Abstract
In the present study, our aim was to determine whether intrafetal glucose infusion increases fetal adiposity, synthesis and secretion of leptin and regulates gene expression of the ‘appetite regulatory’ neuropeptides neuropepetide Y (NPY), agouti-related peptide (AGRP), pro-opiomelanocortin (POMC) and cocaine- and amphetamine-regulated transcript (CART) and receptors (leptin receptor (OB-Rb) and melancortin 3 receptor (MC3R)) within the fetal hypothalamus. Glucose (50% dextrose in saline) or saline was infused (7.5 ml h−1) into fetal sheep between 130 and 140 days gestation (term = 150 ± 3 days gestation). Glucose infusion increased circulating glucose and insulin concentrations, mean lipid locule size (532.8 ± 3.3 μm2 versus 456.7 ± 14.8 μm2) and total unilocular fat mass (11.7 ± 0.6 g versus 8.9 ± 0.6 g) of the perirenal fat depot. The expression of OB-Rb mRNA was higher in the ventromedial nucleus compared to the arcuate nucleus of the hypothalamus in both glucose and saline infused fetuses (F= 8.04; P < 0.01) and there was a positive correlation between expression of OB-Rb and MC3R mRNA in the arcuate nucleus (r= 0.81; P < 0.005). Glucose infusion increased mRNA expression for POMC, but not for the anorectic neuropeptide CART, or the orexigenic neuropeptides NPY and AGRP, in the arcuate nucleus of the fetal hypothalamus. These findings demonstrate that increased circulating glucose and insulin regulate gene expression of the neuropeptides within the fetal hypothalamus that are part of the neural network regulating energy balance in adult life.
In pregnancies complicated by maternal diabetes, the fetus is hyperglycaemic and hyperinsulinaemic, and cord blood leptin concentrations increase in parallel with increased infant adiposity (Cetin et al. 2000; Tapanainen et al. 2001). Furthermore, maternal diabetes mellitus, gestational diabetes or even mildly impaired glucose tolerance during pregnancy are all risk factors for the development of obesity and type II diabetes in the offspring (Plagemann et al. 1997; Silverman et al. 1998). It has therefore been proposed that exposure to excess nutrient supply during critical windows of fetal development may result in permanent changes within either the appetite regulatory system or the adipocyte to result in increased adiposity in adult life (Martin et al. 1998; Plagemann et al. 1999).
Glucose, insulin and leptin act through a range of mechanisms in the adult to alter hypothalamic expression of the orexigenic neuropeptides, neuropeptide Y (NPY) and agouti-related peptide (AGRP), and the anorexigenic precursor, pro-opiomelanocortin (POMC), and neuropeptide cocaine- and amphetamine-regulated transcript (CART), and thereby contribute to energy balance regulation (Ahima et al. 1996; Kalra et al. 1999; Williams et al. 2001). In species, such as the rat, with significant brown fat deposits, leptin also acts centrally to control energy expenditure by regulating expression of the uncoupling protein, UCP1, in brown adipocytes (Scarpace et al. 1997).
NPY containing projections develop between the arcuate nucleus (ARC), the dorsomedial hypothalamus (DMH) and paraventricular nucleus (PVN) of the hypothalamus during the first two weeks of life in the rat (Grove & Smith, 2003). This early postnatal period is also a critical period for the programming of appetite and adiposity, and overnutrition during this time induces permanent alterations in the hypothalamic neurones that express the appetite-regulating neuropeptides, leading to persistent hyperphagia and associated obesity (Plagemann et al. 1999). Whilst the appetite regulatory system develops postnatally in the rat, components of this neural network are expressed before birth in more precocial species, such as humans and sheep (Koutcherov et al. 2003; Muhlhausler et al. 2004). Although fetal glucose and insulin are positive regulators of fat deposition and leptin mRNA expression (Muhlhausler et al. 2003), it is not known whether increases in fetal nutrient supply can alter the expression of ‘appetite regulatory’ neuropeptides in the fetal hypothalamus.
Our aim therefore was to determine whether a 10 day intrafetal glucose infusion during late gestation increased lipid locule size, fetal fat mass, adipose leptin mRNA expression and circulating leptin and regulated the gene expression of the leptin receptor (OB-Rb) and melanocortin receptor (MC3R), and of the appetite regulatory neuropeptides NPY and AGRP (appetite stimulation) and POMC and CART (appetite inhibition) within the fetal sheep hypothalamus.
Methods
Animals and surgery
All procedures were approved by the University of Adelaide Animal Ethics Committee. Surgery was performed on 12 adult Merino ewes between 118 and 120 days (d) gestation (term = 150 ± 3 d) using aseptic techniques. General anaesthesia was induced by intravenous injection of sodium thiopentone (1.25 g i.v., Pentothal, Rhone Merieux, Pinkenba, Qld, Australia) and maintained with 2.5–4% halothane (Fluothane, ICI, Melbourne, Vic., Australia) in oxygen. Vascular catheters were implanted in a jugular vein of the ewe, in a jugular vein and carotid artery of the fetus, and in the amniotic cavity, as previously described (Edwards & McMillen, 2001). During surgery, antibiotics (Norocillin: Norbrook Laboratories Ltd, New Gisborne Vic., Australia and Dihydrostreptomycin in sterile saline: Sigma, St Louis, MO, USA) were administered to each ewe and fetus via intramuscular injection. All catheters were filled with heparinized saline and the fetal catheters exteriorized through an incision in the ewe's flank. The analgesic xylazine (0.02 mg kg−1) was administered to all ewes in the immediate postoperative period.
Ewes were housed in individual pens in animal holding rooms with a 12 h: 12 h light–dark cycle. Ewes were allowed at least 4 days to recover from surgery before experimentation. Ewes were fed a diet comprising lucerne chaff and concentrated pellets (Ridley Agriproducts, Murray Bridge, Australia) calculated to provide 100% of the maintenance energy requirements for a pregnant ewe bearing a singleton fetus (Aldermann et al. 1975). Water was provided ad libitum.
Blood sampling regime
Between 124 and 130 d gestation, maternal (3.0 ml) and fetal (3.0 ml) arterial blood samples were collected three times weekly. Fetal blood was then collected 3 h prior to the start of glucose or saline infusion (130 d gestation), each day for the first 4 days of infusion, and every 2 days thereafter until postmortem (139 ± 1 d gestation). Blood samples were centrifuged at 1500 g for 10 min at 4°C and plasma stored at −20°C for subsequent hormone and metabolite measurements. Fetal arterial blood (0.5 ml) was collected at each time point for measurement of fetal blood gases and pH using an ABL 520 analyser (Radiometer, Copenhagen, Denmark).
