Abstract
Oestrogen dramatically increases uterine blood flow (UBF) in ovariectomized (Ovx) ewes. Both the follicular phase and pregnancy are normal physiological states with elevated levels of circulating oestrogen. ICI 182 780 is a pure steroidal oestrogen receptor (ER) antagonist that blocks oestrogenic actions in oestrogen-responsive tissue. We hypothesized that an ER-mediated mechanism is responsible for in vivo rises in UBF in physiological states of high oestrogen. The purpose of the study was to examine the effect of an ER antagonist on exogenous and endogenous oestradiol-17β (E2β)-mediated elevations in UBF. Sheep were surgically instrumented with bilateral uterine artery blood flow transducers, and uterine and femoral artery catheters. Ovx animals (n = 8) were infused with vehicle (35% ethanol) or ICI 182 780 (0.1–3.0 μg min−1) into one uterine artery for 10 min before and 50 min after E2β was given (1 μg kg−1i.v. bolus) and UBF was recorded for an additional hour. Intact, cycling sheep were synchronized to the follicular phase using progesterone, prostaglandin F2α(PGF2α) and pregnant mare serum gonadotrophin (PMSG). When peri-ovulatory rises in UBF reached near peak levels, ICI 182 780 (1 or 2 μg (ml uterine blood flow)−1) was infused unilaterally (n = 4 sheep). Ewes in the last stages of pregnancy (late pregnant ewes) were also given ICI 182 780 (0.23–2.0 μg (ml uterine blood flow)−1; 60 min infusion) into one uterine artery (n = 8 sheep). In Ovx sheep, local infusion of ICI 182 780 did not alter systemic cardiovascular parameters, such as mean arterial blood pressure or heart rate; however, it maximally decreased ipsilateral, but not contralateral, UBF vasodilatory responses to exogenous E2β by ∼55–60% (P < 0.01). In two models of elevated endogenous E2β, local ICI 182 780 infusion inhibited the elevated UBF seen in follicular phase and late pregnant ewes in a time-dependent manner by ∼60% and 37%, respectively; ipsilateral ≫ contralateral effects (P < 0.01). In late pregnant sheep ICI 182 780 also mildly and acutely (for 5–30 min) elevated mean arterial pressure and heart rate (P < 0.05). We conclude that exogenous E2β-induced increases in UBF in the Ovx animal and endogenous E2β-mediated elevations of UBF during the follicular phase and late pregnancy are partially mediated by ER-dependent mechanisms.
The classic studies of Markee (1932) showed that treatment with crude oestrogen extracts results in the vasodilatation (hypaeremia) of uterine endometrial tissue transplanted to the anterior chamber of the eye. Numerous studies have shown that the response of ovariectomized (Ovx) ewes to a low local uterine arterial (3 μg) or higher systemic (1 μg kg−1i.v.) dose of exogenous estradiol-17β (E2β) will result in a maximal and remarkably predictable pattern of increase in uterine blood flow (UBF); that is, there is a consistent delay of ∼30 min after which time UBF gradually increases and reaches a maximum value by 90–120 min (Greiss & Anderson, 1970; Huckabee et al. 1970; Killam et al. 1973; Resnik et al. 1974, 1977; Magness & Rosenfeld, 1989a; Magness et al. 1993, 1998). Indirect evidence such as the order of potency of various oestrogens suggests that the oestrogen-induced increase in UBF occurs through an oestrogen receptor (ER)-mediated mechanism. Furthermore, the pattern and efficacy of the oestrogen-induced increase in UBF is similar regardless of the oestrogen used including E2β, oestrone, oestriol, Premarin, raloxifene and extremely high doses of the anti-oestrogen trans-clomiphene (Greiss & Anderson, 1970; Killam et al. 1973; Resnik et al. 1974; Still & Greiss, 1976; Rosenfeld & Rivera, 1978; Levine et al. 1984; Zoma et al. 2000; Clark et al. 2000). The assumption that this is a receptor-mediated process was also suggested indirectly by one study in which Lineweaver-Burk plots were developed using the reciprocal of UBF responses versus the dose of oestradiol and catechol oestrogens. Because the y-axis intercepts of the two oestrogens were the same, it was suggested that these oestrogen bind to the same receptors, but have different affinities and thus vasodilatory potency as evidenced by differences in the x-axis intercepts (Rosenfeld & Jackson, 1982).
UBF fluctuates regularly during the oestrous cycle, with a substantial increase followed by a decrease during the peri-ovulatory period (Greiss & Anderson, 1969; Ford et al. 1979a, b; Ford, 1982; Roman-Ponce et al. 1983; Magness, 1990; Magness & Rosenfeld, 1992; Gibson et al. 2004). The follicular phase is the time of follicular development and E2β dominance when UBF reaches maximum levels and progesterone (P4) is virtually undetectable (Ford, 1982; Magness et al. 1991; Souza et al. 1998; Gibson et al. 2004). During pregnancy UBF is also elevated when levels of both E2β and P4 are high (Carnegie & Robertson, 1978; Ford, 1982; Roman-Ponce et al. 1983; Magness et al. 1991; for review see Magness & Rosenfeld, 1998). During the oestrous cycle and pregnancy, oestrogens bind intracellular receptor proteins, which up-regulate both ER and P4 receptor (PR) gene expression in ovine uterine epithelial and myometrial cells (Spencer & Bazer, 1995; Ing & Tornesi, 1997). After ovulation, during the luteal phase, P4 down-regulates the ER and PR in order to alter responsiveness of the uterus to both hormones. We have recently shown that the uterine artery endothelium and vascular smooth muscle of sheep have both ER subtypes, ERα and ERβ, which are regulated by endogenous (follicular and pregnancy) and exogenous steroids, suggesting that they are a target site for fluctuating oestrogen levels during the ovarian cycle (Byers et al. 2005; Liao et al. 2005).
Anti-oestrogens are classified into two major categories based on their mechanism of action. Type I anti-oestrogens are analogues of tamoxifen, also called selective oestrogen receptor modulators (SERMS). Type II are the pure anti-oestrogens, specifically ICI 164 384 and ICI 182 780 (Wakeling & Bowler, 1987; MacGregor & Jordan, 1998). SERMS are non-steroidal compounds that bind both ERα and ERβ and produce weak oestrogen agonist effects in certain tissues, while producing oestrogen antagonist effects in others (Goldstein et al. 2000). ICI 182 780 is a selective steroidal E2β antagonist that blocks oestrogen action by competing for ERs in oestrogen-responsive tissues (Wakeling & Bowler, 1992; Al-Matubsi et al. 1998).
Very limited data are available addressing the effects of ER antagonists on UBF in vivo, specifically ICI 182 780. The elevated UBF in non-pregnant Ovx sheep treated with exogenous tibolone (used as hormone-replacement therapy in postmenopausal women) (Zoma et al. 2001), was completely inhibited by ICI 182 780. There are no studies addressing the local actions of ICI 182 780 on elevated UBF with either exogenous E2β treatments in physiological states of elevated E2β such as the follicular phase of the ovarian cycle or late pregnancy in ewes. The hypothesis tested in the current study was that exogenous E2β-induced rises in UBF occur via activation of a classic ER-mediated mechanism and that the elevated endogenous E2β levels noted in the follicular phase and pregnancy do indeed serve a physiologically relevant role in maintaining elevations in UBF.
Methods
Animal preparation
Procedures for animal handling and protocols for experimental procedures were approved by the University of Wisconsin-Madison Research and Animal Care and Use Committee. As previously described (Magness et al. 1998; Gibson et al. 2004), for non-pregnant ewes, there was no designated day of the ovarian cycle for surgical instrumentation whereas the pregnant sheep underwent surgery on Day 111 ± 1 (109–116) of gestation. Ewes received ketamine (16 mg kg−1, i.m.; Fort Dodge Animal Health, Fort Dodge, IA, USA), atropine (12 μg kg−1; Sigma Chemical Co, St Louis, MO, USA) and antibiotics (400 000 U penicillin G benzathine and penicillin G procaine; H.S. Vet, Syracuse, NY, USA, and 200 mg gentamicin sulphate; Phoenix Pharmaceuticals Inc., St Joseph, MO, USA). A percutaneous jugular venous catheter was then inserted for i.v. administration of ketamine (100 mg ml−1; Fort Dodge Animal Health) in 0.9% saline and 5% dextrose with supplemental pentobarbital sodium (Nembutal; 50 mg ml−1; Sigma Chemical Co.) as needed for additional anaesthesia. Under sterile conditions, via a midventral laparotomy, transonic flow probes (3 or 4 mm in non-pregnant ewes, 6 mm in pregnant ewes; Transonic Systems Inc., Ithaca, NY, USA) were implanted around the middle uterine artery of each uterine horn as previously described (Magness et al. 1993, 1998). Polyvinyl catheters (Tygon, Cleveland, OH, USA) containing heparinized saline (25 U ml−1) were implanted retrogradely into the right and left distal branches of the uterine artery (i.d., 0.23 mm; o.d., 0.47 mm). After closure of the midline incision, a catheter (i.d., 0.40 mm; o.d., 0.70 mm) was inserted into both superficial saphenous femoral arteries through an inguinal incision and was advanced (20 cm) through the femoral circulation into the abdominal aorta. After surgery, ewes were given 75 mg flunixin meglumine (i.m., Phoenix Pharmaceuticals Inc.) analgesia and access to food and water ad libitum.
