Abstract
The mechanisms of sensory transduction of pulmonary reactive oxygen species (ROS) by capsaicin-sensitive vagal lung afferent fibres are unclear. To investigate the role of transient receptor potential vanilloid 1 (TRPV1) receptors and P2X purinoceptors in this sensory transduction, we recorded fibre activity (FA) from 132 fibres of this type in 132 anaesthetized and ventilated rats. Airway challenge of aerosolized H2O2 (0, 0.2 and 0.4%) produced a concentration-dependant fibre stimulation. The fibre responses to 0.4% H2O2 were attenuated by dimethylthiourea (a hydroxyl radical (·OH) scavenger; change in fibre activity (ΔFA), −55 ± 9%) or deferoxamine (an iron-chelator that prevents formation of ·OH; ΔFA, −59 ± 9%), were prevented by catalase (an enzyme catalysing H2O2; ΔFA, −96 ± 3%) and were unaffected by the vehicle for dimethylthiourea, iron-saturated deferoxamine or heat-inactivated catalase. The fibre responses to 0.4% H2O2 were attenuated by capsazepine (a TRPV1 receptor antagonist; ΔFA, −39 ± 9%) or iso-pyridoxalphosphate-6-azophenyl-2′,5′-disulphonate (iso-PPADS, a P2X receptor antagonist; ΔFA, −51 ± 9%), were further reduced by capsazepine and iso-PPADS in combination (ΔFA, −70 ± 13%), and were unaltered by their vehicles. The fibre responses to cigarette smoke (20 ml), an irritant that generates ROS, were attenuated by dimethylthiourea (ΔFA, −61 ± 9%) or capsazepine and iso-PPADS in combination (ΔFA, −67 ± 9%). These results suggest that both the TRPV1 and P2X receptors mediate the sensory transduction of ROS, especially H2O2 and ·OH, by capsaicin-sensitive vagal lung afferent fibres.
Lung pathological conditions such as endotoxin shock (Minamiya et al. 1995), vascular microembolism (Wang et al. 1992) and inhalation of oxidant irritants such as toxic or cigarette smoke (Pryor, 1992), cause increased pulmonary production of reactive oxygen species (ROS). The major ROS are the superoxide anion radical, hydrogen peroxide (H2O2) and hydroxyl radical (·OH) (Comhair & Erzurum, 2002). The superoxide anion radical dismutates to form H2O2, which in the presence of iron can further react to form ·OH via the Fenton reaction (Comhair & Erzurum, 2002). Several animal studies have shown that airway reflexes (Lee, 1990; Kou et al. 1997; Lin & Kou, 1997; Chen & Kou, 2000; Ho & Kou, 2000) and lung afferent responses (Chen et al. 1997; Lai & Kou, 1998a, b; Lai et al. 2005) evoked under these pathological conditions are abolished or attenuated by antioxidant treatments. These observations lead to the notion that ROS are part of a signalling cascade leading to stimulation of lung afferent fibres (Lee & Pisarri, 2001). The findings (Soukhova et al. 1999; Ruan et al. 2003) that exogenous H2O2 challenge evokes airway reflexes, which are mediated through lung vagal afferents, support this notion. However, there is no direct electrophysiological evidence demonstrating the stimulatory effect of ROS on lung vagal afferent fibres, and the mechanisms underlying this sensory transduction remain to be explored.
Capsaicin, a pungent active ingredient of hot pepper, mainly stimulates lung vagal afferent C fibres and some A-δ fibres, which are important to the regulation of respiratory and cardiovascular functions under both normal and pathophysiological conditions (Coleridge & Coleridge, 1986; Lee & Pisarri, 2001; Carr & Undem, 2003). These capsaicin-sensitive vagal lung afferent fibres are sensitive to a variety of chemicals or irritants and are considered as nociceptive-like free nerve endings (Coleridge & Coleridge, 1986; Lee & Pisarri, 2001; Carr & Undem, 2003). Investigations of afferent responses of these vagal fibres to certain agonists reveal that various pharmacological receptors may be present at the membrane of nerve terminals (Undem & Carr, 2001). For example, studies using whole animals (Lee & Lundberg, 1994; Pelleg & Hurt, 1996; Undem & Carr, 2001) or ex vivo airway and/or lung preparations (Fox et al. 1995; Undem & Carr, 2001; Kollarik & Undem, 2002; Carr et al. 2003; Undem et al. 2004) suggested that the transient receptor potential vanilloid 1 (TRPV1) receptors and the P2X purinoceptors play a role in sensory transduction functions of these afferent fibres. Indeed, in vitro electrophysiological and pharmacological studies have characterized TRPV1 and P2X receptors located on the membrane of jugular or nodose vagal neurones (Khakh et al. 1995; Vulchanova et al. 1997; Szallasi & Blumberg, 1999; Dunn et al. 2001; Gu et al. 2003; Ichikawa & Sugimoto, 2003; Undem et al. 2004). The importance of TRPV1 and P2X receptors to pain sensation has also been well documented in somatosensory nociceptors (McCleskey & Gold, 1999). It has been demonstrated that ROS can activate visceral afferent C fibres (Stahl et al. 1993; Ustinova & Schultz, 1994a,b; Huang et al. 1995). Previous investigations in rats suggested that the activation of vagal cardiac C fibres by ROS is mediated through the TRPV1 receptors (Schultz & Ustinova, 1998) and that ROS activate purinergic P2 receptors in aortic smooth muscle cells (Shen et al. 2000). Additionally, ROS may damage cells and cause a rapid release of cytosolic ATP, which activates the P2X receptors resulting in the stimulation of pain nociceptors (Cook & McCleskey, 2002). However, the role of TRPV1 and P2X receptors in the sensory transduction of ROS by capsaicin-sensitive vagal lung afferent fibres is still unclear.
Based upon the abovementioned existing knowledge, we hypothesized that both TRPV1 and P2X receptors located at fibre terminals mediate sensory transduction of ROS by capsaicin-sensitive vagal lung afferent fibres. To test this hypothesis, the present study was undertaken in anaesthetized rats to investigate: (1) the stimulatory effects of airway challenge of aerosolized H2O2 on these afferent fibres; (2) ROS mechanisms underlying H2O2-induced stimulation of these afferent fibres using various antioxidant pretreatments; (3) the involvements of TRPV1 and P2X receptors in H2O2-induced stimulation of these afferent fibres using selective receptor antagonist pretreatments; and (4) the importance of TRPV1 and P2X receptors in the afferent responses of these fibres to airway challenge of cigarette smoke, an irritant that is known to generate ROS (Pryor, 1992).
Methods
Animal preparation
All protocols were in accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals published by the National Institutes of Health, USA and were approved by the Institutional Animal Care and Use Committee of the National Yang-Ming University, Taiwan. Male Sprague-Dawley rats were anaesthetized with an intraperitoneal injection of α-chloralose (100 mg kg−1; Sigma Chemical Co., St Louis, MO, USA) and urethane (500 mg kg−1; Sigma) dissolved in a borax solution (2%; Sigma). A polyethylene catheter was inserted into the jugular vein and advanced until the tip was close to the right atrium for intravenous administration of pharmacological agents. The right femoral artery was cannulated to measure arterial blood pressure. During the course of the experiments, supplemental doses of α-chloralose (20 mg kg−1 h−1) and urethane (100 mg kg−1 h−1) were administered to maintain the abolition of pain reflexes induced by pinching the animal's tail. During the recording of vagal action potentials, the rats were paralysed with pancuronium bromide (0.5 mg kg−1, intravenous; Orgnon Teknika, Boxtel, Holland). Periodically, the effect of pancuronium was allowed to wear off so that the depth of anaesthesia could be checked. Body temperature was maintained at 37°C throughout the experiment by means of a servo-controlled heating blanket. At the end of the experiment, animals were killed by intravenous injection of overdose of the anaesthetics.
Methodologies for other animal preparations and measurements of physiological parameters used in this study have been previously described (Lai & Kou, 1998a; Lai et al. 2005). In brief, the trachea was cannulated and a midline thoracotomy was performed. The lungs were ventilated at a constant tidal volume of 9 ml kg−1 and a constant frequency of 50 breaths min−1 (Fig. 1). Arterial blood pressure, respiratory flow, tidal volume (VT) and tracheal pressure (Ptr; transpulmonary pressure in an open-chest preparation) were monitored. Total lung resistance (RL) and dynamic lung compliance (Cdyn) were determined using the subtraction method (Mead & Whittenberger, 1953).
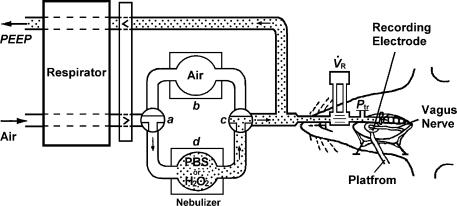
Figure 1. Schematic illustration showing the experimental setup.
During the control period, the respirator delivered room air to the lungs (via a–b–c). To initial the challenge, two 3-way stopcocks were turned quickly during the expiratory phase, so that aerosolized PBS or H2O2 was delivered to the lungs (via a–d–c). To record fibre activity, a fine afferent filament was split from the de-sheathed right nerve trunk lying on a platform and placed on a recording electrode.  , respiratory flow; Ptr, tracheal pressure; PEEP, positive end expiratory pressure.
, respiratory flow; Ptr, tracheal pressure; PEEP, positive end expiratory pressure.
