pregnant adolescents excessive weight gain and preterm delivery are associated (Perry et al. 1996; Hediger et al. 1997), which agrees with our observations of premature delivery in overfed, pregnant adolescent sheep (Wallace et al. 1996, 1999b, 2001). The observation that increased dietary intake during pregnancy is associated with poor pregnancy outcomes, combined with the high incidence of obesity among adolescents, especially in the US (Ogden et al. 2002; NCHS, 2004), emphasizes the importance of this sheep model of the overnourished pregnant adolescent for understanding the physiological mechanisms leading to altered fetal and placental growth.
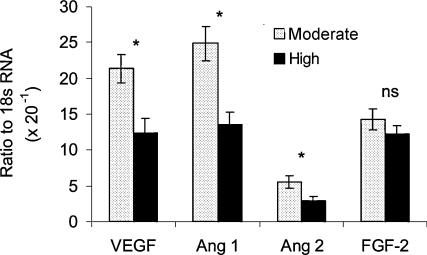
In a recent report (Redmer et al. 2005), pregnant adolescent ewes on a high or moderate plane of nutrition were killed on day 80 of gestation. Placental expression of mRNAs for VEGF, ANG-1 and ANG-2 was significantly reduced in the placentomes of the High compared with the Moderate intake group (Fig. 4). Although placental expression of the receptors for VEGF and ANG-1 and ANG-2 also were evaluated, only VEGFR-1 mRNA was reduced. In addition, Redmer et al. (2004a) observed that in overfed adolescent ewes on day 130 of gestation, capillary size was increased while capillary number density was reduced in maternal and fetal placental tissues. These alterations in placental vascular development are similar to those reported in other models of compromised pregnancy, including environmental heat stress and pregnancy at high altitude, as discussed previously.
Figure 4. Angiogenic factor mRNA expression in placentomes (maternal caruncular + fetal cotyledonary tissue) from adolescent ewes on day 80 of pregnancy.
Ewes were individually offered either a Moderate or High level of a diet calculated to promote moderate or rapid maternal growth rates, respectively. mRNA expression was quantified by real-time RT-PCR. Adapted from Redmer et al. (2005); *indicates effect of diet (P < 0.005), ns = no significant effect of diet.
Taken together with our previous observation that fetal and placental growth are not yet compromised at day 80 but are reduced by day 130 (Wallace et al. 1999a, 2000), these observations strongly support the contention that reduced fetal growth due to overnutrition in growing adolescent ewes results from abnormal expression of placental angiogenic factors leading to alterations in placental vascular development. Although these preliminary observations are very exciting, they clearly need to be expanded to determine the impact of maternal nutritional status on angiogenic growth factor expression at key developmental time points throughout gestation and to relate any changes in their expression to the various measures of placental angiogenesis described in the previous section.
Maternal nutrient deprivation in adults
At the other end of the nutritional spectrum, an association between decreased energy intake in adult women and low birth weight or preterm delivery has been difficult to demonstrate, and large effects have been observed only in extreme situations. However, although the data do not account for maternal age or parity, in humans body mass index and neonatal weight are highly correlated (WHO, 1995; MOD, 2002b). Birth weight also increases in populations as they become more affluent and nutrient intake improves, whereas birth weight decreases during periods of restricted intake or famine (Gruenwald et al. 1967; Kramer, 1987; Dhawan, 1995; Steckel, 1998; Rush, 2001). In addition, providing adult women on poor diets with energy rich and protein balanced supplements has been shown to significantly improve birth weight and pregnancy outcome (Higgins et al. 1989; Kramer & Kakuma, 2003).
In adult sheep severe maternal undernutrition at all stages of pregnancy, and particularly during late gestation, reduces fetal growth by varying degrees (Mellor, 1983; Robinson, 1983; Vincent et al. 1985; Parr et al. 1986). In some studies where moderate to severe maternal nutrient restriction were imposed between early and mid-gestation (Heasman et al. 1998; Steyn et al. 2001) and mid- to late gestation (Oddy & Holst, 1991) lamb birth weight at or near term was unaffected. In contrast, in a recent study in which ewes were restricted to 50% of energy intake requirements from day 28 to 80 of gestation, fetal weights at day 80 were reduced by 32% (Vonnahme et al. 2003). Similarly, the effects of undernutrition during pregnancy on uterine and umbilical blood flows have been variable, with some investigators showing no effect, whereas others have shown a reduction especially in uterine blood flow (Chandler et al. 1985; Leury et al. 1990; Newnham et al. 1991; Kelly et al. 1992). Thus, the effects of nutrient restriction during pregnancy in adult sheep are inconsistent, and may depend on the level and/or length of restriction. Likewise, the precise role of the placenta in mediating nutritional effects in the adult sheep is inconclusive (see review by Kelly, 1992), but may depend on an interaction between maternal live weight at the time of conception and body fat status or nutrient reserves at the onset of nutrient restriction (Russel et al. 1981).
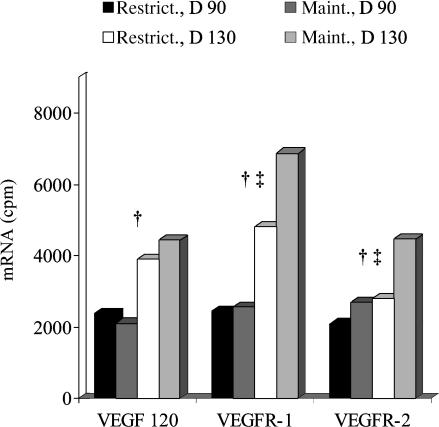
We recently have shown that underfeeding of adult ewes during pregnancy (restriction of feed intake to 60% of recommended levels) results in a 15% reduction in fetal growth from day 90 to 130 of gestation (Arnold et al. 2001b). Although restricted ewes exhibited no decrease in placental VEGF mRNA, placental expression of VEGF receptor mRNA (both VEGFR-1 and VEGFR-2) was reduced by 50% on day 130 compared with that of ewes receiving the maintenance diet (Fig. 5). These observations indicate that reduced fetal growth in undernourished ewes may result from altered placental growth and vascular development or, alternatively, by limiting nutrient availability in the maternal circulation. Comparable data in nutritionally perturbed pregnancies in the human are not currently available due to the obvious difficulties in obtaining anything other than late gestation or term tissues. However, attenuated placental VEGF expression has been reported in both pre-eclamptic and severely intrauterine growth restricted pregnancies (Cooper et al. 1996; Lyall et al. 1997; Chung et al. 2004) implying a central role for this angiogenic factor in the aetiology of these two types of compromised placentas.
Figure 5. Effects of nutrient restriction on placental expression of VEGF and VEGFR mRNAs.
In this study, adult ewes received restricted (Restrict., 60% of NRC requirements) or maintenance (Maint.) diets from day 50 until day 90 (D 90) or day 130 (D 130) of pregnancy (length of gestation approximately 145 days). mRNA expression was quantified by ribonuclease protection assay. †Day effect (P < 0.01) for VEGF, VEGFR-1, and VEGFR-2. ‡Diet effect (P < 0.01) for VEGFR-1 and VEGFR-2. n = 5 ewes per group; s.e.m.= 193.7 for VEGF, 312.0 for VEGFR-1, and 203.1 for VEGFR-2. Taken from Redmer et al. (2004b).
Maternal selenium intake
Selenium (Se) is a trace nutrient that is essential for normal growth and development (Sunde, 1997; McDowell, 2003). Although both Se deficiency and excess have severe health consequences, recent work has shown that ‘supranutritional’ levels (2- to 4-fold above normal dietary requirements) can reduce the combined incidence of lung, colorectal, and prostate cancers by as much as 50% in humans and rats (Clark et al. 1996; Finley et al. 2000; Combs & Lu, 2001). In addition, supranutritional levels of Se have also been shown to reduce angiogenesis and VEGF expression in tumours (Jiang et al. 1999, 2000). However, little data are available regarding the influence of supranutritional levels of Se on growth, cellular proliferation and vascularization of placental and fetal tissues.
