Abstract
Properly regulated interactions among excitatory and inhibitory synapses are critical for brain function. Compared to excitatory synapses, much less is known about the gain control mechanisms at inhibitory synapses. Herein we report a mechanism of noradrenergic long-term potentiation (LTP) at inhibitory synapses following presynaptic β-adrenoceptor activation. Stimulation of β-adrenoceptors elicited LTP of GABA release from terminals of cerebellar interneurones. This action was dependent on the cAMP/protein kinase A signalling cascade and independent of the β-adrenoceptor-mediated acceleration of hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channel. Furthermore, the β-adrenoceptor- and protein kinase A-mediated LTP was triggered by enhancement of the Ca2+ sensitivity of the release machinery and increase in the readily releasable pool. β-Adrenoceptor activation also accelerated the recruitment of GABA into the releasable pool and enhanced synchronous and asynchronous release of GABA from the presynaptic terminal. Thus, the up-regulation of GABA release machinery mediated by noradrenaline and β-adrenoceptor activation provides a likely mechanism of feedforward inhibition of the cerebellar output neurone Purkinje cell, leading to a profound effect on motor control and learning associated with the cerebellum.
Depending on particular patterns of homosynaptic and heterosynaptic activation, central synapses undergo various forms of activity-dependent plasticity that are critical for development of neuronal connections, learning and memory. While the mechanisms underlying long-term modulation of glutamatergic transmission at excitatory synapses have been extensively studied (Malenka & Nicoll, 1999; Sanes & Lichtman, 1999; Malenka & Bear, 2004), much less is known about plasticity of GABAergic transmission at inhibitory synapses. Recent studies have demonstrated that short-term and long-term regulations of inhibitory transmission occur following activation of heterosynaptic inputs to particular inhibitory neurones (Gaiarsa et al. 2002) in the hippocampus (Caillard et al. 1999a,b), the cerebral cortex (Komatsu, 1996; Holmgren & Zilberter, 2001) and the cerebellum (Kano & Konnerth, 1992). However, the molecular mechanisms underlying synaptic plasticity of inhibitory transmission remain obscure.
In the cerebellum, it was previously reported that monoamines, such as noradrenaline (NA) and serotonin, enhance GABAergic transmission between the interneurone basket cells (BCs) and Purkinje cells (PCs) (Llano & Gershenfeld, 1993; Mitoma et al. 1994; Mitoma & Konishi, 1996; Kondo & Marty, 1997). Subsequently, the NA-induced modulation at cerebellar GABAergic synapses has been ascribed to activation of β2-adrecoceptors in presynaptic BC nerve terminals (Mitoma & Konishi, 1999; Saitow et al. 2000), and the actions of exogenously applied NA could be mimicked by synaptically released monoamines following stimulation of afferent fibres in the cerebellar cortex (Mitoma & Konishi, 1999). Furthermore, our recent study has shown that noradrenergic enhancement of cerebellar GABAergic synapses was mediated by two different actions (Saitow et al. 2000): (1) cAMP formation following β2-adrecoceptor activation excited BCs through acceleration of hyperpolarization-activated cyclic nucleotide-gated cation (HCN) channel, resulting in an increase in the frequency of spontaneous inhibitory postsynaptic currents (IPSCs); and (2) there was, additionally, an HCN channel activation-independent increase in the amplitude of evoked IPSCs. It has been demonstrated also that depending on the increase in the amount of NA released into the BC–PC synapse, the presynaptic β-adrenoceptor-mediated modulation of GABA release may cause a shift from short-term to long-term potentiation (LTP) (Mitoma & Konishi, 1999; Saitow et al. 2000).
In this study, we investigated the mechanisms underlying the noradrenergic LTP of GABAergic transmission, including the potential involvement of HCN channels (Beaumont & Zucker, 2000; Mellor et al. 2002). Our findings suggest that noradrenergic and β-adrenoceptor-mediated LTP of GABA release at cerebellar inhibitory synapses involves a cAMP–protein kinase A (PKA)-dependent signalling pathway but does not depend on cAMP-regulated HCN channel modulation. Furthermore, our data provide evidence that PKA-dependent enhancement of Ca2+ sensor function in the GABA release machinery and increase in the number of readily releasable synaptic vesicles contribute to this form of long-term synaptic plasticity of GABAergic transmission in the cerebellar cortex. Part of the present study has appeared in abstract form (Saitow & Konishi, 2002).
Methods
Electrophysiology
Experiments were performed using thin slices of the cerebellum prepared from 12- to 18-day-old Wistar rats (Saitow et al. 2000) with a protocol approved by the Ethics Review of Nippon Medical School. Animals of either sex were deeply anaesthetized with halothane inhalation (approximately 2% v/v) and their brains were rapidly removed. Parasagittal slices (250 μm thick) were cut by using a vibratome (VT1000S, Leica, Germany) at ∼4°C in a Na+-deficient saline that contained (mm): sucrose 299.2, KCl 3.4, CaCl2 0.3, MgCl2 3.0, Hepes 10, NaH2PO4 0.6 and glucose 10. This solution appeared to help avoid tissue damages due to excessive excitation during slicing. The slices were kept for more than 1 h in a humidified and oxygenated chamber with an interface of artificial cerebrospinal fluid (ACSF) that contained (mm): NaCl 138.6, KCl 3.4, CaCl2 2.5, MaCl2 1.0, NaHCO3 21.0, NaH2PO4 0.6 and glucose 10.0. The pH of the ACSF was maintained at 7.4 by bubbling with 95% O2–5% CO2. In most experiments, slices were superfused with ACSF in which 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) was added at 10 μm to eliminate glutamatergic excitatory synaptic responses.
Individual slices were transferred to a recording chamber attached on the stage of a microscope (BX50WI, Olympus, Japan) and continuously perfused with the oxygenated ACSF at a flow rate of 1.5 ml min−1 and temperature of 25–27°C. Patch electrodes used for whole-cell voltage-clamp recordings from PCs had resistances of 2–4 MΩ when filled with an internal solution that contained (mm): CsCl 140.0, KCl 5.0, K-EGTA 0.1, Na-Hepes 5.0, Mg-ATP 3.0, Na-GTP 0.4 (pH 7.4). PCs were visually identified under Nomarski optics with a water-immersion objective (63 ×, NA, 0.90, Olympus, Japan). The membrane potential recordings from BCs were made in the tight-seal whole-cell patch clamp. Glass electrodes used for this purpose had resistance of 5–7 MΩ when filled with an internal solution that contained (mm): potassium methanesulphonate 150.0, KCl 5.0, K-EGTA 0.1, Na-Hepes 5.0, Mg-ATP 3.0, Na-GTP 0.4 (pH 7.4). Membrane currents and potentials were recorded with a patch-clamp amplifier (EPC-9, HEKA Elektronik, Darmastadt, Germany). The series resistance monitored through experiments was in the range from 10 to 20 MΩ and was not compensated. Data were discarded if this value changed by more than 30%. Data were analysed using the software PULSEFIT (HEKA Elektronik) and KYPOLT (http://www.kyence.com). Miniature synaptic currents were visually inspected, and peak amplitudes were determined for analysis using the software Mini analysis (Synaptosoft, Decatur, GA, USA). Data were also continuously stored during experiments on a videotape recorder after digitizing by a pulse code modulation data recorder (NF Electronic Instruments, Japan). All signals were filtered at 1 kHz and sampled at 2 kHz. Membrane currents were recorded from PCs at a holding potential of −50 mV. IPSCs were evoked every 15 or 30 s by stimulation (10–30 V, 60–100 μs) via glass microelectrodes (tip diameter, 1–2 μm) filled with ACSF and placed within the molecular layer. Evoked IPSCs were abolished by bicuculline (10 μm), indicating that they are mediated by GABAA receptor activation.
To examine the properties of the readily releasable pool (RRP) in BC terminals, a hypertonic solution containing 500 mm sucrose in ACSF was puff-applied to the vicinity of recorded PCs through a micropipette controlled by a picospritzer (PV830, WPI, Sarasota, Fl, USA). In these experiments, slices were superfused with an ACSF containing 10 μm CNQX, 50 μm D-2-amino-5-phosphonopentanoic acid (D-APV) and 1 μm CGP55845 to block glutamatergic excitatory and GABAB receptor-mediated inhibitory synaptic responses. In the experiments where the effects of PKA inhibitors were examined, slices were preincubated for at least 1 h in a submerged-type chamber with an ACSF that contained either 5 μm H-89 or 100 μm Rp-Adenosine 3′,5′-cyclic monophosphorothioate (Rp-cAMPS). After transfer to the recording chamber, the slices were continuously superfused with the H-89- or Rp-cAMPS-containing ACSF.