Exogenous E2β experiments in Ovx sheep model
Seven days after surgery, non-pregnant Ovx ewes (n = 8) were given E2β (1 μg (kg body weight)−1i.v.; Sigma Chemical Co) on three separate days to establish a steady uterine response over 2–3 h. For each experiment, baseline control mean arterial pressure (MAP), heart rate (HR) and UBF (Table 1) starting 30 min (averaged at 5-min intervals) prior to and for 130 min after beginning vehicle (35% ethanol in saline) or ICI 182 780 treatment. Vehicle or ICI 182 780 (ZD9238; Tocris Biosciences Inc., Baldwin, MO, USA) was unilaterally infused into one uterine artery 10 min prior to and for 50 min after 1 μg kg−1 E2β was administered i.v. In order to establish dose–response curves, the doses of ICI 182 780 infused (0, 0.1, 0.5, 1.0, 1.5, 2.0, 2.5 and 3.0 μg min−1; infusion rate, 0.103 ml min−1) and the side of infusion were randomized. Only one dose was used on a single day and when possible all doses were used on each animal preparation. ICI 182 780 studies were repeated at intervals averaging 3–4 days. Within each week we confirmed normal bilateral UBF responses to E2β alone (1 μg kg−1) for each Ovx animal, thus confirming that the preparation remained stable. Vehicle infusions did not alter the UBF response to this standard dose of E2β.
Table 1.
Baseline cardiovascular values (means ± s.e.m.) in Ovx non-pregnant, intact follicular non-pregnant, and late pregnant ewes
| Ovx | Intact | Late pregnant | |
|---|---|---|---|
| MAP (mmHg) | 87 ± 2 | 86 ± 5 | 88 ± 5 |
| HR (beats min−1) | 81 ± 3 | 88 ± 5 | 101 ± 4** |
| UVR (mmHg min ml−1) | 8.5 ± 1.5 | 3.9 ± 0.6* | 0.70 ± 0.01** |
| Total UBF (ml min−1) | 16 ± 1 | 24 ± 5* | 1255 ± 136** |
P < 0.01 versus non-pregnant sheep;
P < 0.05 intact versus Ovx;
UBF values are combined ipsilateral and contralateral flows.
Endogenous E2β experiments
Follicular phase model
We used the recently established synchronization method that we developed for studying UBF regardless of season (Gibson et al. 2004). A vaginal progesterone controlled internal drug release (CIDR; 0.3 g; Latinagro de Mexico, Monteurey, Mexico) was placed in the non-pregnant ovary-intact animals 5−7 days post surgery and after 6 days two i.m. injections (4 h apart) of PGF2 (Dinoprost Tromethamine, Lutalyse, Upjohn, Kalamazoo, MI, USA) were given. On the 7th day, baseline haemodynamic measurements were obtained over 30 min and 5-min intervals were averaged (Table 1), the CIDR was removed and 1000 IU pregnant mare serum gonadotrophin (PMSG; Sioux Biochemical Inc., Sioux Center, IA, USA) was injected i.m. Animals were monitored continuously for changes in UBF throughout these studies. ICI 182 780 (in 35% ethanol in saline) was infused at 48–50 h, or when unilateral UBF reached about 100 ml min−1. ICI 182 780 was continuously infused (infusion rate, 0.097 ml min−1) at an initial dose of 1–2 μg ml−1 unilateral calculated uterine blood levels for 2 h. This dose was chosen based on preliminary studies in which lower doses (0.2–0.3 μg ml−1; estimated to match ipsilateral uterine ICI 182 780 concentrations from the above experiments in Ovx sheep) did not reduce UBF (data not shown). Although our intent was to duplicate the studies once per animal, studies were repeated in sequential synchronized ovarian cycles in two sheep, but could not be repeated in the other two sheep due to failure of the animal preparation. The duplicated experiments were both treated statistically as subsamples and also averaged within an animal for comparisons.
Late pregnant model
Ewes in the last stages of pregnancy (late pregnant ewes) were allowed at least 7 days to recover from surgery and the first ICI 182 780 studies commenced on Day 122 ± 1.5 of gestation. Doses of ICI 182 780 (0.1–1.0 mg min−1 for 60 min; infusion rate, 0.103 ml min−1) were randomized and studies were repeated in eight animals at intervals averaging 3–4 days. This dose range was based on the above studies and was chosen in order to achieve an ipsilateral uterine blood concentration ranging from 0.23 to 2.0 μg ml−1. ICI 182 780 (in peanut oil; Astra Zenca, Pharmaceuticals, Macclesfield, Cheshire, UK) was diluted in 70% ethanol and vehicle controls were performed using peanut oil in 70% ethanol and resulted in no change in UBF. For each study, UBF was continuously monitored for 30 min (averaged every 5 min) in order to establish steady-state baseline levels of MAP, HR and UBF during late pregnancy (Table 1). ICI 182 780 was infused into one uterine artery for 120 min and MAP, HR and UBF ipsilateral and contralateral to the ICI 182 780 infusion were monitored for an additional 20–30 min.
Statistical analysis
Data were analysed by one-way or two-way ANOVA as appropriate. When experiments were repeated in the same ewe, these studies were treated as subsamples nested within the experimental unit and also averaged within an animal preparation for statistical comparisons. Both methods yielded similar conclusions. Means were compared using Duncan's multiple comparison test. Data are presented as means ± standard error of the mean (s.e.m.).
Results
Baseline haemodynamic parameters
Table 1 shows baseline systemic and uterine cardiovascular parameters prior to ICI 182 780 infusion. Although no major differences in systemic vascular parameter between Ovx and intact non-pregnant sheep were observed, total uterine perfusion was slightly higher (P < 0.05) in the intact sheep. As expected uterine vascular resistance (UVR) was greatly decreased and UBF and HR were substantially increased by pregnancy (Magness & Rosenfeld, 1988; Magness & Rosenfeld, 1992; Magness & Zheng, 1996; Rosenfeld et al. 1996, 2001).
