Abstract
The time course of cardiac sympathetic nerve activity (CSNA) following acute myocardial infarction (MI) is unknown. We therefore undertook serial direct recordings of CSNA, arterial blood pressure (MAP) and heart rate (HR) in 11 conscious sheep before and after MI, and compared them with 10 controls. Conscious CSNA recordings were taken daily from electrodes glued into the thoracic cardiac nerves. Infarction was induced under pethidine and diazepam analgesia by applying tension to a coronary suture. MI size was assessed by left ventricular planimetry (%) at postmortem, peak troponin T and brain natriuretic peptide levels (BNP). Baroreflex slopes were assessed daily using phenylephrine-nitroprusside ramps. The mean infarcted area was 14.4 ± 2.9%, troponin T 1.88 ± 0.39 μg l−1 and BNP 8.4 ± 1.3 pmol l−1. There were no differences in haemodynamic parameters or CSNA between groups at baseline. MAP and HR remained constant following MI. CSNA burst frequency increased from baseline levels of 55.8 ± 7.1 bursts min−1 to levels of 77.5 ± 8.7 bursts min−1 at 2 h post-MI, and remained elevated for 2 days (P < 0.001). CSNA burst area also increased and was sustained for 7 days following MI (P= 0.016). Baroreflex slopes for pulse interval and CSNA did not change. CSNA increases within 1 h of the onset of MI and is sustained for at least 7 days. The duration of this response may be longer because the recording fields decrease with time. This result is consistent with a sustained cardiac excitatory sympathetic reflex.
Acute myocardial infarction (MI) is the commonest cause of death in industrialized societies (Sleight, 1996). Most deaths occur suddenly, within the first hours of the onset of symptoms, and it is an irony that we remain uncertain about the dominant autonomic mechanisms affecting ischaemic myocardium during this period, when the opportunity to improve outcome is so great. Those who survive to reach hospital, remain at increased risk of heart failure and ventricular arrhythmias. Both of these complications are associated with increased cardiac sympathetic nerve activity (CSNA), which is thought to be of pathogenic importance (Meredith et al. 1991; Rundqvist et al. 1997). CSNA is inversely related to ejection fraction in patients with heart failure, and severely increased in patients surviving ventricular tachycardia. CSNA also increases following MI, but we are uncertain as to the exact time course of this increase (Malliani & Montano, 2003). This is because firstly, MIs are unpredictable, making baseline measurements impossible, and secondly, CSNA cannot be monitored directly in the intact human. Furthermore, previous animal studies of CSNA following MI have not achieved conscious long-term recordings (Ninomiya et al. 1986).
The first recordings of CSNA were from ‘open chest’ anaesthetized animals. Under these experimental conditions, an immediate transient excitatory sympathetic reflex in response to coronary occlusion, was demonstrated 30 years ago (Malliani et al. 1969; Brown & Malliani, 1971). In humans, indirect methods of assessing CSNA, including heart rate variability, baroreflex sensitivity and plasma catecholamine levels, suggest a transient increase in CSNA following MI, but the time course is uncertain (McAlpine et al. 1988; Schwartz et al. 1988; Singh et al. 1996). Furthermore, there is no certainty that these methods actually reflect CSNA. Cardiac norepinephrine spillover, a more direct measure of CSNA, has not been undertaken immediately post-MI (Esler et al. 1988). We therefore report our findings from direct recordings of CSNA in conscious sheep undertaken continuously from 3 days before, to 7 days after, acute MI.
Methods
Animals and procedures
Thirty-nine Coopworth ewes underwent thoracotomy under standard thiopentone, halothane and nitrous oxide general anaesthesia. Data from 10 control animals and 11 animals with MI are reported in this study. The control group underwent identical thoracotomy and recording protocols to the MI group, but did not have a coronary artery suture placed. All procedures were undertaken in accordance with the approval of the Otago University Animal Ethics Committee.
The methods for thoracotomy, ECG recording, carotid artery and jugular vein cannulation, electrode placement, CSNA recording, baroreflex slopes and postoperative analgesia have already been described (Jardine et al. 2002). Briefly, under standard general anaesthesia (thiopentone induction 17 mg kg−1, 2% halothane and 50% nitrous oxide) via a left thoracotomy incision, up to five stainless-steel needle electrodes (0.1 mm diameter) were inserted in the thoracic cardiac nerve(s) and glued with cyanoacrylate. Connecting leads were sutured to the mediastinum, and exteriorized dorsally through the chest wall between the spine and the thoracotomy incision. In the MI sheep, a 3-O proline suture was run under the second diagonal branch of the anterior descending coronary artery using a curved atraumatic needle. Both ends of the suture were threaded into a plastic sheath, and the encircled artery was occluded by applying tension to the suture for 30 s. If no cyanosis of the myocardium was observed or the ECG remained normal, the suture was applied to a larger artery, either the first diagonal or the distal anterior descending coronary artery. The sheath was anchored and run out through the chest wall with the chest drain. Pethidine (50 mg, i.m.) was administered for postoperative analgesia.
