Abstract
Membrane lysis is a common and early defect in muscles experiencing acute injuries or inflammation. Although increased mechanical loading of muscles can induce inflammation and membrane lysis, whether mechanical loads applied to muscle can promote the activation and cytolytic capacity of inflammatory cells and thereby increase muscle damage is unknown. We tested whether mechanical loads applied to mouse muscle cells in vitro can increase membrane lysis, and whether neutrophil-mediated lysis of muscle cells is promoted by mechanical loads applied in vitro and in vivo. Cyclic loads applied to muscle cells for 24 h in vitro produced little muscle cell lysis. Similarly, the addition of neutrophils to muscle cell cultures in the presence of superoxide dismutase (SOD) produced little muscle cell lysis. However, when cyclic mechanical loads were applied to neutrophil–muscle co-cultures in the presence of SOD, there was a synergistic effect on muscle cell lysis, suggesting that mechanical loading activates neutrophil cytotoxicity. However, application of mechanical loads to co-cultures of muscle cells and neutrophils that are null mutants for myeloperoxidase (MPO) showed no mechanical activation of neutrophil cytotoxicity. This indicates that loading promotes neutrophil cytotoxicity via MPO. Activity assays confirmed that mechanical loading of neutrophil–muscle co-cultures significantly increased MPO activity. We further tested whether muscle membrane lysis in vivo was mediated by neutrophils when muscle was subjected to modified loading by using a mouse model of muscle reloading following a period of unloading. We observed that MPO −/− soleus muscles showed a significant 52% reduction in membrane lysis compared to wild-type mice, although the mutation did not decrease inflammatory cell extravasation. Together, these in vitro and in vivo findings show that mechanical loading activates neutrophil-mediated lysis of muscle cells through an MPO-dependent pathway.
Lysis of muscle cell membranes by immune cells can be an early and pivotal event in promoting muscle injury or disease. For example, death of muscle cells in polymyositis, a progressive and debilitating inflammatory myopathy, is initiated by the release of the lytic protein perforin by cytotoxic T-cell lymphocytes onto the surface of muscle fibres (Goebels et al. 1996). Muscle membrane lysis is then followed by T-cell invasion of the lysed fibres, and muscle fibre death (Nakamura et al. 1993; Goebels et al. 1996). In other progressive myopathies, both lymphoid and myeloid cells have been implicated in promoting lysis and death of muscle fibres. Depletion of either cytotoxic T-lymphocytes or macrophages from mdx mice, a model of Duchenne muscular dystrophy, causes a significant reduction in muscle pathology and decreases muscle membrane lysis (Spencer et al. 2001; Wehling et al. 2001).
Myeloid cells also play a key role in promoting the muscle membrane lysis that follows injury. Periods of muscle ischaemia followed by perfusion lead to extensive lysis and death of muscle fibres that can be attenuated by depletion of neutrophils prior to reperfusion (Jolly et al. 1986; Korthuis et al. 1988; Kyriakides et al. 1999). Several observations show that neutrophil-mediated lysis during ischaemia–reperfusion is largely mediated by free radicals. Treatments with superoxide dismutase (SOD) prior to reperfusion to reduce the concentration of the potentially injurious free radical, superoxide, can significantly reduce muscle lysis and damage. Similarly, administration of catalase to decrease hydrogen peroxide concentration can reduce muscle damage during reperfusion (Smith et al. 1989).
Free radicals generated by myeloid cells also promote muscle damage during modified muscle use. Rodents that are subjected to periods of muscle unloading followed by return to normal loading experience muscle inflammation, muscle membrane lysis and necrosis that occur over a stereotypic time course. Significant increases in membrane lysis are detectable within 2 h of the return to muscle loading, and continue to increase for the next 20–24 h (Tidball et al. 1999). Neutrophil populations are significantly elevated within 2 h of reloading, followed by an increase in macrophages within 12–24 h (Tidball et al. 1999). Muscle membrane lysis during reloading was initially thought to be a direct result of the mechanical load placed on the muscle, but more recent experimental observations have shown that the majority of the lysis can be attributed to neutrophil-mediated damage. Membrane lysis induced by neutrophils in this model of muscle injury appears to result directly or indirectly from superoxide because null mutation of gp91phox, the catalytic subunit of NADPH oxidase, yields neutrophils that cannot produce superoxide, and prevents most membrane lesions in muscles experiencing reloading (Nguyen & Tidball, 2003a, b ).
Although neutrophils may cause most muscle membrane lesions that occur during muscle reloading following periods of unloading, neutrophil invasion and subsequent muscle damage are initiated by changes in the mechanical loads applied to muscle. This suggests two potential mechanisms through which mechanical loading can exacerbate muscle injury caused by neutrophils. First, loading could cause the production or release of chemotactic factors that attract neutrophils to the muscle where they then promote muscle membrane lysis. This possibility is supported by previous observations showing that muscle reloading activates complement in muscle, which is necessary for the subsequent invasion of the reloaded muscle by neutrophils (Frenette et al. 2000). Also, reloading could increase the activation of neutrophils, and thereby enhance their cytotoxicity. Several in vivo observations support this possibility. In particular, increased muscle activity can be associated with an increase in myeloperoxidase (MPO) activity in circulating populations of neutrophils (Suzuki et al. 1996), which suggests that loads applied to muscle can influence the activation state of neutrophils, which may in turn affect the subsequent lysis of muscle cells by invading populations of neutrophils.
In the present investigation, we examine the relationships between mechanical loading, neutrophil activation and muscle cell lysis, using in vivo and in vitro models. We use in vitro assays of muscle cell lysis by wild-type or MPO −/− neutrophils in the presence or absence of mechanical loads applied to the cells, to test whether mechanical loading promotes neutrophil lysis of muscle cells, and to assess whether neutrophil cytotoxicity is mediated by MPO. We also test whether mechanical loading increases the activation of neutrophils through the release of soluble factors from the loaded muscle. Finally, we test in vivo whether neutrophil-mediated lysis of muscle cells occurs through MPO-dependent processes by analysing muscle membrane lysis in wild-type and MPO −/− mice subjected to muscle unloading followed by reloading.
Methods
Animals
All experiments involving the use of animals were conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the University of California, Los Angeles Institutional Animal Care and Use Committee. C57BL/6J mice were obtained from the Jackson Laboratories (Bar Harbour, ME, USA). MPO −/− mice were originally generated on the 129/SvJ background and then transferred to the C57BL/6J background by at least 10 generations of back-crossing, as previously described (Brennan et al. 2001a). Mice were maintained in an accredited animal care facility and were monitored daily for signs of distress, injury or disease. At the end of experimentation, mice were killed by an overdose of sodium pentobarbital, according to the Panel on Euthanasia of the American Veterinary Medical Association.
