Abstract
Phosphatidylinositol 3 kinase (PI3-kinase) is activated during and is required for hippocampal glutamate receptor-dependent long-term potentiation. It mediates the delivery of AMPA receptors to the neuronal surface. Among the downstream targets of PI3-kinase are three members of the serum- and glucocorticoid-inducible kinase family, SGK1, SGK2 and SGK3. In Xenopus oocytes expressing the AMPA subunit GluR1, we show that SGK3, and to a lesser extent SGK2, but not SGK1, increase glutamate-induced currents by increasing the abundance of GluR1 protein in the cell membrane. We further show Sgk3 mRNA expression in the hippocampus by RT-PCR and in situ hybridization. According to Western blotting, the hippocampal abundance of GluR1 is significantly lower in gene-targeted mice lacking SGK3 (Sgk3−/−) than in their wild-type littermates (Sgk3+/+). The present observations disclose a novel mechanism in the regulation of GluR1.
Glutamate receptors (GluRs) are the most important mediators of excitatory signal transduction in the central nervous system (Sheng & Kim, 2002; Sheng & Nakagawa, 2002). They can be pharmacologically classified in three distinct classes: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, kainate (KA) receptors, and N-methyl-d-aspartate (NMDA) receptors. The family of AMPA receptors consists of four subunits, GluR1–4. The AMPA-type glutamate receptor opens in response to glutamate binding, and mediates most of the rapid excitatory postsynaptic current. The activation of AMPA receptors leads to stimulation of a multitude of biochemical pathways in postsynaptic neurones eventually leading to postsynaptic neuronal plasticity. Changes in synaptic strength can occur by altering the activity and/or abundance of postsynaptic AMPA receptors. Studies on GluR1-knockout mice suggest that GluR1 is essential for adult hippocampal long-term potentiation (Zamanillo et al. 1999; Mack et al. 2001). Hippocampal phosphatidylinositol 3 kinase (PI3-kinase) is activated during long-term potentiation and is complexed with synaptic AMPA receptors (Passafaro et al. 2001; Sanna et al. 2002; Man et al. 2003). The functional significance of PI3-kinase activation is illustrated by the observation that inhibition of PI3-kinase abrogates the expression of long-term potentiation (Sanna et al. 2002). Moreover, PI3-kinase is required for synaptic plasticity and memory consolidation in the amygdala (Lin et al. 2001). PI3-kinase has been shown to mediate the delivery of AMPA receptors to the cell surface following activation of NMDA receptors (Passafaro et al. 2001). However, the signalling pathway from PI3-kinase to AMPA receptor abundance in the cell membrane remains elusive. Downstream signalling molecules of PI3-kinase include the 3-phosphoinositide-dependent kinases PDK1 and PDK2, which phosphorylate and thus activate protein kinase B and all three members of the serum- and glucocorticoid-inducible kinase (SGK) family, SGK1, SGK2 and SGK3 (Park et al. 1999; Lang & Cohen, 2001). All three members of the SGK family have been shown to regulate the renal epithelial Na+ channel ENaC by increasing the abundance of the channel protein in the plasma membrane (Debonneville et al. 2001; Lang et al. 2003; Pearce, 2003; Verrey et al. 2003). As all three kinases are abundantly expressed in the brain (Waldegger et al. 1997; Kobayashi et al. 1999), we hypothesized that these kinases may participate in the regulation of AMPA receptors.
To examine the potential role of SGK1, SGK2 and SGK3 in the regulation of AMPA receptors, we coexpressed GluR1, a member of the AMPA receptor family, with one of the SGKs in Xenopus oocytes, and compared glutamate-evoked current amplitudes and membrane protein expression. After having identified SGK3 as a kinase that regulates GluR1, we compared the expression of GluR1 in the hippocampus of gene-targeted mice lacking SGK3 (Sgk3−/−) with that of their wild-type littermates (Sgk3+/+) using Western blotting.
Methods
Animals
Age- and sex-matched Sgk3+/+ and Sgk3−/− mouse siblings from heterozygous mating (mixed C57BL/6 and Sv 129 SvJ strain) were used for this study. The generation of Sgk3−/− mice is described in detail elsewhere (McCormick et al. 2004). Briefly, the targeting strategy for disruption of the Sgk3 gene involved removing parts of exon 10, which contains the ATP-binding site necessary for the catalytic activity of SGK3 and 11, deleting intron 10, and introducing an in-frame STOP codon into exon 11.
To obtain the brains, mice were anaesthetized intraperitoneally with ketamine (100 mg kg−1 body weight, Sigma-Aldrich, Munich, Germany) and xylazine (4 mg kg−1 body weight, Sigma-Aldrich) dissolved in 0.9% saline. Mice were killed after loss of pedal reflexes, and terminally bled into the thoracic cavity, placed on ice where the brains were removed, and immediately frozen in liquid nitrogen (Strutz-Seebohm et al. 2005). The experimental protocols were approved by the local councils for animal care, and were conducted according to the German law for the care and use of laboratory animals.
Mutagenesis
The nondesensitizing mutants of the rat AMPA receptor subunits GluR1 and GluR2 (GluR1(L479Y), GluR2(R)(L483Y)) and wild-type GluR3 and GluR4 were used for electrophysiological oocyte recordings and cell-surface expression experiments.
cRNA synthesis
Template DNA was linearized with a suitable restriction enzyme. cRNA was synthesized from 1 μg of linearized DNA using an in vitro transcription kit (mMessage mMachine T7 kit; Ambion Ltd, Cambridgeshire, UK). cRNA concentrations were evaluated by photospectrometry, and transcript quality was checked by agarose gel electrophoresis.