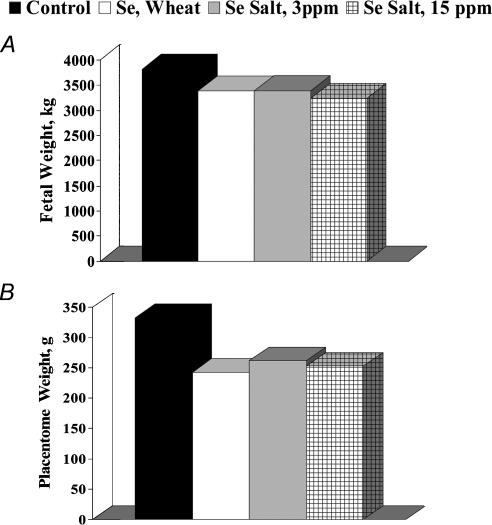
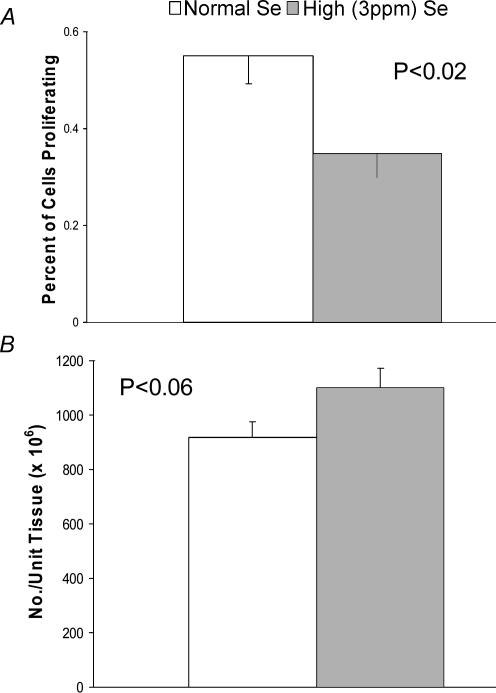
In a recent study (Caton et al. 2004), although supranutritional levels of dietary Se (fed either as high-Se wheat (organic source) or sodium salt (inorganic source)) in pregnant adult ewes had little effect on fetal weight, placental weight was reduced by 24% at day 130 of gestation (Fig. 6). Reduced placental size was associated with a 40% reduction in cell proliferation in the maternal placental tissue, and also with a slight increase in capillary number density in the fetal placenta (Fig. 7). The increased fetal placental capillary number density was associated with increased expression of VEGF and VEGFR-1 mRNAs (Table 2). Thus, although high dietary Se does appear to compromise placental growth and vascular development, and alters placental angiogenic factor expression, much more work needs to be done not only to confirm this response but also to understand the gestational stage-specific mechanisms responsible for these effects.
Figure 6. Impacts of elevated maternal dietary selenium on fetal (A) and placental (B) growth in ewes.
Ewes (n = 8–10 per group) were fed diets with similar nitrogen and energy contents but with normal levels of Se (Control, 0.1 p.p.m), or 3 p.p.m. of Se from an organic (Se, Wheat) or inorganic (Se Salt, 3 p.p.m) source, or 15 p.p.m. of Se from an inorganic source (Se Salt, 15 p.p.m.), beginning on day 50 after mating and continuing until slaughter on day 130. Although fetal weights (A) did not differ among groups, placental weight (B) was less (P < 0.02) in Se-treated versus Control ewes.
Figure 7.
Impacts of elevated maternal dietary selenium on caruncular cell proliferation (A) (BrdU labelling index) and cotyledonary capillary number density (B) in ewes receiving diets containing Normal (0.1 p.p.m) or High (3 p.p.m. from high-Se wheat) levels of Se from day 50 until slaughter on day 130 of gestation. Borowicz, Ward, Vonnahme, Caton, Redmer & Reynolds, unpublished data.
Table 2.
Angiogenic factor mRNA expression in the maternal (caruncular) and fetal (cotyledonary) portion of the placentomes
| Caruncular (n = 6) | Cotyledon (n = 6) | |||
|---|---|---|---|---|
| Gene of interest | Control | Selenium | Control | Selenium |
| VEGF | 0.73 ± 0.16 | 0.74 ± 0.09 | 1.92 ± 0.22 | 2.79 ± 0.54† |
| VEGFR-1 | 1.34 ± 0.32 | 1.53 ± 0.23 | 3.40 ± 0.28 | 4.65 ± 0.84** |
| VEGFR-2 | 0.62 ± 0.05 | 0.87 ± 0.13 | 1.73 ± 0.22 | 1.73 ± 0.23 |
| bFGF | 4.91 ± 1.00 | 5.93 ± 0.76 | 2.80 ± 0.64 | 2.58 ± 0.36 |
| FGF-R | 1.91 ± 0.34 | 1.94 ± 0.36 | 4.33 ± 0.30 | 4.98 ± 0.67 |
| ANG-1 | 0.56 ± 0.13 | 0.63 ± 0.07 | 1.72 ± 0.30 | 1.94 ± 0.27 |
| ANG-2 | 0.87 ± 0.19 | 1.07 ± 0.12 | 2.24 ± 0.41 | 2.65 ± 0.48 |
| TIE-2 | 1.24 ± 0.16 | 1.74 ± 0.31 | 2.51 ± 0.36 | 2.63 ± 0.20 |
Data are for ewes fed Control diets and ewes fed diets containing 3 p.p.m. Se from high-Se wheat, and are expressed relative to the 18S ribosomal mRNA level; significant differences between Control and Selenium are shown in bold.
Means ± standard error of the mean within a tissue and differ, P < 0.09.
Means ± standard error of the mean within a tissue and differ, P < 0.05.
Maternal age
As discussed already, maternal age has a large influence on fetal and placental growth. However, the degree to which this effect is due to maternal immaturity or to numerous confounding factors, including nutritional status, parity, or other risk factors is not known (Ferrell, 1991; Wallace et al. 1996; Bolzan et al. 1999; Rauh & Andrews, 1999). Thus, we developed a model in which the effects of nutritional status and parity were held constant.
For this study (Borowicz et al. 2005), early maturing, highly prolific (Romanov) and relatively late maturing, less prolific (Columbia) ewes were subjected to embryo transfer, using a consistent source of embryos within a breed, just as the ewes reached sexual maturity (i.e. in either of the first three oestrous periods after reaching puberty; designated ‘lambs’) or when they had reached physical maturity (i.e. at approximately 11/2 years of age; designated ‘yearlings’). Thus, regardless of age all dams were primigravida and were fed to meet 100% of the requirements for pregnancy (NRC, 1985). These maternal ages correspond approximately to 10–12 years old compared with 19–20 years old in humans.