Isolation of peritoneal neutrophils
Peritoneal neutrophils were collected 20 h following intraperitoneal injection of 12% sodium caseinate. Cells from the peritoneal exudates were centrifuged at 500 g for 5 min and then resuspended in 0.85% ammonium chloride to lyse erythrocytes. The cells were again pelleted and then resuspended in Hanks' balanced salt solution (HBSS). The suspension was overlaid on Histopaque 1077 (Sigma, St Louis, MO, USA) and then centrifuged at 400 g for 45 min at 4°C. Neutrophils were collected from the pellet. The purity of the neutrophil preparations exceeded 90%, as assessed morphologically in haematoxylin-stained preparations of the isolated cells that adhered to microscope slides by centrifugation (Cytospin, Shandon, USA).
Cytotoxicity assays
C2C12 mouse muscle cells were cultured in 6-well plates in which the bottom of each well consisted of a flexible, collagen-coated, silicon elastomer (Flex 1 plates, Flexcell, Carrsboro, NC, USA). Cells in the mechanical-loading plates were grown in 10% fetal bovine serum (FBS) in Dulbecco's modified Eagle's medium (DMEM) for 7 days, when they formed a confluent monolayer. The cells were then placed in serum-free DMEM overnight to induce fusion, and then returned to a medium containing 10% FBS for 2 days before use in cytotoxicity assays. Myotubes were then co-cultured with neutrophils in HBSS containing 0.25% FBS for 24 h to assess cytotoxicity. Neutrophils in some co-cultures were activated by the addition of 0.64 μm phorbol 12-myristate 13-acetate (PMA). In other co-cultures, SOD (500 units ml−1) was added to remove superoxide from the medium. At the end of 24 h of co-incubation, the medium was collected and lactate dehydrogenase (LDH) release into the medium was assayed as a measure of cell lysis. A 50 μl aliquot of medium was mixed with 50 μl of LDH substrate mix (Promega) which was incubated for 30 min in the dark at room temperature. Absorbance at 490 nm was measured to determine LDH activity, which represented the LDH concentration in the medium. Spontaneous release (0% cytotoxicity) was determined by assaying LDH activity in media collected from cultures containing muscle cells without neutrophils. Total LDH release was determined by measuring LDH activity in the media of muscle cells that were lysed with 0.1% Triton X-100 in HBSS (100% cytotoxicity). Neutrophil density in cytotoxicity cultures was expressed as the number of neutrophils per square millimetre of surface area in the dish, as previously described (Nguyen & Tidball, 2003b). Relative cell numbers were not expressed as effector to target cell ratios because the variability in cell proliferation and fusion that occurs as myoblasts differentiate into myotubes prevents the actual number of target cells being known. Instead, relative target cell numbers were normalized to the surface area of the culture dish, in which they form an adherent and continuous monolayer. All cytotoxicity assays were performed at least 3 times, with six replicates of each condition in each assay, and each value was expressed as the mean with its standard error (s.e.m.).
Cyclic mechanical loading
Mechanical-loading plates containing muscle cells only, neutrophils only or muscle cells and neutrophils together were placed onto a base-plate (Flexcell) housed in a 37°C incubator. Cyclic loads were applied to the flexible bottoms of the wells and to the attached cells by cyclic application of negative pressure. The mean strain of the loaded membrane was 20%, which is within the physiological range experienced by skeletal muscle. The strain cycle consisted of 20 s sinusoidal strain applied at 1.0 Hz followed by 20 s of rest. This strain cycle was continued for 24 h before media were assayed for LDH release cytotoxicity.
MPO activity assay
MPO activity was assayed according to Suzuki et al. (1983). Neutrophils or co-cultures containing neutrophils and muscle cells were incubated in HBSS containing 0.25% FBS with 0.64 μm PMA and 500 units ml−1 SOD. After incubation of cultures containing neutrophils only, muscle cells only, or neutrophils and muscle cell co-cultures, media were collected and centrifuged for 2 min at 12 000 g at 4°C. Mixtures of 50 μl supernatant and 50 μl reaction solution (3.2 mm tetramethylbenzidine, 0.6 mm hydrogen peroxide and 16%N,N-dimethylformamide in 160 mm sodium phosphate buffer, pH 5.4) were incubated for 3 min at 37°C. Reactions were inhibited by the addition of 200 μl of 200 mm sodium acetate buffer (pH 3.0). Absorbance of the reaction mixture was measured immediately at 630 nm. MPO activity was expressed as change in absorbance per minute per millilitre of supernatant.
Assays of neutrophil activation by conditioned media from preloaded cells
Conditioned media were collected from muscle cell cultures at the end of a 24 h period of cyclic mechanical loading, and then transferred to cultures of neutrophils for an additional 24 h. Control neutrophil cultures received conditioned media from muscle cells that were cultured for 24 h in the absence of mechanical loading. The medium was then collected from the neutrophil cultures after 24 h incubation and assayed for MPO activity.
Hindlimb muscle unloading and reloading
Muscle injury and inflammation were induced by subjecting mice to 10 days of hindlimb muscle unloading followed by reloading for 24 h by normal weight bearing. Unloading was performed using a previously described technique (Morey-Holton & Globus, 2002) which has been shown to produce 40% mass loss of the soleus muscle in a 10 day period (Thomason & Booth, 1990). Reloading for 24 h by returning to normal ambulation produces muscle inflammation, fibre injury and membrane lesions in soleus muscle fibres (Krippendorf & Riley, 1993; St Pierre & Tidball, 1994; Kasper, 1995; Tidball et al. 1999). The ‘reloaded’ mice were MPO −/− and wild-type that were subjected to hindlimb unloading followed by reloading for 24 h. The ‘unloaded only’ mice were subjected to hindlimb unloading for 10 days and then immediately killed for tissue collection, without experiencing reloading. The ‘ambulatory control’ mice experienced normal cage activity until they were killed for tissue collection. All mice were 3 months of age. After they had been killed, soleus muscles were dissected from each animal. One soleus muscle from each animal was rapidly frozen in isopentane and used for immunohistochemical analysis. The second soleus from each animal was used for assessment of muscle membrane injury, as described below.
