Abstract
Homeostasis of the central nervous system (CNS) microenvironment is essential for its normal function. It is maintained by the blood–brain barrier (BBB) which regulates the transport of molecules from blood into brain and backwards. The integrity of the BBB is compromised in many disorders of the human CNS; therapeutical strategies for several of these diseases include treatment with glucocorticoids, but the molecular basis of how glucocorticoids regulate BBB permeability is not understood. Here, we report the generation and characterization of a murine immortalized brain (cerebral) capillary endothelial (cEND) cell line which expresses the BBB marker occludin at intercellular tight junctions (TJ). Hydrocortisone at physiological concentrations induced upregulation of occludin, accompanied by a threefold enhancement of transendothelial electrical resistance to values up to 1000 Ωcm2. Insulin enhanced the glucocorticoid response. At the molecular level, hydrocortisone induces increase of occludin at protein and mRNA levels by activation of the glucocorticoid receptor (GR) and its binding to putative glucocorticoid responsive elements in the occludin promoter. At the same time, insulin potentiated the ligand-dependent GR transactivation via induction of the GR in this in vitro system. This study thus provides insights into the molecular processes of barrier genesis, and may help to elucidate mechanisms of brain pathology at the microvascular level.
Homeostasis of the central nervous system (CNS) microenvironment is essential for its normal function, and it is maintained by the blood–brain barrier (BBB) (Pardridge, 1988; Risau & Wolburg, 1990). It accounts for the low extracellular concentrations of amino acids and proteins in the brain relative to the blood, and also restricts access to the immune system and of systemically administered hydrophilic drugs. The cells responsible for the establishment of the barrier are the capillary and venular endothelial cells (blood–brain barrier) and the epithelial cells of the choroid plexus (blood–cerebrospinal fluid barrier). The main structures responsible for this barrier property are the intercellular tight junctions (TJs) (reviewed in Risau & Wolburg, 1990). BBB-forming brain capillary endothelial cells (BCECs) express the TJ proteins occludin and claudin-5.
The integrity of the BBB is compromised in many disorders of the human CNS (Hatashita & Hoff, 1990; McDonald, 1994). Therapeutic strategies for several of these diseases include treatment with glucocorticoids (Engelhardt, 2000; Qizilbash et al. 2002), but the molecular basis of how glucocorticoids regulate BBB permeability is not understood. A major difficulty in the study of the mechanism of differentiation of BCECs into a tight barrier has been the lack of a suitable in vitro cell culture system. Primary cultured BCECs from rat, bovine or porcine origin have been shown to represent a well-differentiated phenotype; nevertheless, they appear to be unable to maintain functions as displayed in vivo (Rubin et al. 1991). One way around this problem was the development of immortalized BCEC systems, mainly derived from rat (Regina et al. 1998; Omidi et al. 2003). However, immortalization of BCECs usually results in a more de-differentiated phenotype, and as in primary cultures, the extremely tight permeability characteristic of brain endothelium in vivo (∼2000–5000 cm2) is also not usually preserved in these cell lines (∼50–100 Ωcm2) (Rubin et al. 1991). However, we believe that studying the permeability regulation of endothelial cells constituting the BBB in the murine model is becoming increasingly important because the mouse is the mammalian model animal of choice for genetic modifications.
In this study, we were able to establish a murine immortalized BCEC cell culture system responsive to glucocorticoids important for inducing the differentiation of BCECs to a phenotype in vitro, which shares principal features of the BBB in vivo. This is in accordance with previous studies showing that glucocorticoid action is essential for the proper induction and maintenance of complex TJs in BCECs and epithelia of various origins (Hoheisel et al. 1998; Nguyen & Neville, 1998; Woo et al. 2000). In the experiments presented herein, we further provide evidence that hydrocortisone increases barrier properties of BCECs by inducing enhanced expression of the TJ transmembrane component occludin via binding of the activated glucocorticoid receptor to putative glucocorticoid responsive elements in the occludin promoter. These findings provide evidence for a possible role of glucocorticoids in direct regulation of the expression of TJ components of the BBB.
Methods
Animals and collection of tissues
Neonatal mice (strain 129Sv) of either sex (3 days old) were killed by CO2 asphyxiation. The brains were immediately removed and transferred into a dissection chamber containing the following solution: 15 mm Hepes (pH 7.4), containing 153 mm NaCl, 5.6 mm KCl, 2.3 mm CaCl2.2H2O, 2.6 mm MgCl2.6H2O, 1% (w/v) BSA (hereafter referred to as buffer A). All experiments were approved by the local Animal Care Committee (Tierschutzbeauftragter).
