Abstract
Calmodulin (CaM) has been shown to modulate different ion channels, including voltage-gated sodium channels (NaChs). Using the yeast two-hybrid assay, we found an interaction between CaM and the C-terminal domains of adult skeletal (NaV1.4) and cardiac (NaV1.5) muscle NaChs. Effects of CaM were studied using sodium channels transiently expressed in CHO cells. Wild type CaM (CaMWT) caused a hyperpolarizing shift in the voltage dependence of activation and inactivation for NaV1.4 and activation for NaV1.5. Intracellular application of CaM caused hyperpolarizing shifts equivalent to those seen with CaMWT coexpression with NaV1.4. Elevated Ca2+ and CaM-binding peptides caused depolarizing shifts in the inactivation curves seen with CaMWT coexpression with NaV1.4. KN93, a CaM-kinase II inhibitor, had no effect on NaV1.4, suggesting that CaM acts directly on NaV1.4 and not through activation of CaM-kinase II. Coexpression of hemi-mutant CaMs showed that an intact N-terminal lobe of CaM is required for effects of CaM upon NaV1.4. Mutations in the sodium channel IQ domain disrupted the effects of CaM on NaV1.4: the I1727E mutation completely blocked all calmodulin effects, while the L1736R mutation disrupted the effects of Ca2+–calmodulin on inactivation. Chimeric channels of NaV1.4 and NaV1.5 also indicated that the C-terminal domain is largely responsible for CaM effects on inactivation. CaM had little effect on NaV1.4 expressed in HEK cells, possibly due to large differences in the endogenous expression of β-subunits between CHO and HEK cells. These results in heterologous cells suggest that Ca2+ released during muscle contraction rapidly modulates NaCh availability via CaM.
Voltage-gated sodium channels (NaChs) are essential for the initiation and propagation of action potentials in both nerve and muscle. They are composed of a large pore-forming α-subunit and one or more auxiliary β-subunits. Nine genes encode the α-subunit in mammals, with the proteins designated NaV1.1–1.9. The majority of these subtypes are expressed in neurones, but NaV1.4 and NaV1.5 are expressed primarily in skeletal and cardiac muscle, respectively.
Activation and inactivation properties of these channels are crucial for determining normal nerve function and muscle contraction. Perhaps the most dramatic illustration of the changes wrought by slight modifications to the activation and inactivation properties are the human diseases caused by mutations that produce myotonias or cardiac arrest (Head & Gardiner, 2003; Goldin, 2003). Mutations in the human sodium channels that disturb activation and/or inactivation are widely distributed over the primary sequence, indicating that many regions of the channel are capable of influencing activation and inactivation.
Auxiliary proteins to the α-subunit can modify activation and inactivation of the channel. NaCh β-subunits have dramatic effects on gating, especially inactivation (Isom, 2000). Calmodulin (CaM) is another protein that probably binds constitutively to the sodium channel. CaM regulates a vast array of different cellular processes, including ion channels (Saimi & Kung, 2002). Evidence for a functional role of CaM binding to NaChs is beginning to emerge. Yeast two-hybrid experiments performed in our laboratory and those of others (Mori et al. 2000) showed that the C-terminal region of NaChs interacts with CaM. This NaCh domain contains an IQ-binding motif, a Ca2+-independent CaM binding site that is highly conserved across all NaCh isoforms (Rhoads & Friedberg, 1997). The IQ domain in L-type calcium channels (CaChs) is known to be important for CaM-mediated inactivation of these channels (Qin et al. 1999; Zuhlke et al. 1999; Erickson et al. 2003; Liang et al. 2003).
A yeast two-hybrid screen using the C-terminal cytoplasmic tails of NaV1.4 and NaV1.5 yielded multiple full-length clones of CaM from a muscle library. Encouraged by the structural similarities of the C-terminal tail of NaChs to those of CaChs, we used whole-cell recordings to search for functional effects of CaM binding to NaV1.4 and NaV1.5. Recent work by Tan et al. (2002), Deschenes et al. (2002) and Herzog et al. (2003) has also shown functional effects of CaM on NaCh function. A perplexing aspect of these three studies is that there are many unexplained discrepancies between them. Our results agree with some aspects of the earlier reports, but other findings of ours are novel. Some of the differences between our results and those of other groups are likely to be due to the different cell lines (CHO versus HEK) used for heterologous expression. We found that coexpression of CaM with NaV1.4 and NaV1.5 affects the voltage dependence of either activation and/or steady-state inactivation. We used mutant CaMs and NaChs with point mutations in the IQ motif to identify regions of CaM and the NaCh required for modulation by CaM. Recordings from chimeric channels made between NaV1.4 and NaV1.5 also confirm the importance of the C-terminal domain for the effects of CaM on steady-state inactivation of the channel.
Methods
Yeast two-hybrid assay
The C-terminal cytoplasmic domains for both the skeletal (rSKM1 or NaV1.4) and cardiac (hH1 or NaV1.5) muscle sodium channels were cloned into pHybLex/Zeo (Invitrogen) for use as bait clones. Each loop was screened against a human-skeletal-muscle library in pGAD10 (Clontech) according to the manufacturer's instructions. Interaction of the bait and prey proteins was assayed by growth on yeast-peptone-dextrose rich medium (YPD)/−leu/−his/+zeo selective media. Autoactivation of the reporter gene was not observed on YPD/−his/+zeo, and autoactivation of the CaM cDNA clones was not observed on YPD/−leu/−his.
Cell culture
CHO-K1 (Chinese hamster ovary) cells (American Type Culture Collection) were used for expression of all of the NaCh and CaM constructs except those for Fig. 7, for which human embryonic kidney cells (HEK293) were used. CHO-K1 cells were grown in F12 (HAMS) medium (Sigma) fortified with 10% FCS and 1% penicillin/streptomycin in a humidified incubator at 37°C and 5% CO2. HEK293 cells were grown in F12 (DMEM) medium, 10% FCS, 1% penicillin/streptomycin and 1% non-essential amino acids (Invitrogen). The following DNAs were transiently transfected in various combinations as described in the Results section: NaV1.4 and NaV1.5 (pZemμ1–2 and pCDNA3.1-hH1, gift of S. R. Levinson, UCHSC), NaV1.4/CT1.5 and NaV1.5/CT1.4 (both in pCDNA3.1, gift of A. George, Vanderbilt University and E. Bennett, University of South Florida), I1727E and L1736R mutated NaV1.4, CaMWT and CaM1234 (gift of K. Beckingham, Rice University), CaM12 and CaM34 (gift of B. Peterson, Penn State University), and β1-subunit (gift of S. Cannon, University Texas, Southwestern). CaMWT and CaM1234 were both cloned into the green fluorescent protein (GFP)-expression vector pEGFPN3 (Clontech). In cases where the DNA was not in a tagged vector, cells were cotransfected with pEGFPN3. Transfections were performed using Lipofectamine (Gibco/BRL) according to the manufacturer's instructions. For each transfection, 1–2 μg total DNA was used. Recordings were made 2–3 days after transfection.
Figure 7. CaM effects and β-subunit mRNA expression in HEK and CHO cells.
A, coexpression of CaM with Nav1.4 did not shift the voltage dependence of activation or inactivation in HEK cells. Nav1.4 (▪, n = 12); Nav1.4 + CaM (○, n = 7). B and C, relative abundance of mRNA of each of the four NaCh β-subunits, expressed as the number of copies relative to 1000 copies of HPRT (an internal control) in HEK and CHO cells, respectively. Note the logarithmic scale.
C2C12 cells (American Type Culture Collection) are derived from a mouse myoblast cell line expressing characteristic muscle proteins. The cells were grown in DMEM high glucose with 10% FCS and 1% penicillin/streptomycin at 37°C and 5% CO2. The medium was switched 24 h prior to recording to DMEM high glucose with 5% horse serum to induce myotube differentiation. After differentiation, C2C12 cells express both Nav1.4 and Nav1.5.
Mutation of NaV1.4
Mutations in the IQ domain were made using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene). The primers used were:
I1727e-5′: AGG AGG TGT GTG CTA TCA AAG AGC AGA GGG CCT ACC GCC G
I1727e-3′: CGG CGG TAG GCC CTC TGC TCT TTG ATA GCA CAC ACC TCC
L1736r-5′: CCG CCA CCT GCG GCA GCG CTC CGT G
The underlined nucleotides show the mutation sites. XL10-Gold Ultracompetent cells were transformed with 2 μl of each reaction to produce the mutated DNA according to the manufacturer's instructions. Positive clones were sequenced by the UCHSC Cancer Center to verify that the mutation was incorporated.
RT-PCR
Primers were designed using GenBank sequences and the Perkin Elmer ABI Primer Express program. Each primer was searched against BLAST (http://www.ncbi.nlm.nih.gov/BLAST) to ensure that it did not match any known gene aside from that for which it was designed, including other family members. The primers for the CHO cells were designed to ensure 100% identity between rat and mouse for each subunit. All amplification products were the same size, and the primers had the same melting temperatures. RNA was made using a Qiagen Rneasy mini kit (Valencia, CA, USA). cDNA was made using Superscript II RT with oligo (dT)12–18 primers (Invitrogen, Carlsbad, CA, USA). For quantitative PCR, PCR was carried out in a GeneAmp Sequence Detection System 5700 (Perkin Elmer, Norwalk, CN, USA) using 40 cycles of 95 to 60°C PCR with a 10 min 95°C initial step. The fluorescent indicator Sybrgreen (Bio-Rad Laboratories, Hercules, CA, USA) was used to allow real-time light detection. Increased fluorescence resulting specifically from amplification of the target sequence was detected real time so that the number of PCR cycles and thus the number of molecules of each β-subunit could be expressed as a percentage of hypoxanthine phosphoribosyltransferase (HPRT) mRNA. Each measurement was made in triplicate and averaged, with two individual replicate experiments used for statistical analysis. Primers used for amplification of β-subunits are presented in the Supplemental material table.
Acquisition of data
Whole-cell patch-clamp recordings were made using standard techniques. Pipettes for recording were pulled from capillary micropipettes (Drummond Scientific Co.) using a 5-stage protocol on a micropipette puller (P-97 Sutter Instruments Co.), coated with blue ski wax, and fire-polished using a micro forge (MF-830 Narishige). Cells were voltage-clamped using an Axopatch 200A integrating amplifier (Axon Instruments). To minimize voltage-clamp errors, low-resistance electrodes (1.5–3 MΩ) were used with series resistance compensation correction > 95%. Voltage pulses were generated and membrane currents were measured using pCLAMP8 software (Axon Instruments). Currents were filtered at 5 kHz and digitized at 20 kHz. Leak current was subtracted using a P/8 protocol. With one exception, cells with currents > 1 nA were analysed to ensure that the records were not contaminated by endogenous currents. The exception was for IQ mutants that interfered with CaM binding; currents in these cells were often low and thus currents greater than 0.8 nA were included in the analysis. For all experiments, recording was started 5 min after gaining whole-cell access to ensure stabilization of currents.
For current–voltage data, cells were held at a holding potential of −120 mV. Voltage-clamp steps of 30 ms were applied from −90 to +100 mV in 10-mV increments. For steady-state inactivation data, a two-pulse protocol was used. A holding potential of −120 mV was used. Prepulse potentials of 100 ms were applied from −150 mV to +30 mV, followed by a test pulse of 30 ms to −10 mV.
Recording solutions
The external solution contained (mm): 132 NaCl, 4 KCl, 1.5 CaCl2, 1.5 MgCl2, 11 glucose and 10 Hepes (pH to 7.4 at room temperature using NaOH). The normal internal solution contained (mm): 115 CsF, 10 CsCl, 10 NaF, 10 Hepes and 5 EGTA (pH to 7.4 at room temperature using CsOH). The 10 μm free-Ca2+ internal solution contained 115 CsF, 10 CsCl, 10 NaF, 10 Hepes, 5 EGTA and 4.9 CaCl2. The intracellular Cl− solution contained (mm): 125 CsCl, 10 NaCl, 10 Hepes and 5 EGTA. Chemicals were purchased from Sigma. Inhibitors were purchased from Calbiochem. CaM-binding peptide (CBP), CaM-inhibitory peptide (CIP) and CaM-inhibitory peptide control (CIPc) stocks were dissolved in H2O. KN93 and KN92 stock solutions were dissolved in DMSO and diluted 1: 1000 prior to use. DMSO at this concentration had no effect on currents. All of the stock inhibitor solutions were stored at −20°C and diluted to their working concentrations immediately prior to use. The liquid junction potential between the internal solutions and the external bath was −8 mV, calculated using pCLAMP8.2. Reported membrane potentials were not corrected for this. All experiments were performed at room temperature.