Recording of afferent activity
Afferent activity arising from capsaicin-sensitive vagal lung afferent fibres was recorded using techniques described elsewhere (Lai & Kou, 1998a; Lai et al. 2005). Briefly, a fine afferent filament of the right vagus nerve was split from the de-sheathed right nerve trunk lying on a platform and placed on a platinum–iridium unipolar recording electrode to record afferent nerve activity (Fig. 1). To search for these afferent fibres, the lungs were hyperinflated in a step-like manner to four or five times VT. Capsaicin-sensitive vagal lung afferent fibres are activated by lung hyperinflation to a high volume (e.g. 3 or 4 ×VT) (Lee & Pisarri, 2001; Carr & Undem, 2003). Once the presence of a suspected single unit was detected, capsaicin (0.75 g kg−1; Sigma) was injected as a bolus into the vein. Only afferent fibres that showed stimulation within 2 s after the injection were studied. The recording of a single fibre was confirmed by matching spike templates. Prior to the end of each experiment, the general locations of the receptors studied were identified within the lung structure by gently probing the tissues with a polyethylene rod (diameter, 2 mm). The conduction velocities of these afferent fibres were not measured due to the limitation of the technique with respect to the experimental set-up (Fig. 1).
Generation and delivery of aerosolized H2O2
Various concentrations (0% (PBS), 0.2% and 0.4%) of a H2O2 solution were prepared just prior to each set of experiments by mixing 35% H2O2 (Shimakyu, Osaka, Japan) with PBS to the desired concentration with the pH adjusted to 7.4. H2O2 aerosol was generated by an active ultrasonic nebulizer (ULTRA-NEB 99, DeVilbiss) containing the H2O2 solution. The particle sizes of the aerosol generated by this nebulizer ranged from 0.5 to 5 µm. The air delivered by the respirator was then directed into a nebulizer cup containing no solution, PBS or an H2O2 solution, as controlled by turning a 3-way stopcock (Fig. 1). These two cups were well sealed to prevent any leakage of air. The outlets to these two cups merged into one piece of tubing (i.d., 8 mm) via another 3-way stopcock, which was connected to the distal end of the trachea cannula (Fig. 1). Airway exposure to aerosolized H2O2 was achieved by adjusting these two 3-way stopcocks for a 30-s period. Using a dye tracer, the time lag between the onset of challenge and the arrival of the aerosolized tracer in the airways was estimated to be 1–2 s. This estimation was based upon post-mortem checks of the presence of the dye tracer in the airways in 10 animals whose tracheal tubes were quickly disconnected from the circuit delivering aerosolized dye tracer 1 s after the onset of challenge.
Generation and delivery of cigarette smoke
Cigarettes (Long Life, Taiwan Tobacco and Liquor Production, Taipei, Taiwan) without filters were combusted with a syringe-driven apparatus. A puff of smoke (20 ml) was drawn into a syringe at a constant rate of 2 ml s−1, injected into a delivery circuit, as previously described (Lee et al. 1989), and delivered directly into the lungs via the inspiratory line during five to six ventilatory cycles (∼6–7 s).
Administration of antioxidant
Catalase (CAT; Sigma) was dissolved in PBS to a concentration of 750 000 IU ml−1. Heat-inactivated catalase (HICAT) was prepared by heating the CAT solution to 100°C for 15 min. Aerosol of CAT or HICAT were generated and delivered into the lower airways for a period of 5 min using the nebulizer and the circuit for delivery of the H2O2 aerosol. Both dimethylthiourea (DMTU; Sigma) and deferoxamine (DEF; Sigma) were dissolved in saline. Iron-saturated DEF (DEF + Fe) was prepared by adding 98 mg FeCl3·6H2O (Fluka) to 1 ml DEF (250 mg ml−1) for 1 h at room temperature. DMTU (1.5 g kg−1), saline, DEF (15 mg kg−1) or DEF + Fe was slowly injected into the vein for 30 s. CAT is an enzyme that catalyses the breakdown of H2O2 into O2 and H2O (Comhair & Erzurum, 2002). DEF is an iron-chelator, which prevents the formation of ·OH derived from H2O2 via the Fenton reaction (Halliwell, 1989), whereas DMTU is a ·OH scavenger (Fox, 1984). The doses of CAT, DMTU and DEF have been used previously in the study of H2O2-evoked airway reflexes (Ruan et al. 2003).
Administration of receptor agonist or antagonist
The stock solution of capsaicin (250 μg ml−1), a TRPV1 receptor agonist, was prepared by dissolving it in a solution containing 1% Tween 80 (Sigma), 1% ethanol and 98% saline. The stock solutions of α,β-methylene-ATP (α,β-meATP, 2 mg ml−1, Sigma), a P2X receptor agonist, phenylbiguanide (2 mg ml−1, Sigma), a serotonin 5-HT3 receptor agonist, or iso-pyridoxalphosphate-6-azophenyl-2′,5′-disulphonate (iso-PPADS, 50 mg ml−1, Tocris Cookson, Ellisville, MO, USA), a P2X receptor antagonist (Ralevic & Burnstock, 1998; Irnich et al. 2001), were prepared by dissolving them in saline. The stock solution of capsazepine (CPZ, 10 mg ml−1, Sigma), a TRPV1 receptor antagonist, was prepared by first dissolving in dimethyl sulfoxide (Sigma) at a concentration of 40 mg ml−1 and further diluting with saline containing 10% Tween 80 and 10% ethanol. Except for the stock solution of CPZ, which was stored at 4°C, the others were stored at −20°C. Injected solutions of these chemical agents at desired concentrations were prepared daily by diluting with saline on the basis of the animal's body weight. Capsaicin, α,β-meATP and phenylbiguanide, in a volume of 0.2 ml, were separately injected into the vein as a bolus at doses of 0.75, 200 and 4 μg kg−1, respectively. CPZ and iso-PPADS, in a volume of 0.2 ml, were slowly injected into the vein over 30 s at doses of 3 and 20 mg kg−1, respectively. Each of these injections was then flushed into the right atrium by an injection of 0.4 ml saline. The doses and effective time of these agents were adopted or modified from studies reported previously (Lee & Lundberg, 1994; Pelleg & Hurt, 1996; Kirkup et al. 1999; Ho et al. 2001). The doses of CPZ and iso-PPADS were chosen also because they could block the afferent responses to capsaicin and α,β-meATP, respectively, in our preliminary study.
Experimental design and protocols
In this study, 132 capsaicin-sensitive vagal lung afferent fibres were recorded from 132 rats (weight, 432 ± 5 g) and were divided into 16 groups to conduct four series of experiments. Group 1 contained 12 afferent fibres, while each of the Groups 2–16 contained eight afferent fibres. In the first study series, the afferent fibre responses to a challenge of PBS, 0.2% or 0.4% H2O2 were studied (Group 1). In the second study series, afferent fibre responses to capsaicin or 0.4% H2O2 were studied before and after pretreatment with CAT (Group 2), HICAT (Group 3), DMTU (Group 4), vehicle of DMTU (Group 5), DEF (Group 6) or DEF + Fe (Group 7). In the third study series, afferent fibre responses to α,β-meATP or 0.4% H2O2 were studied before and after pretreatment with iso-PPADS alone (Group 8). Additionally, afferent fibre responses to capsaicin or 0.4% H2O2 were studied before and after pretreatment with CPZ alone (Group 9), a combination of CPZ and iso-PPADS (CPZ +iso-PPADS; Group 10) or a combination of vehicles of CPZ and iso-PPADS (Group 11). Finally, afferent fibre responses to α,β-meATP or phenylbiguanide were studied before and after pretreatment with a combination of CPZ and iso-PPADS (Group 12). In the fourth study series, afferent fibre responses to capsaicin or cigarette smoke (20 ml) were studied before and after pretreatment with DMTU (Group 13), vehicle of DMTU (Group 14), a combination of CPZ and iso-PPADS (Group 15) or a combination of vehicles of CPZ and iso-PPADS (Group 16). The sequence of two or three challenges of afferent fibre stimulants was alternated to achieve a balanced design. Before each H2O2 challenge, the animal's lungs were hyperinflated (Ptr > 25 cmH2O) to establish a constant volume history. Based upon the results of our preliminary study, at least 60 min was allowed to elapse between two H2O2 or smoke challenges to avoid possible tachyphylaxis. The elapsed time between challenges of PBS and H2O2 was 60 min. The elapsed time between capsaicin and H2O2 challenges, between capsaicin and smoke challenges, between α,β-meATP and H2O2 challenges or between α,β-meATP and phenylbiguanide challenges was 35 min. Pretreatments with CAT, HICAT, DMTU, vehicle of DMTU, DEF, DEF + Fe, CPZ, vehicle of CPZ, iso-PPADS and vehicle of iso-PPADS were made 10, 10, 30, 30, 30, 30, 2, 2, 30 and 30 min, respectively, prior to the challenge of capsaicin, H2O2, cigarette smoke, α,β-meATP or phenylbiguanide. These pretreatment times were different because they have different effective times to reach their drug effects and maximal efficacy as reported by others (Lee & Lundberg, 1994; Ruan et al. 2003) or as indicated by our preliminary study.
Data analysis and statistics
Neural activity of capsaicin-sensitive vagal lung afferent fibres and mean arterial blood pressure were continuously analysed at 1-s intervals, and RL and Cdyn were continuously analysed on a breath-by-breath basis over an interval of at least 2 min before, and 3 min after, each challenge of afferent fibre stimulant. These data were then averaged over 3 s or over three breaths to give mean values for plotting responses over time. Baseline data of these physiological parameters were calculated as the average values over the 30-s or 30-breath period immediately preceding each challenge with afferent fibre stimulant. The peak response was defined as the maximal or minimal value averaged over 3 s or over three breaths after each challenge with the afferent fibre stimulant. As the mean values and variability of their baseline activity were quite small, afferent fibres were judged to be activated by stimulants when the peak response exceeded its baseline activity by at least 1 impulse s−1. Once afferent fibres were judged to be activated, the time of the first mean value of discharge averaged over 3-s period that exceeded the baseline activity by at least 1 impulse s−1 was regarded as the commence time of stimulation. These physiological parameters were analysed using a computer equipped with an analog–digital converter (DASA 4600; Gould, Columbus, OH, USA) and appropriate software (BioCybernatics 1.0; Taipei, Taiwan). Results obtained from the computer analysis were routinely checked with those obtained by manual calculations for accuracy. Results were compared by paired t-test or two-way repeated-measures analysis of variance followed by Fisher's least significant difference procedure where appropriate. A value of P < 0.05 was considered significant. All data are presented as the mean ± s.e.m.