Isolation and culture of brain microvessels/BCECs
Unless otherwise indicated, all isolation procedures were performed at room temperature (22–24°C). Brains (cerebrum without cerebellum and brain stem) were isolated from neonatal mice (3 days post partum) as described above, and after removal of the meninges and capillary fragments, the grey and white matter of the brain was minced in buffer A using a sterile cutter. Fragments were digested in 0.75% (w/v) collagenase A (Roche, Mannheim, Germany) for 30 min at 37°C in a water bath (occasionally shaking). Digestion was stopped by addition of 10 vols ice-cold buffer A. To remove myelin, centrifugation through a 25% (w/v) BSA gradient was carried out for 20 min at 1000 g. The resulting endothelial cell pellet was washed twice with buffer A to remove myelin and BSA. Primary cells were then resuspended in Dulbecco's modified Eagle's medium (DMEM) (Sigma, Taufkirchen, Germany) growth medium (10% heat-inactivated fetal calf serum (FCS)) and plated on 24-well plates (Greiner, Frickenhausen, Germany), freshly coated with collagen IV (Fluka, Taufkirchen, Germany) and transfected with Polyoma middle T after 24 and after 48 h, as previously described for microvascular endothelial cells from brain and heart (Aumailley et al. 1991; Golenhofen et al. 2002). Typically, stable cerebral endothelial (cEND) cell lines were obtained 4–5 weeks later. Thereafter, at confluence, cells were routinely transferred to DMEM differentiation medium (containing 2% heat-inactivated FCS), and were treated for certain experiments with glucocorticoids and insulin, or combinations thereof, as indicated in the figure legends. The immortalized cells were routinely maintained on gelatin.
Cell cultures
HEK 293 and Cos-7 cells were cultured in DMEM medium supplemented with 10% FCS. cEND cells were cultivated as described above. All cultures were supplemented with 100 IU ml−1 penicillin and 100 mg ml−1 streptomycin (1% PEST). Cells were maintained in an atmosphere containing 5.0% CO2 and at 37°C.
Bioelectric and permeability assessments
Cells were plated on top of gelatin-coated transwell chambers for six-well plates (0.4 μm pores) (Falcon, Heidelberg, Germany) at densities of 1 × 104 cells per well. When they had reached confluence at day 5, the different experimental sets of cells were treated with 110 nm hydrocortisone, 110 nm hydrocortisone/1 μm insulin or 1 μm insulin alone. The control set was maintained in basal medium (DMEM, 2% FCS) for an additional 3 days, at which point the permeability and flux measurement assays were performed up to day 21 in culture. All experiments were repeated at least three times.
Resistance measurement
Transendothelial electrical resistance (TER) was measured using an assembly containing current-passing and voltage-measuring electrodes (World Precision Instruments, Inc., New Haven, CT, USA). Resistances of blank filters were subtracted from those of filters with cells before final resistances (Ωcm2) were calculated.
FITC-dextran and fluorescein flux measurement
Confluent monolayers treated with various differentiation media (see figure legends) were washed in prewarmed Hepes buffer (10 mm Hepes, pH 7.2, 0.1% BSA, 4.5% glucose), and subsequently preincubated for 5 min in Hepes buffer at 37°C. Fluorescein-isothiocyanate (FITC)-dextrans (Sigma) were purified from unconjugated FITC by size-exclusion chromatography (Biogel P2 Polyacrylamide gel, Bio-Rad, München, Germany). Paracellular flux measurement was started by adding to the upper chamber of the Transwell system 100 μl of 50 mg ml−1 of 4, 10, 70 or 500 kDa FITC-dextran, or 100 μl of 5 mg ml−1 fluorescein, in Hepes buffer, to a final concentration of 1 mg ml−1 FITC-dextran or 0.1 mg ml−1 fluorescein. Paracellular flux was assessed by taking 100 μl aliquots from the outer chamber every 60 min during the first 4 h of incubation. Fluorescence was measured using a Wallac Victor2 fluorescence spectrophotometer (Perkin-Elmer, Überlingen, Germany) with excitation and emission at 485 and 535 nm, respectively. The fluorescein and FITC-dextran clearance through the monolayer was correlated to control clearance of uncoated wells and the initial amount.