RT-PCR analysis
Total RNA was isolated from tissue by using the Qiashredder and RNeasy Mini Kit from Qiagen. For cDNA first strand synthesis, 1 μg of total RNA in 12.5 μl diethyl pyrocarbonate (DEPC)-H2O was mixed with 1 μl of oligo-dT primer (500 μg ml−1; Invitrogen) and heated for 2 min at 70°C. A RT mix of 2 μl 10× reaction buffer (Biolabs), 1 μl dNTP mix (dATP, dCTP, dGTP, dTTP, 10 mm each, Promega), 0.5 μl recombinant RNase inhibitor (Roche), 0.1 μl M-MuLV reverse transcriptase (Biolabs), and 2.9 μl DEPC-H2O was then added, and the reaction mixture was incubated for 60 min at 42°C. The reaction was stopped by heating the mixture for 5 min at 94°C. The cDNA was stored at −20°C until PCR analysis. PCR analysis was then performed with 1 μl of the reverse transcription product in a total volume of 25 μl of a PCR mix containing 22 μl of sterile H2O, 1 μl of primer 1 (10 pmol μl−1), 1 μl of primer 2 (10 pmol μl−1), and one puReTaq Ready-To-Go PCR bead (Amersham Biosciences) through 40 cycles (30 s at 94°C, 30 s at 60°C, 45 s at 72°C). The following primers were used to amplify a 212 bp stretch of the Sgk3 isoform from exon 10–11: sense primer: 5′-CTTCTTGCAAAACGGAAACTGGATG-3;′ antisense primer: 5′-CCCCTCCATTAACAAAATCCAGACC-3′. PCR products were analysed by agarose gel electrophoresis.
RNA probe labelling by in vitro transcription
DIG-labelled, Sgk3-specific, antisense and sense RNA probes were generated from RT-PCR-derived templates by in vitro transcription. Total cellular RNA was prepared from adult mouse hippocampus by using Trizol reagent (Invitrogen). After treatment with RNase-free DNase, the first-strand oligo(dT)-primed cDNA was synthesized with Superscript II reverse transcriptase (Invitrogen). Afterwards, PCR amplification of a 470 bp specific DNA fragment of Sgk3 sequence was performed with following primers: Sgk3 forward: 5′-CAGAAAACAGCCCTATGACAACAC-3;′ Sgk3 reverse: 5′-GAGGGGCGTAAGAAAAACCAACA-3′. PCR was carried out under following conditions: 95°C for 10 min, 35× (95°C for 30 s, primer annealing at 58°C for 45 s, 72°C for 45 s) and 72°C for 10 min. AmpliTaq DNA polymerase and PCR reagents were purchased from Applied Biosystems (Lincoln, USA). In order to produce a DNA template for the generation of antisense and sense RNA probes by in vitro transcription, T7 RNA polymerase promoter sequence was added to the 5′ end of the PCR product by a further PCR reaction under the same PCR conditions, as described above. Primer sequences are shown below, whereby promoter sequences are in bold: for generation of sense probe: T7_Sgk3 forward: 5′-GCAGTAATACGACTCACTATAGGGCAGAAAACAGCCCTATGACAACAC-3′Sgk3 reverse: 5′-GAGGGGCGTAAGAAAAACCAACA-3′. For production of antisense probe: Sgk3 forward: 5′-CAGAAAACAGCCCTATGACAACAC-3′ Sgk3_T7 reverse: 5′-GCAGTAATACGACTCACTATAGGGGAGGGGCGTAAGAAAAACCAACA-3′. Afterwards, PCR fragments were purified with a PCR purification kit (Qiagen). In vitro transcription was performed with the DIG-labelling kit (Roche Molecular Biochemicals, Mannheim, Germany) by using 100 ng template DNA. After ethanol precipitation, labelled RNA probe was quantified by dot blot according to the protocol of the DIG-labelling kit.
In situ hybridization of Sgk3 mRNA
Adult mice (C57/BL6) were deeply anaesthetized with ketamine/xylazine. Brains were removed, immediately frozen in −25°C cold isopentane, and coronal sections of brain were sliced on a freezing microtome at 12 μm thickness. Brain sections were subsequently mounted on silane-coated slides (2% 3-aminopropyltriethoxy-silane (Sigma-Aldrich) in acetone), dried at 60°C for 30 s, and fixed with 4% phosphate-buffered paraformaldehyde for 20 min. After three washes with phosphate buffered saline (PBS, 0.1 mm, pH 7.4), slides were incubated with TE buffer (100 mm Tris, 50 mm EDTA, pH 8) containing 1 μg ml−1 proteinase K for 10 min at room temperature, and rinsed again three times with PBS. In order to reduce nonspecific background, slides were acetylated with TEA buffer (0.1 m triethanolamine, pH 8.0) containing 0.25% (v/v) acetic anhydride (Sigma-Aldrich) twice for 5 min. After prehybridization with hybridization buffer (50% formamide (Sigma-Aldrich), 10% dextran sulphate, 5 mm EDTA, 20 mm Tris pH 8, 10 mm DTT, 1× Denhardt's solution, 0.05% tRNA, 300 mm NaCl) for 1 h at 62°C, sections were incubated with fresh hybridization buffer containing the denatured DIG-labelled sense or antisense probe (200 ng ml−1) overnight at 62°C. After hybridization, slides were briefly rinsed in 2× SSC (saline–sodium citrate buffer) at room temperature, and three times in 0.1× SSC for 15 min at 62°C. Detection of DIG-labelled RNA probe was performed according to the protocol of the DIG nucleic acid detection kit (Roche). The tissues were blocked for 30 min with blocking buffer (1% blocking reagent (Roche) in maleic acid buffer (0.1 m maleic acid, 0.15 m NaCl, pH 7.5) and then incubated with alkaline phosphatase-conjugated antibody solution (anti-DIG antibody (1:2500, Roche) in blocking buffer containing 0.1% Triton X-100) for 1 h. Following four washes with maleic acid buffer for 15 min, slides were equilibrated for 5 min in Tris buffer pH 9.5 (0.1 m Tris, pH 9.5, 0.1 m NaCl, 50 mm MgCl2). The colour development was carried out with freshly prepared substrate solution (nitroblue tetrazolium salt (NBT) and 5-bromo-4-chloro-3-indolyl phosphate (X-phosphate) (Roche) in Tris buffer pH 9.5). After three washes with PBS, slides were rinsed in distilled water, dried, and coverslipped with Kaiser's solution (Merck, Darmstadt, Germany).