Results
Characteristics of the capsaicin-sensitive vagal lung afferent fibres studied
All 132 capsaicin-sensitive vagal lung afferent fibres studied had irregular and sparse baseline activities (Fig. 2) and were stimulated only when the lungs were hyperinflated to three or four tidal volumes (Fig. 2A). Intravenous injection of capsaicin stimulated each of these afferent fibres (Fig. 2B). Their mean fibre activity (FA) increased from a baseline of 0.03 ± 0.01 impulses s−1 to peaks of 0.37 ± 0.03 and 8.04 ± 0.34 impulses s−1 (n = 132) in response to hyperinflation and capsaicin, respectively. All these afferent fibres were localized within the lung structure.
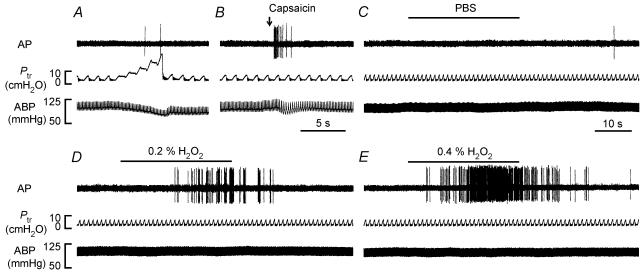
Figure 2. Responses of a rat capsaicin-sensitive vagal lung afferent fibre to lung hyperinflation, intravenous capsaicin and airway challenges of aerosolized PBS or H2O2.
A, the lungs were hyperinflated in a step-like manner to five times tidal volume; B, capsaicin (0.75 μg kg−1, 0.2 ml) was injected into a catheter (dead space, ∼0.3 ml) with its tip close to the right atrium and flushed into the vein with saline (0.4 ml) as indicated by the arrow; C–E, airway delivery of aerosolized PBS, 0.2% H2O2 or 0.4% H2O2 was achieved by directing the air into an active nebulizer cup containing a solution for 30 s using the respirator. The duration of H2O2 challenge is indicated by the horizontal bars. The elapsed time intervals between hyperinflation and capsaicin injection, capsaicin injection and PBS challenge, PBS and H2O2 challenges, and two H2O2 challenges were 5, 15, 60 and 60 min, respectively. AP, action potential; Ptr, tracheal pressure; ABP, arterial blood pressure.
Concentration-dependant afferent stimulation by H2O2
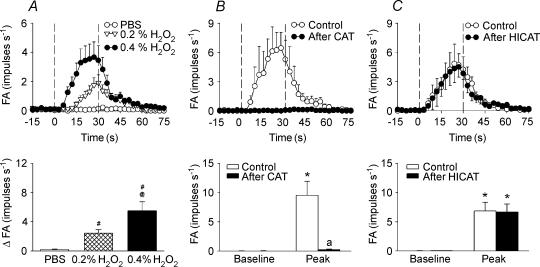
In the first study series, 12 capsaicin-sensitive vagal lung afferent fibres were randomly selected to receive challenges of aerosolized PBS or H2O2. Airway challenge of aerosolized PBS, the vehicle of H2O2, did not affect the activity of any of the 12 afferent fibres tested (Figs 2C and 3A). In contrast, an airway challenge of 0.4% aerosolized H2O2 stimulated 10 of the 12 afferent fibres tested (Fig. 2E). The stimulation began 10.7 ± 6.6 s (range, 5–15 s; n = 10) after H2O2 challenge and lasted for a mean duration of 50.8 ± 13.3 s (range, 39–69 s) (Fig. 3A). When stimulated, these afferent fibres fired irregularly, and the evoked discharge was not in phase with the ventilatory cycle (Fig. 2E). Airway challenge of 0.2% aerosolized H2O2 stimulated only eight of the 12 afferent fibres tested and evoked a milder afferent stimulation with respect to the onset time, amplitude and duration of the afferent responses (Figs 2D and 3 A). As a group (n = 12), the peak change in fibre activity (ΔFA) evoked by 0.4% H2O2 (ΔFA, 5.49 ± 1.23 impulses s−1) was significantly greater than that evoked by 0.2% H2O2 (ΔFA, 2.43 ± 0.05 impulses s−1); both were significantly greater than that evoked by PBS alone (ΔFA, 0.17 ± 0.08 impulses s−1) (Fig. 3A).
Figure 3. Mean responses of capsaicin-sensitive vagal lung afferent fibres to various concentrations of aerosolized H2O2 and to 0.4% aerosolized H2O2 before and after antioxidant pretreatment.
A, responses to aerosolized PBS or H2O2 in one group of fibres; B and C, responses to 0.4% aerosolized H2O2 before and after pretreatment with catalase (CAT) or heat-inactivated catalase (HICAT) in the other two groups. Pretreatments were made 10 min prior to the subsequent challenge by delivery of aerosolized CAT or HICAT (both 750 000 IU ml−1) into lower airways for a period of 5 min using the nebulizer and circuit for delivery of H2O2 aerosol. In the upper panels, the data were averaged over 3 s to give mean values to plot responses over time. The duration of H2O2 challenge is indicated by the interval between dashed lines. #Significantly different from response to PBS; @significantly different from response to 0.2% H2O2; *significantly different from corresponding baseline; asignificantly different from response before pretreatment (control). FA, fibre activity (impulses s−1); ΔFA, difference between peak FA after H2O2 challenge and average baseline activity. Data are mean ± s.e.m. of 12 fibres from 12 rats in A and eight fibres from eight rats in B and C.
ROS mechanisms underlying the afferent stimulation by H2O2
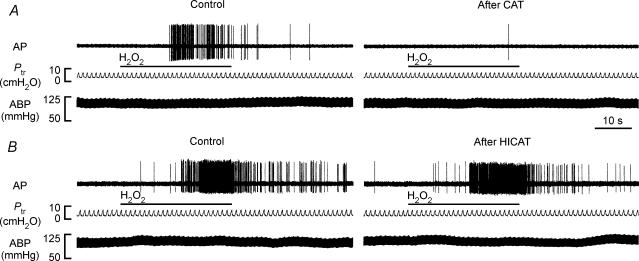
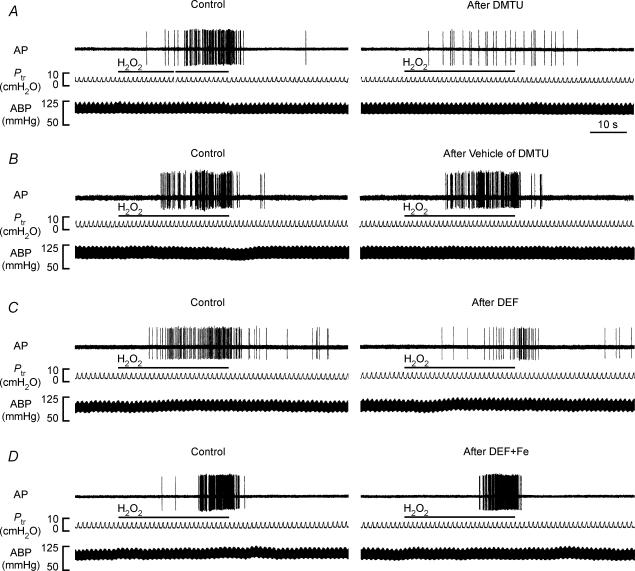
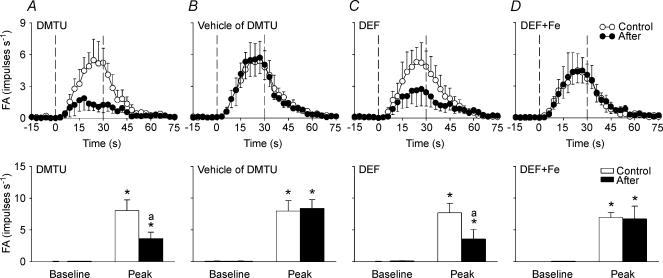
In the second study series, 48 capsaicin-sensitive vagal lung afferent fibres that responded to 0.4% aerosolized H2O2 were divided into six groups (each n = 8) to receive various pretreatments. All pretreatments did not significantly alter either their baseline FA (Figs 3, 4, 5, 6) or their afferent responses to capsaicin (Table 1). The average fibre responses to 0.4% H2O2 were entirely prevented by pretreatment with catalase (ΔFA, −96 ± 3%; control, 9.52 ± 2.39; after treatment, 0.18 ± 0.11 impulses s−1; Figs 3B and 4A). They were also significantly attenuated by pretreatment with dimethylthiourea (ΔFA, −55 ± 9%; control, 7.99 ± 1.69; after treatment, 3.59 ± 1.01 impulses s−1; Figs 5A and 6A) or deferoxamine (ΔFA, −59 ± 9%; control, 7.64 ± 1.46; after treatment, 3.67 ± 1.40 impulses s−1; Figs 5C and 6 C). However, they were not significantly affected by pretreatment with either heat-inactivated catalase (ΔFA, 0 ± 17%; control, 6.78 ± 1.79; after treatment, 6.28 ± 1.41 impulses s−1; Figs 3C and 4B), the vehicle of dimethylthiourea (ΔFA, 16 ± 12%; control, 7.89 ± 1.64; after treatment, 8.13 ± 1.45 impulses s−1; Figs 5B and 6B) or iron-saturated deferoxamine (ΔFA, −7 ± 24%; control, 6.94 ± 0.87; after treatment, 6.45 ± 2.05 impulses s−1; Figs 5 D and 6D).
Figure 4. Responses of two rat capsaicin-sensitive vagal lung afferent fibres to 0.4% aerosolized H2O2 before and after antioxidant pre-treatment.