Glucose infusion
At 130 d gestation, fetuses were randomly assigned to receive either saline (n = 6) or glucose (n = 6) via continuous intravenous infusion. Infusion of glucose (50% dextrose 500 g l−1 in sterile saline) or sterile saline (vehicle) via the fetal jugular vein commenced at an initial rate of 1.9 ml h−1 at 130 d gestation. The infusion rate was increased incrementally by 1.9 ml h−1 per day for the subsequent 3 days, until the final rate of 7.5 ml h−1 was achieved on day 4; this infusion rate was then maintained until postmortem. This infusion rate was derived from a previous protocol in which this infusion level was shown to induce significant fetal hyperglycaemia and hyperinsulinaemia in the absence of any detrimental effects on fetal well-being (Stevens et al. 1990).
Postmortem and tissue collection
Between 138 and 140 d gestation (8–10 days after commencement of glucose or saline infusion), ewes were killed with an overdose of sodium pentobarbitone (Virbac Pty Ltd, Peakhurst, NSW, Australia). Fetal sheep were delivered by hysterotomy, weighed and killed by decapitation. All adipose tissue from the perirenal adipose tissue (PAT) depot was excised and weighed. A sample of PAT was fixed in 4% paraformaldehyde in 0.1 m phosphate buffer for subsequent processing and histological analyses. A second PAT sample was snap frozen in liquid N2 and stored at −80°C (Muhlhausler et al. 2003). The whole brain was excised, frozen in isopentane over dry ice and stored at −80°C.
Glucose, insulin and leptin assays
Maternal and fetal plasma glucose concentrations were determined by enzymatic analysis using the COBAS MIRA automated analysis system (Roche Diagnostica, Basel, Switzerland) previously validated for sheep plasma (Edwards & McMillen, 2001). The intra- and interassay coefficients of variation were both < 5%.
Insulin concentrations were determined in fetal plasma samples using a radioimmunoassay kit (Phadaseph radioimmunoassay kit, Pharmacia & Upjohn, Uppsala, Sweden) previously validated for sheep plasma (Edwards & McMillen, 2001). The inter- and intra-assay coefficients of variation were both < 20%.
A competitive ELISA, previously validated for sheep plasma (Kauter et al. 2000) was used to determine plasma leptin concentrations in fetal plasma samples. The sensitivity of the assay was 0.5 ng ml−1 and the inter- and intra-assay coefficients of variation were both < 10%.
RNA extraction
Total RNA was extracted from ∼100 mg of PAT from each experimental animal following the standard Sigma Tri Reagent protocol as previously described (Yuen et al. 1999; Muhlhausler et al. 2003). The RNA pellet was resuspended in sterile water (20 μl). The ratio of spectrophotometric absorbance at 260 and 280 nm was always > 1.6, RNA yield was 0.13 ± 0.02 μg RNA per mg adipose tissue and RNA integrity was confirmed by agarose gel electrophoresis.
Leptin and β-actin mRNA
Ovine leptin and β-actin cDNA were amplified by reverse transcription (RT)–PCR as previously described (Yuen et al. 1999; Muhlhausler et al. 2003; Yuen et al. 2003). The sequences of the primers for the amplification of the leptin cDNA (183 bp) and β-actin cDNA (349 bp) have been published and validated previously (Yuen et al. 1999). Each PCR product (12 μl) was electrophoresed through a 2.0% (w/v) agarose gel, stained with ethidium bromide, transilluminated with UV radiation, scanned with a Bio-Rad Molecular Imager and quantified using Quantity One Image Analysis Software (Bio-Rad Laboratories, Richmond, CA, USA). The RT-PCR was performed in duplicate on RNA from each adipose tissue sample. The relative abundance of leptin mRNA was calculated by referencing the intensity of the leptin amplicon to the intensity of the β-actin amplicon for each fetus.
UCP1 mRNA and 18S rRNA
The expression of UCP1 mRNA and 18S rRNA in 15 μg of PAT RNA was determined using standard Northern Blotting techniques described in detail elsewhere (Adams et al. 1999; Muhlhausler et al. 2003). Oligonucleotides (Geneworks, Adelaide, SA, Australia) complementary to nucleotides 267–298 of rat UCP1 (GenBank Acc. No. NM012682) (Bouillaud et al. 1986) and nucleotides 151–180 of rat 18S rRNA (Chan et al. 1984) were end-labelled with [32P]dATP (4000 Ci mmol−1; GRA-32 U, Geneworks, Adelaide, Australia) as previously described (Adams et al. 1999; Muhlhausler et al. 2003). The hybridization signal was quantified with Fuji-MacBAS software and the relative abundance of UCP1 mRNA calculated by referencing the intensity of the UCP1 mRNA band to the intensity of the 18S rRNA band for each fetus.
Hypothalamic gene expression
Coronal sections (20 μm) of fetal sheep hypothalami were collected between the mamillary body (caudal) and the optic chiasm (rostral). Sections were thaw-mounted onto slides double-coated with gelatin and poly-l-lysine and stored at −80°C until analysis of mRNA expression.
A riboprobe complementary to fragments of the intracellular domain of OB-Rb was generated from cloned sheep cDNA as previously described (Mercer et al. 1998). The NPY riboprobe was generated from a rat cDNA (Higuchi et al. 1988) and the CART riboprobe from a cloned sheep cDNA (Barrett et al. 2001). AGRP and POMC probes were generated from cloned Siberian hamster cDNA (Mercer et al. 2000) and the MC3R riboprobe was generated from human genomic cDNA (Adam et al. 2000). All probes were previously validated in adult sheep brain tissues (Adam et al. 1997; Adam et al. 2002; Sorensen et al. 2002).
Expression of mRNA for POMC, CART, NPY, AGRP, OB-Rb and MC3R in the fetal hypothalamus was detected by in situ hybridization using methods described in detail elsewhere (Mercer et al. 1995; Adam et al. 1997). All reagents were obtained from Sigma (Sigma UK, Poole, Dorset, UK) unless otherwise stated. Hybridized, radiolabelled slides were air-dried at room temperature, and apposed to Hyperfilm β-max (Amersham Pharmacia Biotech, UK Ltd, Little Chalfont, Buckinghamshire, UK). The resulting autoradiographic films were scanned at high resolution, and localization of specific hybridization signals confirmed in the resulting images using computerized densitometry measurements (Image-Pro Plus, Media Cybergenetics, Silver Spring, MD, USA). The integrated intensity of the hybridization signal was computed using standard curves generated from 14C autoradiographic microscales (Amersham Pharmacia Biotech). For each probe, up to nine sections spanned the caudal, medial and rostral extent of the hypothalamus from each fetus. Since we were interested in overall levels of gene expression, and there was no evidence for consistent differences between these regions, the data were averaged to give a single value for ARC gene expression for each animal.
