Abstract
The epidemiological basis of the developmental origins of disease concept is now widely accepted. The current impetus in research concerns establishing the underlying mechanisms. We discuss the wider biological nature of the phenomenon, with particular reference to ‘maternal effects’, the processes observed in many species by which the mother can induce phenotypic effects in her offspring. Animal models permit investigation of the induction of cardiovascular phenotypic attributes which resemble pathological effects in humans. We discuss the importance of transitions in aspects of the pre- versus the postnatal environment, with emphasis on nutrition and energy expenditure, and the critical role which the timing of environmental cues plays in inducing effects on the offspring. Coupled with the effects of specific maternal dietary components, the effects on the offspring are argued to involve epigenetic mechanisms. In this review we provide a conceptual framework for synthesising experimental and clinical data, important for considering the impact of the developmental origins concept in a life-course approach to the prevention of cardiovascular disease.
Introduction
The effects of unbalanced diet and body composition in pregnancy on the subsequent risk of health and disease in the offspring have been well established from epidemiological studies (Barker, 2001) and are strongly supported by numerous experimental observations. The significance of these early life events to the global patterns of disease and their implications for public health policy have been discussed (Gluckman & Hanson, 2005). In this review we consider the biological nature of this phenomenon, with particular reference to the induction of cardiovascular phenotypes in animals. First, the phenomenon will be considered in the context of ‘maternal effects’, a widespread biological phenomenon in many species. Then, we will discuss the importance of the pre to postnatal environmental transition in mammals, and the role of specific maternal dietary components and timing of effects on underlying mechanisms will be considered. We conclude with a general synthesis and framework for considering experimental and clinical data. Whilst there is a relative dearth of data specifically relating to cardiovascular function in this context, the broader examples we cite and the theoretical basis discussed are directly applicable to this area of physiology.
Maternal effects
The transmission of cues about the nature of the environment from the parental generation to their offspring is a well-established process in developmental biology (see West-Eberhard, 2003). In Daphnia the induction of the ‘helmet phenotype’ as a defence against high predator densities is one example (Laforsch & Tollrian, 2004). A further dramatic example is provided by the desert locust (Schistocerca gregaria) in which the population density detected by the female alters the chemical composition of the fluid secreted around her eggs, determining the percentage of larvae which will develop into the gregarious versus solitarious forms. These are different not only in body colouration but also in wing shape, behaviour, metabolism and food preference (Wilson et al. 2002). In mammals, the meadow vole (Microtus pennsylvanicus) provides an example in which the day length to which the pregnant dam is exposed in early pregnancy determines the coat thickness of the offspring (Lee & Zucker, 1988). This has a later survival advantage but has no value at the time of the ‘choice’ of coat thickness. These examples are important because they demonstrate that phenotypic changes may be made during development which do not convey any immediate advantage during the developmental period, only a later advantage in the environment experienced by the independent mature organism. Because the changes induced, whilst transgenerationally transmitted, are not genomically transmitted but rather involve epigenetic marks such as methylation to DNA, they have been termed epigenetic inheritance. This concept is very well established in both animal and plant kingdoms and its biological importance is increasingly recognized (see Jablonka & Lamb, 1995).
Such responses, whether induced by nutrition, endocrine milieu, thermal or fluid and electrolyte levels, day length, maternal stress, etc. have been termed by us ‘predictive adaptive responses’ (PARs; see Gluckman & Hanson, 2004a, b) building on the concepts of Bateson (2001). We distinguish PARs from the ‘coping’ responses that follow intra-uterine adaptations to a prenatal threatening environment, which are usually considered in the context of the ‘thrifty phenotype’ hypothesis (Hales & Barker, 2001). In the latter the developing organism makes immediate adaptive responses to an immediate environmental challenge by reducing either body growth or growth of individual organs to promote its chances of immediate survival. It then has to cope throughout its life with the consequences of the irreversible components of this prenatal adaptive strategy. Hales & Barker (2001) suggested that such thrifty phenotypes would have an advantage in a postnatally deprived environment but would be disadvantageous in an enriched one. Whether the outcome is advantageous or disadvantageous is purely chance. In some respects this concept is akin to that of exaptation (Gould & Vrba, 1982) by which traits that are part of an adaptive response of an organism to one environment then serve to be beneficial in another: here the two environments would be pre and postnatal (provided the latter is deficient). In contrast, PARs confer no immediate advantage (it is in fact conceptually possible that they could have a small disadvantageous immediate effect) but are induced to confer long-term advantage later in life.