A and B, pretreatment with catalase (CAT) and heat-inactivated catalase (HICAT), respectively. The duration of H2O2 challenge is indicated by horizontal bars. The elapsed time intervals between two H2O2 challenges were 60 min. AP, action potential; Ptr, tracheal pressure; ABP, arterial blood pressure. See legend of Fig. 3 for detail.
Figure 5. Responses of four capsaicin-sensitive vagal lung afferent fibres to 0.4% aerosolized H2O2 before and after various antioxidant pretreatments.
A–D, pretreatment with dimethylthiourea (DMTU), vehicle of DMTU, deferoxamine (DEF) and iron-saturated DEF (DEF + Fe), respectively. Pretreatments were made 30 min prior to the subsequent challenge by slow injection of DMTU (1.5 g kg−1), vehicle of DMTU, DEF (15 mg kg−1) or DEF + Fe (15 mg kg−1) into the vein for 30 s. The duration of H2O2 challenge is indicated by horizontal bars. The elapsed time intervals between two H2O2 challenges were 60 min. AP, action potential; Ptr, tracheal pressure; ABP, arterial blood pressure.
Figure 6. Mean afferent responses to 0.4% aerosolized H2O2 before and after various antioxidant pretreatments in four groups of capsaicin-sensitive vagal lung afferent fibres.
A–D, pretreatment with dimethylthiourea (DMTU), vehicle of DMTU, deferoxamine (DEF) and iron-saturated DEF (DEF + Fe), respectively. In the upper panels, data were averaged over 3 s to give mean values to plot responses over time. The duration of H2O2 challenge is indicated by the interval between the dashed lines. *Significantly different from corresponding baseline; asignificantly different from response before pretreatment (control). FA, fibre activity (impulses s−1). Data are mean ± s.e.m. of eight fibres from eight rats for each group. See legend of Fig. 5 for detail.
Table 1.
Average peak responses of capsaicin-sensitive vagal lung afferent fibres to intravenous injection of capsaicin before and after various antioxidant pretreatments
| Control (impulses s−1) | After pretreatment (impulses s−1) | |||
|---|---|---|---|---|
| Pretreatment | Baseline | Peak response | Baseline | Peak response |
| CAT | 0.01 ± 0.01 | 7.56 ± 0.07 | 0.02 ± 0.02 | 7.44 ± 0.63 |
| HICAT | 0.01 ± 0.01 | 6.65 ± 0.75 | 0.02 ± 0.02 | 6.35 ± 1.07 |
| DMTU | 0.03 ± 0.02 | 8.29 ± 1.43 | 0.04 ± 0.02 | 8.04 ± 1.36 |
| Vehicle of DMTU | 0.01 ± 0.01 | 7.25 ± 0.63 | 0.01 ± 0.01 | 7.77 ± 1.56 |
| DEF | 0.01 ± 0.01 | 7.17 ± 1.33 | 0.00 ± 0.00 | 7.23 ± 1.53 |
| DEF + Fe | 0.01 ± 0.01 | 7.04 ± 0.67 | 0.04 ± 0.02 | 7.65 ± 0.45 |
Baseline values are data averaged over 30 s, whereas peak values are data averaged over 3 s. Data in each group are the mean ± s.e.m. of eight fibres from eight rats. No statistical significance was found between any two groups of mean. CAT, catalase; HICAT, heat-inactivated catalase; DMTU, dimethylthiourea; Vehicle of DMTU, vehicle of dimethylthiourea; DEF, deferoxamine; DEF + Fe, iron-saturated deferoxamine.
Role of TRPV1 and P2X receptors in afferent stimulation by H2O2
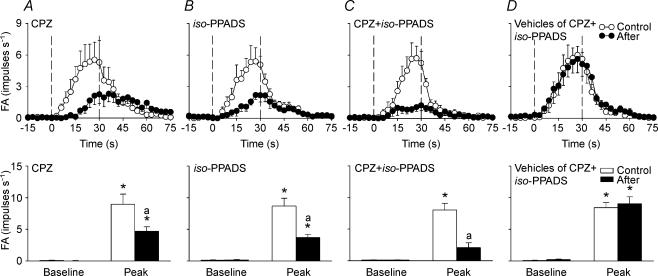
In the third study series, 32 capsaicin-sensitive vagal lung afferent fibres that responded to 0.4% aerosolized H2O2 were divided into four groups (each n = 8) to receive various pretreatments. An additional group of eight capsaicin-sensitive vagal lung afferent fibres that did not receive H2O2 challenge test was employed to receive pretreatment with a combination of capsazepine and iso-PPADS. Among them, intravenous injections of α,β-meATP and phenylbiguanide were also performed to stimulate 16 and eight afferent fibres, respectively (Table 2). All pretreatments did not significantly alter baseline FA (Figs 7 and 8). Pretreatment with capsazepine alone or iso-PPADS alone successfully prevented fibre responses to capsaicin and α,β-meATP, respectively (Table 2). Pretreatment with a combination of capsazepine and iso-PPADS effectively blocked the response of fibres to either capsaicin or α,β-meATP, but it did not significantly affect fibre responses to phenylbiguanide (Table 2). Under these circumstances, the average fibre responses to 0.4% H2O2 were significantly attenuated by pretreatment with capsazepine alone (ΔFA, −39 ± 9%; control, 8.88 ± 1.62; after treatment, 4.65 ± 0.17 impulses s−1; Figs 7A and 8A) or iso-PPADS alone (ΔFA, −51 ± 9%; control, 8.55 ± 1.29; after treatment, 3.55 ± 0.50 impulses s−1; Figs 7B and 8B), but were not significantly affected by pretreatment with a combination of the vehicles of capsazepine and iso-PPADS (ΔFA, 12 ± 19%; control, 8.44 ± 0.78; after treatment, 8.80 ± 1.16 impulses s−1; Figs 7D and 8D). Pretreatment with a combination of capsazepine and iso-PPADS further reduced the average fibre responses to a level (ΔFA, −70 ± 13%; control, 7.91 ± 1.07; after treatment, 1.95 ± 0.75 impulses s−1) where the average H2O2-evoked discharge did not significantly differ from the baseline discharge (Figs 7C and 8C).
Table 2.
Average peak responses of capsaicin-sensitive vagal lung afferent fibres to intravenous injection of three receptor agonists before and after various antagonist pretreatments
| Receptor agonist | Pretreatment | Control (impulses s−1) | After pretreatment (impulses s−1) |
|---|---|---|---|
| Capsaicin | CPZ | 8.14 ± 1.31 | 0.19 ± 0.13* |
| Capsaicin | CPZ +iso-PPADS | 7.74 ± 1.38 | 0.22 ± 0.13* |
| Capsaicin | Vehicles of CPZ +iso-PPADS | 7.52 ± 1.10 | 7.61 ± 1.41 |
| α,β-meATP | iso-PPADS | 8.82 ± 2.39 | 0.24 ± 0.24* |
| α,β-meATP | CPZ +iso-PPADS | 10.83 ± 2.52 | 0.98 ± 0.76* |
| Phenylbiguanide | CPZ +iso-PPADS | 11.79 ± 1.99 | 10.70 ± 1.82 |
Baseline values are data averaged over 30 s, whereas peak values are data averaged over 3 s.
Significantly different from response before pretreatment (control). Data in each group are the mean ± s.e.m. of eight fibres from eight rats. α,β-meATP, α,β-methylene-ATP; CPZ, capsazepine; iso-PPADS, iso-pyridoxalphosphate-6-azophenyl-2′,5′-disulphonate; CPZ +iso-PPADS, a combination of CPZ and iso-PPADS; vehicles of CPZ +iso-PPADS, a combination of vehicles of CPZ and iso-PPADS.
Figure 7. Responses of four rat capsaicin-sensitive vagal lung afferent fibres to 0.4% aerosolized H2O2 before and after various antagonist pretreatments.
A–D, pretreatment with capsazepine (CPZ), iso-pyridoxalphosphate-6-azophenyl-2′,5′-disulphonate (iso-PPADS), a combination of CPZ and iso-PPADS (CPZ +iso-PPADS), and vehicles of CPZ and iso-PPADS (vehicles of CPZ +iso-PPADS). The pretreatments were made 2, 2, 30 and 30 min prior to the subsequent challenge by slow injection of CPZ (3 mg kg−1), vehicle of CPZ, iso-PPADS (20 mg kg−1) and vehicle of iso-PPADS, respectively, into the vein for 30 s. The duration of H2O2 challenge is indicated by the horizontal bars. The elapsed time intervals between two H2O2 challenges were 60 min. AP, action potential; Ptr, tracheal pressure; ABP, arterial blood pressure.
Figure 8. Mean afferent responses to 0.4% aerosolized H2O2 before and after various antagonist pretreatments in four groups of capsaicin-sensitive vagal lung afferent fibres.
A–D, pretreatment with capsazepine (CPZ), iso-pyridoxalphosphate-6-azophenyl-2′,5′-disulphonate (iso-PPADS), a combination of CPZ and iso-PPADS (CPZ +iso-PPADS), and vehicles of CPZ and iso-PPADS (vehicles of CPZ +iso-PPADS). In the upper panels, data were averaged over 3 s to give mean values to plot responses over time. The duration of H2O2 challenge is indicated by the interval between the dashed lines. *Significantly different from corresponding baseline; asignificantly different from response before pretreatment (control). FA, fibre activity (impulses s−1). Data are mean ± s.e.m. of eight fibres from eight rats for each group. See legend of Fig. 7 for detail.
