Abstract
Measurements were made (using fast confocal microscopy) of intracellular Ca2+ levels in fluo-4 loaded interstitial cells isolated from the rabbit urethra. These cells exhibited regular Ca2+ oscillations which were associated with spontaneous transient inward currents recorded under voltage clamp. Interference with d-myo-inositol 1,4,5-trisphosphate (IP3) induced Ca2+ release using 100 μm 2-aminoethoxydiphenyl borate, and the phospholipase C (PLC) inhibitors 2-nitro-4-carboxyphenyl N,N-diphenylcarbamate and U73122 decreased the amplitude of spontaneous oscillations but did not abolish them. However, oscillations were abolished when ryanodine receptors were blocked with tetracaine or ryanodine. Oscillations ceased in the absence of external Ca2+, and frequency was directly proportional to the external Ca2+ concentration. Frequency of Ca2+ oscillation was reduced by SKF-96365, but not by nifedipine. Lanthanum and cadmium completely blocked oscillations. These results suggest that Ca2+ oscillations in isolated rabbit urethral interstitial cells are initiated by Ca2+ release from ryanodine-sensitive intracellular stores, that oscillation frequency is very sensitive to the external Ca2+ concentration and that conversion of the primary oscillation to a propagated Ca2+ wave depends upon IP3-induced Ca2+ release.
The rabbit urethra contains a subpopulation of cells which are distinct both functionally and morphologically from the bulk smooth muscle cells (Sergeant et al. 2000). These ‘interstitial cells’ are noncontractile but are spontaneously active showing regular depolarizations STDs (spontaneous transient depolarizations) under current clamp conditions. The currents or STICs (spontaneous transient inward currents), which underlie the STDs, are believed to result from activation of Ca2+-dependent Cl− channels by Ca2+ released from intracellular stores (Sergeant et al. 2001a, b). In a recent study we demonstrated that interstitial cells fired large STICs and two types of STOCs (spontaneous transient outward currents), ‘fast’ (>100 ms in duration) and ‘slow’ (>1 s in duration). The latter were coupled to STICs, suggesting that they shared the same mechanism, while the former occurred independently at faster rates. All of these currents were abolished by cyclopiazonic acid, caffeine or ryanodine, suggesting that they were activated by Ca2+ release. When d-myo-inositol 1,4,5-trisphosphate (IP3)-sensitive stores were blocked with 2-aminoethoxydiphenyl borate (2-APB), the STICs and slow STOCs were abolished, but the fast STOCs remained. In all of the above experiments it was inferred that spontaneous intracellular Ca2+ oscillations were responsible for the observed changes in membrane potential or currents, so we found it curious that 2-APB appeared to suppress the Ca2+ release necessary for the STICs but not that required for the STOCs. Using purely electrophysiological techniques we were unable to resolve this apparent contradiction, so the purpose of the present investigation was to measure changes in intracellular Ca2+ to shed further light on the nature of spontaneous activity in urethral interstitial cells.
Methods
Bladder and urethra were removed from both male and female rabbits immediately after they had been killed by lethal injection of pentobarbitone. The most proximal 1 cm of the urethra was removed and placed in Krebs solution, and from this, 0.5 cm strips were dissected and cut into 1 mm3 pieces and stored in Hanks' Ca2+-free solution for 30 min before being incubated in an enzyme medium containing (per 5 ml of Hanks' Ca2+-free solution) 15 mg collagenase (Sigma, type 1A), 1 mg protease (Sigma, type XXIV), 10 mg BSA (Sigma), and 10 mg trypsin inhibitor (Sigma) for 5 min at 37°C. They were then placed in Hanks' Ca2+-free solution and stirred for another 5–10 min to release single cells. These were of two types: (1) smooth muscle cells which had the characteristic spindle shape and accounted for about 95% of the total, and (2) interstitial cells which could be readily distinguished from the former by their highly branched appearance under bright field illumination (Sergeant et al. 2000). In the main these latter cells were chosen for study, but in some experiments smooth muscle cells were also studied.
Solutions and drugs
The solutions used were of the following composition (mm): (1) Hanks' Ca2+-free solution (for cell dispersal), 141 Na+, 5.8 K+, 130.3 Cl−, 15.5 HCO3−, 0.34 HPO42−, 0.44 H2PO4−, 10 dextrose, 2.9 sucrose; 10 Hepes (Melford), pH adjusted to 7.4 with NaOH; (2) bath solution, 130 Na+, 5.8 K+, 135 Cl−, 4.16 HCO3−, 0.34 HPO32−, 0.44 H2PO4−, 1.8 Ca2+, 0.9 Mg2+, 0.4 SO42−, 10 dextrose, 2.9 sucrose, 10 Hepes, pH adjusted to 7.4 with NaOH; (3) Cs+ pipette solution, 133 Cs+, 1 Mg2+, 135 Cl−, 0.5 EGTA (Sigma), 10 Hepes, pH adjusted to 7.2 with CsOH. For low-Ca2+ bath solution, CaCl2 was iso-osmotically substituted with MgCl2. It could be argued that the relatively high values of Mg2+ that would be found in the nominally Ca2+-free solutions could block voltage-dependent channels (Fukushima & Hagiwara, 1985) but this would not affect the interpretation of our results. When Ca2+ levels were raised above normal, no osmotic adjustment was made since the change in osmolality would be trivial.
The following drugs were used: caffeine, 2-nitro-4-carboxyphenyl N,N-diphenylcarbamate (NCDC), ryanodine, tetracaine (all Sigma), SKF-96365, U73122 (Calbiochem) 2-APB (Acros) and nifedipine (Bayer). Data are presented as means ± s.e.m., and statistical differences were compared using Student's paired t test, taking P < 0.05 as significant.
