Abstract
The intrauterine environment plays a powerful role in determining the life-long risk of cardiovascular disease. A number of stressors are well known to affect the development of the cardiovascular system in utero including over/under maternal nutrition, excess glucocorticoid and chronic hypoxia. Chronic fetal anaemia in sheep is a complex stressor that alters cardiac loading conditions, causes hypoxic stress and stimulates large changes in flow to specific tissues, including large increases in resting coronary blood flow and conductance. Decreased viscosity can account for approximately half of the increased flow. It appears that immature hearts are ‘plastic’ in that increases in coronary conductance with fetal anaemia persist into adulthood even if the anaemia is corrected before birth. These large changes in conductance are possible only through extensive remodelling of the coronary tree. Adult hearts that were once anaemic in utero are more resistant to hypoxic stress as adults but it is not known whether such an adaptation would be deleterious in later life. These studies indicate the need for investigation into the basic mechanisms of coronary tree remodelling in the immature myocardium. New information on these mechanisms is likely to lead to better prevention of and therapies for adult-onset coronary disease.
It is well established that genetic factors play a central role in life-long risk of cardiovascular disease. In addition, epidemiologic observations support the concept that the intrauterine environment plays an additional powerful role in determining life-long risks of cardiovascular disease (Barker, 2000; Gluckman & Hanson, 2004). Maternal glucocorticoid administration, maternal nutritional deprivation, fetal anaemia, fetal hypoxia and increased fetal cardiac load all affect the fetal heart and may have long-term significance (Dodic et al. 2001; Broberg et al. 2003; Li et al. 2003; Barbera et al. 2000; Han et al. 2004). These factors are likely to initiate multiple interrelated physiological processes. For example, fetal anaemia is associated with lowered blood viscosity, increased blood flow, hypoxaemia, and a series of endocrine responses. Research to date gives only preliminary insight into ‘programming’ of the cardiovascular system. Further research is therefore necessary to understand the mechanisms of fetal origins of adult cardiovascular disease.
Chronic fetal anaemia
Since the study of the fetal origins of adult cardiovascular disease is in its early stages, investigations of the underlying mechanisms are few. Chronic fetal anaemia is one of the best-studied late term fetal manipulations that lend insight into the ‘programming’ of the fetal heart. In fetal sheep, adaptations to chronic anaemia include a 30% increase in heart to body weight ratio, a 50% increase in stroke volume and cardiac output, a sixfold increase in coronary blood flow and a doubling of coronary conductance with preservation of coronary reserve (Copel et al. 1989, Davis et al. 1999). These changes are thought to be adaptive and directed at maintaining myocardial and systemic oxygen supply, oxygen consumption and organ function when oxygen content is reduced. Chronic fetal anaemia also has an effect on the coronary microcirculation. Fetal anaemia is associated with a 40–60% increase in ventricular capillary diameter while capillary density is maintained at the non-anaemic level in the presence of cardiac hypertrophy (Martin et al. 1998). The underlying mechanisms for these effects are not well understood. However, in response to low levels of oxygen seen during fetal anaemia, fetal cardiac concentrations of hypoxia-inducible factor 1 and vascular endothelial growth factor are increased (Martin et al. 1998). Systemic cardiovascular changes seen during fetal anaemia include a fall in the precapillary to postcapillary resistance ratio that increases capillary hydrostatic pressure and results in a nearly threefold increase in thoracic duct lymph flow (Davis & Hohimer, 1991, Davis et al. 1996).
Chronic anaemia in fetal sheep results in a 50% increase in cardiac output and stroke volume (Davis et al. 1999). The compensatory ventricular eccentric hypertrophy that develops appears to normalize wall stress by maintaining a normal ratio of radius to wall thickness while increasing stroke volume. These adaptive changes are similar to the response to anaemia in the adult. For example, anaemic adult rats develop eccentric myocardial hypertrophy with increased coronary capillary length and diameter (Oliverti et al. 1989, 1992). These compensatory responses to fetal anaemia and consequent cardiac adaptations occur over time. When anaemia is rapidly induced in fetal sheep, central venous pressure increases, whereas when anaemia occurs slowly, central venous pressure remains normal (Blair et al. 1994).