CSNA recordings
Sympathetic recordings were made from pairs of electrodes via a preamplifier with an active probe (DAM-80; World Precision Instruments). The raw signal was amplified (×1000), filtered between 300 and 3000 Hz, and integrated using a time constant of 100 ms. Before each recording session, we inspected the signal carefully, both visually and audibly, for signs of interference, including baseline shift, and rapid changes in frequency. The integrated nerve signal was digitally converted using in-house software (sampling rate 200 Hz, 12 bits) and identified by the following characteristics: (a) bursts were synchronized to the arterial pulse; (b) bursts decreased during a 2 mg kg−1i.v. hexamethonium infusion over 2 h on day 3 post-thoracotomy; (c) there was an inverse relationship between burst area and diastolic blood pressure during baroreflex tests undertaken on each recording day (Malpas, 1998). Only recordings with a signal-to-noise ratio of greater than two were analysed. Using these criteria on the first day, the best signal from 10 possible electrode combinations was selected and used for subsequent recordings. CSNA was quantified by: (a) counting the number of bursts per minute (burst frequency); (b) counting the number of bursts per 100 heart beats (burst incidence); and (c) measuring the area under the integrated signal per minute (burst area).
Baseline recordings
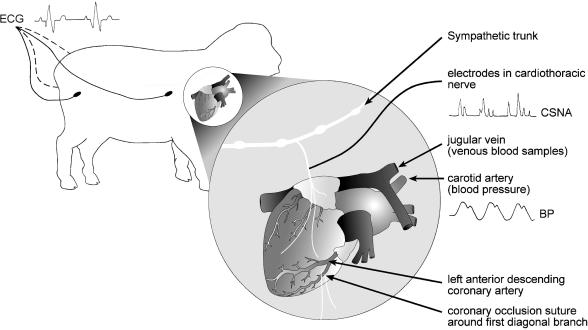
A schematic diagram of the experimental design is shown in Fig. 1. All recordings were undertaken by the same technician in a dedicated room over a 5 min period between 09.00 and 11.00 h each day while the animals were standing quietly in cages. Prior to daily recordings, baroreflex slopes were undertaken, using boluses of intravenous phenylephrine and nitroprusside (150 μg) to achieve a minimum change in mean blood pressure (MBP) of 20 mmHg. Daily recordings consisted of simultaneous blood pressure (BP), heart rate (HR) and CSNA levels measured each minute, and then averaged over the 5 min recording period every day. Care was taken to ensure the animals were not disturbed, and recordings were only used if the variables were stable over the preceding 10 min. After 11.00 h, the animals were disconnected from the recording devices for the subsequent 22 h.
Figure 1. Schematic presentation of the methodology for recording cardiac sympathetic nerve activity (CSNA) from the cardiothoracic nerve in conscious sheep.
Arterial and venous catheters were positioned in the common carotid artery and jugular veins via a neck incision. Intradermal ECG electrodes were positioned at each quarter. The coronary suture was around the first diagonal branch of the left anterior descending artery.
Myocardial infarctions
All infarcts were induced between 4 and 6 days post-thoracotomy. On the morning of infarction, after baseline measurements, the animal received analgesia and sedation while recordings continued. Pethidine 50 mg and diazepam 10 mg were each given i.v. over 1 min, separated by at least 1 min. Pethidine caused a temporary decrease in CSNA, but with no haemodynamic effects. Diazepam caused a sudden increase in BP over 30 s, associated with slowing of the HR and decreased CSNA. If the animal did not appear sedated, further pethidine and diazepam were given before infarction. When parameters returned to baseline and remained stable, usually 5 min later, MI was induced by applying progressive tension to the ends of the coronary suture until ST depression was observed on the ECG. This occurred usually within 30 s. The suture was then fixed inside the sheath with a strong artery clamp to maintain the occlusion. Monitoring continued for 3 h following the onset of MI, and measurements were taken over 5 min periods at 30, 60, 120 and 180 min post-occlusion. The suture remained clamped for 24 h. Further analgesia and sedation were given as required. Post-MI, measurements were undertaken with the animals fully conscious each day for between 5 and 8 days.
Infarct size and postmortem
Venous blood was taken from the jugular venous catheter for measurements of creatine kinase, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) at 2, 4, 6 and 24 h post-MI. ANP, BNP and troponin T levels were measured 2, 3, 4 and 7 days post-MI. A postmortem, following high-dose phenobarbitone euthanasia, was done 7 days post-MI, at which time the infarct was clearly demarcated by visual inspection. This was verified by histological analysis of 1 mm-thick sections through the area of immediately surrounding myocardium. The infarcted area was circumcised, measured by planimetry and expressed as a percentage of the total area of the left ventricle.