Cardiopulmonary responses to H2O2
After airway challenge with aerosolized PBS, the mean arterial blood pressure, total lung resistance and dynamic lung compliance were 88 ± 4 mmHg, 0.13 ± 0.01 cmH2O ml−1 s−1 and 0.41 ± 0.02 ml−1 cmH2O−1, respectively, which did not significantly differ from their corresponding baselines (88 ± 4 mmHg, 0.13 ± 0.01 cmH2O ml−1 s−1 and 0.42 ± 0.02 ml cmH2O−1, respectively; n = 12, P > 0.05). After airway challenge with 0.4% aerosolized H2O2, the mean arterial blood pressure, total lung resistance and dynamic lung compliance were 82 ± 4 mmHg, 0.15 ± 0.01 cmH2O ml−1 s−1 and 0.38 ± 0.02 ml cmH2O−1, respectively, which differs slightly but significantly from their corresponding baselines (89 ± 4 mmHg, 0.13 ± 0.01 cmH2O ml−1 s−1 and 0.41 ± 0.02 ml cmH2O−1, respectively; n = 12, P < 0.05).
Role of TRPV1 and P2X receptors in afferent stimulation by cigarette smoke
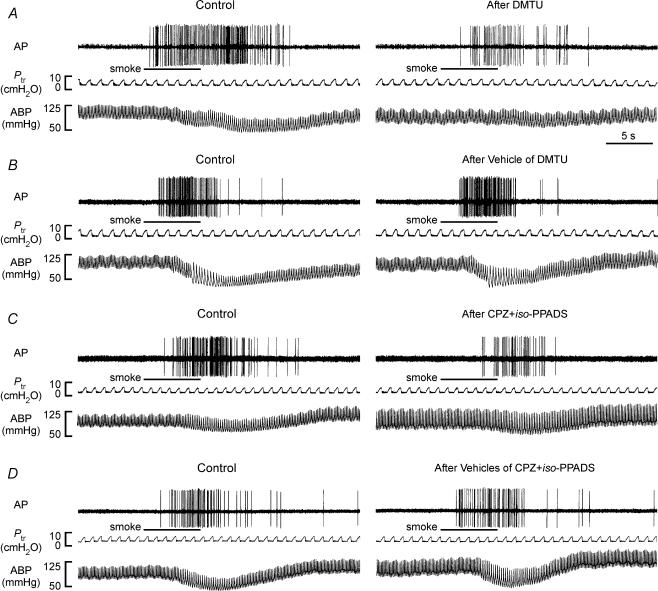
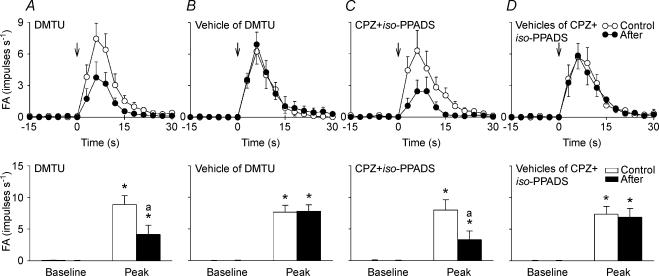
In the fourth study series, 32 capsaicin-sensitive vagal lung afferent fibres that responded to challenge of cigarette smoke were divided into four groups (each n = 8) to receive various pretreatments. Within 1–3 s after smoke delivery, their FA increased from a baseline of 0.04 ± 0.01 impulses s−1 to a peak of 7.96 ± 0.65 impulses s−1 (n = 32). All pretreatments did not significantly alter baseline FA (Figs 9 and 10). Pretreatment with either dimethylthiourea (ΔFA, −61 ± 9%; control, 8.81 ± 1.42; after treatment, 4.10 ± 1.44 impulses s−1; Fig. 9A and 10A) or a combination of capsazepine and iso-PPADS (ΔFA, −67 ± 9%; control, 7.93 ± 1.60; after treatment, 3.25 ± 1.37 impulses s−1; Figs 9C and 10C) significantly reduced the average fibre responses to cigarette smoke, whereas pretreatment with either the vehicle of dimethylthiourea (ΔFA, 7 ± 11%; control, 7.63 ± 1.08; after treatment, 7.79 ± 0.98 impulses s−1; Figs 9B and 10B) or a combination of the vehicles of capsazepine and iso-PPADS (ΔFA, −2 ± 11%; control, 7.31 ± 1.26; after treatment, 7.19 ± 1.48 impulses s−1; Figs 9D and 10D) failed to do so. Furthermore, a combination of capsazepine and iso-PPADS totally prevented the afferent stimulation by capsaicin (ΔFA, −99 ± 1%; control, 9.94 ± 1.84; after treatment, 0.20 ± 0.17 impulses s−1), whereas pretreatment with either dimethylthiourea (ΔFA, −2 ± 7%; control, 7.78 ± 1.36; after treatment, 7.36 ± 1.23 impulses s−1), the vehicle of dimethylthiourea (ΔFA, −1 ± 7%; control, 8.81 ± 0.85 after treatment, 8.73 ± 1.03 impulses s−1) or a combination of the vehicles of capsazepine and iso-PPADS (ΔFA, −1 ± 9%; control, 9.82 ± 1.60; after treatment, 9.70 ± 1.57 impulses s−1) did not significantly alter the average fibre response to capsaicin.
Figure 9. Responses of four capsaicin-sensitive vagal lung afferent fibres to cigarette smoke before and after various pretreatments.
A–D, pretreatment with dimethylthiourea (DMTU), vehicle of DMTU, a combination of capsazepine and iso-pyridoxalphosphate-6-azophenyl-2′,5′-disulphonate (CPZ +iso-PPADS), and vehicles of CPZ and iso-PPADS (vehicles of CPZ +iso-PPADS), respectively. Cigarette smoke (20 ml) was delivered directly into the lungs via the inspiratory line during five to six ventilatory cycles as indicated by the horizontal bars. Pretreatments were made 30, 30, 2, 2, 30 and 30 min prior to the subsequent challenge by slow injection of DMTU (1.5 g kg−1), vehicle of DMTU, CPZ (3 mg kg−1), vehicle of CPZ, iso-PPADS (20 mg kg−1) and vehicle of iso-PPADS, respectively, into the vein for 30 s. The elapsed time intervals between two smoke challenges were 60 min. AP, action potential; Ptr, tracheal pressure; ABP, arterial blood pressure.
Figure 10. Mean afferent responses to cigarette smoke before and after various pretreatments in four groups of capsaicin-sensitive vagal lung afferent fibres.
A–D, pretreatment with dimethylthiourea (DMTU), vehicle of DMTU, a combination of capsazepine and iso-pyridoxalphosphate-6-azophenyl-2′,5′-disulphonate (CPZ +iso-PPADS), and vehicles of CPZ and iso-PPADS (vehicles of CPZ +iso-PPADS), respectively. In the upper panels, data were averaged over 3 s to give mean values to plot responses over time. The onset of smoke challenge is indicated by the arrows. *Significantly different from corresponding baseline; asignificantly different from response before pretreatment (control). FA, fibre activity (impulses s−1). Data are mean ± s.e.m. of eight fibres from eight rats for each group. See legend of Fig. 9 for detail.
Discussion
Results of the first part of this study demonstrate that airway challenge of aerosolized H2O2 stimulated capsaicin-sensitive vagal lung afferent fibres in a concentration-dependent fashion. The afferent responses to H2O2 were totally prevented by catalase and significantly reduced by either dimethylthiourea or deferoxamine. In contrast, the afferent responses to H2O2 were not affected by heat-inactivated catalase, the vehicle of dimethylthiourea or iron-saturated deferoxamine. Catalase is an enzyme that catalyses the breakdown of H2O2 into O2 and H2O (Comhair & Erzurum, 2002). Dimethylthiourea is a ·OH scavenger (Fox, 1984), whereas deferoxamine is an iron-chelator, which prevents the formation of ·OH derived from H2O2 via the Fenton reaction (Halliwell, 1989). The suppressive effects of these antioxidants on afferent responses to H2O2 were unlikely to be due to any anaesthetic or deleterious influence on these afferent fibres, because their afferent responses to capsaicin were unaffected by these pretreatments. It is conceivable that these antioxidants prevented or attenuated afferent responses by lowering the ROS burden. The afferent responses to two H2O2 challenges were similar, suggesting that a prior capsaicin injection did not alter the fibre responsiveness to the subsequent H2O2 challenge. Taken together, these results suggest that the observed stimulation of capsaicin-sensitive vagal lung afferent fibres is a consequence resulting from the specific action of H2O2 and this is in part mediated through the involvement of ·OH derived from H2O2 and possibly the superoxide radical.
Capsaicin-sensitive vagal lung afferent fibres are a subpopulation of pulmonary sensory nerve endings whose afferent activity is conducted through mainly unmyelinated C fibres and some myelinated A-δ fibres (Coleridge & Coleridge, 1986; Lee & Pisarri, 2001; Carr & Undem, 2003). In rats, a right-atrial injection of capsaicin evokes an intense stimulatory effect in 89% of the pulmonary C fibres tested, but only a mild stimulation in 6% of the rapidly adapting pulmonary receptors and none in the slowly adapting pulmonary stretch receptors; the latter two groups are myelinated afferent fibres (Ho et al. 2001). Measurements of conduction velocities of this type of lung vagal afferent fibres in our previous studies (Lai & Kou, 1998a, 1998b; Lai et al. 2005) reveal that they are mainly in the category of C fibres. These ‘nociceptive-like’ afferent fibres are known to play an important role in detecting the onset of pulmonary pathophysiological conditions and triggering various airway reflexes (Coleridge & Coleridge, 1986; Lee & Pisarri, 2001; Carr & Undem, 2003). Excess production of ROS is a common feature of various pulmonary pathophysiological conditions including asthma (Emelyanov et al. 2001), chronic obstructive pulmonary disease (Ferreira et al. 2001), endotoxin shock (Minamiya et al. 1995), vascular microembolism (Wang et al. 1992) and oxidant lung injury (Pryor, 1992). The concept that capsaicin-sensitive vagal lung afferent fibres may detect the existence of excess ROS in the lungs is relatively new (Soukhova et al. 1999). This concept is indirectly supported by the findings that C fibre-mediated airway reflexes are evoked by cigarette smoke (Lee, 1990), inhaled wood smoke (Kou et al. 1997) or pulmonary air embolism (Chen & Kou, 2000) and that the afferent responses of pulmonary C fibres to the latter two insults (Chen et al. 1997; Lai & Kou, 1998a) or endotoxin (Lai et al. 2005) are greatly attenuated by ·OH scavengers. The results of the present study provide the first direct electrophysiological evidence to support the notion that capsaicin-sensitive vagal lung afferent fibres are important in the sensory transduction of ROS in the lungs. Our observations are also in agreement with recent findings that inhalation of aerosolized H2O2 evokes a reflex bradypnoea that is mediated through the action of ROS on vagal lung unmyelinated afferents (Ruan et al. 2003).
