Abstract
This study investigated the developmental and nutritional programming of uncoupling protein-2 (UCP2), glucocorticoid receptor (GR) and 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) mRNA in the sheep lung from the time of uterine attachment to 6 months of age. The effect of maternal nutrient restriction on lung development was determined in early to mid gestation (i.e. 28–80 days gestation, period of maximal placental growth, and embryonic and pseudoglandular stages of fetal lung development) and late gestation (i.e. 110–147 days gestation, period of maximal fetal growth, and canalicular and saccular stages of fetal lung development). Fetal lungs were sampled at 80 and 140 days (term ∼148 days) gestation, and sheep lungs at 1, 7, 30 days and 6 months. GR and 11βHSD1 mRNA were maximal at 140 days gestation, whereas UCP2 mRNA peaked at 1 day of age and then declined with postnatal age. Maternal nutrient restriction in both early-to-mid and late gestation had no effect on lung weight, but increased UCP2, GR and 11βHSD1 mRNA abundance at every sampling age. These findings suggest that the developmental ontogeny of UCP2 mRNA in the ovine lung is under local glucocorticoid hormone action and that maternal nutrient restriction has long-term consequences for UCP2 and GR mRNA abundance in the lung irrespective of its timing.
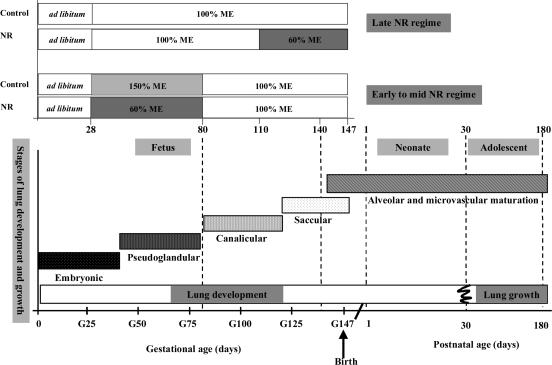
Epidemiological studies of infants, children and adults indicate that prenatal compromises that restrict feto-placental growth and cause low birth weight increase the risk of respiratory deficiencies after birth (Barker et al. 1991). The lung appears to have a limited ability to recover from early compromised development, which can permanently impair lung structures (Harding, 1995; Cock et al. 2001). Lung growth in utero can be adversely affected by factors associated with fetal growth restriction, including fetal hypoxaemia and reduced maternal nutrient supply (Symonds et al. 1995; Harding et al. 2000). The extent to which the timing of maternal nutrient restriction may have differential effects on fetal lung development is not known, although maternal undernutrition in late gestation in the sheep, when lung growth is most rapid, reduces lung growth (Harding & Johnston, 1995; Symonds et al. 1995). It is likely that nutrient restriction targeted between early and mid gestation (period of maximal placental growth, and embryonic and pseudoglandular stages of fetal lung development) may have a different outcome compared with later in gestation (period of maximal fetal growth, and canalicular and saccular stages of fetal lung development), as this represents very different phases of lung development (Kotecha, 2000) (Fig. 1).
Figure 1. Phases of fetal lung development and postnatal lung growth in relation to periods of maternal nutrient restriction in the sheep.
The phases of fetal lung development are as follows: embryonic, 0–40 days; pseudoglandular, 40–80 days; canalicular, 80–120 days; saccular, 120 to term 148 days gestation. In early to mid (28–80 days gestation) maternal nutrient restriction (NR), lungs were sampled in the fetus at 80 and 140 days gestation and sheep at 180 days (6 months), while in late (110–147 days gestation) maternal NR, lungs were sampled at 1 and 30 days postnatal age. Nutrient restricted mothers received 60% of their metabolisable energy (ME) requirements for maternal metabolism and fetal growth (see Methods) and control mothers received 100 (late gestational NR) to 150% (early to mid gestational NR) ME requirements during period of NR. G = gestational age (adapted from Harding, 1994).
Restricted maternal nutrition during pregnancy normally results in a reduction in plasma concentrations of a range of anabolic hormones in the fetus, including insulin, insulin-like growth factors and thyroid hormones (Bauer et al. 1995; Rae et al. 2002). Fetal plasma cortisol does not appear to be substantially affected by maternal nutrient restriction, even when there is a transient rise in maternal plasma cortisol in late gestation (Edwards & McMillen, 2001). The maturation of the fetal lung is dependent on an intact adrenal gland and the effects of glucocorticoid hormones (Fowden et al. 1998). Glucocorticoid hormone action within the lung is regulated by expression of the glucocorticoid receptor (GR) and isoforms of 11β-hydroxysteroid dehydrogenase (11βHSD) at the level of gene transcription. 11βHSD type 1 (11βHSD1) behaves predominantly as an 11-oxoreductase, catalysing the conversion of cortisone to bioactive cortisol and as an intracellular amplifier of glucocorticoid excess to GR (Bamberger et al. 1996; Stewart & Krozowski, 1999). Conversely, 11βHSD type 2 (11βHSD2) behaves as an 11-dehydrogenase, catalysing the inactivation of cortisol to cortisone, and thereby maintains the specificity of the mineralocorticoid receptor for aldosterone (Stewart & Krozowski, 1999). The developmental ontogeny of local glucocorticoid sensitivity in the lung and the impact of maternal nutrient restriction on the fetal and postnatal lung remain undetermined.
Uncoupling protein (UCP) 2, is a member of the of inner mitochondrial membrane carrier subfamily and is highly abundant in the lung (Pecquer et al. 2001). Its function remains a subject of intense debate (Nedergaard & Cannon, 2003) and it has postulated roles in energy balance (Buemann et al. 2001), reactive oxygen species production (Negre-Salvayre et al. 1997), apoptosis (Voehringer et al. 2000) and macrophage-mediated immunity (Arsenijevic et al. 2000). UCP2 shares 56% homology with the brown adipose tissue-specific UCP1 (Erlanson-Albertsson, 2003), whose abundance peaks at birth in the sheep and is no longer detectable after 1 month of postnatal life (Clarke et al. 1997). The extent to which UCP2 shows a similar developmental pattern of expression in the lung is not known. Mitochondrial protein abundance is in part regulated by maternal nutrition (Budge et al. 2003; Mostyn et al. 2003a), with UCP2 and voltage-dependent anion channel being up-regulated in the lungs of offspring born to mothers nutrient restricted in late gestation (Mostyn et al. 2003a). In the rodent, there is an increase in UCP2 mRNA in the lung after birth, which then remains high up to adulthood, and like UCP1 its abundance is strongly influenced by caloric intake (Budge et al. 2004; Xiao et al. 2004). It remains to be established whether similar adaptations occur in large mammals, such as the sheep.
One factor that is established to have a pivotal role in regulating UCP1 abundance in ovine fetal brown adipose tissue is cortisol (Mostyn et al. 2003b), for which the postpartum surge contributes to the rapid increase and activation of UCP1 at birth (Clarke et al. 1997). The potential thermogenic capacity of fetal brown adipose tissue, measured as guanosine diphosphate (GDP) binding, also peaks at birth (Clarke et al. 1997), although this is not affected by manipulation of fetal cortisol (Mostyn et al. 2003b). It is currently unknown whether UCP2 in the fetal lung is similarly regulated by cortisol and whether the fetal or postnatal lung has a potential thermogenic role.