Electrophysiological measurements in Xenopus oocytes
Oocytes of stages V–VI were surgically removed from the ovaries of Xenopus laevis, as described elsewhere (Seebohm et al. 2003). Briefly, female Xenopus laevis frogs were anaesthetized with 0.1% tricaine (Sigma-Aldrich) and pieces of ovary were surgically removed. The incisions were sutured, and the animals were allowed to recover. Frogs were humanely killed after the final collection (Maljevic et al. 2003). The experimental procedures were approved by the Regierungspraesidium Tuebingen, Germany. Oocytes were injected with GluR1 cRNA (4 ng oocyte−1) or together with Sgk cRNA (6 ng oocyte−1) using a Nanoliter 2000 injector (WPI, Berlin, Germany). Standard two-electrode voltage-clamp recordings were performed 5–7 days after cRNA injection with a TurboTec 03 amplifier (npi, Tamm, Germany) and an interface DIGIDATA 1322 A from Axon Instruments (Union City, CA, USA). Data analyses were done with pClamp/Clampex software (Axon Instruments), Chart software (Adinstruments Ltd, Oxfordshire, UK), and Origin 6.0 software (Additive, Friedrichsdorf, Germany). Agonist solutions were prepared in ND-96 buffer (96 mm NaCl, 1.8 mm CaCl2, 2.0 mm KCl, 1.0 mm MgCl2, and 5 mm Hepes–NaOH, pH 7.2 with NaOH, all from Sigma-Aldrich). Current and voltage electrodes were filled with 3 m KCl and had resistances of 0.5–1.5 MΩ. Oocytes were held at −70 mV and agonist (300 μm glutamate; Tocris, Cologne, Germany) was applied by superfusion for 10 s at a flow rate of 10–14 ml min−1.
Electrophysiological measurements in hippocampal slices
Standard techniques were used to prepare transverse acute hippocampal slices (300–400 μm thick) from 15- to 75-day-old mice, which were deeply anaesthetized with halothane. Slices were maintained at room temperature in a storage chamber that was perfused with an artificial cerebrospinal fluid (ACSF) (mm: 119 NaCl, 2.5 KCl, 1.3 MgSO4, 2.5 CaCl2, 1 NaH2PO4, 26 NaHCO3, and 11 glucose, and equilibrated with 95% O2 and 5% CO2) for at least 1 h prior to recording. For synaptic recordings, a cut was made between the CA3 and CA1 region to prevent bursting, and the slices were bathed in a modified ACSF containing 100 μm picrotoxin to block GABAA-receptor-mediated inhibitory postsynaptic currents. Field excitatory postsynaptic potentials (fEPSPs) were recorded with glass pipettes (2–5 MΩ) filled with 1 m NaCl by stimulating Schaffer collaterals in stratum (s) radiatum (0.1 Hz) with a monopolar stimulating electrode. Somatic outside-out patches recordings were made from CA1 pyramidal cells held at −70 mV with patch pipettes (3–5 MΩ) filled with a solution containing (mm): 107.5 caesium gluconate, 20 Hepes, 0.2 EGTA, 8 sodium gluconate, 8 TEA-Cl, 4 MgATP, 0.3 Na3GTP, 5 QX314 (pH 7.2, 272 mosmol l−1). Currents were evoked by local application of 500 μm S-AMPA for 2 s in the presence of 100 μm cyclothiazide. Responses were collected with Axopatch-1D amplifier (Axon Instruments), filtered at 2 kHz, digitized at 5 kHz, and analysed on-line using Igor Pro software (Wavemetrics, Inc.).
Labeling of cell surface proteins using biotinylated ConA
To identify the fraction of receptor protein inserted in the plasma membrane, surface proteins were tagged with biotinylated Concanavalin A (ConA) (Sigma-Aldrich) and isolated by streptavidin/sepharose-mediated precipitation of the biotinyl-ConA/protein complex, as described elsewere (Strutz et al. 2002). Briefly, intact oocytes were incubated in 10 μm biotinyl-ConA (Sigma-Aldrich) for 30 min at room temperature. At this step, the biotinylated ConA binds to glycosylated plasma membrane proteins, e.g. glutamate receptors. To remove excess biotinylated ConA, oocytes were washed five times for 10 min in ND-96 buffer. After washing, 20 intact oocytes were homogenized with a Teflon pestle in H-buffer (20 μl oocyte−1; 100 mm NaCl, 20 mm Tris-HCl, pH 7.4, 1% Triton X-100, plus a mixture of proteinase inhibitors (Complete; Boehringer Mannheim, Mannheim, Germany)) and were kept at 4°C for 1 h on a rotating rod. Since intact oocytes were used for homogenization, only plasma membrane proteins, not proteins of internal membranes, were labelled. A 20 μl aliquot was kept as a total protein sample (T). After centrifugation of the remaining homogenate for 1 min at 16 000 g, the supernatants were supplemented with 20 μl of washed streptavidin-Sepharose beads (Sigma-Aldrich) and incubated at 4°C for 3 h on a rotating rod. During this step, the streptavidin beads bound to the biotinyl-ConA plasma membrane receptor complex. The streptavidin/sepharose beads were then pelleted by a 2 min spin at 16 000 g, and washed three times in H-buffer. A 20 μl volume of the supernatant was kept as supernatant protein sample (SN). The final pellets (P), containing plasma membrane receptors, were boiled in 20 μl of SDS-Page loading buffer (0.8 mβ-mercaptoethanol, 6% SDS, 20% glycerol, 25 mm Tris-HCl, pH 6.8, and 0.1% bromophenol blue).
Homogenization of mouse hippocampal tissue
Pieces of hippocampal tissue were removed from the mouse and immediately placed in liquid nitrogen for storage. The tissue was then placed into a 1.5 ml tube. After adding 500 μl of lysis buffer (50 mm Tris, pH 7.5, 1 mm EDTA, 5 mm NaF, 1 mm sodium pyrophosphate, 1 mm SOV (sodium orthovanadate), 1% Nonidet, 0.1% SDS, 0.1% SDC (sodium deoxycholate), one protease cocktail tablet Complete Mini (Roche)), the tissue was homogenized thoroughly with a polypropylene pestle (Schütt Labortechnik, Goettingen, Germany). The samples were centrifuged for 10 min at 8 000 g at 4°C. The pellet was discarded, and the supernatant aliquoted and stored at −80°C until needed. Prior to gel electrophoresis and Western blotting, a standard Bradford protein assay was performed to determine the total protein concentrations.