That ROS can activate visceral afferent nerve fibres is not unique to the airways and lungs. Topical application of H2O2 to the gastrointestinal tract stimulates abdominal sympathetic afferent C fibres in cats or rats (Stahl et al. 1993; Adelson et al. 1996). Topical application of H2O2 to the heart activates cardiac sympathetic afferent C fibres in cats or rats (Huang et al. 1995; Abe et al. 1998; Schultz & Ustinova, 1998), cardiac vagal afferent C fibres in rats (Ustinova & Schultz, 1994a,b; Schultz & Ustinova, 1996, 1998) and cardiac vagal chemosensitive afferent fibres in guinea pigs (Thompson et al. 2000). In agreement with our findings, the H2O2-induced stimulation of abdominal or cardiac sympathetic (Stahl et al. 1993; Huang et al. 1995) and vagal (Schultz & Ustinova, 1996) afferent C fibres can be attenuated or abolished by either dimethylthiourea or deferoxamine, suggesting the involvement of ·OH. The excitatory effect of ROS has also been demonstrated in other neural tissues. For example, direct application of H2O2 depolarizes brain nerve terminals (Tretter & Adam-Vizi, 1996) and myenteric neurones (Wada-Takahashi & Tamura, 2000) isolated from guinea pigs. Bath application of H2O2 increases the activity of sympathetic preganglionic neurones isolated from rats (Lin et al. 2003). Thus, it appears that ROS has stimulatory effects on a variety of neural tissues.
Results of the second part of this study demonstrate that either capsazepine alone (ΔFA, −39 ± 9%) or iso-PPADS alone (ΔFA, −51 ± 9%) is able to partially attenuate the H2O2-evoked responses of capsaicin-sensitive vagal lung afferent fibres. The specific blocking effects of capsazepine and iso-PPADS on TRPV1 and P2X receptors, respectively, were confirmed by complete blocking of afferent responses to their corresponding receptor agonists, capsaicin and α,β-meATP. Their specific blocking effects were also indicated by the fact that a combination of capsazepine and iso-PPADS did not affect afferent responses to phenylbiguanide (an agonist of 5-HT3 receptors). Capsazepine has been shown to have no scavenger effect on ROS (Schultz & Ustinova, 1998), while iso-PPADS, to our knowledge, also has no such effect and is most probably acting through P2X receptors (Ralevic & Burnstock, 1998; Irnich et al. 2001). Collectively, our observations indicate that both TRPV1 and P2X receptors mediate the stimulation of capsaicin-sensitive vagal lung afferent fibres by ROS. TRPV1 and P2X receptors are two ligand-gated, non-selective cation channels that have been cloned (Valera et al. 1994; Caterina et al. 1999). TRPV1 receptors are activated by stimuli such as capsaicin, noxious heat, acid, anandamide (a cannabinoid receptor agonist) and several products of lipoxygenases (Fox et al. 1995; McCleskey & Gold, 1999; Szallasi & Blumberg, 1999; Hwang et al. 2000; Undem & Carr, 2001; Kollarik & Undem, 2002; Lin & Lee, 2002; Carr et al. 2003) and can be viewed as an integrator of painful chemical and physical stimuli in the somatosensory system (Szallasi & Blumberg, 1999). P2X receptors can be activated by ATP released from damaged cells and their activation causes a pain sensation (McCleskey & Gold, 1999; Dunn et al. 2001; Cook & McCleskey, 2002). It has been suggested that both TRPV1 and P2X receptors are located at the terminals of capsaicin-sensitive vagal lung afferent fibres. Administration of capsaicin or ATP has been shown to stimulate these afferent fibres in whole animals (Lee & Lundberg, 1994; Pelleg & Hurt, 1996; Undem & Carr, 2001) and in ex vivo, vagally innervated, airway and/or lung preparations (Fox et al. 1995; Undem & Carr, 2001; Kollarik & Undem, 2002; Carr et al. 2003; Undem et al. 2004). While the functions of TRPV1 and P2X receptors are relatively established in somatosensory nociceptors (McCleskey & Gold, 1999; Szallasi & Blumberg, 1999; Dunn et al. 2001), their roles in the transduction function of capsaicin-sensitive vagal lung afferent fibres remain to be explored. Our results thus provide evidence to support the notion that the sensory transduction of ROS by these lung afferent fibres is mediated through both the TRPV1 and the P2X receptors.
We further demonstrated that a combination of capsazepine and iso-PPADS provided a more complete blockade of H2O2-evoked responses of capsaicin-sensitive vagal lung afferent fibres (ΔFA, −70 ± 13%), while pretreatment with their vehicles failed to alter the responses. These results suggest that the functional significance of TRPV1 receptors in the ROS-induced afferent stimulation is, at least in part, independent from the P2X receptors. To that end, the stimulation of cardiac vagal or sympathetic afferent fibres by ROS seems to be totally mediated by TRPV1 receptors because capsazepine completely prevents their afferent response to topical application of H2O2 (Schultz & Ustinova, 1998). However, we cannot exclude the possibility that there is a functional interaction between TRPV1 and P2X receptors in ROS-induced afferent stimulation. TRPV1 receptors have been shown to colocalize with P2X3 receptor in rat dorsal root ganglion neurones and the terminals of their axons (Guo et al. 1999). It has been demonstrated that activation of purinoceptors by ATP augments the ionic currents evoked by activation of TRPV1 receptors in rat dorsal root ganglion neurones (Tominaga et al. 2001). Conversely, it has been reported that TRPV1 receptors are important in the regulation of ATP release in mice urinary bladder (Birder et al. 2002).
The mechanisms by which ROS activate TRPV1 and P2X receptors resulting in stimulation of capsaicin-sensitive vagal lung afferent fibres remain unclear. A direct activation of TRPV1 and P2X receptors located on the C-fibre nerve terminals by ROS has been postulated (Schultz & Ustinova, 1998; Shen et al. 2000). Furthermore, H2O2 may act on cardiac sarcolemmal P2 receptors by changing ATP binding (Musat & Dhalla, 1996). As an alternative, ROS may indirectly activate TRPV1 and P2X receptors located at the nerve terminals by the actions of released chemical mediators or receptor ligands. For example, ROS may cause a release of lipoxygenase products in the lung tissues (Matyas et al. 2002), which are activators of TRPV1 receptors (Hwang et al. 2000; Undem & Carr, 2001; Carr et al. 2003). Additionally, ROS may damage cells and can rapidly release cytosolic ATP, which activates P2X receptors of pain nociceptors in the vicinity (McCleskey & Gold, 1999; Cook & McCleskey, 2002). Furthermore, ROS may have non-damaging effects and, upon stimulation, non-damaged epithelial or endothelial cells may release ATP and in this way may exert its paracrine effects on P2X receptors located on other cells (Lazarowski et al. 2003). It is unlikely that the observed ROS-induced sensory stimulation was due to changes in lung mechanics because these afferent fibres are high-threshold mechanoreceptors (Coleridge & Coleridge, 1986; Lee & Pisarri, 2001; Carr & Undem, 2003) and because the airway challenge of 0.4% aerosolized H2O2 only slightly affected total lung resistance and dynamic lung compliance.
In this study, a combination of capsazepine and iso-PPADS did not completely block H2O2-evoked responses of capsaicin-sensitive vagal lung afferent fibres. This incomplete blockade does not seem to be related to the doses of antagonists because, in our preliminary study, doubling their doses did not improve the suppressive effects. The small but residual afferent response observed after pretreatment presumably arises from mechanisms involving ROS, but other than activation of TRPV1 and P2X receptors. To that end, it is known that the nerve terminals of these afferent fibres possess several other pharmacological receptors (Undem & Carr, 2001). Furthermore, although the afferent responses to capsaicin were not influenced with repeated challenges, this does not mean that afferent responses to other stimuli were not altered. In spontaneously breathing rats, inhalation of aerosolized H2O2 produces an early increase and a subsequent decrease in arterial blood pressure (Ruan et al. 2003). In this study, airway challenge of 0.4% aerosolized H2O2 only caused a small drop in arterial blood pressure. Differences in the method of H2O2 challenge, ventilatory mode and animal preparations may account for the discrepancy between the blood pressure responses in our study and in that of Ruan et al. (2003).
Instead of H2O2, we further employed cigarette smoke as the challenge to test the hypothesis that TRPV1 and P2X receptors are important in the sensory transduction of ROS by capsaicin-sensitive vagal lung afferent fibres. Cigarette smoke was chosen as the stimulus because it can generate ROS (Pryor, 1992) and because it triggers vagal C fibre-mediated bradypnoea via a ·OH mechanism (Lee, 1990). Indeed, we found that the afferent responses of these fibres were attenuated by pretreatment with dimethylthiourea suggesting the involvement of ·OH in the sensory activation by cigarette smoke. Furthermore, the afferent responses to cigarette smoke were suppressed by pretreatment with a combination of capsazepine and iso-PPADS, supporting the notion that TRPV1 and P2X receptors play a role in this sensory activation.
In conclusion, our results suggest that ROS, especially H2O2 and ·OH, may stimulate capsaicin-sensitive vagal lung afferent fibres in rats and that this sensory transduction is mediated through both TRPV1 and P2X receptors. The present findings thus enhance our knowledge regarding the functional significance of TRPV1 and P2X receptors in airway afferent physiology and pharmacology. Capsaicin-sensitive vagal lung afferent fibres have been largely implicated in various airway diseases, such as airway hyper-reactivity, cough and bronchoconstriction (Lee & Pisarri, 2001), all of which may be related to excess production of ROS. The potential therapeutic effects of TRPV1 and P2X receptor antagonists in treating these ROS-related airway diseases are mostly unknown and thus require further investigation.