Adipose tissue histology
Sections (4 μm) of PAT were stained with Hematoxylin and Eosin (HE) and examined using an Olympus BH2 microscope (20× objective and 2.5× NFK Photo eyepiece). Standard point counting techniques were used with Video Image Analysis (VIA) using Video Pro software (Leading Edge, Adelaide, SA, Australia) to determine the volume density of unilocular and multilocular tissue in PAT as previously described (Muhlhausler et al. 2002; Yuen et al. 2003). The volume density (Vd) was calculated using the formula: Vd=N/T, where N is the number of points falling on the unilocular or multilocular component, and T is the total number of points counted. The mass of the unilocular and multilocular component of fetal PAT were calculated by multiplying total PAT mass by the appropriate Vd (Muhlhausler et al. 2003).
For each experimental animal, the perimeter of 200 lipid locules with a cross-sectional area > 10 μm2 was manually traced and the Area-Pro software package (Video Pro Image Analysis, Leading Edge, Adelaide, SA, Australia) used to determine the enclosed area (Muhlhausler et al. 2003).
Statistical analysis
Data are presented as the mean ± s.e.m. The effect of glucose infusion on fetal plasma glucose, insulin and leptin concentrations was determined by multifactorial ANOVA with repeated measures using SPSSX (Statistical Package for Social Scientists) on a VAX mainframe computer and Duncan's post hoc test used to identify differences between mean values.
Fetal weight, placental weight and measures of fat mass and fat morphology, mean lipid locule size and relative leptin mRNA and UCP1 mRNA abundance in fetal PAT in the saline and glucose infused fetuses were compared using Student's t test. The effect of glucose infusion on the expression of mRNA for NPY, AGRP, POMC and CART and for OB-Rb and MC3R in the hypothalamus was also determined using a t test.
Fetal plasma concentrations of glucose, insulin and leptin between 135 and 140 d gestation were averaged to obtain a mean value for glucose, insulin and leptin concentrations during this period. Simple linear regression analysis was then used to determine relationships between mean fetal glucose, insulin and leptin concentrations, and mean lipid locule size in PAT, total PAT mass and the leptin: β-actin mRNA or UCP1: 18S rRNA ratios, and hypothalamic gene expression of OB-Rb, MC3R and the ‘appetite regulating’ neuropeptides. Linear regression analysis was also used to determine relationships between expression of NPY, AGRP, POMC, CART, MC3R and OB-Rb mRNA in the fetal ARC or VMH. A probability of 5% (P < 0.05) was taken as the level of significance in all analyses.
Results
Fetal arterial blood gas status and plasma glucose and insulin concentrations
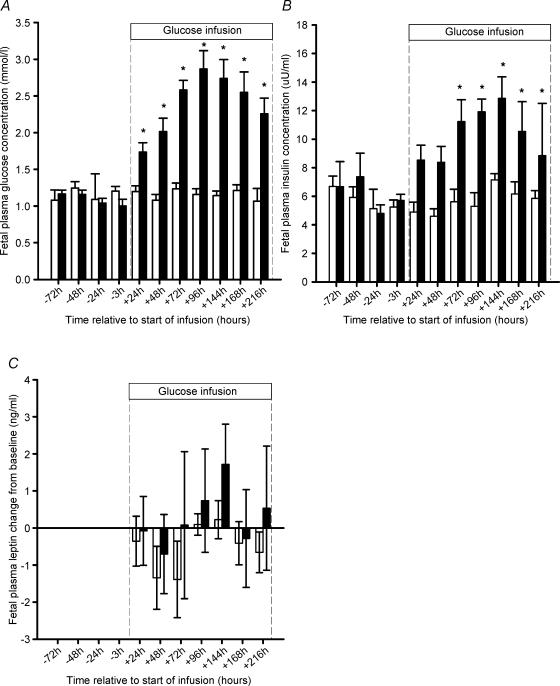
There were no differences in fetal arterial blood gas and pH values between the saline and glucose infused groups either prior to the start of glucose or saline infusion (data not shown) or during the infusion period (Table 1). Before the start of the infusion, there were no differences in fetal plasma glucose and insulin concentrations between fetuses assigned to the saline or glucose infusion protocols. Fetal plasma glucose and insulin concentrations were significantly higher (P < 0.05) than preinfusion concentrations from 24 h and 72 h, respectively, after the start of glucose infusion (Fig. 1A and B). Plasma glucose and insulin concentrations did not change during the saline infusion period.
Table 1.
Fetal arterial blood gas and pH status during the infusion period in saline and glucose infused fetuses
| Saline infused fetuses (n = 6) | Glucose infused fetuses(n = 6) | |
|---|---|---|
| Mean arterial PO2 (mmHg) | 21.0 ± 1.8 | 20.7 ± 0.5 |
| Mean arterial PCO2 (mmHg) | 46.3 ± 1.3 | 45.8 ± 1.4 |
| Mean arterial pH | 7.40 ± 0.01 | 7.39 ± 0.01 |
| Mean arterial oxygen saturation (%) | 63.0 ± 5.0 | 63.7 ± 1.4 |
| Mean arterial haemogloblin content (mg dl−1) | 10.4 ± 0.7 | 9.2 ± 0.4 |
| Total arterial oxygen content (ml dl−1) | 8.9 ± 0.5 | 8.2 ± 0.3 |
Figure 1. Fetal plasma glucose (A), insulin (B) and change in plasma leptin concentrations compared to preinfusion values (C) in saline infused (open bars) and glucose infused (filled bars) groups between −96 and +216 h from the start of saline or glucose infusion.
Asterisks denote a significant increase in plasma glucose and insulin concentrations compared to preinfusion concentrations.
Size of lipid locules and mass of fetal perirenal adipose tissue and fetal and placental growth
Glucose infusion resulted in an increase (P < 0.05) in the mean size of the lipid locules in the PAT depot (Table 2). There was also a direct correlation between the mean size of lipid locules and the mean plasma glucose, but not insulin, concentrations (mean locule size (μm2) = 42.9 (fetal glucose) + 422; r= 0.79, n = 10; P < 0.01). Glucose infusion resulted in a significant increase (P < 0.05) in total unilocular, but not multilocular, fat mass at 140 d gestation (Table 2). There was no dissectible subcutaneous adipose tissue in either saline infused or glucose infused fetuses. There was no effect of glucose infusion on placental weight (saline, 436.3 ± 52.8 g; glucose, 468.9 ± 24.9 g) or fetal weight (saline, 4.84 ± 0.2 kg; glucose, 5.12 ± 0.2 kg).
Table 2.