The aims of the present study were therefore to determine (i) the ontogeny of UCP2, GR and 11βHSD1 mRNA, and GDP binding, in the fetal and postnatal lung up to 6 months of age, and (ii) whether maternal nutritional deprivation in early to mid and late gestation resulted in altered abundance of UCP2, GR and 11βHSD1 mRNA in the fetal and postnatal lung up to 6 months of age.
Methods
Ontogeny of lung development
For the ontogeny study, a mixture of Welsh Mountain and Border Leicester × Swaledale sheep were used. We have previously established that with respect to the molecular measurements made in the present study, there are no distinguishable differences between breeds at the same developmental age (M. G. Gnanalingham, J. Dandrea, M. E. Symonds & T. Stephenson, unpublished data). Lungs were sampled from fetuses at 80 and 140 days gestation (term ∼148 days), and sheep after birth at 1, 7, 30 and 180 days (6 months) (n = 6 at each sampling point, 36 sheep in total), following killing with an overdose of barbiturate (200 mg kg−1 pentobarbital sodium; Euthatal; RMB Animal Health, UK). All sheep were born normally at term to mothers that were fed 100% of their total metabolisable energy (ME) requirements (taking into account requirements for both ewe maintenance and growth of the conceptus in order to produce a 4.5 kg lamb at term; Agricultural Research Council, 1980). The tissues were rapidly dissected, weighed and then placed in liquid nitrogen and stored at −80°C until analysed. Lung dry weights were determined by freeze-drying a representative portion of each lung.
Maternal nutritional manipulation on lung development
Study 1. Early to mid nutritional restriction
This study was designed to examine the effects of early to mid gestational nutrient restriction, coinciding with the period of maximal placental growth, on the fetal and adolescent lung in the sheep, and hence used singleton ewes. Thirty-six singleton bearing Welsh Mountain sheep of similar age (median 3 years) and weight (36.1 ± 0.9 kg (mean ± s.e.m.)) were entered into the study and individually housed at 28 days gestation, as described by Bispham et al. (2003) (Fig. 1). Animals were allocated to one of two nutritional groups using stratified randomization by body weight. They were offered either 60% (i.e. nutrient restricted, NR) or 225% (i.e. allowed to feed to appetite) of their calculated ME requirements for both ewe maintenance and growth of the conceptus on the basis of producing a 4.5 kg lamb at term (Agricultural Research Council, 1980). Feed intakes were measured daily, and NR ewes consumed all of the feed offered, whereas ewes fed to appetite consumed 150% of ME requirements because not all of the hay was eaten. Food consumption between 28 and 80 days gestation was 3.2–3.8 MJ day−1 of ME in the NR group (∼60% of ME requirements) or 8.7–9.9 MJ day−1 of ME in the group fed to appetite (∼150% of ME requirements) (Fig. 1). The amount of feed given to each ewe was increased at 43 and 61 days gestation to meet the higher energy requirements associated with growth of the conceptus (Agricultural Research Council, 1980). The diet comprised chopped hay that had an estimated ME content of 7.91 MJ (kg dry matter)−1 and a crude protein content (nitrogen × 6.25) of 69 g (kg dry matter)−1 and barley-based concentrate that had an estimated ME content of 11.6 MJ (kg dry matter)−1 and a crude protein content of 162 g (kg dry matter)−1. The proportion of hay to concentrate fed was approximately 3: 1, with respect to dry weight. All diets contained adequate minerals and vitamins. After 80 days gestation, ewes were offered sufficient feed to meet 100% of the ME requirements as calculated to produce a 4.5 kg lamb. These animals consumed between 6.5 and 7.5 MJ day−1 of ME. For these animals, the amount of feed provided was increased at 100 and 120 days gestation to meet the increased ME requirements that accompany the increase in fetal weight with gestation. In those sheep allowed to go to term, all gave birth normally and the offspring were weaned at 3 months of age. Throughout lactation, ewes were fed hay ad libitum and 1 kg concentrate.
In order to determine the effect of early to mid gestational maternal nutrient restriction on fetal lung development, six sheep within each nutritional group were randomised to tissue sampling at either 80 or 140 days gestation. Each animal was humanely killed following intravenous administration of 200 mg kg−1 pentobarbital sodium. The fetal lung was rapidly dissected and weighed, and a representative portion placed in liquid nitrogen and stored at −80°C until further analysis. The remaining offspring (n = 6 per nutritional group) had their lungs sampled at 180 days (6 months) after birth.
Study 2. Late nutritional restriction
This study was designed to examine the effects of late gestational nutrient restriction, coinciding with the period of maximal fetal growth, on postnatal lung development, i.e. immediately after birth and at 1 month, when UCP abundance is changing most rapidly in the sheep (Mostyn et al. 2003a). In order to reduce the numbers of pregnant animals recruited into the study, twin bearing ewes were used. This also meant that any potential confounding maternal effects on lung development were minimized. Fourteen twin-bearing Border Leicester × Swaledale sheep of similar weight and body condition score were randomly assigned to receive either 60% (nutrient restricted, n = 8) or 100% of ME requirements (controls, n = 6) for the final (110–147 days) month of gestation, as described by Mostyn et al. (2003a) (Fig. 1). All mothers gave birth normally at term, with one randomly selected twin being humanely killed (intravenous 200 mg kg−1 pentobarbital sodium) within 24 h of birth, while the other was reared with the ewe until it was killed at 30 days of age. The lungs were rapidly dissected and weighed, and a representative portion placed in liquid nitrogen and stored at −80°C until further analysis. The maternal diet composition was identical to that described for the early to mid maternal nutrient restriction. All operative procedures and experimental protocols had the required Home Office approval as designated by the Animals (Scientific Procedures) Act 1986.
