Abstract
Deconditioning is a risk factor for cardiovascular disease. Exercise reduces this risk, possibly by improving the vascular endothelial nitric oxide (NO) pathway. The effect of deconditioning on the NO pathway is largely unknown. This study was designed to assess baseline NO availability in the leg vascular bed after extreme, long-term deconditioning (spinal cord-injured individuals, SCI) as well as after moderate, short-term deconditioning (4 weeks of unilateral lower limb suspension, ULLS). For this purpose, seven SCI were compared with seven matched controls. Additionally, seven healthy subjects were studied pre- and post-ULLS. Leg blood flow was measured by venous occlusion plethysmography at baseline and during infusion of 5 incremental dosages of NG-monomethyl-l-arginine (l-NMMA) into the femoral artery. Sodium nitroprusside (SNP) was infused to test vascular responsiveness to NO. Baseline leg vascular resistance tended to be higher in SCI compared with controls (37 ± 4 versus 31 ± 2 arbitrary units (AU), P = 0.06). Deconditioning altered neither the vasoconstrictor response to l-NMMA (increase in resistance in SCI versus controls: 102 ± 33%versus 69 ± 9%; pre- versus post-ULLS: 95 ± 18%versus 119 ± 15%), nor the vascular responsiveness to NO. In conclusion, two human in vivo models of deconditioning show a preserved baseline NO availability in the leg skeletal muscle vascular bed.
The endothelium plays a crucial role in the regulation of vascular function and structure. Among the various mediators released by the endothelium, nitric oxide (NO) may be considered the most important vasodilator substance. The importance of the NO pathway is demonstrated by the strong link between endothelial dysfunction and cardiovascular disease (Widlansky et al. 2003). Various risk factors for cardiovascular disease, such as arterial hypertension, diabetes, smoking and hypercholesterolaemia, are associated with defects in the NO pathway (Widlansky et al. 2003).
Reduced baseline NO production has been demonstrated in patients with hypertension and their offspring (McAllister et al. 1999), in smokers (McVeigh et al. 1996), and in chronic heart failure patients (Yoshida et al. 1998). Exercise training enhances baseline NO production in healthy subjects (Kingwell et al. 1997), in patients with hypercholesterolaemia (Lewis et al. 1999), and in chronic heart failure patients (Hambrecht et al. 1998). As such, the positive effect of exercise on these conditions may be partly explained by augmentation of baseline NO availability.
A sedentary lifestyle is an independent risk factor for atherosclerosis and cardiovascular disease (Blair et al. 1995). Baseline blood flow to the deconditioned skeletal muscles is reduced (Takenaka et al. 1994; Kamiya et al. 2000; De Groot et al. 2003). In response to a reduction in blood flow, baseline NO synthesis may be attenuated, which triggers vascular remodelling and atherosclerosis (Rudic et al. 1998, 2000). Accordingly, Kamiya et al. (2000) showed a decrease in plasma nitrite/nitrate concentration, an indicator of endogenous NO production, after head-down bed rest, suggesting a diminished release of endothelial NO. Therefore, we hypothesize that the contribution of NO to baseline vascular tone is reduced in deconditioned skeletal muscle. We tested this hypothesis in two human in vivo models of inactivity. The first model concerns spinal cord-injured individuals (SCI). SCI offer a unique model of nature to assess peripheral vascular adaptations to inactivity. In these individuals the part of the body below the level of the lesion is subject to extreme and long-term deconditioning. Extensive vascular adaptations, including a reduction in baseline blood flow, occur in the leg vascular bed in SCI (De Groot et al. 2003; Kooijman et al. 2003). However, the legs of SCI are not only subject to extreme deconditioning, but also to denervation. Therefore, a second model was used: unilateral lower limb suspension (ULLS). This model of deconditioning is less extreme and limited in duration, but is not confounded by denervation. The ULLS model (Berg et al. 1991) is based on the avoidance of all weight-bearing activities of one leg, while the subject uses crutches for locomotion. The ULLS model induces muscle atrophy and a decrease in muscle strength (Berg et al. 1991; Dudley et al. 1992; Berg & Tesch, 1996; Schulze et al. 2002).
The purpose of this study was to assess the effect of extreme, long-term (SCI) and moderate short-term (ULLS) deconditioning on the contribution of NO to baseline vascular tone in the human leg skeletal muscle vascular bed. To address this issue increasing dosages of NG-monomethyl-l-arginine (l-NMMA, a blocker of NO synthase) and sodium nitroprusside (SNP, a NO donor) were infused into the femoral artery in spinal cord-injured individuals and matched controls, and in subjects before and after 4 weeks of ULLS.
Methods
Subjects
In total, 19 subjects participated and underwent the same tests to assess the contribution of NO to baseline vascular tone. In a first study, seven male SCI, with extreme, long-term deconditioning, were compared with seven controls, matched for gender and age. In a second study, seven healthy subjects (three males, four females) were measured twice, once before and once 4 weeks after short-term deconditioning by ULLS. Two of these subjects also served as controls for the SCI.
SCI suffered from a complete motor and sensor spinal cord lesion of traumatic origin varying from cervical 5 to thoracic 12 (American Spinal Injury Association ASIA A). The level of the spinal lesion was assessed by clinical examination. One of the SCI used baclofen (10 mg daily) throughout the study. Three females in the ULLS study used oral contraceptives. During their pre-ULLS and post-ULLS measurements, all females were in the same phase of their menstrual or contraceptive pill cycle.
All subjects met the inclusion criteria: age 18–50 years; non-smokers; diastolic blood pressure below 90 mmHg; normal fasting glucose, cholesterol, and triglyceride values. Thus, individuals with the most important risk factors that could affect endothelial function, in particular baseline NO production, were excluded from the study. Baseline characteristics are shown in Table 1. In the ULLS study, subjects exercised 2.1 ± 0.7 h per week. Exercise in the SCI (4.9 ± 0.8 h per week) consisted of voluntary arm exercise. This type of upper body exercise is limited by the amount of active muscle mass (Hopman et al. 1998) and does not affect the leg vasculature (Huonker et al. 1998). The hospital ethics committee approved the study. All subjects gave their written informed consent prior to the study. The study conforms with the principles outlined in the Declaration of Helsinki.
Table 1.
Baseline characteristics
| Extreme deconditioning | Moderate deconditioning | ||
|---|---|---|---|
| SCI (n = 7) | Controls (n = 7) | ULLS subjects (n = 7) | |
| Age (years) | 38 ± 2 | 32 ± 5 | 24 ± 2 |
| Body mass (kg) | 71.1 ± 5.4 | 81.4 ± 3.6* | 71.8 ± 4.6 |
| Upper leg volume (l) | 5.0 ± 0.3 | 7.1 ± 0.4† | 6.7 ± 0.5 |
| Systolic blood pressure (mmHg) | 123 ± 6 | 124 ± 4 | 114 ± 2 |
| Diastolic blood pressure (mmHg) | 76 ± 2 | 83 ± 3 | 77 ± 3 |
| Triglycerides (mmol l−1) | 1.3 ± 0.3‡ | 1.4 ± 0.3 | 1.2 ± 0.2 |
| Cholesterol (mmol l−1) | 4.6 ± 0.4‡ | 4.5 ± 0.3 | 4.4 ± 0.2 |
| Glucose (mmol l−1) | 4.5 ± 0.3 | 4.7 ± 0.1 | 4.9 ± 0.1 |
| Exercise (hours per week) | 4.9 ± 0.8 | 3.9 ± 0.8 | 2.1 ± 0.7 |
Values represent means ± s.e.m.