Immunohistochemistry
Soleus muscles were stored in isopentane at −80°C. Cross-sections 10 μm thick were taken from the midbelly of one soleus muscle from each animal and used for immunohistochemical analysis. Sections were fixed in acetone and then immunolabelled for neutrophils using rat anti-mouse Ly6G (Pharmingen) or for macrophages using rat anti-mouse F4/80. Anti-F4/80 was prepared by ammonium sulphate precipitation of immunoglobulins from F4/80 hybridoma cultures (ATCC). The concentrations of precipitated immunoglobulins were diluted to 0.1–0.2 μg ml−1 of anti-F4/80 for use in immunohistochemistry. Sections were processed as previously described (Wehling et al. 2001) and immunoreactive cells were identified using a biotinylated mouse anti-rat IgG second antibody and horseradish peroxidase-conjugated avidin before reaction with aminoethylcarbonyl (Vector). The total number of neutrophils or macrophages in each section was counted microscopically. The total volume of each section analysed was determined by measuring the area of each section using a stereological, point-counting technique (Spencer et al. 2001), and then multiplying that value by the section thickness (10 μm). Number of cells per volume of each section was calculated to yield the concentration of cells. The concentrations of neutrophils or macrophages in sections of soleus muscles of each group were expressed as the mean and s.e.m. Values were compared using Student's t test with the level for statistical significance set at P < 0.05.
Assays of muscle membrane injury
Injuries to soleus fibre membranes were assayed by measuring the relative concentration of the fluorescent, extracellular tracer dye, procion orange, in the cytoplasm of fibres from muscles in each experimental and control group. Muscles that are incubated in procion orange solutions exclude the marker dye unless membrane lesions are present. One soleus from each mouse was incubated in 0.5% procion orange dye solution in Krebs–Ringer solution for 1 h followed by two, 5 min washes with Krebs–Ringer solution. The soleus muscles were then rapidly frozen in isopentane, and midbelly cross-sections of each muscle were cut. Two assessments of fibre membrane injury were used. In the first assay, the number of brightly fluorescent, injured fibres in each section was expressed as a percentage of the total fibres in the section. In the second injury assay, the fluorescence intensity of every individual fibre in each muscle cross-section was measured in an 8 μm diameter, circular area that was sampled at the centre of each fibre using a digital imaging system (Bioquant, Nashville, TN, USA). Fluorescence intensity values for each fibre were then corrected for background, by measuring background signal from an area of the slide that contained no tissue, and subtracting the background value from the cytosolic fluorescence measurements.
Results
Mechanical loading promotes neutrophil lysis of muscle cells in vitro through an MPO-mediated pathway
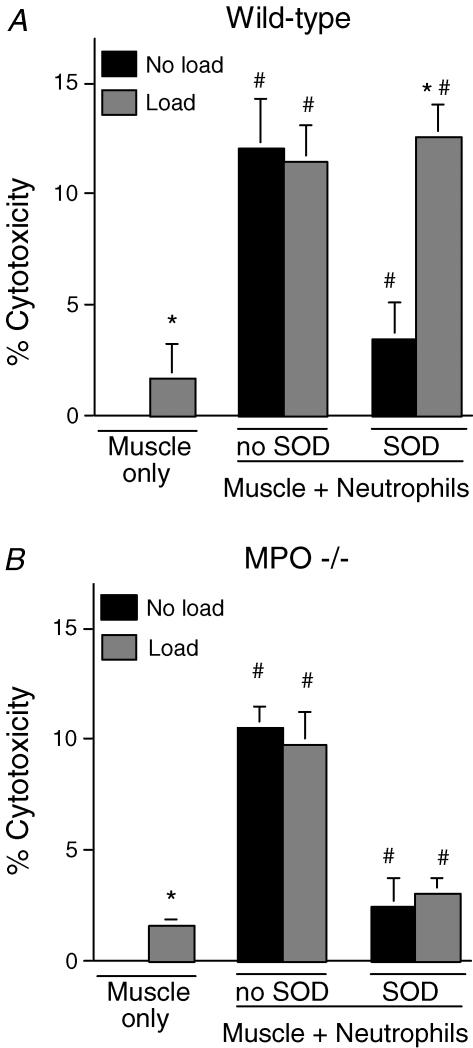
Assays of MPO activity in the media of peritoneal neutrophils confirmed that neutrophil activation produced an increase in MPO release by wild-type neutrophils, but no significant MPO activity was observed in the media of resting or activated neutrophils collected from MPO −/− mice. We then tested whether muscle cell lysis by activated neutrophils occurred through an MPO-dependent pathway. Lysis of muscle by neutrophils (5000 mm−2) did not differ between cultures of wild-type (mean ± s.e.m., 12.1 ± 2.2% lysis; Fig. 1A) and MPO −/− neutrophils (10.5 ± 1.0% lysis; Fig. 1B). Consistent with previous reports (Nguyen & Tidball, 2003b), the addition of superoxide dismutase to the co-cultures significantly reduced neutrophil-mediated lysis by wild-type neutrophils (3.5 ± 1.6% lysis; Fig. 1A). Similar reductions in cytotoxicity were observed in co-cultures with MPO −/− neutrophils when SOD was added (2.5 ± 1.18% lysis; Fig. 1B). These findings indicate that muscle cell lysis by neutrophils occurs through superoxide-dependent, MPO-independent pathways in non-loaded cultures.
Figure 1. Cytolysis of wild-type mouse myotubes in lactate dehydrogenase (LDH) release cytotoxicity assays.
A, muscle cytolysis in the absence (Muscle only) or presence (Muscle + Neutrophils) of wild-type neutrophils. Exogenous superoxide dismutase (SOD) was added to some preparations. All treatments used 5000 neutrophils mm−2. Data from cultures that were not subjected to mechanical loading are shown by black columns. Data from cultures subjected to mechanical loading are shown by grey columns. B, identical treatments as shown in A, but neutrophils from myeloperoxidase (MPO) −/− mice were used in the assays. * Significantly different from corresponding non-loaded treatment. # Significantly different from muscle cells without neutrophils treated under otherwise identical conditions. P < 0.05. n = 6 replicates per treatment. Bars represent s.e.m.
The contribution of mechanical loading to neutrophil-mediated muscle lysis was examined in co-cultures using either wild-type or MPO −/− neutrophils. Application of cyclic mechanical loads to muscle cells in vitro, in the absence of neutrophils, produced small but significant (P < 0.05) increases in muscle cell lysis (1.7 ± 0.35% lysis), compared to non-loaded muscle cells (0.0 ± 0.01% lysis) (Fig. 1). No measureable LDH release was observed in mechanically loaded, neutrophil cultures in the absence of muscle cells. Addition of neutrophils to muscle cultures in the presence of mechanical loading significantly increased lysis to levels that were similar for both wild-type (11.5 ± 1.6% lysis; Fig. 1A) and MPO −/− co-cultures (9.8 ± 1.4% lysis; Fig. 1B). However, muscle cell lysis did not differ between non-loaded and loaded cultures in either wild-type or MPO −/− neutrophil cultures in the absence of SOD (Fig. 1). These findings indicate that mechanical loading does not promote neutrophil-mediated lysis of muscle cells in the absence of exogenous SOD.