Analysis of data
Analysis was performed using Clampfit8 (Axon Instruments), MatLab6.5 (The MathWorks, Inc.) and Origin6.1 (MicroCal Software, Inc., Northampton, MA, USA).
Time constants for inactivation were found by fitting a standard single exponential to the decay phase of the current using Clampfit8.
Conductance was calculated using the equation:
| (1) |
where G/Gmax is the normalized conductance, I is the current measured, Vm is the test potential, and ENa is the reversal potential measured for sodium, determined for each individual current–voltage relationship.
Steady-state inactivation and conductance–voltage plots were fitted with a Boltzmann distribution to the equation:
| (2) |
where I/Imax is the normalized current, V is the test potential or prepulse potential, V1/2 is the measured midpoint of the voltage dependence of activation or inactivation, and k is the slope of the curve.
For producing graphs of the data, the means of the cells for each experimental condition were plotted using Origin6.1. For statistical analysis of the data, the curves for each individual cell were fitted with a Boltzmann distribution. The slope and V1/2 for each treatment group were then analysed using MatLab6.5( Statistics are reported as means ± s.e.m. One-way ANOVA (analysis of variance) multiple comparison tests were used to test the significance of changes within and between treatment groups. This method compensates for the possibility of making at least one incorrect conclusion among pairs (greater than 5%) tested that can result when performing a series of t tests. Whole-cell current amplitudes, fast inactivation, and values of V1/2 and slope for the voltage dependence of activation and steady-state inactivation were compared between different treatment groups where appropriate. Differences were considered statistically significant for P < 0.05.
Results
Using a yeast two-hybrid screen of the C-terminal region of NaV1.4 against a skeletal muscle cDNA library, we isolated seven clones that grew on the medium lacking histidine, and six of the seven were also positive for beta-galactosidase. Three of the beta-galactosidase positive clones were full-length clones for CaM. The C-terminal region of NaV1.5 also bound CaM in the yeast two-hybrid assay. This evidence for direct binding of CaM to the C-terminal region of the muscle NaChs is in agreement with work of others. Mori et al. (2000) found that the C-terminal domain of NaV1.2 binds to CaM using yeast two-hybrid and gel mobility assays. Protein binding assays showed that the C-termini of NaV1.4 and NaV1.5 bind directly to CaM (Deschenes et al. 2002). Using GST-fusion proteins of the full-length C-terminal of NaV1.4, Herzog et al. (2003) showed that CaM binds to NaV1.4 in both low and high Ca2+. CaChs have CaM-binding domains in the C-terminal region and are inactivated by Ca2+–CaM (de Leon et al. 1995; Zuhlke & Reuter, 1998; Peterson et al. 1999). Since all NaChs contain putative Ca2+-independent CaM-binding IQ domains in the C-terminal region, we hypothesized that binding of CaM to the C-terminal of the channel results in modification of channel properties. We anticipated that we would find both Ca2+-independent and Ca2+-dependent effects of CaM upon sodium channel function. Since the binding of CaM to the SK2 channel in the yeast two-hybrid system is Ca2+ independent (Keen et al. 1999) and CaM binding to an IQ motif is typically Ca2+ independent, we predicted that apoCaM and mutants of CaM that are unable to bind Ca2+ would have effects upon NaChs. CaM effects on CaChs and K+ channels can be Ca2+ dependent (Peterson et al. 1999; Schumacher et al. 2001). Thus, our working model was that CaM was tethered to the channel at the IQ domain via Ca2+-independent binding and that CaM changed its conformation upon binding of Ca2+. We used whole-cell voltage-clamp recordings to test for effects of CaM on NaCh function, and we used modulators of CaM, including both Ca2+ and peptides that bind to CaM, to alter this interaction.
Application of CaM shifts the voltage dependence of activation and inactivation of NaV1.4
Currents were recorded from CHO cells transiently transfected with NaV1.4, with Ca2+-buffered intracellular recording solution (5 mm EGTA unless otherwise noted). The CaM inhibitors W5, mastoparan and CaM-inhibitory peptide (CIP, a 17 amino-acid peptide corresponding to the CaM-binding domain of myosin light-chain kinase), were applied to the cells through the pipette and had no effect on whole-cell currents measured (CIP data shown in Fig. 1A and B). Because interactions between CaM and its effector molecules are often mediated by Ca2+, we tested the effect of increasing intracellular Ca2+ on Na+ currents in these cells. Including Ca2+ (buffered to 10 μm) in the intracellular recording solution had no effect on currents measured (Fig. 1A and B).
Figure 1. Effect of CaM and modulators of CaM on Na+ currents in CHO cells transiently transfected with NaV1.4.
A and B, increased Ca2+ and CIP had no effect on currents. A, normalized peak current–voltage (I–V) relationships, elicited by 30 ms voltage-clamp steps from –90 to +100 mV in 10 mV increments (mean ± s.e.m.). NaV1.4 whole-cell currents (▪, n = 21) were unchanged with increased Ca2+ (▵, n = 13) or CIP (⋄, n = 6) included in the pipette. Inset shows one example of current traces used to generate the I–V curve. B, voltage dependence of steady-state activation and inactivation. Steady-state inactivation was measured by a two-pulse protocol with 100 ms conditioning pulses from −150 to +20 mV followed by a 30 ms test pulse to −10 mV. The data were fitted using a Boltzmann distribution. There was no shift in either activation or inactivation with 10 μm Ca2+ (▵) or 75 pm CIP (⋄). C and D, inclusion of CaM in the recording pipette caused hyperpolarizing shifts in the current. C, normalized I–V relationships for NaV1.4 alone (▪, n = 21) and with 10 μm CaM (◃, n = 11), 30 μm CaM (▹, n = 4), or 50 μm CaM (⋄, n = 6) included in the pipette (mean ± s.e.m.). D, activation and steady-state inactivation shifted with CaM included in the pipette. NaV1.4 alone (▪); +10 μm CaM (◃); +30 μm CaM (▹); +50 μm CaM (⋄). E and F, shifts in the V1/2 of activation (E) and steady-state inactivation (F) of NaV1.4 relative to the values for NaV1.4 alone from Fig. 1B and D.
One possible explanation for the lack of effect of high intracellular Ca2+ or CaM inhibitors was that the endogenous CaM concentration was a limiting factor when NaV1.4 was overexpressed. This seemed plausible since the amount of free CaM in HEK293 cells, another mammalian heterologous expression system, has been estimated to lie between 1 and 50 nm (Persechini & Stemmer, 2002). Therefore, we raised levels of CaM in the cell by adding it directly to the Ca2+-buffered intracellular recording solution. The inclusion of CaM protein in the pipette solution caused a hyperpolarizing shift in the peak of the current–voltage (I–V) relationship and in the voltage dependence of both activation and steady-state inactivation (Fig. 1C and D). The highest concentration of CaM used (50 μm) caused a shift of approximately −14 mV for both activation and inactivation. The data are summarized in Fig. 1E and F and in Table 1. These results indicated a functional interaction between CaM and NaV1.4 under conditions of low intracellular Ca2+. The concentrations of CaM required for the effects are in the same range as those required for effects on CaChs in excised patches (see Discussion). The results also suggested that endogenous CaM is a limiting factor when NaChs are transiently overexpressed. Therefore, in the subsequent experiments NaChs were coexpressed with wild type or mutant CaMs to study the interaction between CaM and NaV1.4. Thus, we are assuming that the native condition is NaV1.4 coexpressed with CaMWT rather than NaV1.4 expressed alone.
Table 1.
Functional effects of WT and CaM mutants on NaV1.4 and NaV1.5 expressed in CHO cells
| Steady–state inactivation | Decay | Activation | |||||
|---|---|---|---|---|---|---|---|
| V1/2 (mV) | k | n | (τh in ms) | V1/2 (mV) | k | n | |
| NaV1.4 | −59.8 ± 1.1 | 8.4 ± 0.3 | 25 | 0.72 ± 0.03 | −14.4 ± 1.0 | 7.9 ± 0.3 | 21 |
| +10 μm Ca2+ | −58.9 ± 1.6 | 7.7 ± 0.2 | 12 | 0.85 ± 0.03 | −14.1 ± 0.9 | 7.3 ± 0.3 | 13 |
| +75 pm CIP | −60.8 ± 1.0 | 7.6 ± 0.2 | 5 | 0.76 ± 0.07 | −15.1 ± 1.5 | 7.3 ± 0.5 | 6 |
| +10 μm CaM | −65.3 ± 1.2* | 8.0 ± 0.4 | 10 | 0.56 ± 0.04 | −15.7 ± 1.9 | 7.9 ± 0.4 | 11 |
| +30 μm CaM | −66.7 ± 1.0* | 7.4 ± 0.6 | 3 | 0.90 ± 0.11 | −19.9 ± 0.8 | 7.1 ± 0.6 | 4 |
| +50 μm CaM | −74.4 ± 1.9* | 7.2 ± 0.5 | 5 | 0.65 ± 0.05 | −27.9 ± 2.2* | 7.2 ± 0.5 | 6 |
| NaV1.4 +CaMWT | −71.1 ± 1.8* | 7.6 ± 0.4 | 14 | 0.59 ± 0.03 | −29.3 ± 2.1* | 5.9 ± 0.4 | 13 |
| +10 μm Ca2+ | −64.0 ± 1.7ϕ; | 7.6 ± 0.3 | 16 | 0.69 ± 0.07 | −25.2 ± 1.4 | 6.2 ± 0.4 | 16 |