Statistics
Results are expressed as means ± s.e.m. Two-way ANOVA, with time as a repeated measure, was used to determine time and intervention differences between the MI and control groups. Significance was assumed at P < 0.05. Where significant differences were identified by ANOVA, a priori Fisher's protected least-square difference tests were used to identify in MI animals time points significantly different from time-matched controls. Correlation studies on the MI group were undertaken using Pearson's correlation coefficient and Spearman's nonparametric test. Change in BP during drug ramps did not usually include the hypotension-saturation part of the baroreflex sigmoid curve, thus we used linear regression to calculate slopes. Correlations were not improved by using an exponential curve. For CSNA, 5 s packets of burst area were normalized, and each curve was plotted from greater than 15 co-ordinates. Only curves with R > 0.4 were analysed. For pulse interval, each curve was plotted from greater than 100 co-ordinates, and only curves with R > 0.7 were analysed. For both CSNA and pulse interval, pre- and post-MI slopes were averaged from three consecutive (daily) slopes before and after MI.
Results
Satisfactory CSNA recording fields, lasting for a minimum of 10 days post-thoracotomy, were obtained in 21 of 39 (54%) animals. Data from the remaining animals were not analysed in this study.
Markers of myocardial damage
In the experimental MI group (n = 11), constriction of the coronary artery suture induced ECG changes consistent with MI (data not shown). Both plasma troponin T (baseline: 0.017 ± 0.06 μg l−1versus peak level measured days 1–4: 1.88 ± 0.39 μg l−1) and creatine kinase (baseline: 110 ± 15.7 units l−1versus peak level measured 2–24 h: 1014 ± 328 units l−1) were raised following coronary artery constriction. Plasma ANP (baseline: 15.0 ± 1.6 versus peak: 31.2 ± 8.2 pmol l−1) and BNP (baseline: 4.3 ± 0.7 versus peak: 8.4 ± 1.3 pmol l−1) levels were also increased following coronary artery constriction. Taken together, changes in these biochemical indices are consistent with coronary artery constriction inducing small- to moderate-sized infarcts. Indeed, postmortem macroscopic examination of the hearts revealed well-defined transmural infarcts with a mean size of 14.4 ± 2.9% of the left ventricle (range 4–38%).
Haemodynamic and CSNA response
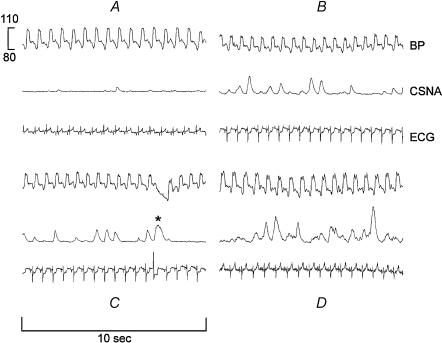
Sample recordings obtained from one sheep demonstrating the effect of experimental MI on haemodynamics and CSNA are shown in Fig. 2. Briefly, BP remained constant post-MI. T-wave flattening was seen on the ECG within 1 min of coronary occlusion, and normalized the following day. CSNA burst amplitude and frequency were relatively constant for the first hour after occlusion, but both were increased at 2 h post-MI. The rise in CSNA was sustained during the 3 h recording period, and remained elevated during the next 7 days.
Figure 2. Effects of myocardial infarction on blood pressure (BP), CSNA and ECG recordings from a single animal.
A, baseline, 20 min before coronary occlusion. B and C, 60 min following occlusion. D, 7 days following occlusion. ST depression was seen during the early phase of infarction (B and C). In C, note the broad burst of CSNA (asterisk) in response to the rapid fall in diastolic BP following a ventricular ectopic beat.
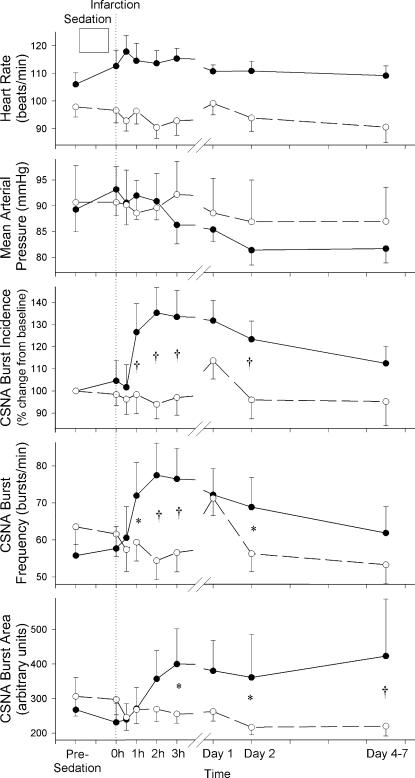
Mean arterial pressure (MAP), HR and CSNA data for the 11 MI and 10 control sheep are summarized in Fig. 3. Baseline levels for HR, MAP, burst frequency and burst area were not significantly different between the two groups. By contrast, baseline levels for burst incidence were significantly higher in the control group compared with the MI group (65.7 ± 5.4 versus 52.7 ± 6.9 bursts (100 beats)−1, P < 0.05). Therefore, burst incidence data are presented as percentage change from baseline. HR tended to be higher in the MI group throughout the study, but there was no significant treatment–time interaction between the groups. MAP tended to be reduced following MI, but again there was no significant treatment–time interaction between the two groups. CSNA burst frequency, which remained relatively stable in the control group, was increased in response to MI (P < 0.001), being raised from baseline levels of 55.8 ± 7.1 bursts min−1 to peak levels at 77.5 ± 8.7 bursts min−1 at 2 h post MI. Burst frequency remained significantly elevated above time-matched control until day 2 post-MI. Similarly CSNA burst area, which was relatively stable in the control group, was significantly raised in response to experimental MI (P= 0.016). Again there was an acute rise following MI (significant by 3 h), with levels remaining higher than time-matched control data for the duration of the recording period. CSNA burst incidence (presented as percentage change from baseline) shows a similar pattern of response to experimental MI (P= 0.046).