The thrifty phenotype model provided the original basis for the widely recognized association between low birthweight and later risk of disease. However, the experimental and clinical data strongly point to a broader phenomenon which has led to further development of the conceptual basis of the impact of early life events on long-term health. Bateson (2001) postulated that the fetus might gain a long-term fitness advantage if it could predict or ‘forecast’ its future environment and Jablonka had indicated the fitness advantage of such ‘phenotypic memory’ (Jablonka et al. 1995). The changes in fetal structure and function during development induced by the fetal environment could occur in the absence of any change in fetal growth. The processes of developmental plasticity involve far more than just effects on body size. Indeed, a plethora of human and animal studies show that the environment during development can affect phenotype in either adverse or beneficial ways in the absence of changes in fetal growth. Sometimes, however, these effects are related to prenatal growth in a graded manner across the entire range of size at birth. We do not believe that this necessarily implies causality between reduced growth and phenotypic change. It may be rather that reducing growth is part of a prenatal strategy to cope with adversity, and occurs in parallel with other phenotypic changes which have a predictive component, or that a reduction is growth is a response invoked only when the prenatal environmental challenge is particularly strong and other adaptive responses are inadequate. If this is true then the association between birth size and later risk of cardiovascular disease need not be viewed as due to a direct causal relationship but could be an epiphenomenon due to independent effects on the fetus in terms of its growth and its long-term development. The consequences of these two types of adaptive response need not be the same.
Further distinctions should also be made from the effects of a very severe environmental challenge in utero: starvation, infectious agents and the administration of glucocorticoids at critical developmental windows can produce a disruption of development which is unrelated to any adaptive advantage either at the time or later. Here, then, a change in phenotypic characteristics, including a possible effect on growth, should be viewed as a direct adverse effect and not an adaptation. This type of effect is not confined to an extremely poor developmental environment: at the other end of the spectrum it should be noted that environmental excess (e.g. during diabetic pregnancy) may also induce detrimental effects including teratogenic change on the developing offspring (see Bateson et al. 2005, for review).
Distinguishing long-term consequences of adaptive responses from those that reflect a disruption of development may be difficult (Bateson et al. 2005). For example, is the reduction in nephron number in utero in response to maternal nutritional manipulation (Langley-Evans et al. 2003) or dexamethasone administration (Wintour et al. 2003) an adaptive response with long-term sequelae or is it a disruptive effect produced by an extreme exposure to steroids or undernutrition during a critical window of renal development? Because fetal renal blood flow is only 3% of combined ventricular output in late gestation, compared to 20% of cardiac output in the adult (Moritz et al. 2005), it is difficult to envisage that any reduction in fetal renal growth constitutes an energy-saving adaptive response. Nonetheless, the relationship between nephron number and maternal vitamin A intake in the rat (Merlet-Benichou et al. 1999) suggests that alteration of this development can have long-term consequences.
The range of long-term effects that can be induced by subtle early (e.g. embryonic) life events is striking. It is difficult to envisage how such latent effects can be considered in terms of immediately adaptive responses, especially as they can persist transgenerationally (see below). It is clear that the phenomenon of predictive adaptive responses is widespread. Theoretical modelling indicates that phenotypic memory, whereby environmental impact on one generation has advantageous effects on the next generation(s), is a powerful tool in a repertoire of processes aimed at promoting species survival through changing environments (Jablonka et al. 1995). We have suggested (Gluckman & Hanson, 2005) that predictive adaptive responses are the basis of this phenotypic memory and that they are likely to be underpinned by epigenetic mechanisms. They are regulated processes and provide the basis for explaining many long-term consequences of early life events, particularly those not associated with a disturbance in fetal growth. We should note, however, that some environmental cues will induce mixed adaptive responses.
It seems probable that all three types of response – developmental disruption, immediate, and predictive adaptation – have been included under the rubric of ‘programming’. We do not find this term helpful as it still echoes its use for the genetic programme of development (Jacob & Monod, 1961) and has deterministic connotations which are not compatible with the more plastic nature of developmental gene–environment interactions in the induction of phenotype. Moreover, it implies that the nature of the programme to be executed is encoded in the command to execute it (Bateson, 2001).