P = 0.047 versus SCI.
P = 0.006 versus SCI.
Data are from 6 subjects.
Experimental procedures
Leg blood flow measurement
All subjects fasted overnight, and refrained from caffeine and alcohol for 24 h. All subjects emptied their bladder before testing to minimize the influence of reflex sympathetic activation on vascular tone. All tests were performed in the morning with the subjects in a supine position in a quiet temperature-controlled room (23–24°C).
The technique of leg blood flow measurements in combination with drug infusions into the femoral artery has been previously described (Kooijman et al. 2003). A cannula (Angiocath 16 gauge, Becton Dickinson, Sandy, Utah, USA) was introduced into the femoral artery of the leg using a modified Seldinger technique. The intra-arterial cannula was used for drug administration and for blood pressure measurement. Heart rate was derived from the electrocardiogram. In control and ULLS subjects, local anaesthesia (4 ml lignocaine (lidocaine) 20 mg ml−1) was applied. Because of the lack of sensibility no anaesthesia was used in SCI.
Bilateral upper leg blood flow was measured by electrocardiography-triggered venous occlusion plethysmography, using mercury-in-silastic strain gauges placed approximately 10 cm proximal to the patella. We have previously shown that the reproducibility of upper leg blood flow measurements is good (Thijssen et al. 2005a). The thigh cuffs were simultaneously inflated to 50 mmHg using a rapid cuff inflator (Hokanson E-20, D.E. Hokanson, Bellevue, Washington, USA) (Groothuis et al. 2003). Cuffs below the knee were inflated to suprasystolic levels (>200 mmHg) in order to occlude the calf circulation. This way, the use of high doses of drugs, with subsequent systemic effects, could be minimized. To prevent discomfort, infusions were interrupted every 10 min and the calf circulation was restored for 5 min.
Drug infusion protocol
The drug infusion protocol is represented at the bottom of Fig. 1. The measurements started at least 30 min after cannulation of the femoral artery. Each drug dose was administered for 5 min. First, baseline leg blood flow was measured during saline (NaCl 0.9%) infusion. Subsequently l-NMMA was infused into the femoral artery at incremental doses of 0.025–0.05–0.1–0.2–0.4 mg min−1 (dl of upper leg volume)−1. In a pilot study with doses up to 0.8 mg min−1 dl−1 maximal vasoconstriction was already achieved at 0.2 and 0.4 mg min−1 dl−1. Subsequently, glucose 5% was infused followed by infusion of increasing dosages of the NO donor sodium nitroprusside (SNP, 0.06–0.2–0.6 μg min−1 dl−1). SNP was infused to explore differences in smooth muscle sensitivity to exogenous NO. A one-hour washout period was scheduled before angiotensin II infusion. Angiotensin II (0.25–0.5–2.0 ng min−1 dl−1) served as a control vasoconstrictor to detect differences in vasoconstrictor capacity due to structural vascular changes induced by deconditioning. During the whole protocol, infusion rate was kept constant at a volume rate of 10 μl min−1 dl−1.
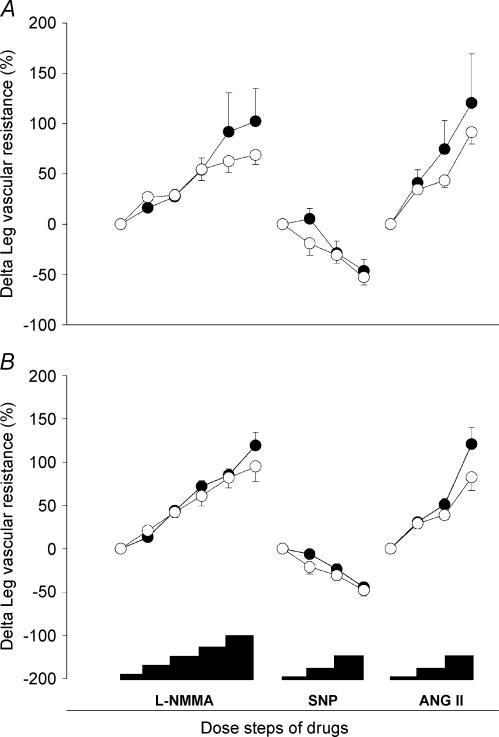
Figure 1. Change in leg vascular resistance in response to l-NMMA (0.025–0.05–0.1–0.2–0.4 mg min−1 dl−1 of leg tissue), SNP (0.06–0.2–0.6 μg min−1 dl−1), and angiotensin II (Ang II 0.25–0.5–2.0 ng min−1 dl−1).
A, spinal cord-injured individuals versus matched controls (• and ○, respectively). B, healthy subjects before and after ULLS (○ and •, respectively). Data represent means and s.e.m.
Drugs and solutions
l-NMMA and angiotensin II (both from Clinalfa, Läuflingen, Switzerland) were dissolved in saline at the beginning of each experiment. SNP (department of Clinical Pharmacy, Radboud University Nijmegen Medical Centre) was dissolved in 5% glucose and protected against light.
Upper leg volume measurement
Upper leg volume was determined by anthropometry as described by Jones & Pearson (1969).
ULLS protocol
In seven subjects, the right leg was exposed to deconditioning induced by 4 weeks of ULLS. We used a ULLS model very similar to the original description by Berg (Berg et al. 1991). The right leg was suspended by attachment of a sling to a non-rigid ankle brace and to a harness on the upper body, and unloaded from all weight bearing. Sole elevation of the contralateral foot was not used, because it produced instability of the leg. The harness was used during all locomotor activity, and the subjects used crutches for walking. Instructions were provided to minimize muscle activity of the suspended leg. Compliance was monitored with a diary, weekly interviews, and measurement of leg skin temperature. Skin temperature was consistently 1–2°C lower in the unloaded thigh and calf versus the control leg.
Strength measurement
In order to test the effectiveness of ULLS to induce deconditioning, the strength of the quadriceps muscle was quantified. Maximum voluntary contraction (Newtons) of the quadriceps muscle of both legs was assessed with an isometric quadriceps dynamometer (Gerrits et al. 2001b) before and after 4 weeks of ULLS. The hips and knees were positioned at 90 deg and 60 deg of flexion, respectively. The highest obtained result of three consecutive measurements represented the maximum voluntary contraction. This method has an acceptable reproducibility with a coefficient of variance of 10.2%.
Data analysis
Upper leg blood flow in ml min−1 dl−1 was calculated as the slope of the plethysmographic volume curve, as previously described (Kooijman et al. 2003). Leg blood flow values of the final 2 min of each 5-minute infusion period were averaged.
Blood pressure significantly changed during the course of the experiment. Therefore, upper leg vascular resistance (LVR) was calculated as mean arterial pressure (MAP, in mmHg) divided by leg blood flow in ml min−1 dl−1 and expressed in arbitrary units (AU = mmHg·min ml−1 dl−1). For these calculations, we assumed that central venous pressure was low and remained constant throughout the protocol.