Effects of ICI 182 780 on UBF vasodilatory responses to exogenous E2β in non-pregnant Ovx ewes
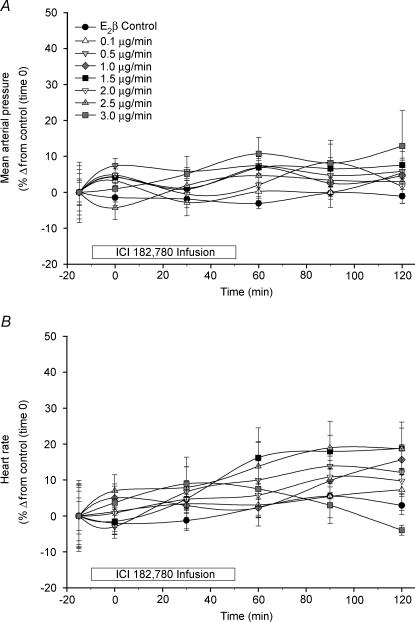
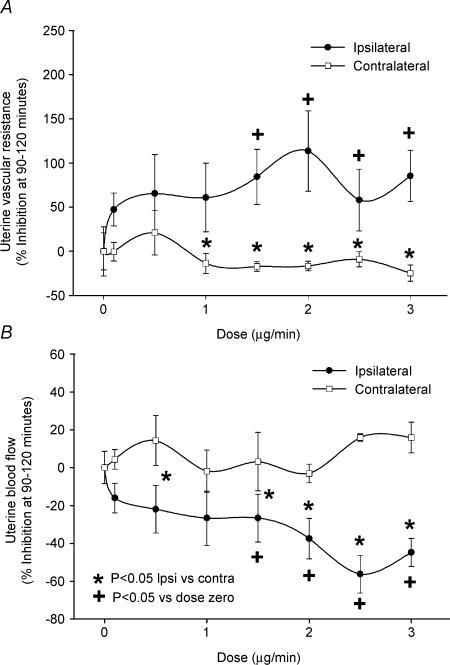
The effects of local unilateral infusion of ICI 182 780 (0.1–3.0 μg min−1) on systemic cardiovascular parameters (MAP and HR) are shown in Fig. 1. During the infusion of ICI 182,780, there were no significant alterations in either MAP or HR in the absence or presence of E2β treatment. The UBF response to systemic E2β (1 μg kg−1) was repeatedly weekly and was relatively consistent throughout the study demonstrating stability of the animal preparation. When all of these standard oestrogen responses were averaged, as expected, systemic administration of E2β alone increased unilateral UBF from 8 ± 1 ml min−1 at control (time zero) to 105 ± 7 ml min−1 (P < 0.01) at 90–120 min (10- to 13-fold increase) (n = 8). Table 2 illustrates the time effects within dose ranges of unilateral ICI 182 780 infusion on ipsilateral versus contralateral UVR and UBF responses to E2β. Unilateral infusion of ICI 182 780 dose-dependently increased the ipsilateral UVR and reduced the ipsilateral UBF vasodilatory response to systemic E2β. At the two highest doses of ICI 182 780, the ipsilateral maximum UVR/UBF inhibition of the oestrogen vasodilatory response was observed to plateau during the 90–120 min steady state demonstrating that maximum inhibition was indeed achieved (P < 0.01). Ipsilateral ICI 182 780 responses were considerably greater than contralateral responses, which ICI 182 780 did not appreciably affect. In order to determine the uterine inhibition by ICI 182 780 relative to maximum oestrogen-mediated vasodilatation, percentage inhibition was also calculated based upon each animal's previous weekly averaged steady-state (90–120 min mean at 5-min intervals) overall responses to the systemic dose of 1 μg kg−1 E2β. We show the relative change in ipsilateral versus contralateral UVR and UBF as a function of dose in Fig. 2. Ipsilateral UBF inhibition responses for ICI 182 780 were dose-dependent (P < 0.05) and exceeded the contralateral responses throughout the study, but were greatest at 2.5–3.0 μg min−1 averaging 56 ± 10%. Maximum ipsilateral ICI 182 780 responses were achieved as no differences were noted between the two highest doses.
Figure 1. The effects of local infusion of ICI 182 780 (0.1–3.0 μg min−1) on MAP (A) and HR (B) in Ovx E2β-treated ewes (n = 8).
ICI 182 780 was infused from time −10 min to 50 min, and E2β (1 μg kg−1i.v.) was given at time zero. ICI 182 780 had no effect on MAP or HR at any dose. Values are means ± s.e.m.
Table 2.
Dose and time course effects of unilateral ICI 182 780 infusion on uterine vascular resistance and uterine blood flow responses in E2β (1 μg kg−1)-treated Ovx non-pregnant sheep (n = 8)
| ICI Dose (μg min−1) | −30 min | 0 | 30 min | 60 min | 90 min | 120 min |
|---|---|---|---|---|---|---|
| Ipsilateral uterine vascular resistance (mmHg min ml−1) | ||||||
| Zero | 8.5 ± 1.5a | 9.8 ± 0.7a | 7.1 ± 0.7a | 1.4 ± 0.1a | 0.8 ± 0.01a | 0.7 ± 0.01a |
| 0.1–0.5 | 7.6 ± 1.4a | 5.8 ± 0.7b | 5.6 ± 0.8ab | 3.3 ± 1.0b | 1.4 ± 0.2b* | 1.2 ± 0.2b* |
| 1.0–1.5 | 10.3 ± 2.0a | 6.0 ± 0.9ab | 5.3 ± 1.1ab | 3.1 ± 0.9b* | 2.2 ± 0.7bc* | 2.6 ± 0.7c* |
| 2.0–3.0 | 7.3 ± 0.8a | 10.4 ± 4.0a | 5.0 ± 0.6b | 2.4 ± 0.4b* | 2.0 ± 0.3c* | 2.2 ± 0.6c* |
| Contralateral uterine vascular resistance (mmHg min ml−1) | ||||||
| Zero | 8.5 ± 1.5a | 9.8 ± 0.7a | 7.1 ± 0.7a | 1.4 ± 0.1a | 0.8 ± 0.01a | 0.7 ± 0.01a |
| 0.1–0.5 | 7.5 ± 1.0a | 9.0 ± 1.2a | 8.1 ± 1.3a | 2.3 ± 0.1b | 0.9 ± 0.1a | 0.7 ± 0.1a |
| 1.0–1.5 | 9.3 ± 1.7a | 8.2 ± 1.1a | 6.9 ± 1.4a | 1.2 ± 0.2a | 1.0 ± 0.4a | 0.7 ± 0.1a |
| 2.0–3.0 | 8.7 ± 1.0a | 8.8 ± 0.9a | 6.1 ± 0.6a | 1.2 ± 0.1a | 0.8 ± 0.1a | 0.7 ± 0.1a |
| Ipsilateral uterine blood flow (ml min−1) | ||||||
| Zero | 8 ± 1a | 9 ± 1a | 14 ± 1a | 65 ± 5a | 102 ± 7a | 107 ± 7a |
| 0.1–0.5 | 13 ± 2b | 17 ± 3b | 14 ± 3ab | 45 ± 9b | 71 ± 11ab | 88 ± 13a |
| 1.0–1.5 | 11 ± 2ab | 16 ± 2b | 22 ± 4b | 48 ± 9b | 63 ± 10b* | 57 ± 10b* |
| 2.0–3.0 | 13 ± 1b | 13 ± 2ab | 18 ± 2ab | 43 ± 6b* | 53 ± 9b* | 52 ± 8b* |
| Contralateral uterine blood flow (ml min−1) | ||||||
| Zero | 8 ± 1a | 9 ± 1a | 14 ± 1a | 65 ± 5a | 102 ± 7a | 107 ± 7a |
| 0.1–0.5 | 10 ± 2a | 9 ± 2a | 10 ± 2a | 41 ± 8b | 87 ± 15a | 109 ± 18a |
| 1.0–1.5 | 9 ± 2a | 9 ± 2a | 13 ± 2a | 65 ± 11a | 103 ± 19a | 107 ± 17a |
| 2.0–3.0 | 9 ± 1a | 9 ± 1a | 13 ± 2a | 67 ± 6a | 106 ± 11a | 119 ± 12a |
Values are means ± s.e.m.;
values with different letter superscripts are significantly different (P < 0.05);
P < 0.05 Ipsilateral ≠ Contralateral.
Figure 2. Relative changes in ipsilateral versus contralateral UVR (A) and UBF (B) as a function of ICI 182 780 dose in E2β-treated Ovx ewes (n = 8).
These data were calculated relative to control UVR and UBF responses averaged (5-min intervals, 90–120 min) across the steady-state 90–120 min plateau in uterine vasodilatory responses to E2β. Ipsilateral ICI 182 780-related inhibition was dose-dependent (+P < 0.05 versus zero dose) and exceeded (*P < 0.05) the contralateral response to E2β but was greatest reaching a plateau at 2.5–3.0 μg min−1 (56 ± 10%). Values are means ± s.e.m.
Effects of ICI 182 780 on endogenous E2β UBF vasodilatory responses in intact sheep
Follicular phase experiments
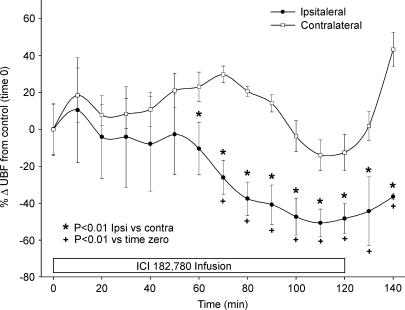
All experiments in the four animals studied resulted in ipsilateral decreases in UBF in response to ICI 182 780 infusions (1–2 μg ml−1 unilateral UBF). Each animal responded somewhat uniquely and with a slightly different percentage change from the time zero control; however, each animal did eventually respond ipsilaterally and to a much lesser degree (or not at all), contralaterally (data not shown). In one experiment ipsilateral UBF did not succumb to the effects of ICI 182 780 until approximately 180 min; that is, approximately 60 min after the infusion had stopped (data not shown). In Fig. 3 we show the mean response (± s.e.m.) for the four sheep (repeated experiments averaged within individual animal) in which ipsilateral UBF was inhibited during the infusion of oestrogen receptor antagonist. ICI 182 780 was infused for 120 min in each experiment and the fall in ipsilateral UBF was observed within 40–80 min; however, significant reductions (P < 0.01) in ipsilateral UBF were first noted at 70 min and were maintained throughout the entire study period. During the administration of ICI 182 780, ipsilateral UBF was reduced by an average of 60 ± 8% (P < 0.01), which was similar (P > 0.05) to the maximum inhibition of 56 ± 10% seen in Ovx E2β–treated sheep at the top of the dose–response curve. The fall in ipsilateral UBF was significantly different from contralateral UBF responses by 60 min of ICI 182 780 infusion and never recovered maximal UBF. Contralateral UBF responses were not reduced below baseline throughout experimentation.