Figure 3. Serial heart rate, mean arterial pressure and CSNA measurements from experimental myocardial infarction and normal control sheep.
Results are expressed as means ± s.e.m. CSNA burst incidence (P= 0.046), burst frequency (P= 0.001) and burst area (P= 0.016) were all raised in the infarcted group (n = 11, •) compared with control sheep (n = 10, ○). Individual time points significantly different from time-matched data (Fisher's protected least-squares difference from two-way ANOVA) are indicated as follows: *P < 0.05, †P < 0.01.
Correlation with infarct size
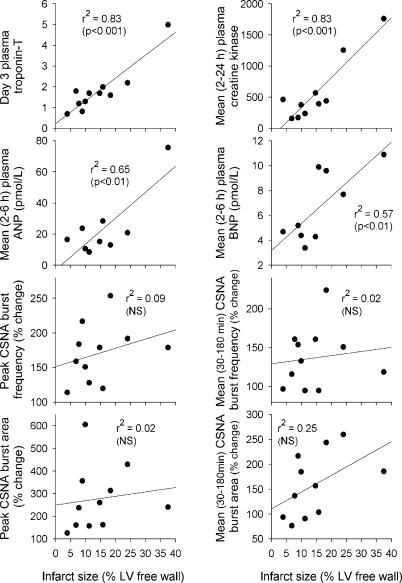
As seen in Fig. 4, infarct size showed a positive correlation with troponin T levels (day 3 post-MI; P < 0.001), creatinine kinase (mean level 2–24 h post-MI; P < 0.001), plasma ANP (mean 2–6 h; P < 0.01) and plasma BNP levels (mean 2–6 h; P < 0.01). By contrast, there was no correlation found between infarct size and any calculated index of the increase in CSNA post-MI.
Figure 4. Plots of neurohumoral markers and CSNA indices with infarct size.
Plasma creatine kinase, troponin T, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP) all correlate with infarct size, whereas CSNA shows no significant correlation to infarct size.
Baroreflex changes post-MI
Using linear correlations, mean normalized CSNA versus MBP slopes were −1.8 ± 0.2 pre-MI and −2.1 ± 0.1 post-MI. Mean pulse interval versus MBP slopes were 10.6 ± 1.5 ms mmHg−1 pre-MI and 9.9 ± 1.2 ms mmHg−1post-MI. For both CSNA and pulse interval, there was no evidence of a change in mean slope following MI. In the control animals, CSNA and pulse interval baroreflex slopes remained constant at equivalent time points.
Discussion
Despite the fact that sympathetic activity has been long implicated in morbidity and mortality associated with MI, little information is available on the time course and degree of sympathetic activation post-MI, and there have been no reports of sequential measurements of cardiac efferent traffic in conscious animals or patients. We have demonstrated, for the first time in a large conscious mammal, a sustained increase in CSNA following experimental MI. This was achieved by obtaining recordings from electrodes glued permanently into postganglionic cardiac thoracic nerves. The increase in CSNA was apparent at 60 min following coronary occlusion, peaked at 120 min, and was sustained for 7 days post-MI in most animals. All indices of CSNA were affected (including burst area, burst frequency and burst incidence) consistent with increased recruitment of postganglionic sympathetic fibres independent of HR, secondary to a central increase in sympathetic tone (Sundlof & Wallin, 1978; Malpas, 1998). We did not observe a simultaneous increase in BP or HR associated with the onset of this activity, probably reflecting a highly differentiated sympathetic response directed only to the myocardium. Coronary occlusion resulted in well-defined transmural anteroapical infarcts, the size of which correlated with creatine kinase, troponin T and plasma ANP and BNP, but not with any indices of CSNA measured.
The majority of information on autonomic function post-MI has been derived from the use of indirect indices. In earlier studies, venous catecholamines were measured sequentially post-MI, and a minor immediate increase was demonstrated which normalized within 12 h unless heart failure supervened (Karlsberg et al. 1981; McAlpine et al. 1988; Sigurdsson et al. 1993; Foy et al. 1995). The contribution of the heart, versus other tissues and organs, to this increase in venous catecholamine levels remains uncertain and, given that plasma levels may be affected by regional variations in their release and by changes in their clearance rate, plasma catecholamines are a crude estimate of sympathetic output (Esler et al. 1990). Cardiac catecholamine spillover studies are more reliable indicators of CSNA, but are too invasive to be used immediately post-MI in patients (Esler et al. 1988). Despite their widespread use, the results of HR variability analysis post-MI are difficult to interpret, and it should be remembered that changes in HR are end-organ effects, reflecting integrated changes in autonomic activity directed to the sinus node, not its components. Time-domain parameters appear to decrease post-MI, consistent with vagal withdrawal. However, low-frequency HR variability, an indicator of CSNA, is also decreased (Casolo et al. 1992; Singh et al. 1996). Baroreflex activity, a measure of cardiac vagal activity, has been shown to correlate inversely with sympathetic activity, and is transiently decreased after MI (Schwartz et al. 1988).