Ca2+ imaging
Cells were incubated for 15–30 min in 2–10 μm fluo-4/AM (Molecular Probes) in Hanks' solution containing 100 μm Ca2+ at 37°C. They were then allowed to settle in glass-bottomed Petri dishes and placed on the stage of either a Nikon TE3000 or a Nikon TE2000 microscope. After the cells had been allowed to stick down for 30 min, they were perfused at 37°C in normal Hanks' solution and imaged using ×40, ×60 or ×100 oil immersion lenses with either an iXon 887 EMCCD camera (Andor Technology, Belfast; 512 × 512 pixels, pixel size 16 × 16 μm) coupled to a Nipkow spinning disk confocal head (CSU22, Yokogawa, Japan) or a MegaXR10 GenIII + ICCD (Stanford Photonics, USA; 1280 × 1024 pixels, pixel size 7 × 7 μm) attached to a Nipkow spinning disk confocal head (CSU10, Visitech UK). A krypton–argon laser (Melles Griot Ltd, UK) at 488 nm was used to excite the Fluo 4, and the emitted light was detected at wavelengths >510 nm. Images were usually acquired at 5 or 15 frames s−1 and analysed using ImageJ. Image grabbing and data sampling were performed on either a 2.4 Ghz Intel Xeon Dell PC using Andor software or a Power Macintosh using QED software (version 1.6). Post-hoc analysis and figure preparation were carried out using either NIH Image 1.62 or ImageJ (National Institutes of Health, MD, USA). Where indicated, the fluorescence intensity was normalized by dividing the Ca2+ signal in the region of interest by the average fluorescence intensity found in the resting cell (F/F0). During experiments the dish containing cells was perfused with bath solution. In addition, the cell under study was continuously superfused by means of a close delivery system consisting of a pipette (tip diameter 200 μm) placed approximately 300 μm away. This could be changed, with a dead space time of around 10 s, to a solution containing a drug. All experiments were carried out at 37°C.
Patch clamping
In some experiments, cells were simultaneously imaged and voltage clamped. Recordings were made using the amphotericin B perforated patch method as previously described (Sergeant et al. 2000). Voltage clamp commands were delivered with an Axopatch 1D patch-clamp amplifier (Axon Instruments), and currents were recorded by means of a 12-bit AD/DA converter (Labmaster, Scientific Solutions) interfaced to an Intel computer running pCLAMP software (Axon Instruments). Recordings were synchronized by placing a light-emitting diode within the light path and giving a light pulse at the beginning of the voltage-clamp sweep, thus enabling the two records to be lined up afterwards.
Results
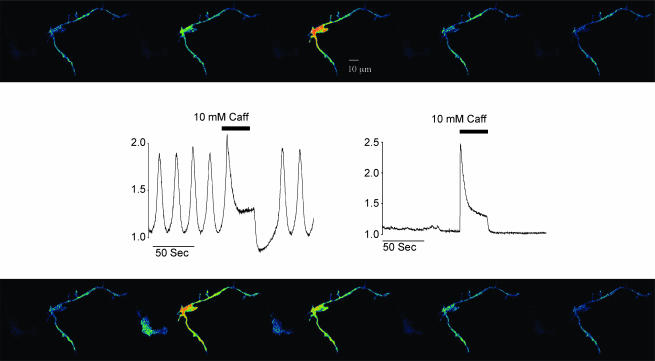
In any given dispersal, about 5% of the cells showed (under bright field illumination) the highly branched appearance typical of interstitial cells (Sergeant et al. 2000). Of these, more than 60% showed regular spontaneous increases in fluorescence intensity when viewed with the confocal microscope. In contrast the smooth muscle cells, which again were readily identified under bright field illumination by their smooth spindle-shape, rarely exhibited spontaneous Ca2+ oscillations. Figure 1 illustrates this point. Each of the 10 frames shows a highly branched cell on the right of the frame (a typical interstitial cell) and a darker spindle-shaped cell (smooth muscle cell) on the left, scarcely visible under control conditions. Regular spontaneous increases in fluorescence intensity were apparent in the perinuclear region of the branched cell, while the smooth muscle cell remained dark. However, when 10 mm caffeine was added to the perfusate, both cells lit up simultaneously. When the caffeine-induced transient was complete, the smooth muscle cell again became dark and remained so for the rest of the experiment, while the interstitial cell, after a brief pause, resumed its oscillatory behaviour. This experiment also illustrated the different contractile properties of both cells. Thus, while the smooth muscle cell contracted vigorously in response to caffeine addition, the interstitial cell did not contract either in response to the spontaneous increases in intracellular Ca2+ or in response to the caffeine-induced Ca2+ transient.
Figure 1. Ca2+ changes in interstitial and smooth muscle cells.
Sequence of 10 frames before (top panel, taken at 4 s intervals) and during addition of 10 mm caffeine (bottom panel, at 3 s intervals). The highly branched cell on the right of each frame is a typical interstitial cell, while the darker spindle-shaped cell on the left, scarcely visible under control conditions, is a smooth muscle cell. Regular spontaneous increases in fluorescent intensity were apparent in the perinuclear region of the branched cell, while the smooth muscle cell remained dark. However, when 10 mm caffeine was added both cells lit up simultaneously (frame taken at 64 s). When the caffeine-induced transient was complete, the smooth muscle cell again became dark, and remained so for the rest of the experiment, while the interstitial cell, after a brief pause, resumed its oscillatory behaviour. This experiment also illustrated the different contractile properties of both cells. Thus, while the smooth muscle cell contracted vigorously in response to caffeine addition, the interstitial cell did not contract either in response to the spontaneous increases in intracellular Ca2+ or in response to the caffeine-induced Ca2+ transient. Scale bar, 10 μm.
The oscillations described above were spontaneous (i.e. they occurred in the absence of external agonist) but we have demonstrated previously (Sergeant et al. 2001a, b, 2002) that agonists such as noradrenaline that are known to raise intracellular IP3 levels have the effect of increasing the frequency of spontaneous depolarizations or spontaneous inward currents. It was therefore of interest to examine the effects of three substances that are known to inhibit IP3-induced Ca2+ release.
Effect of 2-APB
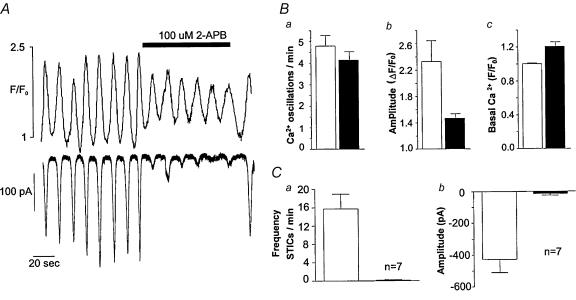
This drug is known (among its other actions) to inhibit Ca2+ release from IP3R-modulated channels (Bootman et al. 2002; Peppiatt et al. 2003), and we have demonstrated previously (Sergeant et al. 2001a) that a dose of 100 μm completely abolished STICs. In the experiment shown in Fig. 2A, the upper record shows Ca2+ oscillations imaged in the perinuclear region of the cell, while the lower record shows the STICS resulting from the intracellular increases in Ca2+. Addition of 100 μm 2-APB had the effect of almost abolishing the STICS, but the underlying Ca2+ oscillations, while reduced in amplitude, retained their normal rhythm and returned to control amplitude after washout of the drug. In 11 such experiments (Fig. 2B), the frequency of Ca2+ oscillation was 4.8 ± 0.49 (s.e.m.) per minute before, and 4.1 ± 0.39 during drug perfusion (not significant, paired t test). Oscillation amplitude decreased from 1.3 ± 0.32 (ΔF/F0) before, to 0.47 ± 0.07 during drug perfusion (P < 0.05, paired t test). The effect of 2-APB on STICs was more dramatic than its effects on Ca2+ oscillations. In seven such experiments (Fig. 2C), average STIC amplitude was reduced from −427 ± 88 to −14 ± 9 pA during drug perfusion.