Chemicals used in this study were obtained from the following sources: bicuculline, isoprenaline (isoproterenol; ISP), Rp-cAMPS and H-89 from Sigma (St Louis, MO, USA); CNQX, ZD7288, D-APV and CGP55845 from Tocris Cookson (Bristol, UK); tetrodotoxin (TTX) from Sankyo (Tokyo, Japan); and picrotoxin from Wako (Tokyo, Japan).
Measurements of presynaptic Ca2+ influx
Digital fluorescence imaging was carried out using the water-immersion objective (63 ×, NA, 0.90) on a Fluoview confocal microscope system (Olympus, Tokyo, Japan) mounted on the microscope. BCs were loaded with either 400 μm Calcium Green-5N, a low-affinity Ca2+ indicator, or 200 μm Oregon Green 488 BAPTA-1, a high-affinity indicator (Molecular Probes, Eugene, OR, USA) added in the potassium-based patch-pipette solution (see above). Approximately 15–20 min after indicator loading into BCs with the whole-cell mode, local areas of contacts between stained BC axons and PC somata were identified by visual inspection during image acquisition. Fluorescence was excited using the 488-nm line of an argon laser, and emissions were detected through a 515-nm low-pass filter. Fluorescence time courses were then recorded in line-scan mode by positioning the scan line across the identified synaptic contacts. Fluorescence signals were sampled at rate of 2.5 ms per line. Image acquisition was started while the cell was held at −60 mV and a train of three pulses (each 3 ms to 0 mV) was given at 150 ms after the onset of imaging. This protocol was repeated 5–10 times every 1 min before and during application of ISP. Recorded signals were averaged to reduce the signal-to-noise ratio. [Ca2+]i changes are expressed as the ratio of the change in fluorescence over the resting fluorescence level (ΔF/F0). Under these conditions, there was no significant attenuation of resting fluorescence level (F0) during the recording if BCs were not stimulated by depolarization pulses. Data were discarded when F0 was too low to detect even with detectable signal increases following depolarization-pulse stimulation (see Fig. 3A).
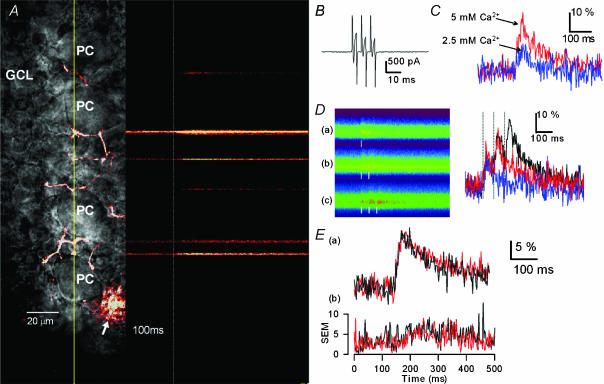
Figure 3. Action potential-induced Ca2+ transients in individual BC buttons and effects of ISP.
A, a representative image of a single BC and its neurites stained by a Ca2+-sensitive fluorescent indicator, Oregon Green 488 BAPTA-1 loaded through the recording patch electrode, and confocal line scans over the trajectory indicated by a yellow line passing over some of the contact sites between BC axonal boutons and PC bodies. Action potentials were evoked in the BC by injecting a train of three depolarizing pulses (from −60 to 0 mV for 3 ms) from the time point indicated by a dotted white line, and Ca2+ transients at individual BC buttons were determined by using a laser scanning confocal microscope. B, current responses induced in a BC by a train of three depolarizing voltage steps (from −60 to 0 mV for 3 ms). C, effects of changes in extracellular Ca2+ concentration ([Ca2+]o) from 2.5 to 5 mm on the action potential-triggered Ca2+ transients. The peak values of fluorescence signal changes (ΔF/F0, averaged trace of five trials) determined with a low-affinity Ca2+ indicator, Calcium Green 5N, increased from 13.8% in 2.5 mm [Ca2+]o to 27.9% in 5 mm [Ca2+]o. D, fluorescence images of line scanning at a single bouton recorded in response to single, two and three depolarizing pulses (left) and corresponding Ca2+ transients (right, averaged traces of three trials). Time points of depolarizing pulses are indicated by white vertical bars. The peak values of ΔF/F0 were 11.7% (single depolarizing pulse, blue), 27.4% (two pulses, red) and 31.5% (three pulses, black). Note that the signal intensity increased depending on the increase in the number of pulses, indicating no saturation in the detection capability. E, effects of ISP on the action potential-triggered Ca2+ transients determined by Calcium Green 5N. Ca2+ transients (ΔF/F0) were recorded in the absence (black trace) and presence of 20 μm ISP (red trace). Traces labelled (a) and (b) represent the mean and s.e.m., respectively, determined at different boutons derived from individually stained BCs (n = 8). The peak values and decay time constants of ΔF/F0 were 19.3 ± 3.4% and 92.5 ± 28 ms before ISP application, and 18.7 ± 4.2% and 102.3 ± 20 ms after ISP application, respectively.
Statistics
Numerical data are given as means ±s.e.m., where n represents the number of independent experiments. Statistical differences were evaluated using Student's paired t test unless otherwise noted. For multiple comparisons between experimental groups and comparison of cumulative curves, Tukey's parametric multiple comparison test and Kolmogorov-Smirnov (K-S) test were used, respectively.
Results
β-Adrenoceptor-mediated LTP of GABA release depended on a cAMP-dependent PKA cascade and did not involve HCN channel activation
As previously reported, stimulation of BCs with the β-adrenoceptor agonist ISP elicited two actions on the GABAergic transmission that were recorded in PCs: a short-term increase in the frequency of spontaneous IPSCs (sIPSCs) and LTP of stimulation-evoked IPSCs (eIPSCs) (Mitoma & Konishi, 1999; Saitow et al. 2000). The ISP-induced LTP of GABAergic IPSCs was illustrated in Fig. 1A: the amplitude of eIPSCs increased to 131.2 ± 7.5% of baseline following application of ISP (20 μm, n = 19, determined at 5–10 min after starting the ISP application), and the enhancement persisted for at least 40 min after washing out the β-agonist. The ISP-induced LTP was unlikely to have resulted from persistent activation of β-adrenoceptors by the β-agonist, because application of the β-adrenoceptor antagonist propranolol (10 μm) did not attenuate the long-lasting effect of ISP on eIPSCs (• in Fig. 1A). There was no significant difference in changes of the eIPSC amplitude between ISP alone and ISP plus propranolol determined at 35–40 min after the ISP application (P > 0.5, unpaired t test, n = 7).
Figure 1. β-Adrenergic agonist-induced enhancement of GABA release, and effects of the HCN channel blocker on BC membrane properties and GABAergic transmission onto BCs and PCs.
A, ISP (20 μm)-induced long-term enhancement of eIPSCs in PCs (○) and the effect of β-adrenoceptor antagonist propranolol (•). ISP was applied by superfusion during the period indicated by a black horizontal bar (n = 19). Propranolol (10 μm) was applied during the period indicated by a grey horizontal bar after the ISP-induced enhancement of eIPSCs was fully developed (n = 7). B, the HCN channel blocker ZD7288 abolished a voltage-sag (1) and increased the input resistance (2), resulting in hyperpolarization of a BC (upper trace). Constant current pulses of −200 pA for 400 ms were injected into the BC at 20-s intervals, and ZD7288 was applied by superfusion during the period indicated by a horizontal bar. Records in 1 and 2 were obtained at a and b in the upper trace and displayed on expanded time scales. BCs exhibited repetitive spike firing at the mean resting potential of −51 ± 4 mV (n = 4), which was suppressed by the HCN inhibitor. Note also inhibition by ZD7288 of spontaneous synaptic responses in the BC (see traces in 2). C, inhibition by ZD7288 of spontaneous IPSCs in a PC. Sample records (a and b) represent sIPSCs, obtained at the time points labelled a and b in the upper trace, displayed on an expanded scale. D, the effect of ZD7288 (50 μm) on the ISP-induced enhancement of eIPSCs in PCs. ZD7288 itself caused a partial suppression of eIPSCs (○, n = 7). ISP (20 μm) increased the amplitude of eIPSCs in the presence of ZD7288 (•, n = 7). The amplitude of eIPSCs was expressed as a percentage of the control (determined before application of the HCN inhibitor) in each series. ZD7288 and ISP were applied by superfusion during the periods indicated by open and filled horizontal bars, respectively. Lower traces represent eIPSCs recorded at the time points labelled a–c in the graph. E, ISP-induced increase in the eIPSC amplitude after blockade of HCN by ZD7288. The graph was obtained by subtracting the amplitude of eIPSCs recorded in the presence of ZD7288 alone (○ in D) from that in ZD7288 + ISP (• in D). ZD7288 and ISP were applied as in D.