Laboratory analyses
Messenger RNA detection
Total RNA was isolated from lung tissue using Tri-Reagent (Sigma). In order to maximize sensitivity, a two-tube approach to reverse transcription (RT) was adopted. The conditions used to generate first-strand cDNA RT were: 72°C (5 min), 4°C (2 min), 25°C (5 min), 25°C (10 min), 42°C (60 min), 70°C (10 min) and 8°C (hold). The RT reaction (final volume 20 μl) contained: 5 × cDNA (first-strand), buffer (250 mm Tris-HCl, 40 mm MgCl2, 150 mm KCl, 5 mm dithioerythritol pH 8.5), 2 mm dNTPs, 1 × hexanucleotide mix, 10 units RNase inhibitor, 10 units M-MLV reverse transcriptase and 1 μg total RNA. All these commercially available products were purchased from Roche. The expression of UCP2, GR (type 2) and 11βHSD1 mRNA was determined as described by Brennan et al. (2005). The analysis used oligonucleotide cDNA primers to ovine UCP2, GR and 11βHSD1 genes, generating specific intron spanning products (Table 1). QuantumRNA alternate 18S internal standards (Ambion, catalogue number 1717, Cambridge, UK) were included in the multiplex PCRs. Ribosomal RNA makes up > 80% of total RNA samples, with the majority of that comprised by the 28S and 18S ribosomal RNA species (in mammalian species) (http://ambion.com/techlib). Briefly the PCR programme consisted of an initial denaturation (95°C (15 min)), amplification (stage I, 94°C (30 s); stage II, annealing temperature (30 s); stage III, 72°C (60 s)) and final extension (72°C (7 min); 8°C ‘hold’). The PCR mixture (final volume 20 μl) contained 7 μl diethylpyrocarbonate (DEPC) H2O, 10 μl Thermo-Start PCR Master Mix (50 μl contains 1.25 units Thermo-Start DNA polymerase, 1 × Thermo-Start reaction buffer, 1.5 mm MgCl2 and 0.2 mm each of dATP, dCTP, dGTP and dTTP, catalogue number AB-0938-DC-15 ABgene, Epsom, UK), 1 μl forward primer, 1 μl reverse primer and 1 μl RT (cDNA) product. The annealing temperature and cycle number of all primers were optimized for the relevant tissue (see Table 1). Agarose gel electrophoresis (2.0–2.5%) and ethidium bromide staining confirmed the presence of both the product and 18S at the expected sizes. Densitometric analysis was performed on each gel following image detection using a Fujifilm LAS-1000 cooled charge-coupled device camera (Fuji Photo Film Co. Ltd, Tokyo, Japan) and UCP2, GR, 11βHSD1 and 18S mRNA abundance determined. Consistency of lane loading for each sample was verified and all results expressed as a ratio of a reference sample to 18S ribosomal abundance. All analyses and gels were conducted in duplicate, with appropriate positive and negative controls, and a range of molecular mass markers. The resultant PCR product was extracted (QIAquick gel extraction kit, Qiagen, catalogue number 28704, West Sussex, UK), sequenced and results cross-referenced against the GenBank website to determine specificity of the target gene.
Table 1.
Primer sequences and optimal PCR conditions used in the sheep lung
| Primer set | Product size (BP) | Primer sequence | Annealing temp. (°C) | Cycle number |
|---|---|---|---|---|
| UCP2 | 513 | F 5′-GGG ACT CTG GAA AGG GAC AT-3′ | 59.2 | 27 |
| R 5′-AAG AGA GGG ATG GGG AGA GA-3′ | ||||
| GR (type 2) | 150 | F 5′-ACT GCC CCA AGT GAA AAC AGA-3′ | 58.9 | 30 |
| R 5′-ATG AAC AGA AAT GGC AGA CAT T-3′ | ||||
| 11βHSD1 | 160 | F 5′-GTG CCA GAT CCC TGT CTG GAT-3′ | 58.2 | 31 |
| R 5′-AGC GGG ATA CCA CCT TCT TT-3′ | ||||
| 11βHSD2 | 260 | F 5′-CGC ATT GTG ACC GTA AGC-3′ | 58.5 | 38 |
| R 5′-CAG GCA GGC AGG ATG ATG-3′ | ||||
| 18S | 324 | Ambion Classic II 18S internal standards Catalogue no. 1717 |
UCP2, uncoupling protein-2; GR, glucocorticoid receptor; 11βHSD1, 11β-hydroxysteroid dehydrogenase type 1; 11βHSD2, 11β-hydroxysteroid dehydrogenase type 2.
Guanosine diphosphate (GDP) binding
Mitochondria were prepared from 1 g frozen lung tissue as previously described (Symonds et al. 1992) and the protein content of each preparation determined (Lowry et al. 1951). The thermogenic activity of lung tissue at 140 days gestation, 1 and 7 days postnatal age (n = 4 per time point), was assessed from the in vitro activity of the mitochondrial conductance pathway using GDP at a concentration of 2 μm, with non-specific binding measured using a 200 μm concentration of GDP (Symonds et al. 1992). Mitochondrial protein prepared from perirenal adipose tissue from a 1-day-old lamb acted as the positive control on this assay, since adipose tissue is conventionally used to determine the potential thermogenic capacity. All measurements were made in triplicate.
Statistical analysis
All data are presented as the means ± s.e.m. Statistical analysis with respect to significant differences (P < 0.05) between values obtained from the different ages was determined by one-way analysis of variance with post hoc Bonferroni analysis and between control and nutrient restricted groups by the Mann–Whitney U test. Statistically significant correlations between mRNA abundance were determined by Spearman's rank order test (SPSS v11.0, SPSS Inc., Chicago, IL, USA).
Results
Ontogeny of mRNA abundance and GDP binding in the lung
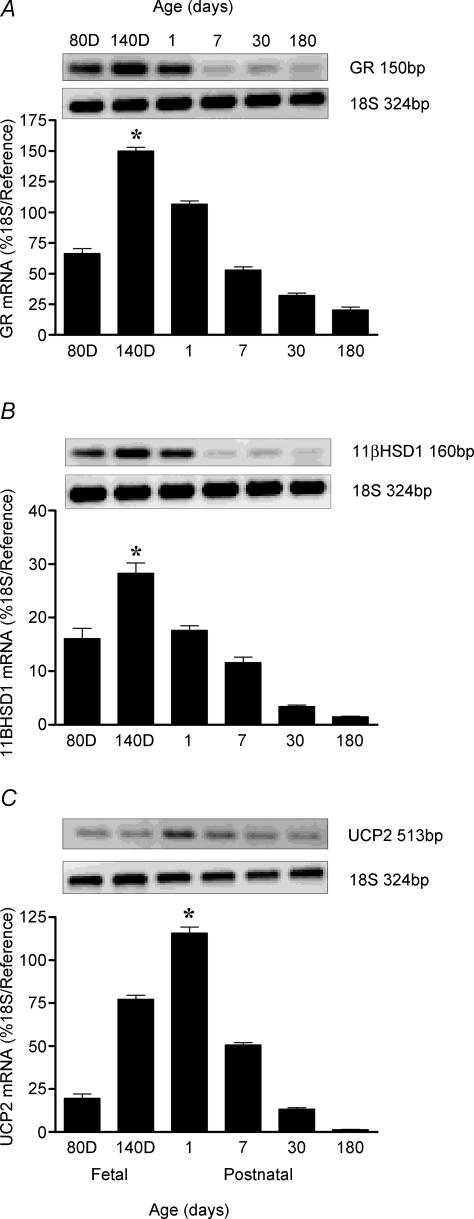
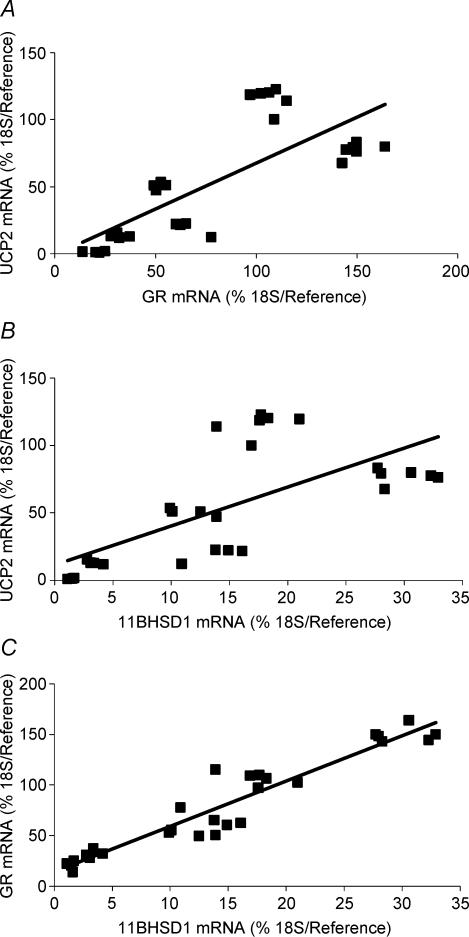
UCP2, GR and 11βHSD1 mRNA was detected in the lung at all sampling ages, with their abundance being developmentally regulated. UCP2 mRNA peaked 1 day after birth, while in contrast both GR and 11βHSD1 mRNA were maximal at 140 days gestation (Fig. 2A–C). The mRNA abundance of all three then decreased with postnatal age. Overall, there was a positive correlation between UCP2 and GR mRNA (P < 0.001), UCP2 and 11βHSD1 mRNA (P < 0.001), and between GR and 11βHSD1 mRNA (P < 0.001) in all lung samples, irrespective of age (Fig. 3A–C). Partial correlation analysis revealed that both GR and 11βHSD1 mRNA regulated UCP2 mRNA independently of each other, although this effect was more pronounced with GR than with 11βHSD1 mRNA (GR: correlation coefficient 0.68, P < 0.0001; 11βHSD1: correlation coefficient 0.41, P < 0.0001).