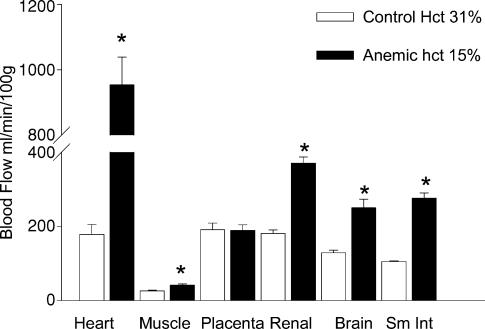
The effects of chronic anaemia on regional blood flow within the ovine fetus are illustrated in Fig. 1. In these experiments the haematocrit was reduced from 35 to 15% over 1 week. Coronary blood flow increased to a greater extent than did flow to other organs (Davis & Hohimer 1991). These findings are thought to be due at least in part, to local metabolic regulation at the organ level. Since oxygen extraction in the fetal sheep heart is normally 65% and 34% in the brain (Fisher et al. 1982, Davis et al. 1999), the heart has less extraction reserve. Thus, blood flow to the heart would need to increase severalfold under hypoxaemic conditions, while the cerebral blood flow would need to only double, in order to maintain oxygen consumption at resting levels. This has consequences in regards to programming. At the same degree of hypoxia, endothelial changes that are regulated by increased blood flow may not be apparent in some vascular beds but present in others.
Figure 1. Regional blood flow after 7 days of anaemia.
Regional blood flow in chronically anaemic and control fetal sheep at near term as determined by microspheres. Data taken from Davis & Hohimer (1991).
Coronary pressure–flow relationships
The fetal coronary flow–perfusion pressure relationship has yielded significant insight into the effects of chronic fetal anaemia on the regulation of the coronary tree. Maximal coronary conductance is the slope of the relationship between myocardial blood flow and coronary perfusion pressure during maximal coronary vasodilatation with adenosine (Hoffman, 1984). This relationship is a physiological measure of the total resistance vessel area of the coronary circulation and can be used to index vascular ‘growth’ of resistance vessels. This relationship is also useful as it allows the determination of coronary reserve at a specific pressure if resting blood flow is known. In the adult, maximal coronary conductance does not increase in response to stress (Hoffman & Spaan, 1990). For example, in response to an increase in afterload accompanied by left ventricular hypertrophy in the adult, coronary conductance actually decreases (Flanagan et al. 1994, Kalkman et al. 1996). This fall in conductance is not well understood and is ascribed to vascular rarefaction as myocyte growth outstrips resistance vessel growth, to medial hypertrophy of resistance vessels leading to decreased conductance, or to functional vascular changes secondary to increased transmission of pressure.
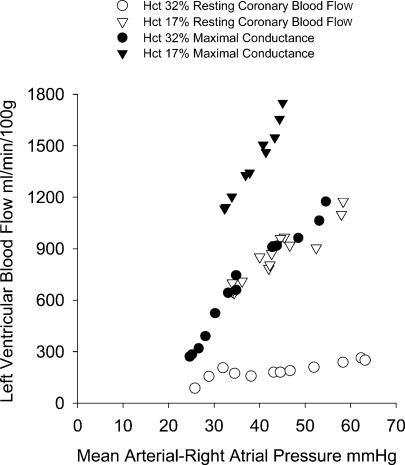
Coronary conductance can be approximated by altering central arterial pressure by variable occlusion of the aorta or inferior vena cava. The effects of chronic anaemia on coronary conductance in the fetus are illustrated in Fig. 2. The coronary flow–perfusion pressure relationships are shown in the same fetus studied at a normal haematocrit and following 1 week of anaemia. Autoregulation of resting flow appears to be intact in the normal fetus, in that coronary flow increases very little in response to increased perfusion pressure (open circles). Resting flow in the anaemic animal at its normal arterial pressure (45 mmHg) is at about the same level as maximal flow conditions in the non-anaemic state. However maximal coronary conductance is approximately double during anaemia compared to the non-anaemic state (Davis et al. 1999). Maximal coronary conductance increases from ∼18 to 33 ml min−1 (100 g left ventricle)−1 mmHg−1 (Fig. 3). Even after adjustment for changes in viscosity and haematocrit by transfusion in the anaemic fetus, coronary blood flow is 43% greater at normal coronary perfusion pressures (Davis et al. 1999). Thus, approximately half of the increase in maximal coronary conductance can be attributed to angiogenesis and half to physical forces in the vasculature.