We used the first baseline measurement (during saline infusion) to calculate the percentage change in outcome parameters during infusion of l-NMMA and SNP. Glucose 5% and SNP were infused immediately after infusion of l-NMMA. The NO donor SNP directly affects the vascular smooth muscle cell and overrules the effect of l-NMMA, so the effect of l-NMMA during infusion of SNP can be ignored. To calculate the percentage change during angiotensin II infusion, the baseline measurement directly prior to angiotensin II infusion was used. In order to control for changes in vasoconstrictor capacity by deconditioning, the vasoconstrictor response to l-NMMA was normalized to the average vasoconstrictor response to angiotensin II.
Statistics
Results represent means ± s.e.m. Differences in baseline characteristics between SCI and controls were tested using the Mann–Whitney U-test. Changes in strength, leg volume, and body mass after ULLS were tested with Wilcoxon signed rank test. For SCI and matched controls, differences in the response to infusion of drugs were analysed using two-factor repeated measures ANOVA with the drug dose as within subject factor and the presence of a spinal cord lesion as between group factor. For ULLS, a two-factor repeated measures ANOVA was used with the drug dose and pre- or post-ULLS as within subject factors. Differences in response between drug doses were tested with the least significant difference post hoc test (Statistical Package for Social Sciences (SPSS) 11.0). Differences were considered to be statistically significant at a two-sided P-value of less than 0.05.
Results
Effects of extreme, long-term deconditioning (SCI) on baseline parameters
SCI and matched controls did not differ with respect to age and blood pressure. Related to their spinal cord lesion, thigh volume and body mass were lower in SCI compared with controls (Table 1). Baseline leg blood flow tended to be lower (2.8 ± 0.5 versus 3.3 ± 0.2 ml min−1 dl−1, P = 0.064) and LVR tended to be higher (37 ± 4 versus 31 ± 2 AU, P = 0.064) in SCI compared with controls.
Effects of moderate, short-term deconditioning (ULLS) on baseline parameters
Body mass and thigh volume did not change after ULLS (pre versus post: 71.8 ± 4.6 versus 72.1 ± 4.6 kg, and 6.7 ± 0.5 versus 6.7 ± 0.5 l, respectively). After 4 weeks of ULLS, strength of the quadriceps muscle decreased by 22.2 ± 8.3% (P < 0.05). Strength of the control leg quadriceps muscle did not change (−1.8 ± 14.0%). ULLS did not alter baseline leg blood flow, or LVR (pre versus post: 3.9 ± 0.7 versus 4.5 ± 0.9 ml min−1 dl−1 and 26 ± 3 versus 23 ± 3 AU, respectively).
Response to l-NMMA
Leg vascular resistance increased significantly in all groups during l-NMMA infusion (P < 0.01). The responses of LVR to l-NMMA, expressed as percentage of baseline LVR, were not different between SCI (extreme, long-term deconditioning) and matched controls (Fig. 1A). The response of LVR to l-NMMA in SCI was also compared to the data of all 12 controls (including five additional control subjects from the pre-ULLS study), but again no difference in response was detected. Likewise, moderate short-term deconditioning (ULLS), did not affect the response of LVR to infusion of l-NMMA (Fig. 1B). All these observations were similar if the absolute instead of the relative (%) changes in the LVR were analysed. After normalizing the response to l-NMMA to the individual mean response to angiotensin II (for data of angiotensin II response see next paragraph), again no differences were observed between SCI and controls, nor between pre- and post-ULLS.
In SCI, the matched controls and in the pre-ULLS tests, the maximal vasoconstrictor response to l-NMMA was already achieved at the dose of 0.2 mg min−1 dl−1. As a consequence, doubling of the dose to 0.4 mg min−1 dl−1 did not induce a further vasoconstrictor response, indicating that maximal NO inhibition was achieved. In the post-ULLS-tests, LVR increased further at the dose of 0.4 mg min−1 dl−1 (P = 0.031). However, pilot studies in healthy volunteers showed that increasing the l-NMMA dose to 0.8 mg min−1 dl−1 did not further increase LVR (LVR 46 ± 4, 45 ± 5, and 48 ± 4 AU during 0.2, 0.4 and 0.8 mg l-NMMA min−1 dl−1, respectively, n = 3).
l-NMMA induced a significant and dose-dependent increase in MAP (P < 0.001), which did not significantly differ between SCI and controls, and between pre- and post-ULLS tests (Tables 2 and 3). SCI and their controls and the ULLS subjects showed a decrease in HR during l-NMMA infusion (Tables 2 and 3).
Table 2.
Effect of l-NMMA, SNP and angiotensin II on blood pressure, heart rate, and vascular tone in SCI and controls
| SCI (n = 7) | Controls (n = 7) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAP | HR | LBF | LVR | MAP | HR | LBF | LVR | |||
| Drug (Does min−1 dl−1 | Infusion | Control | Infusion | Control | ||||||
| l-NMMA | ||||||||||
| Saline | 91 ± 4 | 57 ± 4 | 2.8 ± 0.5 | 37 ± 4 | 34 ± 5 | 98 ± 4 | 57 ± 3 | 3.3 ± 0.2 | 31 ± 2 | 33 ± 2 |
| 0.025 mg | 91 ± 4 | 58 ± 5 | 2.4 ± 0.4 | 43 ± 5 | 36 ± 4 | 100 ± 5 | 56 ± 3 | 2.6 ± 0.2 | 40 ± 3 | 35 ± 2 |
| 0.05 mg | 94 ± 4 | 56 ± 5 | 2.2 ± 0.4 | 47 ± 5 | 33 ± 4 | 99 ± 4 | 58 ± 3 | 2.6 ± 0.1 | 40 ± 3 | 35 ± 2 |
| 0.1 mg | 95 ± 5 | 55 ± 6 | 1.9 ± 0.3 | 56 ± 7 | 36 ± 5 | 100 ± 4 | 57 ± 3 | 2.3 ± 0.2 | 47 ± 4 | 33 ± 3 |
| 0.2 mg | 96 ± 4 | 53 ± 4 | 1.8 ± 0.3 | 71 ± 16 | 37 ± 6 | 102 ± 4 | 55 ± 3 | 2.2 ± 0.1 | 49 ± 3 | 33 ± 3 |
| 0.4 mg | 101 ± 4 | 54 ± 4 | 1.6 ± 0.2 | 71 ± 11 | 39 ± 5 | 105 ± 4 | 57 ± 3 | 2.1 ± 0.1 | 52 ± 3 | 30 ± 2 |
| * | * | * | * | † | * | * | * | * | ‡ | |
| SNP | ||||||||||
| Glucose | 104 ± 4 | 53 ± 4 | 2.0 ± 0.2 | 54 ± 5 | 40 ± 7 | 109 ± 3 | 55 ± 5 | 2.9 ± 0.2 | 39 ± 2 | 30 ± 3 |
| 0.06 μg | 105 ± 4 | 52 ± 5 | 3.1 ± 0.5 | 37 ± 4 | 42 ± 5 | 106 ± 4 | 58 ± 4 | 4.9 ± 0.6 | 25 ± 4 | 34 ± 3 |
| 0.2 μg | 92 ± 4 | 56 ± 5 | 4.4 ± 0.8 | 25 ± 3 | 35 ± 5 | 101 ± 5 | 58 ± 4 | 5.3 ± 0.6 | 21 ± 3 | 39 ± 6 |
| 0.6 μg | 83 ± 4 | 64 ± 5 | 5.5 ± 1.0 | 18 ± 2 | 33 ± 7 | 96 ± 5 | 67 ± 5 | 7.7 ± 0.9 | 15 ± 3 | 35 ± 6 |
| * | * | * | * | * | * | * | * | |||
| Ang II | ||||||||||
| Saline | 103 ± 4 | 56 ± 5 | 2.2 ± 0.4 | 56 ± 12 | 43 ± 7 | 105 ± 4 | 60 ± 5 | 3.1 ± 0.4 | 37 ± 5 | 34 ± 3 |
| 0.25 ng | 106 ± 3 | 56 ± 5 | 1.7 ± 0.3 | 80 ± 18 | 46 ± 9 | 109 ± 5 | 61 ± 6 | 2.4 ± 0.3 | 51 ± 6 | 34 ± 3 |
| 0.5 ng | 106 ± 4 | 55 ± 5 | 1.5 ± 0.3 | 105 ± 32 | 55 ± 20 | 109 ± 5 | 63 ± 6 | 2.3 ± 0.2 | 53 ± 6 | 33 ± 3 |
| 2.0 ng | 112 ± 5 | 57 ± 6 | 1.4 ± 0.3 | 124 ± 36 | 55 ± 17 | 112 ± 5 | 62 ± 5 | 1.8 ± 0.2 | 73 ± 12 | 32 ± 4 |
| * | * | * | * | * | * | |||||
MAP, mean arterial pressure (mmHg); HR, heart rate (beats min−1); LBF, leg blood flow (ml min−1 dl−1); LVR, leg vascular resistance (AU); Infusion, the infused leg; Control, the non-infused leg. Ang II, angiotensin II.