Figure 3. Effects of unilateral uterine artery infusion of ICI 182 780 on ipsilateral versus contralateral UBF in follicular phase ewes (n = 4).
The studies that were repeated during the unilateral infusion of ICI 182 780 (1–2 μg ml−1 ipsilateral uterine blood concentrations) were averaged within an animal and then meaned. Ipsilateral UBF was decreased (≥ 70 min; P > 0.05) by a maximum average of 60 ± 8% (P < 0.01) and became significantly different (*P < 0.01) from contralateral UBF at approximately 60 min. Values are means ± s.e.m.
Late pregnancy experiments
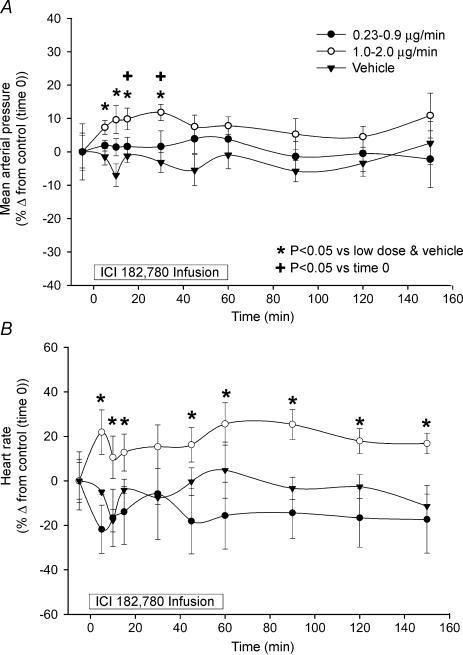
The systemic cardiovascular effects of unilateral infusion of ICI 182 780 in pregnant sheep are shown (Fig. 4). MAP and HR were not altered during infusion of either the vehicle or low doses of ICI 182 780. However, with the high doses of ICI 182 780, MAP began to increase by 5 min reaching significance, versus time 0 control, at 15 and 30 min (P < 0.05), but returned to control values by 45 min even though the ICI 182 780 infusion continued until 60 min. When MAP was compared both to the low doses of ICI 182 780 and vehicle, it was elevated from 5 to 30 min (P < 0.05). For the HR responses to the high doses of ICI 182 780, when compared to the time zero control, it was unaltered throughout the study in all groups. In contrast, HR values were elevated during high dose ICI 182 780 infusion compared to low dose ICI 182 780 and vehicle infusion, and remained so throughout the study (P < 0.05).
Figure 4. The systemic cardiovascular effects of unilateral uterine artery infusion of ICI 182 780 in late pregnant sheep (n = 7).
A, with the higher doses of ICI 182 780, MAP began to increase by 5 min reaching significance versus time 0 control at 15 and 30 min (*P < 0.05), but returned to control values by 45 min (P > 0.05). When compared both to the low doses of ICI 182 780 and vehicle, MAP was elevated from 5 to 30 min (+P < 0.05). B, when compared to the zero control, HR was unaltered throughout the study in all groups (P > 0.05). However, HR values were elevated during infusion of high dose ICI 182 780 compared to low dose ICI 182 780 and vehicle (*P < 0.05). Values are means ± s.e.m.
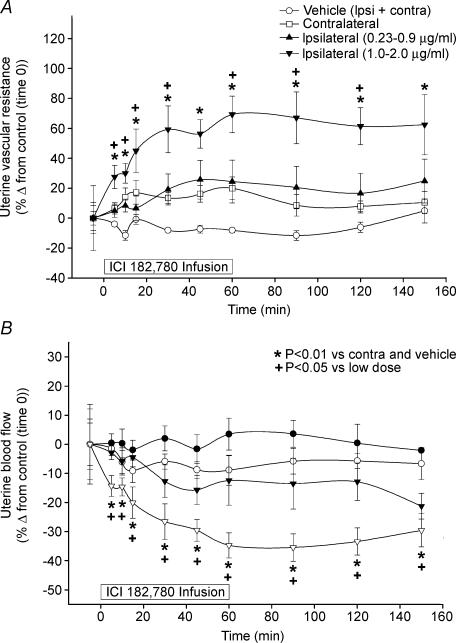
Uterine vascular responses to unilateral infusion of vehicle or ICI 182 780 are shown in Fig. 5. Neither UVR nor UBF were significantly altered by either the vehicle or the lower doses of ICI 182 780. In contrast with the high doses of ICI 182 780, UVR was elevated and UBF was decreased, but only in the ipsilateral uterine artery (P < 0.05). The increases in UVR and decreases in UBF were observed as early as 5 min, increased to significant levels by 30 min and were maintained throughout the remainder of the experiment (P < 0.01). These ipsilateral uterine responses not only exceeded those of the contralateral responses, but also the vehicle responses throughout the studies. Using the high doses of ICI 182 780, we were able to reduce ipsilateral UBF by 37 ± 4% (P < 0.01) from an average baseline (control) ipsilateral UBF of 607 ± 39 ml min−1. Moreover in these studies, two of the pregnant sheep had singleton pregnancies and the rest carried twins. There were no overt trends for a gravid versus non-gravid uterine horn effect of responses to ICI 182 780.
Figure 5. Time course of UVR responses to unilateral uterine artery infusion of ICI 182 780 (for 60 min) in late pregnant ewes (n = 8).
A, the higher doses of 1.0–2.0 μg ml−1 resulted in significantly higher UVR almost immediately compared to the lower doses (0.23–0.9 μg ml−1) as well as vehicle and contralateral controls. B, all ewes (n = 8) exhibited significant ipsilateral decreases in UBF in response to the higher doses of unilateral ICI 182 780 infusions. The average baseline (control) ipsilateral UBF was 607 ± 39 ml min−1. We were able to inhibit ipsilateral UBF by an overall average of 37 ± 4% (P < 0.01). Values are means ± s.e.m.
Discussion
Using three physiological animal models for studying the effects of elevated oestrogen on UBF in sheep we have shown that the pure ER antagonist ICI 182 780, when locally infused into the uterine artery, partially (∼35–60%) inhibits ipsilateral, but not contralateral UBF responses to both exogenous and endogenous oestrogen. This is the first report showing that ICI 182 780 locally inhibits UBF responses to exogenous E2β as well as to endogenous ovarian (follicular) and/or placental (pregnant) oestrogen. Zoma et al. (2001) reported that the UBF vasodilatory effects of tibolone, an oestrogenic hormone-replacement therapy (HRT) compound with androgen and progestagen properties were completely inhibited by systemic ICI 182 780 in Ovx sheep. In contrast to these tibolone responses, we only observed a maximum ipsilateral inhibition of 56 ± 10% of the UBF response to systemic E2β at the highest two local doses of infused ICI 182 780. Differences are likely to be because tibilone is not a pure oestrogenic compound and Zoma et al. (2001) administered ICI 182 780 systemically.
There are several other studies addressing other vascular effects of ICI 182 780 in rabbits, dogs, pigs, mice and rats (Sudhir et al. 1995; Hegele-Hartung et al. 1997; Teoh et al. 1999; Sawada et al. 2000; Zhai et al. 2001). Zhai et al. (2001) showed in rats that the cardiovascular protective effects of phyto-oestrogens, natural non-steroidal plant-derived compounds with structures similar to oestrogen which bind ERs (Kuiper et al. 1998), were partially blocked by ICI 182 780. Sudhir et al. (1995) used dogs and showed using high doses of E2β that l-NAME, indomethacin or ICI 182 780 did not attenuate the in vivo vasodilatory effects of E2β-induced conductance resistance coronary vessel dilatation, and suggested that the effects of E2β were not mediated via NO or prostaglandin release in the epicardial circulation nor the classic intracellular oestrogen receptor. However this conclusion is diametrically opposed to the present observations and those of Zoma et al. (2001) who observed that ICI 182 780 fully blocked the tibilone-related responses in both coronary and uterine circulation. In addition, Mershon et al. (2002) reported that E2β-induced increases in coronary blood flow were completely inhibited by systemic administration of ICI 182 780. Hegele-Hartung et al. (1997) also used ICI 182 780 in Ovx oestrogen-treated rabbits and observed that ICI 182 780 dose-dependently reversed the effects of long-term treatment (14 days) of E2β on rises in aortic blood flow.