The effect of intrafetal glucose infusion on the total and relative mass of unilocular and multilocular perirenal fat
| Saline infused fetuses (n = 6) | Glucose infused fetuses (n = 6) | |
|---|---|---|
| Mean lipid locule size in perirenal adipose tissue (μm2) | 456.7 ± 14.8 | 532.8 ± 3.3* |
| Total mass of unilocular perirenal fat (g) | 8.9 ± 0.6 | 11.7 ± 0.6* |
| Relative mass of unilocular perirenal fat (g kg−1) | 1.9 ± 0.2 | 2.3 ± 0.1 |
| Total mass of multilocular perirenal fat (g) | 12.0 ± 1.3 | 16.6 ± 2.7 |
| Relative mass of multilocular perirenal fat (g kg−1) | 2.5 ± 0.2 | 3.2 ± 0.5 |
| Total mass of perirenal fat (g) | 22.1 ± 1.4 | 27.4 ± 2.4 |
| Relative mass of total perirenal fat (g kg−1) | 4.6 ± 0.3 | 5.4 ± 0.4 |
Asterisks denote significant differences compared to the saline infused group (P < 0.05).
Leptin mRNA and UCP 1 mRNA expression in fetal perirenal adipose tissue and circulating leptin concentrations
There was no effect of glucose infusion on the relative expression of leptin mRNA (saline, 0.58 ± 0.06; glucose, 0.66 ± 0.12) or UCP1 mRNA (saline, 0.46 ± 0.06; glucose, 0.49 ± 0.07) in fetal PAT. In the glucose but not saline infused fetuses, however, there was a positive correlation between the relative abundance of leptin mRNA in fetal PAT and the mean plasma concentrations of either insulin (leptin mRNA: β actin mRNA = 0.07 (fetal insulin) − 0.096; r= 0.81; n = 6; P < 0.05) or glucose (leptin mRNA: β actin mRNA = 0.52 (fetal glucose) − 0.70; r= 0.94; n = 6; P < 0.01).
Before the start of the infusion, there was no difference in fetal plasma leptin concentrations between fetuses assigned to either the saline (3.5 ± 0.6 ng ml−1) or the glucose (4.7 ± 0.8 ng ml−1) infusion protocols. There was no difference in plasma leptin concentrations between saline and glucose infused fetuses at any time point either prior to or after the start of infusion (Table 3). Fetal plasma leptin concentrations did not change during the infusion period when compared with baseline concentrations in either the glucose or saline infused fetuses (Fig. 1C). There was no relationship between circulating leptin concentrations and either the total or relative fat or unilocular fat mass in either the glucose and saline infused fetuses.
Table 3.
The effect of intrafetal glucose infusion on plasma leptin concentrations
| − 72 h | − 48 h | − 24 h | − 3 h | + 24 h | + 48 h | + 72 h | + 96 h | + 144 h | + 192 h | + 216 h | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Saline infusion (n = 6) | 3.9 ± 0.6 | 3.8 ± 1.0 | 3.2 ± 0.8 | 2.6 ± 0.6 | 3.1 ± 0.8 | 2.1 ± 0.5 | 2.1 ± 0.6 | 3.6 ± 0.5 | 3.7 ± 0.7 | 3.1 ± 0.3 | 2.7 ± 1.0 |
| Glucose infusion n = 6) | 4.8 ± 1.1 | 3.9 ± 0.9 | 4.4 ± 1.0 | 3.9 ± 0.9 | 4.0 ± 0.9 | 4.1 ± 0.6 | 4.1 ± 2.0 | 4.4 ± 1.8 | 5.2 ± 1.7 | 3.9 ± 1.5 | 5.5 ± 2.0 |
The table shows plasma leptin (ng ml−1) at times relative to the start of infusion.
OB-Rb, MC3R, POMC, CART, NPY and AGRP mRNA expression in the fetal hypothalamus
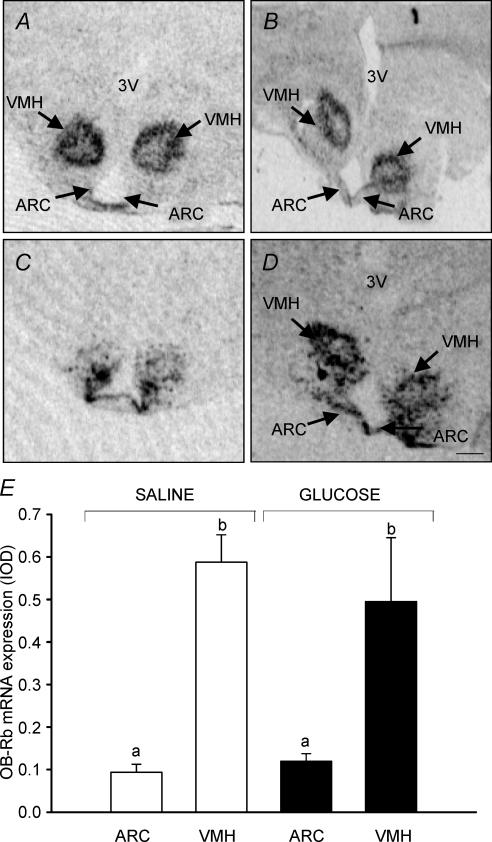
OB-Rb and MC3R mRNAs were localized predominantly within the ARC and VMH (Fig. 2A–D). The level of OB-Rb mRNA expression was higher (F= 8.04; P < 0.01) in the VMH than in the ARC in both glucose and saline infused fetuses (Fig. 2E). There was no effect of glucose infusion on the level of OB-Rb or MC3R mRNA expression in the ARC or VMH. There was a positive correlation between the expression of mRNA for OB-Rb and MC3R within the ARC (r= 0.81; n = 11; P < 0.005).
Figure 2. Autoradiographs showing the distribution of expression of OB-Rb mRNA (A and B) and MC3R mRNA (C and D) in the hypothalamus of the sheep fetus at 140 ± 1 d gestation. OB-Rb and MC3R mRNA was expressed predominately in the arcuate nucleus (ARC) and ventromedial hypothalmaus (VMH) in both saline infused (left panel) and glucose infused (right panel) fetuses. The expression of OB-Rb mRNA was higher in the VMH compared to the ARC in both saline infused and glucose infused fetuses. E, different letters denote significant differences between mRNA expression of OB-Rb in the VMN and ARC. Scale bar, 1.3 mm.