Recent studies by Graham et al. (2002, 2004) have reported MSNA (direct microneurography of the peroneal nerve) measurements at 2–4 days post-MI, and at 3 and 6 months post-MI in humans. Levels were compared with matched normals and patients with cardiac disease. MSNA was increased 2–4 days post-MI, and remained high for at least 6 months. This sympathetic hyperactivity was inversely related to left ventricular ejection fraction (LVEF) and was not significantly related to changes in arterial pressure or body weight, suggesting mechanisms related to central and reflex control from cardiac receptors. The degree of activation observed in these studies (50–60% higher burst incidence in MI patients compared with normal control subjects) is similar to the order of magnitude seen in the present study (30–40% increment from baseline). However, it should be noted that MSNA measurement is restricted to sympathetic outflow directed to the periphery. Although sympathetic excitation is sometimes rather generalized, this is not always the case, and direct experimental studies have shown that under certain circumstances there is great selectivity of sympathetic reflex activity highlighting the danger of extrapolating from sympathetic hyperactivity in one tissue bed to another (Pagani et al. 1974).
Although demonstrating a protracted (6–9 months) rise in MSNA following MI, the earliest time-point measured in the studies by Graham et al. (2002, 2004) was 2–4 days post-MI. This highlights the difficulties in obtaining early (hours) data post-MI in patients who invariably suffer their MI out of hospital. In contrast, the present study, performed in an ovine model, documented sequential measurement of sympathetic activity before and after (hourly for 3 h and then daily to 7 days) experimental MI in a controlled laboratory setting. Hence, we were able to document an acute rise (within an hour) in sympathetic activity that was maintained for the duration of measurements. We cannot comment on the likely total duration of the CSNA response because our recording fields tended to fail from 10 days post-implantation. If anything, this would have caused an apparent decrease in burst amplitude and under-estimation of the increase in CSNA (Jardine et al. 2002). For practical purposes, CSNA recordings for all animals were only undertaken over a limited time (09.00–11.00 h each day), but to capture the circadian rhythms of sympathetic activity, longer recording times will be necessary.
A number of studies have reported cardiac afferent and efferent sympathetic (and vagal) responses to acute coronary artery occlusion in anaesthetized cats (Brown, 1967; Brown & Malliani, 1971; Malliani et al. 1969; Lombardi, 1984; Ninomiya et al. 1986) and dogs (Felder & Thames, 1981). Coronary occlusion in all of these studies has been of very short duration (90–100 s), representing transient ischaemia. Despite these studies being reported over 30 years ago, to date there have been no studies examining CSNA response to permanent coronary occlusion resulting in MI, such has been reported here. Also of note is that there are important differences in the CSNA responses to short-term coronary occlusion between conscious and anaesthetized cats (Ninomiya et al. 1986; Matsukawa et al. 1993). In the conscious state, the CSNA and vagal activity increased simultaneously at the early stage of occlusion, whereas in anaesthetized animals there was no rise in CSNA recorded. These findings highlight the need to perform studies in the conscious state. In the present study, sheep were conscious, but sedated and given analgesia at the time of infarct with diazepam and pethidine. Both pethidine and diazepam had short-lived (1–2 min) effects on BP, HR and CSNA, but no effects were evident beyond 3 min post bolus injection (data not shown). Therefore, we took care not to analyse data recorded within a 5 min window of drug administration. In addition, drugs were not administered beyond 60 min post-MI. Ninomiya et al. (1986) also reported sudden increases in CSNA and tachyarrhythmia in conscious animals are associated with excitement and/or body movement indicative of pain or discomfort of the acute occlusion. Similarly, in the present study, ‘spiking’ of CSNA was routinely observed during the first 1–2 min of occlusion concurrent with generalized excitability and agitation of the sheep (data not shown). Given that it was unclear whether these effects were in response to coronary ischaemia or merely a response to the obtrusive physical manipulation of tightening and clamping the coronary artery snare, these data were not analysed.