Figure 2. Coronary pressure–flow relationships.
Coronary pressure–flow relationships in a singleton fetus before and after anaemia induced over 1 week. Resting coronary blood flow shows normal autoregulation. Resting flow when anaemic is at the maximal flow in response to adenosine 1 week earlier. Reproduced with permission from Davis et al. (1999).
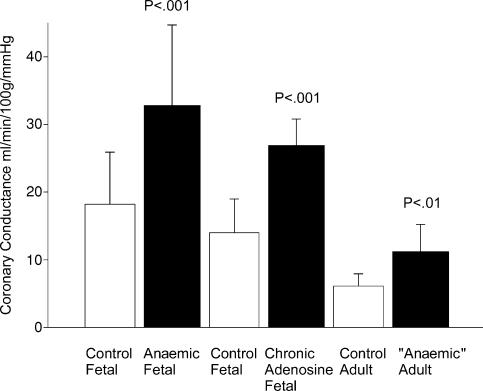
Figure 3. Maximal coronary conductance in response to adenosine.
Maximal left ventricular coronary conductance measured in ovine fetuses that were anaemic as compared to controls, in ovine fetuses infused with adenosine into the coronary circulation to increase flow without altering oxygen content as compared to controls, and in adult sheep that were anaemic in utero only as compared to control twin siblings. Data from Davis et al. (1999) and Wothe et al. (2002); reproduced from Davis et al. (2003).
Increased flow, perhaps via shear forces, appears to stimulate growth in both the adult and fetal coronary circulation (Wothe et al. 2002; Helisch & Schaper, 2003). Chronic infusion of adenosine into the fetal coronary artery that does not change oxygen content, arterial pressure or cardiac output increases maximal coronary conductance. However, the excess in maximal coronary conductance can be largely blocked by NG-nitro-l-arginine methyl ester (l-NAME) (Wothe et al. 2002). Because maximal coronary conductance and flow in the presence of l-NAME are not less than the response to adenosine alone, differential regulation of larger proximal resistance vessels by nitric oxide and more distal metabolic regulation of smaller arterioles by adenosine as suggested by Kuo et al. (1995) may occur in the fetal coronary tree. Similarly, nitric oxide-dependent coronary dilatation in fetal guinea pigs raised in conditions of chronic hypoxia is also increased (Thompson, 2000). However, it is not currently known where in the fetal coronary vascular tree resistance occurs or how flow affects total cross-sectional area.
From these experiments, it is clear that fetal anaemia can alter the coronary vasculature during the fetal period and that the fetal coronary tree is highly plastic. In terms of fetal ‘programming’ and the fetal origins of adult cardiovascular disease, it is important to know whether the effects of anaemia on the fetal coronary circulation persist into adulthood. To address this, twin fetal sheep were instrumented to make one of the fetal twins anaemic in utero and the other twin to serve as a non-anaemic control. Fetal anaemia was induced in late gestation for about a week. Just before delivery, the anaemia was corrected by transfusion to allow the anaemic fetus to survive a natural delivery. At 7 months of age the postpubertal sheep that were once anaemic in utero had a maximal coronary conductance twice that of their non-anaemic twins, ∼11 versus 6 ml min−1 (100 g−1) mmHg−1 even though their resting coronary blood flows were not different (Davis et al. 2003).