Because extracellular SOD is present in vivo and its concentration is influenced by mechanical loading in vivo (Hollander et al. 2001), we assayed the effect of SOD on neutrophil-mediated lysis of muscle cells experiencing mechanical loading. Addition of SOD to mechanically loaded muscle cells in the presence of MPO −/− neutrophils reduced muscle lysis to levels that did not differ significantly from the levels observed in non-loaded MPO −/− co-cultures in the absence of mechanical loading (loaded: 2.5 ± 1.2% lysis; non-loaded: 3.1 ± 0.6% lysis; Fig. 1B). However, addition of SOD to mechanically loaded muscle cells in the presence of wild-type neutrophils greatly increased muscle lysis compared to levels observed in non-loaded, wild-type neutrophil co-cultures in the absence of mechanical loading (loaded: 12.6 ± 1.4% lysis; non-loaded: 3.5 ± 1.6% lysis; Fig. 1A). These findings show that mechanical loading activates MPO-mediated cytotoxicity of neutrophils in the presence of SOD.
Mechanical loading promotes neutrophil MPO activity in vitro
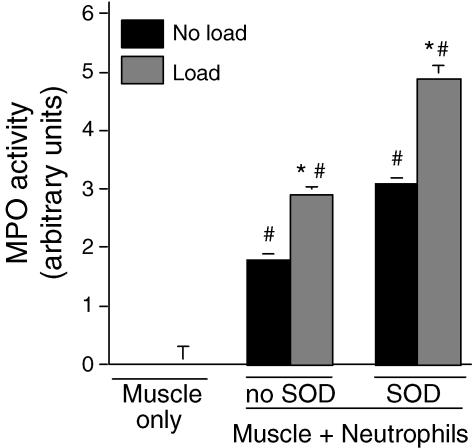
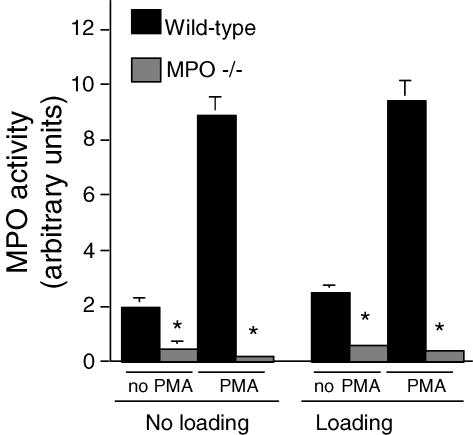
Our finding that mechanical loading increased neutrophil-mediated, MPO-dependent cytotoxicity suggested that loading could increase MPO activity. Assays of MPO activity in co-cultures of muscle cells and neutrophils showed that loading increased activity by 60% in the absence of exogenous SOD in the culture media (loaded: 2.9 ± 0.14 units; non-loaded: 1.8 ± 0.1 units) (Fig. 2). Similarly, loading increased MPO activity in the presence of exogenous SOD by 58% (loaded: 4.9 ± 0.2 units; non-loaded: 3.1 ± 3.1 units). In addition, exogenous SOD caused significant increases in MPO activity for both non-loaded (72% increase) and loaded cultures (70%) (Fig. 2). Mechanical loading of neutrophils in the absence of muscle cells did not significantly affect MPO activity (Fig. 3).
Figure 2. MPO activity in supernatants of muscle cells or muscle cells with neutrophil co-cultures.
Conditions were identical to those used in Fig. 1A except that all treatments used 25 000 neutrophils mm−2. * Significantly different from corresponding non-loaded treatment. # Significantly different from muscle cells without neutrophils treated under otherwise identical conditions. P < 0.05. n = 6 per treatment. Bars represent s.e.m.
Figure 3. MPO activity of neutrophil cultures in the absence of muscle cells.
Cultures were either subjected to mechanical loading or not loaded. Some preparations were activated with phorbol 12-myristate 13-acetate (PMA). Mechanical loading did not significantly increase MPO activity in the supernatant of any treatment compared to identically treated samples in the absence of mechanical loading. * Significantly different from wild-type neutrophils subjected to identical experimental treatments. P < 0.05. n = 6 per treatment. Bars represent s.e.m. Data sets without apparent error bars had errors that were too small to appear at the scale of this graph.
Conditioned media from mechanically loaded muscle cells do not increase MPO activity in neutrophils
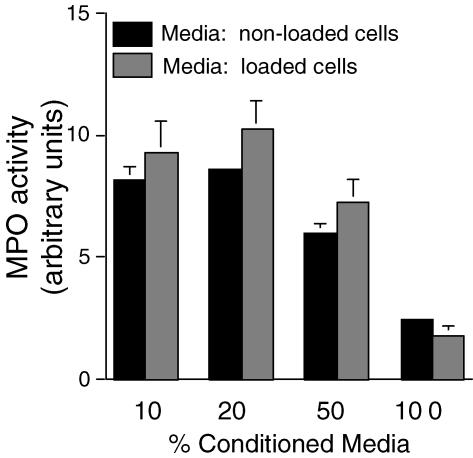
We tested whether the increase in MPO activity in neutrophils was mediated by a soluble factor whose release from muscle was stimulated by loading. However, we found no difference in MPO activity in neutrophil cultures receiving conditioned media from muscle cultures that had experienced mechanical loading or were non-loaded. Unexpectedly, we found that as we titrated different concentrations of conditioned media from muscle cultures to fresh media in the neutrophil cultures, there was a decrease in MPO activity as the proportion of conditioned media was increased (Fig. 4). These findings indicate that muscle cells may release a soluble factor that decreases MPO activity, but the release of that factor is not affected by mechanical loading.
Figure 4. Transfer of conditioned media from muscle cells subjected to loading or not subjected to loading does not increase MPO activity in neutrophil cultures.
Medium was collected from muscle cultures after 24 h of cyclic loading or non-loading, transferred to neutrophil cultures and then MPO activity measured after 24 h. Neutrophil cultures contained 5 × 106 neutrophils ml−1. ‘% Conditioned Media’ indicates the percentage of the total media in the cultures that consisted of conditioned media. There was no significant difference in MPO activity between cultures receiving conditioned media from loaded or non-loaded muscle cell cultures at any concentration of conditioned media. Bars represent s.e.m.n = 6.