Figure 2. The effect of 2-APB on Ca2+ oscillations and STICs.
A, the upper record shows Ca2+ oscillations imaged in the perinuclear region of the cell, while the lower record shows the spontaneous transient inward currents (STICs) resulting from the intracellular increases in Ca2+. Addition of 100 μm 2-aminoethoxydiphenyl borate (2-APB) had the effect of almost abolishing the STICS, but the underlying Ca2+ oscillations, while reduced in amplitude, retained their normal rhythm and returned to control amplitude after washout of the drug. The summarized results (B and C) show essentially the same pattern as the original record in A.
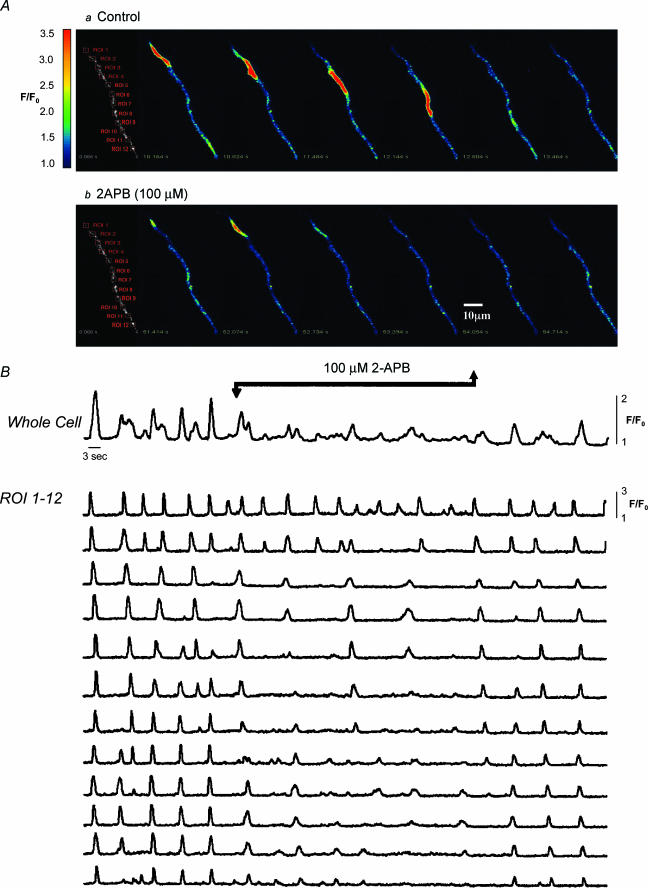
The differential effects of 2-APB on Ca2+ oscillations and STICs can be explained, perhaps, by the experiment shown in Fig. 3. The upper panel of Fig. 3 shows a series of images of the initiation and spread of a Ca2+ wave. Under control conditions this arose at the top of the cell (region of interest 1(ROI 1)) and propagated without decrement to the other end of the cell. Occasionally the wave originated at ROI 9 and propagated in the opposite direction but generally the global event was well co-ordinated throughout the cell. However in the presence of 2-APB, the pattern was quite different. Oscillations continued in all parts of the cell, but these were now propagated only to one or two of the neighbouring regions of interest, with the result that activity in the cell as a whole was now very poorly coordinated. Upon washout of 2-APB the well-coordinated global events were quickly restored. These experiments would suggest that the role of IP3 in these cells was to amplify the Ca2+ signal and ensure propagation of the wave. Such a global event appears to be necessary for the production of STICs but not apparently for the generation of STOCs (Sergeant et al. 2001a).
Figure 3. Effect of 2-APB on wave propagation.
Series of images of the initiation and spread of a Ca2+ wave under control conditions (A, upper panel) The wave arose at the top of the cell (region of interest (ROI) 1) and propagated without decrement to the other end of the cell. Occasionally the wave originated at ROI 9 and propagated in the opposite direction, but generally the global event was well coordinated throughout the cell (as is apparent from the plotted regions of interest in B). In the presence of 2-APB (A, lower panel) the pattern was quite different. Oscillations continued in all parts of the cell, but these were now propagated to only one or two of the neighbouring ROIs, with the result that activity in the cell as a whole was now very poorly coordinated. Upon washout of 2-APB, the well-coordinated global events were quickly restored. Scale bar, 10 μm.
Effect of PLC inhibitors
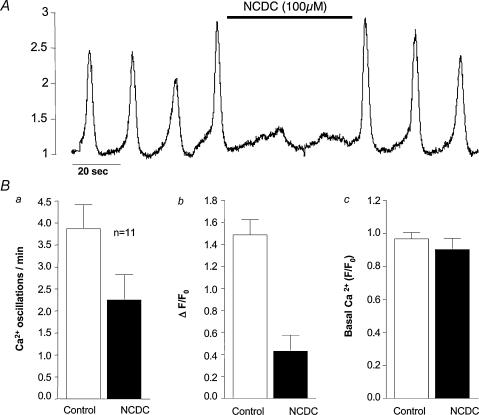
Another approach to assessing the role of IP3 in the genesis and modulation of Ca2+ oscillations in the intersitial cells was to inhibit the enzyme necessary for its production. We used two different compounds for this purpose, NCDC (Cremaschi & Sterin-Borda, 1989; Gotoh et al. 1993) and U73122 (Smith et al. 1990). Figure 4A shows the effect of 100 μm NCDC on frequency and amplitude of Ca2+ oscillations. Before drug addition, oscillations were occurring at a frequency of about 3 min−1. After drug addition, the amplitude of oscillations was much reduced, but they continued at the slightly slower frequency of 2 min−1. In 11 such experiments (Fig. 4B), frequency was reduced from a mean of 3.86 ± 0.52 before drug addition to 2.3 ± 0.55 oscillations min−1 during drug addition (P < 0.05). Normalized change in amplitude (ΔF/F0) was reduced from a control value of 1.58 ± 0.13 to 0.41 ± 0.14 in the presence of drug (P < 0.05). Basal normalized amplitude (F/F0) was little affected. Similar results were obtained with U73122. In six experiments, mean frequency of oscillation was 3.9 ± 0.9 in control conditions and this decreased to 3.4 ± 1.2 oscillations min−1 during drug addition (P < 0.05). Normalized amplitude was reduced from a control value of 1.3 ± 0.18 to 0.78 ± 0.17 in the presence of 1 μm U73122.