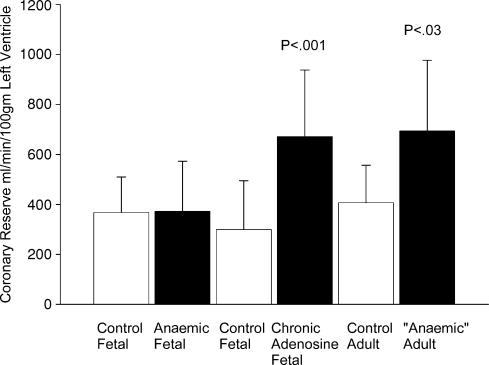
The effects of anaemia and adenosine infusion on coronary perfusion are summarized in Fig. 3. Several issues are worthy of note. (1) Maximal coronary conductance increases in fetal anaemia. Thus, an increase in coronary conductance allows the anaemic fetus to maintain coronary reserve at normal pressures (Fig. 4). (2) Chronic adenosine infusion via flow-modulated growth also increases maximal coronary conductance over time even if the fetus is not anaemic. (3) Fetal anaemia, even if corrected before delivery, results in an excess coronary vasodilatory reserve that persists into young adulthood. It is important to note that maximal coronary blood flow when expressed per unit weight of heart has been shown to decrease with age, being higher in the fetus than the newborn, and lower in the adult (Toma et al. 1985; Flanagan et al. 1994; Davis et al. 1999; Davis et al. 2003). With ageing, there are further decreases in coronary blood flow and coronary reserve as measured in the rat (Hachamovitch et al. 1989). The effects of haemoconcentration on coronary conductance however, have not been studied in the fetus. We are aware that experiments in adult dogs show a linear decrease in the maximal incremental conductance as haematocrit increases (Baer et al. 1987). While the general concept would likely hold true for the fetus, it has not yet been investigated.
Figure 4. Coronary reserve.
Coronary reserve ovine fetuses that were anaemic as compared to controls, in ovine fetuses infused with adenosine into the coronary circulation to increase flow without altering oxygen content as compared to controls, and in adult sheep that were anaemic in utero only as compared to control twin siblings. Data from Davis et al. (1999) and Wothe et al. (2002); reproduced from Davis et al. (2003).
It would be valuable to know if the additional coronary reserve seen in adults that were anaemic in late gestation confers functional advantage during the adult period. In keeping with the concept of programming the heart for disease risk, one might also wonder whether the once anaemic adult heart is at a functional disadvantage in spite of an increased conductance. Eight-month-old sheep that were anaemic only in utero were studied under conditions of 5-min hypoxic episodes and were compared to their twins which were not anaemic in utero. The sheep that were anaemic in utero had higher indices of left ventricular systolic function including end-systolic elastance (Emax) and dP/dt compared to their twin controls (Broberg et al. 2003). These findings show that in utero events can induce changes that persist into adulthood and in this case, confer a function advantage in terms of coronary vasodilatory reserve and cardiac function.
Responses that are unique to the fetal–neonatal period
Changes in coronary vascular reserve, however, are not limited to the fetal period. Several studies suggest that maximal coronary conductance in the adult can be modified by events that occur in the perinatal period. Exercise training in young swine results in an initial increase in capillary density that subsequently normalizes as the number of small (20–30 μm) arterioles increase (White et al. 1998). These investigators suggested that the ‘extra’ capillaries developed into arterioles (White et al. 1998). Flanagan et al. (1994) found that in adult sheep, aortic banding for 6 weeks results in a 67% decrease in left ventricular coronary conductance and a 17% decrease in capillary density. When aortic banding is performed in month-old lambs, coronary conductance as well as capillary density is maintained. Similarly, capillary to myocyte density is increased in congenital aortic stenosis in humans and decreased in acquired aortic stenosis (Rakusan et al. 1992). Functionally, Aoyagi et al. (1992) found that aortic banding in adult sheep decreased left ventricular shortening indices by 35%, whereas month-old lambs submitted to 6 weeks of aortic banding maintained LV function. These investigators found that there was no difference in baseline dP/dtmax. However, heart rate-corrected circumferential shortening decreased in aortic-banded adults and increased above normal in aortic-banded lambs. Levels of Ca2+-ATPase mRNA and Na+–Ca2+ exchanger protein were decreased in adult sheep with hypertrophy compared to newborns with aortic banding (Aoyagi et al. 2001). Taken together, these data show that maximal coronary conductance and accompanying function decrease in the adult left ventricle in response to aortic banding, whereas in young lambs coronary conductance and function are maintained. These data are consistent with the observation that maximal coronary conductance is greater in adults that were anaemic in utero. However, the functional significance of whether this increase in conductance confers a similar advantage when the heart remodels after a pressure load is applied to the left ventricle in the adult is not known. Finally, there is the recent observation that in arterioles of female hearts transplanted into male recipients, 45% of the smooth muscle cells show the presence of a Y chromosome (Quaini et al. 2002). This suggests that under conditions of immunosupression and increased workload (the female hearts were significantly smaller than the male recipient hearts) resistance vessels may be reprogrammed and/or remodelled.