To investigate the role of HCN channels in the β-agonist-induced enhancement of GABAergic transmission, we sought to determine the effects of blocking HCN channels in BCs on these two actions of ISP. BCs have been reported to display a prominent HCN activity (Saitow & Konishi, 2000; Southan et al. 2000): a signature of the HCN channel activation is the ‘membrane potential sag’ during injection of hyperpolarizing current into BCs (see Fig. 1B, inset 1). At 50 μm, the HCN channel inhibitor, ZD7288, completely suppressed this membrane potential sag and caused a gradual hyperpolarization that was accompanied by an increase in the membrane resistance (Fig. 1B), indicating that HCN channels in BCs were completely blocked by the inhibitor. Concomitantly, ZD7288 inhibited repetitive firing of BCs as well as sIPSCs in both BCs and PCs (Fig. 1B and C). The reduction of neuronal activities by HCN channel blockade suggested that tonic activation of HCN channels contributed to repetitive action potential generation in BCs, which then led to spontaneous, action potential-dependent GABAergic synaptic activity due to BC–BC and BC–PC couplings. When the HCN channels were blocked by ZD7288, the ISP-induced transient increase in the frequency of sIPSCs in PCs was markedly suppressed, suggesting that HCN channel activation in BCs contributed to the ISP-induced increase in the sIPSC frequency. In contrast, in the absence of HCN channel activation, the effects of ISP on the amplitude of eIPSCs in PCs remained the same (Fig. 1D and E).
ZD7288 itself reduced the eIPSC to 76.5 ± 1.1% when determined at 15–25 min after ZD7288 application (•, Fig. 1D; n = 7). This effect was attributable to its presynaptic action on the BCs, because the inhibitor significantly increased the paired-pulse ratio (PPR) of eIPSCs from 0.96 ± 0.05 to 1.36 ± 0.11 (n = 7, P < 0.01; see traces labelled a and b in Fig. 1D). Furthermore, the HCN channel inhibitor hyperpolarized BCs (10.7 ± 1.5 mV, n = 4), resulting in inhibition of repetitive firings (Fig. 1B). On the other hand, there were no significant changes in the extent of ISP-induced enhancement in the presence or absence of ZD7288 (Fig. 1D and E); the percentage increases in eIPSC amplitude were 149 ± 10% in the control solution (n = 12) and 154 ± 12% in the presence of ZD7288 (n = 7, determined at 5–10 min after starting the ISP application; P > 0.8, unpaired t test). Although the amplitude of eIPSCs slightly decreased following ISP washout and again increased after washing out ZD7288 (Fig. 1E), it is not clear whether the HCN inhibitor has a reversible suppressive action on GABAergic synapses. Together, these findings showed that HCN channel activation in BCs contributed only to the ISP-induced increase in the sIPSC frequency but not to the enhancement of stimulation-evoked GABA release at BC–PC inhibitory synapses.
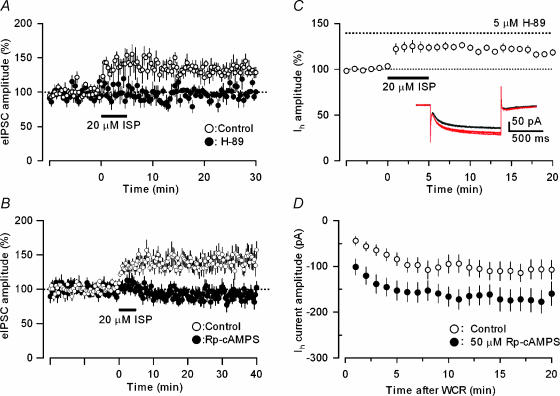
As β-adrenoceptors are known to be linked with cAMP–PKA-dependent mechanisms, we then examined the effects of the PKA inhibitor, H-89, and the membrane permeable cAMP analogue, Rp-cAMPS, on the ISP-induced LTP of GABAergic transmission at the BC–PC synapse. Pretreatment with either H-89 (5 μm) or Rp-cAMPS (100 μm) almost completely abolished the ISP-induced long-term enhancement of eIPSCs in PCs (142.8 ± 2.1 (Fig. 2A, n = 6) and 140.8 ± 1.9% of baselines (Fig. 2B, n = 19) in the control solution, and 96.6 ± 1.8 (n = 8, P < 0.01) or 97.0 ± 1.7% of baselines (n = 12, P < 0.001, unpaired t test, assessed at 5–10 min after starting the ISP application) in the presence H-89 or Rp-cAMPS, respectively). The results point to the involvement of the cAMP–PKA cascade in this form of β-adrenoceptor-mediated LTP at inhibitory synapses. In contrast, H-89 at the same concentration did not affect the ISP-induced increase in HCN channel activation (Fig. 2C, 125.6 ± 16% of control, n = 9), suggesting that HCN channel modulation by β-adrenoceptors depended on a direct action of cAMP on HCN channels. This interpretation was supported by the results presented in Fig. 2D. Rp-cAMPS (50 μm), a cAMP analogue and PKA inhibitor, mimicked the action of ISP in increasing the amplitude of HCN currents in BCs: the HCN current amplitudes were 105.7 ± 2.0 pA in the control condition (n = 14) and 168.8 ± 1.8 pA after Rp-cAMP injection (n = 18, P < 0.001 assessed during the period 11–20 min after the initiation of recordings). Taken together, these findings indicated that the β-adrenoceptor activation at BC–PC inhibitory synapses elicited two distinct actions on GABAergic transmission: (1) the increase in eIPSC amplitude that depended on a cAMP–PKA cascade; and (2) the increase in sIPSC frequency that resulted from a direct action of cAMP on HCN channels to accelerate firing rate due to the excitability increase in presynaptic BCs (see also Saitow & Konishi, 2000).
Figure 2. Effects of the PKA inhibitors H-89 and Rp-cAMPS on ISP-induced enhancement of eIPSCs in PCs and hyperpolarization-activated HCN currents in BCs.
A and B, ISP (20 μm), applied during the period indicated by a horizontal bar, increased the eIPSC amplitude in the control solution (○; A, n = 6; B, n = 19), and this effect was abolished by 5 μm H-89 or 100 μm Rp-cAMPS treatment (•; n = 8 and 12 in A and B, respectively). C, ISP increased the amplitude of HCN currents induced by hyperpolarization of BCs from −50 to −100 mV in the presence of the PKA inhibitor H-89 (5 μm, n = 9). D, enhancement of HCN currents of BCs by Rp-cAMPS, a cAMP analogue and PKA inhibitor. Rp-cAMPS (50 μm) was infused into BCs via the recording patch pipette. The graph represents the time course of change in the amplitude of HCN currents induced by a hyperpolarizing voltage step from −50 to −100 mV and recorded with the control internal solution (○, n = 14) and a Rp-cAMPS-containing internal solution (•, n = 18) after the initiation of whole-cell recording (WCR).
β-Adrenergic LTP of GABA release is not associated with changes in Ca2+ influx into BC terminals
β-Adrenoceptor activation increases the activity of voltage-dependent Ca2+ channels in various excitable cells (Muraki et al. 1993; Gao et al. 1997) and enhances excitatory synaptic transmission by increasing the open probability of P/Q-type Ca2+ channels in presynaptic terminals (Huang et al. 1998). Therefore, we examined whether the β-agonist ISP could enhance Ca2+ influx into BC axon terminals by measuring the action potential-triggered Ca2+ transients in individual boutons of BC axons loaded with Ca2+-sensitive fluorescence indicators and measuring [Ca2+]i with fast confocal imaging in a line scan mode. A representative image of action potential-induced Ca2+ transients, measured by using a high-affinity Ca2+-indicator, Oregon Green BAPTA-1 (OGB-1), is illustrated in Fig. 3A. In agreement with a previous report (Llano et al. 1997), PC somata were surrounded by fluorescent boutons along the trajectory of the labelled BC axon. Depolarization of BC soma (from −60 mV to 0 mV for 3 ms) caused escaping currents (Fig. 3B) that induced action potentials, which in turn triggered reproducible Ca2+ transients in these boutons (Fig. 3A). The peak amplitude of depolarization-induced Ca2+ transients (ΔF/F0) augmented with increasing either extracellular Ca2+ concentrations from 2.5 mm to 5 mm (Fig. 3C) or the number of depolarizing pulses to stimulate BCs (Fig. 3D). However, when [Ca2+]i was determined by the use of either OGB-1 (data not shown) or a low-affinity indicator, Calcium Green 5N (CG5N), the β-agonist ISP did not alter Ca2+ transients at individual boutons (Fig. 3E): the peak amplitudes were 19.3 ± 3.4 and 18.7 ± 4.2% in the absence and presence of ISP, respectively, n = 8, P > 0.7. Furthermore, there was no significant change in the decay time constants of Ca2+ transients before and after the application of ISP (92.5 ± 28 and 102.3 ± 20 ms in the absence and presence of ISP, respectively; n = 8, P > 0.6). The resting level of fluorescence was not significantly changed by ISP (n = 4, data not shown). These results indicated that the long-term modulation of GABA release following β-adrenoceptor activation at BC–PC synapses was independent of calcium influx and, perhaps, of changes in [Ca2+]i dynamics, such as buffering and removal of Ca2+ ions in BC axon terminals.