Null mutation of MPO reduces fibre membrane injury in muscle experiencing modified loading in vivo
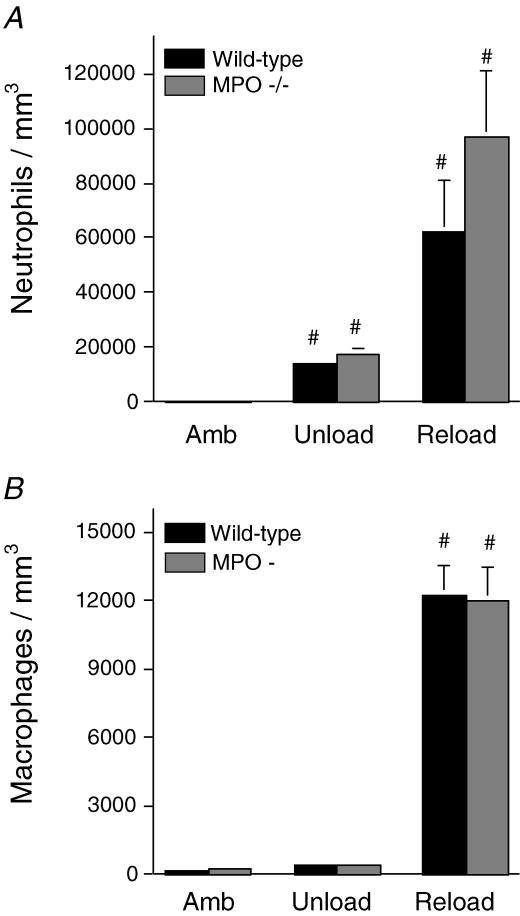
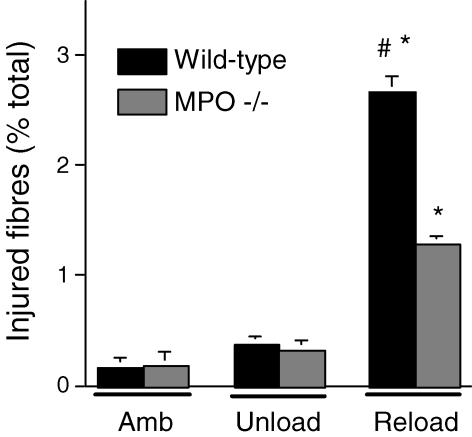
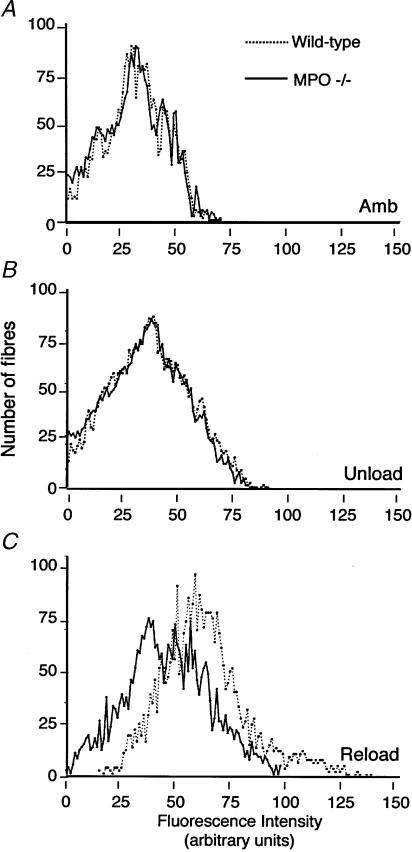
Soleus muscles of wild-type mice experiencing 24 h of muscle reloading following 10 days of muscle unloading showed elevated concentrations of neutrophils and macrophages (Fig. 5), and increased concentrations of injured fibres (Fig. 6). Injured fibres were identified as brightly fluorescent fibres after incubation in fluorescent, extracellular marker dye. However, MPO −/− mice that had experienced identical muscle unloading–reloading protocols showed a significant 52% reduction in the percentage of fibres that were injured (Fig. 6), indicating that fibre injury is mediated by MPO in this model. We also observed that the frequency distribution of intracellular fluorescence for all soleus muscle fibres in MPO −/− mice that had experienced reloading was shifted significantly towards lower values (Fig. 7). This indicates a general reduction of membrane lysis during reloading in MPO −/− mice, compared to controls. Although null mutation of MPO significantly reduced membrane lysis and fibre injury during reloading, the concentration of neutrophils and macrophages did not differ significantly between wild-type and MPO −/− mice (Fig. 5). This observation indicates that MPO-mediated damage to muscle is not necessary to promote leucocyte invasion, and supports the possibility that the null mutation of MPO reduces muscle membrane damage because it reduces the cytolytic capacity of myeloid cells, rather than affecting their ability to target and invade muscle experiencing modified loading.
Figure 5. Null mutation of MPO does not reduce myeloid cell invasion of reloaded muscle.
Soleus muscles from ambulatory control mice (Amb), mice subjected to 10 days of hindlimb unloading only (Unload) or unloading followed by 24 h reloading (Reload) were assayed for neutrophil concentration (A) and macrophage concentration (B). # Significantly different from ambulatory mice of the same genotype. No significant differences were found between MPO −/− and wild-type mice in any experimental group. Bars represent s.e.m.n = 6.
Figure 6. Soleus muscle fibre injury assessed by bright intracellular fluorescence of procion orange.
Treatment groups are the same as for Fig. 5. * Significantly different from ambulatory mice of the same genotype. # Significantly different from MPO −/− mice in the same experimental group. Bars represent s.e.m.n = 6.
Figure 7. Frequency distributions of muscle fibres over the range of intensities of intracellular fluorescence of procion orange.
Intracellular fluorescence was measured in individual fibres in entire cross-sections of soleus muscles from each treatment group (n = 6 per group). A total of more than 34 000 individual fibres was analysed. A rightward shift of peaks on the abscissa indicates an increase in the frequency of fibres with membrane lesions. Frequency distribution of intracellular fluorescence for wild-type (dashed line) or MPO −/− (continuous line) muscle fibres of ambulatory control (A), unloaded (B) or reloaded (C) soleus muscle fibres.
Discussion
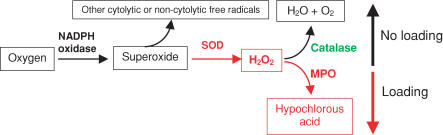
Recent investigations have shown that superoxide or a superoxide-derivative plays a central role in the lysis of muscle cell membranes in vitro or in muscle experiencing modified loading in vivo (Nguyen & Tidball, 2003b). However, superoxide itself seems unlikely to cause muscle membrane lysis directly, because its toxicity is relatively low, and it would be rapidly converted to other free radicals (Hampton et al. 1998). In the present investigation, we show that null mutation of MPO greatly reduces neutrophil-mediated lysis of muscle cells in vitro and in vivo, and yields a protective effect against muscle cell lysis similar to that previously reported for gp91phox null mutants (Nguyen & Tidball, 2003b), which are deficient in superoxide production. Together, those findings indicate that the toxic effects of superoxide may occur through its conversion first to hydrogen peroxide by SOD, followed by conversion to the highly reactive and toxic hypochlorous acid by MPO (Fig. 8). Because MPO competes with catalase for hydrogen peroxide (Hampton et al. 1998), and catalase converts hydrogen peroxide to benign water and oxygen, our results suggest that increased expression of catalase would have a protective effect against muscle membrane lysis that would be similar to null mutation of MPO. In agreement with this expectation, other investigators have shown that the transgenic over-expression of catalase protects cardiac muscle from injury that is caused by superoxide derivatives (Li et al. 1997). Collectively, these findings emphasize the fact that the relative activities of catalase and MPO may play a central role in determining the extent of muscle damage during inflammation or oxidative stress.