The relationship between vascular growth and cardiac myocyte growth is not well understood in the fetus. In models of hypertrophy in young animals, including aortic banding (Flanagan et al. 1999), arteriovenous anastomoses (Chen et al. 1994), and anaemia (Rakusan et al. 2001) arterial density does not change. In these studies, arterial growth is presumed to have occurred since vascular density did not decrease with concurrent hypertrophy. Accurate measurements of arterial size and density in the heart are difficult to perform sterologically due to the rarity of the vessels by comparison to capillaries and due to anisotropy of arterioles as arterioles change direction within a planar section. A direct approach to quantifying properties of coronary vascular trees that gives a more complete picture has been to analyse vascular casts as developed by Kassab et al. (1993). Kassab compared the casts of the coronary arterial tree of the hypertrophied adult pig right ventricle resulting from pulmonary artery banding compared to the normal right ventricle. The coronary vascular parameters from sectioned casts were compared. Kassab found an increase in the diameter of the trunk of the right coronary artery and substantial increases in the total number of vessels in each branching order in the hypertrophied right ventricle. However, following normalization to gram of right ventricle, the hypertrophied ventricle was not different from the control ventricle. Thus, the vascular growth measured was appropriate for the increased muscle mass in this adult model of hypertrophy. This analysis has never been applied to a fetal heart under control or under hypertrophic conditions much less to an adult heart which is no longer hypertrophied yet has increased conductance (adult that was anaemic in utero). Other investigators (Tomanek et al. 1999) have suggested that vascular growth is regulated at least in part by the rate of myocardial growth.
In fetal sheep, increased right ventricular systolic pressure load results in increased heart mass, right ventricular myocyte size and binucleation of cardiac myocytes suggesting accelerated terminal differentiation (Barbera et al. 2000). In addition the estimated number of cardiac myocytes increased, suggesting that right ventricular systolic pressure load stimulates both hypertrophic and hyperplasic growth. Chronic hypoxia in pregnant rats resulted in increased size and proportion of binucleated myocytes and increased numbers of apoptotic cells in the fetal heart (Bae et al. 2003). The long-term consequences of the effects in increased cardiac load or chronic fetal hypoxia on the cardiac myocyte are not known and remain an important area of investigation.
Evidence for endothelial dysfunction in cardiac programming
There is evidence for endothelial dysfunction in a number of animal models that lead to programming and in humans of low birth weight. Exposure to corticosteroids at 27–28 days of gestation in sheep results in adult-onset hypertension and decreased cardiac function in response to dobutamine (Dodic et al. 2001). Recently, Roghair et al. (2005) gave dexamethasone at a similar time period and evaluated vascular function in 4-month-old lambs. Conduit coronary arteries of steroid-exposed fetuses showed increased constriction to acetylcholine, angiotensin II and U46619 compared to controls. Mesenteric vessel reactivity, however, was not altered. Thus, glucocorticoid-induced tissue responses may be different in various tissues. This is an interesting observation in view of the recent finding by Bugiardini et al. (2004) that in women with angina but normal angiograms, vasoconstriction in response to acetylcholine predicted an eventual abnormal angiogram in some 60%, whereas in women who showed vasodilatation in response to acetylcholine resolved their symptoms. These data and others suggest that endothelial dysfunction, which is common to most models of intrauterine programming, is a powerful risk factor for coronary disease later in life.
In a Langendorff model of total coronary ischaemia–reperfusion injury, Li et al. (2003) found that adult rats that were exposed to hypoxic conditions before birth had increased area of infarct compared to normal controls. Hypoxia-exposed animals had decreased ventricular levels of heat shock protein 70 and endothelial nitric oxide synthase. A recent review (Zhang, 2005) gives greater detail. Taken together, these observations suggest the possibility of long-term endothelial dysfunction in response to intrauterine programming.
Our understanding of the fetal origins of adult cardiovascular disease is in its earliest stages. Over the next decades major advances will be made in understanding the mechanisms underlying ‘programming’ of the cardiovascular system during the fetal and neonatal period. Understanding these mechanisms will advance the prevention and the treatment of cardiovascular diseases in the adult. In addition, understanding these mechanisms may give insight into the adaptive processes of congenital heart disease that result from erroneous developmental processes earlier in gestation.
Acknowledgments
This study was supported by the NHLBI grant HL45043 and NICHD grant P01HD34430.