Figure 2. Ontogeny of (A) glucocorticoid receptor (GR) mRNA (B) 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) mRNA and (C) uncoupling protein-2 (UCP2) mRNA, between the fetus at 80- and 140-days (D) gestation (term ∼148 days) and 6 months of postnatal age in the sheep lung.
Examples of each gene mRNA expression are given. Values are means and s.e.m. (n = 6 per time point). *Maximal abundance detected, significantly (P < 0.01) different from all other age groups, greatest at 180 days (6 months).
Figure 3. Relationships between uncoupling protein-2 (UCP2), glucocorticoid receptor (GR) and 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) mRNA in the sheep lung.
Positive relationships (Spearman's rank order test) were found in all lung samples irrespective of age between the following. A, uncoupling protein-2 (UCP2) mRNA and glucocorticoid receptor (GR) mRNA: r2= 0.59, P < 0.001, where y= 0.68x− 0.75. B, UCP2 mRNA and 11β-hydroxysteroid dehydrogenase type 1 (11βHSD1) mRNA: r2= 0.47, P < 0.001, where y= 2.88x+ 11.64. C, GR and 11BHSD1 mRNA: r2= 0.91, P < 0.001, where y= 4.49x+ 14.15.
There was minimal GDP binding activity in the fetal or postnatal lung (140-day fetal lung: 3.73 ± 1.29; 1-day lung: 3.93 ± 1.31; 7-day lung: 3.47 ± 1.41 pmol (mg mitochondrial protein)−1; NS). The level of activity was therefore approximately 5% of that found in brown adipose tissue (BAT) from the newborn sheep (1-day BAT, 90.45 ± 2.32 pmol (mg mitochondrial protein)−1).
Effect of early to mid gestational maternal nutrient restriction on mRNA abundance in the fetal and sheep lung
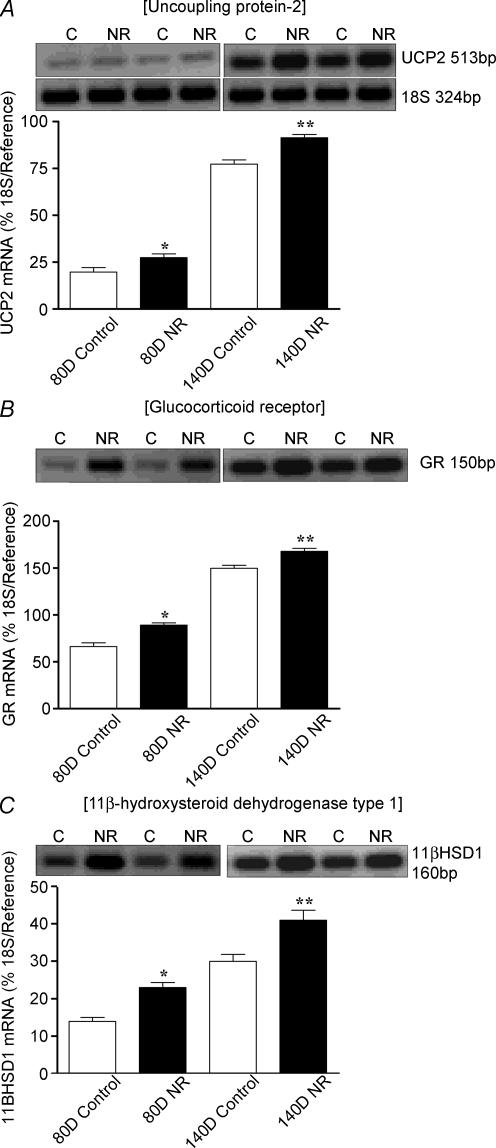
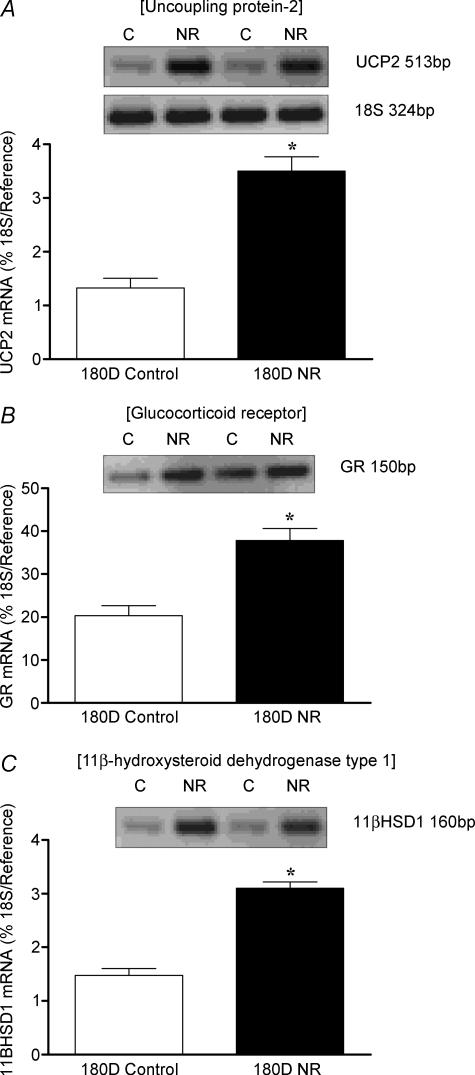
Total body and absolute and relative fresh and dry lung weights (Table 2) were similar between fetuses or offspring born to controls and NR mothers. Early to mid gestational NR up-regulated (P < 0.05) UCP2, GR and 11βHSD1 mRNA at both fetal sampling ages (Fig. 4A–C), an effect that persisted up to 6 months postnatal age (Fig. 5A–C).