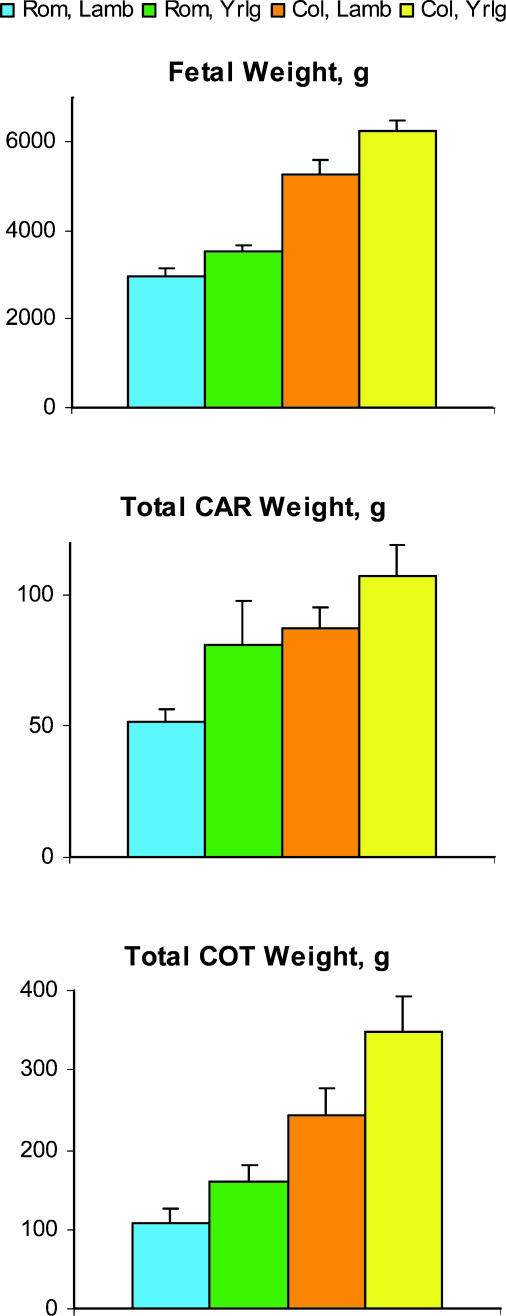
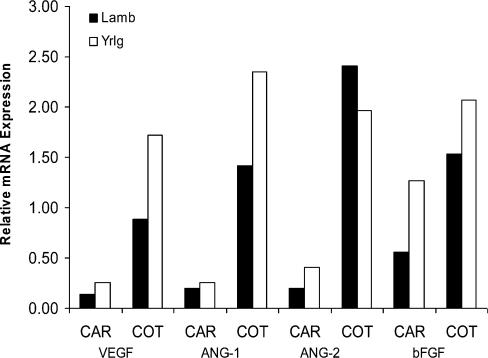
At day 130 of gestation, fetal, cotyledonary and caruncular weights were reduced in the immature dams (lambs) compared with the mature dams (yearlings), regardless of breed (Fig. 8). These effects were associated with a decrease in expression of VEGF and ANG-1 in cotyledonary but not caruncular placental tissues (Fig. 9). No significant effects of maternal age were observed for ANG-2 or bFGF (Fig. 9). Thus, although fetal and placental size were less in Romanov compared with Columbia ewes, as expected (the highly prolific Romanovs are well known to have smaller offspring), in both breed groups a profound effect of maternal age on fetal size as well as cotyledonary size and angiogenic factor expression was observed. These effects confirm that gynaecological maturity per se influences fetal size and placental angiogenesis.
Figure 8.
Fetal weight, caruncular (CAR; maternal placental) weight, and cotyledonary (COT; fetal placental) weight in Romanov and Columbia lambs (Lamb; peripubertal) and yearlings (Yrlg; early adult) on day 130 of gestation. n = 5–7 per group; Lamb versus Yearling, P < 0.006 for fetal weight, P < 0.05 for total CAR weight, and P < 0.03 for total COT weight.
Figure 9.
Expression of angiogenic factors in caruncular (CAR; maternal placental) and cotyledonary (COT; fetal placental) tissues of Romanov and Columbia lambs (Lamb; peripubertal) and yearlings (Yrlg; early adult) on day 130 of gestation. n = 7–12 per group; Lamb versus Yearling, P < 0.05 for VEGF and ANG-1 for COT only.
Maternal and fetal genotype
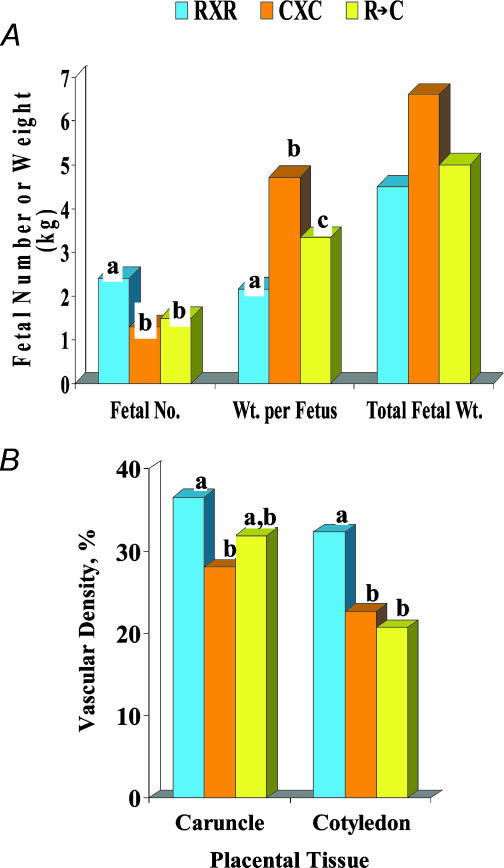
To begin to understand the influence of fetal and maternal genotype on placental angiogenesis, we have utilized Romanov and Columbia ewes, which represent highly prolific (3–5 fetuses per pregnancy) and more standard (1–3 fetuses per pregnancy) breeds, as described above, combined with embryo transfer. Thus, we evaluated fetal weight and placental angiogenesis in straight-bred Romanov and Columbia ewes and also in Columbia dams carrying Romanov embryos (Arnold et al. 2001a; L. P. Reynolds, J. S. Caton & D. A. Redmer, unpublished observations; Fig. 10).
Figure 10.
Fetal number, weight per fetus, total fetal weight, and caruncular and cotyledonary capillary area density (vascular density) on day 130 after mating in straight-bred Romanov (R × R) and Columbia (C × C) pregnancies or when Romanov embryos were transferred to Columbia dams (R → C). n = 4–9 per group. Bars with different superscripts differ, P < 0.04 for fetal no., P < 0.10 for fetal wt, P < 0.01 for vascular density.
Although fetal number at day 130 of gestation was greater in the Romanov ewes, fetal weights were less, resulting in a similar fetal mass among all three groups (Fig. 10A). Capillary area density was greater in Romanov compared with Columbia ewes for both maternal and fetal placental tissues (Fig. 10B). However, when Romanov embryos were placed into Columbia dams (Romanov × Columbia group), caruncular capillary area density was increased compared with straight-bred Columbias (Fig. 10B). This observation indicates that the Romanov embryos influenced vascularity of the maternal placenta in these Columbia ewes. Similarly, in the Romanov × Columbia group cotyledonary capillary area density was decreased (Fig. 10B), indicating that the Columbia uterus negatively influenced fetal placental vascularity in these Romanov embryos. Surprisingly, no differences were observed among these groups for placental VEGF, VEGFR-1 or VEGFR-2 mRNA expression. Thus elucidating the mechanism(s) by which the maternal and fetal placental tissues influence each other's microvascular development will require further investigation.
Numbers of fetuses
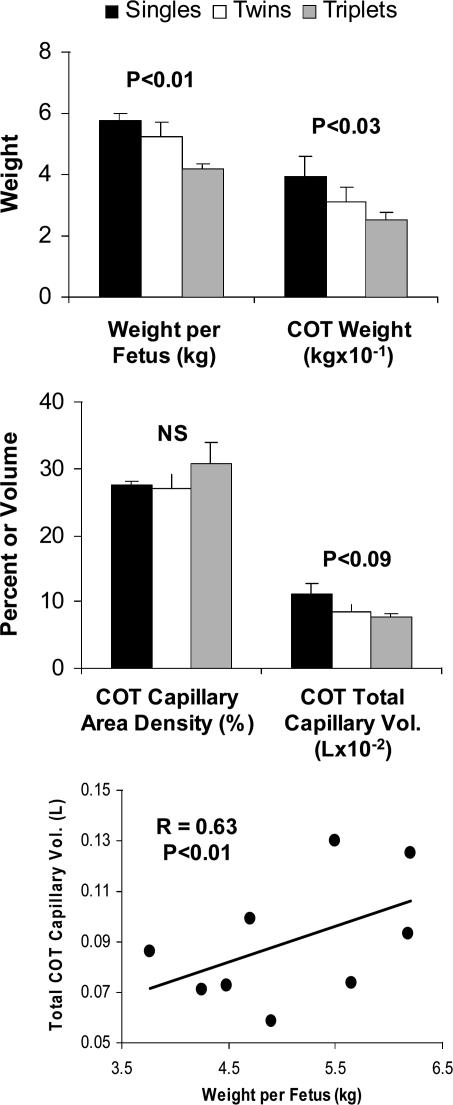
It is well known that multiple fetuses are associated with reduced fetal size and birth weights as well as increased neonatal morbidity and mortality (Huffman et al. 1985). To examine the response of placental angiogenesis to multiple fetuses, we evaluated fetal and placental weights and placental vascular development in late pregnancy in ewes bearing single, twin, or triplet fetuses (Pant et al. 2003; Wirrenga et al. 2004).