Figure 4. The effect of NCDC on Ca2+ oscillations.
A, before drug addition oscillations were occurring at a frequency of about 3 min−1. In the presence of 100 μmN,N-diphenylcarbamate (NCDC), the amplitude of oscillations was much reduced, but they continued but at the slightly slower frequency of 2 min−1. The summarized results (B) showed a decrease in both frequency and amplitude, but there was little effect on basal Ca2+ levels.
Effect of ryanodine receptor (RyR) blockade
Ryanodine
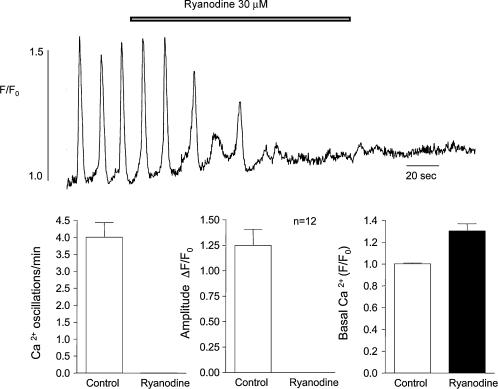
Figure 5 shows the effect on Ca2+ oscillations of 30 μm ryanodine. Within 80 s of drug addition, oscillations had ceased completely and this was accompanied by an increase in basal Ca2+ concentration within the cell. The effects of ryanodine were irreversible even on prolonged washout. In 12 such experiments, this concentration of ryanodine invariably abolished spontaneous oscillations. The effect on basal Ca2+ level was also a consistent one with normalized level increasing from 1.0 ± 0.007 under control conditions to 1.30 ± 0.065 in the presence of 30 μm ryanodine.
Figure 5. The effect of ryanodine.
The effect of 30 mm ryanodine on Ca2+ oscillations is shown. Within 80 s of drug addition, oscillations had ceased completely, and this was accompanied by an increase in basal Ca2+ concentration within the cell. The effects of ryanodine were irreversible even on prolonged washout. In 12 such experiments this concentration of ryanodine invariably abolished spontaneous oscillations. The effect on basal Ca2+ level was also a consistent one with normalized level increasing from 1.0 ± 0.007 before drug addition to 1.30 ± 0.065 in the presence of 30 mm ryanodine.
Tetracaine
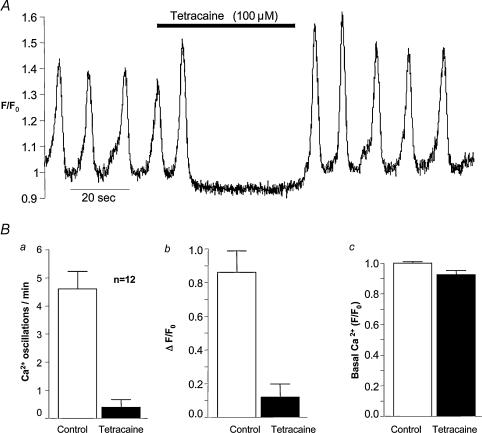
Tetracaine in concentrations greater than 50 μm is known to inhibit Ca2+ release from ryanodine-sensitive stores in skeletal (Csernoch et al. 1999) cardiac (Lukyanenko et al. 1996; Overend et al. 1997, 1998) and smooth muscle (Hyvelin et al. 2000; Cheranov & Jaggar, 2002). Figure 6 shows the effect on Ca2+ oscillations of 100 μm tetracaine. Within 10 s of drug addition, oscillations had ceased completely, and within 10 s of washout they had returned at control frequency and greater amplitude. In 12 such experiments, mean frequency was reduced from a control value of 4.8 ± 0.67 to 0.5 ± 0.25 oscillations min−1 during tetracaine application, while mean amplitude was reduced from a control value of 0.85 ± 0.6 to 0.12 ± 0.45 ΔF/F0. It should be noted that during tetracaine addition, the basal signal intensity fell below the normal resting value, and this was evident on visual inspection of the movie where the cell became noticeably darker during tetracaine application.
Figure 6. The effect of tetracaine.
A, tetracaine at 100 μm abolished oscillations within 10 s of drug addition, and these were just as quickly restored when the drug was washed out. The summarized results (B) show that both frequency and amplitude of oscillations were profoundly depressed by this concentration of tetracaine.