Table 2.
Total body, absolute and relative fresh and dry lung weights in fetuses at 80- and 140-days fetus (term ∼148 days), and sheep at 1, 30, 180 days (6 months) in controls (C) and nutrient-restricted (NR; early to mid (28–80 days gestation) or late (110–147 days gestation)) groups
| Controls | Nutrient-restricted | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age (days) | 80D Fetal | 140D Fetal | 1 | 30 | 180 | Early NR 80D Fetal | Early NR 140D Fetal | Late NR 1 | Late NR 30 | Early NR 180 |
| Number | 5 | 6 | 6 | 5 | 6 | 6 | 6 | 6 | 5 | 5 |
| Body weight (kg) | 0.22 ± 0.01 | 4.78 ± 0.22 | 4.28 ± 0.28 | 17.25 ± 1.83 | 33.25 ± 1.41 | 0.24 ± 0.01 | 4.82 ± 0.30 | 4.64 ± 0.48 | 13.92 ± 1.01 | 32.10 ± 2.09 |
| Absolute fresh lung weight (g) | 12.46 ± 0.61 | 144.33 ± 14.27 | 76.05 ± 2.28 | 187.22 ± 34.33 | 311.07 ± 38.58 | 13.13 ± 0.65 | 136.28 ± 9.85 | 92.19 ± 1.97* | 160.30 ± 23.54 | 290.94 ± 38.94 |
| Relative fresh lung weight (per kg body weight) | 56.30 ± 2.43 | 30.21 ± 2.72 | 17.97 ± 0.68 | 10.75 ± 1.64 | 9.52 ± 1.31 | 56.08 ± 2.51 | 28.46 ± 1.66 | 20.40 ± 1.70 | 11.94 ± 1.90 | 9.18 ± 1.21 |
| Absolute dry lund weight (g) | 1.38 ± 0.11 | 13.44 ± 1.18 | 16.04 ± 0.56 | 36.24 ± 7.88 | 62.00 ± 6.94 | 1.46 ± 0.12 | 12.86 ± 0.90 | 19.52 ± 0.55* | 31.47 ± 3.92 | 59.28 ± 7.94 |
Values are means and their standard errors and
P < 0.05, significant difference from control group.
Figure 4. Effect of early to mid maternal nutrient restriction on the abundance of (A) UCP2 (B) GR and (C) 11βHSD1 mRNA in fetal lungs sampled at 80 and 140 days (D) gestation (term ∼148 days), from ewes that consumed 60% (nutrient restricted, NR) or 100% (control) of their metabolisable energy requirements for maternal metabolism and fetal growth between 28 and 80 days gestation.
Examples of mRNA expression are given in each nutritional group. Values are means and s.e.m. (n = 6 per group). *P < 0.05, **P < 0.01, mean value significantly different from control group.
Figure 5. Effect of early to mid maternal nutrient restriction on the abundance of (A) UCP2 (B) GR (C) and 11βHSD1 mRNA in sheep lungs sampled at 180 days (D; 6 months) postnatal age, from ewes that consumed 60% (nutrient restricted, NR) or 100% (control) of their metabolisable energy requirements for maternal metabolism and fetal growth between 28 and 80 days gestation.
Examples of mRNA expression are given in each nutritional group. Values are means and s.e.m. (n = 6 per group). *P < 0.05, mean value significantly different from control group.
Effect of late gestational maternal nutrient restriction on mRNA abundance in the sheep lung
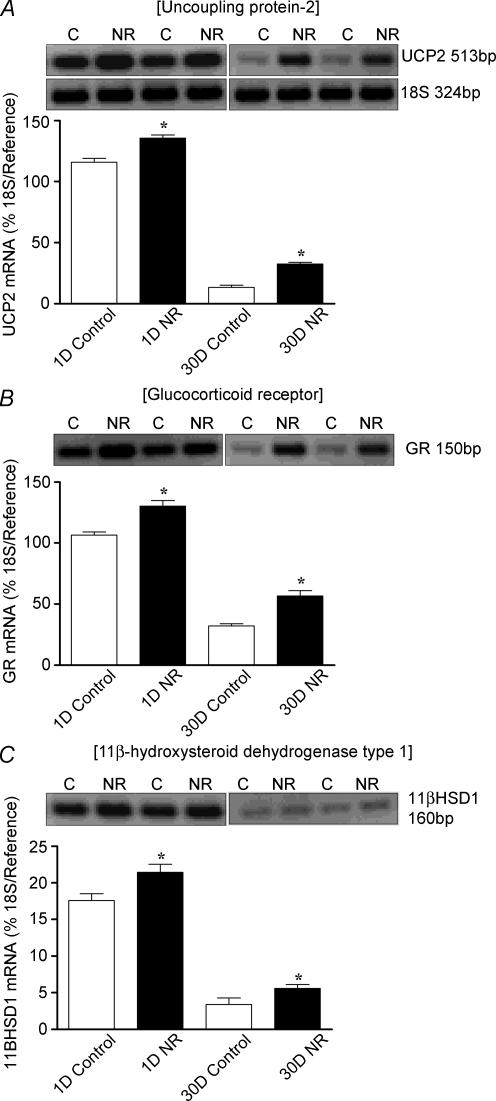
Total body and absolute and relative fresh and dry lung weights were similar between controls and NR, except in the 1-day age group, where there was a significant increase in fresh and dry lung weight in the NR group (Table 2). Late gestational maternal NR resulted in up-regulation (P < 0.05) of UCP2, GR and 11βHSD1 mRNA abundance at 1-day, an effect that persisted at 30 days postnatal age (Fig. 6A–C).
Figure 6. Effect of late maternal nutrient restriction on the abundance of (A) UCP2 (B) GR (C) and 11βHSD1 mRNA in lungs sampled at 1 and 30 days (D) of age, from ewes that consumed 60% (nutrient restricted, NR) or 100% (control) of their metabolisable energy requirements for maternal metabolism and fetal growth between 110 and 147 days gestation (term ∼148 days).
Examples of mRNA expression are given in each nutritional group. Values are means and s.e.m. (n = 6 per group). *P < 0.05, mean value significantly different from control group.
Discussion
Ontogeny of mRNA in the ovine lung
We have shown for the first time the developmental ontogeny of UCP2 and local glucocorticoid action in the fetal and postnatal lung in the sheep, as determined by the abundance of GR and 11βHSD1 mRNA. The current study has emphasized the developmental link between UCP2 and cortisol, with peak abundance in GR and 11βHSD1 mRNA close to term, and UCP2 mRNA just after birth. This suggests a critical role for UCP2 in the peripartum period and that the effect on the ovine lung is directly linked to fetal development, since UCP2 mRNA is abundant in the fetal lung prior to the appearance of large amounts of protein (Mostyn et al. 2003a). The developmental ontogeny of UCP2 mRNA mirrors that of the brown adipose tissue-specific UCP1, which has an essential role in non-shivering thermogenesis in the newborn (Clarke et al. 1997). A thermogenic role is very unlikely for UCP2, considering the low GDP binding activity in the fetal and postnatal lung. The developmental ontogeny of UCP2 mRNA in the ovine lung appears to be markedly different from that in the rodent lung, where the concentration of UCP2 mRNA was low and unchanged during late gestation, doubled within 6 h after birth, to remain high to adulthood (Xiao et al. 2004). This discrepancy in the developmental ontogeny of UCP2 between small and large mammals may be due to rodents having an immature hypothalamic–pituitary axis at birth, as further indicated by increasing thermogenesis with postnatal age in the rodent, which is not apparent in the human infant or lamb (Giralt et al. 1990; Nedergaard et al. 1999).