As we had expected, fetal and cotyledonary weights were reduced as the number of fetuses increased (Fig. 11). Although cotyledonary capillary area density was not affected by number of fetuses, the total cotyledonary capillary volume was reduced as number of fetuses increased. In addition, total cotyledonary capillary volume of individual fetuses was highly correlated with their weight (Fig. 11). These observations indicate that the reduction in placental weight, and not vascular development per se explain the reduction in fetal weight with increasing numbers of fetuses. Thus, multiple fetuses is a clear case in which compensatory mechanisms do not seem to be operating, at least in terms of placental vascularity of individual fetuses.
Figure 11.
Fetal and cotyledonary (COT) weights, cotyledonary capillary area density and total capillary volume, and correlation between cotyledonary total capillary volume and fetal weight on day 130 of gestation in mature ewes bearing singles, twins, or triplets. n = 15, 12, and 3 ewes per group, respectively.
Concluding remarks
As we have discussed, development of the placental vascular beds is essential to normal placental function, and thus in supporting normal fetal growth. Compromised fetal growth and development, in turn, have adverse health consequences not only during the neonatal period and infancy but in fact have a profound influence on health and well-being throughout adulthood. We have described a variety of sheep models that have been developed to evaluate the effects on fetal growth of various factors, including maternal nutrient excess or deprivation and specific nutrients; maternal age; maternal and fetal genotype; increased numbers of fetuses; environmental thermal stress; and high altitude (hypobaric) conditions. Although placental angiogenesis is altered in each of these models in which fetal growth is adversely affected, the specific effect on placental angiogenesis depends on the type of ‘stress’ to which the pregnancy is subjected, and also differs between the fetal and maternal systems and between genotypes. We believe that the models of compromised pregnancy and the methods described in this review will enable us to develop a much better understanding of the mechanisms responsible for alterations in placental vascular development.
Acknowledgments
We gratefully acknowledge the many individuals who have made valuable and important contributions to the progress of our work in this area, including our collaborators and colleagues (Dr Calvin Ferrell, Dr Stephen Ford, Dr Derek Killilea, Dr Ronald Magness, Dr Robert Moor, and Dr Russell Anthony), current and former graduate students (Mr Daniel Arnold, Mr Justin Luther, Dr Abraham Scheaffer, Ms Marcy Ware, and Dr Jing Zheng), numerous undergraduate students, and laboratory personnel (Mr James Kirsch, Mr Kim Kraft, Mr Robert Weigl, Dr Jerzy Bilski, Ms Dana Millaway, and Dr Joan Infeld). We also gratefully acknowledge the North Dakota Agricultural Experiment Station, the Scottish Executive Environmental and Rural Affairs Department, the US Department of Agriculture, the US National Institutes of Health, and the US National Science Foundation, without whose funding we could not have accomplished nor continue this work.
References
- Abrams B, Altman SL, Pickett KE. Pregnancy weight gain: still controversial. Am J Clin Nutr. 2000;71(5 Suppl.):1233S–1241S. doi: 10.1093/ajcn/71.5.1233s. [DOI] [PubMed] [Google Scholar]
- Adair TH, Gay WJ, Montani J-P. Growth regulation of the vascular system: evidence for a metabolic hypothesis. Am J Physiol. 1990;259:R393–R404. doi: 10.1152/ajpregu.1990.259.3.R393. [DOI] [PubMed] [Google Scholar]
- Alexander G. Studies on the placenta of sheep (Ovis aries L.).Effect of surgical reduction in the number of caruncles. J Reprod Fertil. 1964;7:307–322. doi: 10.1530/jrf.0.0070307. [DOI] [PubMed] [Google Scholar]
- Alexander G, Williams D. Heat stress and development of the conceptus in domestic sheep. J Agric Sci (Cambridge) 1971;76:53–72. [Google Scholar]
- Anthony RV, Scheaffer AN, Wright CD, Regnault TR. Ruminant models of prenatal growth restriction. Reproduction. 2003;61(Suppl.):183–194. [PubMed] [Google Scholar]
- Armitage JA, Khan IY, Taylor PD, Nathanielsz PW, Poston L. Developmental programming of the metabolic syndrome by maternal nutritional imbalance: How strong is the evidence from experimental models in mammals? J Physiol. 2004;561:355–377. doi: 10.1113/jphysiol.2004.072009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold DR, Kirsch JD, Kraft KC, Redmer DA, Reynolds LP. Relationship among placental vascularity, fetal growth, and offspring number in sheep. J Anim Sci. 2001a;79(Suppl. 2):90. (Abstract 258) [Google Scholar]
- Arnold DR, Scheaffer AN, Redmer DA, Caton JS, Reynolds LP. Effect of nutrient restriction on placental vascularity and fetal growth in sheep. Biol Reprod. 2001b;64(Suppl. 1):352. (Abstract. 625) [Google Scholar]
- Assheton R. The morphology of the ungulate placenta, particularly the development of that organ in sheep, and notes upon the placenta of the elephant and hyrax. Philos Trans R Soc Lond B Biol Sci. 1906;198:143–220. [Google Scholar]
- Barker DJ. The developmental origins of well-being. Philos Trans R Soc Lond B Biol Sci. 2004;359:1359–1366. doi: 10.1098/rstb.2004.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron DH. Oxygen Supply to the Human Fetus, a Symposium. Oxford: Blackwell; 1959. Epilogue. [Google Scholar]
- Bassingthwaighte JB, Goresky CA. Modeling in the analysis of solute and water exchange in the microvasculature. In: Renkin EM, Michel CC, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol IV, Microcirculation. Bethesda, MD, USA: American Physiological Society; 1984. pp. 549–626. [Google Scholar]
- Bolzan A, Guimarey L, Norry M. Relationship between the nutritional status of pregnancy adolescents and fetal growth. Medicina (Buonos Aires) 1999;59:254–258. [PubMed] [Google Scholar]
- Borowicz PP, Arnold DR, Grazul-Bilska AT, Redmer DA, Reynolds LP. Modeling vascular growth in the sheep placentome. Biol Reprod. 2003;68(Suppl.1):150. [Google Scholar]
- Borowicz PP, Johnson ML, Grazul-Bilska AT, Soto-Navarro SA, Borowicz MA, Redmer DA, Reynolds LP. Modeling the relationships among quantitative measures of placental angiogenesis and expression of mRNA for vascular endothelial growth factor (VEGF), angiopoietins (ANG), and their receptors (R) Biol Reprod. 2004a;70(Suppl.1):207. [Google Scholar]
- Borowicz PP, Johnson ML, Grazul-Bilska AT, Soto-Navarro SA, Redmer DA, Reynolds LP. Modeling changes in expression of mRNA for angiopoietins-1 (ANG-1) and ANG-2, and their receptor TIE-2 in the sheep placenta throughout the last two-thirds of pregnancy. J Soc Gynecol Invest. 2004b;11(Suppl.):300A. [Google Scholar]
- Borowicz PP, Vonnahme KA, Grazul-Bilska AT, Redmer DA, Johnson JL, Reynolds LP. The effect of maternal age (age at first pregnancy) on placental expression of the major angiogenic factors and their receptors. J Soc Gynecol Invest. 2005;12(suppl.):327A. [Google Scholar]