Importance of the pre- to postnatal transition
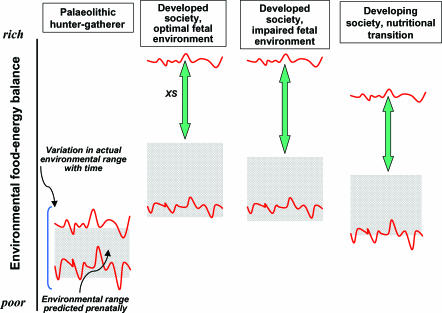
These concepts emphasize the importance of environmental transitions in development. Most focus has been on the transition between the prenatal and postnatal environment but this may be better considered as a transition between the environment during the plastic phase of development and the mature, post-plastic phase. Birth itself may be incidental to this transition because different organ systems have different windows of plasticity, some of which are postnatal. Further transitions are not dichotomous – each stage of development can affect the next and responses in one phase may thus affect the capacity to respond to the environment in later stages. Thus we have an increasing awareness that potential transitions between the embryonic or early fetal and late gestation environments, and indeed between the pre- and post-weaning postnatal environments, maybe of great importance (Harding, 1997; Bloomfield et al. 2003, 2004; Singhal et al. 2003; Wlodek et al. 2003; Cleal et al. 2004; Hambidge et al. 2005; Edwards et al. 2005). The recent studies of Ozanne & Hales (2004) demonstrate the importance of the transition elegantly, because mice whose dams were fed a low protein diet during pregnancy and then a cafeteria diet post weaning lived significantly less long than those whose dams were fed a normal diet during pregnancy. Conversely, feeding a low protein diet to the dams during suckling extended the longevity of their offspring. How the precise nature, magnitude and speed of such transitions affects the offspring has not yet been established – this is an important, if inevitably expensive, study to perform. A clue that there must be an upper limit to the deleterious effects of the poor–rich transition also comes from the study of Ozanne & Hales (2004) because longevity in their mouse offspring was similarly reduced in those weaned onto normal chow and a cafeteria diet. A more detailed consideration of the characteristics of such transitions has been proposed (Gluckman & Hanson, 2004b). Their relevance to humans is shown in Fig. 1.
Figure 1. Diagram to show a conceptual framework for the influence of predictive adaptive responses (PARs) under various food availability–energy expenditure conditions in the postnatal environment.
Red lines show the range of environmental conditions occurring over time. Stippled regions show the range for which mature individuals have maximal fitness as determined during early development by PARs. Those living in a food–energy environment outside their PARs range are at increased risk of ‘lifestyle’ disease such as metabolic syndrome. For a Palaeolithic hunter-gatherer society the level of food–energy balance is low and the range narrow, and this period of evolutionary adaptiveness ensured an approximate match between the physiology of the adult and the range of environments experienced. PARs allowed the individual to cope with the extremes of this range. The risk of disease such as metabolic syndrome was irrelevant given that low life expectancy resulted from other causes. In contemporary developed societies, the range of food–energy balance has moved upwards, especially at the upper limit. However, the intra-uterine environment remains relatively constrained due to the limits on maternal size. Therefore the persistent presence of PARs leaves the individual with a set of predictive adaptations mismatched with the actual environment faced and this leads to a gap between the upper limit of the range of optimal fitness and the environment actually faced. Such individuals are not well-adapted to live in this range and this confers increased risk of metabolic disorder with accompanying morbidity and mortality. The XS range is even larger following pregnancies with excessive constraint, due, for example, to unbalanced maternal diet or body composition, disease or placental insufficiency; under these conditions the PARs range shifts downwards and is narrower. The XS range is nearly as wide in developing societies undergoing economic transition, even if the food–energy balance has not yet reached the level of developed societies, because for many generations small maternal size and relatively poor nutrition have constrained the fetus and the processes of PARs have set the postnatal fitness range lower. Such societies are particularly at risk as the size of the food–energy transition increases further.
Maternal diet
Whilst a range of experimental manipulations before and during pregnancy have been utilized, including exposure to nicotine, xenoestrogens, pesticides, bacterial endotoxins, glucocorticoids and manipulation of maternal stress responses (Barbazanges et al. 1996; Thiruchelvan et al. 2003; Ling et al. 2004; Newbold et al. 2004; Akingbemi et al. 2004; Levin, 2005) the majority of studies have involved alterations to the maternal diet during pregnancy. Particular emphasis has been placed on reduction of global nutrient intake (e.g. Ozaki et al. 2000, 2001), feeding a high fat diet (Norman & LeVeen, 2001; Khan et al. 2004a) or a low protein diet (Langley & Jackson, 1994; Brawley et al. 2003). The effects of a high fat diet are reviewed in this issue (Armitage et al. 2005). The focus here will be on the interpretation of the effects of a low protein diet. The widespread consumption of foods of high calorific content, especially those of high glycaemic index, is proposed to exacerbate the effects of early life environments in the aetiology of obesity. Because humans, like rodents and even invertebrates, defend their protein intake within narrow limits, the consumption of a diet with a reduced protein to carbohydrate and fat ratio necessitates ingestion of an increased non-protein calorific load (see Simpson et al. 2003). In our evolutionary past, the hunter-gatherer diet was high in protein, even if its availability was uncertain. The precise size of the contemporary effect varies across the globe, but FAO statistics indicate that the fat and carbohydrate content of the Western diet has increased from 86 to 87.5% between 1961 and 2000, with the corresponding fall in protein content from 14% to 12.5%. This necessitates a 14% increase in energy intake from carbohydrate and fat to maintain dietary protein intake (reviewed in Simpson et al. 2003). In animals, feeding a diet with 50% reduction in protein in early gestation, the first half of gestation or throughout pregnancy induces cardiovascular abnormalities in the offspring (Langley & Jackson, 1994; Kwong et al. 2000; Brawley et al. 2003; Nishina et al. 2003) including elevated blood pressure, endothelial dysfunction, glucose intolerance, disturbances in metabolism and specific tissue gene expression. The precise component of the low protein diet which mediates the effect is not known, although attention has focused recently on glycine because, although a non-essential amino acid, it is required in large quantities in the human fetus in late gestation and thus becomes ‘conditionally essential’. Supplementation of the low protein diet with glycine in the rat prevents hypertension in the offspring (Jackson et al. 2002) and also the disordered placental lipid levels (Burdge et al. 2002). In addition it rectifies the abnormalities in responses of the uterine arteries of the pregnant dam in late gestation (Brawley et al. 2004).