Recently we reported that UBF responses to exogenous E2β are considerably more rapid and higher in both Ovx and intact ewes than those during the follicular phase in response to elevated levels of ovarian-derived oestrogen (Gibson et al. 2004). We also demonstrated that this peri-ovulatory model could be utilized to test local antagonism of vasodilator pathways (e.g. NO) in the uterine circulation. The current study provides the first report that unilateral UBF in follicular phase ewes is reduced by ∼60% using doses of ICI 182 780 ranging from 1 to 2 μg ml−1 ipsilateral UBF with no significant contralateral (i.e. systemic) UBF effects. Because we did not observe differences in the peri-ovulatory UBF responses to the aforementioned dose range of ICI 182 780, and the relative inhibition of the elevated UBF in the follicular model by 60 ± 8% is similar to the maximum dose-related inhibition in the Ovx E2β-treated sheep of 56 ± 10%, these data collectively suggest that under these experimental conditions we were indeed at the top of the dose–response relationship. Although it is unknown at what dose ICI 182 780 will show consistent and substantial contralateral effects, due to systemic recirculation of ICI 182 780, in either the follicular or Ovx E2β-treated sheep, both of these non-pregnant models demonstrate that exogenous E2β and endogenous ovarian-derived oestrogen are indeed responsible for the observed ER-mediated UBF responses. However, as a caveat to the relatively modest systemic concentrations of E2β achieved during the follicular phase of the ovarian cycle (Gibson et al. 2004), we cannot rule out the possibly that there are local mechanisms for maintaining local oestrogen levels in the uterine horns adjacent to the ovaries containing oestrogen-producing follicles as previously suggested (Magness & Ford, 1982; Magness et al. 1991).
Pregnancy is another physiological state of elevated oestrogen; however, P4 is increased as well (Carnegie & Robertson, 1978; Ford, 1982; Magness et al. 1991; for review see Magness & Rosenfeld, 1989). It is unlikely, however, that P4 plays a major role in the uterine vasodilatation seen in pregnancy except to maintain normal uterine quiescence (and thus a healthy gestation; Magness & Zheng, 1996) or to elevate/maintain uterine artery ERα and ERβ endothelial and vascular smooth muscle (VSM) receptors (Byers et al. 2005). This derives from numerous studies, which showed that P4 does not increase UBF in non-pregnant or pregnant sheep (Greiss & Anderson, 1970; Ford, 1982; Resnik et al. 1977; Magness & Rosenfeld, 1989a; Magness, 1990). As seen in the two non-pregnant animal models, in late pregnant ewes we report partial local unilateral inhibition of UBF, but no contralateral effect of ICI 182 780. Inhibition was shown to be dose-dependent because the lower doses (0.23–1.0 μg ml−1) had no effects whatsoever and the higher doses (1.0–2.0 μg ml−1) only reduced ipsilateral, but not contralateral UBF by ∼30–35%. When we calculated the maximum steady-state decrease in ipsilateral UBF 60–120 min after beginning the ICI 182 780 infusion, we observed an average decrease of 37%. This reduction of ipsilateral UBF with doses of 1–2 μg ml−1 ipsilateral uterine blood levels was not different, demonstrating that we were indeed at the upper end of a rather narrow dose–response curve and that we did achieve maximum falls in UBF. Although no contralateral UBF effects were observed in the late pregnant sheep studies, we observed that ICI 182 780 increased MAP and HR acutely (5–30 min), and both parameters returned to control levels by ∼45 min. Thus elevated levels of oestrogen in late pregnancy (Carnegie & Robertson, 1978; Magness, 1998; Magness et al. 1991) partly maintain MAP at a relatively reduced level. This systemic cardiovascular effect of ICI 182 780 was specific to pregnancy, as we did not observe an increase in MAP during ICI 182 780 infusion in Ovx non-pregnant sheep. During infusion of the high dose range of ICI 182 780, HR increased and remained so throughout the study. Thus, acute increases in MAP may be due to an increase in cardiac output, and decreases in MAP back to non-significant levels may be a peripheral reflex action in the systemic circulation to buffer further rises in cardiac output (Magness & Rosenfeld, 1988; Magness et al. 1993). Because we do not know what cardiac output changes occurred, we are unable to make definitive conclusions as to whether the systemic cardiovascular changes we observed in the pregnant sheep are due to peripheral or cardiac responses to infused ICI 182 780.
Contrasting the two endogenous oestrogen models (follicular and pregnant) with the Ovx exogenous oestrogen-treated model, the latter Ovx animals were pretreated with ICI 182 780, in studies designed to block the ERs from being occupied prior to pharmacological treatment with E2β. In contrast with the endogenous oestrogen models, because it is not practical to infuse the inhibitor for extended periods of time due to a limited supply of the drug, the ICI 182 780 was infused after UBF was already elevated. We believe this difference accounts for the observation that it only required 0.2–0.3 μg ml−1 ICI 182 780 in the ipsilateral uterine blood in the Ovx E2β-treated sheep, in contrast to the 1–2 μg ml−1 ICI 182 780 in the two endogenous oestrogen models to attain the observed maximum reductions of UBF; the reason for this nearly 8-fold difference in the doses of ICI 182 780 is unclear. However, our recent data showing that protein expression of uterine artery endothelial ER-β in Ovx sheep is lower than in intact luteal, follicular and pregnant sheep and that ERα and/or ER-β are elevated in follicular and pregnant sheep (Byers et al. 2005) suggests that it simply may be a reflection of the fact that in Ovx sheep less ICI 182 780 is required to compete with the bound E2β on ERs due to the lower level of E2β. Additionally, during the 2-h time course of UBF measurement, uterine artery ERs may be degraded by exogenous oestrogen stimulation, whereas in follicular phase and pregnant sheep cyclically and chronically primed with endogenous oestrogen and P4, their receptors may not be degraded to as great an extent. It has been recently established that upon ligand stimulation, rapid (< 2 h) degradation of ERs occurs via the proteasome-mediated ubiquitination pathway (Preisler-Mashek et al. 2002). A similar proteasome-dependent ERα and ERβ turnover has been recently reported in cultured human uterine artery endothelial cells (Tschugguel et al. 2003) and fetal sheep pulmonary artery endothelial cells (Ihionkhan et al. 2002). It is likely that in the follicular and pregnant models, the antagonist either displaced ER occupied by ligand, or with the rapid turnover of the ERs, ICI 182 780 in turn occupied these ERs. Regardless, in an analogous fashion, infusions of l-NAME, like ICI 182 780, will decrease UBF whether given before exogenous oestrogen treatment or after UBF was elevated by exogenous E2β (Van Buren et al. 1992; Rosenfeld et al. 1996) and endogenous follicular (Gibson et al. 2004) and pregnancy-related (Miller et al. 1999) oestrogen. Thus the oestogen-induction and maintenance of the rise in UBF is via both an ER and NO-mediated mechanism.
One intriguing question remains: if the vasodilatory effects of oestrogen are mediated by ERs, why can only 50–65% inhibition of the E2β-induced rise in UBF be achieved with ICI 182 780? Our data suggest that classical ER-mediated and NO-dependent mechanisms cannot fully explain the rapid uterine vasodilator effects of oestrogen. A recent report has shown that a seven transmembrane G-protein-coupled receptor (GPR30) functions as a membrane receptor for oestrogens. Ligand-binding analysis revealed that GPR30 interacts with various oestrogens (E2β, oestrone, oestriol and phytoestrogens) and anti-oestrogens (ICI 182 780 and tamoxifen) with high affinity (Qiu et al. 2003; Thomas et al. 2005). Similar to the classical ERs (α and β) localized on the plasma membrane (Chambliss et al. 2002; Chen et al. 2004), upon ligand binding this receptor can also initiate various rapid oestrogen signalling mechanisms (Thomas et al. 2005) that are inhibited by ICI 182 780. Thus the remaining non-ICI 182 780-dependent, oestrogen-induced UBF responses suggest a non-ER mediated aetiology.