- Breier BH, Vickers MH, Ikenasio BA, Chan KY, Wong WP. Fetal programming of appetite and obesity. Molec Cell Endocrinol. 2001;20:73–79. doi: 10.1016/s0303-7207(01)00634-7. [DOI] [PubMed] [Google Scholar]
- Castro LC, Avina RL. Maternal obesity and pregnancy outcomes. Curr Opin Obstet Gynecol. 2002;14:601–606. doi: 10.1097/00001703-200212000-00005. [DOI] [PubMed] [Google Scholar]
- Caton JS, Ward MA, Taylor B, Reynolds LP, Redmer DA. Effect of level and source of selenium on size of gravid uterine tissues in growing pregnant lambs. J Soc Gynecol Invest. 2004;11(Suppl.):216A. [Google Scholar]
- Chandler KD, Leury BJ, Bird AR, Bell AW. Effects of undernutrition and exercise during late pregnancy on uterine, fetal and uteroplacental metabolism in the ewe. Br J Nutr. 1985;53:625–635. doi: 10.1079/bjn19850072. [DOI] [PubMed] [Google Scholar]
- Charnock-Jones DS, Kaufmann P, Mayhew TM. Aspects of human fetoplacental vasculogenesis and angiogenesis. I. Molecular regulation. Placenta. 2004;25:103–113. doi: 10.1016/j.placenta.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Chung J-Y, Song Y, Wang Y, Magness RR, Zheng J. Differential expression of vascular endothelial growth factor (VEGF), endocrine gland derived-VEGF, and VEGF receptors in human placentas from normal and preeclamptic pregnancies. J Clin Endocrinol Metab. 2004;89:2484–2490. doi: 10.1210/jc.2003-031580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LC, Combs GF, Jr, Turnbull BW, Slate EH, Chalker DK, Chow J, Davis LS, Glover RA, Graham GF, Gross EG, Krongard A, Lesher JL, Jr, Park HK, Sanders BB, Smith CL, Taylor JE. Effect of selenium supplementation for cancer prevention in patients with carcinoma of the skin. J Am Med Assoc. 1996;276:1957–1962. [PubMed] [Google Scholar]
- Cnattingius S, Bergstrom R, Lipworth L, Kramer MS. Prepregnancy weight and the risk of adverse pregnancy outcomes. N Engl J Med. 1998;338:191–192. doi: 10.1056/NEJM199801153380302. [DOI] [PubMed] [Google Scholar]
- Combs GF, Jr, Junxuan L. Selenium as a cancer preventive agent. In: Hatfield DL, editor. Selenium: Its Molecular Biology & Role in Human Health. Norwell, MA, USA: Kluwer Academic Publishers; 2001. pp. 205–217. [Google Scholar]
- Cooper JC, Sharkey AM, Charnock-Jones DS, Palmer CR, Smith SK. VEGF mRNA levels in placentae from pregnancies complicated by pre-eclampsia. Br J Obstet Gynaecol. 1996;103:1191–1196. doi: 10.1111/j.1471-0528.1996.tb09627.x. [DOI] [PubMed] [Google Scholar]
- Corah LR, Dunn TG, Kaltenbach CC. Influence of prepartum nutrition on the reproductive performance of beef females and the performance of their progeny. J Anim Sci. 1975;41:819–824. doi: 10.2527/jas1975.413819x. [DOI] [PubMed] [Google Scholar]
- Dhawan S. Birth weights of infants of first generation Asian women in Britain compared with second generation Asian women. Br Med J. 1995;311:876. doi: 10.1136/bmj.311.6997.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebbs JH, Tisdall FF, Scott WA, Moyle WJ, Bell M. Nutrition in pregnancy. Can Med Assoc J. 1942;46:1–6. [PMC free article] [PubMed] [Google Scholar]
- Eckstein P, McKeown T, Record RG. Variation in placental weight according to litter size in the guinea-pig. J Endocrinol. 1955;12:108–114. doi: 10.1677/joe.0.0120108. [DOI] [PubMed] [Google Scholar]
- Faber JJ, Thornburg KL. Placental Physiology. Structure and Function of Fetomaternal Exchange. New York: Raven Press; 1983. [Google Scholar]
- Ferrazzi E, Rigano S, Bozzo M, Bellotti M, Giovannini N, Galan H, Battaglia FC. Umbilical vein blood flow in growth-restricted fetuses. Ultrasound Obstet Gynecol. 2000;16:432–438. doi: 10.1046/j.1469-0705.2000.00208.x. [DOI] [PubMed] [Google Scholar]
- Ferrell CL. Maternal and fetal influences on uterine and conceptus development in the cow. I. Growth of tissues in the gravid uterus. J Anim Sci. 1991;69:1945–1953. doi: 10.2527/1991.6951945x. [DOI] [PubMed] [Google Scholar]
- Ferrell CL, Reynolds LP. Uterine and umbilical blood flows and net nutrient uptake by fetuses and uteroplacental tissues of cows gravid with either single or twin fetuses. J Anim Sci. 1992;70:426–433. doi: 10.2527/1992.702426x. [DOI] [PubMed] [Google Scholar]
- Finley JW, Davis CD, Feng Y. Selenium from high selenium broccoli protects rats from colon cancer. J Nutr. 2000;130:2384–2389. doi: 10.1093/jn/130.9.2384. [DOI] [PubMed] [Google Scholar]
- Folkman J, Klagsbrun MA. Angiogenic factors. Science. 1987;235:442–447. doi: 10.1126/science.2432664. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305:1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep. I. Body growth and composition, and some aspects of energetic efficiency. J Anim Sci. 1998;76:2354–2367. doi: 10.2527/1998.7692354x. [DOI] [PubMed] [Google Scholar]
- Greenwood PL, Hunt AS, Hermanson JW, Bell AW. Effects of birth weight and postnatal nutrition on neonatal sheep. II. Skeletal muscle growth and development. J Anim Sci. 2000;78:50–61. doi: 10.2527/2000.78150x. [DOI] [PubMed] [Google Scholar]
- Gruenwald P, Funakawa H, Mitani S, Nishimura T, Takeuchi S. Influence of environmental factors on foetal growth in man. Lancet. 1967;1:1026–1028. doi: 10.1016/s0140-6736(67)91541-3. [DOI] [PubMed] [Google Scholar]
- Heasman L, Clarke L, Firth K, Stephenson T, Symonds ME. Influence of restricted maternal nutrition in early to mid gestation on placental and fetal development at term in sheep. Paediatric Res. 1998;44:546–551. doi: 10.1203/00006450-199810000-00013. [DOI] [PubMed] [Google Scholar]
- Hediger ML, Scholl TO, Schall JI, Krueger PM. Young maternal age and preterm labor. Ann Epidemiol. 1997;7:400–406. doi: 10.1016/s1047-2797(97)00046-x. [DOI] [PubMed] [Google Scholar]
- Higgins AC, Moxley JE, Pencharz PB, Mikolainis D, Dubois S. Impact of the Higgins Nutrition Intervention Program on birth weight: a within-mother analysis. J Am Diet Assoc. 1989;89:1097–1103. [PubMed] [Google Scholar]
- Hudlicka O. Development of microcirculation: Capillary growth and adaptation. In: Renk in EM, Michel CC, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol IV, Microcirculation. Bethesda, MD, USA: American Physiological Society; 1984. pp. 165–216. [Google Scholar]
- Huffman EM, Kirk JH, Pappaioanou M. Factors associated with neonatal lamb mortality. Theriogenology. 1985;24:163–171. doi: 10.1016/0093-691x(85)90180-3. [DOI] [PubMed] [Google Scholar]
- Hunter GL. The maternal influence on size in sheep. J Agric Sci (Cambridge) 1956;48:36–64. [Google Scholar]
- Jiang C, Ganther H, Lu J. Monomethyl selenium-specific inhibition of MMP-2 and VEGF expression: implications for angiogenic switch regulation. Mol Carcinog Dec. 2000;29:236–250. doi: 10.1002/1098-2744(200012)29:4<236::aid-mc1006>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Jiang C, Jiang W, Ip C, Gather H, Lu J. Selenium-induced inhibition of angiogenesis in mammary cancer at chemopreventive levels of intake. Mol Carcinog Dec. 1999;26:213–225. doi: 10.1002/(sici)1098-2744(199912)26:4<213::aid-mc1>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- Joubert DM, Hammond J. A crossbreeding experiment with cattle with special reference to the maternal effect in South Devon-Dexter crosses. J Agric Sci (Cambridge) 1958;51:325–341. [Google Scholar]