Despite the many differences in experimental design between this study and the aforementioned transient myocardial ischaemia studies performed in anaesthetized animals, the results do provide important insight into possible mechanisms contributing to cardiac sympathetic hyperactivity following myocardial ischaemia and MI, particularly with regard to the proposed cardiocardiac reflex (Malliani et al. 1969; Brown & Malliani, 1971; Felder & Thames, 1981; Matsukawa et al. 1993). Briefly summarized, cardiovascular neural regulation is likely to result from a complex interaction of central integration and peripheral inhibitory and excitatory reflexes. Brown (1967) demonstrated that cardiac sympathetic afferent fibres are excited by coronary occlusion. Malliani et al. 1969) found that efferent activity also increased and introduced the concept of an excitatory sympathetic reflex. Such reflexes remain active after vagotomy and sinoaortic denervation, and thus appear to be independent of baroreflex mechanisms (Malliani et al. 1969; Brown & Malliani, 1971). These and other studies (Felder & Thames, 1981; Lombardi et al. 1984) make it clear that both excitatory (afferent sympathetic) and inhibitory (afferent vagal) reflexes arise from the heart during myocardial ischaemia, and the net result may be no activation of cardiac efferents unless the vagal input is interrupted. Therefore, evidence exists for a cardiocardiac sympathetic reflex operating during acute coronary occlusion, and we suggest that this may be a major factor in the sustained CSNA response to MI.
Studies in humans suggest that within the first hours of developing infarction, excessive vagal discharge may occur, often with concurrent bradycardia (Adgey et al. 1968; Rundqvist et al. 1997). Furthermore, HR variability studies performed at approximately 3 h of infarction suggest there is a shift to sympathetic activation and diminishing vagal modulation of the HR (Lombardi et al. 1996). Taken together, these reports suggest that vagal overactivity and its confounding effect on sympathetic activation is short-lived (perhaps restricted to the first hour of a developing infarct), and thereafter sympatho-excitatory pathways predominate. Data from the present study would support such a hypothesis, since net efferent CSNA was not raised 30 min post occlusion, but had begun to rise by 60 min post-MI and peaked at 2–3 h post-MI.
Other possible mechanisms contributing to the raised CSNA include pain, modulation by the central nervous system (CNS) and baroreceptor-mediated effects. During myocardial ischaemia, sympathetic afferents are known to transmit pain to the sensory cortex and thalamus, which in turn stimulate CSNA via descending cortico-medullary pathways (Albutahi et al. 2003; Floras, 2003). However, this mechanism is unlikely to have contributed during the first hour of coronary occlusion in the present study, because the sheep were given adequate sedation and analgesia, and this would not explain the sustained CSNA response. We cannot exclude the possibility that afferent cardiac sympathetic activity is increased following MI and that we may have recorded this in addition to postganglionic efferent CSNA. This is unlikely because the features of the integrated CSNA signal did not change post-MI, in particular burst timing and baroreflex control of burst area. Further studies, in which hexamethonium is given before and after MI, will be undertaken in order to confirm that only efferent CSNA is increased.
Most deaths from acute MI occur within hours of the onset of symptoms. As early as the 1970s, it was demonstrated that autonomic disturbance is apparent within an hour of the onset of infarction consistent with a pattern of both vagal and sympathetic overactivity (Webb et al. 1972). Autonomic disturbance appears to facilitate life-threatening arrhythmias, and its pharmacological correction is highly beneficial (Pantridge et al. 1981). Furthermore, dorsal root section in experimental animals has been shown to decrease arrhythmias during coronary occlusion (Schwartz et al. 1976). Thus, the deleterious effects of sympathetic reflexes from the heart during acute myocardial ischaemia/infarction are widely accepted (Malliani & Montano, 2003). Chronic β-blockade in patients post-MI has been shown to have remarkable therapeutic benefit (Gottlieb et al. 1998). One mechanism of β-blockade action may be the attenuation of sympathetic excitatory reflexes during subsequent ischaemic episodes, leading to the reduction of life-threatening arrhythmias and reinfarction. Our results indicate that the most important time to treat patients is during the first hours of infarction, when the major increase in CSNA occurs and sudden arrhythmic death is most likely. Future research will be directed towards studying how CSNA can be decreased rapidly during MI (for example using centrally acting sympatholytic agents such as natriuretic peptides, angiotensin II blockers, α2 adrenoreceptor agonists and lipophyllic β-blockers) without causing cardiovascular decompensation.
In conclusion, despite the fact that it has been known for more than 30 years that transient myocardial ischaemia increases sympathetic traffic to the heart, only indirect methods have examined sympathetic hyperactivity during and following MI. Using microneurographic recordings from postganglionic cardiac efferent nerves, we provide the first direct evidence that CSNA is increased in the first hours of developing infarct secondary to coronary artery occlusion in conscious sheep, and that the rise in CSNA is sustained for up to 7 days post-MI. This model can be utilized to examine the role of augmented CSNA post-MI in arrhythmogenesis and how it is affected by drug treatment.
Acknowledgments
Figures were prepared by The Department of Medical Illustration, Christchurch Hospital. This research was funded by Health Research Council of New Zealand, Lotteries Health and National Heart Foundation grants.