References
- Aoyagi T, Fujii A, Flanagan M, Arnold L, Mirsky I, Izumo S. Maturation-dependent differences in regulation of sarcoplasmic reticulum Ca2+ ATPase in sheep myocardium in response to pressure overload: a possible mechanism for maturation-dependent systolic and diastolic dysfunction. Ped Research. 2001;50:246–253. doi: 10.1203/00006450-200108000-00014. [DOI] [PubMed] [Google Scholar]
- Aoyagi T, Mirsky I, Flanagan M, Currier J, Colan S, Fujii A. Myocardial function in immature and mature sheep with pressure-overload hypertrophy. Am J Physiol. 1992;262:H1036–H1148. doi: 10.1152/ajpheart.1992.262.4.H1036. [DOI] [PubMed] [Google Scholar]
- Bae S, Xiao Y, Li G, Casiano C, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol. 2003;285:H983–H990. doi: 10.1152/ajpheart.00005.2003. [DOI] [PubMed] [Google Scholar]
- Baer RW, Vlahakes GJ, Uhlig Pn, Hoffman JI. Maximum myocardial oxygen transport during anemia and polycythemia in dogs. Am J Physiol. 1987;252:H1086–H1085. doi: 10.1152/ajpheart.1987.252.6.H1086. [DOI] [PubMed] [Google Scholar]
- Barbera A, Giraud G, Reller M, Maylie J, Morton M, Thornburg K. Right ventricular systolic pressure load alters myocyte maturation in fetal sheep. Am J Physiol. 2000;279:R1157–R1164. doi: 10.1152/ajpregu.2000.279.4.R1157. [DOI] [PubMed] [Google Scholar]
- Barker DJP, editor. Fetal Origins of Cardiovascular and Lung Disease. New York: Preface. Marcel Dekker, Inc.; 2000. [Google Scholar]
- Blair D, Vander Straten M, Gest A. Hydrops in fetal sheep from rapid induction of anemia. Pediatr Res. 1994;35:560–564. [PubMed] [Google Scholar]
- Broberg C, Giraud G, Schultz J, Thornburg K, Hohimer A, Davis L. Fetal anemia leads to augmented contractile response to hypoxic stress in adulthood. Am J Physiol. 2003;285:R649–R655. doi: 10.1152/ajpregu.00656.2002. [DOI] [PubMed] [Google Scholar]
- Bugiardini RO, Manfrini C, Pizzi C, Fontana F, Morgagni G. Endothelial function predicts future development of coronary artery disease. Circulation. 2004;109:2518–2523. doi: 10.1161/01.CIR.0000128208.22378.E3. [DOI] [PubMed] [Google Scholar]
- Chen Y, Torry R, Baumbach G, Tomanek R. Proportional arteriolar growth accompanies cardiac hypertrophy induced by volume overload. Am J Physiol. 1994;267:H2132–H2137. doi: 10.1152/ajpheart.1994.267.6.H2132. [DOI] [PubMed] [Google Scholar]
- Copel J, Grannum P, Green J, Belanger K, Hanna N, Jaffe C, Hobbins J, Kleinman C. Fetal cardiac output in the isoimmunized pregnancy: a pulsed Doppler-echocardiographic study of patients undergoing intravascular intrauterine transfusion. Am J Obstet Gynecol. 1989;161:361–365. doi: 10.1016/0002-9378(89)90520-6. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hohimer AR. Hemodynamics and organ blood flow in fetal sheep subjected to chronic anemia. Am J Physiol. 1991;261:R1542–R1548. doi: 10.1152/ajpregu.1991.261.6.R1542. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hohimer AR, Brace RA. Changes in left thoracic duct lymph flow during progressive anemia in the ovine fetus. Am J Obstet Gynecol. 1996;174:1469–1476. doi: 10.1016/s0002-9378(96)70590-2. [DOI] [PubMed] [Google Scholar]
- Davis LE, Hohimer AR, Morton MJ. Myocardial blood flow and coronary reserve in chronically anemic fetal lambs. Am J Physiol. 1999;277:R306–R313. doi: 10.1152/ajpregu.1999.277.1.R306. [DOI] [PubMed] [Google Scholar]