Significant dose effect for both groups(P < 0.05, two-way-ANOVA).
Trend to significant interaction for dose × group (P = 0.069).
Significant dose effect(P<0.05, one-way ANOVA).
Table 3.
Effect of l-NMMA, SNP and angiotensin II on blood pressure, heart rate, and vascular tone pre- and post-ULLS
| Pre-ULLS (n = 7) | Post-ULLS (n = 7) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MAP | HR | LBF | LVR | MAP | HR | LBF | LVR | |||
| Drug dose (Does min−1 dl−1 | Infusion | Control | Infusion | Control | ||||||
| l-NMMA | ||||||||||
| Saline | 90 ± 2 | 62 ± 3 | 3.9 ± 0.7 | 26 ± 3 | 29 ± 4 | 90 ± 2 | 67 ± 4 | 4.5 ± 0.9 | 23 ± 3 | 26 ± 3 |
| 0.025 mg | 92 ± 2 | 62 ± 3 | 3.3 ± 0.6 | 32 ± 4 | 30 ± 4 | 91 ± 2 | 67 ± 4 | 4.2 ± 0.9 | 27 ± 4 | 28 ± 4 |
| 0.05 mg | 92 ± 2 | 60 ± 3 | 2.8 ± 0.4 | 37 ± 4 | 30 ± 4 | 93 ± 2 | 65 ± 3 | 3.3 ± 0.7 | 34 ± 5 | 30 ± 5 |
| 0.1 mg | 94 ± 2 | 63 ± 3 | 2.5 ± 0.3 | 41 ± 4 | 28 ± 4 | 95 ± 3 | 66 ± 4 | 2.8 ± 0.5 | 40 ± 6 | 29 ± 4 |
| 0.2 mg | 97 ± 2 | 60 ± 3 | 2.2 ± 0.2 | 46 ± 4 | 30 ± 4 | 96 ± 2 | 63 ± 4 | 2.6 ± 0.4 | 43 ± 6 | 30 ± 5 |
| 0.4 mg | 99 ± 2 | 60 ± 3 | 2.1 ± 0.2 | 49 ± 4 | 28 ± 3 | 99 ± 3 | 64 ± 3 | 2.3 ± 0.3 | 51 ± 7 | 29 ± 5 |
| * | * | * | * | * | * | * | * | |||
| SNP | ||||||||||
| Glucose | 101 ± 2 | 61 ± 4 | 3.0 ± 0.3 | 36 ± 3 | 27 ± 4 | 98 ± 2 | 59 ± 3 | 2.9 ± 0.4 | 37 ± 4 | 28 ± 4 |
| 0.06 μg | 100 ± 1 | 62 ± 4 | 5.7 ± 0.9 | 21 ± 3 | 28 ± 4 | 98 ± 2 | 65 ± 4 | 5.3 ± 0.9 | 22 ± 3 | 29 ± 4 |
| 0.2 μg | 95 ± 2 | 62 ± 3 | 6.3 ± 1.4 | 19 ± 3 | 30 ± 3 | 93 ± 2 | 66 ± 4 | 6.4 ± 1.5 | 18 ± 3 | 31 ± 4 |
| 0.6 μg | 90 ± 1 | 72 ± 4 | 8.0 ± 1.5 | 14 ± 3 | 34 ± 5 | 90 ± 3 | 72 ± 3 | 8.3 ± 1.7 | 13 ± 2 | 35 ± 6 |
| * | * | * | * | * | * | * | * | * | * | |
| Ang II | ||||||||||
| Saline | 97 ± 2 | 65 ± 4 | 3.5 ± 0.6 | 32 ± 4 | 31 ± 4 | 97 ± 2 | 66 ± 5 | 4.4 ± 1.1 | 31 ± 6 | 32 ± 7 |
| 0.25 ng | 101 ± 2 | 67 ± 3 | 2.9 ± 0.5 | 41 ± 5 | 28 ± 5 | 101 ± 3 | 71 ± 4 | 3.5 ± 0.8 | 40 ± 8 | 29 ± 6 |
| 0.5 ng | 100 ± 2 | 66 ± 4 | 2.6 ± 0.4 | 44 ± 5 | 26 ± 4 | 99 ± 2 | 69 ± 4 | 2.9 ± 0.8 | 46 ± 8 | 29 ± 6 |
| 2.0 ng | 106 ± 3 | 68 ± 4 | 2.1 ± 0.2 | 55 ± 5 | 27 ± 5 | 105 ± 2 | 72 ± 5 | 2.0 ± 0.4 | 63 ± 10 | 28 ± 6 |
| * | * | * | * | * | * | * | * | |||
MAP, mean arterial pressure (mmHg); HR, heart rate (beats min−1); LBF, leg blood flow (ml min−1 dl−1); LVR, leg vascular resistance (AU); Infusion, the infused leg; Control, the non-infused leg. Ang II, angiotensin II.
Significant dose effect for both groups (P <0.05, two-way ANOVA).
Response to SNP
Neither the absolute nor the relative (%) response of LVR to SNP differed between SCI and controls (Table 2, Fig. 1A). This also holds for the pre- versus post-ULLS response to SNP (Table 3, Fig. 1B). MAP decreased in all groups during the higher dosages of SNP (P < 0.001, Tables 2 and 3).