The maturation of the fetal lung is dependent on an intact fetal adrenal and thyroid gland (Symonds & Clarke, 1996; Fowden et al. 1998). Potential glucocorticoid and thyroid response elements have also been identified in the promoter region of human UCP2 (Tu et al. 1999), suggesting that the developmental ontogeny of UCP2 mRNA expression in the sheep lung could be directly or indirectly regulated by glucocorticoids and thyroid hormones, as is the case for brown adipose tissue-specific UCP1 (Mostyn et al. 2003b). In the sheep, fetal plasma cortisol increases with gestational age, peaking at the time of birth, before declining rapidly with postnatal age (Fowden et al. 1998; Bispham et al. 2003; Mostyn et al. 2003b). While, there has been no comparative study examining the developmental ontogeny of UCP2 and its potential regulation by local glucocorticoid hormone action via GR and 11βHSD1, there is some indirect evidence that glucocorticoids and thyroid hormones are important in the regulation of UCP2 expression in the lung. Triiodothyronine administration up-regulates lung UCP2 mRNA 6 h after birth, whereas the antithyroid drug, propylthiouracil, partially blocks the early postnatal rise in UCP2 mRNA in rodents (Xiao et al. 2004). In sheep, the increase in maternal and fetal plasma thyroxine with gestational age towards term (Bispham et al. 2003; Fraser & Liggins, 1989) may contribute to the increase in UCP2 mRNA in the peripartum period. However, in the case of maternal nutrient restriction, both maternal and fetal plasma thyroid hormones are reduced (Rae et al. 2002; Bispham et al. 2003), and so are unlikely in this case to cause the up-regulation of UCP2 in NR fetuses and offspring. In the case of glucocorticoids directly or indirectly regulating UCP2 expression in the lung, successive injections of lipopolysaccharide and dexamethasone, or N-acetyl-cysteine prevented the induction of UCP2 in the mouse lung, suggesting that oxygen free radical generation plays a role in UCP2 regulation (Pecquer et al. 2001). Further studies are obviously warranted to determine the exact function and regulation of UCP2 in the lung.
Maternal nutrient restriction and the programming of lung development
In addition, we have also shown for the first time, the long-term programming of the ovine lung at the level of the mitochondria and GR by reduced maternal nutrition through pregnancy. Up-regulation in lung UCP2 mRNA abundance with maternal nutrient restriction extends results from previous acute nutritional studies in adult rodents, where lung UCP2 mRNA and protein abundance increased fivefold within 12 h of calorie restriction or 24 h of fasting (Pecqueur et al. 2001; Xiao et al. 2004). Furthermore, there is an up-regulation of UCP2 protein in the lungs of offspring at 30 days of age born to nutrient restricted mothers in late gestation (Mostyn et al. 2003a), which is positively correlated (r2= 0.57, P= 0.009) with UCP2 mRNA abundance, as determined in this study. The exact mechanism mediating this up-regulation in UCP2 mRNA with maternal nutrient restriction is uncertain, but our present findings suggest a potential role for local glucocorticoid action, with GR and 11βHSD1 mRNA also increased in the fetal and sheep lung up to 6 months of age. The increased local glucocorticoid action in the fetal and sheep lung at 6 months of postnatal age following early to mid maternal nutrient restriction is in accord with Whorwood et al. (2001), who demonstrated increased GR mRNA in the neonatal sheep lung following a similar nutritional insult. In the present study, this adaptation within the lung was irrespective of the timing of maternal nutrient restriction in early to mid (period of maximal placental growth, and embryonic and pseudoglandular stages of fetal lung development) or late (period of maximal fetal growth, and canalicular and saccular stages of fetal lung development) gestation. Xiao et al. (2004) proposed that free fatty acids (FFA) regulate lung UCP2 mRNA in both adult and neonatal rodents, since calorie restriction caused a rapid increase in FFA, and lung UCP2 mRNA was increased by FFA administration to fed animals. In sheep, whilst there is an increase in maternal plasma FFA between 80 and 140 days gestation, there were no differences in maternal or fetal plasma NEFA after 80 days gestation following early to mid maternal nutrient restriction (Clarke et al. 1998; Bispham et al. 2003). Other potential nutritional mediators include glutathione, for which there is a marked reduction in plasma concentration with starvation (Smith & Anderson, 1992). This may up-regulate UCP2 expression in the lung by increasing levels of intracellular reactive oxygen species (Pecqueur et al. 2001). A similar mechanism has been proposed for the increase in lung UCP2 with lipopolysaccharide injection, whereby macrophage receptors stimulate the production of proinflammatory cytokines, such as tumour necrosis factor α (Ryan et al. 1997), which activates the nuclear factor-κB pathway, again increasing levels of intracellular reactive oxygen species (Pecqueur et al. 2001). However, it is currently uncertain what role glutathione has on the fetus at the time of nutrient restriction, in particular within the lung (Gauthier et al. 2004).
In the present nutrient restriction studies, despite the 60% reduction in total ME requirements in either early to mid or late gestation, there were no consistent inhibitory effects on whole body or fresh lung weights between controls and NR groups, which is in accord with other studies using a comparable magnitude of nutrient restriction (Edwards et al. 2001; Yuen et al. 2002). Similarly, the transition from consuming 150% to 100 or 150% of total ME requirements after 80 days gestation, has no effect on lung weight at term (M. E. Symonds & T. Stephenson, unpublished results). Compensatory responses to maternal and fetal nutrient restriction are therefore occurring to maintain fetal growth. One consequence of these adaptations is an up-regulation of mitochondrial protein and receptor abundance, which is dissociated from any effects on tissue growth. The increased mitochondrial protein and receptor abundance within the perinatal period could be inferred as a beneficial response to extrauterine adaptation within the lung, especially with the establishment of independent breathing. However, the increased abundance with postnatal life may have deleterious consequences in terms of lung function (Symonds et al. 1993), as an increased abundance of UCP2 has been shown to result in enhanced susceptibility to infection and death from toxoplasmosis in rodents (Arsenijevic et al. 2000).
A limitation of our UCP2 mRNA results is the lack of corresponding protein levels, since subsequent bleeds of rabbits producing the UCP2 antibody described by Pecqueur et al. (2001) no longer detected ovine UCP2. While UCP2 mRNA is expressed in a variety of tissues (Ricquier & Bouillaud, 2000), UCP2 protein expression is limited to the spleen, lungs, stomach and white adipose tissue, due to translational regulation of the UCP2 mRNA by an upstream open reading frame located in exon 2 of the UCP2 gene, which strongly inhibits the expression of the protein (Pecqueur et al. 2001). However, when we compared our UCP2 mRNA data with the UCP2 protein published by Mostyn et al. (2003a), we found a significant positive correlation between controls and NR offspring at 30 days of life, confirming the translation of mRNA into protein.