- Kaufmann P, Mayhew TM, Charnock-Jones DS. Aspects of human fetoplacental vasculogenesis and angiogenesis. II. Changes during normal pregnancy. Placenta. 2004;25:114–126. doi: 10.1016/j.placenta.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Kelly RW. Nutrition and placental development. Proc Nutr Soc Australia. 1992;17:203–211. [Google Scholar]
- Kelly RW, Speijers EJ, Ralph IG, Newnham JP. Lambing performances and wool production of maiden and adult merino ewes fed different amounts of lupin seed in mid-pregnancy. Aust J Agric Res. 1992;43:339–354. [Google Scholar]
- King GJ, Atkinson BA, Robertson HA. Implantation and early placentation in domestic ungulates. J Reprod Fertil. 1982;31(Suppl.):17–30. [PubMed] [Google Scholar]
- Knight JW, Bazer FW, Thatcher WW, Franke DE, Wallace HD. Conceptus development in intact and unilaterally hysterectomized-ovariectomized gilts: Interrelations among hormonal status, placental development, fetal fluids and fetal growth. J Anim Sci. 1977;44:620–637. doi: 10.2527/jas1977.444620x. [DOI] [PubMed] [Google Scholar]
- Koh GY, Kim I, Kwak HJ, Yun MJ, Leem JC. Biomedical significance of endothelial cell specific growth factor, angiopoietin. Exp Molec Med. 2002;34:1–11. doi: 10.1038/emm.2002.1. [DOI] [PubMed] [Google Scholar]
- Konje JC, Howarth ES, Kaufmann P, Taylor DJ. Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. Br J Obstet Gynecol. 2003;110:301–305. [PubMed] [Google Scholar]
- Kramer MS. Determinants of low birthweight: methodological assessment and meta-analysis. Bull World Health Organiz. 1987;65:663–737. [PMC free article] [PubMed] [Google Scholar]
- Kramer MS, Kakuma R. Energy and protein intake in pregnancy. The Cochrane Database of Systematic Reviews 2003. 2003;(Issue 4) doi: 10.1002/14651858.CD000032. Art No.:CD000032 10.1002/14651858.CD000032. [DOI] [PubMed] [Google Scholar]
- Krebs C, Longo LD, Leiser R. Term ovine placental vasculature: Comparison of sea level and high altitude conditions by corrosion cast and histomorphometry. Placenta. 1997;18:43–51. doi: 10.1016/s0143-4004(97)90070-9. [DOI] [PubMed] [Google Scholar]
- Leury BJ, Bird AR, Chandler KD, Bell AW. Glucose partitioning in the pregnant ewe: Effects of undernutrition and exercise. Br J Nutr. 1990;64:449–462. doi: 10.1079/bjn19900045. [DOI] [PubMed] [Google Scholar]
- Longo LD. Disorders of placental transfer. In: Assali NS, Brinkman SR III, editors. Pathophysiology of Gestation. II. New York: Academic Press; 1972. pp. 1–76. [Google Scholar]
- Longo LD. Respiratory gas exchange in the placenta. In: Farhi LE, Tenney SM, editors. Handbook of Physiology, section 3, The Respiratory System, vol. IV, Gas Exchange. Bethesda, MD, USA: American Physiological Society; 1987. pp. 351–401. [Google Scholar]
- Luke B, Hediger ML, Nugent C, Newman RB, Mauldin JG, Witter FR, O'Sullivan MJ. Body mass index – specific weight gains associated with optimal birth weights in twin pregnancies. J Reprod Med. 2003;48:217–224. [PubMed] [Google Scholar]
- Lyall F, Young A, Boswell F, Kingdom JC, Greer IA. Placental expression of vascular endothelial growth factor in placentae from pregnancies complicated by pre-eclampsia and intrauterine growth restriction does not support placental hypoxia at delivery. Placenta. 1997;18:269–276. doi: 10.1016/s0143-4004(97)80061-6. [DOI] [PubMed] [Google Scholar]
- Marconi AM, Paolini C, Buscaglia M, Zerbe G, Battaglia FC, Pardi G. The impact of gestation age and foetal growth upon the maternal-foetal glucose concentration difference. Obstet Gynecol. 1996;87:937–942. doi: 10.1016/0029-7844(96)00048-8. [DOI] [PubMed] [Google Scholar]
- Mayhew TM, Charnock-Jones DS, Kaufmann P. Aspects of human fetoplacental vasculogenesis and angiogenesis. III. Changes in complicated pregnancies. Placenta. 2004;25:127–139. doi: 10.1016/j.placenta.2003.10.010. [DOI] [PubMed] [Google Scholar]
- McDowell LR. Minerals in Animal and Human Nutrition. 2. Amsterdam: Elsevier; 2003. [Google Scholar]
- Mckeown T, Record RG. The influence of placental size on foetal growth in man, with special reference to multiple pregnancy. J Endocrinol. 1953;9:418–426. doi: 10.1677/joe.0.0090418. [DOI] [PubMed] [Google Scholar]
- Mellor DJ. Nutritional and placental determinants of foetal growth rate in sheep and consequences for the newborn lamb. Br Vet J. 1983;139:307–324. doi: 10.1016/s0007-1935(17)30436-0. [DOI] [PubMed] [Google Scholar]
- Meschia G. Circulation to female reproductive organs. In: Shepherd JT, Abboud FM, editors. Handbook of Physiology, section 2, The Cardiovascular System, vol. III, part 1, Peripheral Circulation and Organ Blood Flow. Bethesda, MD, USA: American Physiological Society; 1983. pp. 241–269. [Google Scholar]
- MOD. March of Dimes. Teenage Pregnancy. 2000a http://www.marchofdimes.com/professionals/681_1159.asp.
- MOD. March of Dimes. Nutrition Report. Nutrition Today Matters Tomorrow. 2000b http://www.marchofdimes.com/professionals/681_2198.asp.
- Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert T, Parra E, Vargas E. Maternal adaptation to highaltitude pregnancy: An experiment of nature – A review. Placenta. 2004;25(Suppl. A):S60–S71. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Mossman HW. Vertebrate Fetal Membranes. New Brunswick, NJ, USA: Rutgers University Press; 1987. [Google Scholar]
- Naeye RL. Maternal body weight and pregnancy outcome. Am J Clin Nutr. 1990;52:273–279. doi: 10.1093/ajcn/52.2.273. [DOI] [PubMed] [Google Scholar]
- NCHS. Prevalence of Overweight Among Children and Adolescents: United States. National Center for Health Statistics; 2004. pp. 1999–2000. http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overwght99.htm. [Google Scholar]
- NCPTP. Teen Pregnancy – A Decade of Decline. National Campaign to Prevent Teen Pregnancy; 2004. http://www.teenpregnancy.org/about/announcements/pdf/teenpregnancyrates2004.pdf. [Google Scholar]
- Newnham JP, Kelly RW, Patterson L, James I. The influence of maternal undernutrition in ovine twin pregnancy on fetal growth and Doppler flow-velocimetry waveforms. J Dev Physiol. 1991;16:277–282. [PubMed] [Google Scholar]
- NLM. Adolescent Pregnancy. National Library of Medicine, MEDLINEplus; 2002a. http://www.nlm.nih.gov/medlineplus/ency/article/001516.htm#prognosis. [Google Scholar]
- NLM. Intrauterine Growth Restriction. National Library of Medicine, MEDLINEplus; 2000b. http://www.nlm.nih.gov/medlineplus/ency/article/001500.htm. [Google Scholar]
- North RA, Ferrier C, Long D, Townend K, Kincaid-Smith P. Uterine artery Doppler flow velocity waveforms in the second trimester for the prediction of preeclampsia and fetal growth retardation. Obstet Gynecol. 1994;83:378–386. [PubMed] [Google Scholar]
- Nowak RM. Walker's Mammals of the World. 5. Baltimore: The Johns Hopkins University Press; 1991. [Google Scholar]
- NRC. Nutrient Requirements of Sheep. 6th revised edn. Washington, DC: National Academy Press; 1985. [Google Scholar]
- NVSR. National Vital Statistics Reports. 2001;49 no. 10 DHHS Pub. No. (PHS) 2001-1120 PRS 01-0580 (9/2001) http://www.cdc.gov/nchs/data/nvsr/nvsr49/nvsr49_10.pdf.