Timing of effects
The original concept of the fetal origins of adult disease (Barker, 2001) focused attention on reductions in fetal growth in late gestation as a direct response to an adverse intra-uterine environment, which then produced detrimental long-term consequences. Recent research has, however, drawn attention to the ways in which the environment of the early embryo or the fetus in the first trimester of pregnancy can induce phenotypic changes. Kwong et al. (2000) demonstrated that the low protein diet fed during only the first 4.25 days of pregnancy (pre-implantation) in the rat produced hypertension in the offspring. Nutritional manipulation in the pre-implantation period in the sheep produces effects on the length of gestation and maturation of the hypothalamic–pituitary–adrenal (HPA) axis (Bloomfield et al. 2003, 2004), blood pressure in late gestation and the endocrine and cardiovascular responses of lambs at 21/2 years of age (Cleal et al. 2004; Poore et al. 2004). In addition, a follow-up study of offspring of mothers who suffered the Dutch ‘hunger winter’ in 1944/5 reveals that famine exposure during the first trimester produced effects on the offspring which were not accompanied by a reduction in birth weight (Roseboom et al. 2001). Recently we showed in the guinea pig (Khan et al. 2004b) that global undernutrition during the first half of pregnancy produced elevated blood pressure and left ventricular hypertrophy in the absence of a reduction in birth weight; undernutrition in late pregnancy reduced birthweight, as expected, but did not lead to hypertension or significant left ventricular hypertrophy in the adult offspring.
That such effects can be produced in early gestation emphasizes their later predictive, rather than immediate, adaptive value. Indeed, in the sheep, thickening of the cardiac interventricular septum in adults was seen in offspring whose ewes received an early undernutrition challenge, as in the guinea pig, but the effect was absent in offspring who also received undernutrition in the postweaning period (Boullin et al. 2005). Hence the predictive value of the first insult appears to protect against the detrimental effects of the second. These effects are most striking in male offspring, with changes in body composition and fat deposition being more evident in female offspring. It would appear that males preserve growth, but trade off cardiovascular and renal function, thus maintaining body size, whereas females trade off growth for metabolic responses and fat deposition. Each of these processes can be interpreted as giving a reproductive advantage.
Mechanisms
The importance of methyl group provision, derived especially from dietary glycine, is referred to above. Glycine supplementation of the low protein diet prevents hypertension in the adult offspring in the rat, although supplementation with casein, alanine or urea does not (Jackson et al. 2002). Apart from its effects on the maternal cardiovascular adaptations to pregnancy, glycine supplementation also reduces the elevation of maternal plasma homocysteine levels in animals fed the low protein diet during pregnancy, and this may give an additional cardiovascular protective effect on both the pregnant dam and her fetuses. The effects on the uterine artery appear to be mediated primarily via promotion of vasodilator function, and glycine supplementation restores uterine artery NO production (Brawley et al. 2004). These effects may partially explain the effect of the low protein diet on the offspring, as presumably it would promote nutrient supply to them in utero. However, considerable interest is also focused on the ways in which altered methyl group provision may affect epigenetic processes in the developing offspring. Such processes may affect both histone acetylation, and thus the accessibility of regions of the genome for transcription, and DNA methylation at CpG islands, which is known to act at gene promoter regions to regulate their expression. Such processes are well known for imprinted genes (Constancia et al. 2004). The provision of methyl groups for the process depends on the availability of cofactors including folate, vitamin B6 and B12. Dietary folate supplementation affects the expression of the agouti mutation in the mouse (Waterland & Jirtle, 2003) preventing obesity and cancer as well as the yellow coat colour in the offspring. We have now shown that folate supplementation prevents elevated blood pressure, endothelial dysfunction and the reduction in eNOS expression in the vasculature of the offspring in the rat (Torrens C, Brawley L, Anthony FW, Dance CS, Dunn R, Jackson AA, Poston L and Hanson MA, unpublished observation). The precise timing of the window when changes in DNA methylation may produce permanent effects on the offspring is not known, but in the rat it would appear to extend beyond the embryonic phase: Pham et al. (2003) showed that uterine artery ligation in late gestation produced alterations in the methylation of the p53 gene in the kidney of the adult offspring. This is interesting because p53 is involved in tumour suppression, monitoring DNA damage and apoptosis and could therefore mediate some of the effects on nephron number of the low protein diet.