Gel electrophoresis and Western blotting
Proteins from homogenized oocytes were separated by SDS polyacrylamide gel electrophoresis and transferred to nitrocellulose filters. Blots were blocked in PBS containing 5% milk powder for at least 1 h at room temperature. For the detection of GluR1, primary rabbit immunoaffinity-purified anti-GluR1 antibody (1 μg μl−1; Upstate, Biomol, Hamburg, Germany) and secondary horseradish peroxidase-conjugated donkey anti-rabbit antibody (1:1000 dilution; Amersham Biosciences, Freiburg, Germany) were used. For the detection of β-tubulin, primary mouse monoclonal anti-β-tubulin antibody (1:250; Santa Cruz, CA, USA) and secondary horseradish peroxidase-conjugated sheep anti-mouse antibody (1:1000 dilution; Amersham Biosciences) were used. For verification of protein levels, Ponceau Red staining was performed.
Statistical analysis
For the immunoblotting studies, representative immunoblots are shown, and a quantitative assessment of plasma membrane abundance was carried out by densitometric analysis of immunoblots from similar experiments. Before pooling the results from different blots, the result from each blot was expressed as a percentage of the control value (relative abundance). The combined results from all blots were then expressed as the means ± s.e.m. Statistical analyses of the data were performed by Origin 6.0. Student's t test was applied for unpaired data and P < 0.05 was considered statistically significant. Oocyte experiments were analysed by Student's t test or ANOVA, as applicable.
Results
Regulation of GluR1 by SGK isoforms in Xenopus oocytes
To test for regulation of GluR1 by the SGK isoforms, we expressed the AMPA receptor subunit GluR1 in Xenopus oocytes with or without coexpression of either SGK1, SGK2 or SGK3. We used a nondesensitizing GluR1 mutant, GluR1(L479Y) (Stern-Bach et al. 1998), for our experiments. The membrane abundance of GluR1 was determined by performing an oocyte plasma membrane preparation and subsequent SDS gel and Western Blot. For quantification, we calculated the mean intensity from three different blots. As illustrated in Fig. 1, the protein membrane abundance of GluR1 was significantly increased in Xenopus oocytes expressing GluR1 together with SGK3, as compared with the GluR1 protein abundance in oocytes expressing GluR1 alone. GluR1 protein abundance tended to be higher following coexpression of SGK2, but was not appreciably affected by coexpression of SGK1. The coexpression of GluR1 with the SGK-related kinase protein kinase (PKB), which we used as a control for specificity of the regulatory mechanism, also did not result in an increase in GluR1 membrane abundance. As shown in a different study by our group, SGK3 has only a slight effect on the KA receptor subunit GluR6, whereas SGK1 increases significantly the protein membrane abundance of GluR6 (Strutz-Seebohm et al. 2005).
Figure 1. Increase of GluR1 subunit protein abundance in the plasma membranes of Xenopus oocytes coexpressing serum- and glucocorticoid-inducible kinase (SGK).
A, representative Western blot. Glycosylated plasma membrane proteins expressed in oocytes were labelled with biotinyl-ConA. Oocytes were homogenized and plasma membrane proteins were streptavidin-precipitated. Samples including controls from uninjected oocytes were separated on a SDS gel, Western-blotted and probed with a primary rabbit immunoaffinity-purified anti-GluR1 antibody. GluR1 protein has an apparent molecular mass of ∼105 kDa. P, plasma membrane protein (n = 18); SN, supernatant fraction containing intracellular protein (n = 1); T, total protein (n = 1). B, bar graph showing relative abundance of GluR1 plasma membrane protein. The band intensity was quantified by arithmetric analysis using the software Scion image. The values of three different blots from different batches were used for the statistical analysis. Due to the saturating conditions of the Western Blot procedure, the analysis represents only an estimation of relative abundance of GluR1 plasma membrane protein.
Note that the quantification of Western blots (Fig. 1B) can only serve as an estimation of protein ratios due to the saturating conditions of the technique.
The protein abundance was paralleled by similar effects on glutamate-induced currents. Figure 2 shows glutamate-induced current amplitudes of GluR1 expressed alone or coexpressed with either SGK isoform or PKB in Xenopus oocytes. The glutamate-induced currents were significantly larger in oocytes expressing GluR1 together with SGK3 than in oocytes expressing GluR1 alone. The glutamate-induced current in oocytes coexpressing SGK2 was significantly smaller than that in SGK3-expressing oocytes, but significantly larger than in oocytes expressing GluR1 alone. Again, coexpression of SGK1 or PKB did not significantly modify GluR1-mediated currents. The PKB used in all experiments was always a constitutively active mutant (T308D/S473D). To also assure that the SGK kinases are active in the oocyte system, we repeated the experiments with the constitutively active SGK1(S422D) and SGK3(S419D), as well as inactive SGK1(K127N) and SGK3(K124N) mutants. The results showed that wild-type SGK1 and wild-type SGK3 are active when injected into oocytes. Coexpression of SGK3(S419D) with GluR1 leads to a current amplitude that was comparable with wild-type SGK3 (3.2 ± 0.4 nA, and 3.0 ± 0.3 nA, respectively; n = 15), whereas coexpression of GluR6 with the inactive form SGK3(K124N) resulted in reduced current amplitudes (0.8 ± 0.1 nA; n = 15). For SGK1, no effect was seen for either the active (0.9 ± 0.2 nA; n = 15) or the inactive form (1.0 ± 0.2 nA; n = 15) compared with the wild-type SGK1 (1.3 ± 0.2 nA; n = 16).
Figure 2. Increase in GluR1 currents by SGK2 and SGK3 isoforms but not by SGK1 and PKB.
A, representative current traces measured in Xenopus oocytes in response to superfusion with 300 μm glutamate. All currents were measured at −70 mV. Horizontal scale bars indicate 5 s, and vertical scale bars represent 1 μA. B, GluR1 current amplitudes in oocytes expressing GluR1(L479Y) + DEPC-H2O, GluR1(L479Y) + SGK1, GluR1(L479Y) + SGK2, GluR1(L479Y) + SGK3 and GluR1(L479Y) + PKB normalized to the GluR1(L479Y) + DEPC-H2O currents. Numbers of oocytes are shown in parenthesis, and significant changes (P < 0.001) are indicated by ***.