References
- Adgey AA, Geddes JS, Mulholland HC, Keegan DA, Pantridge JF. Incidence, significance and management of early arrhythmia complicating myocardial infarction. Lancet. 1968;2:1097–1101. doi: 10.1016/s0140-6736(68)91577-8. [DOI] [PubMed] [Google Scholar]
- Albutahi IAM, DeJongste MJL, Foreman RD. Angina pectoris: a neuroanatomical review of mechanisms and pathways. Int J Pain Med Pall Care. 2003;3:12–17. [Google Scholar]
- Brown AM. Excitation of afferent cardiac sympathetic nerve fibres during myocardial ischaemia. J Physiol. 1967;190:35–53. doi: 10.1113/jphysiol.1967.sp008191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AM, Malliani A. Spinal sympathetic reflexes initiated by coronary receptors. J Physiol. 1971;212:685–705. doi: 10.1113/jphysiol.1971.sp009350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casolo G, Stroder P, Signorini C, Calzolari F, Zucchini M, Balli E, et al. Heart rate variability during the acute phase of myocardial infarction. Circulation. 1992;85:2073–2079. doi: 10.1161/01.cir.85.6.2073. [DOI] [PubMed] [Google Scholar]
- Esler M, Jennings G, Korner P, Willet I, Dudley F, Hasking G, et al. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11:3–20. doi: 10.1161/01.hyp.11.1.3. [DOI] [PubMed] [Google Scholar]
- Esler MD, Jennings G, Lambert G, Meredith I, Horne M, Eisenhofer G. Overflow of catecholamine neurotransmitters to the circulation: source, fate and functions. Physiol Rev. 1990;70:963–985. doi: 10.1152/physrev.1990.70.4.963. [DOI] [PubMed] [Google Scholar]
- Felder RB, Thames MD. The cardiocardiac sympathetic reflex during coronary occlusion in anesthetized dogs. Circ Res. 1981;48:685–692. doi: 10.1161/01.res.48.5.685. [DOI] [PubMed] [Google Scholar]
- Floras J. Sympathetic activation in human heart failure: diverse mechanisms, therapeutic opportunities. Acta Physiol Scand. 2003;177:391–398. doi: 10.1046/j.1365-201X.2003.01087.x. 10.1046/j.1365-201X.2003.01087.x. [DOI] [PubMed] [Google Scholar]
- Foy SG, Crozier IG, Richards AM, Nicholls MG, Turner JG, Frampton CM, et al. Neurohormonal changes after acute myocardial infarction. Eur Heart J. 1995;16:770–778. doi: 10.1093/oxfordjournals.eurheartj.a060995. [DOI] [PubMed] [Google Scholar]
- Gottlieb SS, McCarter RJ, Vogel RA. Effect of beta-blockade on mortality among high-risk and low-risk patients after myocardial infarction. N Eng J Med. 1998;339:489–497. doi: 10.1056/NEJM199808203390801. 10.1056/NEJM199808203390801. [DOI] [PubMed] [Google Scholar]
- Graham LN, Smith PA, Stoker JB, MacKintosh AF, Mary DA. Time course of sympathetic neural activity after uncomplicated acute myocardial infarction. Circulation. 2002;106:793–797. doi: 10.1161/01.cir.0000025610.14665.21. [DOI] [PubMed] [Google Scholar]
- Graham LN, Smith PA, Stoker JB, MacKintosh AF, Mary DA. Sympathetic neural hyperactivity and its normalization following unstable angina and acute myocardial infarction. Clin Sci. 2004;106:605–611. doi: 10.1042/CS20030376. 10.1042/CS20030376. [DOI] [PubMed] [Google Scholar]
- Jardine DL, Charles CJ, Melton IC, May CN, Forrester MD, Frampton CM, Bennett SI, Ikram H. Continual recordings of cardiac sympathetic nerve activity in conscious sheep. Am J Physiol Heart Circ Physiol. 2002;282:H93–99. doi: 10.1152/ajpheart.2002.282.1.H93. [DOI] [PubMed] [Google Scholar]
- Karlsberg RP, Cryer PE, Roberts R. Serial plasma catecholamine response early in the course of clinical acute myocardial infarction: relationship to infarct extent and mortality. Am Heart J. 1981;102:24–29. doi: 10.1016/0002-8703(81)90408-7. 10.1016/0002-8703(81)90408-7. [DOI] [PubMed] [Google Scholar]
- Lombardi F, Casalone C, Della Bella P, Malfatto G, Pagani M, Malliani A. Global versus regional myocardial ischaemia: differences in cardiovascular and sympathetic responses in cats. Cardiovasc Res. 1984;18:14–23. doi: 10.1093/cvr/18.1.14. [DOI] [PubMed] [Google Scholar]
- Lombardi F, Sandrone G, Spinnler MT, Torzillo D, Lavezzaro GC, Brusca A, Malliani A. Heart rate variability in the early hours of an acute myocardial infarction. Am J Cardiol. 1996;77:1037–1044. doi: 10.1016/s0002-9149(96)00127-0. 10.1016/S0002-9149(96)00127-0. [DOI] [PubMed] [Google Scholar]