| +75 pm CIP | −62.8 ± 2.0ϕ; | 7.1 ± 0.6 | 9 | 0.75 ± 0.05 | −25.0 ± 1.9 | 5.9 ± 0.5 | 9 |
| +75 pm CIPc | −68.8 ± 2.7 | 6.6 ± 0.3 | 6 | 0.67 ± 0.06 | −29.6 ± 3.0 | 6.3 ± 0.7 | 5 |
| +25 μm CBP | −61.5 ± 1.3ϕ; | 6.4 ± 0.2 | 14 | 0.74 ± 0.04 | −23.5 ± 1.9 | 6.1 ± 0.5 | 11 |
| +10 μm KN93 | −69.2 ± 2.3 | 6.8 ± 0.3 | 10 | 0.54 ± 0.05 | −29.7 ± 1.4 | 5.8 ± 0.5 | 8 |
| NaV1.4 +CaM1234 | −64.5 ± 1.6 | 7.4 ± 0.3 | 8 | 0.61 ± 0.04 | −25.5 ± 1.9* | 7.7 ± 0.5 | 8 |
| +10 μm Ca2+ | −66.4 ± 1.3 | 7.6 ± 0.2 | 14 | 0.65 ± 0.03 | −23.1 ± 1.2 | 7.0 ± 0.5 | 10 |
| +75 pm CIP | −65.6 ± 2.5 | 7.4 ± 0.6 | 9 | 0.74 ± 0.05 | −24.6 ± 1.2 | 6.5 ± 0.6 | 6 |
| +25 μm CBP | −64.7 ± 1.4 | 6.8 ± 0.3 | 7 | 0.63 ± 0.03 | −23.9 ± 1.9 | 6.1 ± 0.6 | 6 |
| NaV1.4 +CaM12 | −58.6 ± 1.8ϕ; | 6.5 ± 0.5 | 10 | 0.66 ± 0.03 | −16.6 ± 1.0ϕ; | 6.8 ± 0.4 | 9 |
| +10 μm Ca2+ | −59.6 ± 2.9ϕ; | 7.4 ± 0.5 | 8 | 0.69 ± 0.08 | −14.8 ± 1.6ϕ; | 6.1 ± 0.4 | 7 |
| +75 pm CIP | −61.0 ± 1.3ϕ; | 6.2 ± 0.3 | 9 | 0.80 ± 0.05 | −17.3 ± 1.1ϕ; | 5.9 ± 0.4 | 9 |
| NaV1.4 +CaM34 | −68.4 ± 1.6* | 7.3 ± 0.2 | 9 | 0.72 ± 0.06 | −24.1 ± 2.7* | 7.5 ± 0.4 | 9 |
| +10 μm Ca2+ | −54.2 ± 1.3† | 7.6 ± 0.6 | 10 | 0.71 ± 0.06 | −15.3 ± 1.7† | 6.2 ± 0.4 | 7 |
| +75 pm CIP | −57.4 ± 2.2† | 7.3 ± 0.4 | 8 | 0.70 ± 0.06 | −16.8 ± 1.2† | 7.5 ± 0.4 | 7 |
| I1727E | −61.3 ± 1.2 | 11.9 ± 0.7 | 5 | 0.69 ± 0.05 | −12.3 ± 1.6 | 9.1 ± 0.5 | 5 |
| I1727E +CaMWT | −62.4 ± 1.5 | 8.2 ± 0.6 | 9 | 0.77 ± 0.07 | −15.2 ± 1.7 | 8.1 ± 0.4 | 9 |
| +10 μm Ca2+ | −63.7 ± 1.2 | 7.9 ± 0.4 | 8 | 0.66 ± 0.07 | −12.7 ± 0.6 | 8.9 ± 0.5 | 7 |
| +75 pm CIP | −62.0 ± 2.2 | 7.5 ± 0.5 | 9 | 0.64 ± 0.04 | −16.1 ± 1.8 | 8.4 ± 0.4 | 9 |
| I1727E +CaM1234 | −63.8 ± 1.3 | 7.9 ± 0.3 | 10 | 0.56 ± 0.04 | −16.0 ± 1.8 | 8.2 ± 0.4 | 10 |
| L1736R | −61.3 ± 1.2 | 8.4 ± 0.7 | 6 | 0.79 ± 0.07 | −17.9 ± 1.1 | 7.2 ± 0.4 | 6 |
| L1736R +CaMWT | −70.1 ± 2.0‡ | 7.5 ± 0.4 | 7 | 0.71 ± 0.05 | −29.6 ± 2.3‡ | 6.2 ± 0.4 | 10 |
| +10 μm Ca2+ | −70.7 ± 2.8‡ | 6.7 ± 0.3 | 8 | 0.58 ± 0.04 | −26.3 ± 1.8‡ | 6.1 ± 0.5 | 7 |
| +75 pm CIP | −69.2 ± 1.5‡ | 6.4 ± 0.2 | 9 | 0.66 ± 0.03 | −28.0 ± 1.2‡ | 6.0 ± 0.4 | 10 |
| L1736R +CaM1234 | −66.3 ± 2.0 | 8.1 ± 0.4 | 10 | 0.64 ± 0.04 | −22.0 ± 1.3 | 7.8 ± 0.4 | 9 |
| NaV1.5 | −66.0 ± 2.0 | 10.1 ± 0.4 | 20 | 1.16 ± 0.07 | −32.7 ± 1.3 | 8.7 ± 0.5 | 20 |
| NaV1.5 +CaMWT | −72.4 ± 1.7 | 9.1 ± 0.3 | 21 | 1.04 ± 0.07 | −39.1 ± 1.3 Θ | 7.8 ± 0.4 | 20 |
| +10 μm Ca2+ | −69.5 ± 1.1 | 9.4 ± 0.3 | 17 | 1.15 ± 0.07 | −39.2 ± 1.8 Θ | 8.4 ± 0.5 | 17 |
| +75 pm CIP | −73.4 ± 2.0 | 8.7 ± 0.1 | 15 | 1.14 ± 0.08 | −38.6 ± 1.4 Θ | 9.0 ± 0.6 | 13 |
| +25 μm CBP | −74.0 ± 2.3 | 10.6 ± 0.7 | 9 | 1.09 ± 0.10 | −39.8 ± 1.6 Θ | 8.7 ± 0.8 | 7 |
| +10 μm KN93 | −77.2 ± 1.6 Θ | 9.1 ± 0.3 | 16 | 0.98 ± 0.08 | −48.1 ± 1.7 Θσ | 6.2 ± 0.5 | 14 |
| +10 μm KN92 | −70.6 ± 2.7 | 8.8 ± 0.6 | 5 | 0.89 ± 0.14 | −39.6 ± 2.2 | 7.7 ± 0.9 | 5 |
| NaV1.5 +CaM1234 | −69.4 ± 1.7 | 8.4 ± 0.2 | 12 | 1.17 ± 0.09 | −33.7 ± 1.6 | 8.7 ± 0.6 | 11 |
| +10 μm Ca++ | −71.1 ± 1.3 | 9.9 ± 0.3 | 16 | 1.16 ± 0.07 | −35.0 ± 1.1 | 8.9 ± 0.5 | 17 |
| +25 μm CBP | −69.8 ± 2.3 | 9.1 ± 0.2 | 9 | 1.16 ± 0.11 | −35.5 ± 1.8 | 8.2 ± 0.7 | 8 |
| +10 μm KN93 | −69.6 ± 2.0 | 8.0 ± 0.4 | 11 | 1.15 ± 0.10 | −34.0 ± 1.7 | 9.9 ± 0.7 | 8 |
| NaV1.4/CT1.5 | −46.3 ± 1.3 | 8.9 ± 0.4 | 6 | 1.24 ± 0.13 § | −20.7 ± 1.4 | 7.9 ± 0.5 | 6 |
| NaV1.4/CT1.5+CaMWT | −54.2 ± 1.7ζ | 9.1 ± 0.3 | 16 | 1.23 ± 0.10 § | −29.0 ± 1.6ζ | 6.1 ± 0.3 | 16 |
| +10 μm Ca2+ | −53.3 ± 2.2ζ | 8.7 ± 0.4 | 8 | 1.06 ± 0.07 § | −19.6 ± 1.5Ψ | 6.7 ± 0.5 | 8 |
| +75 pm CIP | −54.2 ± 1.5ζ | 9.9 ± 0.6 | 10 | 1.09 ± 0.07 § | −21.2 ± 1.3Ψ | 7.3 ± 0.5 | 8 |
| NaV1.5/CT1.4 | −69.1 ± 1.8 | 9.3 ± 0.3 | 21 | 0.86 ± 0.05 ɛ | −22.8 ± 1.2 | 11.0 ± 0.4 | 21 |
| NaV1.5/CT1.4+CaMWT | −87.2 ± 1.2 ! | 8.8 ± 0.1 | 19 | 0.72 ± 0.03 ɛ | −32.6 ± 1.6! | 10.1 ± 0.4 | 18 |
| +10 μm Ca2+ | −80.6 ± 1.5!ρ | 9.4 ± 0.3 | 14 | 0.79 ± 0.03 ɛ | −32.9 ± 1.4! | 9.6 ± 0.5 | 11 |
| +75 pm CIP | −82.6 ± 1.8!ρ | 8.9 ± 0.3 | 11 | 0.83 ± 0.03 ɛ | −32.5 ± 2.2! | 9.5 ± 0.5 | 11 |
Significantly different from NaV1.4 expressed alone;
significantly different from NaV1.4 coexpressed with CaMWT;
significantly different from NaV1.4 coexpressed with CaM34;
significantly different from L1736R expressed alone;
significantly different from NaV1.5 expressed alone;
significantly different from NaV1.5 coexpressed with CaMWT;
significantly different from NaV1.4/CT1.5 expressed alone;
significantly different from NaV1.4/CT1.5 coexpressed with CaMWT;
significantly different from NaV1.5/CT1.4 expressed alone;
significantly different from NaV1.5/CT1.4 coexpressed with CaMWT;
significantly different from the decay for NaV1.4, but not for NaV1.5:
significantly different from the decay for NaV1.5, but not for NaV1.4.
CaM coexpression with NaV1.4
CaMWT and CaM1234 were expressed as fusion proteins with GFP at the amino-terminal end of CaM. For CaM1234, a glutamate required for Ca2+ binding was mutated to glutamine in all four Ca2+-binding EF hands, diminishing the Ca2+ affinity of CaM by several orders of magnitude (Mukherjea et al. 1996). Addition of the GFP tag to CaM does not change its cellular distribution or its functional properties when GFP-CaM is expressed in HEK293 cells (Erickson et al. 2001).
Coexpression of either CaMWT or CaM1234 with NaV1.4 did not significantly affect the current density (data not shown) or the time constant of rapid inactivation (Fig. 2A). However, coexpression of either CaMWT or CaM1234 with NaV1.4 did cause a hyperpolarizing shift in the peak of the I–V relationship (Fig. 2B). In addition, there was a hyperpolarizing shift of between 10 and 15 mV in the voltage dependence of activation (for both CaMWT and CaM1234) and inactivation (for CaMWT) of NaV1.4 current (Fig. 2C). The shift seen with CaMWT coexpression was similar to that observed with 50 μm CaM added to the intracellular recording solution (Fig. 1E and F), suggesting that endogenous CaM was indeed limiting in CHO cells with NaV1.4 overexpression. The hyperpolarizing shifts in both activation and steady-state inactivation by CaMWT (Fig. 2C) were statistically significant compared with NaV1.4 expressed alone. For reasons that are not clear, it is easier to obtain good whole-cell recordings with large sodium currents when the intracellular recording anion is fluoride (e.g. see Qu et al. 2000). For that reason most studies are done with high intracellular F−. Since Cl− is the physiological intracellular anion, we repeated these measurements with F− replaced by Cl− in the pipette. The voltage dependence of inactivation is shifted relative to that obtained with F−, but a hyperpolarizing shift still occurred when CaM was coexpressed (V1/2=−52.0 ± 0.7 mVversus−44.5 ± 1.1 mV for control). Thus, the effect of CaM was independent of the intracellular anion.
Figure 2. Coexpression of NaV1.4 with CaMWT or CaM1234.
A, normalized representative sodium currents through NaV1.4 in the absence (continuous line) and presence of coexpressed CaMWT (dashed line) or Ca2+-binding deficient mutant CaM1234 (dotted line). Currents were elicited by a 30 ms step to −10 mV from a holding potential of −120 mV. Time constants for inactivation (control = 0.67 ± 0.02 ms; + CaMWT= 0.59 ± 0.03 ms; + CaM1234= 0.61 ± 0.04 ms) were not significantly different. CaMWT is designated by WT in all panels. B, normalized I–V relationships for NaV1.4 (▪, n = 21) coexpressed with CaMWT (□, n = 13) or CaM1234 (▵, n = 8), as described in Fig. 1A. C, the voltage dependence of both activation and inactivation for NaV1.4 shifted significantly with coexpression of CaMWT (−14.9 mV and −11.3 mV, respectively). CaM1234 caused a significant shift in activation (−11.1 mV), but had no significant effect on inactivation. NaV1.4 alone (▪); + CaMWT (□); + CaM1234 (▵). D, modulators of CaM (10 μm Ca2+, 25 μm CBP or 75 pm CIP) caused a depolarizing shift in V1/2 for steady-state inactivation. + CaMWT+10 μm Ca2+ (▵, n = 16); + CaMWT+ 25 μm CBP (◃, n = 14); + CaMWT+ 75 pm CIP (⋄, n = 9). E, shifts in the V1/2 of activation relative to the values for NaV1.4 coexpressed with CaMWT (filled bars) or CaM1234 (open bars). Ca2+, CBP and CIP included in the pipette were not significantly different from CaMWT alone. Likewise, the addition of these modulators of CaM to cells expressing CaM1234 did not produce any changes in V1/2 of activation. CIPc (75 pm; a non-functional peptide analogue of CIP) and 10 μm KN93 (a blocker of CaM activation of CKII) had no effect. F, shifts in the V1/2 of steady-state inactivation relative to the values for NaV1.4 coexpressed with CaMWT (filled bars) or CaM1234 (open bars). Ca2+, CBP and CIP caused significant shifts from CaMWT alone (*). 75 pm CIPc and 10 μm KN93 had no effect. CaM modulators had no effect on the voltage dependence of inactivation of currents with CaM1234 coexpression.