- Oddy VH, Holst PJ. Maternal-foetal adaptation to mid pregnancy feed restriction in single-bearing ewes. Aust J Agric Res. 1991;42:969–978. [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. J Am Med Assoc. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Ogunyemi D, Hullett S, Leeper J, Risk A. Prepregnancy body mass index, weight gain during pregnancy, and perinatal outcome in a rural black population. J Matern Fetal Med. 1998;7:190–193. doi: 10.1002/(SICI)1520-6661(199807/08)7:4<190::AID-MFM5>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Oken E, Gillman MW. Fetal origins of obesity. Obesity Res. 2003;11:496–506. doi: 10.1038/oby.2003.69. [DOI] [PubMed] [Google Scholar]
- Pant D, Choi JT, Luther JS, Borowicz P, Weigl RM, Kirsch JD, Kraft KC, Redmer DA, Reynolds LP, Grazul-Bilska AT. Comparison of gravid uterine parameters in naturally bred ewes and ewes after embryo transfer of in vitro produced embryos, and in single, twin and triplet pregnancies. J Anim Sci. 2003;80(Suppl.2) doi: 10.1016/j.anireprosci.2005.06.013. Abstract 254 http://www.asas.org/abstracts/2003sectuibak/sec19.pdf. [DOI] [PubMed] [Google Scholar]
- Pardi G, Cetin I, Marconi AM, Lanfranchi A, Bozzetti P, Ferrazzi E, Buscaglia M, Battaglia FC. Diagnostic value of blood sampling in fetuses with growth retardation. N Engl J Med. 1993;328:692–696. doi: 10.1056/NEJM199303113281004. [DOI] [PubMed] [Google Scholar]
- Parr RA, Williams AH, Campbell IP, Witcome GF, Roberts AM. Low nutrition of ewes in early pregnancy and the residual effect on the offspring. J Agric Sci, Cambr. 1986;106:81–87. [Google Scholar]
- Perry RL, Mannino B, Hediger ML, Scholl TO. Pregnancy in early adolescence: are there obstetric risks? J Matern Fetal Med. 1996;5:333–339. doi: 10.1002/(SICI)1520-6661(199611/12)5:6<333::AID-MFM8>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Ramsey EM. The Placenta, Human and Animal. New York: Praeger; 1982. [Google Scholar]
- Rattray PV, Garrett WN, East NE, Hinman N. Growth, development and composition of the ovine conceptus and mammary gland during pregnancy. J Anim Sci. 1974;38:613–626. doi: 10.2527/jas1974.383613x. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Andrews HF. Birth outcomes for Asian American adolescents: a high risk group? J Am Med Womens Assoc. 1999;54:121–125. [PubMed] [Google Scholar]
- Redmer DA, Aitken RP, Milne JS, Borowicz PP, Borowicz MA, Kraft KD, Reynolds LP, Luther JS, Wallace JM. Influence of maternal nutrition on placental vascularity during late pregnancy in adolescent ewes. Biol Reprod. 2004a;70(Suppl. 1):150. [Google Scholar]
- Redmer DA, Aitken RP, Milne JS, Reynolds LP, Wallace JM. Influence of maternal nutrition on messenger RNA expression of placental angiogenic factors and their receptors at mid-gestation in adolescent sheep. Biol Reprod. 2005;72:1004–1009. doi: 10.1095/biolreprod.104.037234. [DOI] [PubMed] [Google Scholar]
- Redmer DA, Wallace JM, Reynolds LP. Effects of nutrient intake during pregnancy on fetal and placental growth and vascular development. Domestic Anim Endocrinol. 2004b;27:199–217. doi: 10.1016/j.domaniend.2004.06.006. [DOI] [PubMed] [Google Scholar]
- Regnault TR, de Vrijer B, Galan HL, Davidsen ML, Trembler KA, Battaglia FC, Wilkening RB, Anthony RV. The relationship between transplacental O2 diffusion and placental expression of PlGF, VEGF and their receptors in a placental insufficiency model of fetal growth restriction. J Physiol. 2003;550:641–656. doi: 10.1113/jphysiol.2003.039511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regnault TR, Galan HL, Parker TA, Anthony RV. Placental development in normal and compromised pregnancies – a review. Placenta. 2002a;23(Suppl. A):S119–S129. doi: 10.1053/plac.2002.0792. [DOI] [PubMed] [Google Scholar]
- Regnault TR, Orbus RJ, de Vrijer B, Davidsen ML, Galan HL, Wilkening RB, Anthony RV. Placental expression of VEGF, PlGF and their receptors in a model of placental insufficiency-intrauterine growth restriction (PI-IUGR) Placenta. 2002b;23:132–144. doi: 10.1053/plac.2001.0757. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Biondini ME, Borowicz PP, Vonnahme KA, Caton JS, Grazul-Bilska AT, Redmer DA. Functional significance of developmental changes in placental microvascular architecture: The sheep as a model. Endothelium. 2005a doi: 10.1080/10623320590933734. (in press) [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Borowicz PP, Vonnahme KA, Johnson ML, Grazul-Bilska AT, Wallace JM, Caton JS, Redmer DA. Animal models of placental angiogenesis. Placenta. 2005b doi: 10.1016/j.placenta.2004.11.010. 10.1016/j.placenta.2004.11.010. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Ferrell CL, Nienaber JA, Ford SP. Effects of chronic environmental heat-stress on blood flow and nutrient uptake of the gravid bovine uterus and foetus. J Agric Sci (Cambridge) 1985;104:289–297. [Google Scholar]
- Reynolds LP, Grazul-Bilska AT, Redmer DA. Angiogenesis in the female reproductive system: Pathological implications. Internat J Exp Pathol. 2002;83:151–164. doi: 10.1046/j.1365-2613.2002.00277.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds L, Kirsch J, Kraft KC, Knutson DL, McClaflin WJ, Redmer D. Time-course of the uterine response to estradiol-17β in ovariectomized ewes: Uterine growth and microvascular development. Biol Reprod. 1998;59:606–612. doi: 10.1095/biolreprod59.3.606. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Growth and microvascular development of the uterus during early pregnancy in ewes. Biol Reprod. 1992;47:698–708. doi: 10.1095/biolreprod47.5.698. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Utero-placental vascular development and placental function. J Anim Sci. 1995;73:1839–1851. doi: 10.2527/1995.7361839x. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Minireview: Angiogenesis in the placenta. Biol Reprod. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- Robinson JJ. Nutrition of the pregnant ewe. In: Haresign W, editor. Sheep Production. London: Butterworths; 1983. pp. 111–131. [Google Scholar]
- Rush D. Maternal nutrition and perinatal survival. Nutr Rev. 2001;59:315–326. doi: 10.1111/j.1753-4887.2001.tb06956.x. [DOI] [PubMed] [Google Scholar]
- Russel AJF, Foot JZ, White IR. The effect of weight at mating and of nutrition during mid-pregnancy on the birthweight of lambs from primiparous ewes. J Agric Sci (Cambridge) 1981;97:723–729. [Google Scholar]
- Schieve LA, Cogswell ME, Scanlon KS. Maternal weight gain and preterm delivery: differential effects by body mass index. Epidemiology. 1999;10:141–147. doi: 10.1097/00001648-199903000-00007. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Cronk CE, Schall JI. Maternal growth during pregnancy and lactation. Hormone Res. 1993;39(Suppl.):59–67. doi: 10.1159/000182785. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Schall JI. Maternal growth and foetal growth: pregnancy course and outcome in the Camden Study. Ann NY Acad Sci. 1997;81:292–301. doi: 10.1111/j.1749-6632.1997.tb48215.x. [DOI] [PubMed] [Google Scholar]