Effects of β-adrenoceptor activation on the GABA release machinery
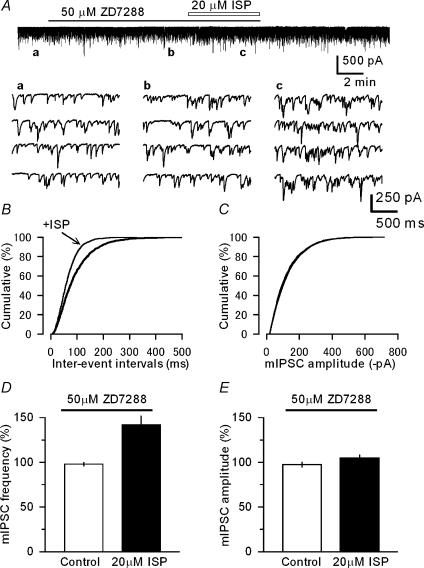
Based on the above results, we then examined whether the β-adrenoceptor activation could modulate the exocytotic machinery in GABAergic presynaptic terminals. It has been previously reported that serotonin increases the frequency of miniature excitatory synaptic responses in the crayfish neuromuscular junction through activation of HCN channels in the presynaptic terminals (Beaumont & Zucker, 2000). However in the cerebellar BC–PC inhibitory synapses, ISP increased the frequency of mIPSCs even in the presence of the HCN channel inhibitor ZD7288 (Fig. 4A), and this effect was statistically significant (Fig. 4B, P < 0.05 K-S test and Fig. 4D, P < 0.001, n = 14). ISP did not significantly affect the mean amplitude of mIPSCs (Fig. 4C, P > 0.5 K-S test and Fig. 4E, P > 0.3, n = 14). These observations suggested that β-adrenoceptor activation directly modulates the release machinery in presynaptic BC nerve terminals.
Figure 4. Effects of ISP on mIPSCs recorded in PCs in the presence of the HCN channel blocker.
A, sample records of membrane currents in a PC in the presence of TTX (1 μm), and traces labelled a to c obtained at the corresponding time points in the upper continuous record and displayed on expanded scales, showing an increase in the frequency of mIPSCs after ISP application. ZD7288 (50 μm) and ISP (20 μm) were applied during the periods indicated horizontal bars. B and C, cumulative curves for the inter-event intervals (B) and amplitude (C) of mIPSCs recorded in a PC before and after application of 20 μm ISP in the presence of 50 μm ZD7288. mIPSCs, recorded from the same PC shown in A, were counted for a constant period (180 s) before (2198 events, mean amplitude 126.3 ± 2.1 pA) and after application of ZD7288 alone (2189 events, 123.0 ± 2.1 pA) and ZD7288 plus ISP (3070 events, 126.6 ± 1.7 pA). The inter-event curve was significantly changed (shifted leftwards) by ISP (P < 0.05, K-S test) in B. ISP did not significantly alter the amplitude of mIPSCs in C: three curves overlapped (P > 0.5, before versus after ISP application, K-S test). The threshold of inter-event interval and amplitude of mIPSC were set at 8 ms and −18 pA, respectively. D and E, pooled data for the effects of ISP on the mIPSC frequency (D) and amplitude (E). Treatment with ZD7288 (50 μm) was started at least 10 min before ISP application as in A. The mean amplitude and frequency of mIPSCs were determined before (control, open columns) and after ISP application (filled columns) in the presence of ZD7288 and expressed as percentage changes from the controls (determined before the ZD7288 treatment: ISP significantly increased the mIPSC frequency, P < 0.001 without altering the amplitude, P > 0.3, n = 14).
As the degree of miniature synaptic activity depends on the vesicle pool size in presynaptic terminals (Prange & Murphy, 1999), we aimed to determine whether β-adrenoceptor stimulation would increase the size of readily releasable pool (RRP) responsible for GABA exocytosis from BC terminals. Application of a hypertonic solution (HS) has been shown to cause tonic release of glutamate at hippocampal excitatory synapses (Rosenmund & Stevens, 1996). Therefore, we tested the effect of HS (500 mm sucrose added in normal ACSF) on cerebellar GABAergic inhibitory synapses. When applied by pressure pulses to the vicinity of recorded PCs, HS reproducibly produced GABAergic synaptic currents and increased the frequency of mIPSCs, both of which were completely abolished by 50 μm picrotoxin (Fig. 5A). Increasing [Ca2+]o from 0 to 2.5 mm did not significantly alter the extent of HS-induced currents (Fig. 5B, P > 0.4 Tukey's multiple comparison test, n = 4), indicating that HS induced exocytotic GABA release at BC–PC synapses by stimulating the RRP downstream of Ca2+ influx in a manner independent of changes in [Ca2+]i. We next examined the effects of β-adrenoceptor stimulation on the HS-induced GABA currents by comparing the amount of charge transfer induced by puff-application of HS before and after ISP application. As shown in Fig. 5B, the β-agonist ISP markedly increased the HS-induced charge transfer to 139 ± 13% of the control (P < 0.01, Tukey's multiple comparison test, n = 12). This effect was abolished by the PKA inhibitor, H-89 (5 μm) (109 ± 4%; P < 0.05 Tukey's multiple comparison test, n = 7), but not significantly affected by the HCN inhibitor ZD7288 (50 μm) (150 ± 13%; P > 0.8, Tukey's multiple comparison test, n = 5). Moreover, there was a strong correlation between the increase in the HS-induced charge transfer and the increase in the mIPSC frequency following application of ISP (Fig. 5C, P < 0.01 non-parametric Spearman rank test, r= 0.76, n = 12). These results thus strongly suggested that the β-adrenoceptor activation enhanced GABA exocytosis from BC terminals through direct stimulation of the release machinery in a cAMP–PKA-dependent manner, leading to the increase in the RRP of GABA (see below).
Figure 5. ISP-induced enhancement of GABAergic currents in PCs produced in response to focal application of a hypertonic solution (HS).
A, a continuous trace of membrane current responses in a PC. Sucrose (500 mm)-containing HS was puff-applied from a micropipette for 5 s indicated by the arrowhead to the vicinity of the recorded PC, and ISP (20 μm) was applied by perfusion during the period indicated by a horizontal bar. Sample records of HS-induced current response and increases in mIPSCs before (a) and during ISP application (b) obtained at the corresponding time points in the upper continuous trace. The HS response was completely suppressed by picrotoxin (50 μm, PicroTX). The time course of ISP-induced increase in the HS-evoked GABA current (determined as an integral of superimposed mIPSCs during 20 s for each point) was plotted (lower right panel): ISP was applied by superfusion during the period indicated by a horizontal bar. B, effects of changes in [Ca2+]o on the HS-evoked GABA current, and H-89 and ZD7288 on the ISP-induced enhancement of the HS response. The magnitude of HS-evoked response in each column was expressed as a ratio to each control: 0Ca2+ for the 2.5 mm effect, and 2.5 mm Ca2+ (labelled as ‘Control’) for both H-89 (5 μm) and ZD7288 (50 μm). Changing [Ca2+]o from 0 to 2.5 did not significantly alter the HS-induced response (P > 0.4, n = 4). The PKA inhibitor H-89 almost completely suppressed the effect of ISP on the HS-evoked GABA current (P < 0.05 Tukey's multiple comparison test, n = 7), while the HCN inhibitor ZD7288 had no significant effects (P > 0.8, Tukey's multiple comparison test, n = 5). C, relationship between the ISP-induced enhancement of HS current response and increase in the mIPSC frequency. The effects of ISP on both responses were determined as the amount of charge transfer in individual PCs as in A. The straight line represents y = 1.3x − 0.3, showing a strong correlation (correlation coefficient = 0.76) between the ISP effects on the HS-evoked current and the mIPSC frequency (P < 0.01 simple regression analysis, n = 12).