Epigenetic changes inducible during the suckling period are demonstrated by the findings of Weaver et al. (2004) who showed changes in histone acetylation and transcription factor (NGFI-A) binding to the promoter of the glucocorticoid receptor gene in the hippocampus in offspring of mothers who showed different degrees of licking and grooming of their pups. Administration of a histone deacetylase inhibitor restored histone acetylation, DNA methylation, NGFI-A binding, glucocorticoid receptor expression and HPA responses to stress. These results are interesting because changes in the HPA stress responses in humans may lead to aspects of the metabolic syndrome (Flanagan et al. 1999). Changes in the expression of imprinted genes during suckling may also be important for offspring growth and metabolism (see Constancia et al. 2004, for review). Finally, Lillycrop et al. (2004) have shown changes in the expression of peroxisome proliferator-activated receptor alpha (PPARα) in the liver of offspring of dams fed the low protein diet which are accompanied by a reduction in DNA methylation of the gene and are reversible by dietary folate supplementation of the dam. Such effects would be expected to alter fatty acid metabolism in the liver and are targeted to specific parts of the genome as changes in PPARγ were not observed in those experiments.
There is clearly considerably more work to be done on the area of epigenetic regulation of early development, and one of the most intriguing aspects of the phenomenon is that recent work demonstrates that the methylation patterns may be in part transmitted to more than one subsequent generation. Thus the effects of the low protein diet or of glucocorticoid administration during pregnancy can be seen on cardiovascular function and on glucose homeostasis in the F2 offspring, even in the absence of an additional nutritional challenge during the pregnancy of the F1 offspring (Torrens et al. 2002; Drake et al. 2005). Possible underlying mechanisms include effects on the oocytes developing in the fetus in utero, effects on maternal adaptations to pregnancy in the F1 generation or direct transmission of methylation patterns to subsequent generations (see Drake & Walker, 2004, for review). The association of such processes with cardiovascular and metabolic dysfunction makes it clear that non-genomic inheritance of factors for risk of disease needs considerably more attention.
Conclusion
The genotype of the early embryo contains a record of the evolutionary past of the species, including changes which have been manifest by mutation, natural and sexual selection, genetic drift, etc. It also contains echoes of the environmental exposures of recent antecedent generations, in the form of persistent epigenetic marks on the genome. Transition from the genotype of the early embryo to the phenotype of the offspring by the time of weaning involves a range of epigenetic processes, including predictive adaptive responses, developmental plasticity and, under some circumstances, developmental disruption. Further transitions in phenotype occur in childhood and into adult life. The level of matching between developmental and later environments, or more specifically between the predicted and actual later environments, influences these transitions in phenotype and, in turn, the risk of disease. Understanding these processes will help us to devise interventions to limit the progression to disease in susceptible individuals and sections of the population.
Acknowledgments
M.A.H. is supported by the British Heart Foundation.