Some mechanisms other than the ones stimulated by acute ER activation may account for the remaining rises in UBF. We and others have mainly used the Ovx model to test the role of numerous mechanisms on E2β-dependent rises in UBF (Magness & Rosenfeld, 1989a). Only l-NAME (Van Buren et al. 1992; Rosenfeld et al. 1996), cycloheximide (Killam et al. 1973), glucocorticoids (Monheit & Resnik, 1981) and the potassium channel blocker TEA (Rosenfeld et al. 2000, 2002) have been shown to greatly inhibit the E2β-induced rise in UBF in the Ovx model. Moreover, we reported that in follicular phase ewes, endothelial nitric oxide synthase (eNOS) activation to increase NO production is also significantly, but not totally, responsible for rises in UBF (Gibson et al. 2004). Specifically, in these studies only ∼60% inhibition of UBF was noted in follicular phase (Gibson et al. 2004) and Ovx E2β-treated (Van Buren et al. 1992; Rosenfeld et al. 1996) ewes infused with l-NAME. It is remarkable that these decreases are similar to the currently reported ∼55–60% decrease in UBF with ICI 182 780, suggesting that the ER activation may be responsible for the l-NAME-sensitive, NO-mediated component of the E2β-induced elevation of UBF. Support for this includes observations that eNOS is localized mainly to uterine artery (UA) endothelium (Magness et al. 1996, 1997, 2001; Vagnoni et al. 1998; Rupnow et al. 2001) and we recently showed that ICI 182 780 inhibits the E2β-induced rises in uterine artery endothelial cell (UAEC) de novo NO production (Chen et al. 2004). It was suggested that the additional 40–45% of the UBF response was mediated by inducible NOS (iNOS) as oestrogen increases iNOS expression in the coronary artery from Ovx ewes (Mershon et al. 2002). These investigators also showed that ICI 182 780 or dexamethasone (a putative non-specific inhibitor of iNOS transcription) alone did not completely obliterate the E2β-induced rises in coronary blood flow; however, combination therapy completely eliminated this response. Similar responses may be seen in the uterine vascular bed (K.E. Clark, personal communication). Additionally, Monheit & Resnik (1981) infused hydrocortisone and reduced UBF in Ovx E2β-treated ewes by 44%. However, further studies are needed as iNOS is not expressed in substantial quantities in UA endothelium or VSM in non-pregnant oestrogen-treated sheep (Vagnoni et al. 1998; Salhab et al. 2000; Zheng et al. 2000). Others have suggested that the remaining 30–40% of UBF that cannot be inhibited by l-NAME might be due to calcium-activated potassium channels expressed on uterine VSM cells (Rosenfeld et al. 2000, 2002). They found that the combination of TEA, an inhibitor of the calcium-activated potassium channel (BKca), and l-NAME completely inhibited the E2β-induced rise in UBF in Ovx (Rosenfeld et al. 2000). In a related study using E2β treated late pregnant ewes, they reported that TEA inhibited basal uteroplacental blood flow as well as approximately 50% of the rise in exogenous E2β-mediated UBF (Rosenfeld et al. 2001). Moreover as seen for the non-pregnant ovine models, data for the current ICI 182 780 study and those using l-NAME in the pregnant sheep (Miller et al. 1999) showed similar decreases in UBF of 30–35%. Therefore we believe that the ER-mediated activation of eNOS during the latter portion of gestation can account for only a limited amount of the elevations in UBF in pregnancy. As suggested above, either iNOS or BKca via a TEA-sensitive mechanism may regulate the additional 60–70% of the oestrogen-related UBF elevation in pregnancy. The former supposition may be unlikely as dexamethasone does not appreciably decrease UBF in pregnant sheep (Edelstone et al. 1978). Alternatively the majority of the rise in UBF in pregnancy (Magness & Zheng, 1996; Reynolds & Redmer, 2001), unlike the follicular phase (Reynolds et al. 1998), is due to angiogenic and vascular growth processes, which occur many months prior to the time of gestation at which we performed the current studies and are unlikely to be affected by acute ICI 182 780 treatments.
Acknowledgments
We thank Cindy Goss for secretarial help in the preparation of this manuscript and Dr Alan E. Wakeling for the gift of the ICI 182 780 preparation for the pregnancy studies. This work was supported, in part, by National Institutes of Health Grants HL49210, HL57653, HD33255 and HD38843 (R.R.M.), and HL70562 and HL74947 (D.-b.C.). This study is in partial fulfillment for an MS degree (T.C.G.) in the Endocrinology–Reproductive Physiology Training Program (http://www.erp.wisc.edu).
References
- Al-Matubsi HY, Fairclough RJ, Jenkin G. Oestrogenic effects of ICI 182,780, a putative anti-oestrogen, on the secretion of oxytocin and prostaglandin F2 alpha during oestrous cycle in the intact ewe. Anim Reprod Sci. 1998;51:81–96. doi: 10.1016/s0378-4320(98)00068-2. 10.1016/S0378-4320(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Byers MJ, Zangl A, Phernetton TM, Lopez G, Chen DB, Magness RR. Endothelial vasodilator production by uterine and systemic arteries X: ovarian steroid and pregnancy control of ERa and ERb levels. J Physiol. 2005;000:000–000. doi: 10.1113/jphysiol.2005.085753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnegie JA, Robertson HA. Conjugated and unconjugated estrogens in fetal and maternal fluids of the pregnant ewe: a possible role for estrone sulfate during early pregnancy. Biol Reprod. 1978;19:202–211. doi: 10.1095/biolreprod19.1.202. [DOI] [PubMed] [Google Scholar]
- Chambliss KL, Yuhanna IS, Anderson RG, Mendelsohn ME, Shaul PW. ERbeta has nongenomic action in caveolae. Mol Endocrinol. 2002;16:938–946. doi: 10.1210/mend.16.5.0827. [DOI] [PubMed] [Google Scholar]
- Chen DB, Bird IM, Zheng J, Magness RR. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology. 2004;145:113–125. doi: 10.1210/en.2003-0547. [DOI] [PubMed] [Google Scholar]
- Clark KE, Baker RS, Lang U. Premarin-induced increases in coronary and uterine blood flow in nonpregnant sheep. Am J Obstet Gynecol. 2000;183:12–17. doi: 10.1067/mob.2000.105200. [DOI] [PubMed] [Google Scholar]
- Edelstone DI, Botti JJ, Mueller-Heubach E, Caritis SN. Response of the circulation of pregnant sheep to angiotensin and norepinephrine before and after dexamethasone. Am J Obstet Gynecol. 1978;130:689–692. doi: 10.1016/0002-9378(78)90329-0. [DOI] [PubMed] [Google Scholar]
- Ford SP. Control of uterine and ovarian blood flow throughout the estrous cycle and pregnancy of ewes, sows and cows. J Anim Sci. 1982;55(Suppl.2):32–42. [PubMed] [Google Scholar]
- Ford SP, Chenault JR, Echternkamp SE. Uterine blood flow of cows during the oestrous cycle and early pregnancy: effect of the conceptus on the uterine blood supply. J Reprod Fertil. 1979a;56:53–62. doi: 10.1530/jrf.0.0560053. [DOI] [PubMed] [Google Scholar]
- Ford SP, Christenson RK, Chenault JR. Patterns of blood flow to the uterus and ovaries of ewes during the period of luteal regression. J Anim Sci. 1979b;49:1510–1516. doi: 10.2527/jas1979.4961510x. [DOI] [PubMed] [Google Scholar]
- Gibson TC, Phernetton TM, Wiltbank MC, Magness RR. Development and use of an ovarian synchronization model to study the effects of endogenous estrogen and nitric oxide on uterine blood flow during ovarian cycles in sheep. Biol Reprod. 2004;70:1886–1894. doi: 10.1095/biolreprod.103.019901. 10.1095/biolreprod.103.019901. [DOI] [PubMed] [Google Scholar]