β-Adrenoceptor stimulation enhances the Ca2+ sensitivity of the release machinery
Because neurotransmitter release is triggered by the action potential-induced rise of intracellular Ca2+ concentrations in presynaptic terminals (Augustine et al. 1999), we addressed how the dependency of GABAergic transmission on Ca2+ concentration could be modulated by the β-adrenoceptor activation. Thus we examined the relationship between the average amplitudes of eIPSCs in PCs and changes in [Ca2+]o before and after application of ISP. Fitting the Hill equation to this relationship in the absence of ISP showed that the change of eIPSC amplitude depended on the second power of [Ca2+]o (Fig. 6A). To date the efficacy of excitatory transmission has been shown to depend on the fourth power of [Ca2+]o at the neuromuscular junction (Dodge & Rahamimoff, 1967) and central excitatory synapses (Yawo, 1996), whereas GABAergic transmission at collicular inhibitory synapses has been reported to display an approximately third-power dependency on presynaptic Ca2+ concentration (Kirischuk et al. 1999). As illustrated in Fig. 6A, the extent of β-adrenergic agonist-induced enhancement of eIPSCs was consistently larger at lower [Ca2+]o (210 ± 12% and 123 ± 3% at 1 and 5 mm[Ca2+]o, respectively, n = 8–9). Consequently, ISP shifted the relationship between the eIPSC amplitude and [Ca2+]o in a leftward direction with a decrease in Kd for Ca2+ from 1.9 to 1.0 mm (Fig. 6A), suggesting that the increase in Ca2+ sensitivity of the release machinery contributed to the β-adrenoceptor-mediated enhancement of inhibitory transmission at BC–PC synapses. Furthermore, the ISP-induced enhancement of the Ca2+ sensor function depended on a cAMP–PKA cascade, because the PKA inhibitor Rp-cAMPS (100 μm) markedly suppressed the ISP-induced increase in the eIPSC amplitude at 1.5 and 2.5 mm[Ca2+]o (Fig. 6B): 210.0 ± 12.0% (n = 10) and 144.3 ± 13.0% (n = 5) increases in the absence and presence of Rp-cAMPS at 1.5 mm[Ca2+]o, respectively (P < 0.01, unpaired t test).
Figure 6. Dependency of GABAergic transmission at BC–PC synapses on [Ca2+]o in the absence and presence of ISP.
A, the amplitude of eIPSCs was first determined while changing [Ca2+]o and expressed as a relative amplitude (percentage) of that determined at 2.5 mm[Ca2+]o in the control solution (○). *P < 0.05 and ***P < 0.001, between control and ISP treatment. Data are least-mean square fits to Hill equation: y =ymax[Ca2+]on/(Kdn+[Ca2+]on), with n (Hill coefficient), ymax and Kd (dissociation constant) as free parameters. The best fitting of data revealed a Kd of 1.9 mm, ymax of 1.6 and Hill coefficient (n) of 1.9 in the control solution, and Kd of 1.0 mm, ymax of 1.7 and n of 2.0 in the presence of ISP, indicating that ISP significantly decreased the Kd value (n = 7–12, P < 0.05). B, relationships of the ISP-induced enhancement of eIPSCs and [Ca2+]o (open column), and the effect of the PKA inhibitor Rp-cAMPS (filled column). The enhancement ratio decreased when [Ca2+]o was increased from 1 to 5 mm. The dependency of the ISP-induced enhancement on [Ca2+]o was significantly suppressed by treatment with 100 μm Rp-cAMPS (P < 0.01 and 0.001 unpaired t test at 1.5 and 2.5 mm[Ca2+]o, respectively). The number in each column represents the number of cells where the effects were determined.
β-Adrenoceptor stimulation also increases the number of releasable synaptic vesicles
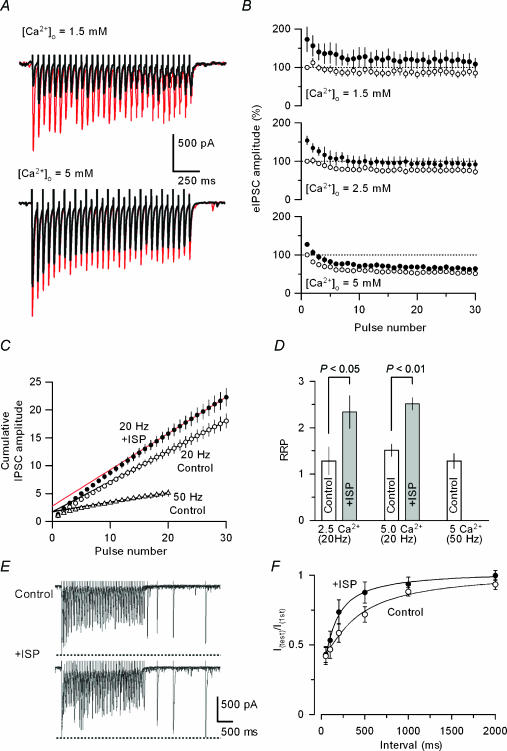
We next examined whether β-adrenergic agonist stimulation would affect the process of neurotransmitter recruitment that regulates the size of RRP (namely, the number of releasable vesicles) in GABAergic terminals. In the experiments illustrated in Fig. 7, the time course of changes in GABAergic transmission following repeated activation of the presynaptic terminals was compared before and after ISP application. Tetanic stimulation at 20 Hz for 1.5 s (30 stimuli) in the control solution transiently reduced the amplitude of eIPSCs within the initial 10 stimuli, reaching a steady level of its amplitude with only partial suppression. This effect was prominent in 5 mm[Ca2+]o (Fig. 7A and B). Possible explanations for such changes in GABA release kinetics following tetanic stimulation are that: (1) the presynaptic terminals of cerebellar interneurones might be endowed with relatively large size of the RRP, which could be replenished during repeated activation; and/or (2) the Ca2+ sensor in the release machinery of BC terminals could support sustained GABA exocytosis. Under these conditions, application of ISP not only reinforced GABA release induced by individual stimuli but also enhanced the extent, as well as the speed, of transmission depression during repeated stimulation at 1.5 and 2.5 mm[Ca2+]o, but the effects were less prominent at 5 mm[Ca2+]o (Fig. 7A and B).
Figure 7. Effects of changes in [Ca2+]o and ISP on depression of GABA release, the size of the RRP and refilling of neurotransmitter pools at BC–PC synapses following tetanic stimulation.
A, sample records of eIPSCs evoked by tetanic stimulation (20 Hz for 1.5 s) at 1.5 (upper) and 5 mm[Ca2+]o (lower) before (black) and during application of 20 μm ISP (red). Each record was obtained by averaging five successive traces. B, the amplitude of eIPSCs evoked by repetitive stimulation as in A at different levels of [Ca2+]o and expressed as relative amplitude to the initial IPSCs in each [Ca2+]o before (○) and during the application of ISP (•) (n = 5–6). C, estimation of the RRP from the relationship between the cumulative eIPSC amplitude (determined in B) and the pulse number during tetanic stimulation. Cumulative curves were compiled from the amplitudes of successive IPSCs during repetitive stimulation normalized to the first IPSC amplitude recorded in each trial in 5 mm[Ca2+]o. Data points during a steady-state phase (from the 21st to the 30th and the 16th to the 20th pulses for 20 Hz, and 50 Hz stimulation, respectively) were fitted by linear regression, and extrapolated to the y-axis, giving values of the RRP size in the control solution (○ for 20 Hz, ▵ for 50 Hz, and lines in black). ISP (20 μm) significantly increased the RRP size (• and line in red). D, pooled data of the PPR sizes in the absence (open column) and the presence of ISP (grey column) in 2.5 and 5 mm[Ca2+]o, and the pool size determined with 50-Hz tetanic stimulation. ISP (20 μm) significantly increased the size of the RRP under both [Ca2+]o conditions (2.5 mm[Ca2+]o, P < 0.05, n = 8; 5 mm[Ca2+]o, P < 0.01, n = 8). E, effects of ISP on the rate of transmitter refilling. After conditioning with high frequency stimulation (20 Hz, 30 pulses) in 5 mm[Ca2+]o, single test stimuli with different intervals were applied to evoke IPSCs in a PC and superimposed before (upper trace) and during ISP (20 μm) application (lower trace). ISP accelerated the refilling process. F, pooled data of averaged time course for the recovery of synaptic depression after the tetanic stimulation (20 Hz, 30 pulses) in the control solution (○, n = 10) and in the presence of 20 μm ISP (•, n = 9). Smooth curves represent single exponential fits to the averaged data. ISP application significantly shortened the time constant of IPSC recovery from 210.4 ± 21.4 ms (n = 10) to 86 ± 11.6 ms (n = 9) (P < 0.05, unpaired t test).