Since CaMWT had an effect on NaV1.4 at low internal free-Ca2+ concentration (5 mm EGTA with no added Ca2+ in the recording solution), apoCaM must be the predominant CaM species present. Therefore, we predicted that CaM1234 would similarly affect voltage-dependent properties. However, application of CaM1234 produced a significant shift only for activation, not inactivation (Table 1 and Fig. 2). There are at least two possible explanations for this difference between the actions of CaMWT and CaM1234. First, it is conceivable that the C lobe of CaMWT has one or two Ca2+ ions bound even at very low Ca2+ concentrations since the affinity for Ca2+ is strongly influenced by the protein environment of the Ca2+-binding sites (Saimi & Kung, 2002; Putkey et al. 2003). However, even if the C lobe has one or two Ca2+ ions bound, it is extremely unlikely that the N lobe has any Ca2+ bound under these conditions. Second, CaM1234 may not be equivalent to apoCaM because the amino acid substitutions that reduce Ca2+ binding (glutamate to glutamine) may also lead to conformational changes that alter CaM binding to the sodium channel. In summary, these recordings under low-Ca2+ conditions are consistent with a Ca2+-independent interaction between CaM and NaV1.4.
The essential underlying question in these studies was whether changes in CaM conformation elicited by molecules or proteins that bind to CaM would affect the biophysical properties of the channel. A change in Ca2+ concentration is the most obvious modulator of CaM conformation. Increased Ca2+ in the cells expressing NaV1.4 and CaMWT caused a depolarizing shift in steady-state inactivation but not activation (the depolarizing shift in activation in Fig. 2D was not statistically significant). We reasoned that other factors that would produce a conformational change in CaM might also alter the voltage-dependent properties of the channel. Two CaM-binding peptides, 75 pm CIP and 25 μm CBP (a 29-amino-acid peptide corresponding to the CaM-binding domain of CaM-kinase II (CKII)) also caused significant depolarizing shifts of inactivation in the CaM-cotransfected cells (Fig. 2D and F). One explanation for this depolarizing shift is that CIP and CBP compete with the IQ domain for binding to CaM and could cause CaM to dissociate from the channel producing voltage dependence of inactivation similar to that of the channel expressed without CaM. The depolarizing shifts of 7–10 mV seen with Ca2+, CIP and CBP compared with CaMWT alone were significant for the voltage dependence of steady-state inactivation (Fig. 2F), but despite a similar trend, the shifts were not significant for activation (Fig. 2E). It seems likely that binding of Ca2+ produces a different conformational change in CaM than the binding of the peptides. Nevertheless, both types of ligands produced a depolarizing voltage shift of inactivation when CaMWT was coexpressed. The inclusion of 10 μm Ca2+, 25 μm CBP or 75 pm CIP in the pipette had no effect on either activation or inactivation when the Ca2+-insensitive mutant CaM1234 was coexpressed with NaV1.4 (Fig. 2E and F). We conclude that Ca2+ and CaM-binding peptides modulate the effects of CaM and that activation and inactivation are not equally regulated by CaM.
CaM often affects target proteins through effector molecules such as phosphatases or kinases. NaV1.4 is not affected by cAMP-dependent protein kinase, despite the presence of consensus sequences for phosphorylation in the α-subunit (Yang & Barchi, 1990; Smith & Goldin, 1992). NaChs in rat cerebellar granule cells are regulated by CKII (Carlier et al. 2000). Therefore, it was possible that CaM was acting on NaV1.4 through phosphorylation of the channel by CKII. To test this hypothesis, we applied KN93, a specific inhibitor of CKII that competes for the binding site of CaM on the kinase. KN93 had no effect on the hyperpolarizing shifts in both activation and inactivation seen with CaMWT coexpression (Fig. 2E and F). This suggests that the voltage-dependent shifts caused by CaM were not due to phosphorylation of NaV1.4 by CKII.
The yeast two-hybrid interaction and the failure of a kinase inhibitor to modify the effects of CaM coexpression indicate that the hyperpolarizing shifts in the V1/2 of activation and inactivation of the Na+ current when CaM is coexpressed are a result of CaM binding directly to the channel. This interaction is likely to be Ca2+ independent, since the Ca2+ concentration in these cells was held in the low nanomolar range by including EGTA in the intracellular recording solution. Further tests, described below, were designed to identify the regions of CaM and NaV1.4 that are required for this interaction.
Effects of mutations in the N and C lobes of CaM
The N- and C-terminal halves of CaM have different effects on ion channels. For example, an intact C lobe is required for Ca2+-dependent inactivation of the L-type CaCh (Peterson et al. 1999) while an intact N lobe is required for the effects of CaM on SK Ca2+-activated K+ channels (Keen et al. 1999). Yue and colleagues (Liang et al. 2003) have recently proposed a unifying model for CaM's effects on CaChs: the N lobe detects global Ca2+ and the C lobe responds to high local Ca2+. In addition, the N lobe of CaM is important for voltage-dependent inactivation and the C lobe for Ca2+-dependent inactivation. The structural basis for these effects must rely on differing affinities for Ca2+ of the two lobes of CaM with different conformational changes of the protein (Tjandra et al. 1995; Finn et al. 1995; Kuboniwa et al. 1995; Zhang et al. 1995). By perturbing states of Ca2+ binding between the lobes, we can alter specific conformational changes of CaM that could affect its ability to bind to NaV1.4. Therefore, NaV1.4 was coexpressed with either CaM12 or CaM34, which have reduced affinity for Ca2+ in either the N (CaM12) or C (CaM34) lobes of CaM (an aspartate to alanine mutation in each pair of EF hands in either the N or C lobe of CaM) (Putkey et al. 1989; Peterson et al. 1999; DeMaria et al. 2001). These CaM mutants allowed us to test the relative importance of the individual lobes of CaM for the shifts in the voltage dependence of activation and inactivation.
The biophysical properties of NaV1.4 did not change when the channel was coexpressed with CaM12 compared with the channel expressed alone. In contrast to the shift in the voltage dependence of currents seen with CaMWT coexpression with NaV1.4, CaM12 coexpression had no effect on the voltage dependence of activation or inactivation (Fig. 3A and filled bars in C). This mutation of the Ca2+ binding sites in the N lobe of CaM produced an inactive CaM when coexpressed with the channel. Increased Ca2+ and CIP had no further effect on the voltage dependence of activation or inactivation when CaM12 was expressed (Fig. 3D and E). The loss of Ca2+ binding in the N lobe of CaM abolished not only the voltage shifts in activation and inactivation, but also the modulatory effects of Ca2+ and CIP, providing further evidence that the effects of Ca2+ and CIP (Fig. 2D – F) depend on the association of a Ca2+-sensitive CaM with the channel. Since CaM12 had no effects on measured channel properties, we used anti-CaM antibodies for immunolabelling of transfected cells (cotransfected with GFP) to ensure that the mutant CaM was being expressed. Labelling of GFP and CaM cotransfected cells was 2- to 5-fold greater than non-transfected cells and control GFP- transfected cells. This increase in CaM labelling was similar to that observed for wild type and the other CaM mutants (data not shown). In summary, these effects of mutations in the N lobe were simple, i.e. a complete loss of action by CaM.
Figure 3. Coexpression of NaV1.4 with mutant CaMs lacking Ca+ binding in either the N-terminal (CaM12) or C-terminal (CaM34) lobes.
A, currents from cells with coexpression of CaM12 with NaV1.4 were unchanged from NaV1.4 expressed alone. CaMWT coexpression is shown for comparison: + CaMWT (▪, n = 13); + CaM12 (▵, n = 10). Addition of Ca2+ (10 μm, ▵, n = 8) or CIP (75 pm, ⋄, n = 9) was not different from CaM12. Cartoons depict mutations in the Ca2+-binding sites of CaM (N-terminal for panel A and C-terminal for panel B). B, coexpression of CaM34 caused a hyperpolarizing shift in the voltage dependence of both activation and inactivation, similar to CaMWT. + CaMWT (▪); + CaM34 (▵, n = 9); + CaM34+ 10 μm Ca2+ (▵, n = 10); + CaM34+ 75 pm CIP (⋄, n = 8). C, shifts in the V1/2 of activation and inactivation of NaV1.4 coexpressed with CaM12 or CaM34 relative to coexpression with CaMWT. Coexpression of CaM12 (filled bars) was significantly different from CaMWT, and not significantly different from NaV1.4 expressed alone. The shifts in both activation and inactivation with CaM34 (open bars) were not significantly different compared with CaMWT. D, Ca2+ or CIP induced shifts in the V1/2 of activation relative to coexpression with either CaM12 (filled bars) or CaM34 (open bars). Ca2+ and CIP had no effect on currents with CaM12. The depolarizing shifts with 10 μm Ca2+ and 75 pm CIP were significant compared with CaM34 alone. E, Ca2+ or CIP induced shifts in the V1/2 of steady-state inactivation relative to coexpression with either CaM12 or CaM34. Ca2+ and CIP had no effect on currents with CaM12. The depolarizing shifts seen with Ca2+ and CIP were significant compared with CaM34 alone.
CaM34 coexpressed with NaV1.4, on the other hand, produced hyperpolarizing shifts in both activation and inactivation, similar to those seen with CaMWT coexpression (Fig. 3B and C; open bars were not significantly different from CaMWT coexpression). The CaM modulators Ca2+ and CIP caused statistically significant depolarizing shifts in both activation and steady-state inactivation (Fig. 3B, and open bars in 3D and 3E), in contrast to CaMWT coexpression for which only inactivation was affected. Thus, this mutant CaM which did not bind Ca2+ in the C lobe was almost equivalent to wild type CaM (but was surprisingly more responsive to Ca2+). Because the depolarizing shifts seen with Ca2+ and CIP were larger with CaM34 than with CaMWT coexpression, we conclude that Ca2+ binding by the C-terminal domain of CaM is not required for CaM/Ca2+–CaM effects, but that it contributes in a more subtle way to this interaction.
Mutations of two amino acids in the IQ domain
The IQ-binding motif is a known consensus sequence for CaM binding in a variety of different proteins (Rhoads & Friedberg, 1997) and is present in all known NaChs in the C-terminal domain. Mutations in this domain in CaChs alter or abolish CaM binding and its functional effects on the channel (Peterson et al. 1999; Zuhlke et al. 1999). CaM has been shown to bind NaV1.2 in this region by yeast two-hybrid and gel mobility-shift assays (Mori et al. 2000). To define the region of the C-terminal required for binding between NaV1.4 and CaM, two mutations (expressed separately) in the IQ domain (IQRAYRRHLLQRSKV; I1727E and L1736R) were made at residues known to participate in CaM binding in CaChs and NaV1.5 (Tan et al. 2002).
When the channel was expressed by itself, these mutations did not change channel properties compared with native NaV1.4 (data not shown). However, when coexpressed with CaMWT or CaM1234, the I1727E mutation blocked all effects of CaMWT (Fig. 4A and B) and CaM1234 (data not shown). Although currents tended to be smaller for the mutated channel, there was no significant difference in the current density between native NaV1.4 and the I1727E mutant channel coexpressed with CaMWT (data not shown). Thus, the mutation does not affect surface expression even though the channel behaves as if CaM is not associated with it. The addition of Ca2+ or CIP in the recording solution also had no effect on voltage dependence (filled bars in Fig. 4E and F). Therefore, this mutation in the IQ domain of NaV1.4 abolished the shifts in voltage dependence caused by CaMWT and by modulators of CaM. This is consistent with a model in which the proximal end of the IQ domain (which includes the IQ) is required for CaM binding to the sodium channel (see Discussion).
Figure 4. Coexpression of NaV1.4 IQ-domain mutants with CaMWT.