- Davis L, Roullet JB, Thornburg KL, Shokry M, Hohimer AR, Giraud GD. Augmentation of coronary conductance in adult sheep made anemic during fetal life. J Physiol. 2003;547:53–59. doi: 10.1113/jphysiol.2002.023283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodic M, Samuel C, Moritz K, Wintour M, Morgan J, Grigg L, Wong J. Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res. 2001;89:623–629. doi: 10.1161/hh1901.097086. [DOI] [PubMed] [Google Scholar]
- Fisher D, Heyman J, Rudolph A. Myocardial oxygen and carbohydrate consumption in fetal lambs and in adult sheep. Am J Physiol. 1982;238:H399–H405. doi: 10.1152/ajpheart.1980.238.3.H399. [DOI] [PubMed] [Google Scholar]
- Flanagan M, Aoyagi T, Arnold L, Maute C, Fujii A, Currier J, Bergau D, Warren H. Effects of chronic heparin administration on coronary vascular adaptation to hypertension and ventricular hypertrophy in sheep. Circulation. 1999;100:981–987. doi: 10.1161/01.cir.100.9.981. [DOI] [PubMed] [Google Scholar]
- Flanagan M, Aoyagi T, Currier J, Colan S, Fujii A. Effect of young age on coronary adaptations to left ventricular pressure overload hypertrophy in sheep. J Am Coll Cardiol. 1994;24:1786–1796. doi: 10.1016/0735-1097(94)90188-0. [DOI] [PubMed] [Google Scholar]
- Gluckman P, Hanson M. Living with the past: evolution, development and patterns of disease. Science. 2004;305:1733–1734. doi: 10.1126/science.1095292. 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Hachamovitch R, Wicker P, Capasso J, Anversa P. Alterations of coronary blood flow and reserve with aging in Fischer 344 rats. Am J Physiol. 1989;256:H66–H73. doi: 10.1152/ajpheart.1989.256.1.H66. [DOI] [PubMed] [Google Scholar]
- Han H, Austin K, Nathanielsz P, Ford S, Nijland M, Hansen T. Maternal nutrient restriction alters gene expression in the ovine fetal heart. J Physiol. 2004;558:111–121. doi: 10.1113/jphysiol.2004.061697. 10.1113/jphysiol.2004.061697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helisch A, Schaper W. Arteriogenesis: the development and growth of collateral arteries. Microcirculation. 2003;10:83–97. doi: 10.1038/sj.mn.7800173. 10.1038/sj.mn.7800173. [DOI] [PubMed] [Google Scholar]
- Hoffman JI. Maximal coronary flow and the concept of coronary vascular reserve. Circulation. 1984;70:153–159. doi: 10.1161/01.cir.70.2.153. [DOI] [PubMed] [Google Scholar]
- Hoffman J, Spaan J. Pressure-flow relations in coronary circulation. Physiol Rev. 1990;70:331–390. doi: 10.1152/physrev.1990.70.2.331. [DOI] [PubMed] [Google Scholar]
- Kalkman E, Bilgin Y, van Haren P, van Suylen R, Saxena P, Schoemaker R. Determinants of coronary reserve in rats subjected to coronary artery ligation or aortic banding. Cardiovascular Res. 1996;32:1088–1095. doi: 10.1016/s0008-6363(96)00166-6. 10.1016/S0008-6363(96)00166-6. [DOI] [PubMed] [Google Scholar]
- Kassab G, Imoto K, White F, Rider C, Fung Y, Bloor C. Coronary arterial tree remodeling in right ventricular hypertrophy. Am J Physiol. 1993;265:H366–H375. doi: 10.1152/ajpheart.1993.265.1.H366. [DOI] [PubMed] [Google Scholar]
- Kuo K, Davis M, Chilian W. Longitudinal gradients for endothelium-dependent and -independent vascular responses in the coronary microcirculation. Circulation. 1995;92:518–525. doi: 10.1161/01.cir.92.3.518. [DOI] [PubMed] [Google Scholar]
- Li G, Xiao Y, Estrella J, Ducsay C, Gilbert R, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Invest. 2003;10:265–274. doi: 10.1016/s1071-5576(03)00074-1. 10.1016/S1071-5576(03)00074-1. [DOI] [PubMed] [Google Scholar]