We also tested the current–voltage (I–V) relationship of GluR1 coexpressed with one of the SGK isoforms or PKB. Neither kinase significantly modified the (I–V) relationship of GluR1, indicating that the ion selectivity for the major charge-carrying ions (Na+ and K+) is not changed by SGK isoforms or PKB.
We also coexpressed SGK3 with GluR2(R), GluR3, GluR4, GluR1 plus GluR2(R), andGluR1 plus GluR3. Interestingly, GluR2 and GluR3 receptor subunits coexpressed with SGK3 showed reduced current amplitudes (0.51 ± 0.04 nA, n = 36 for GluR2; 0.67 ±0.07 nA, n = 17 for GluR3) compared with single expression of either receptor subunit, and GluR4 amplitudes were unchanged when coexpressed with SGK3 (0.82 ± 0.10 nA, n = 25). Coexpression of GluR1 plus GluR2 plus SGK3 resulted in increased current amplitudes (2.24 ± 0.59 nA, n = 30). Similarily, coexpression of GluR1 plus GluR3 plus SGK3 showed increased current amplitudes (3.5 ± 1.3 nA, n = 15) compared with expression of GluR1 plus GluR3 (1.0 ± 0.3 nA, n = 15). GluR1 appears to be the only AMPA subunit that is upregulated by SGK3. Therefore, we focused on GluR1 for further studies on the SGK3-knockout mice.
SGK3 is expressed in hippocampal tissue
GluR1 is abundantly expressed in the brain (Hollmann & Heinemann, 1994; Wenthold et al. 1996). It plays a major role in the generation of long-term potentiation which occurs in the hippocampus. Therefore, regulation of GluR1 by SGK3 appeared particularly interesting in the hippocampus. For SGK3, however, it had not been shown that mRNA is expressed in the hippocampus. Therefore, total RNA of hippocampal tissue was collected from SGK3-knockout mice (Sgk3−/−) and their wild-type littermates (Sgk3+/+). RT-PCR experiments were performed to confirm that Sgk3 transcripts are present in hippocampal tissue. A 211 bp Sgk3-specific PCR product was amplified and revealed Sgk3 mRNA expression in the hippocampus of Sgk3+/+ but not Sgk3−/− mice (Fig. 3A). To determine the exact localization of SGK3 in the hippocampus, we performed in situ hybridization, showing Sgk3 mRNA expression in the CA1, CA3 and dentate gyrus of hippocampus (Fig. 3B). These regions overlap with GluR1 mRNA expression (Hollmann & Heinemann, 1994). Sgk3 mRNA is mostly localized in the pyramidal cell layers of CA3, on hilar cells and in the granular cell layer of the dentate gyrus (Fig. 3Bb). Also, the neuronal cortex cell layers (Fig. 3Be) and the thalamus (Fig. 3Ba, b, d) are Sgk3-mRNA-positive. In the cornu ammonis and stratum radiatum, hybridization signals are present in the pyramidal cell layer and interneurones (Fig. 3Bf).
Figure 3. Detection of Sgk3 transcripts in hippocampal tissue by RT-PCR and in situ hybridization.
A, amplification of hippocampal Sgk3 cDNA by RT-PCR. RNA from the hippocampus of Sgk3−/− and Sgk3+/+ mice was collected and subjected to RT-PCR. As a control, RNA from kidney and heart was used. Further controls were samples without reverse transcriptase and without template. B, in situ hybridization of Sgk3 mRNA on frontal cryostat sections of the adult hippocampus. Overview (a) and closeup (d) of Sgk3 expression in the hippocampal system. c, sense control of Sgk3 of an area similar to the one shown in d. Dotted lines indicate the principal cell layers of the hippocampus. b, Sgk3 mRNA is detected in the pyramidal cell layers of CA3, on hilar cells and in the granular cell layer of the dentate gyrus (rectangle in d indicates magnified area). Medium (e) and high (f) magnification photomicrographs of Sgk3 expression in neocortex and CA1. (e) The neuronal cortex cell layers are Sgk3 mRNA positive. (f) In the cornu ammonis, hybridization signals are present in the pyramidal cell layer. Furthermore, a few cells, probably interneurones, are labelled in the stratum radiatum (arrows). Also, in the thalamus (a, b, d), neurones were stained by the antisense probe. NC, neocortex; CA, cornu ammonis; DG, dentate gyrus; gcl, granular cell layer; hi, hilus; ml, molecular layer; SO, stratum oriens; SR, stratum radiatum; TH, thalamus. Scale bars: a, 400 μm; b, 50 μm; c, d and e, 100 μm; f, 25 μm.
SGK3 regulates GluR1 abundance in mouse hippocampal neurones
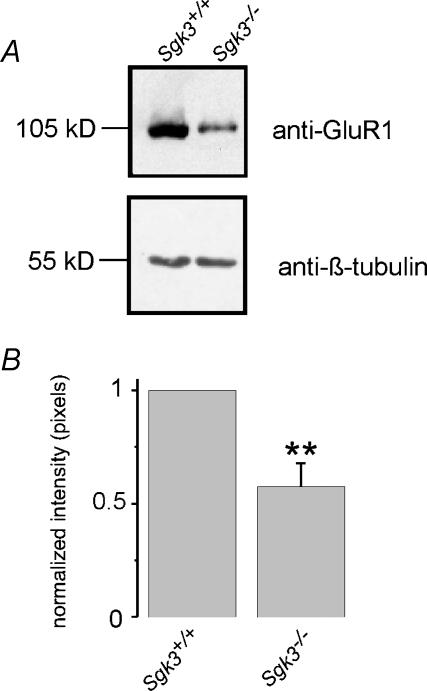
To explore whether the regulation of GluR1 by SGK3 is relevant for receptor density in hippocampal neurones, hippocampal tissue from SGK3-knockout mice (Sgk3−/−) was compared with that of wild-type littermates (Sgk3+/+) (McCormick et al. 2004). Western blotting indeed revealed significantly reduced protein expression of GluR1 in Sgk3−/− as compared with Sgk3+/+ total hippocampal tissue (Fig. 4). In contrast, β-tubulin as a control did not show any differences in protein expression in Sgk3−/− as compared with Sgk3+/+ hippocampal tissue (Fig. 4A).