Effect of external Ca2+ concentration on spontaneous oscillations
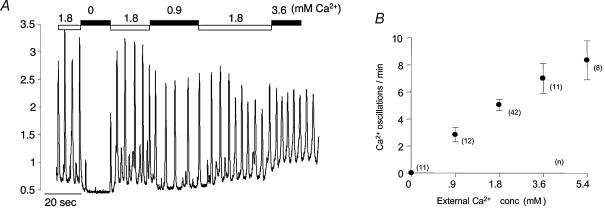
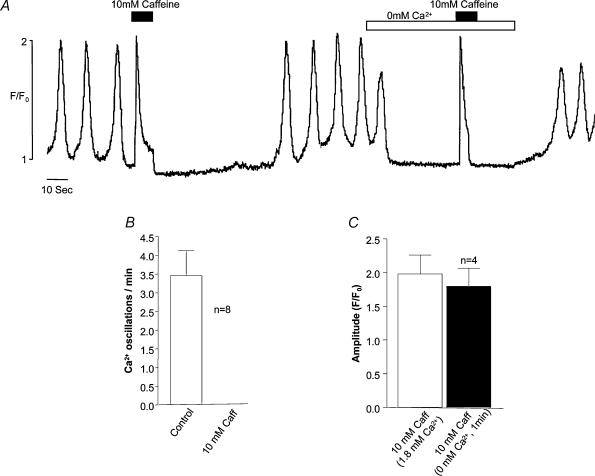
The experiment shown in Fig. 7 was designed to test the dependency of oscillation frequency on external Ca2+ concentration. Reduction of [Ca2+]o from the normal value of 1.8 mm to 0 immediately caused cessation of oscillations and a decrease in basal cytosolic Ca2+. Frequency and amplitude of oscillations were promptly restored on readmission of normal Ca2+. When this solution was changed to one containing 0.9 mm Ca2+, frequency of oscillation was markedly reduced. On the other hand increasing [Ca2+]o to 3.6 mm had the effect of increasing frequency of oscillation. The graph in Fig. 7B shows the averaged results (numbers of experiments at each concentration are shown alongside each point) ± s.e.m. at five different Ca2+ concentrations. It can be seen that there is a roughly linear relationship between oscillation frequency and external Ca2+ concentration. It could be argued that cessation of calcium oscillation in nominally Ca2+-free solution was due to rapid depletion of the intracellular stores, but the experiment shown in Fig. 8 would suggest that this is not the case. When 10 mm caffeine was added in 1.8 mm Ca2+ solution, a maximal Ca2+ transient was induced followed by inhibition of spontaneous oscillations (eight such experiments are summarized in Fig. 8B). When nominally Ca2+-free solution was introduced after normal oscillations had returned, there was the usual cessation of oscillation. However a second introduction of 10 mm caffeine still evoked a maximal Ca2+ transient, suggesting that the stores had not been depleted in the Ca2+-free solution. In four such experiments there was no significant difference between the transient evoked in 1.8 mm Ca2+ as compared with that evoked in Ca2+-free solution (Fig. 8C).
Figure 7. Effect of external Ca2+ concentration.
Reduction of [Ca2+]o from the normal value of 1.8–0 mm (nominally Ca2+-free, since no chelator was present) immediately caused cessation of oscillations and a decrease in basal cytosolic Ca2+. Frequency and amplitude of oscillations were promptly restored on readmission of normal Ca2+. When this solution was changed to one containing 0.9 mm Ca2+, frequency of oscillation was markedly reduced. On the other hand increasing [Ca2+]o to 3.6 mm had the effect of increasing frequency of oscillation. B, the averaged results (numbers of experiments at each concentration are shown alongside each point) (± s.e.m.) at five different Ca2+ concentrations. It can be seen that there is a roughly linear relationship between oscillation frequency and external Ca2+ concentration.
Figure 8. Removing external Ca2+ does not rapidly deplete stores.
When 10 mm caffeine was added in 1.8 mm Ca2+ solution, a maximal Ca2+ transient was induced followed by inhibition of spontaneous oscillations (eight such experiments are summarized in B). A second introduction of 10 mm caffeine a minute after exposure to 0 mm[Ca2+]o still evoked a maximal Ca2+ transient suggesting that the stores had not been depleted in the nominally Ca2+-free solution. In four such experiments there was no significant difference between the transient evoked in 1.8 mm Ca2+ as compared with that evoked in nominally Ca2+-free solution (C).
Effect of blockers of Ca2+ influx
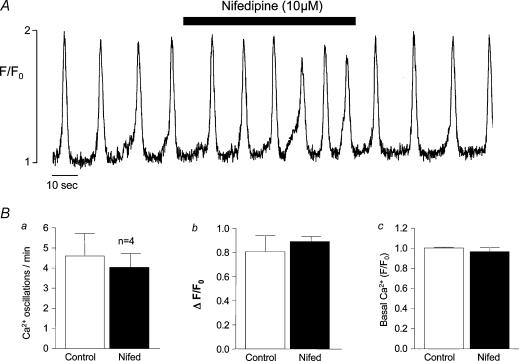
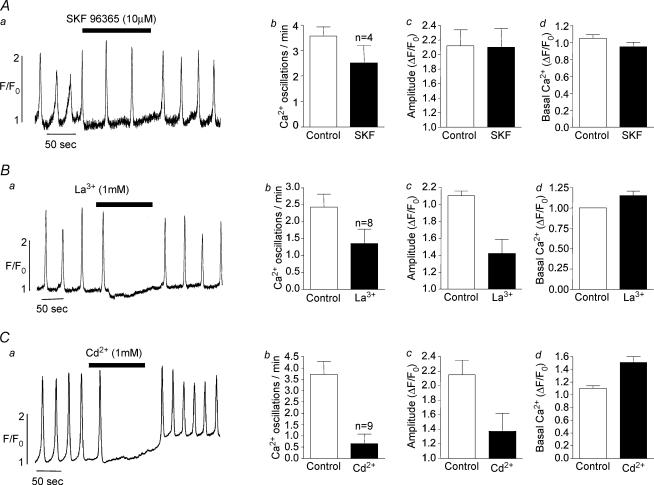
The sensitivity of oscillation frequency to external Ca2+ concentration would suggest that Ca2+ influx was essential for the maintenance of normal oscillations. These cells are known to possess L-type Ca2+ channels, and these may be one possible influx pathway. The experiment illustrated in Fig. 9A was designed to test this possibility. Prior to the addition of 10 μm nifedipine (an L-type channel blocker), frequency of oscillation was 4 min−1 and this slightly increased (to about 6 min−1) rather than decreased in the presence of nifedipine. In four similar experiments, mean frequency decreased slightly from a control value of 4.56 ± 1.06 to 4.1 ± 0.68 oscillations min−1 in the presence of nifedipine (non significant, paired t test). These results would suggest that influx through L-type Ca2+ channels does not significantly modulate frequency of oscillation. In Fig. 10, the effects of three other Ca2+ influx blockers are illustrated. In Fig. 10Aa, 10 μm SKF-96365 (a blocker of store operated Ca2+ entry, Wayman et al. 1996) decreased frequency from a control value of 2.2 to 1.1 oscillations min−1 during drug addition. In four similar experiments, frequency was reduced from a control value of 3.57 ± 0.37 to 2.52 ± 0.68 oscillations min−1 (P < 0.05) in the presence of SKF-96365, suggesting that Ca2+ influx through store-operated Ca2+ channels played a role in store refilling. The nonspecific blockers lanthanum and cadmium chloride had a more dramatic blocking effect. In Fig. 10Ba and Ca, oscillations were completely inhibited when either lanthanum or cadmium were present, Fig. 10Bb shows a summary of eight experiments where 1 mm lanthanum decreased mean oscillation frequency from 2.87 ± 0.44 to 1.57 ± 0.48 (P < 0.05). Figure 10Cb shows a summary of nine experiments where 1 mm cadmium decreased mean frequency from 1.09 ± 0.05 to 0.42 ± 0.17 oscillations min−1 (P < 0.05).