Acknowledgments
The authors are grateful to Dr Tien Huan Hsu for his help in statistical analysis of data. This study was supported by grants NSC93-2320-B-010-014 and NSC93-2320-B-010-028 from the National Science Council, Taiwan, and a grant VGHUST 93-P7-32 from the joint programme of the Veterans General Hospitals and the University System of Taiwan.
References
- Abe T, Morgan D, Sengupta JN, Gebhart GF, Gutterman DD. Attenuation of ischemia-induced activation of cardiac sympathetic afferents following brief myocardial ischemia in cats. J Auton Nerv Syst. 1998;71:28–36. doi: 10.1016/s0165-1838(98)00060-5. 10.1016/S0165-1838(98)00060-5. [DOI] [PubMed] [Google Scholar]
- Adelson DW, Wei JY, Kruger L. H2O2 sensitivity of afferent splanchnic C fiber units in vitro. J Neurophysiol. 1996;76:371–380. doi: 10.1152/jn.1996.76.1.371. [DOI] [PubMed] [Google Scholar]
- Birder LA, Nakamura Y, Kiss S, Nealen ML, Barrick S, Kanai AJ, Wang E, Ruiz G, De Groat WC, Apodaca G, Watkins S, Caterina MJ. Altered urinary bladder function in mice lacking the vanilloid receptor TRPV1. Nat Neurosci. 2002;5:856–860. doi: 10.1038/nn902. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Kollarik M, Meeker SN, Undem BJ. A role for TRPV1 in bradykinin-induced excitation of vagal airway afferent nerve terminals. J Pharmacol Exp Ther. 2003;304:1275–1279. doi: 10.1124/jpet.102.043422. [DOI] [PubMed] [Google Scholar]
- Carr MJ, Undem BJ. Bronchopulmonary afferent nerves. Respirology. 2003;8:291–301. doi: 10.1046/j.1440-1843.2003.00473.x. [DOI] [PubMed] [Google Scholar]
- Caterina MJ, Rosen TA, Tominaga M, Brake AJ, Julius D. A capsaicin-receptor homologue with a high threshold for noxious heat. Nature. 1999;398:436–441. doi: 10.1038/18906. 10.1038/18906. [DOI] [PubMed] [Google Scholar]
- Chen HF, Kou YR. Vagal and mediator mechanisms underlying the tachypnea caused by pulmonary air embolism in dogs. J Appl Physiol. 2000;88:1247–1253. doi: 10.1152/jappl.2000.88.4.1247. [DOI] [PubMed] [Google Scholar]
- Chen HF, Lee BP, Kou YR. Mechanisms of stimulation of vagal pulmonary C fibers by pulmonary air embolism in dogs. J Appl Physiol. 1997;82:765–771. doi: 10.1152/jappl.1997.82.3.765. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JCG. Reflexes evoked from tracheobronchial tree and lungs. In: Cherniack NS, Widdicombe JG, editors. Handbook of Physiology, Section 3, the Respiratory System,vol. II Control of Breathing. Bethesda, MD: American Physiological Society; 1986. pp. 395–429. part I chap. 12. [Google Scholar]
- Comhair SA, Erzurum SC. Antioxidant responses to oxidant-mediated lung diseases. Am J Physiol Lung Cell Mol Physiol. 2002;283:L246–L255. doi: 10.1152/ajplung.00491.2001. [DOI] [PubMed] [Google Scholar]
- Cook SP, McCleskey EW. Cell damage excites nociceptors through release of cytosolic ATP. Pain. 2002;95:41–47. doi: 10.1016/s0304-3959(01)00372-4. [DOI] [PubMed] [Google Scholar]
- Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. 10.1016/S0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- Emelyanov A, Fedoseev G, Abulimity A, Rudinski K, Fedoulov A, Karabanov A, Barnes PJ. Elevated concentrations of exhaled hydrogen peroxide in asthmatic patients. Chest. 2001;120:1136–1139. doi: 10.1378/chest.120.4.1136. [DOI] [PubMed] [Google Scholar]
- Ferreira IM, Hazari MS, Gutierrez C, Zamel N, Chapman KR. Exhaled nitric oxide and hydrogen peroxide in patients with chronic obstructive pulmonary disease: effects of inhaled beclomethasone. Am J Respir Crit Care Med. 2001;164:1012–1015. doi: 10.1164/ajrccm.164.6.2012139. [DOI] [PubMed] [Google Scholar]
- Fox AJ, Urban L, Barnes PJ, Dray A. Effects of capsazepine against capsaicin- and proton-evoked excitation of single airway C-fibres and vagus nerve from the guinea-pig. Neuroscience. 1995;67:741–752. doi: 10.1016/0306-4522(95)00115-y. 10.1016/0306-4522(95)00115-Y. [DOI] [PubMed] [Google Scholar]
- Fox RB. Prevention of granulocyte-mediated oxidant lung injury in rats by a hydroxyl radical scavenger, dimethylthiourea. J Clin Invest. 1984;74:1456–1464. doi: 10.1172/JCI111558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Q, Kwong K, Lee LY. Ca2+ transient evoked by chemical stimulation is enhanced by PGE2 in vagal sensory neurons: role of cAMP/PKA signaling pathway. J Neurophysiol. 2003;89:1985–1993. doi: 10.1152/jn.00748.2002. [DOI] [PubMed] [Google Scholar]
- Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11:946–958. doi: 10.1046/j.1460-9568.1999.00503.x. 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Protection against tissue damage in vivo by desferrioxamine: what is its mechanism of action. Free Radic Biol Med. 1989;7:645–651. doi: 10.1016/0891-5849(89)90145-7. 10.1016/0891-5849(89)90145-7. [DOI] [PubMed] [Google Scholar]
- Ho CY, Gu Q, Lin YS, Lee LY. Sensitivity of vagal afferent endings to chemical irritants in the rat lung. Respir Physiol. 2001;127:113–124. doi: 10.1016/s0034-5687(01)00241-9. 10.1016/S0034-5687(01)00241-9. [DOI] [PubMed] [Google Scholar]
- Ho CY, Kou YR. Protective and defensive airway reflexes evoked by nasal exposure to wood smoke in anesthetized rats. J Appl Physiol. 2000;88:863–870. doi: 10.1152/jappl.2000.88.3.863. 10.1063/1.373748. [DOI] [PubMed] [Google Scholar]
- Huang HS, Pan HL, Stahl GL, Longhurst JC. Ischemia- and reperfusion-sensitive cardiac sympathetic afferents: influence of H2O2 and hydroxyl radicals. Am J Physiol. 1995;269:H888–H901. doi: 10.1152/ajpheart.1995.269.3.H888. [DOI] [PubMed] [Google Scholar]
- Hwang SW, Cho H, Kwak J, Lee SY, Kang CJ, Jung J, Cho S, Min KH, Suh YG, Kim D, Oh U. Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc Natl Acad Sci U S A. 2000;97:6155–6160. doi: 10.1073/pnas.97.11.6155. 10.1073/pnas.97.11.6155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichikawa H, Sugimoto T. The co-expression of VR1 and VRL-1 in the rat vagal sensory ganglia. Brain Res. 2003;980:293–296. doi: 10.1016/s0006-8993(03)02998-6. 10.1016/S0006-8993(03)02998-6. [DOI] [PubMed] [Google Scholar]
- Irnich D, Burgstahler R, Bostock H, Grafe P. ATP affects both axons and Schwann cells of unmyelinated C fibres. Pain. 2001;92:343–350. doi: 10.1016/S0304-3959(01)00277-9. 10.1016/S0304-3959(01)00277-9. [DOI] [PubMed] [Google Scholar]
- Khakh BS, Humphrey PP, Surprenant A. Electrophysiological properties of P2X-purinoceptors in rat superior cervical, nodose and guinea-pig coeliac neurones. J Physiol. 1995;484:385–395. doi: 10.1113/jphysiol.1995.sp020672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkup AJ, Booth CE, Chessell IP, Humphrey PP, Grundy D. Excitatory effect of P2X receptor activation on mesenteric afferent nerves in the anaesthetised rat. J Physiol. 1999;520:551–563. doi: 10.1111/j.1469-7793.1999.00551.x. 10.1111/j.1469-7793.1999.00551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollarik M, Undem BJ. Mechanisms of acid-induced activation of airway afferent nerve fibres in guinea-pig. J Physiol. 2002;543:591–600. doi: 10.1113/jphysiol.2002.022848. 10.1113/jphysiol.2002.022848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kou YR, Lai CJ, Hsu TH, Lin YS. Involvement of hydroxyl radical in the immediate ventilatory responses to inhaled wood smoke in rats. Respir Physiol. 1997;107:1–13. doi: 10.1016/s0034-5687(96)02507-8. 10.1016/S0034-5687(96)02507-8. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Kou YR. Stimulation of vagal pulmonary C fibers by inhaled wood smoke in rats. J Appl Physiol. 1998a;84:30–36. doi: 10.1152/jappl.1998.84.1.30. [DOI] [PubMed] [Google Scholar]
- Lai CJ, Kou YR. Stimulation of pulmonary rapidly adapting receptors by inhaled wood smoke in rats. J Physiol. 1998b;508:597–607. doi: 10.1111/j.1469-7793.1998.597bq.x. 10.1111/j.1469-7793.1998.597bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CJ, Ruan T, Kou YR. The involvement of hydroxyl radical and cyclooxygenase metabolites in the activation of lung vagal sensory receptors by circulatory endotoxin in rats. J Appl Physiol. 2005;98:620–628. doi: 10.1152/japplphysiol.00539.2004. [DOI] [PubMed] [Google Scholar]