Figure 4. Decreased GluR1 protein abundance in mice deficient of SGK3.
A, original, representative Western blot of mouse hippocampal tissue. Immunostaining of protein fractions of hippocampal tissue of wild-type mice and SGK3-knockout mice using an immunoaffinity-purified rabbit anti-GluR1 antibody. GluR1 receptor expression was less pronounced in Sgk3−/− than in Sgk3+/+ hippocampal tissue. The same blot was stripped and reprobed with monoclonal anti-β-tubulin antibody. β-(ubulin protein expression was tested as a control, and was comparable in SGK3-knockout and wild-type mice. B, bar graph showing arithmetic means ± s.e.m. of relative abundance of GluR1 protein. Protein was isolated from three wild-type mice and five SGK3-knockout mice. **Significant difference between Sgk3+/+ and Sgk3−/−(P < 0.01). Due to the saturating conditions of the Western blot the analysis represents only an estimation of relative abundance of GluR1 plasma membrane protein.
We then investigated whether the reduction in GluR1 expression in Sgk3−/− mice affects basal AMPA receptor-mediated transmission in hippocampal slices. To assess the strength of synaptic transmission, we compared the size of the presynaptic fibre volley (input) to the slope of the EPSP (output) in stratum radiatum and found no significant difference in Sgk3−/−(n= 5) as compared with Sgk3+/+ (n = 22) mice (data not shown). As GluR1−/− mice show unaltered synaptic, but strongly reduced extrasynaptic AMPA receptor currents (Zamanillo et al. 1999), we also assessed the pool of extrasynaptic receptors. Extrasynaptic AMPA receptor-mediated currents were recorded in outside out somatic membrane patches, since excitatory synapses do not contact the soma of pyramidal cells, and were similar in Sgk3−/− (−652 ± 181 pA, n = 6) and Sgk3+/+ mice (−601 ± 72 pA, n = 12).
Discussion
Dynamic regulation of AMPA-type receptors at the synapse is proposed to play a critical role in alterations of the synaptic strength seen in cellular models of learning and memory, such as long-term potentiation in the hippocampus. Protein phosphorylation plays a central role in controlling AMPA receptor expression at the synapse and in regulating synaptic strength (Soderling & Derkach, 2000; Ahmadian et al. 2004). The signalling underlying the regulation of AMPA receptor trafficking involves the PI3-kinase (Passafaro et al. 2001). Downstream targets of the PI3-kinase include phosphoinositide-dependent kinases PDK1 and PDK2, protein kinase B, and the serum- and glucocorticoid-inducible kinases SGK1, SGK2 and SGK3. All three members of the SGK family are excellent candidates for the regulation of ion channels. All three have been been shown to regulate the renal epithelial Na+ channel ENaC by increasing the abundance of the channel protein in the plasma membrane (Lang & Cohen, 2001; Pearce, 2003; Verrey et al. 2003). All three kinases are abundantly expressed in the brain (Soderling & Derkach, 2000; Kobayashi et al. 1999), and are thus in a position to regulate the abundance of neuronal ion channels. Therefore, we explored the possible regulatory role of the serum- and glucocorticoid-inducible kinases on the AMPA receptor subunit GluR1 by applying various methods. The results indeed suggest a regulatory role of SGK3 on GluR1 abundance. By using constitutively active mutants of SGK1, SGK3 and PKB for oocyte recordings, we verified that the observed effect of SGK3 on GluR1 is not due to different activation of the kinases. The effect of SGK1, SGK2 and SGK3 on the epithelial Na+ channel ENaC (Lang & Cohen, 2001; Pearce, 2003; Verrey et al. 2003) is similar, which also indicates that the lack of effect of SGK1 on GluR1 is not due to inactivity of the kinases in oocytes. Nevertheless the abundance and activation of the kinases may be different in neuronal cells than in oocytes. Moreover, although coexpression of GluR1 with either GluR2 or GluR3 in oocytes did not result in significant changes of the SGK3 effect, we cannot exclude that the effect of the kinases may be different in homomerically and heteromerically expressed glutamate receptors in vivo. In any case, the data on SGK3-knockout mice are in line with the oocyte data, and illustrate the significance of SGK3-dependent regulation of GluR1. The present observations reveal a novel mechanism in the regulation of the GluR1 subunit of AMPA receptors. The delivery of GluR1 to the neuronal surface is regulated by activation of NMDA receptors (Passafaro et al. 2001), which may involve the following signalling: Activation of NMDA receptors leads to Ca2+ entry (Sheng & Kim, 2002) with subsequent activation of the small G-protein Ras (Yun et al. 1998; Cullen & Lockyer, 2002), which may trigger a signalling cascade including PI3-kinase, PDK1/PDK2 and SGK3 (Lang & Cohen, 2001). SGK3 then enhances the protein abundance of GluR1 in the cell membrane. SGK3 may stabilize GluR1 in the membrane thus preventing its retrieval and subsequent degradation and/or enhance trafficking of the GluR1 protein to the cell membrane. The decrease of total GluR1 protein in tissues of Sgk3−/− mice suggests decreased expression or enhanced degradation of GluR1 protein. Clearly, lack of SGK3 does not abrogate GluR1 trafficking to the cell membrane pointing to additional, parallel mechanisms serving the same or similar functions such as calmodulin-dependent kinase II and protein kinase A (Sheng & Kim, 2002; Esteban et al. 2003). However, the present observations strongly suggest that SGK3 substantially contributes to the fine-tuning of GluR1 abundance. SGK3 is thus expected to participate in GluR1-dependent neuronal function. GluR1 is required for hippocampal CA1 long-term potentiation (Zamanillo et al. 1999), participates in the generation of spatial memory (Reisel et al. 2002; Lee et al. 2003), and has also been associated with major depressive disorders (Meador-Woodruff et al. 2001).