Figure 9. The effect of nifedipine.
Nifedipine at 10 μm increased rather than decreased oscillation frequency while having little effect on amplitude (an effect that was consistent in four preparations as shown in the summarized data, B) suggesting that Ca2+ influx through L-type channels is not important for store refilling.
Figure 10. The effect of blockers of Ca2+ influx.
SKF-96365 at 10 μm approximately halved the frequency of oscillation (Aa) and, although this was a greater reduction than the average of four such experiments (30% reduction, Ab), it would suggest that Ca2+ entry through store-operated channels does play a part in modulating oscillation frequency. The nonspecific blockers lanthanum and cadmium chloride had a more dramatic blocking effect confirming the importance of Ca2+ influx in the modulation of intracellular oscillations.
Discussion
The main conclusion of this study is that spontaneous electrical events in the interstitial cells of the rabbit urethra are generated by the oscillatory release of Ca2+ from intracellular stores as was inferred from our previous studies of these cells (Sergeant et al. 2000, 2001a, b). The ‘prime oscillator’ would appear to be the ryanodine-sensitive store rather than the IP3-sensitive, store since blockade of the former with tetracaine or with ryanodine (Sergeant et al. 2001a) stopped oscillations completely, whereas inhibition of the action or production of IP3 decreased the amplitude or propagation of the Ca2+ wave but did not entirely prevent Ca2+ oscillations. These observations help to explain our earlier observations (Sergeant et al. 2001a) that STICs were almost completely blocked by 2-APB and by PLC inhibitors, whereas STOCs were not. The channels underlying STICs, low conductance Ca2+-activated Cl− channels (Large & Wang, 1996; Piper & Large, 2003), appear not to be clustered sufficiently for small local increases in Ca2+ to produce measurable currents. Thus it appears that large STICs result only from global increases in Ca2+. The channels underlying STOCs, on the other hand, are large conductance Ca2+-activated K+ channels which can produce measureable currents in response to small local changes in Ca2+ concentration. Recent studies by Rossi et al. (2002) and Aoyama et al. (2004) in HEK 293 cells (and by the latter authors in isolated Interstitial cells of Cajal (ICC) from the mouse small intestine) would support the idea that spontaneous Ca2+ oscillations (as distinct from agonist-induced oscillations) require the presence of functional ryanodine receptors. HEK 293 cells, which expressed all three subtypes of IP3 receptor (IP3R) as well as RyR1, did not exhibit spontaneous Ca2+ oscillations although they were responsive to caffeine and carbachol. On the other hand those cells that expressed the RyR3 subtype did show spontaneous Ca2+ oscillations. Many of the observations of Aoyama et al. (2004) on the properties of mouse ICC would concur with the results of our present study. For example, they also showed that nifedipine did not affect spontaneous Ca2+ oscillations, while tetracaine and ryanodine abolished them. However, in contrast to the results we report here, they found that 2-APB in doses as low as 1 μm could abolish spontaneous oscillations. An interesting aspect of this finding is that the effects of even this low dose of 2-APB were not reversible on washout. Some of the actions of 2-APB other than that of preventing IP3-induced Ca2+ release (sarcoplasmic reticulum Ca2+-ATPase pump (SERCA) inhibition, blockade of store-operated Ca2+ channels, swelling of mitochondria, Bootman et al. 2002; Peppiatt et al. 2003) are irreversible or reversed only slowly. In the present study, we found that a much higher concentration of 2-APB (100 μm) was very rapidly reversed on washout.
We are well aware that 2-APB is not as ‘clean’ a drug as we would like it to be. It is known to have three actions in addition to its effect of inhibiting IP3-mediated Ca2+ release (Peppiatt et al. 2003; Ma et al. 2003). These are: (1) inhibition of store-operated Ca2+ entry; (2) inhibition of SERCA pumps (both of these could result in store rundown while the latter effect could slow the recovery of the Ca2+ transient); and (3) inhibition of the plasma membrane Ca2+ pump. However, we have several reasons for believing that these three actions are of much less significance than the inhibition of IP3-mediated Ca2+ release under the conditions of the present study. (1) We have demonstrated that caffeine-induced Ca2+ transients (G. P. Sergeant, K. D. Thornbury, M. A. Hollywood and N. G. McHale, unpublished data) and caffeine-induced STICs (Sergeant et al. 2001a) persisted in the presence of 100 μm 2-APB (suggesting that store filling and SR uptake were working normally) while noradrenaline-induced transients were abolished. (2) Direct measurement of capacitative Ca2+ entry showed that 2-APB decreased this by less than 20% (G. P. Sergeant, K. D. Thornbury, M. A. Hollywood and N. G. McHale, unpublished data) and that a reduction of this magnitude was not enough to affect STICs. (3) We have observed that basal Ca2+ does increase in the presence of 2-APB and this is consistent with inhibition of the plasma membrane Ca2+-ATPase. However this does not pose a serious challenge to our interpretation of our results. The above observations and the fact that oscillation frequency was not significantly decreased (as it would be if influx through store-operated channels were blocked) would suggest that in isolated urethral interstitial cells 2-APB is acting preferentially on IP3-induced Ca2+ release.
Although Ca2+ release via the IP3R may not be the primary oscillator in urethral interstitial cells, the results of the present study bear out the importance of IP3-induced Ca2+ release in the amplification and coordination of the Ca2+ wave. A well-coordinated wave appears to be necessary for the STICs which underlie the normal spontaneous electrical activity in these cells. This means that both RyR and IP3R are necessary for normal electrical activity. Several other studies have shown that calcium oscillations require both RyR and IP3R. For example, Koizumi et al. (1999) showed that elementary Ca2+ signals in PC12 cells appeared to arise from clusters containing both RyR and IP3R since they had the spatial spread or kinetics of neither Ca2+ sparks nor Ca2+ puffs. This is very similar to the pattern of elementary events observed in urethral interstitial cells. We never observed events that had the spatiotemporal properties of sparks such as those seen in myocytes isolated from the portal vein (Boittin et al. 1998), although we have observed such events in isolated bladder myocytes under the same experimental conditions as those of the present study (unpublished observations). Bayguinov et al. (2000) reported that spontaneous localized Ca2+-release events (insensitive to ryanodine and mediated by IP3Rs) were generated in murine colonic myocytes in the absence of external agonists presumably due to basal activity of PLC. These were referred to as ‘Ca2+ puffs’ similar to those observed in Xenopus oocytes (Berridge, 1997; Boittin et al. 2000). Similarly Boittin et al. (2000) observed Ca2+ puffs discharged through IP3-gated channels in response to low concentrations of IP3 which was photo-released from its caged precursor. On the other hand, Gordienko & Bolton (2002) did not observe discrete Ca2+-release events arising from coordinated opening of IP3R clusters in rabbit portal vein myocytes. All spontaneous Ca2+-release events observed in these myocytes were completely abolished by high (50–100 μm) concentrations of ryanodine. Nevertheless these authors also confirmed the importance of IP3-induced Ca2+ release in the amplification and coordination of the Ca2+ wave.