- Lazarowski ER, Boucher RC, Harden TK. Mechanisms of release of nucleotides and integration of their action as P2X-and P2Y-receptor activating molecules. Mol Pharmacol. 2003;64:785–795. doi: 10.1124/mol.64.4.785. 10.1124/mol.64.4.785. [DOI] [PubMed] [Google Scholar]
- Lee LY. Inhibitory effect of gas phase cigarette smoke on breathing: role of hydroxyl radical. Respir Physiol. 1990;82:227–238. doi: 10.1016/0034-5687(90)90037-y. 10.1016/0034-5687(90)90037-Y. [DOI] [PubMed] [Google Scholar]
- Lee LY, Kou YR, Frazier DT, Beck ER, Pisarri TE, Coleridge HM, Coleridge JC. Stimulation of vagal pulmonary C-fibers by a single breath of cigarette smoke in dogs. J Appl Physiol. 1989;66:2032–2038. doi: 10.1152/jappl.1989.66.5.2032. 10.1063/1.344342. [DOI] [PubMed] [Google Scholar]
- Lee LY, Lundberg JM. Capsazepine abolishes pulmonary chemoreflexes induced by capsaicin in anesthetized rats. J Appl Physiol. 1994;76:1848–1855. doi: 10.1152/jappl.1994.76.5.1848. [DOI] [PubMed] [Google Scholar]
- Lee LY, Pisarri TE. Afferent properties and reflex functions of bronchopulmonary C-fibers. Respir Physiol. 2001;125:47–65. doi: 10.1016/s0034-5687(00)00204-8. 10.1016/S0034-5687(00)00204-8. [DOI] [PubMed] [Google Scholar]
- Lin HH, Chen CH, Hsieh WK, Chiu TH, Lai CC. Hydrogen peroxide increases the activity of rat sympathetic preganglionic neurons in vivo and in vitro. Neuroscience. 2003;121:641–647. doi: 10.1016/s0306-4522(03)00517-7. 10.1016/S0306-4522(03)00517-7. [DOI] [PubMed] [Google Scholar]
- Lin YS, Kou YR. Reflex apneic response evoked by laryngeal exposure to wood smoke in rats: neural and chemical mechanisms. J Appl Physiol. 1997;83:723–730. doi: 10.1152/jappl.1997.83.3.723. [DOI] [PubMed] [Google Scholar]
- Lin YS, Lee LY. Stimulation of pulmonary vagal C-fibres by anandamide in anaesthetized rats: role of vanilloid type 1 receptors. J Physiol. 2002;539:947–955. doi: 10.1113/jphysiol.2001.013290. 10.1113/jphysiol.2001.013290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCleskey EW, Gold MS. Ion channels of nociception. Annu Rev Physiol. 1999;61:835–856. doi: 10.1146/annurev.physiol.61.1.835. 10.1146/annurev.physiol.61.1.835. [DOI] [PubMed] [Google Scholar]
- Matyas S, Pucovsky V, Bauer V. Effects of various reactive oxygen species on the guinea pig trachea and its epithelium. Jpn J Pharmacol. 2002;88:270–278. doi: 10.1254/jjp.88.270. 10.1254/jjp.88.270. [DOI] [PubMed] [Google Scholar]
- Mead J, Whittenberger JL. Physical properties of human lungs measured during spontaneous respiration. J Appl Physiol. 1953;5:779–796. [Google Scholar]
- Minamiya Y, Abo S, Kitamura M, Izumi K, Kimura Y, Tozawa K, Saito S. Endotoxin-induced hydrogen peroxide production in intact pulmonary circulation of rat. Am J Respir Crit Care Med. 1995;152:348–354. doi: 10.1164/ajrccm.152.1.7599844. [DOI] [PubMed] [Google Scholar]
- Musat S, Dhalla NS. Alteration in cardiac sarcolemmal ATP receptors by oxyradicals. Ann N Y Acad Sci. 1996;793:1–12. doi: 10.1111/j.1749-6632.1996.tb33500.x. [DOI] [PubMed] [Google Scholar]
- Pelleg A, Hurt CM. Mechanism of action of ATP on canine pulmonary vagal C fibre nerve terminals. J Physiol. 1996;490:265–275. doi: 10.1113/jphysiol.1996.sp021142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor WA. Biological effects of cigarette smoke, wood smoke, and the smoke from plastics: the use of electron spin resonance. Free Radic Biol Med. 1992;13:659–676. doi: 10.1016/0891-5849(92)90040-n. 10.1016/0891-5849(92)90040-N. [DOI] [PubMed] [Google Scholar]
- Ralevic V, Burnstock G. Receptors for purines and pyrimidines. Pharmacol Rev. 1998;50:413–492. [PubMed] [Google Scholar]
- Ruan T, Ho CY, Kou YR. Afferent vagal pathways mediating respiratory reflexes evoked by ROS in the lungs of anesthetized rats. J Appl Physiol. 2003;94:1987–1998. doi: 10.1152/japplphysiol.01047.2002. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Ustinova EE. Cardiac vagal afferent stimulation by free radicals during ischaemia and reperfusion. Clin Exp Pharmacol Physiol. 1996;23:700–708. doi: 10.1111/j.1440-1681.1996.tb01762.x. [DOI] [PubMed] [Google Scholar]
- Schultz HD, Ustinova EE. Capsaicin receptors mediate free radical-induced activation of cardiac afferent endings. Cardiovasc Res. 1998;38:348–355. doi: 10.1016/s0008-6363(98)00031-5. 10.1016/S0008-6363(98)00031-5. [DOI] [PubMed] [Google Scholar]
- Shen JZ, Zheng XF, Kwan CY. Evidence for P2-purinoceptors contribution in H2O2-induced contraction of rat aorta in the absence of endothelium. Cardiovasc Res. 2000;47:574–585. doi: 10.1016/s0008-6363(00)00123-1. 10.1016/S0008-6363(00)00123-1. [DOI] [PubMed] [Google Scholar]
- Soukhova GK, Ahmed M, Fletcher EC, Yu J. Hydrogen peroxide in the lung parenchyma stimulates vagally mediated phrenic activity. Chest. 1999;116:1365–1368. doi: 10.1378/chest.116.5.1365. [DOI] [PubMed] [Google Scholar]
- Stahl GL, Pan HL, Longhurst JC. Activation of ischemia- and reperfusion-sensitive abdominal visceral C fiber afferents. Role of hydrogen peroxide and hydroxyl radicals. Circ Res. 1993;72:1266–1275. doi: 10.1161/01.res.72.6.1266. [DOI] [PubMed] [Google Scholar]
- Szallasi A, Blumberg PM. Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol Rev. 1999;51:159–212. [PubMed] [Google Scholar]
- Thompson GW, Horackova M, Armour JA. Chemotransduction properties of nodose ganglion cardiac afferent neurons in guinea pigs. Am J Physiol Regul Integr Comp Physiol. 2000;279:R433–R439. doi: 10.1152/ajpregu.2000.279.2.R433. [DOI] [PubMed] [Google Scholar]
- Tominaga M, Wada M, Masu M. Potentiation of capsaicin receptor activity by metabotropic ATP receptors as a possible mechanism for ATP-evoked pain and hyperalgesia. Proc Natl Acad Sci U S A. 2001;98:6951–6956. doi: 10.1073/pnas.111025298. 10.1073/pnas.111025298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter L, Adam-Vizi V. Early events in free radical-mediated damage of isolated nerve terminals: effects of peroxides on membrane potential and intracellular Na+ and Ca2+ concentrations. J Neurochem. 1996;66:2057–2066. doi: 10.1046/j.1471-4159.1996.66052057.x. [DOI] [PubMed] [Google Scholar]
- Undem BJ, Carr MJ. Pharmacology of airway afferent nerve activity. Respir Res. 2001;2:234–244. doi: 10.1186/rr62. 10.1186/rr62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Undem BJ, Chuaychoo B, Lee MG, Weinreich D, Myers AC, Kollarik M. Subtypes of vagal afferent C-fibres in guinea-pig lungs. J Physiol. 2004;556:905–917. doi: 10.1113/jphysiol.2003.060079. 10.1113/jphysiol.2003.060079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ustinova EE, Schultz HD. Activation of cardiac vagal afferents in ischemia and reperfusion. Prostaglandins versus oxygen-derived free radicals. Circ Res. 1994b;74:904–911. doi: 10.1161/01.res.74.5.904. [DOI] [PubMed] [Google Scholar]
- Ustinova EE, Schultz HD. Activation of cardiac vagal afferents by oxygen-derived free radicals in rats. Circ Res. 1994a;74:895–903. doi: 10.1161/01.res.74.5.895. [DOI] [PubMed] [Google Scholar]
- Valera S, Hussy N, Evans RJ, Adami N, North RA, Surprenant A, Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;371:516–519. doi: 10.1038/371516a0. 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vulchanova L, Riedl MS, Shuster SJ, Buell G, Surprenant A, North RA, Elde R. Immunohistochemical study of the P2X2 and P2X3 receptor subunits in rat and monkey sensory neurons and their central terminals. Neuropharmacology. 1997;36:1229–1242. doi: 10.1016/s0028-3908(97)00126-3. 10.1016/S0028-3908(97)00126-3. [DOI] [PubMed] [Google Scholar]
- Wada-Takahashi S, Tamura K. Actions of reactive oxygen species on AH/type 2 myenteric neurons in guinea pig distal colon. Am J Physiol Gastrointest Liver Physiol. 2000;279:G893–G902. doi: 10.1152/ajpgi.2000.279.5.G893. [DOI] [PubMed] [Google Scholar]
- Wang D, Li MH, Hsu K, Shen CY, Chen HI, Lin YC. Air embolism-induced lung injury in isolated rat lungs. J Appl Physiol. 1992;72:1235–1242. doi: 10.1152/jappl.1992.72.4.1235. [DOI] [PubMed] [Google Scholar]