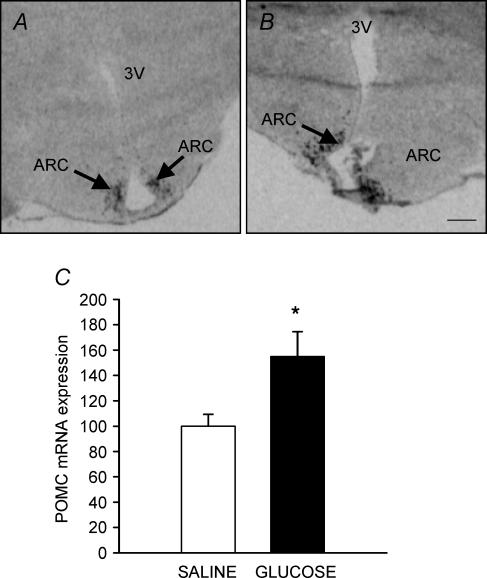
Expression of POMC mRNA was localized within the ARC in both glucose and saline infused fetuses (Fig. 3A and B). The level of POMC mRNA expression in the ARC was higher (P < 0.04) in the hypothalami of glucose infused compared with saline infused fetuses at 140 d gestation (Fig. 3C). There was no relationship, however, between circulating glucose, insulin or leptin concentrations and POMC mRNA expression in either group.
Figure 3. Autoradiographs showing the distribution of expression of POMC mRNA in the saline infused (A) and glucose infused (B) fetuses at 140 d gestation. POMC mRNA expression at 140 ± 1 d gestation was higher (P < 0.05) in glucose infused compared to saline infused fetuses (C). ARC, arcuate nucleus. Scale bar, 1.3 mm.
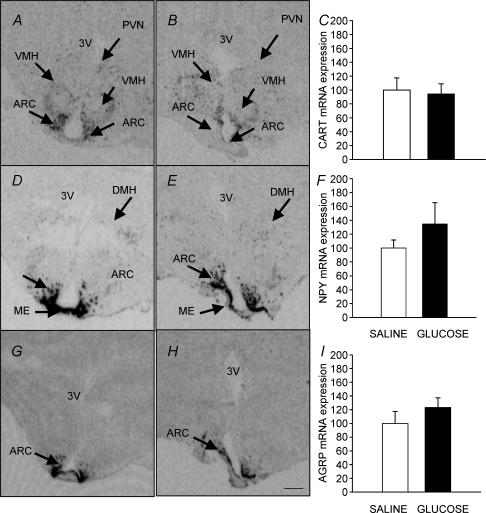
CART mRNA was expressed in the ARC, VMH and PVN and there was no effect of glucose infusion on the level of CART mRNA expression (Fig. 4A, B and C). NPY and AGRP mRNAs were localized within the ARC, dorsomedial hypothalamus (DMH) and median eminence (ME) and there was no effect of glucose infusion on their level of expression (Fig. 4D–I).
Figure 4. Autoradiographs showing the distribution of expression of CART mRNA (A and B), NPY mRNA (D and E) and AGRP mRNA (G and H) in saline infused (left panel) and glucose infused (right panel) fetuses at 140 ± 1 d gestation. There was no difference in the localization or level of expression of CART, AGRP or NPY mRNA between the saline and glucose infused groups (C, F and I). ARC, arcuate nucleus; PVN, paraventricular nucleus; VMH, ventromedial hypothalamus; ME, median eminence; DMH, dorsomedial hypothalamus. Scale bar, 1.3 mm.
Discussion
We have demonstrated that infusion of glucose into the late gestation fetal sheep increases fetal glucose and insulin concentrations, increases the size of the lipid locules within the major fetal fat depot, and increases the expression of mRNA for the precursor molecule POMC in the arcuate nucleus of the hypothalamus at 140 d gestation. These results provide evidence that the expression of a hypothalamic neuropeptide known to regulate energy balance in adult life also responds to changes in nutritional status before birth.
Intrafetal glucose infusion for 10 days in late gestation resulted in an increase in the size of the dominant lipid locules and in the total mass of unilocular perirenal adipose tissue. The mean lipid locule size was also directly related to fetal glucose, but not insulin, concentrations during the infusion period in both saline and glucose infused fetuses. This is consistent with previous findings (Muhlhausler et al. 2003), and suggests that glucose, rather than insulin, is the principal determinant of lipid storage in fetal adipocytes during late gestation. This is supported by previous reports that the abundance of the insulin independent glucose transporter (GLUT1) is greater than that of the insulin dependent glucose transporter (GLUT4) in the adipose tissue of the sheep fetus in late gestation (Das et al. 1998).
Whilst the 10-day period of glucose infusion resulted in an increase in lipid locule size, it did not result in a significant increase in relative fetal adiposity. It has previously been reported that a 30 day infusion of glucose in fetal sheep increased total fetal fat mass (Stevens et al. 1990) and that the increased adiposity in infants of diabetic mothers is directly related to maternal circulating glucose concentrations (Catalano et al. 2003). It may be that the fetus is able to adapt to a 10 day, but not a more prolonged, period of hyperglycaemia through mechanisms which limit the impact of an increase in fetal glucose on fetal adiposity. There is evidence that prolonged fetal hyperglycaemia is associated with a decline in adipose GLUT-1 levels in the sheep fetus (Das et al. 1999) which may be one mechanism which acts to counter a chronic increase in fetal nutrient supply.
In the present study, there was no effect of chronic glucose infusion on leptin mRNA expression in the fetal perirenal adipose tissue. There was, however, a strong positive relationship between either plasma insulin or glucose concentrations and leptin mRNA expression in fetal adipose tissue in the glucose infused, but not saline infused fetuses. This same direct relationship is also present in fetuses of ewes fed at ∼55% above maintenance energy requirements in late pregnancy (Muhlhausler et al. 2003). Insulin-responsive elements have been identified within the promoter of the leptin gene in the adult mouse and insulin up-regulates leptin gene transcription in adipocytes of adult rodents (Saladin et al. 1995; Wang et al. 2000). In the fetal sheep it has recently been reported that intrafetal infusion of insulin for 24 h, resulting in fetal insulin concentrations higher than 50 μU ml−1, stimulates a significant increase in leptin mRNA expression in the perirenal adipose tissue of the sheep fetus (Devaskar et al. 2002). One possibility is that fetal adipose tissue may be relatively insensitive to the actions of insulin or that in the presence of moderate elevations in fetal insulin, other mechanisms limit the actions of insulin on leptin expression in the fetal adipocyte.
In the present study, there was no significant relationship between plasma leptin concentrations and either total or relative unilocular fat mass. This is in contrast to our previous findings of a positive relationship between fat mass and leptin concentrations in fetuses of ewes fed at or above maintenance energy requirements in late gestation (Muhlhausler et al. 2002). Whilst maternal overnutrition was not associated with an increase in fetal fat deposition, however, intrafetal glucose infusion significantly increased lipid storage within fetal adipose depots without altering plasma leptin. These results therefore suggest that increasing fetal plasma glucose concentrations beyond those achieved by modest maternal overnutrition acts to alter the regulation of leptin secretion and adipose tissue mass. One possible explanation is that there may be a counter-regulatory mechanism which acts to limit leptin synthesis and secretion from fetal adipose cells when fetal glucose concentrations are increased beyond a given threshold.