It is well known that external Ca2+ concentration affects oscillation frequency in many cells types (Kawanishi et al. 1989; Bootman et al. 1996; Shuttleworth & Thompson, 1996; Sneyd et al. 2004). For example, Bootman et al. (1996) found that raising external Ca2+ from 0 to 1.3 mm increased both frequency of oscillations and intracellular [3H]inosityl phosphate levels in HeLa cells. When external Ca2+ concentrations were increased above this level, oscillation frequency continued to rise even though the PLC response had already saturated. They took this to mean that external Ca2+ (at least at levels above 1.3 mm) could control the release of Ca2+ from intracellular stores by modulating the rate of store refilling between each Ca2+ spike. Since PLC activity was essentially saturated they argued that the positive feedback responsible for Ca2+ spiking must be generated by calcium-induced Ca2+ release (CICR). The obvious mechanism for such an oscillator would be cyclical filling and emptying of a ryanodine-sensitive store. Sneyd et al. (2004) argued that the Ca2+ influx pathway which was responsible for modulating frequency of oscillation did not depend on store depletion or capacitative Ca2+ entry, since oscillations could occur in the absence of significant store depletion (Park et al. 2000). This would accord with the results of the present study that blockade of store-operated Ca2+ entry with SKF-96365, although it decreased frequency, did not abolish oscillations. This contrasted with the effect of nonspecific blockers of Ca2+ entry such as lanthanum or cadmium.
In conclusion, the results presented here confirm our previous inference (Sergeant et al. 2001a) that regular intracellular Ca2+ oscillations underlie the spontaneous electrical activity of the interstitial cells of the rabbit urethra. This activity can be modulated by substances which raise intracellular levels of IP3, and IP3-induced Ca2+ release appears to be necessary for the coordination of the global Ca2+ event underlying STICs (and thus pacemaking); however, localized Ca2+ oscillations (and thus STOCs) can occur in an IP3-independent manner.
Acknowledgments
The study was supported by Grant no. 064212 from the Wellcome Trust. L.J. is in receipt of a DEL(NI) PhD studentship.
References
- Aoyama M, Yamada A, Wang J, Ohya S, Furuzono S, Goto T, Hotta S, Ito Y, Matsubara T, Shimokata K, Chen SR, Imaizumi Y, Nakayama S. Requirement of ryanodine receptors for pacemaker Ca2+ activity in ICC and HEK293 cells. J Cell Sci. 2004;117:2813–2825. doi: 10.1242/jcs.01136. 10.1242/jcs.01136. [DOI] [PubMed] [Google Scholar]
- Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol Cell Physiol. 2000;279:C126–135. doi: 10.1152/ajpcell.2000.279.1.C126. [DOI] [PubMed] [Google Scholar]
- Berridge MJ. Elementary and global aspects of calcium signalling. J Physiol. 1997;499:291–306. doi: 10.1113/jphysiol.1997.sp021927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boittin FX, Coussin F, Macrez N, Mironneau C, Mironneau J. Inositol 1,4,5-trisphosphate-and ryanodine-sensitive Ca2+ release channel-dependent Ca2+ signalling in rat portal vein myocytes. Cell Calcium. 1998;23:303–311. doi: 10.1016/s0143-4160(98)90026-4. [DOI] [PubMed] [Google Scholar]
- Boittin FX, Coussin F, Morel JL, Halet G, Macrez N, Mironneau J. Ca2+ signals mediated by Ins(1,4,5)P3-gated channels in rat ureteric myocytes. Biochem J. 2000;349:323–332. doi: 10.1042/0264-6021:3490323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. Faseb J. 2002;16:1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- Bootman MD, Young KW, Young JM, Moreton RB, Berridge MJ. Extracellular calcium concentration controls the frequency of intracellular calcium spiking independently of inositol 1,4,5-trisphosphate production in HeLa cells. Biochem J. 1996;314:347–354. doi: 10.1042/bj3140347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheranov SY, Jaggar JH. Sarcoplasmic reticulum calcium load regulates rat arterial smooth muscle calcium sparks and transient K(Ca) currents. J Physiol. 2002;544:71–84. doi: 10.1113/jphysiol.2002.025197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cremaschi GA, Sterin-Borda L. Stimulation of phosphoinositide hydrolysis via class I antigen-specific recognition in murine cardiac tissue. FEBS Lett. 1989;249:302–306. doi: 10.1016/0014-5793(89)80646-5. [DOI] [PubMed] [Google Scholar]
- Csernoch L, Szentesi P, Sarkozi S, Szegedi C, Jona I, Kovacs L. Effects of tetracaine on sarcoplasmic calcium release in mammalian skeletal muscle fibres. J Physiol. 1999;515:843–857. doi: 10.1111/j.1469-7793.1999.843ab.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukushima Y, Hagiwara S. Currents carried by monovalent cations through calcium channels in mouse neoplastic B lymphocytes. J Physiol. 1985;358:255–284. doi: 10.1113/jphysiol.1985.sp015550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordienko DV, Bolton TB. Crosstalk between ryanodine receptors and IP3 receptors as a factor shaping spontaneous Ca2+-release events in rabbit portal vein myocytes. J Physiol. 2002;542:743–762. doi: 10.1113/jphysiol.2001.015966. 10.1113/jphysiol.2001.015966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh H, Kamiyama A, Shibayama R, Sawada M, Kashimoto T. Involvement of phosphoinositide turnover in ouabain inotropism. Biochem Biophys Res Commun. 1993;194:72–78. doi: 10.1006/bbrc.1993.1786. 10.1006/bbrc.1993.1786. [DOI] [PubMed] [Google Scholar]