In conclusion, the developmental ontogeny of UCP2 mRNA suggests a critical role in the newborn period and potential regulation by local glucocorticoid hormone action via GR and 11βHSD. Critically, the rate of change of UCP2 mRNA in the fetal and sheep lung can be significantly altered by maternal nutrient restriction in either early to mid or late gestation, which in the lung may compromise its function in later life.
Acknowledgments
This work was funded in part by the Special Trustees for Nottingham University Hospitals.
References
- Agricultural Research Council. The Nutritional Requirements of Ruminant Livestock. Slough, UK: Commonwealth Agricultural Bureau; 1980. Requirements for energy; pp. 115–119. [Google Scholar]
- Arsenijevic D, Onuma H, Pecquer C, Raimbault S, Manning BS, Miroux B, et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Godfrey KM, Fall C, Osmond C, Winter PD, Shaheen SO. Relation of birth weight and childhood respiratory infection to adult lung function and disease from obstructive airways disease. BMJ. 1991;303:671–675. doi: 10.1136/bmj.303.6804.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer MK, Breier BH, Harding J, Veldhuis JD, Gluckman PD. The fetal somatotrophic axis during long term maternal undernutrition in sheep; evidence of nutritional regulation in utero. Endocrinology. 1995;136:1250–1257. doi: 10.1210/endo.136.3.7867579. [DOI] [PubMed] [Google Scholar]
- Bispham J, Gopalakrishnan GS, Dandrea J, Wilson V, Budge H, Keisler DH, et al. Maternal endocrine adaptation throughout pregnancy to nutritional manipulation: consequences for maternal plasma leptin and cortisol and the programming of fetal adipose tissue development. Endocrinology. 2003;144:3575–3585. doi: 10.1210/en.2003-0320. [DOI] [PubMed] [Google Scholar]
- Brennan KA, Gopalakrishnan GS, Rhind SM, Kyle CE, Brooks AN, Rae MT, et al. Impact of maternal undernutrition and fetal number on glucocorticoid, growth hormone and insulin-like growth factor receptor mRNA abundance in the ovine fetal kidney. Reproduction. 2005;129:151–159. doi: 10.1530/rep.1.00229. [DOI] [PubMed] [Google Scholar]
- Budge H, Dandrea J, Mostyn A, Evens Y, Watkins R, Sullivan C, et al. Differential effects of fetal number and maternal nutrition in late gestation on prolactin receptor abundance and adipose tissue development in the neonatal lamb. Pediatr Res. 2003;53:302–308. doi: 10.1203/01.PDR.0000047653.73271.C4. [DOI] [PubMed] [Google Scholar]
- Budge H, Edwards LJ, McMillen IC, Bryce A, Warnes K, Pearce S, et al. Nutritional manipulation of fetal adipose tissue deposition and uncoupling protein 1 messenger RNA abundance in the sheep: differential effects of timing and duration. Biol Reprod. 2004;71:359–365. doi: 10.1095/biolreprod.103.018986. [DOI] [PubMed] [Google Scholar]
- Buemann B, Schierning B, Toubro S, Bibby BM, Sørensen T, Dalgaard L, et al. The association between the val/ala-55 polymorphism of the uncoupling protein 2 gene and exercise efficiency. Int J Obes Relat Metab Disord. 2001;25:467–471. doi: 10.1038/sj.ijo.0801564. [DOI] [PubMed] [Google Scholar]
- Clarke L, Heasman L, Firth K, Symonds ME. Influence of route of delivery and ambient temperature on thermoregulation in newborn lambs. Am J Physiol. 1997;272:R1931–R1939. doi: 10.1152/ajpregu.1997.272.6.R1931. [DOI] [PubMed] [Google Scholar]
- Clarke L, Heasman L, Juniper DT, Symonds ME. Maternal nutrition in early-mid gestation and placental size in sheep. Br J Nutr. 1998;79:359–364. doi: 10.1079/bjn19980060. [DOI] [PubMed] [Google Scholar]
- Cock ML, Camm EJ, Louey S, Joyce BJ, Harding R. Postnatal outcomes in term and preterm lambs following fetal growth restriction. Clin Exp Pharmacol Physiol. 2001;28:931–937. doi: 10.1046/j.1440-1681.2001.03552.x. [DOI] [PubMed] [Google Scholar]
- Edwards LJ, McMillen IC. Maternal undernutrition increases arterial blood pressure in the sheep fetus during late gestation. J Physiol. 2001;533:561–570. doi: 10.1111/j.1469-7793.2001.0561a.x. 10.1111/j.1469-7793.2001.0561a.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards LJ, Symonds ME, Warnes K, McMillen IC. Responses of the fetal pituitary-adrenal axis to acute and chronic hypoglycaemia during late gestation in the sheep. Endocrinology. 2001;142:1778–1785. doi: 10.1210/endo.142.5.8143. 10.1210/en.142.5.1778. [DOI] [PubMed] [Google Scholar]
- Erlanson-Albertsson C. The role of uncoupling proteins in the regulation of metabolism. Acta Physiol Scand. 2003;178:405–412. doi: 10.1046/j.1365-201X.2003.01159.x. 10.1046/j.1365-201X.2003.01159.x. [DOI] [PubMed] [Google Scholar]
- Fowden AL, Li J, Forhead AJ. Glucocorticoids and the preparation for life after birth: are there long-term consequences of the life insurance? Proc Nutr Soc. 1998;57:113–122. doi: 10.1079/pns19980017. [DOI] [PubMed] [Google Scholar]
- Fraser M, Liggins The effect of cortisol on thyroid hormone kinetics in the ovine fetus. J Dev Physiol. 1989;11:207–211. [PubMed] [Google Scholar]
- Gauthier TW, Ping XD, Harris FL, Wong M, Elbahesh H, Brown LA. Fetal alcohol exposure impairs alveolar macrophage function via decreased glutathione availability. Pediatr Res. 2004;57:76–81. doi: 10.1203/01.PDR.0000149108.44152.D3. 10.1203/01.PDR.0000149108.44152.D3. [DOI] [PubMed] [Google Scholar]
- Giralt M, Martin I, Iglesias R, Vinas O, Villarroya F, Mampel T. Ontogeny and perinatal modulation of gene expression in rat brown adipose tissue. Eur J Biochem. 1990;193:297–302. doi: 10.1111/j.1432-1033.1990.tb19336.x. 10.1111/j.1432-1033.1990.tb19336.x. [DOI] [PubMed] [Google Scholar]
- Harding R. Development of the respiratory system. In: Thorburn GD, Harding R, editors. Textbook of Fetal Physiology. 1. Oxford Medical Publications, Oxford University Press; 1994. pp. 140–167. [Google Scholar]
- Harding R. Sustained alterations in postnatal respiratory function following sub-optimal intra-uterine conditions. Reprod Fertil Dev. 1995;7:431–441. doi: 10.1071/rd9950431. [DOI] [PubMed] [Google Scholar]