To estimate the effect of β-agonist on the RRP size, the cumulative amplitude of eIPSCs during tetanic stimulation was compared before and after ISP application (Fig. 7C). Based on the assumption that depression of synaptic transmission is mainly caused by the reduction of the RRP size, it has been demonstrated that a linear slope of the cumulative amplitude relationship reflects vesicle recycling at the steady-state level during repeated transmitter release and that extrapolation of the linear slope to the ordinate would provide an estimate of the size of RRP (Kirischuk & Grantyn, 2003). Using this procedure, we also estimated the RRP at BC–PC inhibitory synapses in the experiments illustrated in Fig. 7C, where the amplitudes of eIPSCs evoked by repetitive stimulation at 20 or 50 Hz were normalized to that of the first response. There was no significant difference in the size of RRP for GABA release observed with different stimulation frequencies (Fig. 7C and D, 1.5 ± 0.1 and 1.3 ± 0.2 when determined at 20 and 50 Hz, respectively, P > 0.6, unpaired t test, n = 6–8), indicating that the estimated RRP size did not depend on the frequency of stimulation. While the effect of ISP on the amplitude of eIPSCs was marginal in 5 mm[Ca2+]o as compared to the effects in 1.5 and 2.5 mm Ca2+ (Fig. 7B), the β-agonist ISP significantly increased the size of the RRP even in the higher-Ca2+ medium (Fig. 7D, 1.3 ± 03 and 1.5 ± 0.1 in 2.5 and 5 mm Ca2+, respectively; n = 8 for both cases, P < 0.05 for 2.5 mm and P < 0.01 for 5 mm Ca2+).
In addition to increasing the RRP size, ISP also increased the rate at which the transmitter pool was refilled following the high frequency stimulation (Fig. 7E and F). To determine the time course of refilling, we measured the amplitude of IPSCs evoked by single test stimuli at different intervals after termination of high-frequency train stimulation (20 Hz with 30 stimuli). While the overall time course of IPSC recovery in the control experiment was fitted by a single-exponential function with a time constant of 210.4 ± 21.4 ms (n = 10), the ISP application significantly accelerated the refilling kinetics with a time constant of 86 ± 11.6 ms (P < 0.05, unpaired t test, n = 9). These observations imply that (1) β-adrenoceptor activation could principally modulate the Ca2+ sensitivity of the release machinery, contributing to the increase in eIPSC amplitude and (2) that the ISP-induced increase in the RRP would serve as a secondary mechanism for compensating vesicle depletion following the increment of release probability. Based on these results, we suggest that the β-adrenoceptor stimulation at BC–PC synapses enhanced GABA release through the up-regulation of both the Ca2+ sensor in the exocytotic machinery and the size of transmitter pools immediately available for release.
β-Adrenoceptor stimulation enhances synchronous and asynchronous GABA release
Finally, we explored the effects of β-adrenoceptor activation on GABA release kinetics. The process of neurotransmitter release is divided into phasic synchronous and tonic asynchronous phases. The tonic release is manifest when Ca2+ ions are replaced by Sr2+ ions at both excitatory (Atluri & Regehr, 1996) and inhibitory synapses (Rumpel & Behrends, 1999). A previous study on the knockout mice lacking synaptotagmin I, a presynaptic Ca2+-binding protein, has demonstrated that this Ca2+-binding protein contributes to synchronous release of glutamate at hippocampal excitatory synapses without affecting asynchronous release (Geppert et al. 1994). It is therefore possible that the two release mechanisms could be differentially modulated by the β-adrenoceptor activation. This possibility was tested in the experiments shown in Fig. 8. When Ca2+ in the ACSF was replaced by an equimolar amount of Sr2+, the amplitude of eIPSCs (generated by synchronous GABA release) was largely suppressed (Fig. 8A and B, 31 ± 4% of the control; P < 0.001, Tukey's multiple comparison test, n = 11), whereas asynchronous GABA release became prominent (Fig. 8D and F, 0.4 ± 0.7 and 9.6 ± 1.2 Hz in Ca2+- and Sr2+-containing ACSF, respectively, P < 0.001 Tukey's multiple comparison test, n = 11). ISP (20 μm) not only increased the synchronous component of GABA release in the Sr2+-containing ACSF (Fig. 8A – C, 153.8 ± 8.6% of baseline determined at 3 min after ISP application; P < 0.05, Tukey's multiple comparison test, n = 11) but also enhanced asynchronous GABA release as determined by comparing inhibitory synaptic events during a time window of 500 ms before (Pre) and after (Post) single shock stimulation (Fig. 8D – F, 9.1 ± 1.1 and 13.0 ± 1.3 Hz before and after ISP application in Sr2+-containing ACSF, respectively; P < 0.05, Tukey's multiple comparison test, n = 10). These results indicated that both Ca2+- and Sr2+-mediated release mechanisms, namely synchronous and asynchronous GABA release at BC–PC inhibitory synapses, were under the control of β-adrenoceptor-mediated modulation.
Figure 8. Effects of ISP on synchronous and asynchronous GABA release from BCs.
A, stimulation-evoked synchronous IPSCs recorded in a PC in the control solution containing normal [Ca2+]o (top trace), and in a solution in which Ca2+ was replaced by equimolar Sr2+ (middle trace) and during 20 μm ISP application in the Sr2+-containing solution (bottom trace). Three successive eIPSCs were superimposed in each record. B, relative amplitudes of synchronous eIPSCs in control (Ca2+) and Sr2+-containing (Sr2+, 31.0 ± 4%) solutions and during the ISP application in the Sr2+-containing solution (Sr2++ ISP, 53.3 ± 8.6%). The IPSC amplitude was expressed as a percentage of that determined in the control Ca2+-containing solution. C, time course of ISP-induced enhancement of synchronous eIPSCs in the Sr2+-containing solution (n = 11). The amplitude of eIPSC was expressed as a percentage of the control (determined before ISP application). D and E, effects of ISP on asynchronous GABA release. In the Sr2+-containing solution, a single stimulation (at arrowhead) induced a marked increase in the frequency of asynchronous IPSCs during the time window of 500 ms (indicated by a horizontal bar and shaded area) (D), and the asynchronous IPSCs were further increased by 20 μm ISP application (E). F, pooled data for increases in the occurrence of asynchronous IPSCs in the Sr2+-containing solution and its enhancement by ISP application. Asynchronous IPSCs were recorded as in D and expressed as a frequency difference of events detected between pre- and poststimulus time windows (as in D and E) in the control ACSF (Ca2+), and Sr2+-containing ACSF (Sr2+) and during 20 μm IPS application in Sr2+-containing ACSF (Sr2++ ISP) (n = 10 for each condition).
Discussion
Our findings that activation of HCN channels did not contribute to the enhancement of stimulation-evoked GABA release at BC–PC inhibitory synapses provided an interesting contrast to the roles played by activation of HCN channels in mediating LTP of neurotransmitter release at excitatory synapses where the long-term enhancement of glutamate release has been proposed to involve both HCN channel- and PKA-dependent mechanisms (Mellor et al. 2002). Recently, however, cAMP has been reported to facilitate neurotransmitter release via a PKA-independent mechanism (Zhong & Zucker, 2004; Kaneko & Takahashi, 2004). Furthermore, our data were in contrast to the previous finding that serotonin, another monoamine, accelerates activation of HCN channels and thereby increases miniature synaptic responses in the crayfish neuromuscular junction (Beaumont & Zucker, 2000). Thus, it seems that excitatory and inhibitory synapses rely on different signalling mechanisms for short-term and long-term modulation of the efficacy of neurotransmission at individual synapses.
For cerebellar GABAergic interneurones, the short-term modulation of their excitability by β-adrenoceptor stimulation depended only on the cAMP-mediated HCN channel acceleration but not on the cAMP–PKA pathway (Saitow & Konishi, 2000), whereas, as the present results suggested, increase in eIPSC amplitude (i.e. LTP of GABA release), largely relied on a cAMP–PKA cascade. Stimulation of afferent fibres in the cerebellar cortex mimicked the β-adrenoceptor-mediated LTP of GABAergic transmission at BC–PC synapses (Mitoma & Konishi, 1999). Therefore, depending on different time windows and signalling pathways following monoaminergic afferent activation, the β-adrenergic short- and long-lasting forms of plasticity in the GABAergic inhibitory synapse provides a likely mechanism of feedforward inhibition onto PCs, the sole output from the cerebellar cortex, thereby critically influencing motor coordination in the cerebellar function.