- McAlpine HM, Morton JJ, Leckie B, Rumley A, Gillen G, Dargie HJ. Neuroendocrine activation after acute myocardial infarction. Br Heart J. 1988;60:117–124. doi: 10.1136/hrt.60.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malliani A, Montano N. Emerging excitatory role of cardiovascular sympathetic afferents in pathophysiological conditions. Hypertension. 2002;39:63–68. doi: 10.1161/hy0102.099200. 10.1161/hy0102.099200. [DOI] [PubMed] [Google Scholar]
- Malliani A, Montano N. Time course of sympathetic neural hyperactivity after uncomplicated myocardial infarction. Circulation. 2003;107:e53. doi: 10.1161/01.cir.0000055543.21433.24. [DOI] [PubMed] [Google Scholar]
- Malliani A, Schwartz PJ, Zanchetti A. A sympathetic reflex elicited by experimental coronary occlusion. Am J Physiol. 1969;217:703–709. doi: 10.1152/ajplegacy.1969.217.3.703. [DOI] [PubMed] [Google Scholar]
- Malpas SC. The rhythmicity of sympathetic nerve activity. Prog Neurobiol. 1998;55:65–96. doi: 10.1016/s0301-0082(98)00030-6. 10.1016/S0301-0082(98)00030-6. [DOI] [PubMed] [Google Scholar]
- Matsukawa K, Ninomiya I, Nishikura N. Effects of anesthesia on cardiac and renal sympathetic nerve activities and plasma catecholamines. Am J Physiol Regul Integr Comp Physiol. 1993;265:R792–797. doi: 10.1152/ajpregu.1993.265.4.R792. [DOI] [PubMed] [Google Scholar]
- Meredith IT, Esler MD, Jennings GL, Broughton A. Evidence of a selective increase in sympathetic activity in patients with sustained ventricular arrhythmias. N Eng J Med. 1991;325:618–624. doi: 10.1056/NEJM199108293250905. [DOI] [PubMed] [Google Scholar]
- Ninomiya I, Matsukawa K, Honda T, Nishiura N, Shirai M. Cardiac sympathetic nerve activity and heart rate during coronary occlusion in awake cats. Am J Physiol Heart Circ Physiol. 1986;251:H528–537. doi: 10.1152/ajpheart.1986.251.3.H528. [DOI] [PubMed] [Google Scholar]
- Pagani M, Schwartz PJ, Banks R, Lombardi F, Malliani A. Reflex responses of sympathetic preganglionic neurones initiated by different cardiovascular receptors in spinal animals. Brain Res. 1974;68:215–225. doi: 10.1016/0006-8993(74)90391-6. 10.1016/0006-8993(74)90391-6. [DOI] [PubMed] [Google Scholar]
- Pantridge JF, Webb SF, Adgey AA. Arrhythmias in the first hours of acute myocardial infarction. Prog Cardiovasc Dis. 1981;23:265–278. doi: 10.1016/0033-0620(81)90016-5. 10.1016/0033-0620(81)90016-5. [DOI] [PubMed] [Google Scholar]
- Rundqvist B, Elam M, Bergman-Sverrirsdottir Y, Eisenhofer G, Friberg P. Increased cardiac adrenergic drive precedes generalized sympathetic activation in human heart failure. Circulation. 1997;95:169–175. doi: 10.1161/01.cir.95.1.169. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Foreman RD, Stone HL, Brown AM. Effect of dorsal root section on the arrhythmias associated with coronary occlusion. Am J Physiol. 1976;231:923–928. doi: 10.1152/ajplegacy.1976.231.3.923. [DOI] [PubMed] [Google Scholar]
- Schwartz PJ, Zaza A, Pala M, Locati E, Bera G, Zanchetti A. Baroreflex sensitivity and its evolution during the first year after myocardial infarction. J Am Coll Cardiol. 1988;12:629–636. doi: 10.1016/s0735-1097(88)80048-2. [DOI] [PubMed] [Google Scholar]
- Sigurdsson A, Held P, Swedberg K. Short- and long-term neurohormonal activation following myocardial infarction. Am Heart J. 1993;126:1068–1076. doi: 10.1016/0002-8703(93)90656-t. 10.1016/0002-8703(93)90656-T. [DOI] [PubMed] [Google Scholar]
- Singh N, Mironov D, Armstrong PW, Ross AM, Langer A. Heart rate variability assessment early after acute myocardial infarction. Circulation. 1996;93:1388–1395. doi: 10.1161/01.cir.93.7.1388. [DOI] [PubMed] [Google Scholar]
- Sleight P. Myocardial infarction. In: Weatherall DJ, Ledingham JGG, Warrell DA, editors. The Oxford Textbook of Medicine. 3. UK: Oxford University Press; 1996. pp. 2331–2349. [Google Scholar]
- Sundlof G, Wallin BG. Human muscle nerve sympathetic activity at rest. Relationship to blood pressure and age. J Physiol. 1978;274:621–637. doi: 10.1113/jphysiol.1978.sp012170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb SW, Adgey AAJ, Pantridge JF. Autonomic disturbance at onset of acute myocardial infarction. BMJ. 1972;3:89–92. doi: 10.1136/bmj.3.5818.89. [DOI] [PMC free article] [PubMed] [Google Scholar]