- Hyvelin JM, Martin C, Roux E, Marthan R, Savineau JP. Human isolated bronchial smooth muscle contains functional ryanodine/caffeine-sensitive Ca-release channels. Am J Respir Crit Care Med. 2000;162:687–694. doi: 10.1164/ajrccm.162.2.9911025. [DOI] [PubMed] [Google Scholar]
- Kawanishi T, Blank LM, Harootunian AT, Smith MT, Tsien RY. Ca2+ oscillations induced by hormonal stimulation of individual fura-2-loaded hepatocytes. J Biol Chem. 1989;264:12859–12866. [PubMed] [Google Scholar]
- Koizumi S, Bootman MD, Bobanovic LK, Schell MJ, Berridge MJ, Lipp P. Characterization of elementary Ca2+ release signals in NGF-differentiated PC12 cells and hippocampal neurons. Neuron. 1999;22:125–137. doi: 10.1016/s0896-6273(00)80684-4. 10.1016/S0896-6273(00)80684-4. [DOI] [PubMed] [Google Scholar]
- Large WA, Wang Q. Characteristics and physiological role of the Ca2+-activated Cl− conductance in smooth muscle. Am J Physio Cell Physioll. 1996;271:C435–454. doi: 10.1152/ajpcell.1996.271.2.C435. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Gyorke I, Gyorke S. Regulation of calcium release by calcium inside the sarcoplasmic reticulum in ventricular myocytes. Pflugers Arch. 1996;432:1047–1054. doi: 10.1007/s004240050233. 10.1007/s004240050233. [DOI] [PubMed] [Google Scholar]
- Ma HT, Venkatachalam K, Rys-Sikora KE, He LP, Zheng F, Gill DL. Modification of phospholipase C-gamma-induced Ca2+ signal generation by 2-aminoethoxydiphenyl borate. Biochem J. 2003;376:667–676. doi: 10.1042/BJ20031345. 10.1042/BJ20031345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overend CL, Eisner DA, O'Neill SC. The effect of tetracaine on spontaneous Ca2+ release and sarcoplasmic reticulum calcium content in rat ventricular myocytes. J Physiol. 1997;502:471–479. doi: 10.1111/j.1469-7793.1997.471bj.x. 10.1111/j.1469-7793.1997.471bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Overend CL, O'Neill SC, Eisner DA. The effect of tetracaine on stimulated contractions, sarcoplasmic reticulum Ca2+ content and membrane current in isolated rat ventricular myocytes. J Physiol. 1998;507:759–769. doi: 10.1111/j.1469-7793.1998.759bs.x. 10.1111/j.1469-7793.1998.759bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park MK, Petersen OH, Tepikin AV. The endoplasmic reticulum as one continuous Ca2+ pool: visualization of rapid Ca2+ movements and equilibration. EMBO J. 2000;19:5729–5739. doi: 10.1093/emboj/19.21.5729. 10.1093/emboj/19.21.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peppiatt CM, Collins TJ, Mackenzie L, Conway SJ, Holmes AB, Bootman MD, Berridge MJ, Seo JT, Roderick HL. 2-Aminoethoxydiphenyl borate (2-APB) antagonises inositol 1,4,5-trisphosphate-induced calcium release, inhibits calcium pumps and has a use-dependent and slowly reversible action on store-operated calcium entry channels. Cell Calcium. 2003;34:97–108. doi: 10.1016/s0143-4160(03)00026-5. [DOI] [PubMed] [Google Scholar]
- Piper AS, Large WA. Multiple conductance states of single Ca2+-activated Cl− channels in rabbit pulmonary artery smooth muscle cells. J Physiol. 2003;547:181–196. doi: 10.1113/jphysiol.2002.033688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi D, Simeoni I, Micheli M, Bootman M, Lipp P, Allen PD, Sorrentino V. RyR1 and RyR3 isoforms provide distinct intracellular Ca2+ signals in HEK 293 cells. J Cell Sci. 2002;115:2497–2504. doi: 10.1242/jcs.115.12.2497. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, McHale NG, Thornbury KD. Role of IP3 in modulation of spontaneous activity in pacemaker cells of rabbit urethra. Am J Physiol Cell Physiol. 2001a;280:C1349–1356. doi: 10.1152/ajpcell.2001.280.5.C1349. [DOI] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McCloskey KD, Thornbury KD, McHale NG. Specialised pacemaking cells in the rabbit urethra. J Physiol. 2000;526:359–366. doi: 10.1111/j.1469-7793.2000.t01-2-00359.x. 10.1111/j.1469-7793.2000.t01-2-00359.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeant GP, Hollywood MA, McHale NG, Thornbury KD. Spontaneous Ca2+-activated Cl− currents in isolated urethral smooth muscle cells. J Urol. 2001b;166:1161–1166. 10.1097/00005392-200109000-00101. [PubMed] [Google Scholar]
- Sergeant GP, Thornbury KD, McHale NG, Hollywood MA. Characterization of norepinephrine-evoked inward currents in interstitial cells isolated from the rabbit urethra. Am J Physiol Cell Physiol. 2002;283:C885–894. doi: 10.1152/ajpcell.00085.2002. [DOI] [PubMed] [Google Scholar]
- Shuttleworth TJ, Thompson JL. Ca2+ entry modulates oscillation frequency by triggering Ca2+ release. Biochem J. 1996;313:815–819. doi: 10.1042/bj3130815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Sam LM, Justen JM, Bundy GL, Bala GA, Bleasdale JE. Receptor-coupled signal transduction in human polymorphonuclear neutrophils: effects of a novel inhibitor of phospholipase C-dependent processes on cell responsiveness. J Pharmacol Exp Ther. 1990;253:688–697. [PubMed] [Google Scholar]
- Sneyd J, Tsaneva-Atanasova K, Yule DI, Thompson JL, Shuttleworth TJ. Control of calcium oscillations by membrane fluxes. Proc Natl Acad Sci U S A. 2004;101:1392–1396. doi: 10.1073/pnas.0303472101. 10.1073/pnas.0303472101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wayman CP, McFadzean I, Gibson A, Tucker JF. Two distinct membrane currents activated by cyclopiazonic acid-induced calcium store depletion in single smooth muscle cells of the mouse anococcygeus. Br J Pharmacol. 1996;117:566–572. doi: 10.1111/j.1476-5381.1996.tb15228.x. [DOI] [PMC free article] [PubMed] [Google Scholar]