A, normalized I–V relationships (mean ± s.e.m.). I1727E whole-cell currents (▪, n = 5) were unchanged by coexpression with CaMWT (○, n = 9). Whole-cell currents from I1727E were not significantly different from native NaV1.4 (data not shown). CaMWT is designated WT in all panels. B, the I1727E mutation blocked all shifts in V1/2 of activation or inactivation seen with coexpression of CaMWT. Curves for I1727E were not significantly different compared with native NaV1.4 (data not shown). I1727E (▪); I1727E + CaMWT (○); I1727E + CaMWT+ 10 μm Ca2+ (▵, n = 8); I1727E + CaMWT+ 75 pm CIP (⋄, n = 9). C, normalized I–V relationships for L1736R (mean ± s.e.m.). L1736R whole-cell currents (▪, n = 5) were shifted by coexpression with CaMWT (○, n = 10). Whole-cell currents from L1736R were not significantly different from native NaV1.4 (data not shown). D, the L1736R mutated channel retained its responsiveness to CaMWT coexpression but activation and inactivation were no longer affected by Ca2+ and CIP with CaMWT coexpression. The V1/2 of activation and inactivation for L1736R was not significantly different compared with native NaV1.4 (data not shown). L1736R (▪); L1736R + CaMWT (○); L1736R + CaMWT+ 10 μm Ca2+ (▵, n = 8); L1736R + CaMWT+ 75 pm CIP (⋄, n = 9). E, shifts in the V1/2 of activation for I1727E (filled bars) and L1736R (open bars) coexpressed with CaMWT, relative to NaV1.4. Data for coexpression of native NaV1.4 coexpressed with CaMWT (cross-hatched bar) were replotted from Fig. 2 for comparison. The I1727E mutation abolished the effects of CaMWT coexpression (ϕ). For the L1736R mutation, V1/2 of activation with CaMWT was significantly shifted compared with L1736R alone, but not different from native NaV1.4 coexpressed with CaMWT. Ca2+ and CIP caused no significant shift compared with L1736R + CaMWT. F, shifts in the V1/2 of steady-state inactivation for I1727E and L1736R coexpressed with CaMWT, relative to NaV1.4. Data for coexpression of native NaV1.4 coexpressed with CaMWT were replotted from Fig. 2 for comparison. The I1727E mutation abolished the effects of CaMWT coexpression (ϕ; P < 0.05). For the L1736R mutation, activation with CaMWT coexpression was significantly shifted compared with L1736R alone. Addition of Ca2+ and CIP caused no significant shift compared with L1736R + CaMWT.
The L1736R mutation produced more subtle effects on channel properties in that it altered some CaM effects but not others. It did not disrupt effects on channel activation or inactivation with coexpression of CaMWT (Fig. 4C and D) or CaM1234 (data not shown). However, inclusion of 10 μm Ca2+ or CIP in the intracellular recording solution in the pipette no longer caused significant depolarizing shifts in the voltage dependence of inactivation with CaMWT coexpression (open bars in Fig. 4F; compare with filled bars in Fig. 2F).
One mutation (I1727E) blocked all effects of CaM, as though CaM was no longer bound to or was able to interact with the channel. The other mutation (L1736R) preserved some effects, presumably due to association of apoCaM with the channel, while abolishing others, as though it interferes with a conformational change or shift in the binding of CaM to the channel in the presence of increased Ca2+ or CIP. These results suggest that the amino-terminal end of the IQ domain is essential for apoCaM binding. The carboxyl end of the IQ domain is more important for effects caused by increased Ca2+ in the cell.
Effects of CaM coexpression on the cardiac channel, NaV1.5
In the yeast two-hybrid assay, CaM interacted with the C-terminal domains of both NaV1.4 and NaV1.5. We therefore tested whether CaM affected channel properties in NaV1.5 as well. The time constants of fast inactivation were not significantly different for NaV1.5 coexpressed with CaMWT or CaM1234 compared with NaV1.5 alone (Table 1). Coexpression of CaMWT with NaV1.5 caused a shift in the I–V curve toward negative potentials, accompanied by a significant shift in the voltage dependence of activation (Fig. 5A and B). There was no significant shift in the voltage dependence of inactivation (Fig. 5B). CaM1234 coexpression had no significant effect on either activation (unlike its effect on NaV1.4) or inactivation. In contrast to NaV1.4, including 10 μm Ca2+, 75 pm CIP or 25 μm CBP in the pipette had no effect on activation or inactivation of NaV1.5 currents coexpressed with CaMWT (Fig. 5C, E and F). Increased Ca2+ had a slight depolarizing effect on the voltage dependence of inactivation, but this shift was not significant (Fig. 5F).
Figure 5. Coexpression of NaV1.5 with CaMWT or CaM1234.
A, normalized I–V relationships for NaV1.5 (▪, n = 19) coexpressed with CaMWT (○, n = 20) or CaM1234 (▵, n = 11) (mean ± s.e.m.). Inset shows one example of current traces used to generate the I–V curve. CaMWT is designated WT in all panels. B, coexpression of CaMWT with NaV1.5 caused a significant hyperpolarizing shift in the voltage dependence of activation, but not inactivation. CaM1234 had no significant effect on currents. NaV1.5 (▪); + CaMWT (○); + CaM1234 (▵). C, modulators of CaM (10 μm Ca2+, 25 μm CBP or 75 pm CIP) had no significant effect on activation or steady-state inactivation of NaV1.5 compared with CaMWT coexpression. + CaMWT+ 10 μm Ca2+ (▵, n = 17); + CaMWT+ 25 μm CBP (◃, n = 9); + CaMWT+ 75 pm CIP (⋄, n = 15). D, blocking CKII with KN93 caused a shift in activation, but had no effect on inactivation of NaV1.5: + 10 μm KN93 (▵, n = 16); + 10 μm KN92 (*, n = 5). KN92 was unchanged from CaMWT alone. E, shifts in the V1/2 of activation for NaV1.5 relative to CaMWT coexpression. Coexpression with CaMWT caused a significant hyperpolarizing shift. Ca2+, CBP and CIP had no significant effect compared with CaMWT alone. 10 μm KN93 caused a further hyperpolarizing shift compared with CaMWT (σ). F, shifts in the V1/2 of steady-state inactivation for NaV1.5 relative to CaMWT coexpression. Coexpression with CaMWT had no significant effect on NaV1.5. CaM modulators had no significant effect compared with CaMWT alone. KN93 (10 μm) caused a hyperpolarizing shift compared with CaMWT coexpression alone (Θ).
As mentioned above, KN93 specifically inhibits activation of CKII by binding to the CaM-binding domain of the kinase, blocking CaM binding to this domain (Sumi et al. 1991). KN93 included in the pipette caused a further hyperpolarizing shift in the voltage dependence of activation with CaMWT coexpression (Fig. 5D). KN92 (an inactive structural analogue of KN93) showed no effect. This suggests that the effects of KN93 on this channel are through the inactivation of CKII. Despite its effect on activation, KN93 had no significant effect on the shift in inactivation (Fig. 5D and F). Although we cannot exclude the possibility that KN93 has a direct effect on NaV1.5 that shifts the voltage dependence of activation, the fact that KN93 had no effect on currents when CaM1234 was coexpressed with NaV1.5 argues against this possibility (data not shown).
A comparison of the results seen with CaMWT coexpressed with either NaV1.4 (Fig. 2) or NaV1.5 (Fig. 5) suggests that CaMWT acts on these channel isoforms through different pathways. CaMWT affected the voltage-dependent properties of both channels. However, CaM modulators caused depolarizing shifts on NaV1.4 currents, but had no effect on NaV1.5 currents, while KN93 only affected NaV1.5 currents.
Chimeric sodium channels formed by swapping the C-terminal domains
Studies using chimeric channels between NaV1.2 and NaV1.5 (Mantegazza et al. 2001) showed that the cytoplasmic C-terminus is important in determining the voltage dependence of steady-state inactivation but has no effect on activation. Cytoplasmic loops in the amino terminal half of the protein are important in distinguishing the activation properties of NaV1.4 and NaV1.5 from one another (Bennett, 2001). Since the C-terminal region of NaV1.4 was required for CaM's effects on the channel and coexpression of CaM had different effects on NaV1.4 and NaV1.5, chimeric channels of these two NaCh isoforms were used to test the possibility that some of the differences in the effects of CaM between NaV1.4 and NaV1.5 were based solely on the C-terminal tail. Recordings were made with NaV1.4/CT1.5 (NaV1.4 parent channel with NaV1.5 C terminal) and NaV1.5/CT1.4 (NaV1.5 parent channel with NaV1.4 C terminal; see inset in Fig. 6) coexpressed with CaMWT. The time constants of fast inactivation for the chimeric channels shifted to values of the C-terminal donor (Table 1), consistent with Deschenes et al. (2001) who showed that the donor of the C-terminal domain determined the time constant of fast inactivation for these channel isoforms. Coexpression of CaMWT had no further effect on fast inactivation.
Figure 6. Coexpression of C-terminal chimeric sodium channels with CaMWT.
A, coexpression of NaV1.4/CT1.5 with CaMWT caused a hyperpolarizing shift in the voltage dependence of activation and inactivation. V1/2 of inactivation was not shifted by 10 μm Ca2+ or 75 pm CIP. Activation was shifted by these CaM modulators. Chimera (▪, n = 6); chimera + CaMWT (○, n = 16); chimera + CaMWT+ 10 μm Ca2+ (▵, n = 8); chimera + CaMWT+ 75 pm CIP (⋄, n = 10). Inset shows cartoon of chimeric channels, with portions contributed by NaV1.4 depicted by thin lines and by NaV1.5 by thick lines. CaMWT is designated WT in all panels. B, coexpression of NaV1.5/CT1.4 with CaMWT caused a hyperpolarizing shift in the voltage dependence of activation and steady-state inactivation. V1/2 of activation was not shifted by 10 μm Ca2+ or 75 pm CIP. Steady-state inactivation was shifted by these CaM modulators. Chimera (▪, n = 21); chimera + CaMWT (○, n = 19); chimera + CaMWT+ 10 μm Ca2+ (▵, n = 14); chimera + CaMWT+ 75 pm CIP (⋄, n = 11). C, shifts in the V1/2 of activation of chimeric channels compared with native NaV1.4 + CaMWT (filled bars) and NaV1.5 + CaMWT (cross-hatched bars). The depolarizing shifts seen with the addition of Ca2+ and CIP were significantly different for NaV1.4/CT1.5+ CaMWT (open bars), similar to the activation properties of NaV1.4 (filled bars). Ca2+ or CIP had no effect on NaV1.5/CT1.4. Chimeric channels behaved like the parent channel. Lettering at the top of the panel indicates the chimeric constructs. D, shifts in the V1/2 of steady-state inactivation of chimeric channels compared with native NaV1.4 (filled bars) and NaV1.5 (cross-hatched bars). Ca2+ and CIP had no effect on inactivation in NaV1.4/CT1.5. Ca2+ and CIP produced significant depolarizing shifts of V1/2 compared with NaV1.5/CT1.4+ CaMWT (striped bars), similar to the effects of Ca2+ and CIP on the inactivation properties of native NaV1.4 (filled bars). Chimeric channels behaved like the channel contributing the carboxyl tail. Lettering at the top of the panel indicates the chimeric constructs.
CaMWT caused a hyperpolarizing shift in both activation and steady-state inactivation when coexpressed with NaV1.4/CT1.5 (Fig. 6A). Recordings of Na+ currents from NaV1.4/CT1.5 channels with Ca2+ and CIP included in the pipette showed statistically significant depolarizing shifts in the voltage dependence of activation (Fig. 6A and C), unlike the native NaV1.4 currents (compare the open bars to the filled bars in Fig. 6C). However, these modulators had no effect on steady-state inactivation, more similar to native NaV1.5 currents (compare the open bars to the cross-hatched bars in Fig. 6D). Thus, the inactivation but not the activation properties of this chimera were predicted from a simple summation of the properties of the separate pieces of the channel.