- Scholl TO, Hediger ML, Schall JI, Khoo CS, Fischer RL. Maternal growth during pregnancy and the competition for nutrients. Am J Clin Nutr. 1994;60:183–188. doi: 10.1093/ajcn/60.2.183. [DOI] [PubMed] [Google Scholar]
- Sreenan JM, Beehan D. Embryonic survival and development at various stages of gestation after bilateral egg transfer in the cow. J Reprod Fertil. 1976;47:127–128. doi: 10.1530/jrf.0.0470127. [DOI] [PubMed] [Google Scholar]
- Steckel RH. Birth weights and stillbirths in historical perspective. Eur J Clin Nutr. 1998;52(Suppl.1):S16–S20. [PubMed] [Google Scholar]
- Stegeman JHJ. Placental development in sheep and its relation to fetal development. BiJdragen Tot Dierkunde (Contributions Zool) 1974;44:3–72. [Google Scholar]
- Stephansson O, Dickman PW, Johansson A, Cnattingius S. Maternal weight, pregnancy weight gain, and the risk of antepartum stillbirth. Am J Obstet Gynecol. 2001;184:463–469. doi: 10.1067/mob.2001.109591. [DOI] [PubMed] [Google Scholar]
- Steyn C, Hawkins P, Saito T, Noakes DE, Kingdom JCP, Hanson MA. Undernutrition during the first half of gestation increases the predominance of foetal tissue in late gestation ovine placentomes. Eur J Obst Gynaecol Reprod Biol. 2001;98:165–170. doi: 10.1016/s0301-2115(01)00321-9. [DOI] [PubMed] [Google Scholar]
- Sunde RA. Selenium. In: O'Dell BL, Sunde RA, editors. Handbook of Nutritionally Essential Minerals. New York: Marcel Dekker; 1997. [Google Scholar]
- Thompson GE, Bassett JM, Samson DE, Slee J. The effects of cold exposure of pregnant sheep on foetal plasma nutrients, hormones and birth weight. Br J Nutr. 1982;48:59–64. doi: 10.1079/bjn19820087. [DOI] [PubMed] [Google Scholar]
- Trahair JF, DeBarro TM, Robinson JS, Owens JA. Restriction of nutrition in utero selectively inhibits gastrointestinal growth in fetal sheep. J Nutr. 1997;127:637–641. doi: 10.1093/jn/127.4.637. [DOI] [PubMed] [Google Scholar]
- Trudinger BJ, Giles WB, Cook CM. Uteroplacental blood flow velocity-time waveforms in normal and complicated pregnancy. Br J Obstet Gynecol. 1985;92:39–45. doi: 10.1111/j.1471-0528.1985.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Turman EJ, Laster DB, Renbarger RE, Stevens DF. Multiple births in beef cows treated with equine gonadotropin (PMS) and chorionic gonadotropin (HCG) J Anim Sci. 1971;32:962–967. doi: 10.2527/jas1971.325962x. [DOI] [PubMed] [Google Scholar]
- Vincent IC, Williams HLI, Hill R. The influence of a low-nutrient intake after mating on gestation and perinatal survival of lambs. Br Vet J. 1985;141:611–617. doi: 10.1016/0007-1935(85)90008-9. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Ford SP, Nijland MJ, Reynolds LP. Alteration in cotyledonary (COT) vascular responsiveness to angiotensin II (ANG II) in beef cows undernourished during early gestation. Bio Reprod. 2004a;70(Suppl. 1):110. [Google Scholar]
- Vonnahme KA, Hess BW, Hansen TR, McCormick RJ, Rule DC, Moss GE, Murdoch WJ, Nijland M, Skinner DC, Nathanielsz PW, Ford SP. Maternal undernutrition from early- to mid-gestation leads to growth retardation cardiac ventricular hypertrophy, and increased liver weight in the fetal sheep. Biol Reprod. 2003;69:133–140. doi: 10.1095/biolreprod.102.012120. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Reynolds LP, Nijland MJ, Ford SP. Impacts of undernutrition during early to mid gestation on basal vascular tone of the cotyledonary and caruncular arterial beds in the bovine placentome. J Soc Gynecol Invest. 2004b;11(Suppl.):222A. [Google Scholar]
- Vonnahme KA, Wilson ME, Ford SP. Relationship between placental vascular endothelial growth factor expression and placental/endometrial vascularity in the pig. Biol Reprod. 2001;64:1821–1825. doi: 10.1095/biolreprod64.6.1821. [DOI] [PubMed] [Google Scholar]
- Vonnahme KA, Wilson ME, Ford SP. Conceptus competition for uterine space: Different strategies exhibited by the Meishan and Yorkshire pig. J Anim Sci. 2002;80:1311–1316. doi: 10.2527/2002.8051311x. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Aitken RP, Cheyne MA. Nutrient partitioning and fetal growth in rapidly growing adolescent ewes. J Reprod Fertil. 1996;107:183–190. doi: 10.1530/jrf.0.1070183. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP. Nutrition and fetal growth: paradoxical effects in the overnourished adolescent sheep. J Reprod Fertil Supplement. 1999a;54:385–399. [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Cruickshank MA. Switching maternal dietary intake at the end of the first trimester has profound effects on placental development and fetal growth in adolescent ewes carrying singleton fetuses. Biol Reprod. 1999b;61:101–110. doi: 10.1095/biolreprod61.1.101. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Aitken RP, Palmer RM, Da Silva P, Cruickshank MA. Relationship between nutritionally mediated placental growth restriction and foetal growth, body composition and endocrine status during late gestation in adolescent sheep. Placenta. 2000;21:100–108. doi: 10.1053/plac.1999.0440. [DOI] [PubMed] [Google Scholar]
- Wallace JM, Bourke DA, Da Silva P, Aitken RP. Nutrient partitioning during adolescent pregnancy. Reproduction. 2001;122:347–357. doi: 10.1530/rep.0.1220347. [DOI] [PubMed] [Google Scholar]
- Walton A, Hammond J. The maternal effects on growth and conformation in Shire horse – Shetland pony crosses. Proc R Soc Lond B Biol Sci. 1938;125:311–335. [Google Scholar]
- Weibel ER. Point counting methods. J Microscopy. 1972;95:373–378. [Google Scholar]
- Wheeler T, Anthony FW, Young DA, Moore LG, Zamudio S. Maternal circulating vascular endothelial growth factor (VEGF) is elevated in high altitude pregnancy. J Soc Gynecol Invest. 2002;9(Suppl.):301A. [Google Scholar]
- WHO. Maternal anthropometry and pregnancy outcomes: A WHO collaborative study. Bull World Health Organiz. 1995;73(Suppl.) parts 1 & 2. [PMC free article] [PubMed] [Google Scholar]
- Wirrenga JM, Borowicz PP, Luther JS, Pant D, Redmer DA, Grazul-Bilska AT, Reynolds LP. Vascular development of fetal placental cotyledons (COT) in single, twin and triplet pregnancies in sheep. J Anim Sci. 2004;80(Suppl. 2) Abstract 325 http://www.asas.org/abstracts/2004sectional/S33.PDF. [Google Scholar]
- Wooding FBP, Flint APF. Placentation. In: Lamming GE, editor. Marshall's Physiology of Reproduction. 4. Vol. 3. London: Chapman & Hall; 1994. pp. 233–460. part I. [Google Scholar]
- Wulff C, Weigland M, Kreienberg R, Fraser HM. Angiogenesis during primate placentation in health and disease. Reproduction. 2003;126:569–577. doi: 10.1530/rep.0.1260569. [DOI] [PubMed] [Google Scholar]