Immunocytochemistry
Antibodies were purchased from Zymed Laboratories, CA, USA (zy) and Santa Cruz Biotechnology, CA, USA, unless otherwise indicated. Rabbit polyclonal VE-cadherin antibody directed against the cytoplasmic domain of mouse VE-cadherin was kindly provided by D. Vestweber, Münster, Germany. cEND cells were grown to confluence on glass coverslips (diameter 12 mm) coated with gelatin cross-linked with glutaraldehyde in polystyrene tissue-culture plates (Greiner, Germany), and treated as indicated in the figure legends. For immunocytochemistry, cells were washed twice with PBS and fixed in ice-cold methanol for 15 min, and blocked with PBS containing 1% (w/v) BSA in 1% (w/v) normal goat serum for 30 min. Immunostainig for PECAM-1 (1:100; Pharmingen/BD Biosciences, Heidelberg, Germany), VE-cadherin (Gotsch et al. 1997), claudin-5 (zy-34–1600; 1:100), occludin (zy-71–1500; 1:100), claudin-1 (zy-71–7800; 1:100) and claudin-3 (zy-18–7340; 1:100) was performed by incubation with primary antibodies (in PBS) overnight at 4°C. PBS was used in place of primary antibodies in negative controls. Slides were washed with PBS, and incubated with the respective FITC- or Cy3-conjugated secondary antibodies (Jackson ImmunoResearch Laboratory, West Grove, PA, USA) for 45 min at 37°C. Coverslips were mounted onto slides using 60% (w/v) glycerol, and 1.5% (w/v) n-propylgallate (Fluka) as an antifading component. The slides were analysed using a Zeiss Axioscop2 microscope. All pictures within each experiment were captured and manipulated identically with SpotAdvanced software and Adobe Photoshop. Cell areas and junctional lenght were measured on the captured images using SpotAdvanced software for Windows from Diagnostic Instruments.
Analysis and statistics
Cell areas and junctional length were averaged to esthablish a single value for control and after 3 days of HC treatment. Throughout, averaged values are reported as means ±s.d. The indicated statistical test (Mann–Whitney U-test) was performed assuming significance for P < 0.05.
Electrophoresis and immunoblotting
cEND cells were dissolved in Laemmli sample buffer (Laemmli, 1970) and subjected to sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE, 15% gels). Protein contents were quantified by protein estimation directly from SDS-PAGE loading buffer using 0.1% (w/v) Amidoschwarz (AppliChem, Darmstadt, Germany) in 25% (v/v) methanol/5% (v/v) acetic acid. For immunoblotting, proteins were transferred in Kyhse-Andersen transfer buffer (Kyhse-Andersen, 1984) to Hybond nitrocellulose membranes (Amersham, Braunschweig, Germany), which were blocked with 10% (w/v) low-fat milk in PBS (pH 7.4) and incubated overnight at 4°C with the respective primary antibody in PBS plus 10% low-fat milk. The polyclonal rabbit antibodies against occludin and claudin-5 were used at a dilution of 1:1000. The glucocorticoid receptor (GR) antibody was obtained from Santa Cruz Biotechnology and diluted 1:1000. As secondary antibody, horseradish-peroxidase-labelled goat antirabbit IgG (Jackson ImmunoResearch Laboratory) was used diluted 1:3000 with PBS. Bound immunoglobulins were visualized by the enhanced chemiluminescence technique (ECL; Amersham). Densitometric analysis using Scion Image Beta 4.02 (Scion Corp., MD, USA) was performed for quantification.
Semiquantitative RT-PCR
Total RNA was extracted from confluent cEND BCEC monolayers after treatment with various differentiation media (for further details see legends to Figs 3A and B, and 4A) using the RNeasy Reagent (Quiagen, Hilden, Germany) according to the manufacturer's instructions. RNA was digested with DNase I (Roche) 10 units (μg RNA)−1 for 1 h at 37°C, followed by heat inactivation 15 min at 80°C. A 2 μg quantity of total RNA was then reverse-transcribed and amplified using the Titan One Tube RT-PCR System (Roche) in a final volume of 50 μl according to the manufacturer's instructions, using 10 mm dNTP (Gibco, Karlsruhe, Germany) and 30 pmol of each primer (seeTable 1; MWG Biotech, Ebersberg, Germany).