For the other chimera (NaV1.5/CT1.4), the voltage dependence of activation and steady-state inactivation of NaV1.5/CT1.4 were also shifted with coexpression of CaMWT (Fig. 6B). Ca2+ and CIP included in the recording solution did not cause a depolarizing shift in activation, similar to the lack of effect on NaV1.5 currents (Fig. 6C). However, Ca2+ and CIP caused a significant shift in the V1/2 of steady-state inactivation, similar to NaV1.4 (compare striped bars to filled bars in Fig. 6D). Thus, this chimera with a NaV1.4 carboxyl tail had inactivation properties similar to the native NaV1.4.
Dependence upon the expression system for CaM effects
One of the disturbing aspects of studies of the functional effects of CaM upon voltage-gated sodium channels is that the results are inconsistent from one laboratory to another, despite efforts to duplicate the recording solutions and cell lines used. Three previous studies used HEK293 cells (Deschenes et al. 2002; Tan et al. 2002; Herzog et al. 2003) and, in some cases, identical solutions and still reported results different from each other. We used CHO cells for the experiments described above. To determine if the use of a different cell line contributed to the effects we observed, we also recorded from HEK cells and tested the effect of coexpression of CaM and Nav1.4 upon the voltage dependence of activation and inactivation. In contrast to the shift in voltage dependence seen in CHO cells (Fig. 2C), we found no evidence of a shift in HEK cells (Fig. 7A).
Although many differences between CHO and HEK cells could account for this difference, we tested one of the most obvious possibilities, namely that β-subunit expression differs between these two cell lines. β-Subunits are not required for channel formation but do affect kinetics, voltage dependence and surface expression. Four β-subunit genes have been described. We used quantitative PCR to measure the relative abundance of the mRNA for each of these genes in CHO and HEK cells. Mouse and human brain RNA were used as controls (data not shown). The primers were chosen in coding regions that are strictly conserved for each subtype in mouse, rat and human. mRNA levels were normalized to the copies of HPRT (hypoxanthine phosphoribosyltransferase) mRNA in every case (Fig. 7B and C). Three conclusions can be drawn. First, β4 expression is non-existent in CHO cells. Second, β1 expression is absent in HEK cells. Finally, assuming that the primers have the same amplification efficiency, the overall levels of mRNA for β-subunits are much lower in HEK cells than in CHO cells (roughly a 10-fold difference). To ensure that the lack of expression of β4 in CHO cells and of β1 in HEK cells was not due to a poor choice of PCR primers, a second set of PCR primers to different coding regions was generated and used for amplification. The results were the same: β1 mRNA is missing in HEK cells and β4 mRNA is missing in CHO cells (data from both sets of primers are included in Fig. 7B and C). Thus, some of the differences between the results presented here using CHO cells and those of other laboratories using HEK cells could be due to different levels of β-subunits in the cell types (see Discussion). We tested the possibility that the absence of β1 in HEK cells was responsible for the lack of a CaM effect. Coexpression of β1 with Nav1.4 and CaM did not produce a shift in voltage dependence of inactivation (data not shown). Thus, β1 cannot explain the failure to observe an effect in HEK cells. The involvement of β4 has not been tested.
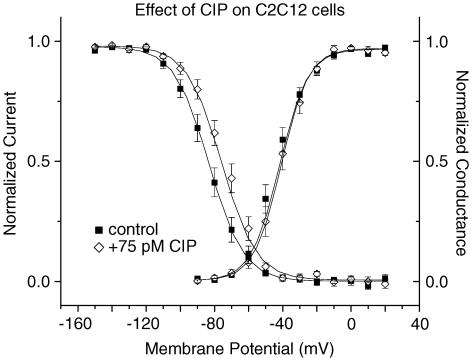
Effects of a CaM-binding peptide on Na++ currents in C2C12 cells
Endogenous muscle sodium channels are expressed by the muscle-derived C2C12 cell line. Inclusion of CIP (75 pm) in the pipette caused a significant depolarizing shift (7.4 mV, n = 10, P < 0.01) in the voltage dependence of inactivation (Fig. 8). There was no effect on the I–V relationship or the voltage dependence of activation. The magnitude of this effect is close to that observed for Nav1.4 (8.3 mV, see Table 1). Since CIP had no effect on inactivation in CHO cells when CaM1234 was expressed with Nav1.4 (Fig. 2F), the shift in C2C12 cells implies that it acts via CaM bound to the endogenous sodium channel.
Figure 8. Voltage dependence of steady-state activation and inactivation in C2C12 cells.
There was a shift in steady-state inactivation with 75 pm CIP (⋄, n = 9, V1/2=−75.8 ± 1.1 mV) compared with control (▪, n = 10, V1/2=−83.2 ± 1.2 mV) (P = 0.009). CIP had no effect on activation (control: ▪, n = 11, Va=−41.8 ± 1.0 mV; + 75 pm CIP: ⋄, n = 9, Va=−40.8 ± 1.2 mV).
Discussion
The importance of CaM regulation of a wide variety of ion channels has recently been established (Levitan, 1999; Saimi & Kung, 2002). Here we describe functional effects of CaM and Ca2+ on sodium channels NaV1.4 (skeletal muscle channel) and NaV1.5 (cardiac muscle channel). Elevation of CaM concentration by inclusion in the recording pipette produced hyperpolarizing shifts in the voltage dependence of both activation and steady-state inactivation. Transient coexpression of CaM and NaChs in CHO cells produced similar hyperpolarizing shifts of voltage dependence, whereas addition of Ca2+ and CaM-binding peptides caused depolarizing shifts of voltage dependence. CaM directly regulates the voltage dependence of NaV1.4, specifically through an IQ domain in the C-terminal. Point mutations in the IQ domain abolished all CaM effects or eliminated the modulation by Ca2+ and CaM-binding peptides. The N-terminal lobe of CaM is essential for this regulation. The effects of CaM on NaV1.5 are likely through activation of a CaM-activated kinase. Chimeric NaV1.4 and NaV1.5 channels had inactivation properties and responses to CaM that closely resembled the donor of the C-terminal domain.
These results are consistent with the following simple model (Fig. 9). The C lobe of CaM binds to a region near to or including the IQ portion of this motif at low resting levels of Ca2+. Mori et al. (2003) used circular dichroism to show that apoCaM binds to the C-terminal of Nav1.2, suggesting that the binding of apoCaM is a general feature of NaChs. Our assumption is that the Ca2+-free form of CaM is similar to the crystal structure of myosin light chain bound to IQ domains (Terrak et al. 2003). The canonical sequence (IQxxxRGxxxR) of the IQ domain of both skeletal and cardiac muscle NaChs is missing the conserved glycine (replaced by an R) and the conserved arginine at the end (replaced by a Q). The muscle IQ sequences are very similar to an IQ sequence of myosin that causes myosin light chain to adopt an extended conformation, with the C lobe binding to the IQ domain and the N lobe in a position that is away from and not bound to the IQ domain (Terrak et al. 2003). Therefore, we propose that CaM is in an extended conformation in which the N lobe of CaM binds to a different domain (the N lobe binding domain, NLBD). The NLBD could be another part of the carboxyl terminal of the sodium channel, another cytoplasmic domain of the NaCh, or another protein associated with the NaCh. Increased Ca2+ or CaM-binding peptides cause a conformational change in CaM and a shift in CaM binding that is likely to be different for Ca2+versus the CaM-binding peptides. When Ca2+ is elevated, this CaM-binding shift could be a movement of the N lobe from the NLBD to the distal IQ (containing the leucine that we mutated to arginine, which blocked the Ca2+ effect). It is unlikely that the N lobe can bind to the IQ domain when CaM-binding peptides are applied because they would interfere with this interaction. This would imply that it is the release from the NLBD that produces shifts in voltage dependence of inactivation. This conformational change in the channel/CaM interaction results in a depolarizing shift in the V1/2 of inactivation of NaV1.4. The binding of the C lobe of CaM to the IQ domain is Ca2+ independent since the CaM34 mutant retains the ability to shift the V1/2 curve. However, depolarizing shifts seen with increased Ca2+ or CIP were greatest with CaM34, indicating that these effects are primarily due to Ca2+ binding to the N lobe of CaM.
Figure 9. Model of CaM interactions with the IQ domain.
CaM is in an extended conformation with the C lobe bound to the proximal IQ region under low Ca2+ conditions (apoCaM). The sequence of the sodium channel IQ domain is similar to that of myosin IQ domains that crystallize with myosin light chain (a protein similar to CaM) in an extended conformation (Terrak et al. 2003). The N lobe interacts with another region of the carboxyl-terminal (or alternatively, other domains of the sodium channel or its subunits) termed the NLBD (N-lobe binding domain). When Ca2+ is elevated or CaM-binding peptides are added, the N lobe of CaM is released from the NLBD and, in the case of increased Ca2+, binds to the distal IQ domain (since mutation of the second leucine of RYLL abolished the Ca2+ effects).
We found that elevated Ca2+ and application of CaM-binding peptides and CaM inhibitors had no effect in cells transiently overexpressing sodium channel NaV1.4. However, micromolar CaM added to the interior of the cell through the patch pipette shifted the voltage dependence of both activation and inactivation. This suggested to us that CaM might be limiting in the cell, especially if CaM is associated with the channel during synthesis, as appears to be the case for CaChs (Erickson et al. 2001). Is the concentration of applied CaM excessive, given the measured average free-CaM concentration in HEK cells (50 nm, Persechini & Stemmer, 2002)?Dzhura et al. (2003) used 2–40 μm CaM applied to the cytoplasmic face of excised patches. The requirement for high concentrations of CaM are consistent with CaM becoming bound to the sodium channel during synthesis and with exogenous CaM having reduced access once the channel is in the plasma membrane. In this regard, it is notable that recently Mori et al. (2004) have estimated that the effective concentration of CaM in the vicinity of the carboxyl tail of the CaCh is 2.5 mm. This could be simply explained by a non-uniform and highly localized variation in CaM concentration. Given the fact that there are many CaM binding proteins in a cell, including both cytoskeletal-associated and membrane-bound proteins, free-CaM concentration could be dynamically regulated throughout the cell. Moreover, tethering of CaM in the vicinity of the NaCh or to the channel itself would be the equivalent of a high local concentration. Thus, the concentrations of CaM (10–50 μm) required for changes in voltage dependence of activation and inactivation of NaV1.4 are consistent with the results of others.
We would have predicted that coexpression of CaMWT (in low Ca2+), CaM1234 and CaM12 would have produced equal shifts in the voltage dependence, but each one had a different effect. ApoCaMWT shifted both activation and inactivation, CaM1234 shifted only activation, and CaM12 shifted neither activation nor inactivation. The differences between apoCaMWT, CaM1234 and CaM12 cannot be simply explained since they should ideally all bind if apoCaMWT is able to bind to the channel. Since NMR studies have shown considerable flexibility of CaM in solution (Chou et al. 2001) we speculate that the mutations in the N lobe and/or C lobe to lower Ca2+ affinity produce CaMs that are not structurally equivalent to apoCaMWT or to each other.
Based on our assumption that CaM is in an extended conformation, we anticipated an effect of peptides that are CaM binding sites in CaM kinase II and myosin light chain kinase. Nevertheless, the close similarity to the effects of elevated Ca2+ was remarkable. The effects of these peptides raise the possibility that CaM might coordinate a complex of the NaCh with other CaM-binding proteins such as CKII. This would place CKII in a position that could allow rapid control of phosphorylation of the channel.
Two point mutations in the IQ domain of the NaV1.4 sodium channel altered the effects of CaM. A mutation of I to E at the IQ of this domain (I1727) abolished all effects of CaM. This is a highly conserved amino acid (hence the name of the motif) and this region has been shown to be essential for binding of CaM to the IQ motif (Deschenes et al. 2002; Herzog et al. 2003). The second point mutation occurred in a distal part of the IQ motif and at a position that tends to be hydrophobic but is not strictly conserved (L1736). The L to R mutation retained the shift in voltage dependence with CaM coexpression, but the responses to Ca2+ and the CaM-binding peptide CIP were abolished. The conformations of CaM and of the NaCh at the IQ domain and the surrounding domains are not known, but we speculate on the CaM conformation based on recent crystal structure results with myosin light chain (MLC), a molecule that binds to repeated IQ domains in the myosin heavy chain and has a structure similar to CaM (Terrak et al. 2003). An IQ motif with the L substituted by a positively charged amino acid (R or K) is also associated with MLC binding in an extended conformation (Terrak et al. 2003), suggesting that the mutation L1736R could prevent the N lobe from binding to the IQ domain when Ca2+ is elevated (see Fig. 9).