References
- Akingbemi BT, Sottas CM, Koulova AI, Klindfelter GR, Hardy MP. Inhibition of testicular steroidogenesis by the xenoestrogen bisphenol A is associated with reduced pituitary luteinizing hormone secretion and decreased steroidogenic enzyme gene expression in rat Leydig cells. Endo. 2004;145:592–603. doi: 10.1210/en.2003-1174. [DOI] [PubMed] [Google Scholar]
- Armitage JA, Taylor PD, Poston L. Experimental models of developmental programming: consequences of exposure to an energy rich diet during development. J Physiol. 2005;565:3–8. doi: 10.1113/jphysiol.2004.079756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbazanges A, Piazza PV, Le Moal M, Maccari S. Maternal glucocorticoid secretion mediates long-term effects of prenatal stress. J Neurosci. 1996;16:3943–3949. doi: 10.1523/JNEUROSCI.16-12-03943.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Fetal Origins of Cardiovascular and Lung Disease. New York: Dekker; 2001. [Google Scholar]
- Bateson P. Fetal experience and good adult design. Int J Epid. 2001;30:928–934. doi: 10.1093/ije/30.5.928. [DOI] [PubMed] [Google Scholar]
- Bateson P, Gluckman PD, Spencer HG, Hanson MA. Environmental influences during development and their later consequences: implications for the interpretation of empirical studies. Proc R Soc Lond B Biol Sci. 2005 doi: 10.1098/rspb.2004.3001. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomfield FH, Oliver MH, Hawkins P, Campbell M, Phillips DJ, Gluckman PD, Challis JR, Harding JE. A periconceptional nutritional origin for noninfectious preterm birth. Science. 2003;300:606. doi: 10.1126/science.1080803. [DOI] [PubMed] [Google Scholar]
- Bloomfield FJ, Oliver MH, Hawkins P, Holloway AC, Campbell M, Gluckman PD, Harding JE, Challis JR. Periconceptional undernutrition in sheep accelerates maturation of the fetal hypothalamic-pituitary-adrenal axis in late gestation. Endocrinology. 2005;145:4278–4285. doi: 10.1210/en.2004-0424. [DOI] [PubMed] [Google Scholar]
- Boullin JP, Green LR, Khan OA, Cleal JK, Poore KR, Newman JP, Noakes D, Poston L, Morgan JM, Hanson MA. The effect of moderate early gestation undernutrition with or without undernutrition in early postnatal life on cardiac morphology in adult male sheep. Br J Obstet Gynaecol. 2005 (in press) [Google Scholar]
- Brawley L, Poston L, Hanson MA. Mechanisms underlying the programming of small artery dysfunction: review of the model using low protein diet in pregnancy in the rat. Arch Physiol Biochem. 2003;111:23–35. doi: 10.1076/apab.111.1.23.15138. [DOI] [PubMed] [Google Scholar]
- Brawley L, Torrens C, Anthony FW, Itoh S, Wheeler T, Jackson AA, Clough GF, Poston L, Hanson MA. Glycine rectifies vascular dysfunction induced by dietary protein imbalance during pregnancy. J Physiol. 2004;554:497–504. doi: 10.1113/jphysiol.2003.052068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdge GC, Dunn RL, Jackson AA. The effect of reduced protein intake during pregnancy on placental lipid composition in the rat: effect of glycine supplementation of the low protein diet. Nutr Res. 2002;24:909–921. [Google Scholar]
- Cleal J, Newman JP, Poore KR, Forhead AJ, Noakes D, Hanson MA, Green LR. The renin-angiotensin system in adult sheep following moderate postconceptional undernutrition and undernutrition in early postnatal life. J Physiol. 2004;555.P:C92. [Google Scholar]
- Constancia M, Kelsey G, Reik W. Resourceful imprinting. Nature. 2004;432:53–57. doi: 10.1038/432053a. 10.1038/432053a. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR. The intergenerational effects of fetal programming: non-genomic mechanisms for the inheritance of low birth weight and cardiovascular risk. J Endocrinol. 2004;180:1–16. doi: 10.1677/joe.0.1800001. 10.1677/joe.0.1800001. [DOI] [PubMed] [Google Scholar]
- Drake AJ, Walker BR, Seckl J. Intergenerational consequences of fetal programming by in utero exposure to glucocorticoids in rats. Am J Physiol Regul Integr Comp Physiol. 2005;288:R34–R38. doi: 10.1152/ajpregu.00106.2004. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McFarlane JR, Kauter KG, McMillen IC. Impact of periconceptional nutrition on maternal and fetal leptin and fetal adiposity in singleton and twin pregnancies. Am J Physiol Regul Integr Comp Physiol. 2005;288:R39–R45. doi: 10.1152/ajpregu.00127.2004. [DOI] [PubMed] [Google Scholar]