Response to angiotensin II
The absolute and relative (%) responses of LVR to angiotensin II (Table 2, Fig. 1) were not different in SCI compared with controls. The absolute responses of LVR to angiotensin II (Table 3) was not different in pre- versus post-ULLS. In all groups, MAP increased significantly during the higher dosages of angiotensin II (P < 0.001, Tables 2 and 3). This increase in MAP was not different in SCI versus controls nor in pre-ULLS versus post-ULLS. HR did not change significantly in either group.
Adverse effect of ULLS
Originally eight subjects participated in the ULLS protocol. One subject developed a deep venous thrombosis of the suspended leg during ULLS and was excluded from the study. We have reported separately on this serious adverse effect of ULLS and have proposed precautionary measures (Bleeker et al. 2004).
Discussion
The present study showed that, in contrast to our hypothesis, the contribution of NO to baseline vascular tone is not affected by deconditioning of human skeletal muscle. This is remarkable since exercise increases the contribution of NO to baseline vascular tone (Kingwell et al. 1997). Therefore, the effects of deconditioning are not the inverse of the effects of exercise training. Preserved contribution of NO to baseline vascular tone was confirmed in both extreme, long-term (SCI) and moderate, short-term (ULLS) deconditioning. Infusion of l-NMMA into the femoral artery increased basal LVR to a similar extent in SCI versus matched controls, as well as in pre- versus post-ULLS. Second, the response to the NO donor sodium nitroprusside was similar in SCI versus controls, and in pre- versus post-ULLS, indicating equal smooth muscle responsiveness to NO. Third, the vasoconstrictor response to angiotensin II did not differ between SCI and controls or between pre- and post-ULLS, indicating that there were no non-specific changes in vasoconstrictive capacity in either of the models. The latter observation is further strengthened by the fact that the response to l-NMMA normalized to the individual mean angiotensin II response did not differ in SCI versus controls, nor in pre- versus post-ULLS.
Deconditioning below the spinal cord lesion is an important consequence in SCI. In the SCI subjects in this study, leg volume was lower, indicating muscle atrophy, leg blood flow tended to be lower and LVR tended to be higher compared with controls. This is in accordance with previous observations of a significantly higher leg vascular resistance in SCI as compared with controls (Hopman et al. 2002). While in our study, ULLS did not lower baseline leg blood flow, as assessed by plethysmography, we are confident that deconditioning did occur. Using echo Doppler ultrasound, we demonstrated that the diameter of the common and superficial femoral artery significantly decreased after ULLS in the same group of subjects, while overall blood flow in these arteries was unchanged (Bleeker et al. 2005). This corresponds with ultrasound data on diameter and blood flow in SCI (De Groot et al. 2004). Changes in diameter without alterations in baseline blood flow have also been shown in training studies, where an increase in femoral artery diameter occurred without changes in baseline blood flow (Dinenno et al. 2001). Finally, since there was a significant decrease of 22% in strength of the quadriceps muscle, the ULLS model evidently caused deconditioning of the leg. This decrease in maximum voluntary contraction closely agrees with previous reports of a 13–21% reduction after 10 days to 6 weeks of unloading, which was invariably accompanied by muscular atrophy as assessed by magnetic resonance imaging (MRI) or computed tomography (CT) measurements (Dudley et al. 1992; Hather et al. 1992; Ploutz-Snyder et al. 1995; Berg & Tesch, 1996; Schulze et al. 2002).
We assessed the contribution of NO to baseline vascular tone by quantifying the vasoconstrictor response to l-NMMA. Fundamental to this technique is achievement of maximal inhibition of NO synthase. In our SCI, their matched controls, and in the pre-ULLS-tests, LVR did not further increase during the final l-NMMA dose, which indicates that a maximal vasoconstrictor effect was achieved. This maximal vasoconstrictor response to l-NMMA (l-NMMA caused a 35–40% decrease in leg blood flow in the controls of the SCI study and before ULLS) is very similar to a 31% decrease in forearm blood flow when l-NMMA is infused into the brachial artery of healthy subjects (Vervoort et al. 1999). So, despite the dependent position of the leg to the heart during standing, the contribution of NO to baseline vascular tone seems comparable in the vascular beds of the leg and arm. The maximal dose of l-NMMA used in previous studies was comparable to our 0.1 mg min−1 dl−1 dose (Green et al. 1997; Kingwell et al. 1997). If, in analogy with these studies, we limited the analysis to the lower three doses, our results and conclusion did not change. After ULLS, the vasoconstrictor response to the final l-NMMA dose (0.4 mg min−1 dl−1) was higher than with the previous dose (0.2 mg min−1 dl−1). Although this may indicate that maximal vasoconstriction was not achieved, data from a pilot study showed that the higher dose of 0.8 mg min−1 dl−1 of l-NMMA did not cause further vasoconstriction in the leg of healthy volunteers. Finally, if the vasoconstrictor response to l-NMMA was not maximal in the post-ULLS test, then this would point towards augmented NO-mediated effects by moderate deconditoning. Augmented NO-mediated effects by deconditioning would be a strong argument against our hypothesis.
The results of the present study indicate that short-term and long-term deconditioning of skeletal muscle does not reduce the contribution of NO to baseline vascular tone in humans. Therefore, the observed increase in vascular resistance after deconditioning (Takenaka et al. 1994; Kamiya et al. 2000; Hopman et al. 2002) cannot be explained by a reduced role of NO in baseline vascular tone. Training, i.e. the opposite of deconditioning, caused an increased basal NO production in the forearm vascular bed of both healthy individuals (Kingwell et al. 1997) and hypercholesterolaemic patients (Lewis et al. 1999; Walsh et al. 2003), and an increase in nitrite–nitrate level after 8 weeks of cycle training (Maeda et al. 2001). Since these studies suggest that exercise can improve baseline NO availability in humans, the results of the present study were unexpected. Data on the effects of long-term training (months–years) on endothelial function are limited. However, baseline NO production was similar in endurance-trained athletes and controls (Kingwell et al. 1996). It has been proposed that changes in endothelial function represent short-term adaptations to training and are eventually replaced by structural or other adaptations (Green et al. 2004a). This is illustrated by the observation that the endothelial function in forearm vessels of long-term tennis players was similar in the dominant and non-dominant arm (Green et al. 1996). One should take notice that these subjects played tennis for 13 h per week and that this may have resulted in concomitant training of the non-dominant arm.
Data on the effects of deconditioning on the NO pathway are scarce. In agreement with our results, deconditioning induced by casting for arm fractures, does not change the vasoconstrictor response to infusion of l-NMMA in the forearm in a cross-sectional study after cast removal (Green et al. 1997). Our data provide additional information, since they are derived from both a cross-sectional and a longitudinal intervention study, and are not biased by the effects of trauma and fracture healing on baseline blood flow. Plasma nitrite–nitrate concentration decreased in one bed rest study (Kamiya et al. 2000), suggesting an impaired endothelial NO production, while in another bed rest study no changes in urinary nitrite–nitrate excretion occurred (Bonnin et al. 2001). As compared with the nitrite–nitrate method, which reflects total body NO metabolism, our approach more specifically quantifies the role of NO in baseline vascular tone after deconditioning.