We have demonstrated that an increase in fetal glucose concentrations between 130 and 140 days gestation resulted in a significant increase in the expression of mRNA for the precursor molecule POMC in the arcuate nucleus of the fetal sheep hypothalamus. This occurred despite the fact that glucose infusion did not increase circulating concentrations of leptin, which is a key regulator of POMC expression in the adult (Mizuno et al. 1998). There is recent evidence that there are subpopulations of POMC-containing neurones in the mouse hypothalamus that are glucose responsive, responding to increases in extracellular glucose by an increase in activity (Dunn-Meynell et al. 2002; Ibrahim et al. 2003). Furthermore, populations of POMC-containing neurones have also been found to coexpress insulin receptors and administration of insulin into the third ventricle of fasted rats increases POMC mRNA expression (Air et al. 2002). The results of the current study suggest that expression of POMC in the arcuate neurones of the fetal hypothalamus may be responsive to increases in glucose or insulin, acting either alone or in combination.
The direct relationship between the expression of OB-Rb and MC3R expression in the fetal arcuate nucelus is consistent with the known coexpression of these receptors on POMC neurones in the arcuate nucleus in the adult (Cone et al. 2001). In the adult, cleavage of POMC produces the melanocortin agonist, α-MSH, which acts via melanocortin receptors to limit weight gain by reducing appetite and promoting thermogenic activation of brown adipose tissue. Whilst there was no difference in UCP1 mRNA expression in adipose tissue from glucose and saline infused fetuses in the present study, this may not indicate the absence of any thermogenic activation, since the abundances of UCP1 mRNA and protein within the same adipose depot are not directly related (Yuen et al. 2003). It has been demonstrated that maternal overnutrition in late pregnancy results in an increase in the thermogenic activity and abundance of UCP1 protein in perirenal adipose tissue and a decrease in adipose tissue mass in the fetal sheep (Budge et al. 2000). It is possible therefore that an increase in hypothalamic POMC expression may act to limit accumulation of fat in the fetus when the nutrient supply is excessive.
There was no effect of intrafetal glucose infusion and concomitant increases in circulating glucose and insulin concentrations on gene expression for the orexigenic neuropeptides NPY and AGRP. Conversely, fetal hypothalamic NPY content is increased following maternal undernutrition in sheep (Warnes et al. 1998). Therefore, fetal hypothalamic NPY and AGRP gene expression may be relatively insensitive to increases in glucose or insulin concentrations, but relatively sensitive to decreases in nutrient supply, thus preserving an orexigenic pathway that may be important to neonatal survival (Ross et al. 2003).
We have demonstrated that the expression of OB-Rb is higher in the ventromedial compared to the arcuate nucleus in the fetal sheep hypothalamus, in contrast to the adult sheep or rodent in which OB-Rb mRNA is predominantly expressed within the arcuate nucleus (Guan et al. 1998; Williams et al. 1999). This suggests that the leptin axis undergoes further development after birth in the sheep. In the adult rodent, the ventromedial nucleus has been implicated as important for the regulation of thermogenesis in the brown adipose tissue (Cannon & Nedergaard, 2003), and the higher level of OB-Rb expression in the fetal ventromedial hypothalamus may indicate that leptin has a greater role in the regulation of the thermogenic activity of brown adipose tissue, rather than ‘energy intake’ during the perinatal period. Consistent with this role is the observation that infusion of leptin into fetal sheep in late gestation resulted in an increase in the proportion of multilocular tissue and UCP1 protein abundance in fetal perirenal adipose tissue (Yuen et al. 2003).
In summary, we have demonstrated that intrafetal glucose infusion regulates gene expression of neuropeptides in the fetal hypothalamus that normally play a role in the regulation of energy intake and energy expenditure in adult life. An alteration in the regulation of appetite regulatory neuropeptides within the fetal hypothalamus is clearly one potential mechanism whereby a transplacental increase in substrate supply, such as occurs in pregnancies complicated by maternal glucose intolerance, may lead to a subsequent increase in childhood and adult obesity.
Acknowledgments
This work was supported by an NHMRC Program Grant. We gratefully acknowledge the National Health and Medical Research Council (NHMRC) for financial support of this work. We are also grateful to Anne Jurisevic and Frank Carbone for their expert assistance with the sheep surgery and to Laura O'Carroll for her invaluable assistance with experimental animal protocols.