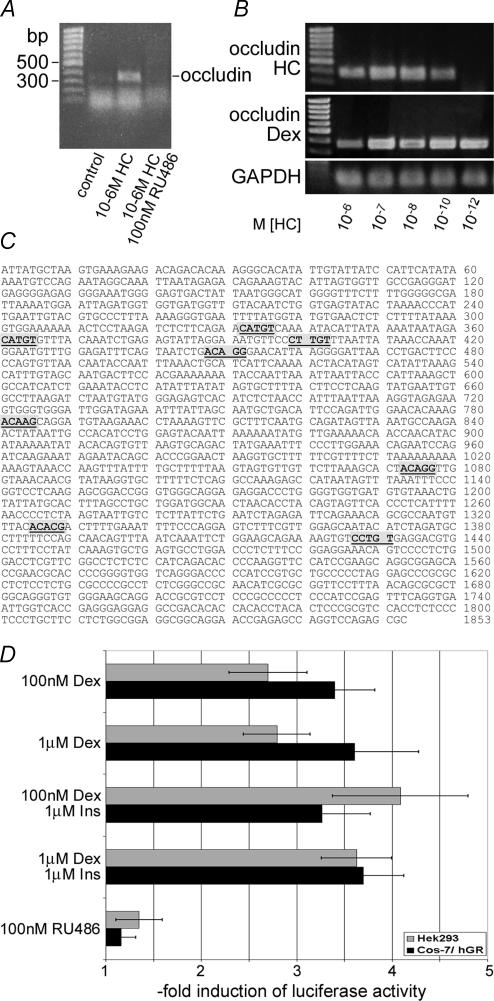
Figure 3. GC effects on barrier properties.
A, induction of occludin mRNA transcription by glucocorticoids in cEND cells. After reaching confluence, cEND cells were treated with 1 μm HC or the GR antagonist RU486 for 3 days, and mRNA expression was assessed. 1 μm HC upregulated occludin mRNA expression (1.6-fold), while the antagonist RU486 abolished transcription of the occludin mRNA. B, concentration requirements for HC and dexamethasone (Dex) for induction of occludin expression in cEND cells. Cells pretreated as described in A were incubated with hormones for 3 days. Dependence of occludin mRNA induction on the concentration of glucorticoids was determined in the presence of 10−6–10−12μm HC or Dex, respectively. A maximum glucocorticoid effect on occludin mRNA transcription was observed at 10−7m HC and Dex. C, putative glucocorticoid receptor (GR)-binding sites in the human occludin promoter. The human occludin promoter contains several pentamer sequences located 332–336, 361–365, 399–403, 448–452, 781–785, 1073–1077, 1325–1329 and 1427–1431 bp upstream of the 5′ flanking region, which might belong to imperfect glucocorticoid-responsive elements (GREs) (bold letters/shadowed boxes) and on which glucocorticoids can influence gene expression. D, transactivation of the luciferase-linked human occludin promoter (pOCLNproluc7) by glucocorticoids and insulin. Cos-7 cells were cotransfected with expression vectors for human GR (hGR), pOCLNproluc7 (Mankertz et al. 2000), and the renilla luciferase reporter vector pRL-TK (Promega) for normalization of transfection efficiency. Cells were then grown with or without various concentrations of Dex, 1 μm Ins and 100 nm RU486 as indicated for 24 h. HEK 293 cells were treated accordingly but without hGR cotransfection. Cell extracts were assayed for luciferase and renilla activity. The results are displayed as the ratio of activity with and without ligands (means and s.d. from at least 4 experiments).
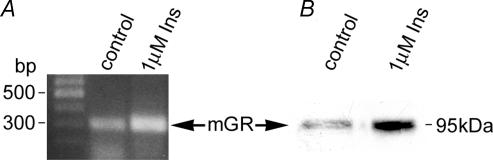
Figure 4. Insulin regulation of GR expression in cEND BCECs.
A, after reaching confluence, cEND cells were treated with 1 μm insulin for 3 days, and mRNA expression was determined. 1 μm insulin upregulated GR mRNA expression (1.5-fold). The panel is representative of three independent experiments. B, confluent monolayers of cEND BCECs were grown in gelatin-coated cell-culture flasks, and subsequently differentiated in DMEM differentiation medium containing 2% FCS in the presence and absence of 1 μm insulin for 3 days. Cell lysates were analysed by Western blot for GR expression. Densitometric evaluation resulted in an estimated threefold higher expression of GR protein by insulin treatment. The result is representative of three independent experiments.