Previous animal studies have mimicked physical inactivity by hindlimb unloading, and report conflicting results. Some studies demonstrate a decreased endothelial nitric oxide synthase (eNOS) expression in the endothelial cells of both conductance and resistance vessels, and an attenuated maximal vasodilation to acetylcholine suggesting a decrease of endothelial NO release by hindlimb unloading (Jasperse et al. 1999; Delp et al. 2000; Schrage et al. 2000). In contrast, another study demonstrated no alterations of eNOS expression, but an increase in aortic inducible NOS (iNOS) content following hindlimb unloading (Vaziri et al. 2000).
After reduction of blood flow in animals by partial ligation of conduit arteries, baseline NO synthesis is reduced, which may trigger arterial remodelling and atherosclerosis (Rudic et al. 1998, 2000). However, the present study suggests that a reduction in blood flow or increase in vascular tone in humans is not explained by a diminished endogenous NO release under baseline conditions. Although, in the present study baseline NO synthesis is not affected, the NO release upon different stimuli may still play a role in arterial remodelling and in the vulnerability to atherosclerosis. Nevertheless, application of a stimulus is an artificial setting, since it is highly unlikely that the leg vascular bed of SCI is exposed to triggers that increase NO release. In this regard, it is interesting to realize that flow-mediated dilation of the superficial femoral artery, based on endothelial NO release in response to a shear stress stimulus, is preserved and seems to be enhanced in the chronically inactive legs of SCI (de Groot et al. 2004) and after 4 weeks of ULLS (Bleeker et al. 2005). However, these data are derived from a conduit artery in response to a shear stress stimulus and cannot predict the response to acetylcholine or comparable drugs on the arteriolar level (Green et al. 2004b). Moreover, in animals the short-term and long-term effects of exercise training on the NO pathway differ between conduit and resistance arteries (Laughlin et al. 2001).
In the SCI and the ULLS model, the physical inactivity is most prominent in the part of the body below the spinal cord lesion and in the suspended leg, respectively. One might argue that increased activity of the upper body and of the non-suspended leg could have systemic effects on endothelial function, masking the effect of inactivity on endothelial NO release. However, in the ULLS subjects the physical activity score tended to be lower after ULLS, arguing against a training effect of walking with crutches. It has been debated whether the effects of training on endothelial function are localized or systemic in nature. Leg training causes changes in baseline NO production (Kingwell et al. 1997) and improves endothelial function (Linke et al. 2001) in the arms. However, evidence exists that changes in endothelial function occur predominantly locally in the exercised limbs (Gokce et al. 2002; Allen et al. 2003; Kobayashi et al. 2003). In SCI neither systemic cardiovascular effects (Phillips et al. 1998) nor local vascular effects of deconditioning (Huonker et al. 1998) can be normalized with upper body training. Recently, Thijssen et al. (2005b) demonstrated that the adaptation to leg exercise is a local phenomenon and only occurs in the stimulated leg muscles. Collectively, these data provide evidence that upper arm exercise and even exercise of adjacent muscle by electrical stimulation does not affect the vasculature in the non-exercised leg muscles in SCI. Based on the previous arguments we believe that our results are not confounded by systemic effects of increased upper body physical activity.
In the present study we used spinal cord injury as a unique model of nature to investigate adaptations in the peripheral circulation in response to extreme deconditioning. As valuable as information is from this patient population, one should be cautious to extrapolate this information to the general population, since other conditions unique to spinal cord injury can influence the results, such as impaired supraspinal sympathetic control. However, recent human studies have shown that endothelial function does not change after chronic sympathectomy (Eisenach et al. 2002). In addition, adaptations in the circulatory system in SCI are reversible by functional electrostimulation training (Gerrits et al. 2001a; Hopman et al. 2002), providing more evidence that these adaptations primarily result from deconditioning. We used ULLS as a second model of deconditioning. Since healthy subjects participated in this part of the study, loss of supraspinal control was not a confounder. The observations on the NO pathway in the ULLS studies are in close agreement with the observations in the SCI study.
In conclusion, the results of the present study demonstrate a preserved contribution of NO to baseline vascular tone in deconditioned leg skeletal muscle in man.
Acknowledgments
We thank Jos Evers and Heleen Schijvens for support during measurements and data analysis. The contribution of G.A. Rongen has been made possible by a fellowship of the Royal Netherlands Academy of Arts and Sciences.
References
- Allen JD, Geaghan JP, Greenway F, Welsch MA. Time course of improved flow-mediated dilation after short-term exercise training. Med Sci Sports Exerc. 2003;35:847–853. doi: 10.1249/01.MSS.0000064931.62916.8A. [DOI] [PubMed] [Google Scholar]
- Berg HE, Dudley GA, Haggmark T, Ohlsen H, Tesch PA. Effects of lower limb unloading on skeletal muscle mass and function in humans. J Appl Physiol. 1991;70:1882–1885. doi: 10.1152/jappl.1991.70.4.1882. 10.1063/1.349490. [DOI] [PubMed] [Google Scholar]
- Berg HE, Tesch PA. Changes in muscle function in response to 10 days of lower limb unloading in humans. Acta Physiol Scand. 1996;157:63–70. doi: 10.1046/j.1365-201X.1996.476217000.x. [DOI] [PubMed] [Google Scholar]
- Blair SN, Kohl HW, 3rd, Barlow CE, Paffenbarger RS, Jr, Gibbons LW, Macera CA. Changes in physical fitness and all-cause mortality. A prospective study of healthy and unhealthy men. JAMA. 1995;273:1093–1098. [PubMed] [Google Scholar]
- Bleeker MW, De Groot PC, Poelkens F, Rongen GA, Smits P, Hopman MT. Vascular adaptation to 4 wk of deconditioning by unilateral lower limb suspension. Am J Physiol Heart Circ Physiol. 2005;288:H1747–H1755. doi: 10.1152/ajpheart.00966.2004. [DOI] [PubMed] [Google Scholar]