References
- Adam CL, Archer ZA, Findlay PA, Thomas L, Marie M. Hypothalamic gene expression in sheep for cocaine- and amphetamine-regulated transcript, pro-opiomelanocortin, neuropeptide Y, agouti-related peptide and leptin receptor and responses to negative energy balance. Neuroendocrinology. 2002;75:250–256. doi: 10.1159/000054716. 10.1159/000054716. [DOI] [PubMed] [Google Scholar]
- Adam CL, Findlay PA, Kyle CE, Young P, Mercer JG. Effect of chronic food restriction on pulsatile luteinizing hormone secretion and hypothalamic neuropeptide Y gene expression in castrated male sheep. J Endocrinol. 1997;152:329–337. doi: 10.1677/joe.0.1520329. 10.1677/joe.0.1520329. [DOI] [PubMed] [Google Scholar]
- Adam CL, Moar KM, Logie TJ, Ross AW, Barrett P, Morgan DE, Mercer JG. Photoperiod regulates growth, puberty and hypothalamic neuropeptide and receptor gene expression in female Siberian hamsters. Endocrinology. 2000;141:4349–4356. doi: 10.1210/endo.141.12.7807. 10.1210/en.141.12.4349. [DOI] [PubMed] [Google Scholar]
- Adams MB, Ross JT, Butler TG, McMillen IC. Glucocorticoids decrease phenylethanolamine N-methyltransferase mRNA expression in the immature foetal sheep adrenal. J Neuroendocrinol. 1999;11:569–575. doi: 10.1046/j.1365-2826.1999.00359.x. 10.1046/j.1365-2826.1999.00359.x. [DOI] [PubMed] [Google Scholar]
- Ahima R, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier J. Role of leptin in the neuroendocrine response to fasting. Nature. 1996;382:250–252. doi: 10.1038/382250a0. 10.1038/382250a0. [DOI] [PubMed] [Google Scholar]
- Air EL, Benoit SC, Clegg DJ, Seeley RJ, Woods SC. Insulin and leptin combine additively to reduce food intake and body weight in rats. Endocrinology. 2002;143:2449–2452. doi: 10.1210/endo.143.6.8948. 10.1210/en.143.6.2449. [DOI] [PubMed] [Google Scholar]
- Aldermann GA, Morgan DE, Harvard A, Edwards RE, Todd JR. Ministry of Agriculture, Fisheries and Food: Technical Bulletin 33. London: Her Majesty's Stationery Office; 1975. Energy allowances and feeding systems for ruminants. [Google Scholar]
- Barrett P, Morris MA, Moar KM, Mercer JG, Davidson JA, Findlay PA, Adam CL, Morgan DE. The differential regulation of CART gene expression in a pituitary cell line and primary cell cultures of ovine pars tuberalis cells. J Neuroendocrinol. 2001;13:347–352. doi: 10.1046/j.1365-2826.2001.00634.x. [DOI] [PubMed] [Google Scholar]
- Bouillaud F, Weissenbach J, Riquier D. Complete cDNA-derived amino acid sequence of rat brown uncoupling proteins. J Biochem. 1986;261:1487–1490. [PubMed] [Google Scholar]
- Budge H, Bispham J, Dandrea J, Evans E, Heasman L, Ingleton PM, Sullivan C, Wilson V, Stephenson T, Symonds ME. Effect of maternal nutrition on brown adipose tissue and its prolactin receptor status in the fetal lamb. Pediatr Res. 2000;47:781–786. doi: 10.1203/00006450-200006000-00017. [DOI] [PubMed] [Google Scholar]
- Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. 2003;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- Catalano PM, Thomas A, Huston-Presley L, Amini SB. Increased fetal adiposity: a very sensitive marker of abnormal in utero development. Am J Obstet Gynecol. 2003;189:1698–1704. doi: 10.1016/s0002-9378(03)00828-7. [DOI] [PubMed] [Google Scholar]
- Cetin I, Morpurgo PS, Radaelli T, Taricco E, Cortelazzi D, Bellotti M, Pardi G, Beck-Peccoz P. Fetal plasma leptin concentrations: relationship with different fetal growth patterns from 19 weeks up to term. Pediatr Res. 2000;48:646–651. doi: 10.1203/00006450-200011000-00016. [DOI] [PubMed] [Google Scholar]
- Chan Y, Gutell R, Noller H, Wool I. The nucleotide sequence of a rat 18S ribosomal ribonucleic acid gene and a proposal for the secondary structure of 18S ribosomal ribonucleic acid. J Biol Chem. 1984;259:224–230. [PubMed] [Google Scholar]
- Cone RD, Cowley MA, Butler AA, Fan W, Marks DL, Low MJ. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int J Obes Relat Metab Disord. 2001;25:S63–S67. doi: 10.1038/sj.ijo.0801913. [DOI] [PubMed] [Google Scholar]
- Das UG, Sadiq HF, Soares J, Hay WWJ, Devaskar SU. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Physiol Regul Integr Comp Physiol. 1998;274:R339–R347. doi: 10.1152/ajpregu.1998.274.2.R339. [DOI] [PubMed] [Google Scholar]
- Das UG, Schroeder RE, Hay WWJ, Devaskar SU. Time-dependent and tissue-specific effects of circulating glucose on fetal ovine glucose transporters. Am J Physiol Regul Integr Comp Physiol. 1999;276:R809–R817. doi: 10.1152/ajpregu.1999.276.3.R809. [DOI] [PubMed] [Google Scholar]
- Devaskar SU, Anthony RV, Hay WWJ. Ontogeny and insulin regulation of fetal ovine white adipose tissue leptin expression. Am J Physiol Regul Integr Comp Physiol. 2002;282:R431–R418. doi: 10.1152/ajpregu.2002.282.2.R431. [DOI] [PubMed] [Google Scholar]
- Dunn-Meynell AA, Routh VH, Kang L, Gaspers L, Levin BE. Glucokinase is the likely mediator of glucosensing in both glucose-excited and glucose-inhibited central neurons. Diabetes. 2002;51:2056–2065. doi: 10.2337/diabetes.51.7.2056. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol. 2001;533:561–570. doi: 10.1111/j.1469-7793.2001.0561a.x. 10.1111/j.1469-7793.2001.0561a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grove KL, Smith MS. Ontogeny of the hypothalamic neuropeptide Y system. Physiol Behav. 2003;79:47–63. doi: 10.1016/s0031-9384(03)00104-5. 10.1016/S0031-9384(03)00104-5. [DOI] [PubMed] [Google Scholar]
- Guan XMYuH, Trumbauer M, Frazier E, Van der Ploeg LH, Chen H. Induction of neuropeptide Y expression in dorsomedial hypothalamus of diet-induced obese mice. Neuroreport. 1998;9:3415–3419. doi: 10.1097/00001756-199810260-00015. [DOI] [PubMed] [Google Scholar]
- Higuchi H, Yang H, Sabol S. Rat neuropeptide Y precursor gene expression. mRNA structure, tissue distribution, and regulation by glucocorticoids, cyclic AMP, and phorbol ester. J Biol Chem. 1988;263:6288–6295. [PubMed] [Google Scholar]
- Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Ronnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- Kalra SP, Dube MG, Pu S, Xu B, Horvath TL, Kalra PS. Interacting appetite-regulating pathways in the hypothalamic regulation of body weight. Endocr Rev. 1999;20:68–100. doi: 10.1210/edrv.20.1.0357. 10.1210/er.20.1.68. [DOI] [PubMed] [Google Scholar]