- Goldstein SR, Siddhanti S, Ciaccia AV, Plouffe L., Jr A pharmacological review of selective oestrogen receptor modulators. Hum Reprod Update. 2000;6:212–224. doi: 10.1093/humupd/6.3.212. 10.1093/humupd/6.3.212. [DOI] [PubMed] [Google Scholar]
- Greiss FC, Anderson SG. Uterine vascular changes during the ovarian cycle. Am J Obstet Gynecol. 1969;103:629–640. doi: 10.1016/0002-9378(69)90560-2. [DOI] [PubMed] [Google Scholar]
- Greiss FC, Jr, Anderson SG. Effect of ovarian hormones on the uterine vascular bed. Am J Obstet Gynecol. 1970;107:829–836. doi: 10.1016/s0002-9378(16)34033-9. [DOI] [PubMed] [Google Scholar]
- Hegele-Hartung C, Fritzemeier KH, Diel P. Effects of a pure antiestrogen and progesterone on estrogen-mediated alterations of blood flow and progesterone receptor expression in the aorta of ovariectomized rabbits. J Steroid Biochem Mol Biol. 1997;63:237–249. doi: 10.1016/s0960-0760(97)00125-8. 10.1016/S0960-0760(97)00125-8. [DOI] [PubMed] [Google Scholar]
- Huckabee WE, Crenshaw C, Curet LB, Mann L, Barron DH. The effect of exogenous oestrogen on the blood flow and oxygen consumption of the uterus of the non-pregnant ewe. Q J Exp Physiol Cogn Med Sci. 1970;55:16–24. doi: 10.1113/expphysiol.1970.sp002046. [DOI] [PubMed] [Google Scholar]
- Ihionkhan CE, Chambliss KL, Gibson LL, Hahner LD, Mendelsohn ME, Shaul PW. Estrogen causes dynamic alterations in endothelial estrogen receptor expression. Circ Res. 2002;91:814–820. doi: 10.1161/01.res.0000038304.62046.4c. 10.1161/01.RES.0000038304.62046.4C. [DOI] [PubMed] [Google Scholar]
- Ing NH, Tornesi MB. Estradiol up-regulates estrogen receptor and progesterone receptor gene expression in specific ovine uterine cells. Biol Reprod. 1997;56:1205–1215. doi: 10.1095/biolreprod56.5.1205. [DOI] [PubMed] [Google Scholar]
- Killam AP, Rosenfeld CR, Battaglia FC, Makowski EL, Meschia G. Effect of estrogens on the uterine blood flow of oophorectomized ewes. Am J Obstet Gynecol. 1973;115:1045–1052. doi: 10.1016/0002-9378(73)90552-8. [DOI] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–4263. doi: 10.1210/endo.139.10.6216. 10.1210/en.139.10.4252. [DOI] [PubMed] [Google Scholar]
- Levine MG, Miodovnik M, Clark KE. Uterine vascular effects of estetrol in nonpregnant ewes. Am J Obstet Gynecol. 1984;148:735–738. doi: 10.1016/0002-9378(84)90557-x. [DOI] [PubMed] [Google Scholar]
- Liao WX, Magness RR, Chen DB. Expression of estrogen receptors-α and β in the pregnant ovine uterine artery endothelial cells in vivo and in vitro. Biol Reprod. 2005;72:530–537. doi: 10.1095/biolreprod.104.035949. 10.1095/biolreprod.104.035949. [DOI] [PubMed] [Google Scholar]
- MacGregor JI, Jordan VC. Basic guide to the mechanisms of antiestrogen action. Pharmacol Rev. 1998;50:151–196. [PubMed] [Google Scholar]
- Magness RR. Ovarian Secretions and Cardiovascular and Neurological Function, Serono Foundation Symposia. Vol. 80. Norwell, MA, USA: Raven Press; 1990. Ovarian secretion and vascular function; pp. 93–125. [Google Scholar]
- Magness RR, Ford SP. Steroid concentrations in uterine lymph and uterine arterial plasma of gilts during the estrous cycle and early pregnancy. Biol Reprod. 1982;27:871–877. doi: 10.1095/biolreprod27.4.871. [DOI] [PubMed] [Google Scholar]
- Magness RR, Ford SP. Estrone, estradiol-17 beta and progesterone concentrations in uterine lymph and systemic blood throughout the porcine estrous cycle. J Anim Sci. 1983;57:449–455. doi: 10.2527/jas1983.572449x. [DOI] [PubMed] [Google Scholar]
- Magness RR, Parker CR, Jr, Rosenfeld CR. Systemic and uterine responses to chronic infusion of estradiol-17 beta. Am J Physiol. 1993;265:E690–E698. doi: 10.1152/ajpendo.1993.265.5.E690. [DOI] [PubMed] [Google Scholar]
- Magness RR, Phernetton TM, Zheng J. Systemic and uterine blood flow distribution during prolonged infusion of 17beta-estradiol. Am J Physiol. 1998;275:H731–H743. doi: 10.1152/ajpheart.1998.275.3.H731. [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR. Mechanisms for attenuated pressor responses to alpha-agonists in ovine pregnancy. Am J Obstet Gynecol. 1988;159:252–261. doi: 10.1016/0002-9378(88)90531-5. [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR. The role of steroid hormones in the control of uterine blood flow. In: Rosenfeld CR, editor. Reproductive and Perinatal Medicine. X. Ithaca, NY: Perinatoogy Press; 1989a. pp. 234–271. [Google Scholar]
- Magness RR, Rosenfeld CR. Local and systemic estradiol-17 beta: effects on uterine and systemic vasodilation. Am J Physiol. 1989b;256:E536–E542. doi: 10.1152/ajpendo.1989.256.4.E536. [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR. Endometrial Function and Dysfunctional Uterine Bleeding. Washington DC: American Association for the Advancement of Science Press; 1992. Steroid control of blood vessel function; pp. 107–120. Meeting NACDA-NIH. [Google Scholar]
- Magness RR, Rosenfeld CR, Carr BR. Protein kinase C in uterine and systemic arteries during ovarian cycle and pregnancy. Am J Physiol. 1991;260:E464–E470. doi: 10.1152/ajpendo.1991.260.3.E464. [DOI] [PubMed] [Google Scholar]
- Magness RR, Rosenfeld CR, Hassan A, Shaul PW. Endothelial vasodilator production by uterine and systemic arteries. I. Effects of ANG II on PGI2 and NO in pregnancy. Am J Physiol. 1996;270:H1914–H1923. doi: 10.1152/ajpheart.1996.270.6.H1914. [DOI] [PubMed] [Google Scholar]
- Magness RR, Shaw CE, Phernetton TM, Zheng J, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. II. Pregnancy effects on NO synthase expression. Am J Physiol. 1997;272:H1730–H1740. doi: 10.1152/ajpheart.1997.272.4.H1730. [DOI] [PubMed] [Google Scholar]
- Magness RR, Sullivan JA, Li Y, Phernetton TM, Bird IM. Endothelial vasodilator production by uterine and systemic arteries. VI. Ovarian and pregnancy effects on eNOS and NO(x) Am J Physiol Heart Circ Physiol. 2001;280:H1692–H1698. doi: 10.1152/ajpheart.2001.280.4.H1692. [DOI] [PubMed] [Google Scholar]
- Magness RR, Zheng J. Maternal Cardiovascular Alterations During Pregnancy. London: Arnold Publishing; 1996. [Google Scholar]
- Markee JE. Rhythmic uterine vascular changes. Am J Physiol. 1932;100:32–39. [Google Scholar]
- Mershon JL, Baker RS, Clark KE. Estrogen increases iNOS expression in the ovine coronary artery. Am J Physiol Heart Circ Physiol. 2002;283:H1169–H1180. doi: 10.1152/ajpheart.00397.2000. [DOI] [PubMed] [Google Scholar]
- Miller SL, Jenkin G, Walker DW. Effect of nitric oxide synthase inhibition on the uterine vasculature of the late-pregnant ewe. Am J Obstet Gynecol. 1999;180:1138–1145. doi: 10.1016/s0002-9378(99)70607-1. [DOI] [PubMed] [Google Scholar]
- Monheit AG, Resnik R. Corticosteroid suppression of estrogen-induced uterine blood flow in nonpregnant sheep. Am J Obstet Gynecol. 1981;139:454–458. doi: 10.1016/0002-9378(81)90324-0. [DOI] [PubMed] [Google Scholar]
- Preisler-Mashek MT, Solodin N, Stark BL, Tyriver MK, Alarid ET. Ligand-specific regulation of proteasome-mediated proteolysis of estrogen receptor-alpha. Am J Physiol Endocrinol Metab. 2002;282:E891–E898. doi: 10.1152/ajpendo.00353.2001. [DOI] [PubMed] [Google Scholar]
- Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnik R, Brink GW, Plumer MH. The effect of progesterone on estrogen-induced uterine blood flow. Am J Obstet Gynecol. 1977;128:251–254. doi: 10.1016/0002-9378(77)90617-2. [DOI] [PubMed] [Google Scholar]