Figure 8. Schematic diagram of free radical production that may be influenced by mechanical loading of neutrophils in skeletal muscle.
Black boxes indicate substrates or products that are benign or have low toxicity. Red boxes indicate substrates or products that are cytotoxic. Red lettering indicates enzymes that are activated by mechanical loading. Green lettering indicates enzymes that are inhibited by mechanical loading.
We were intrigued to find that there was a substantial, synergistic effect on the level of muscle cell lysis when both mechanical loading and neutrophils were applied to muscle cells in vitro. Loading alone caused only a 1.7% lysis of muscle cells, while co-culturing with neutrophils in the absence of loading resulted in only 3.5% lysis. However, loading in the presence of neutrophils resulted in 12.6% lysis of muscle cells under otherwise identical culture conditions in the presence of SOD. In vivo observations have provided conflicting interpretations concerning the potential roles of neutrophils and mechanical loading in muscle injury during modified muscle loading. Some investigations have reported that neutrophils are present at elevated concentrations at the time of muscle injury during modified loading (Frenette et al. 2000) while others using the same model report that neutrophils do not invade until after injury occurs (Frenette et al. 2002). If our in vitro model resembles events that occur in vivo, our findings would indicate that neutrophils can greatly amplify muscle damage caused by modified muscle use. That interpretation is consistent with the finding that MPO −/− mice showed significantly reduced muscle membrane lysis during muscle reloading in vivo, and previous findings that gp91phox−/− mice experienced no significant increase in muscle membrane lysis during reloading in vivo (Nguyen & Tidball, 2003b).
The observations that mechanical loading of muscle cells in vitro increases the release of MPO from neutrophils present in co-cultures provides direct evidence for a link between the mechanical environment and neutrophil activity and cytotoxicity. Several studies conducted in vivo have shown a relationship between exercise and levels of MPO in sera (Bury & Pirnay, 1995; Belcastro et al. 1996) but conclusions could not be made concerning whether mechanical loading experienced during exercise or another variable experienced during exercise stimulated the increase in MPO. In addition, alternative explanations for the increase in MPO in serum following exercise have emerged from in vivo studies. Elevations in the concentration of neutrophils in the serum that were caused by increased neutrophil de-margination during exercise (Muir et al. 1984) may be responsible for part of the observed increase in MPO. In addition, other investigators have observed that exercise increased the amount of MPO released per neutrophil (Suzuki et al. 1996), so that exercise could elevate both the numbers and activity of neutrophils in vivo. Our in vitro findings show that the increased release of MPO per neutrophil may reflect, at least in part, the mechanical stimulation of muscle that leads to neutrophil activation.
The stimulation of neutrophil cytotoxicity by mechanical loading that we observed in vitro occurred only if exogenous SOD was present in the culture medium. This suggests that in the absence of exogenous SOD, the slower conversion of superoxide to hydrogen peroxide did not yield sufficient quantities of toxic intermediates to cause muscle membrane damage. However, we observed in vivo that null mutation of MPO greatly reduced muscle membrane lysis by neutrophils in muscle experiencing reloading. That finding indicates that the levels of endogenous, extracellular SOD in muscle during reloading in vivo are sufficient to produce cytotoxic levels of hydrogen peroxide and hypochlorous acid. Previous investigators have reported the expression of extracellular SOD in skeletal muscle (Folz & Crapo, 1994), including a muscle-specific transcript that is present at high levels (Folz & Crapo, 1994). Increased muscle activation and loading during either acute or endurance exercise can increase levels of SOD (Lawler et al. 1994; Powers et al. 1994) and MPO activity in muscle (Bury & Pirnay, 1995; Belcastro et al. 1996), which is consistent with the model that we propose in which increased muscle use during reloading drives superoxide conversion to hypochlorous acid by SOD and MPO. Furthermore, exercise can decrease the activity of catalase, which competes with MPO for its substrate, hydrogen peroxide (Laughlin et al. 1990; Leeuwenburgh et al. 1994; Hong & Johnson, 1995), although under some exercise treatments catalase activity is unaffected or increased in some muscles (Alessio & Goldfarb, 1988; Ji et al. 1992; Powers et al. 1994). Thus, the addition of MPO-rich neutrophils to muscle experiencing increased loading in the presence of elevated SOD activity and decreased catalase activity, would promote muscle membrane lysis.
Our model of the role of mechanical loading in neutrophil-mediated lysis of muscle cells (Fig. 8) can be expected to represent only a part of the complex and potentially cytolytic interactions between the mechanical environment and free-mediated events in muscle in vivo. For example, increased activation of skeletal muscle also increases the release of nitric oxide by muscle (Balon & Nadler, 1994; Tidball et al. 1998; Patwell et al. 2004), which can then react with superoxide to produce a more highly cytotoxic radical, peroxynitrite. Furthermore, the release of superoxide from muscle is also influenced by muscle activation (Patwell et al. 2004). This scenario may be even more complex because the stoichiometry of superoxide and nitric oxide may determine the reactivity and cytotoxicity of the final reactant that is generated (Miles et al. 1996). In addition, hydrogen peroxide can be converted to hypochlorous acid by MPO or to other free radicals or non-radical oxidants in vivo, depending on the presence of other enzymes or cofactors. The extent to which changes in mechanical loading can effect the expression, activity or availability of these other variables has only barely begun to be explored.
The reduction in tissue damage that was observed in reloaded skeletal muscle of MPO −/− mice differs from the increased pathology in MPO −/− mice that has been observed in other models of injury and disease. For example, tissue damage in experimental autoimmune encephalomyelitis and atherosclerosis is exacerbated in MPO −/− mice (Brennan et al. 2001b). Similarly, we reported previously that null mutation of gp91phox, the catalytic subunit of NADPH oxidase, reduced muscle damage in the unloading–reloading model (Nguyen & Tidball, 2003b), although gp91phox−/− mice experienced increased tissue damage in experimental arthritis models (van de Loo et al. 2003). Although there are numerous differences between the mechanisms of tissue damage in each of these models of injury and disease, important differences may include whether the mutations affect myeloid cell function in acute or chronic disease, and whether the pathology primarily involves an innate or acquired immune response. The protective effects of null mutation of MPO or gp91phox were observed in an acute injury in which there is exclusively an innate, inflammatory response to tissue damage (Nguyen & Tidball, 2003b; present investigation). The injurious effects of null mutation of MPO or gp91phox were observed in chronic diseases that were driven by T-lymphocytes (Brennan et al. 2001b; van de Loo et al. 2003). Loss of MPO or gp91phox expression by myeloid cells in that context could result in loss of suppression of T-cell function, leading to increased pathology (Brennan et al. 2001b).