Comparisons between the native and chimeric channels made by switching the C-termini of NaV1.4 and NaV1.5 (Fig. 6) showed that the effects of CaM on steady-state inactivation were determined primarily by the C-terminal donor, while activation properties of the channels were more complex. For example, activation of the NaV1.4/CT1.5 chimera was shifted by Ca2+ and CIP but neither native channel showed this shift. Activation of NaV1.5 was selectively affected by KN93, further strengthening the argument that activation and inactivation are separable processes and that CaM modulates these channel properties through different pathways. These results highlight the fact that activation and inactivation are not confined solely to the S4 loop (activation) and the inactivation loop between domains III and IV (Goldin, 2003). Our data suggest that the C-terminal region of the channel is the cytoplasmic domain necessary for the interaction of CaM with the NaCh and for its effects on steady-state inactivation. However, CaM affects both the voltage dependence of activation and steady-state inactivation (Fig. 2), despite the fact that it is binding primarily in the carboxyl tail. This suggests that it may also bind other cytoplasmic domains or modify the interaction of the C-terminal with the other cytoplasmic regions.
Three recent papers have reported functional effects of CaM coexpressed with NaChs (Deschenes et al. 2002; Tan et al. 2002; Herzog et al. 2003). Our results are similar in some regards, but are novel and different with respect to key properties of the channels. Focusing first on data for NaV1.4, Deschenes et al. (2002) found that coexpression of NaV1.4 with CaMWT caused a hyperpolarizing shift in HEK293 cells when intracellular Ca2+ was not buffered through the internal solution, but no shift when Ca2+ was buffered by BAPTA included in the pipette. Herzog et al. (2003) were unable to replicate the Deschenes et al. findings (also using HEK293 cells and using the same internal and external solutions). Herzog et al. (2003) did not find any changes in the electrical properties of NaV1.4 with buffered, unbuffered or 10 μm Ca2+; however, they did find a significant increase in peak amplitude of Na+ current when CaMWT was coexpressed, implying an increase in cell surface expression. This increase in peak current was blocked by a mutation (Q to E) in the IQ domain. We found a significant shift in the voltage dependence of inactivation with CaMWT coexpression in CHO cells, which was modulated by Ca2+ and CaM-binding peptides, but we found no shift in HEK293 cells. We did not find a significant difference in the current density between native NaV1.4 and the I1727E mutant coexpressed with CaMWT in CHO cells (data not shown).
Differences between ion channel properties that are due to the use of different expression systems have been reported. For example, Kv1.5 currents expressed in HEK293 cells differ from those expressed in mouse L-cells due to expression of endogenous K+ channel β2.1 in L-cells but not in HEK293 cells (Uebele et al. 1996). We did not find a shift in voltage dependence of inactivation in HEK cells (Fig. 7A) and hypothesized that differential endogenous expression of β subunits by CHO and HEK cells might account for these differences. Quantitative RT-PCR was used to measure the relative amounts of mRNA for each of the four known β genes in CHO and HEK cells. Two major differences were found: CHO cells had no β4-subunits and HEK cells had no β1-subunits. β-Subunits could act in either a positive or negative way to permit the shift in voltage dependence that we found in CHO cells. HEK cells might require β1 to generate a shift with CaM coexpression or β4 might prevent the shift. Deschenes et al. (2002) compared expression of Nav1.4 with coexpression of β1 and Nav1.4 and reported no difference in the results. We also found that CaM had no effect on steady-state inactivation in HEK cells even when we coexpressed β1 with Nav1.4. These results indicate that β4 may be the critical subunit. An additional difference between cell types is post-translational modifications such as glycosylation. Decreased sialylation of NaV1.4 resulted in shifts in the voltage dependence of activation and inactivation (Bennett et al. 1997). Such differences in the background expression system could lead to differences in the electrical properties of measured Na+ currents.
The recording solutions and control of intracellular ion concentrations such as calcium can differ between laboratories. Our experiments were performed with intracellular solutions containing CsF and different buffered concentrations of Ca2+. We also tested Cl−- containing intracellular solutions and found that the voltage dependence was shifted (see Meadows et al. 2002), but more importantly, the shift in steady-state inactivation caused by CaM coexpression was still present. Deschenes et al. (2002) used high intracellular concentrations of CsF and repeated the experiments with CsCl; they obtained the same results for both Cl− and F− solutions. Thus, differences between our work and theirs are unlikely to be due to the anion used. Recent studies of CaM interactions with CaChs have shown that the concentration of Ca2+ buffer and free Ca2+ in the pipette during whole-cell recording can drastically affect CaM modulation of inactivation (Liang et al. 2003). Differences in Ca2+ buffers and intracellular free Ca2+ could account for some of the inconsistencies between earlier papers and our work.
Our results for CaM coexpression with NaV1.5 show much smaller effects than for NaV1.4 and suggest that the action of CaM on NaV1.5 is via CKII. This conclusion is similar to that of Deschenes et al. (2002) but not Tan et al. (2002) who found direct effects of CaM upon NaV1.5. Our results and those of Tan et al. (2002) and Deschenes et al. (2002) each differ in some respect from each other. Changes in slow inactivation of NaV1.5 (not studied by us) were either due to Ca2+-CaM (Tan et al. 2002) or to inhibition of CKII (Deschenes et al. 2002). Tan et al. (2002) observed a depolarizing shift in the voltage dependence of inactivation with the peptide CBP on NaV1.5 currents recorded from tsA201 cells (HEK293 cells that are stably transfected with the SV40 large T antigen), while in CHO cells (our results) this peptide had no effect on NaV1.5 coexpressed with CaMWT. Deschenes et al. (2002) found no shift in either inactivation or activation when CaMWT was coexpressed with NaV1.5 in HEK293 cells; we found a shift in activation but not inactivation in CHO cells. Both we and Deschenes et al. (2002) recorded a shift in voltage dependence when CKII was blocked with KN93, but the shift we observed was for activation and was hyperpolarizing while Deschenes et al. (2002) observed the shift for inactivation and it was depolarizing. In addition, there are disagreements about the binding of CaM to NaV1.5. Both Deschenes et al. (2002) and Tan et al. (2002) showed binding of a peptide containing the IQ domain of NaV1.5 to CaM. We observed binding of CaM to the full-length C-terminal region of NaV1.5 in the yeast two-hybrid assay. Using biochemical assays and fusion proteins, Kim et al. (2004) showed that CaM binds to the IQ domain of NaV1.5 and that Ca2+ binds to the CaM– NaV1.5 complex. However, Herzog et al. (2003) reported that the full-length C-terminal region of NaV1.5 did not bind to CaM under conditions of high or low Ca2+in vitro. In summary, none of the published results is wholly consistent with any of the others. The simplest explanation for the variability in the results from one laboratory to another is that there are unknown components (e.g. additional binding proteins or different states of phosphorylation) or differences in free-Ca2+ concentration that vary between the different expression systems. The difference in β-subunit expression that we found between CHO and HEK cells could account for some of the variable findings. Moreover, it is possible that β-subunit expression differs between different HEK subclones (e.g. tsA201 and HEK293).
Despite the disagreements described above regarding the specific effects of CaM upon NaChs, it is clear that CaM modulates all NaCh subtypes that have been tested. What are the physiological implications of NaCh modulation by CaM? Muscle fibres undergo large calcium concentration changes (roughly 100-fold, from approximately 100 nm to tens of μm) each time contraction occurs. CaM will therefore cycle between apoCaM and Ca2+-CaM during contraction and relaxation. Our results predict that the properties of NaChs in skeletal muscle are dynamically changing as internal Ca2+ varies. The shift in V1/2 of steady-state inactivation to more depolarized values would ensure that when threshold is reached, there would be an adequate number of channels for action potential initiation and propagation. Thus, the regulation of NaChs by intracellular Ca2+ via Ca2+-CaM enables NaCh voltage dependence to be tuned to the activity of the muscle fibre. Moreover, the predicted mode of action of CaM (direct and fast in skeletal muscle versus indirect and relatively slow in cardiac muscle because it works via kinases) is congruent with the speed of action of these muscles. Skeletal muscle responds to stimulation rates as high as 100–200 Hz with brief action potentials, while cardiac muscle action potentials occur at much slower rates (∼5–10 Hz for a rodent) with long-duration action potentials.
Why should this fine-tuning of the biophysical properties of muscle NaChs be required? Skeletal muscle fibres have depolarizing after-potentials (∼10 mV) due both to the electrical propagation of action potentials in the t-tubule membrane and to K+ accumulation in the t-tubules. The depolarizing after-potential in skeletal muscle lasts for tens of milliseconds and will inactivate some of the NaChs. The muscle could compensate for the inactivation by having a high density of NaChs to ensure initiation and propagation of the action potential, and in fact, at the neuromuscular junction there is a local, high concentration of NaChs. However, there is an alternative strategy. Numerical simulations of NaChs found in cortical pyramidal neurones showed that the biophysical properties of axonal channels, instead of the high density of channels in the initial segment, were most likely what determined the threshold for initiating an action potential (Colbert & Pan, 2002). In their model, a change of 7 mV in the V1/2 of activation was equivalent to increasing the channel density 100-fold. They concluded that shifts in the voltage dependence of activation and inactivation (like those reported here) could exert larger effects than altering channel density. Our results show that CaM is normally bound to and acts directly on the skeletal muscle NaCh. Elevation of internal Ca2+ will produce a shift in V1/2 that changes the percentage of channels available to open. For example, the shift of 7 mV in V1/2 for steady-state inactivation for NaV1.4 + CaMWT with elevated Ca2+ results in 50% of the channels being available at −64 mV, compared with only approximately 35% of the channels at resting internal Ca2+ (Fig. 2D). This shift in the voltage dependence of channel inactivation by Ca2+-CaM will ensure the electrical excitability of skeletal muscle, which could be especially important during intense activity or fatigue when the cell undergoes long-lasting depolarization.
Acknowledgments
We thank Drs Blaise Peterson and Kate Beckingham for the CaM mutants and Eric Bennett and Al George for the chimeric channel cDNAs. We thank Dr Rock Levinson for native sodium channel cDNAs, Dr Steve Cannon for β1 cDNA, and Dr Kristin Schaller for assistance with the yeast two-hybrid assay K.A.Y. was supported by Neuroscience Training Grant HD41697-03. This work was supported by NIH R01 NS26505 and by the Muscular Dystrophy Association.
Supplemental material
The online version of this paper can be accessed at: DOI:10.1113/jphysiol.2004.081422
http://jp.physoc.org/cgi/content/full/jphysiol.2004.081422/DC1 and contains supplemental material entitled: Table of PCR Primers.