- Harding R, Cock ML, Louey S, Joyce BJ, Davey MG, Albuquerque CA, et al. The compromised intrauterine environment: implications for future lung health. Clin Exp Pharmacol Physiol. 2000;27:965–974. doi: 10.1046/j.1440-1681.2000.03379.x. 10.1046/j.1440-1681.2000.03379.x. [DOI] [PubMed] [Google Scholar]
- Harding JE, Johnston BM. Nutrition and fetal growth. Reprod Fertil Dev. 1995;7:539–547. doi: 10.1071/rd9950539. [DOI] [PubMed] [Google Scholar]
- Kotecha S. Lung growth: implications for the newborn infant. Arch Dis Child Fetal Neonatal Edition. 2000;82:F69–F74. doi: 10.1136/fn.82.1.F69. 10.1136/fn.82.1.F69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mostyn A, Pearce S, Budge H, Elmes M, Forhead AJ, Fowden AL, et al. Influence of cortisol on adipose tissue development in the fetal sheep during late gestation. J Endocrin. 2003b;176:23–30. doi: 10.1677/joe.0.1760023. 10.1677/joe.0.1760023. [DOI] [PubMed] [Google Scholar]
- Mostyn A, Wilson V, Dandrea J, Yakubu DP, Budge H, Alves-Guerra MC, et al. Ontogeny and nutritional manipulation of mitochondrial abundance in adipose tissue and the lungs of postnatal sheep. Br J Nutr. 2003a;90:323–328. doi: 10.1079/bjn2003912. 10.1079/BJN2003912. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Cannon B. The ‘novel’‘uncoupling’ proteins UCP2 and UCP3: what do they really do? Pros and cons for suggested functions. Exp Physiol. 2003;88:65–84. doi: 10.1113/eph8802502. [DOI] [PubMed] [Google Scholar]
- Nedergaard J, Matthias A, Golozoubova V, Jacobsson A, Cannon B. UCP1: the original uncoupling protein and perhaps the only one? New perspectives on UCP1, UCP2 and UCP3 in the light of the bioenergetics of the UCP1-ablated mice. J Bioenerg Biomemb. 1999;31:475–491. doi: 10.1023/a:1005400507802. 10.1023/A:1005400507802. [DOI] [PubMed] [Google Scholar]
- Negre-Salvayre A, Hirtz C, Carrera G, Cazenave R, Troly M, Salvayre R, et al. A role for uncoupling protein-2 as a regulator of mitochondrial hydrogen peroxide generation. FASEB J. 1997;11:809–815. [PubMed] [Google Scholar]
- Pecquer C, Alves-Guerra M-C, Gelly C, Lévi-Meyrueis C, Couplan E, Collins S, et al. Uncoupling protein-2: in vivo distribution, induction upon oxidative stress and evidence for translational regulation. J Biol Chem. 2001;276:8705–8712. doi: 10.1074/jbc.M006938200. 10.1074/jbc.M006938200. [DOI] [PubMed] [Google Scholar]
- Rae MT, Rhind SM, Kyle CE, Miller DW, Brooks AN. Maternal undernutrition alters triiodothyronine concentrations and pituitary response to GnRH in fetal sheep. J Endocrinol. 2002;173:449–455. doi: 10.1677/joe.0.1730449. 10.1677/joe.0.1730449. [DOI] [PubMed] [Google Scholar]
- Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3, StUCP and AtUCP. Biochem J. 2000;345:161–179. 10.1042/0264-6021:3450161. [PMC free article] [PubMed] [Google Scholar]
- Ryan LK, Golenbock DT, Wu J, Vermeulen MW. Characterisation of proinflammatory cytokine production and expression by murine alveolar macrophage cell lines. In Vitro Cell Dev Biol Anim. 1997;33:647–653. doi: 10.1007/s11626-997-0116-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith LJ, Anderson J. Oxygen-induced lung damage. Relationship to lung mitochondrial glutathione levels. Am Rev Respir Dis. 1992;146:1452–1457. doi: 10.1164/ajrccm/146.6.1452. [DOI] [PubMed] [Google Scholar]
- Stewart PM, Krozowski ZS. 11β-Hydroxysteroid dehydrogenase. Vitam Horm. 1999;57:249–324. [PubMed] [Google Scholar]
- Symonds ME, Bird JA, Clarke L, Gate JJ, Lomax MA. Nutrition, temperature and homeostasis during perinatal development. Exp Physiol. 1995;80:907–940. doi: 10.1113/expphysiol.1995.sp003905. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Bryant MJ, Clarke L, Darby CJ, Lomax MA. Effect of maternal cold exposure on brown adipose tissue and thermogenesis in the neonatal lamb. J Physiol. 1992;455:487–502. doi: 10.1113/jphysiol.1992.sp019313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symonds ME, Clarke L. Influence of thyroid hormones and temperature on adipose tissue development and lung maturation. Proc Nutr Soc. 1996;55:561–569. doi: 10.1079/pns19960048. [DOI] [PubMed] [Google Scholar]
- Symonds ME, Lomax MA, Kenward MG, Andrews DC, Johnson P. Effect of the prenatal maternal environment on the control of breathing during non-rapid eye movement sleep in the developing lamb. J Dev Physiol. 1993;19:43–50. [PubMed] [Google Scholar]
- Tu N, Chen H, Winnikes U, Reinert I, Marmann G, Pirke KM, et al. Molecular cloning and functional characterization of the promoter region of the human uncoupling protein-2 gene. Biochem Biophys Res Commun. 1999;265:326–334. doi: 10.1006/bbrc.1999.1663. 10.1006/bbrc.1999.1663. [DOI] [PubMed] [Google Scholar]
- Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, et al. Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. Proc Natl Acad Sci U S A. 2000;97:2680–2685. doi: 10.1073/pnas.97.6.2680. 10.1073/pnas.97.6.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whorwood CB, Firth KM, Budge H, Symonds ME. Maternal undernutrition during early to midgestation programs tissue-specific alterations in the expression of the glucocorticoid receptor, 11β-hydroxysteroid dehydrogenase isoforms, and type 1 angiotensin II receptor in neonatal sheep. Endocrinology. 2001;142:2854–2864. doi: 10.1210/endo.142.7.8264. 10.1210/en.142.7.2854. [DOI] [PubMed] [Google Scholar]
- Xiao H, Massaro D, DeCarlo Massaro G, Clerch LB. Expression of lung uncoupling protein-2 mRNA is modulated developmentally and by caloric intake. Exp Biol Med. 2004;229:479–485. doi: 10.1177/153537020422900605. [DOI] [PubMed] [Google Scholar]
- Yuen BSJ, Owens PC, McFarlane J, Symonds ME, Edwards LJ, Kauter KG, et al. Circulating leptin concentrations are positively related to leptin mRNA expression in fetus adipose tissue in the pregnant ewe fed at or below maintenance energy requirements during late gestation. Biol Reprod. 2002;67:911–916. doi: 10.1095/biolreprod.101.002931. 10.1095/biolreprod.101.002931. [DOI] [PubMed] [Google Scholar]