Our findings revealed two independent mechanisms underlying the noradrenergic and β-adrenoceptor-mediated LTP of inhibitory GABAergic transmission at BC–PC synapses: β-adrenoceptor stimulation not only up-regulated the efficacy of the Ca2+-sensor in the GABA release machinery, it also increased the number of presynaptic vesicles that were immediately available for release.
β-Adrenoceptor activation regulates the Ca2+ sensor function and the number of releasable synaptic vesicles
Using two independent tests, we have shown that the β-adrenoceptor stimulation enhanced the GABA release machinery in the presynaptic nerve terminals: (1) the β-agonist ISP increased the frequency of mIPSCs in PCs without significantly changing the mean amplitude, and this effect persisted in the presence of the HCN channel inhibitor ZD7288 (Fig. 4); and (2) ISP also increased GABA release produced in response to hypertonic stimulation of the nerve terminal in a manner sensitive to PKA-linked pathways but insensitive to HCN channels (Fig. 5).
Our data from two other experiments contributed to the characterization of the targets involved in the noradrenergic LTP of GABA release at BC–PC synapses. First, the dependency of GABAergic transmission on [Ca2+]o was reduced by ISP (Fig. 6), indicating that β-adrenoceptor stimulation enhanced the efficacy of the Ca2+ sensor in the GABA release machinery. The Hill equation fitted to the relationship between the eIPSC amplitudes and [Ca2+]o indicated that the only parameter affected by ISP application was Kd, the dissociation constant of the release machinery to Ca2+ ions, which was reduced by β-adrenoceptor activation. Second, the estimation of the RRP in GABAergic nerve terminals using the high-frequency stimulation protocol showed that the β-agonist increased the RRP both in normal- and high-Ca2+-containing solutions (Fig. 7), suggesting that the increase in the number of releasable synaptic vesicles also contributed to this noradrenergic LTP at BC–PC synapses. The increases in both Ca2+ sensor function and RRP following β-adrenoceptor stimulation were suppressed by the PKA inhibitors H-89 and Rp-cAMPS (Figs 5 and 6), suggesting that a cAMP–PKA pathway contributes to β-adrenoceptor-mediated LTP of GABA release. The effect of ISP on the eIPSC amplitude was larger in low Ca2+ while ISP significantly increased the size of RRP even in 5 mm[Ca2+]o. This discrepancy suggests that the Ca2+ sensor was almost saturated in a high-Ca2+ medium, while the replenishment of GABA to releasable pools still occurred during repeated synaptic activation. Alternatively, a high-Ca2+ medium might reduce the excitability at presynaptic nerve terminals, resulting in a less prominent effect of the β-agonist on the eIPSCs in 5 mm[Ca2+]o than the change in RRP size: the increase in the RRP size could prevent the depletion of releasable GABA associated with massive increases in excitability and release probability following β-adrenoceptor activation. This interpretation would be consistent with the finding that the β-agonist also accelerated the recovery from synaptic depression (namely, the refilling of transmitter pools) following high-frequency stimulation (Fig. 7F).
The analysis of GABA release kinetics indicated that ISP facilitated both synchronous and asynchronous GABA release (Fig. 8), which indicated that ISP enhanced the sensitivity of vesicles to both Ca2+ and Sr2+ ions. It is therefore suggested that β-adrenoceptor stimulation increased the number of synaptic vesicles with both high- and low-release probability and that fast and slow components of GABA exocytosis could be modulated by a shared molecular mechanism underlying the noradrenergic LTP. A similar mechanism has been proposed at chick ciliary ganglion synapses, where noradrenergic protein kinase C (PKC)-dependent mechanism up-regulates the Ca2+ and Sr2+ sensitivities of vesicular proteins, leading to the increase in vesicle fusion probability for synchronous and asynchronous transmitter release (Yawo, 1996). cAMP–PKA cascade has been shown to selectively increase the number of vesicles with higher release probability (Sakaba & Neher, 2001). A synaptic vesicular protein, synaptotagmin, has been shown to regulate only the process associated with fast transmitter release (Geppert et al. 1994). It is therefore unlikely that this vesicular protein could be involved in the β-adrenoceptor-mediated modulation of asynchronous GABA release.
Possible mechanisms of PKA-dependent enhancement of GABA release
The finding that the PKA inhibitor abolished β-adrenoceptor-mediated increases in the amplitudes of eIPSCs and HS-induced GABA currents, as well as in the frequency of mIPSCs in PCs, suggested that the noradrenergic LTP at BC–PC synapses was associated with cAMP–PKA-dependent phosphorylation of vesicular proteins that could enhance the translocation of synaptic vesicles towards the active sites in GABAergic presynaptic terminals. Among various vesicular proteins, synapsins, the major substrates of PKA- and Ca2+/calmodulin-dependent protein kinases (Hosaka et al. 1999), have been reported to regulate the synaptic vesicle translocation upon phosphorylation (Hilfiker et al. 1999). Moreover, synapsins have been implicated in modulation of PKA-dependent LTP at hippocampal mossy fibre–CA3 synapses (Rosahl et al. 1995). However, the noradrenergic LTP at cerebellar GABAergic synapses could be induced in synapsin-knockout mice (Saitow et al., unpublished observations). Two other vesicular proteins that serve as PKA substrates are RIM1 and rab3. These two proteins are also unlikely to contribute to the β-adrenoceptor-mediated LTP of GABA release, because ISP enhanced GABAergic transmission at BC–PC synapses in cerebellar slices derived from knockout mice of these proteins (F. Saitow & S. Konishi, unpublished observations).
Snapin is another PKA target protein of the release machinery members (Ilardi et al. 1999). Although it has been reported in adrenal chromaffin cells that association of synaptotagmin with SNARE complex is enhanced by PKA-dependent phosphorylation of snapin, leading to an increase in both the number of releasable vesicles and the kinetics of the vesicle priming to the release site (Chheda et al. 2001), its roles at central synapses remain unclear. The β-adrenergic-dependent LTP of GABAergic transmission that we observed at cerebellar BC–PC synapses is likely to result from modulation of vesicular protein interactions via PKA-dependent phosphorylation at the presynaptic active site. Further studies are required to determine the molecular target involved in this form of LTP at inhibitory synapses.
Acknowledgments
We thank F. Wong for critical reading of the manuscript, H. Ohnishi, M. Takahashi, T. Murakoshi and K. Kobayashi for discussions, D. A. Rusakov for suggestions for Ca2+ imaging and helpful comments, and Y. Ikebuchi for technical assistance. This work was supported by the Core Research for Evolutional Science and Technology, Japan Science and Technology Agency (CREST, JST).