We conclude that neutrophils play a significant role in injuring muscle cell membranes during muscle reloading following periods of unloading, and that this membrane damage is mediated by MPO. Our findings further show that mechanical activation of neutrophil cytolysis can occur in vivo and in vitro, by stimulating the release of MPO. These observations provide a direct link between changes in mechanical loads applied to muscle, and an increase in muscle damage that is induced by inflammatory cells. Our continuing investigations are directed towards identifying the specific, muscle-derived signals that produce an increase in neutrophil cytotoxicity and MPO release.
Acknowledgments
This work was supported by grants from the NIH (AR47721, AR47855, HL28481) and NASA (NAG5-4837).
References
- Alessio HM, Goldfarb AH. Lipid peroxidation and scavenger enzymes during exercise: adaptive response to training. J Appl Physiol. 1988;64:1333–1336. doi: 10.1152/jappl.1988.64.4.1333. [DOI] [PubMed] [Google Scholar]
- Balon TW, Nadler JL. Nitric oxide release is present from incubated skeletal muscle preparations. J Appl Physiol. 1994;77:2519–2521. doi: 10.1152/jappl.1994.77.6.2519. [DOI] [PubMed] [Google Scholar]
- Belcastro AN, Arthur GD, Albisser TA, Raj DA. Heart, liver, and skeletal muscle myeloperoxidase activity during exercise. J Appl Physiol. 1996;80:1331–1335. doi: 10.1152/jappl.1996.80.4.1331. [DOI] [PubMed] [Google Scholar]
- Brennan ML, Anderson MM, Shih DM, Qu XD, Wang X, Mehta AC, Lim LL, Shi W, Hazen SL, Jacob JS, Crowley JR, Heinecke JW, Lusis AJ. Increased atherosclerosis in myeloperoxidase-deficient mice. J Clin Invest. 2001a;107:419–430. doi: 10.1172/JCI8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan ML, Gaur A, Pahuja A, Lusis AJ, Reynolds WF. Mice lacking myeloperoxidase are more susceptible to experimental autoimmune encephalomyelitis. J Neuroimmunol. 2001b;112:97–105. doi: 10.1016/s0165-5728(00)00392-1. [DOI] [PubMed] [Google Scholar]
- Bury TB, Pirnay F. Effect of prolonged exercise on neutrophil myeloperoxidase secretion. Int J Sports Med. 1995;16:410–412. doi: 10.1055/s-2007-973029. [DOI] [PubMed] [Google Scholar]
- Folz RJ, Crapo JD. Extracellular superoxide dismutase (SOD3): tissue-specific expression, genomic characterization, and computer-assisted sequence analysis of the human EC SOD gene. Genomics. 1994;22:162–171. doi: 10.1006/geno.1994.1357. 10.1006/geno.1994.1357. [DOI] [PubMed] [Google Scholar]
- Frenette J, Cai B, Tidball JG. Complement activation promotes muscle inflammation during modified muscle use. Am J Pathol. 2000;156:2103–2110. doi: 10.1016/S0002-9440(10)65081-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenette J, St-Pierre M, Cote C, Mylona E, Pizza F. Muscle impairment occurs rapidly and precedes inflammatory cell accumulation after mechanical loading. Am J Physiol Regul Integr Comp Physiol. 2002;282:R351–R357. doi: 10.1152/ajpregu.00189.2001. [DOI] [PubMed] [Google Scholar]
- Goebels N, Michaelis D, Engelhardt M, Huber S, Bender A, Pongratz D, Johnson MA, Wekerle H, Tschopp J, Jenne D, Hohlfeld R. Differential expression of perforin in muscle-infiltrating T cells in polymyositis and dermatomyositis. J Clin Invest. 1996;97:2905–2910. doi: 10.1172/JCI118749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampton MB, Kettle AJ, Winterbourn CC. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- Hollander J, Fiebig R, Gore M, Ookawara T, Ohno H, Ji LL. Superoxide dismutase gene expression is activated by a single bout of exercise in rat skeletal muscle. Pflugers Arch. 2001;442:426–434. doi: 10.1007/s004240100539. 10.1007/s004240100539. [DOI] [PubMed] [Google Scholar]
- Hong H, Johnson P. Antioxidant enzyme activities and lipid peroxidation levels in exercised and hypertensive rat tissues. Int J Biochem Cell Biol. 1995;27:923–931. doi: 10.1016/1357-2725(95)00057-v. 10.1016/1357-2725(95)00057-V. [DOI] [PubMed] [Google Scholar]
- Ji LL, Fu R, Mitchell EW. Glutathione and antioxidant enzymes in skeletal muscle: effects of fiber type and exercise intensity. J Appl Physiol. 1992;73:1854–1859. doi: 10.1152/jappl.1992.73.5.1854. [DOI] [PubMed] [Google Scholar]
- Jolly SR, Kane WJ, Hook BG, Abrams GD, Kunkel SL, Lucchesi BR. Reduction of myocardial infarct size by neutrophil depletion: effect of duration of occlusion. Am Heart J. 1986;112:682–690. doi: 10.1016/0002-8703(86)90461-8. 10.1016/0002-8703(86)90461-8. [DOI] [PubMed] [Google Scholar]
- Kasper CE. Sarcolemmal disruption in reloaded atrophic skeletal muscle. J Appl Physiol. 1995;79:607–614. doi: 10.1152/jappl.1995.79.2.607. [DOI] [PubMed] [Google Scholar]
- Korthuis RJ, Grisham MB, Granger DN. Leukocyte depletion attenuates vascular injury in postischemic skeletal muscle. Am J Physiol. 1988;254:H823–H827. doi: 10.1152/ajpheart.1988.254.5.H823. [DOI] [PubMed] [Google Scholar]
- Krippendorf BB, Riley DA. Distinguishing unloading- versus reloading-induced changes in rat soleus muscle. Muscle Nerve. 1993;16:99–108. doi: 10.1002/mus.880160116. 10.1002/mus.880160116. [DOI] [PubMed] [Google Scholar]
- Kyriakides C, Austen W, Wang Y, Favuzza J, Kobzik L, Moore FD, Hechtman HB. Skeletal muscle reperfusion injury is mediated by neutrophils and the complement membrane attack complex. Am J Physiol. 1999;277:C1263–C1268. doi: 10.1152/ajpcell.1999.277.6.C1263. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Simpson T, Sexton WL, Brown OR, Smith JK, Korthuis RJ. Skeletal muscle oxidative capacity, antioxidant enzymes, and exercise training. J Appl Physiol. 1990;68:2337–2343. doi: 10.1152/jappl.1990.68.6.2337. [DOI] [PubMed] [Google Scholar]