- Bleeker MW, Hopman MT, Rongen GA, Smits P. Unilateral lower limb suspension can cause deep venous thrombosis. Am J Physiol Regul Integr Comp Physiol. 2004;286:R1176–R1177. doi: 10.1152/ajpregu.00718.2003. [DOI] [PubMed] [Google Scholar]
- Bonnin P, Ben Driss A, Benessiano J, Maillet A, Pavy le Traon A, Levy BI. Enhanced flow-dependent vasodilatation after bed rest, a possible mechanism for orthostatic intolerance in humans. Eur J Appl Physiol. 2001;85:420–426. doi: 10.1007/s004210100483. [DOI] [PubMed] [Google Scholar]
- De Groot PCE, Poelkens F, Kooijman M, Hopman MTE. Preserved flow-mediated dilation in the inactive legs of spinal cord-injured individuals. Am J Physiol Heart Circ Physiol. 2004;287:H374–H380. doi: 10.1152/ajpheart.00958.2003. 10.1152/ajpheart.00958.2003. [DOI] [PubMed] [Google Scholar]
- De Groot PC, Van Kuppevelt DH, Pons C, Snoek G, Van Der Woude LH, Hopman MT. Time course of arterial vascular adaptations to inactivity and paralyses in humans. Med Sci Sports Exerc. 2003;35:1977–1985. doi: 10.1249/01.MSS.0000099088.21547.67. [DOI] [PubMed] [Google Scholar]
- Delp MD, Colleran PN, Wilkerson MK, McCurdy MR, Muller-Delp J. Structural and functional remodeling of skeletal muscle microvasculature is induced by simulated microgravity. Am J Physiol Heart Circ Physiol. 2000;278:H1866–H1873. doi: 10.1152/ajpheart.2000.278.6.H1866. [DOI] [PubMed] [Google Scholar]
- Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, Seals DR. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534:287–295. doi: 10.1111/j.1469-7793.2001.00287.x. 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley GA, Duvoisin MR, Adams GR, Meyer RA, Belew AH, Buchanan P. Adaptations to unilateral lower limb suspension in humans. Aviat Space Environ Med. 1992;63:678–683. [PubMed] [Google Scholar]
- Eisenach JH, Clark ES, Charkoudian N, Dinenno FA, Atkinson JL, Fealey RD, Dietz NM, Joyner MJ. Effects of chronic sympathectomy on vascular function in the human forearm. J Appl Physiol. 2002;92:2019–2025. doi: 10.1152/japplphysiol.01025.2001. [DOI] [PubMed] [Google Scholar]
- Gerrits HL, de Haan A, Sargeant AJ, van Langen H, Hopman MT. Peripheral vascular changes after electrically stimulated cycle training in people with spinal cord injury. Arch Phys Med Rehabil. 2001a;82:832–839. doi: 10.1053/apmr.2001.23305. 10.1053/apmr.2001.23305. [DOI] [PubMed] [Google Scholar]
- Gerrits HL, Hopman MTE, Sargeant AJ, de Haan A. Reproducibility of contractile properties of the human paralysed and non-paralysed quadriceps muscle. Clin Physiol. 2001b;21:105–113. doi: 10.1046/j.1365-2281.2001.00293.x. 10.1046/j.1365-2281.2001.00293.x. [DOI] [PubMed] [Google Scholar]
- Gokce N, Vita JA, Bader DS, Sherman DL, Hunter LM, Holbrook M, O'Malley C, Keaney J, John F, Balady GJ. Effect of exercise on upper and lower extremity endothelial function in patients with coronary artery disease. Am J Cardiol. 2002;90:124–127. doi: 10.1016/s0002-9149(02)02433-5. 10.1016/S0002-9149(02)02433-5. [DOI] [PubMed] [Google Scholar]
- Green DJ, Fowler DT, O'Driscoll JG, Blanksby BA, Taylor RR. Endothelium-derived nitric oxide activity in forearm vessels of tennis players. J Appl Physiol. 1996;81:943–948. doi: 10.1152/jappl.1996.81.2.943. [DOI] [PubMed] [Google Scholar]
- Green DJ, Maiorana A, O'Driscoll G, Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004a;561:1–25. doi: 10.1113/jphysiol.2004.068197. 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, O'Driscoll JG, Blanksby BA, Taylor RR. Effect of casting on forearm resistance vessels in young men. Med Sci Sports Exerc. 1997;29:1325–1331. doi: 10.1097/00005768-199710000-00008. [DOI] [PubMed] [Google Scholar]
- Green DJ, Walsh JH, Maiorana A, Burke V, Taylor RR, O'Driscoll JG. Comparison of resistance and conduit vessel nitric oxide-mediated vascular function in vivo: effects of exercise training. J Appl Physiol. 2004b;97:749–755. doi: 10.1152/japplphysiol.00109.2004. 10.1152/japplphysiol.00109.2004. [DOI] [PubMed] [Google Scholar]
- Groothuis JT, van Vliet L, Kooijman M, Hopman MT. Venous cuff pressures from 30 mmHg to diastolic pressure are recommended to measure arterial inflow by plethysmography. J Appl Physiol. 2003;95:342–347. doi: 10.1152/japplphysiol.00022.2003. [DOI] [PubMed] [Google Scholar]
- Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, Yu J, Adams V, Niebauer J, Schuler G. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation. 1998;98:2709–2715. doi: 10.1161/01.cir.98.24.2709. [DOI] [PubMed] [Google Scholar]
- Hather BM, Adams GR, Tesch PA, Dudley GA. Skeletal muscle responses to lower limb suspension in humans. J Appl Physiol. 1992;72:1493–1498. doi: 10.1152/jappl.1992.72.4.1493. [DOI] [PubMed] [Google Scholar]
- Hopman MT, Dueck C, Monroe M, Philips WT, Skinner JS. Limits to maximal performance in individuals with spinal cord injury. Int J Sports Med. 1998;19:98–103. doi: 10.1055/s-2007-971889. [DOI] [PubMed] [Google Scholar]
- Hopman MT, Groothuis JT, Flendrie M, Gerrits KH, Houtman S. Increased vascular resistance in paralyzed legs after spinal cord injury is reversible by training. J Appl Physiol. 2002;93:1966–1972. doi: 10.1152/japplphysiol.00897.2001. [DOI] [PubMed] [Google Scholar]
- Huonker M, Schmid A, Sorichter S, Schmidt-Trucksab A, Mrosek P, Keul J. Cardiovascular differences between sedentary and wheelchair-trained subjects with paraplegia. Med Sci Sports Exerc. 1998;30:609–613. doi: 10.1097/00005768-199804000-00020. [DOI] [PubMed] [Google Scholar]
- Jasperse JL, Woodman CR, Price EM, Hasser EM, Laughlin MH. Hindlimb unweighting decreases ecNOS gene expression and endothelium- dependent dilation in rat soleus feed arteries. J Appl Physiol. 1999;87:1476–1482. doi: 10.1152/jappl.1999.87.4.1476. [DOI] [PubMed] [Google Scholar]
- Jones PR, Pearson J. Anthropometric determination of leg fat and muscle plus bone Volumes in young male and female adults. J Physiol. 1969;204:63P–66P. [PubMed] [Google Scholar]
- Kamiya A, Iwase S, Michikami D, Fu Q, Mano T, Kitaichi K, Takagi K. Increased vasomotor sympathetic nerve activity and decreased plasma nitric oxide release after head-down bed rest in humans: disappearance of correlation between vasoconstrictor and vasodilator. Neurosci Lett. 2000;281:21–24. doi: 10.1016/s0304-3940(00)00804-1. 10.1016/S0304-3940(00)00804-1. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Sherrard B, Jennings GL, Dart AM. Four weeks of cycle training increases basal production of nitric oxide from the forearm. Am J Physiol Heart Circ Physiol. 1997;272:H1070–H1077. doi: 10.1152/ajpheart.1997.272.3.H1070. [DOI] [PubMed] [Google Scholar]