Lack of SGK3 presumably does not result in a phenotype identical to complete lack of GluR1 (Reisel et al. 2002). In GluR1-knockout mice, synaptic AMPA receptor currents are unchanged, but AMPA-evoked extrasynaptic currents are reduced (Zamanillo et al. 1999). We would expect that the knockout of a kinase such as SGK that modulates but not abrogates GluR1 trafficking should result in an even more subtle phenotype that could be difficult to detect. Furthermore, the modulation of GluR1 trafficking in Sgk3−/− mice could be compensated by other AMPA receptor subunits. It is not possible to distinguish between AMPA subunits when recording AMPA-evoked currents in patches. Finally, molecules other than SGK could additionally modulate GluR1 receptors, e.g. by increasing the conductance, which might compensate for reduced surface expression, thereby resulting in no differences in current amplitudes after activation by AMPA. The same could be true for extrasynaptic subunits: other upstream molecules could compensate for the lack of modulation by SGK3.
Conversely, the function of SGK3 is most likely not limited to the regulation of GluR1, and lack of SGK3 may affect neuronal functions other than those dependent on GluR1. For example, SGK3 has been shown to upregulate the voltage-gated K+ channel Kv1.3 (Gamper et al. 2002a, b), which may also affect memory function (Kourrich et al. 2001). Downstream targets of SGK3 further include the Na+,K+-ATPase which has been shown to be activated by SGK3 (Henke et al. 2002). Impaired Na+,K+-ATPase activity in hippocampal synaptic plasma membranes has been suggested to foster depressive disorders (Li et al. 1997; Gamaro et al. 2003).
Additional experimentation will be required to disclose the participation of SGK3 and of SGK3-dependent regulation of GluR1 in memory consolidation, or further neuronal functions, and to disclose the compensatory mechanisms maintaining AMPA-receptor-mediated current in the absence of SGK3. In summary, SGK3 enhances the abundance of GluR1 in the plasma membrane and increases GluR1-mediated glutamate-induced currents. Thus, it is likely to participate in the PI3-kinase-dependent regulation of AMPA receptor trafficking.
References
- Ahmadian G, Ju W, Liu L, Wyszynski M, Lee SH, Dunah AW, Taghibiglou C, Wang Y, Lu J, Wong TP, Sheng M, Wang YT. Tyrosine phosphorylation of GluR2 is required for insulin-stimulated AMPA receptor endocytosis and LTD. EMBO J. 2004;23:1040–1050. doi: 10.1038/sj.emboj.7600126. 10.1038/sj.emboj.7600126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen PJ, Lockyer PJ. Integration of calcium and Ras signalling. Nat Rev Mol Cell Biol. 2002;3:339–348. doi: 10.1038/nrm808. [DOI] [PubMed] [Google Scholar]
- Debonneville C, Flores SY, Kamynina E, Plant PJ, Tauxe C, Thomas MA, Munster C, Chraibi A, Pratt JH, Horisberger JD, Pearce D, Loffing J, Staub O. Phosphorylation of Nedd4–2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J. 2001;20:7052–7059. doi: 10.1093/emboj/20.24.7052. 10.1093/emboj/20.24.7052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Gamaro GD, Streck EL, Matte C, Prediger ME, Wyse AT, Dalmaz C. Reduction of hippocampal Na+, K+-ATPase activity in rats subjected to an experimental model of depression. Neurochem Res. 2003;28:1339–1344. doi: 10.1023/a:1024988113978. 10.1023/A:1024988113978. [DOI] [PubMed] [Google Scholar]
- Gamper N, Fillon S, Feng Y, Friedrich B, Lang PA, Henke G, Huber SM, Kobayashi T, Cohen P, Lang F. K+ channel activation by all three isoforms of serum- and glucocorticoid-dependent protein kinase SGK. Pflugers Arch. 2002a;445:60–66. doi: 10.1007/s00424-002-0873-2. 10.1007/s00424-002-0873-2. [DOI] [PubMed] [Google Scholar]
- Gamper N, Fillon S, Huber SM, Feng Y, Kobayashi T, Cohen P, Lang F. IGF-1 up-regulates K+ channels via PI3-kinase, PDK1 and SGK1. Pflugers Arch. 2002b;443:625–634. doi: 10.1007/s00424-001-0741-5. 10.1007/s00424-001-0741-5. [DOI] [PubMed] [Google Scholar]
- Henke G, Setiawan I, Bohmer C, Lang F. Activation of Na+/K+-ATPase by the serum and glucocorticoid-dependent kinase isoforms. Kidney Blood Press Res. 2002;25:370–374. doi: 10.1159/000068699. 10.1159/000068699. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Deak M, Morrice N, Cohen P. Characterization of the structure and regulation of two novel isoforms of serum- and glucocorticoid-induced protein kinase. Biochem J. 1999;344:189–197. 10.1042/0264-6021:3440189. [PMC free article] [PubMed] [Google Scholar]
- Kourrich S, Mourre C, Soumireu-Mourat B. Kaliotoxin, a Kv1.1 and Kv1.3 channel blocker, improves associative learning in rats. Behav Brain Res. 2001;120:35–46. doi: 10.1016/s0166-4328(00)00356-9. 10.1016/S0166-4328(00)00356-9. [DOI] [PubMed] [Google Scholar]
- Lang F, Cohen P. Regulation and physiological roles of serum- and glucocorticoid-induced protein kinase isoforms. Sci STKE. 2001;2001:RE17. doi: 10.1126/stke.2001.108.re17. [DOI] [PubMed] [Google Scholar]