- Martin C, Yu A, Jiang B, Davis L, Kimberly D, Hohimer A, Semenza G. Cardiac hypertrophy in chronically anemic fetal sheep: increased vascularization is associated with increased myocardial expression of vascular endothelial growth factor and hypoxia-inducable factor 1. Am J Obstet Gynecol. 1998;178:527–534. doi: 10.1016/s0002-9378(98)70433-8. [DOI] [PubMed] [Google Scholar]
- Oliverti G, Lagrasta C, Quaini F, Ricci R, Moccia G, Capasso J, Anversa P. Capillary growth in anemia induced ventricular wall remodeling in the rat heart. Cir Res. 1989;65:1182–1192. doi: 10.1161/01.res.65.5.1182. [DOI] [PubMed] [Google Scholar]
- Olivetti G, Quaini F, Lagrasta C, Ricci R, Tiberti G, Capasso J, Anversa P. Myocyte cellular hypertrophy and hyperplasia contribute to ventricular wall remodeling in anemia induced cardiac hypertrophy in rats. Am J Path. 1992;141:227–239. [PMC free article] [PubMed] [Google Scholar]
- Quaini F, Urbanek K, Beltrami A, Finato N, Beltrami C, Nadal-Ginard B, Kajstura J, Leri A, Anversa P. Chimerism of the transplanted heart. N Engl J Med. 2002;346:5–15. doi: 10.1056/NEJMoa012081. 10.1056/NEJMoa012081. [DOI] [PubMed] [Google Scholar]
- Rakusan K, Cicutti N, Kolar F. Effect of anemia on cardiac function, microvascular structure, and capillary hematocrit in rat hearts. Am J Physiol. 2001;280:H1407–H1414. doi: 10.1152/ajpheart.2001.280.3.H1407. [DOI] [PubMed] [Google Scholar]
- Rakusan K, Flanagan M, Geva T, Southern J, Van Praagh R. Morphometry of human coronary capillaries during normal growth and the effect of age in left ventricular pressure-overload hypertrophy. Circulation. 1992;86:38–46. doi: 10.1161/01.cir.86.1.38. [DOI] [PubMed] [Google Scholar]
- Roghair R, Lamb F, Miller F, Scholz T, Segar J. Early gestation dexamethasone programs enhanced postnatal ovine coronary artery vascular reactivity. Am J Physiol. 2005;288:R46–R53. doi: 10.1152/ajpregu.00165.2004. [DOI] [PubMed] [Google Scholar]
- Toma B, Wangler R, DeWitt D, Sparks H. Effect of development on coronary vasodilator reserve in the isolated guinea pig heart. Circ Res. 1985;57:538–544. doi: 10.1161/01.res.57.4.538. [DOI] [PubMed] [Google Scholar]
- Tomanek R, Hu B, Phan B, Clark E. Rate of coronary vascularization during embryonic chicken development is influenced by the rate of myocardial growth. Cardiovasclar Res. 1999;41:663–671. doi: 10.1016/s0008-6363(98)00330-7. 10.1016/S0008-6363(98)00330-7. [DOI] [PubMed] [Google Scholar]
- Thompson L, Aguan K, Pinkas G, Weiner C. Chronic hypoxia increases the NO contribution of acetylcholine vasodilation of the fetal guinea pig heart. Am J Physiol. 2000;279:R1813. doi: 10.1152/ajpregu.2000.279.5.R1813. [DOI] [PubMed] [Google Scholar]
- White F, Bloor C, McKirnan M, Carroll S. Exercise training in swine promotes growth of arteriolar bed and capillary angiogenesis in heart. J Appl Physiol. 1998;85:1160–1168. doi: 10.1152/jappl.1998.85.3.1160. [DOI] [PubMed] [Google Scholar]
- Wothe D, Hohimer A, Morton M, Thornburg K, Giraud G, Davis L. Increased coronary blood flow signals growth of coronary resistance vessels in near term ovine fetuses. Am J Physiol. 2002;282:R295–R302. doi: 10.1152/ajpregu.2002.282.1.R295. [DOI] [PubMed] [Google Scholar]
- Zhang L. Prenatal hypoxia and cardiac programming. J Soc Gynecol Invest. 2005;12:2–13. doi: 10.1016/j.jsgi.2004.09.004. 10.1016/j.jsgi.2004.09.004. [DOI] [PubMed] [Google Scholar]