Table 1.
Primers used for RT-PCR
| Target gene | Primer sequence | Product size (bp) | Annealing temperature (°C) | Reference |
|---|---|---|---|---|
| Occludin | 369 | 60 | This study | |
| F | CAC ACT TGC TTG GGA CAG AGG C | |||
| R | TGA GCC GTA CAT AGA TCC AGA AGC | |||
| Claudin-5 | 153 | 56 | Wang et al. 2003 | |
| F | GCT GGC GCT GGT GGC ACT CTT TGT | |||
| R | GGC GAA CCA GCA GAG CGG CAC | |||
| GR | 314 | 56 | Gupta & Wagner, 2003 | |
| F | CAA AGC CGT TTC ACT GTC C | |||
| R | ACA ATT TCA CAC TGC CAC C | |||
| GAPDH | 59 | Hosoya et al. 2000 | ||
| F | CAA GAC GGA CCA GAG CGA AAG C | |||
| R | CAA TCT CGG GTG GCT GAA CGC |
GR, glucocorticoid receptor.
The PCR reactions were performed in a mastercycler gradient thermocycler (Eppendorf) under the following conditions: 94°C 30 min, annealing temperature as indicated in Table 1 for 30 min, 72°C 1 min, with 33 cycles for each target gene. A negative control reaction with water as the template was included. GAPDH RNA was used as the housekeeping gene. All PCR reactions were done in triplicate for each target.
PCR products were separated on a 1.5% agarose gel stained with ethidium bromide. A 100 bp DNA ladder (Generuler, Fermentas) was used for calibration. Band densities were compared under UV transillumination. Densitometric analysis using Scion Image Beta 4.02 was performed for quantification.
Transfection and luciferase assay
Essentially, transfection and luciferase assays were carried out as described (Mankertz et al. 2000). Briefly, HEK 293 and Cos-7 cells were seeded on six-well cell culture plates 24 h before transfection in DMEM containing 10% dextran-coated-charcoal (DCC)-treated FCS (Fagart et al. 1998), and 1% PEST at a density of 2 × 106 cells per well. Transient transfection experiments utilizing the Effectene reagent (Quiagen) were performed as described by the manufacturer, using 2 μg of pOCDN-proLUC7 (Mankertz et al. 2000), 1 μg of pTRL-TK (Promega), and, in the case of Cos-7 cells, 0.4 μg of GR expression vector pCMVhGRα (Almlof et al. 1995), in the absence or presence of ligands (as indicated in the figure legends).
To assess glucocorticoid effects on occludin promoter transactivation, after addition of the DNA/Effectene mixture, cells were incubated overnight at 37°C and 5% CO2. After this, 4 ml fresh DMEM containing 10% DCC-treated FCS/1% PEST and ligands, or vehicle alone (as indicated in the figure legends), were added. After 24 h, cells were washed once with PBS and harvested with 500 μl lysis buffer. Thereafter, cellular extracts were prepared and analysed for luciferase activity. Measurement of both firefly and renilla luciferase activity was performed with the Dual-Luciferase assay kit (Promega) according to the manufacturer's instructions. Protein concentration was estimated by standard Bradford protein assay (Bradford, 1976). A 20 μl volume of cell lysate was used for assaying the enzymatic activities, using a LB9507 luminometer with dual injector (Berthold, Bad Wildbad, Germany). Each lysate was measured twice. Promoter activities were expressed as relative light units (RLU), normalized for the protein content and the activity of renilla luciferase in each extract. The data were calculated as the mean of five identical setups.