References
- Atluri PP, Regehr WG. Determinants of the time course of facilitation at the granule cell to Purkinje cell synapse. J Neurosci. 1996;16:5661–5671. doi: 10.1523/JNEUROSCI.16-18-05661.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustine GJ, Burns ME, DeBello WM, Hilfiker S, Morgan JR, Schweizer FE, Tokumaru H, Umayahara K. Proteins involved in synaptic vesicle trafficking. J Physiol. 1999;520:33–41. doi: 10.1111/j.1469-7793.1999.00033.x. 10.1111/j.1469-7793.1999.00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaumont V, Zucker RS. Enhancement of synaptic transmission by cyclic AMP modulation of presynaptic Ih channels. Nat Neurosci. 2000;3:133–141. doi: 10.1038/72072. [DOI] [PubMed] [Google Scholar]
- Caillard O, Ben-Ari Y, Gaiarsa JL. Long-term potentiation of GABAergic synaptic transmission in neonatal rat hippocampus. J Physiol. 1999a;518:109–119. doi: 10.1111/j.1469-7793.1999.0109r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillard O, Ben-Ari Y, Gaiarsa JL. Mechanisms of induction and expression of long-term depression at GABAergic synapses in the neonatal rat hippocampus. J Neurosci. 1999b;19:7568–7577. doi: 10.1523/JNEUROSCI.19-17-07568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chheda MG, Ashery U, Thakur P, Rettig J, Sheng ZH. Phosphorylation of Snapin by PKA modulates its interaction with the SNARE complex. Nat Cell Biol. 2001;3:331–338. doi: 10.1038/35070000. [DOI] [PubMed] [Google Scholar]
- Dodge FA, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. 10.1016/S0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- Gao T, Yatani A, Dell'Acqua ML, Sako H, Green SA, Dascal N, Scott JD, Hosey MM. cAMP-dependent regulation of cardiac L-type Ca2+ channels requires membrane targeting of PKA and phosphorylation of channel subunits. Neuron. 1997;19:185–196. doi: 10.1016/s0896-6273(00)80358-x. 10.1016/S0896-6273(00)80358-X. [DOI] [PubMed] [Google Scholar]
- Geppert M, Goda Y, Hammer RE, Li C, Rosahl TW, Stevens CF, Sudhof TC. Synaptotagmin I: a major Ca2+ sensor for transmitter release at a central synapse. Cell. 1994;79:717–727. doi: 10.1016/0092-8674(94)90556-8. [DOI] [PubMed] [Google Scholar]
- Hilfiker S, Pieribone VA, Czernik AJ, Kao HT, Augustine GJ, Greengard P. Synapsins as regulators of neurotransmitter release. Philos Trans R Soc Lond B Biol Sci. 1999;354:269–279. doi: 10.1098/rstb.1999.0378. 10.1098/rstb.1999.0378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren CD, Zilberter Y. Coincident spiking activity induces long-term changes in inhibition of neocortical pyramidal cells. J Neurosci. 2001;21:8270–8277. doi: 10.1523/JNEUROSCI.21-20-08270.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosaka M, Hammer RE, Sudhof TC. A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron. 1999;24:377–387. doi: 10.1016/s0896-6273(00)80851-x. 10.1016/S0896-6273(00)80851-X. [DOI] [PubMed] [Google Scholar]
- Huang CC, Wang SJ, Gean PW. Selective enhancement of P-type calcium currents by isoproterenol in the rat amygdala. J Neurosci. 1998;18:2276–2282. doi: 10.1523/JNEUROSCI.18-06-02276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilardi JM, Mochida S, Sheng ZH. Snapin: a SNARE associated protein implicated in synaptic transmission. Nat Neurosci. 1999;2:119–124. doi: 10.1038/5673. 10.1038/5673. [DOI] [PubMed] [Google Scholar]
- Kaneko M, Takahashi T. Presynaptic mechanism underlying cAMP-dependent synaptic potentiation. J Neurosci. 2004;24:5202–5208. doi: 10.1523/JNEUROSCI.0999-04.2004. 10.1523/JNEUROSCI.0999-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kano M, Konnerth A. Potentiation of GABA-mediated currents by cAMP-dependent protein kinase. Neuroreport. 1992;3:563–566. doi: 10.1097/00001756-199207000-00004. [DOI] [PubMed] [Google Scholar]
- Kirischuk S, Grantyn R. Intraterminal Ca2+ concentration and asynchronous transmitter release at single GABAergic boutons in rat collicular cultures. J Physiol. 2003;548:753–764. doi: 10.1113/jphysiol.2002.037036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirischuk S, Veselovsky N, Grantyn R. Relationship between presynaptic calcium transients and postsynaptic currents at (-aminobutyric acid (GABA) ergic boutons. Proc Natl Acad Sci U S A. 1999;96:7520–7525. doi: 10.1073/pnas.96.13.7520. 10.1073/pnas.96.13.7520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y. GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J Neurosci. 1996;16:6342–6352. doi: 10.1523/JNEUROSCI.16-20-06342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo S, Marty A. Protein kinase A-mediated enhancement of miniature IPSC frequency by noradrenaline in cerebellar stellate cells. J Physiol. 1997;498:165–176. doi: 10.1113/jphysiol.1997.sp021849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Gershenfeld HM. β-Adrenergic enhancement of inhibitory synaptic activity in rat cerebellar stellate and Purkinje cells. J Physiol. 1993;468:201–224. doi: 10.1113/jphysiol.1993.sp019767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llano I, Tan YP, Caputo C. Spatial heterogeneity of intracellular Ca2+ signals in axons of basket cells from rat cerebellar slices. J Physiol. 1997;502:509–519. doi: 10.1111/j.1469-7793.1997.509bj.x. 10.1111/j.1469-7793.1997.509bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: An embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation—A decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Mellor J, Nicoll RA, Schmitz D. Mediation of hippocampal mossy fiber long-term potentiation by presynaptic Ih channels. Science. 2002;295:143–147. doi: 10.1126/science.1064285. 10.1126/science.1064285. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Kobayashi T, Song SY, Konishi S. Enhancement by seratonin of GABA-mediated inhibitory synaptic currents in rat cerebellar Purkinje cells. Neurosci Lett. 1994;173:127–130. doi: 10.1016/0304-3940(94)90165-1. 10.1016/0304-3940(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Konishi S. Long-lasting facilitation of inhibitory transmission by monoaminergic and cAMP-dependent mechanism in rat cerebellar GABAergic synapses. Neurosci Lett. 1996;217:141–144. 10.1016/0304-3940(96)13090-1. [PubMed] [Google Scholar]
- Mitoma H, Konishi S. Monoaminergic long-term facilitation of GABA-mediated inhibitory transmission at cerebellar synapses. Neuroscience. 1999;88:871–883. doi: 10.1016/s0306-4522(98)00260-7. 10.1016/S0306-4522(98)00260-7. [DOI] [PubMed] [Google Scholar]
- Muraki K, Bolton TB, Imaizumi Y, Watanabe M. Effect of isoprenaline on Ca2+ channel current in single smooth muscle cells isolated from taenia of the guinea-pig caecum. J Physiol. 1993;471:563–582. doi: 10.1113/jphysiol.1993.sp019916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prange O, Murphy TH. Correlation of miniature synaptic activity and evoked release probability in cultures of cortical neurons. J Neurosci. 1999;19:6427–6438. doi: 10.1523/JNEUROSCI.19-15-06427.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC. Essential functions of synapsins I and II in synaptic vesicle regulation. Nature. 1995;375:488–493. doi: 10.1038/375488a0. 10.1038/375488a0. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Stevens CF. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron. 1996;16:1197–1207. doi: 10.1016/s0896-6273(00)80146-4. 10.1016/S0896-6273(00)80146-4. [DOI] [PubMed] [Google Scholar]
- Rumpel E, Behrends JC. Sr2+-dependent asynchronous evoked transmission at rat striatal inhibitory synapses in vitro. J Physiol. 1999;514:447–458. doi: 10.1111/j.1469-7793.1999.447ae.x. 10.1111/j.1469-7793.1999.447ae.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitow F, Konishi S. Excitability increase induced by β-adrenergic receptor-mediated activation of hyperpolarization-activated cation channels in rat cerebellar basket cells. J Neurophysiol. 2000;84:2026–2034. doi: 10.1152/jn.2000.84.4.2026. [DOI] [PubMed] [Google Scholar]
- Saitow F, Konishi S. Roles of Ih in β-adrenergic receptor-mediated enhancement of inhibitory transmission at cerebellar basket cell-Purkinje cell synapses. Soc Neurosci Abstr. 2002;837:15. [Google Scholar]
- Saitow F, Satake S, Yamada J, Konishi S. β–Adrenergic receptor-mediated presynaptic facilitation of inhibitory GABAergic transmission at cerebellar interneuron-Purkinje cell synapses. J Neurophysiol. 2000;84:2016–2025. doi: 10.1152/jn.2000.84.4.2016. [DOI] [PubMed] [Google Scholar]
- Sakaba T, Neher E. Preferential potentiation of fast-releasing synaptic vesicles by cAMP at the calyx of Held. Proc Natl Acad Sci U S A. 2001;98:331–336. doi: 10.1073/pnas.021541098. 10.1073/pnas.021541098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanes JR, Lichtman JW. Can molecules explain long-term potentiation? Nat Neurosci. 1999;2:597–604. doi: 10.1038/10154. 10.1038/10154. [DOI] [PubMed] [Google Scholar]
- Southan AP, Morris NP, Stephens GJ, Robertson B. Hyperpolarization-activated currents in presynaptic terminals of mouse cerebellar basket cells. J Physiol. 2000;526:91–97. doi: 10.1111/j.1469-7793.2000.t01-1-00091.x. 10.1111/j.1469-7793.2000.t01-1-00091.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yawo H. Noradrenaline modulates transmitter release by enhancing the Ca2+ sensitivity of exocytosis in the chick ciliary presynaptic terminal. J Physiol. 1996;493:385–391. doi: 10.1113/jphysiol.1996.sp021390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong N, Zucker RS. Roles of Ca2+, hyperpolarization and cyclic nucleotide-activated channel activation, and actin in temporal synaptic tagging. J Neurosci. 2004;24:4205–4212. doi: 10.1523/JNEUROSCI.0111-04.2004. 10.1523/JNEUROSCI.0111-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]