- Lawler JM, Powers SK, Van Dijk H, Visser T, Kordus MJ, Ji LL. Metabolic and antioxidant enzyme activities in the diaphragm: effects of acute exercise. Respir Physiol. 1994;96:139–149. doi: 10.1016/0034-5687(94)90122-8. 10.1016/0034-5687(94)90122-8. [DOI] [PubMed] [Google Scholar]
- Leeuwenburgh C, Fiebig R, Chandwaney R, Ji LL. Aging and exercise training in skeletal muscle: responses of glutathione and antioxidant enzyme systems. Am J Physiol. 1994;267:R439–R445. doi: 10.1152/ajpregu.1994.267.2.R439. [DOI] [PubMed] [Google Scholar]
- Li G, Chen Y, Saari JT, Kang YJ. Catalase-overexpressing transgenic mouse heart is resistant to ischemia-reperfusion injury. Am J Physiol. 1997;273:H1090–H1095. doi: 10.1152/ajpheart.1997.273.3.H1090. [DOI] [PubMed] [Google Scholar]
- Miles AM, Bohle DS, Glassbrenner PA, Hansert B, Wink DA, Grisham MB. Modulation of superoxide-dependent oxidation and hydroxylation reactions by nitric oxide. J Biol Chem. 1996;271:40–47. doi: 10.1074/jbc.271.1.40. [DOI] [PubMed] [Google Scholar]
- Morey-Holton ER, Globus RK. Hindlimb unloading rodent model: technical aspects. J Appl Physiol. 2002;92:1367–1377. doi: 10.1152/japplphysiol.00969.2001. 10.1063/1.1492860. [DOI] [PubMed] [Google Scholar]
- Muir AL, Cruz M, Martin BA, Thommasen H, Belzberg A, Hogg JC. Leukocyte kinetics in the human lung: role of exercise and catecholamines. J Appl Physiol. 1984;57:711–719. doi: 10.1152/jappl.1984.57.3.711. [DOI] [PubMed] [Google Scholar]
- Nakamura K, Arahata K, Ishiura S, Osame M, Sugita H. Degradative activity of granzyme A on skeletal muscle proteins in vitro: a possible molecular mechanism for muscle fiber damage in polymyositis. Neuromuscul Disord. 1993;4:303–310. doi: 10.1016/0960-8966(93)90023-d. 10.1016/0960-8966(93)90023-D. [DOI] [PubMed] [Google Scholar]
- Nguyen HX, Tidball JG. Interactions between neutrophils and macrophages promote macrophage killing of muscle cells in vitro. J Physiol. 2003a;547:125–132. doi: 10.1113/jphysiol.2002.031450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen HX, Tidball JG. Null mutation of gp91phox reduces muscle membrane lysis during muscle inflammation in mice. J Physiol. 2003b;553:833–841. doi: 10.1113/jphysiol.2003.051912. 10.1113/jphysiol.2003.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patwell DM, McArdle A, Morgan JE, Partridge TA, Jackson MJ. Release of reactive oxygen and nitrogen species from contracting skeletal muscle cells. Free Rad Biol Med. 2004;37:1064–1072. doi: 10.1016/j.freeradbiomed.2004.06.026. 10.1016/j.freeradbiomed.2004.06.026. [DOI] [PubMed] [Google Scholar]
- Powers SK, Criswell D, Lawler J, Ji LL, Martin D, Herb RA, Dudley G. Influence of exercise and fiber type on antioxidant enzyme activity in rat skeletal muscle. Am J Physiol. 1994;266:R375–R380. doi: 10.1152/ajpregu.1994.266.2.R375. [DOI] [PubMed] [Google Scholar]
- St Pierre B, Tidball JG. Differential response of macrophage subpopulations to soleus muscle reloading after rat hindlimb suspension. J Appl Physiol. 1994;77:290–297. doi: 10.1152/jappl.1994.77.1.290. [DOI] [PubMed] [Google Scholar]
- Smith J, Grisham M, Granger N, Korthuis R. Free radical defense mechanisms and neutrophil infiltration in postischemic skeletal muscle. Am J Physiol. 1989;256:H789–H793. doi: 10.1152/ajpheart.1989.256.3.H789. [DOI] [PubMed] [Google Scholar]
- Spencer MJ, Montecino-Rodriguez E, Dorshkind K, Tidball JG. Helper (CD4 (+)) and cytotoxic (CD8 (+)) T-cells promote the pathology of dystrophin-deficient muscle. Clin Immunol. 2001;98:235–243. doi: 10.1006/clim.2000.4966. 10.1006/clim.2000.4966. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Ota H, Sasagawa S, Sakatani T, Fujikura T. Assay method for myeloperoxidase in human polymorphonuclear leukocytes. Anal Biochem. 1983;132:345–352. doi: 10.1016/0003-2697(83)90019-2. 10.1016/0003-2697(83)90019-2. [DOI] [PubMed] [Google Scholar]
- Suzuki K, Sato H, Kikuchi T, Abe T, Nakaji S, Sugawara K, Totsuka M, Sato K, Yamaya K. Capacity of circulating neutrophils to produce reactive oxygen species after exhaustive exercise. J Appl Physiol. 1996;81:1213–1222. doi: 10.1152/jappl.1996.81.3.1213. [DOI] [PubMed] [Google Scholar]
- Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. 10.1063/1.347115. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Berchenko E, Frenette J. Macrophage invasion does not contribute to muscle membrane injury during inflammation. J Leukocyte Biol. 1999;65:492–498. [PubMed] [Google Scholar]
- Tidball JG, lavergne E, Lau KS, Spencer MJ, Stull JT, Wehling M. Mechanical loading regulates nitric oxide synthase expression and activity in developing and adult skeletal muscle. Am J Physiol. 1998;275:C260–C266. doi: 10.1152/ajpcell.1998.275.1.C260. [DOI] [PubMed] [Google Scholar]
- van de Loo FA, Bennink MB, Arntz OJ, Smeets RL, Lubberts E, Joosten LA, van Lent PL, Coenen-de Roo CJ, Cuzzocrea S, Segal BH, Holland SM, van den Berg WB. Deficiency of NADPH oxidase components p47phox and gp91phox caused granulomatous synovitis and increased connective tissue destruction in experimental arthritis models. Am J Pathol. 2003;163:1525–1537. doi: 10.1016/S0002-9440(10)63509-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]