- Kauter K, Ball M, Kearney P, Tellam R, McFarlane JR. Adrenaline, insulin and glucagon do not have acute effects on plasma leptin levels in sheep: development and characterisation of an ovine leptin ELISA. J Endocrinol. 2000;166:127–135. doi: 10.1677/joe.0.1660127. 10.1677/joe.0.1660127. [DOI] [PubMed] [Google Scholar]
- Koutcherov Y, Mai JK, Paxinos G. Hypothalamus of the human fetus. J Chem Neuroanat. 2003;26:253–270. doi: 10.1016/j.jchemneu.2003.07.002. 10.1016/j.jchemneu.2003.07.002. [DOI] [PubMed] [Google Scholar]
- Martin RJ, Hausman GJ, Hausman DB. Regulation of adipose cell development in utero. Proc Soc Exp Biol Med. 1998;219:200–210. doi: 10.3181/00379727-219-44333. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Lawrence CB, Beck B, Burlet A, Atkinson T, Barrett P. Hypothalamic NPY and prepro-NPY mRNA in Djungarian hamsters: effects of food deprivation and photoperiod. Am J Physiol Regul Integr Comp Physiol. 1995;269:R1099–R1106. doi: 10.1152/ajpregu.1995.269.5.R1099. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Moar KM, Findlay PA, Hoggard N, Adam CL. Association of leptin receptor (OB-Rb), NPY and GLP-1 gene expression in the ovine and murine brainstem. Regul Pept. 1998;75–76:271–278. doi: 10.1016/s0167-0115(98)00078-0. [DOI] [PubMed] [Google Scholar]
- Mercer JG, Moar KM, Ross AW, Hoggard N, Morgan PJ. Photoperiod regulates arcuate nucleus POMC, AGRP, and leptin receptor mRNA in Siberian hamster hypothalamus. Am J Physiol Regul Integr Comp Physiol. 2000;278:R271–R281. doi: 10.1152/ajpregu.2000.278.1.R271. [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV. Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and in ob/ob and db/db mice, but is stimulated by leptin. Diabetes. 1998;47:294–297. doi: 10.2337/diab.47.2.294. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, McMillen IC, Rouzaud G, Findlay PA, Marrocco EM, Rhind SM, Adam CL. Appetite regulatory neuropeptides are expressed in the sheep hypothalamus before birth. J Neuroendocrinol. 2004;16:502–507. doi: 10.1111/j.1365-2826.2004.01197.x. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Roberts CT, McFarlane JR, Kauter KG, McMillen IC. Fetal leptin is a signal of fat mass independent of maternal nutrition in ewes fed at or above maintenance energy requirements. Biol Reprod. 2002;67:493–499. doi: 10.1095/biolreprod67.2.493. [DOI] [PubMed] [Google Scholar]
- Muhlhausler BS, Roberts CT, Yuen BSJ, Marrocco E, Budge H, Symonds ME, McFarlane JR, Kauter KG, Stagg P, Pearse JK, McMillen IC. Determinants of fetal leptin synthesis, fat mass, and circulating leptin concentrations in well-nourished ewes in late pregnancy. Endocrinology. 2003;144:4947–4954. doi: 10.1210/en.2003-0555. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Kohlhoff R, Rhode W, Dorner G. Overweight and obesity in infants of mothers with long-term insulin-dependent diabetes or gestational diabetes. Int J Obes Rel Metabol Disord. 1997;21:451–456. doi: 10.1038/sj.ijo.0800429. [DOI] [PubMed] [Google Scholar]
- Plagemann A, Harder T, Rake A, Waas T, Melchior K, Ziska T, Rohde W, Dorner G. Observations on the orexigenic hypothalamic neuropeptide Y-system in neonatally overfed weanling rats. J Neuroendocrinol. 1999;11:541–546. doi: 10.1046/j.1365-2826.1999.00357.x. [DOI] [PubMed] [Google Scholar]
- Ross MG, El-Haddad M, DeSai M, Gayle D, Beall MH. Unopposed orexic pathways in the developing fetus. Physiol Behav. 2003;79:79–88. doi: 10.1016/s0031-9384(03)00107-0. [DOI] [PubMed] [Google Scholar]
- Saladin R, De Vos P, Guerre-Millo M, Leturque A, Girard J, Staels B, Auwerx J. Transient increase in obese gene expression after food intake or insulin administration. Nature. 1995;377:527–529. doi: 10.1038/377527a0. [DOI] [PubMed] [Google Scholar]
- Scarpace PJ, Matheny M, Pollock BH, Tumer N. Leptin increases uncoupling protein expression and energy expenditure. Am J Physiol Endocrinol Metabol. 1997;273:E226–E230. doi: 10.1152/ajpendo.1997.273.1.E226. [DOI] [PubMed] [Google Scholar]
- Silverman BL, Rizzo TA, Cho NH, Metzger BE. Long-term effects of the intrauterine environment. Diabetes Care. 1998;21:B142–B149. [PubMed] [Google Scholar]
- Sorensen A, Adam CL, Findlay PA, Marie M, Thomas L, Travers MT, Vernon RG. Leptin secretion and hypothalamic neuropeptide and receptor gene expression in sheep. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1227–R1235. doi: 10.1152/ajpregu.00595.2001. [DOI] [PubMed] [Google Scholar]
- Stevens D, Alexander G, Bell AW. Effect of prolonged glucose infusion into fetal sheep on body growth, fat deposition and gestation length. J Dev Physiol. 1990;13:277–281. [PubMed] [Google Scholar]
- Tapanainen P, Leinonen E, Ruokonen A, Knip M. Leptin concentrations are elevated in newborn infants of diabetic mothers. Horm Res. 2001;55:185–190. doi: 10.1159/000049993. [DOI] [PubMed] [Google Scholar]
- Wang FN, Ma CG, Zhang YL, Zhang NX, Chen YM, Tang QQ, Song HY. Identification of glucose-responsive and insulin-responsive elements in promoter of mouse ob gene. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 2000;32:541–544. [PubMed] [Google Scholar]
- Warnes KE, Morris MJ, Symonds ME, Phillips ID, Clarke IJ, Owens JA, McMillen IC. Effects of increasing gestation, cortisol and maternal undernutrition on hypothalamic neuropeptide Y expression in the sheep fetus. J Neuroendocrinol. 1998;10:51–57. doi: 10.1046/j.1365-2826.1998.00172.x. [DOI] [PubMed] [Google Scholar]
- Williams LM, Adam CL, Mercer JG, Moar KM, Slater D, Hunter L, Findlay PA, Hoggard N. Leptin receptor and neuropeptide Y gene expression in the sheep brain. J Neuroendocrinol. 1999;11:165–169. doi: 10.1046/j.1365-2826.1999.00293.x. [DOI] [PubMed] [Google Scholar]
- Williams G, Bing C, Cai XJ, Harrold JA, King PJ, Liu XH. The hypothalamus and the control of energy homeostasis: different circuits, different purposes. Physiol Behav. 2001;74:683–701. doi: 10.1016/s0031-9384(01)00612-6. [DOI] [PubMed] [Google Scholar]
- Yuen BSJ, McMillen IC, Symonds ME, Owens PC. Abundance of leptin mRNA in fetal adipose tissue is related to fetal body weight. J Endocrinol. 1999;163:R11–R14. doi: 10.1677/joe.0.163r011. [DOI] [PubMed] [Google Scholar]
- Yuen BSJ, Owens PC, Muhlhausler BS, Roberts CK, Symonds ME, Keisler DH, McFarlane JR, Kauter K, Evens Y, McMillen IC. Leptin alters the structural and functional characteristics of adipose tissue before birth. FASEB J. 2003;17:1102–1104. doi: 10.1096/fj.02-0756fje. [DOI] [PubMed] [Google Scholar]