References
- Bennett ES. Channel cytoplasmic loops alter voltage-dependent sodium channel activation in an isoform-specific manner. J Physiol. 2001;535:371–381. doi: 10.1111/j.1469-7793.2001.t01-1-00371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett ES, Urcan MS, Tinkle SS, Koszowski AG, Levinson SR. Contribution of sialic acid to the voltage dependence of sodium channel gating. A possible electrostatic mechanism. J General Physiol. 1997;109:327–343. doi: 10.1085/jgp.109.3.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier E, Dargent B, De Waard M, Couraud F. Na+ channel regulation by calmodulin kinase II in rat cerebellar granule cells. Biochem Biophys Res Commun. 2000;274:394–399. doi: 10.1006/bbrc.2000.3145. [DOI] [PubMed] [Google Scholar]
- Chou JJ, Li S, Klee CB, Bax A. Solution structure of Ca2+-calmodulin reveals flexible hand-like properties of its domains. Nature Struc Biol. 2001;8:990–996. doi: 10.1038/nsb1101-990. [DOI] [PubMed] [Google Scholar]
- Colbert CM, Pan E. Ion channel properties underlying axonal action potential initiation in pyramidal neurons. Nat Neurosci. 2002;5:533–538. doi: 10.1038/nn0602-857. [DOI] [PubMed] [Google Scholar]
- de Leon M, Wang Y, Jones L, Perez-Reyes E, Wei X, Soong TW, et al. Essential Ca2+-binding motif for Ca2+-sensitive inactivation of L-type Ca2+ channels. Science. 1995;270:1502–1506. doi: 10.1126/science.270.5241.1502. [DOI] [PubMed] [Google Scholar]
- DeMaria CD, Soong TW, Alseikhan BA, Alvania RS, Yue DT. Calmodulin bifurcates the local Ca2+ signal that modulates P/Q-type Ca2+ channels. Nature. 2001;411:484–489. doi: 10.1038/35078091. [DOI] [PubMed] [Google Scholar]
- Deschenes I, Neyroud N, DiSilvestre D, Marban E, Yue DT, Tomaselli GF. Isoform-specific modulation of voltage-gated Na+ channels by calmodulin. Circ Res. 2002;90:E49–E57. doi: 10.1161/01.res.0000012502.92751.e6. [DOI] [PubMed] [Google Scholar]
- Deschenes I, Trottier E, Chahine M. Implication of the C-terminal region of the alpha-subunit of voltage-gated sodium channels in fast inactivation. J Membr Biol. 2001;183:103–114. doi: 10.1007/s00232-001-0058-5. [DOI] [PubMed] [Google Scholar]
- Dzhura I, Wu Y, Zhang R, Colbran RJ, Hamilton SL, Anderson ME. C terminus L-type Ca2+ channel calmodulin-binding domains are ‘auto-agonist’ ligands in rabbit ventricular myocytes. J Physiol. 2003;550:731–738. doi: 10.1113/jphysiol.2003.043778. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Erickson MG, Alseikhan BA, Peterson BZ, Yue DT. Preassociation of calmodulin with voltage-gated Ca2+ channels revealed by FRET in single living cells. Neuron. 2001;31:973–985. doi: 10.1016/s0896-6273(01)00438-x. [DOI] [PubMed] [Google Scholar]
- Erickson MG, Liang H, Mori MX, Yue DT. FRET two-hybrid mapping reveals function and location of L-type Ca2+ channel CaM preassociation. Neuron. 2003;39:97–107. doi: 10.1016/s0896-6273(03)00395-7. [DOI] [PubMed] [Google Scholar]
- Finn BE, Evenas J, Drakenberg T, Waltho JP, Thulin E, Forsen S. Calcium-induced structural changes and domain autonomy in calmodulin. Nat Struct Biol. 1995;2:777–783. doi: 10.1038/nsb0995-777. [DOI] [PubMed] [Google Scholar]
- Goldin AL. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol. 2003;13:284–290. doi: 10.1016/s0959-4388(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Head C, Gardiner M. Paroxysms of excitement: sodium channel dysfunction in heart and brain. Bioessays. 2003;25:981–993. doi: 10.1002/bies.10338. [DOI] [PubMed] [Google Scholar]
- Herzog RI, Liu C, Waxman SG, Cummins TR. Calmodulin binds to the C terminus of sodium channels Nav1.4 and Nav1.6 and differentially modulates their functional properties. J Neurosci. 2003;23:8261–8270. doi: 10.1523/JNEUROSCI.23-23-08261.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isom LL. I. Cellular and molecular biology of sodium channel beta-subunits: therapeutic implications for pain? Am J Physiol Gastrointest Liver Physiol. 2000;278:G349–353. doi: 10.1152/ajpgi.2000.278.3.G349. [DOI] [PubMed] [Google Scholar]
- Keen JE, Khawaled R, Farrens DL, Neelands T, Rivard A, Bond CT, et al. Domains responsible for constitutive and Ca2+–dependent interactions between calmodulin and small conductance Ca2+-activated potassium channels. J Neurosci. 1999;19:8830–8838. doi: 10.1523/JNEUROSCI.19-20-08830.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ghosh S, Liu H, Tateyama M, Kass RS, Pitt GS. Calmodulin mediates Ca2+ sensitivity of sodium channels. J Biol Chem. 2004;279:45004–45012. doi: 10.1074/jbc.M407286200. [DOI] [PubMed] [Google Scholar]
- Kuboniwa H, Tjandra N, Grzesiek S, Ren H, Klee CB, Bax A. Solution structure of calcium-free calmodulin. Nat Struct Biol. 1995;2:768–776. doi: 10.1038/nsb0995-768. [DOI] [PubMed] [Google Scholar]
- Levitan IB. It is calmodulin after all! Mediator of the calcium modulation of multiple ion channels. Neuron. 1999;22:645–648. doi: 10.1016/s0896-6273(00)80722-9. [DOI] [PubMed] [Google Scholar]
- Liang H, DeMaria CD, Erickson MG, Mori MX, Alseikhan BA, Yue DT. Unified mechanisms of Ca2+ regulation across the Ca2+ channel family. Neuron. 2003;39:951–960. doi: 10.1016/s0896-6273(03)00560-9. [DOI] [PubMed] [Google Scholar]
- Mantegazza M, Yu FH, Catterall WA, Scheuer T. Role of the C-terminal domain in inactivation of brain and cardiac sodium channels. Proc Natl Acad Sci U S A. 2001;98:15348–15353. doi: 10.1073/pnas.211563298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meadows LS, Chen YH, Powell AJ, Clare JJ, Ragsdale DS. Functional modulation of human brain Nav1.3 sodium channels, expressed in mammalian cells, by auxiliary beta 1, beta 2 and beta 3 subunits. Neuroscience. 2002;114:745–753. doi: 10.1016/s0306-4522(02)00242-7. [DOI] [PubMed] [Google Scholar]
- Mori MX, Erickson MG, Yue DT. Functional stoichiometry and local enrichment of calmodulin interacting with Ca2+ channels. Science. 2004;304:432–435. doi: 10.1126/science.1093490. [DOI] [PubMed] [Google Scholar]
- Mori M, Konno T, Morii T, Nagayama K, Imoto K. Regulatory interaction of sodium channel IQ-motif with calmodulin C-terminal lobe. Biochem Biophys Res Comm. 2003;307:290–296. doi: 10.1016/s0006-291x(03)01183-5. [DOI] [PubMed] [Google Scholar]
- Mori M, Konno T, Ozawa T, Murata M, Imoto K, Nagayama K. Novel interaction of the voltage-dependent sodium channel (VDSC) with calmodulin: does VDSC acquire calmodulin-mediated Ca2+-sensitivity? Biochemistry. 2000;39:1316–1323. doi: 10.1021/bi9912600. [DOI] [PubMed] [Google Scholar]
- Mukherjea P, Maune JF, Beckingham K. Interlobe communication in multiple calcium-binding site mutants of Drosophila calmodulin. Protein Sci. 1996;5:468–477. doi: 10.1002/pro.5560050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persechini A, Stemmer PM. Calmodulin is a limiting factor in the cell. Trends Cardiovasc Med. 2002;12:32–37. doi: 10.1016/s1050-1738(01)00144-x. [DOI] [PubMed] [Google Scholar]
- Peterson BZ, DeMaria CD, Adelman JP, Yue DT. Calmodulin is the Ca2+ sensor for Ca2+-dependent inactivation of L-type calcium channels. Neuron. 1999;22:549–558. doi: 10.1016/s0896-6273(00)80709-6. [DOI] [PubMed] [Google Scholar]
- Putkey JA, Kleerekoper O, Gaertner TR, Waxham MN. A new role for IQ motif proteins in regulating calmodulin function. J Biol Chem. 2003;278:49667–49670. doi: 10.1074/jbc.C300372200. [DOI] [PubMed] [Google Scholar]
- Putkey JA, Sweeney HL, Campbell ST. Site-directed mutation of the trigger calcium-binding sites in cardiac troponin C. J Biol Chem. 1989;264:12370–12378. [PubMed] [Google Scholar]
- Qin N, Olcese R, Bransby M, Lin T, Birnbaumer L. Ca2+-induced inhibition of the cardiac Ca2+ channel depends on calmodulin. Proc Natl Acad Sci U S A. 1999;96:2435–2438. doi: 10.1073/pnas.96.5.2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu W, Moorhouse AJ, Rajendra S, Barry PH. Very negative potential for half-inactivation of, and effects of anions on, voltage-dependent sodium currents in acutely isolated rat olfactory receptor neurons. J Membr Biol. 2000;175:123–138. doi: 10.1007/s002320001061. [DOI] [PubMed] [Google Scholar]
- Rhoads AR, Friedberg F. Sequence motifs for calmodulin recognition. FASEB J. 1997;11:331–340. doi: 10.1096/fasebj.11.5.9141499. [DOI] [PubMed] [Google Scholar]
- Saimi Y, Kung C. Calmodulin as an ion channel subunit. Ann Rev Physiol. 2002;64:289–311. doi: 10.1146/annurev.physiol.64.100301.111649. [DOI] [PubMed] [Google Scholar]
- Schumacher MA, Rivard AF, Bachinger HP, Adelman JP. Structure of the gating domain of a Ca2+-activated K+ channel complexed with Ca2+/calmodulin. Nature. 2001;410:1120–1124. doi: 10.1038/35074145. [DOI] [PubMed] [Google Scholar]
- Smith RD, Goldin AL. Protein kinase A phosphorylation enhances sodium channel currents in Xenopus oocytes. Am J Physiol. 1992;263:C660–666. doi: 10.1152/ajpcell.1992.263.3.C660. [DOI] [PubMed] [Google Scholar]
- Sumi M, Kiuchi K, Ishikawa T, Ishii A, Hagiwara M, Nagatsu T, et al. The newly synthesized selective Ca2+/calmodulin dependent protein kinase II inhibitor KN-93 reduces dopamine contents in PC12h cells. Biochem Biophys Res Commun. 1991;181:968–975. doi: 10.1016/0006-291x(91)92031-e. [DOI] [PubMed] [Google Scholar]
- Tan HL, Kupershmidt S, Zhang R, Stepanovic S, Roden DM, Wilde AA, et al. A calcium sensor in the sodium channel modulates cardiac excitability. Nature. 2002;415:442–447. doi: 10.1038/415442a. [DOI] [PubMed] [Google Scholar]
- Terrak M, Wu G, Stafford WF, Lu RC, Dominguez R. Two distinct myosin light chain structures are induced by specific variations within the bound IQ motifs-functional implications. EMBO J. 2003;22:362–371. doi: 10.1093/emboj/cdg058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjandra N, Kuboniwa H, Ren H, Bax A. Rotational dynamics of calcium-free calmodulin studied by 15N-NMR relaxation measurements. Eur J Biochem. 1995;230:1014–1024. doi: 10.1111/j.1432-1033.1995.tb20650.x. [DOI] [PubMed] [Google Scholar]
- Uebele VN, England SK, Chaudhary A, Tamkun MM, Snyders DJ. Functional differences in Kv1.5 currents expressed in mammalian cell lines are due to the presence of endogenous Kv beta 2.1 subunits. J Biol Chem. 1996;271:2406–2412. doi: 10.1074/jbc.271.5.2406. [DOI] [PubMed] [Google Scholar]
- Yang J, Barchi R. Phosphorylation of the rat skeletal muscle sodium channel by cyclic AMP-dependent protein kinase. J Neurochem. 1990;54:954–962. doi: 10.1111/j.1471-4159.1990.tb02343.x. [DOI] [PubMed] [Google Scholar]
- Zhang M, Tanaka T, Ikura M. Calcium-induced conformational transition revealed by the solution structure of apo calmodulin. Nat Struct Biol. 1995;2:758–767. doi: 10.1038/nsb0995-758. [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Pitt GS, Deisseroth K, Tsien RW, Reuter H. Calmodulin supports both inactivation and facilitation of L-type calcium channels. Nature. 1999;399:159–162. doi: 10.1038/20200. [DOI] [PubMed] [Google Scholar]
- Zuhlke RD, Reuter H. Ca2+-sensitive inactivation of L-type Ca2+ channels depends on multiple cytoplasmic amino acid sequences of the alpha1C subunit. Proc Natl Acad Sci U S A. 1998;95:3287–3294. doi: 10.1073/pnas.95.6.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.