- Flanagan DE, Vaile JC, Petley GW, Moore VM, Godsland IF, Cockington RA, Robinson JS, Phillips DI. The autonomic control of heart rate and insulin resistance in young adults. J Clin Endocrinol Metab. 1999;84:1263–1267. doi: 10.1210/jcem.84.4.5592. 10.1210/jc.84.4.1263. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. Living with the past: evolution, development, and disease. Science. 2004a;305:1733–1736. doi: 10.1126/science.1095292. 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. The developmental origins of the metabolic syndrome. Trends Endocrinol Metab. 2004b;15:183–187. doi: 10.1016/j.tem.2004.03.002. 10.1016/j.tem.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA. The Fetal Matrix – Evolution, Development and Disease. Cambridge, UK: Cambridge University Press; 2005. [Google Scholar]
- Gould SJ, Vrba E. Exaptation – a missing term in the science of form. Paleobiology. 1982;8:4–14. [Google Scholar]
- Hales CN, Barker DJP. The thrifty phenotype hypothesis. Br Med Bull. 2001;60:5–20. doi: 10.1093/bmb/60.1.5. [DOI] [PubMed] [Google Scholar]
- Hambidge ORH, Torrens C, Noakes D, Poston L, Hanson MA, Green LR. The effect of moderate early gestation undernutrition with or without undernutrition in early postnatal life on renal vascular function in adult sheep. Br J Obstet Gynaecol. 2005 (in press) [Google Scholar]
- Harding JE. Periconceptional nutrition determines the fetal growth response to acute maternal undernutrition in fetal sheep of late gestation. Prenat Neonatal Med. 1997;2:310–319. [Google Scholar]
- Jablonka E, Lamb MJ. Epigenetic Inheritance and Evolution: the Lamarckian Dimension. Oxford: Oxford University Press; 1995. [Google Scholar]
- Jablonka E, Oborny B, Molnar I, Kisdi E, Hofbauer J, Czaran T. The adaptive advantage of phenotypic memory in changing environments. Philos Trans R Soc Lond B Biol Sci. 1995;350:133–141. doi: 10.1098/rstb.1995.0147. [DOI] [PubMed] [Google Scholar]
- Jackson AA, Dunn RL, Marchand MC, Langley-Evans SC. Increased systolic blood pressure in rats induced by a maternal low-protein diet is reversed by dietary supplementation with glycine. Clin Sci (Lond) 2002;103:633–639. doi: 10.1042/cs1030633. [DOI] [PubMed] [Google Scholar]
- Jacob F, Monod J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- Khan I, Dekou V, Hanson M, Poston L, Taylor P. Predictive adaptive responses to maternal high-fat diet prevent endothelial dysfunction but not hypertension in adult rat offspring. Circulation. 2004a;110:1097–1102. doi: 10.1161/01.CIR.0000139843.05436.A0. [DOI] [PubMed] [Google Scholar]
- Khan OA, Loades C, Boullin J, Bertram C, Ohri SK, Hanson MA. Early pre-natal undernutrition causes cardiac hypertrophy and hypertension. Proceedings of the American Heart Association. 2004b (in press) [Google Scholar]
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development. 2000;127:4195–4202. doi: 10.1242/dev.127.19.4195. [DOI] [PubMed] [Google Scholar]
- Laforsch C, Tollrian R. Embryological aspects of inducible morphological defenses in Daphnia. J Morphol. 2004;262:701–707. doi: 10.1002/jmor.10270. [DOI] [PubMed] [Google Scholar]
- Langley SC, Jackson AA. Increased systolic blood pressure in adult rats induced by fetal exposure to maternal low protein diets. Clin Sci (Lond) 1994;86:217–222. doi: 10.1042/cs0860217. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Langley-Evans AJ, Marchand MC. Nutritional programming of blood pressure and renal morphology. Arch Physiol Biochem. 2003;111:8–16. doi: 10.1076/apab.111.1.8.15136. [DOI] [PubMed] [Google Scholar]
- Lee TM, Zucker I. Vole infant development is influenced perinatally by maternal photoperiodic history. Am J Physiol. 1988;255:R831–R838. doi: 10.1152/ajpregu.1988.255.5.R831. [DOI] [PubMed] [Google Scholar]
- Levin ED. Fetal nicotinic overload, blunted sympathetic responsivity and weight gain. Teratology. 2005 doi: 10.1002/bdra.20162. (in press) [DOI] [PubMed] [Google Scholar]
- Lillycrop KA, Phillips ES, Jackson AA, Hanson MA, Burdge GC. Maternal dietary restriction during pregnancy in the rat alters hepatic gene expression in the offspring by a promoter methylation-dependent mechanism. J Physiol. 2004;563 (in press) [Google Scholar]
- Ling ZD, Chang Q, Lipiton JW, Tong CW, Landers TM, Carvey PM. Combined toxicity of prenatal bacterial endotoxin exposure and postnatal 6-hydroxydopamine in the adult rat midbrain. Neuroscience. 2004;124:619–628. doi: 10.1016/j.neuroscience.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Merlet-Benichou C, Gilbert T, Vilar J, Moreau E, Freund N, Lelievre-Pegorier M. Nephron number: variability is the rule. Causes and consequences. Lab Invest. 1999;79:515–527. [PubMed] [Google Scholar]