- Resnik R, Killam AP, Barton MD, Battaglia FC, Makowski EL, Meschia G. The effect of various vasoactive compounds upon the uterine vascular bed. Am J Obstet Gynecol. 1976;125:201–206. doi: 10.1016/0002-9378(76)90593-7. [DOI] [PubMed] [Google Scholar]
- Resnik R, Killam AP, Battaglia FC, Makowski EL, Meschia G. The stimulation of uterine blood flow by various estrogens. Endocrinology. 1974;94:1192–1196. doi: 10.1210/endo-94-4-1192. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Kirsch JD, Kraft KC, Redmer DA. Time-course of the uterine response to estradiol-17beta in ovariectomized ewes: expression of angiogenic factors. Biol Reprod. 1998;59:613–620. doi: 10.1095/biolreprod59.3.613. [DOI] [PubMed] [Google Scholar]
- Reynolds LP, Redmer DA. Angiogenesis in the placenta. Biol Reprod. 2001;64:1033–1040. doi: 10.1095/biolreprod64.4.1033. [DOI] [PubMed] [Google Scholar]
- Roman-Ponce H, Caton D, Thatcher WW, Lehrer R. Uterine blood flow in relation to endogenous hormones during estrous cycle and early pregnancy. Am J Physiol. 1983;245:R843–R849. doi: 10.1152/ajpregu.1983.245.6.R843. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, Cornfield DN, Roy T. Ca2+-activated K+ channels modulate basal and E(2)beta-induced rises in uterine blood flow in ovine pregnancy. Am J Physiol Heart Circ Physiol. 2001;281:H422–H431. doi: 10.1152/ajpheart.2001.281.1.H422. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, Cox BE, Roy T, Magness RR. Nitric oxide contributes to estrogen-induced vasodilation of the ovine uterine circulation. J Clin Invest. 1996;98:2158–2166. doi: 10.1172/JCI119022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld CR, Jackson GM. Induction and inhibition of uterine vasodilation by catechol estrogen in oophorectomized, nonpregnant ewes. Endocrinology. 1982;110:1333–1339. doi: 10.1210/endo-110-4-1333. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, Rivera R. Circulatory responses to systemic infusions of estrone and estradiol-17alpha in nonpregnant, oophorectomized ewes. Am J Obstet Gynecol. 1978;132:442–448. doi: 10.1016/0002-9378(78)90782-2. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, Roy T, Cox BE. Mechanisms modulating estrogen-induced uterine vasodilation. Vascul Pharmacol. 2002;38:115–125. doi: 10.1016/s0306-3623(02)00135-0. 10.1016/S0306-3623(02)00135-0. [DOI] [PubMed] [Google Scholar]
- Rosenfeld CR, White RE, Roy T, Cox BE. Calcium-activated potassium channels and nitric oxide coregulate estrogen-induced vasodilation. Am J Physiol Heart Circ Physiol. 2000;279:H319–H328. doi: 10.1152/ajpheart.2000.279.1.H319. [DOI] [PubMed] [Google Scholar]
- Rupnow HL, Phernetton TM, Shaw CE, Modrick ML, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. VII. Estrogen and progesterone effects on eNOS. Am J Physiol Heart Circ Physiol. 2001;280:H1699–H1705. doi: 10.1152/ajpheart.2001.280.4.H1699. [DOI] [PubMed] [Google Scholar]
- Salhab WA, Shaul PW, Cox BE, Rosenfeld CR. Regulation of types I and III NOS in ovine uterine arteries by daily and acute estrogen exposure. Am J Physiol Heart Circ Physiol. 2000;278:H2134–H2142. doi: 10.1152/ajpheart.2000.278.6.H2134. [DOI] [PubMed] [Google Scholar]
- Sawada M, Alkayed NJ, Goto S, Crain BJ, Traystman RJ, Shaivitz A, Nelson RJ, Hurn PD. Estrogen receptor antagonist ICI182,780 exacerbates ischemic injury in female mouse. J Cereb Blood Flow Metab. 2000;20:112–118. doi: 10.1097/00004647-200001000-00015. 10.1097/00004647-200001000-00015. [DOI] [PubMed] [Google Scholar]
- Souza CJ, Campbell BK, Baird DT. Follicular waves and concentrations of steroids and inhibin A in ovarian venous blood during the luteal phase of the oestrous cycle in ewes with an ovarian autotransplant. J Endocrinol. 1998;156:563–572. doi: 10.1677/joe.0.1560563. 10.1677/joe.0.1560563. [DOI] [PubMed] [Google Scholar]
- Spencer TE, Bazer FW. Temporal and spatial alterations in uterine estrogen receptor and progesterone receptor gene expression during the estrous cycle and early pregnancy in the ewe. Biol Reprod. 1995;53:1527–1543. doi: 10.1095/biolreprod53.6.1527. [DOI] [PubMed] [Google Scholar]
- Still JG, Greiss FC. Effects of Cis- and trans-clomiphene on the uterine blood flow of oophorectomized ewes. Gynecol Invest. 1976;7:187–200. doi: 10.1159/000301381. [DOI] [PubMed] [Google Scholar]
- Sudhir K, Chou TM, Mullen WL, Hausmann D, Collins P, Yock PG, Chatterjee K. Mechanisms of estrogen-induced vasodilation: in vivo studies in canine coronary conductance and resistance arteries. J Am Coll Cardiol. 1995;26:807–814. doi: 10.1016/0735-1097(95)00248-3. 10.1016/0735-1097(95)00248-3. [DOI] [PubMed] [Google Scholar]
- Teoh H, Leung SW, Man RY. Short-term exposure to physiological levels of 17 beta-estradiol enhances endothelium-independent relaxation in porcine coronary artery. Cardiovasc Res. 1999;42:224–231. doi: 10.1016/s0008-6363(98)00265-x. 10.1016/S0008-6363(98)00265-X. [DOI] [PubMed] [Google Scholar]
- Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- Tschugguel W, Dietrich W, Zhegu Z, Stonek F, Kolbus A, Huber JC. Differential regulation of proteasome-dependent estrogen receptor alpha and beta turnover in cultured human uterine artery endothelial cells. J Clin Endocrinol Metab. 2003;88:2281–2287. doi: 10.1210/jc.2002-021165. 10.1210/jc.2002-021165. [DOI] [PubMed] [Google Scholar]
- Vagnoni KE, Shaw CE, Phernetton TM, Meglin BM, Bird IM, Magness RR. Endothelial vasodilator production by uterine and systemic arteries. III. Ovarian and estrogen effects on NO synthase. Am J Physiol. 1998;275:H1845–H1856. doi: 10.1152/ajpheart.1998.275.5.H1845. [DOI] [PubMed] [Google Scholar]
- Van Buren GA, Yang DS, Clark KE. Estrogen-induced uterine vasodilatation is antagonized by L-nitroarginine methyl ester, an inhibitor of nitric oxide synthesis. Am J Obstet Gynecol. 1992;167:828–833. doi: 10.1016/s0002-9378(11)91597-x. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Bowler J. Steroidal pure antioestrogens. J Endocrinol. 1987;112:R7–R10. doi: 10.1677/joe.0.112r007. [DOI] [PubMed] [Google Scholar]
- Wakeling AE, Bowler J. ICI 182,780, a new antioestrogen with clinical potential. J Steroid Biochem Mol Biol. 1992;43:173–177. doi: 10.1016/0960-0760(92)90204-v. 10.1016/0960-0760(92)90204-V. [DOI] [PubMed] [Google Scholar]
- Zhai P, Eurell TE, Cotthaus RP, Jeffery EH, Bahr JM, Gross DR. Effects of dietary phytoestrogen on global myocardial ischemia-reperfusion injury in isolated female rat hearts. Am J Physiol Heart Circ Physiol. 2001;281:H1223–H1232. doi: 10.1152/ajpheart.2001.281.3.H1223. [DOI] [PubMed] [Google Scholar]
- Zheng J, Li Y, Weiss AR, Bird IM, Magness RR. Expression of endothelial and inducible nitric oxide synthases and nitric oxide production in ovine placental and uterine tissues during late pregnancy. Placenta. 2000;21:516–524. doi: 10.1053/plac.1999.0504. 10.1053/plac.1999.0504. [DOI] [PubMed] [Google Scholar]
- Zoma WD, Baker RS, Clark KE. Coronary and uterine vascular responses to raloxifene in the sheep. Am J Obstet Gynecol. 2000;182:521–528. doi: 10.1067/mob.2000.104205. [DOI] [PubMed] [Google Scholar]
- Zoma W, Baker RS, Lang U, Clark KE. Hemodynamic response to tibolone in reproductive and nonreproductive tissues in the sheep. Am J Obstet Gynecol. 2001;184:544–551. doi: 10.1067/mob.2001.111098. 10.1067/mob.2001.111098. [DOI] [PubMed] [Google Scholar]