- Kingwell BA, Tran B, Cameron JD, Jennings GL, Dart AM. Enhanced vasodilation to acetylcholine in athletes is associated with lower plasma cholesterol. Am J Physiol Heart Circ Physiol. 1996;270:H2008–H2013. doi: 10.1152/ajpheart.1996.270.6.H2008. [DOI] [PubMed] [Google Scholar]
- Kobayashi N, Tsuruya Y, Iwasawa T, Ikeda N, Hashimoto S, Yasu T, Ueba H, Kubo N, Fujii M, Kawakami M, Saito M. Exercise training in patients with chronic heart failure improves endothelial function predominantly in the trained extremities. Circ J. 2003;67:505–510. doi: 10.1253/circj.67.505. 10.1253/circj.67.505. [DOI] [PubMed] [Google Scholar]
- Kooijman M, Rongen GA, Smits P, Hopman MT. Preserved alpha-adrenergic tone in the leg vascular bed of spinal cord-injured individuals. Circulation. 2003;108:2361–2367. doi: 10.1161/01.CIR.0000096480.55857.3C. [DOI] [PubMed] [Google Scholar]
- Laughlin MH, Pollock JS, Amann JF, Hollis ML, Woodman CR, Price EM. Training induces nonuniform increases in eNOS content along the coronary arterial tree. J Appl Physiol. 2001;90:501–510. doi: 10.1152/jappl.2001.90.2.501. [DOI] [PubMed] [Google Scholar]
- Lewis TV, Dart AM, Chin-Dusting JP, Kingwell BA. Exercise training increases basal nitric oxide production from the forearm in hypercholesterolemic patients. Arterioscler Thromb Vasc Biol. 1999;19:2782–2787. doi: 10.1161/01.atv.19.11.2782. [DOI] [PubMed] [Google Scholar]
- Linke A, Schoene N, Gielen S, Hofer J, Erbs S, Schuler G, Hambrecht R. Endothelial dysfunction in patients with chronic heart failure: systemic effects of lower-limb exercise training. J Am Coll Cardiol. 2001;37:392–397. doi: 10.1016/s0735-1097(00)01108-6. 10.1016/S0735-1097(00)01108-6. [DOI] [PubMed] [Google Scholar]
- McAllister AS, Atkinson AB, Johnston GD, Hadden DR, Bell PM, McCance DR. Basal nitric oxide production is impaired in offspring of patients with essential hypertension. Clin Sci (Lond) 1999;97:141–147. [PubMed] [Google Scholar]
- McVeigh GE, Lemay L, Morgan D, Cohn JN. Effects of long-term cigarette smoking on endothelium-dependent responses in humans. Am J Cardiol. 1996;78:668–672. doi: 10.1016/s0002-9149(96)00391-8. 10.1016/S0002-9149(96)00391-8. [DOI] [PubMed] [Google Scholar]
- Maeda S, Miyauchi T, Kakiyama T, Sugawara J, Iemitsu M, Irukayama-Tomobe Y, Murakami H, Kumagai Y, Kuno S, Matsuda M. Effects of exercise training of 8 weeks and detraining on plasma levels of endothelium-derived factors, endothelin-1 and nitric oxide, in healthy young humans. Life Sci. 2001;69:1005–1016. doi: 10.1016/s0024-3205(01)01192-4. 10.1016/S0024-3205(01)01192-4. [DOI] [PubMed] [Google Scholar]
- Phillips WT, Kiratli BJ, Sarkarati M, Weraarchakul G, Myers J, Franklin BA, Parkash I, Froelicher V. Effect of spinal cord injury on the heart and cardiovascular fitness. Curr Probl Cardiol. 1998;23:641–716. doi: 10.1016/s0146-2806(98)80003-0. [DOI] [PubMed] [Google Scholar]
- Ploutz-Snyder LL, Tesch PA, Crittenden DJ, Dudley GA. Effect of unweighting on skeletal muscle use during exercise. J Appl Physiol. 1995;79:168–175. doi: 10.1152/jappl.1995.79.1.168. [DOI] [PubMed] [Google Scholar]
- Rudic RD, Bucci M, Fulton D, Segal SS, Sessa WC. Temporal events underlying arterial remodeling after chronic flow reduction in mice: correlation of structural changes with a deficit in basal nitric oxide synthesis. Circ Res. 2000;86:1160–1166. doi: 10.1161/01.res.86.11.1160. [DOI] [PubMed] [Google Scholar]
- Rudic RD, Shesely EG, Maeda N, Smithies O, Segal SS, Sessa WC. Direct evidence for the importance of endothelium-derived nitric oxide in vascular remodeling. J Clin Invest. 1998;101:731–736. doi: 10.1172/JCI1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrage WG, Woodman CR, Laughlin MH. Hindlimb unweighting alters endothelium-dependent vasodilation and ecNOS expression in soleus arterioles. J Appl Physiol. 2000;89:1483–1490. doi: 10.1152/jappl.2000.89.4.1483. [DOI] [PubMed] [Google Scholar]
- Schulze K, Gallagher P, Trappe S. Resistance training preserves skeletal muscle function during unloading in humans. Med Sci Sports Exerc. 2002;34:303–313. doi: 10.1097/00005768-200202000-00019. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Suzuki Y, Kawakubo K, Haruna Y, Yanagibori R, Kashihara H, Igarashi T, Watanabe F, Omata M, Bonde-Petersen F, et al. Cardiovascular effects of 20 days bed rest in healthy young subjects. Acta Physiol Scand Suppl. 1994;616:59–63. [PubMed] [Google Scholar]
- Thijssen DH, Bleeker MW, Smits P, Hopman MT. Reproducibility of blood flow and post-occlusive reactive hyperaemia as measured by venous occlusion plethysmography. Clin Sci (Lond) 2005a;108:151–157. doi: 10.1042/CS20040177. [DOI] [PubMed] [Google Scholar]
- Thijssen DHJ, Heesterbeek P, van Kuppevelt DJM, Duysens J, Hopman MTE. Local vascular adaptations after hybrid training in spinal cord-injured subjects. Med Sci Sports Exerc. 2005b doi: 10.1249/01.mss.0000170126.30868.fb. in press. [DOI] [PubMed] [Google Scholar]
- Vaziri ND, Ding Y, Sangha DS, Purdy RE. Upregulation of NOS by simulated microgravity, potential cause of orthostatic intolerance. J Appl Physiol. 2000;89:338–344. doi: 10.1152/jappl.2000.89.1.338. [DOI] [PubMed] [Google Scholar]
- Vervoort G, Wetzels JF, Lutterman JA, van Doorn LG, Berden JH, Smits P. Elevated skeletal muscle blood flow in noncomplicated type 1 diabetes mellitus: role of nitric oxide and sympathetic tone. Hypertension. 1999;34:1080–1085. doi: 10.1161/01.hyp.34.5.1080. [DOI] [PubMed] [Google Scholar]
- Walsh JH, Yong G, Cheetham C, Watts GF, O'Driscoll GJ, Taylor RR, Green DJ. Effects of exercise training on conduit and resistance vessel function in treated and untreated hypercholesterolaemic subjects. Eur Heart J. 2003;24:1681–1689. doi: 10.1016/s0195-668x(03)00384-1. 10.1016/S0195-668X(03)00384-1. [DOI] [PubMed] [Google Scholar]
- Widlansky ME, Gokce N, Keaney JF, Jr, Vita JA. The clinical implications of endothelial dysfunction. J Am Coll Cardiol. 2003;42:1149–1160. doi: 10.1016/s0735-1097(03)00994-x. 10.1016/S0735-1097(03)00994-X. [DOI] [PubMed] [Google Scholar]
- Yoshida H, Nakamura M, Akatsu T, Arakawa N, Hiramori K. Effects of nitric oxide inhibition on basal forearm blood flow in patients with nonischemic chronic heart failure. Heart Vessels. 1998;13:142–146. doi: 10.1007/BF01747831. [DOI] [PubMed] [Google Scholar]