- Moritz KM, Wintour EM, Dodic M. The developmental environment, renal function and disease. In: Gluckman PD, Hanson MA, editors. Developmental Origins of Health and Disease – a Biomedical Perspective. Cambridge,UK: Cambridge University Press; 2005. (in press) [Google Scholar]
- Newbold RR, Jefferson WJ, Padilla-Banks E, Haseman J. Developmental exposure to diethylstilbestrol (DES) alters uterine response to estrogens in prepubescent mice: low versus high dose effects. Report Toxicol. 2004;18:399–406. doi: 10.1016/j.reprotox.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Nishina H, Green LR, McGarrigle HH, Noakes DE, Poston L, Hanson MA. Effect of nutritional restriction in early pregnancy on isolated femoral artery function in mid-gestation fetal sheep. J Physiol. 2003;553:637–647. doi: 10.1113/jphysiol.2003.045278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman, LeVeen Maternal atherogenic diet in swine is protective against early atherosclerosis development in offspring consuming an atherogenic diet post-natally. Atherosclerosis. 2001;157:41–47. doi: 10.1016/s0021-9150(00)00668-7. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Hawkins P, Nishina H, Steyn C, Poston L, Hanson MA. Effects of undernutrition in early pregnancy on systemic small artery function in late-gestation fetal sheep. Am J Obstet Gynecol. 2000;183:1301–1307. doi: 10.1067/mob.2000.107463. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hanson MA, Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozanne SE, Hales CN. Lifespan: catch-up growth and obesity in male mice. Nature. 2004;427:411–412. doi: 10.1038/427411b. [DOI] [PubMed] [Google Scholar]
- Pham TD, MacLennan NK, Chiu CT, Laksana GS, Hsu JL, Lane RH. Uteroplacental insufficiency increases apoptosis and alters p53 gene methylation in the full-term IUGR rat kidney. Am J Physiol Regul Integr Comp. 2003;285:R962–R970. doi: 10.1152/ajpregu.00201.2003. [DOI] [PubMed] [Google Scholar]
- Poore KR, Cleal JK, Newman JP, Boullin J, Noakes D, Hanson MA, Green LR. Glucose metabolism and body composition in mature adult sheep following early life undernutrition. J Physiol. 2004;563 (in press) [Google Scholar]
- Roseboom TJ, van der Meulen JH, Ravelli AC, Osmond C, Barker DJ. Effects of prenatal exposure to the Dutch famine on adult disease in later life: an overview. Mol Cell Endocrinol. 2001;185:93–98. doi: 10.1016/s0303-7207(01)00721-3. [DOI] [PubMed] [Google Scholar]
- Simpson SJ, Batley R, Raubenheimer D. Geometric analysis of macronutrient intake in humans: the power of protein? Appetite. 2003;41:123–140. doi: 10.1016/s0195-6663(03)00049-7. [DOI] [PubMed] [Google Scholar]
- Singhal A, Wells J, Cole TJ, Fewtrell M, Lucas A. Programming of lean body mass: a link between birth weight, obesity, and cardiovascular disease? Am J Clin Nutr. 2003;77:726–730. doi: 10.1093/ajcn/77.3.726. [DOI] [PubMed] [Google Scholar]
- Thiruchelvan M, McCormack A, Richfield EK, Baggs RB, Tank AW, DiMonte DA, Cory-Slechta DA. Age-related irreversible progressive nigrostriatal dopaminergic neurotoxicity in the paraquat and maneb model of the Parkinson's disease phenotype. Eur J Neurosci. 2003;18:589–600. doi: 10.1046/j.1460-9568.2003.02781.x. [DOI] [PubMed] [Google Scholar]
- Torrens C, Brawley L, Dance CS, Itoh S, Poston L, Hanson MA. First evidence for transgenerational programming of the rat protein restriction model. J Physiol. 2002;543.P:41P. [Google Scholar]
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–5300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver IC, Cervoni N, Champagne FA, D'Alessio AC, Sharma S, Seckl JR, Dymov S, Szyf M, Meaney MJ. Epigenetic programming by maternal behavior. Nat Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- West-Eberhard MJ. Developmental Plasticity and Evolution. New York: Oxford University Press; 2003. [Google Scholar]
- Wilson K, Thomas MB, Blanford S, Doggett M, Simpson SJ, Moore SL. Coping with crowds: density-dependent disease resistance in desert locusts. Proc Natl Acad Sci U S A. 2002;99:5471–5475. doi: 10.1073/pnas.082461999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintour EM, Johnson K, Koukoulas I, Moritz K, Tersteeg M, Dodic M. Programming the cardiovascular system, kidney and the brain – a review. Placenta. 2003;24(Suppl. A):S65–S71. doi: 10.1053/plac.2002.0927. [DOI] [PubMed] [Google Scholar]
- Wlodek ME, Westcott KT, Serruto A, O'Dowd R, Wassef L, Ho PW, Moseley JM. Impaired mammary function and parathyroid hormone-related protein during lactation in growth-restricted spontaneously hypertensive rats. J Endocrinol. 2003;178:233–245. doi: 10.1677/joe.0.1780233. [DOI] [PubMed] [Google Scholar]