- Lang F, Henke G, Embark HM, Waldegger S, Palmada M, Bohmer C, Vallon V. Regulation of channels by the serum and glucocorticoid-inducible kinase – implications for transport, excitability and cell proliferation. Cell Physiol Biochem. 2003;13:41–50. doi: 10.1159/000070248. 10.1159/000070248. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of the AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Li R, el Mallakh RS, Harrison L, Changaris DG, Levy RS. Lithium prevents ouabain-induced behavioral changes. Toward an animal model for manic depression. Mol Chem Neuropathol. 1997;31:65–72. doi: 10.1007/BF02815161. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. 10.1016/S0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Mack V, Burnashev N, Kaiser KM, Rozov A, Jensen V, Hvalby O, Seeburg PH, Sakmann B, Sprengel R. Conditional restoration of hippocampal synaptic potentiation in Glur-A-deficient mice. Science. 2001;292:2501–2504. doi: 10.1126/science.1059365. 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- Maljevic S, Lerche C, Seebohm G, Alekov AK, Busch AE, Lerche H. C–terminal interaction of KCNQ2 and KCNQ3 K+ channels. J Physiol. 2003;548:353–360. doi: 10.1113/jphysiol.2003.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man HY, Wang Q, Lu WY, Ju W, Ahmadian G, Liu L, D'Souza S, Wong TP, Taghibiglou C, Lu J, Becker LE, Pei L, Liu F, Wymann MP, MacDonald JF, Wang YT. Activation of PI3-kinase is required for AMPA receptor insertion during LTP of mEPSCs in cultured hippocampal neurons. Neuron. 2003;38:611–624. doi: 10.1016/s0896-6273(03)00228-9. 10.1016/S0896-6273(03)00228-9. [DOI] [PubMed] [Google Scholar]
- McCormick JA, Feng Y, Dawson K, Behne MJ, Yu B, Wang J, Wyatt AW, Henke G, Grahammer F, Mauro TM, Lang F, Pearce D. Targeted disruption of the protein kinase SGK3/CISK impairs postnatal hair follicle development. Mol Biol Cell. 2004;15:4278–4288. doi: 10.1091/mbc.E04-01-0027. 10.1091/mbc.E04-01-0027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meador-Woodruff JH, Hogg AJ, Jr, Smith RE. Striatal ionotropic glutamate receptor expression in schizophrenia, bipolar disorder, and major depressive disorder. Brain Res Bull. 2001;55:631–640. doi: 10.1016/s0361-9230(01)00523-8. 10.1016/S0361-9230(01)00523-8. [DOI] [PubMed] [Google Scholar]
- Park J, Leong ML, Buse P, Maiyar AC, Firestone GL, Hemmings BA. Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI3-kinase-stimulated signaling pathway. EMBO J. 1999;18:3024–3033. doi: 10.1093/emboj/18.11.3024. 10.1093/emboj/18.11.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passafaro M, Piech V, Sheng M. Subunit-specific temporal and spatial patterns of AMPA receptor exocytosis in hippocampal neurons. Nat Neurosci. 2001;4:917–926. doi: 10.1038/nn0901-917. 10.1038/nn0901-917. [DOI] [PubMed] [Google Scholar]
- Pearce D. SGK1 regulation of epithelial sodium transport. Cell Physiol Biochem. 2003;13:13–20. doi: 10.1159/000070245. 10.1159/000070245. [DOI] [PubMed] [Google Scholar]
- Reisel D, Bannerman DM, Schmitt WB, Deacon RM, Flint J, Borchardt T, Seeburg PH, Rawlins JN. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5:868–873. doi: 10.1038/nn910. 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- Sanna PP, Cammalleri M, Berton F, Simpson C, Lutjens R, Bloom FE, Francesconi W. Phosphatidylinositol 3-kinase is required for the expression but not for the induction or the maintenance of long-term potentiation in the hippocampal CA1 region. J Neurosci. 2002;22:3359–3365. doi: 10.1523/JNEUROSCI.22-09-03359.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seebohm G, Chen J, Strutz N, Culberson C, Lerche C, Sanguinetti MC. Molecular determinants of KCNQ1 channel block by a benzodiazepine. Mol Pharmacol. 2003;64:70–77. doi: 10.1124/mol.64.1.70. 10.1124/mol.64.1.70. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Sheng M, Nakagawa T. Neurobiology: glutamate receptors on the move. Nature. 2002;417:601–602. doi: 10.1038/417601a. 10.1038/417601a. [DOI] [PubMed] [Google Scholar]
- Soderling TR, Derkach VA. Postsynaptic protein phosphorylation and LTP. Trends Neurosci. 2000;23:75–80. doi: 10.1016/s0166-2236(99)01490-3. 10.1016/S0166-2236(99)01490-3. [DOI] [PubMed] [Google Scholar]
- Stern-Bach Y, Russo S, Neuman M, Rosenmund C. A point mutation in the glutamate binding site blocks desensitization of AMPA receptors. Neuron. 1998;21:907–918. doi: 10.1016/s0896-6273(00)80605-4. 10.1016/S0896-6273(00)80605-4. [DOI] [PubMed] [Google Scholar]
- Strutz N, Villmann C, Breitinger HG, Werner M, Wenthold RJ, Kizelsztein P, Teichberg VI, Hollmann M. Kainate-binding proteins are rendered functional ion channels upon transplantation of two short pore-flanking domains from a kainate receptor. J Biol Chem. 2002;277:48035–48042. doi: 10.1074/jbc.M209647200. 10.1074/jbc.M209647200. [DOI] [PubMed] [Google Scholar]
- Strutz-Seebohm N, Seebohm G, Shumilina S, Mack AF, Wagner H-J, Lampert A, Grahammer F, Henke G, Just L, Skutella T, Hollmann M, Lang F. Glucocorticoid adrenal steroids and glucocorticoid inducible kinase isoforms in the regulation of GluR6 expression. J Physiol. 2005 doi: 10.1113/jphysiol.2004.079624. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verrey F, Loffing J, Zecevic M, Heitzmann D, Staub O. SGK1: aldosterone-induced relay of Na+ transport regulation in distal kidney nephron cells. Cell Physiol Biochem. 2003;13:021–028. doi: 10.1159/000070246. 10.1159/000070246. [DOI] [PubMed] [Google Scholar]
- Waldegger S, Barth P, Raber G, Lang F. Cloning and characterization of a putative human serine/threonine protein kinase transcriptionally modified during anisotonic and isotonic alterations of cell Volume. Proc Natl Acad Sci U S A. 1997;94:4440–4445. doi: 10.1073/pnas.94.9.4440. 10.1073/pnas.94.9.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, II, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun HY, Gonzalez-Zulueta M, Dawson VL, Dawson TM. Nitric oxide mediates N-methyl-d-aspartate receptor-induced activation of p21ras. Proc Natl Acad Sci U S A. 1998;95:5773–5778. doi: 10.1073/pnas.95.10.5773. 10.1073/pnas.95.10.5773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]