Results
Immunocytochemical characterization of immortalised murine brain endothelial cells
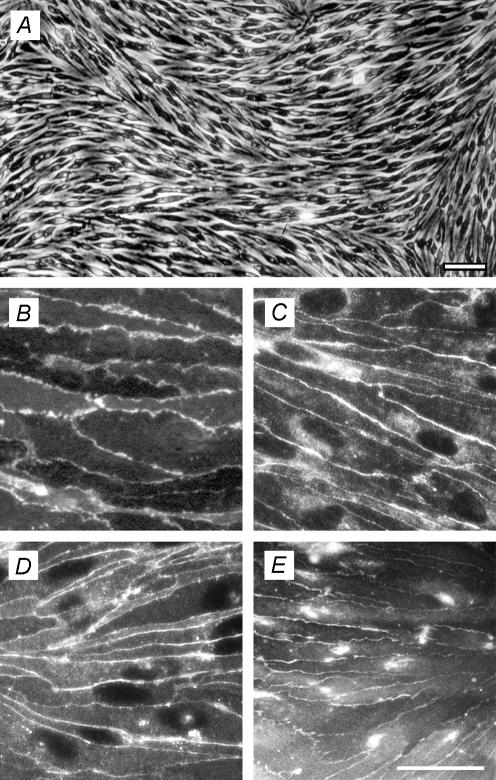
The immortalized mouse brain capillary endothelial, (cEND) cell line, was obtained from six wild-type mice (129 Sv strain). Morphologically, cEND cells showed spindle-shaped morphology (Fig. 1A) and retained their endothelial cell (EC) phenotype when cultured on coverslips coated with glutaraldehyde-cross-linked gelatin, as judged by PECAM-1 antibody staining (Fig. 1B). They displayed junctional staining with anti-VE-cadherin antibody (Fig. 1C), and expressed the endothelial TJ marker protein claudin-5 (Fig. 1D) along their intercellular junctions, as did a mouse myocardial microvascular (MyEND) cell line generated by the same technique (Golenhofen et al. 2002). Importantly, in contrast to peripheral ECs, cEND cells also displayed junctional immunostaining for the TJ-associated protein occludin (Fig. 1E). Expression of the TJ proteins claudin-1 and claudin-3 could not be detected by immunocytochemistry or Western blotting, or by using reverse transcription-polymerase chain reaction (RT-PCR) analysis, although they were detectable at the mRNA level in brain homogenates from whole animals in vivo (data not shown). Similarly, mdr1a protein, mdr1b protein and mRNA were not detected in cEND BCECs, although they were present in vivo (data not shown). Contamination with astrocytes, pericytes and smooth muscle cells was ruled out by staining with anti-glial fibrillary acidic protein and anti-smooth-muscle α-actin as primary antibodies (data not shown).
Figure 1. Murine immortalized cEND cultures exhibit endothelial markers and express tight-junction (TJ)-associated proteins and blood–brain barrier (BBB) markers.
A, phase microscopic image of cEND brain capillary endothelial cells (BCECs). Bar, 100 μm. B–E, cEND BCECs grown on gelatin-coated coverslips were labelled with antibodies against (B) PECAM-1, (C) VE-cadherin, (D) claudin-5 and (E) occludin. E, bar, 50 μm (valid for B–E).
Effects of glucocorticoids on brain EC resistance
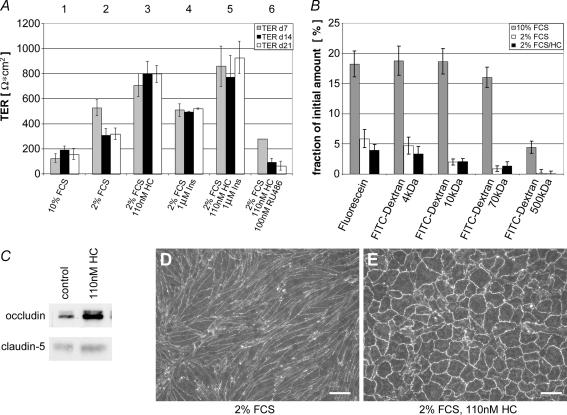
We were able to develop a serum-reduced but hormone-supplemented cell differentiation medium: in the presence of 10% serum contained in the growth medium TER was about 100–150 Ωcm2. Reduction of serum to 2% caused increase of TER to the range of 300–500 Ωcm2. The establishment of this BBB in vitro model further benefited from glucocorticoid supplementation of the serum-reduced endothelial differentiation medium to induce and maintain the BBB phenotype in vitro by mimicking a brain-like microenvironment: we tested several supplements and found that selected corticosteroids are potent stimulators for the formation of barrier properties without the need of a coculture system (the benefit of coculture systems has been discussed controversially in the literature: Rubin et al. 1991; Hamm et al. 2004). TERs up to 800 Ωcm2 were measured in the presence of physiological hydrocortisone concentrations in serum-reduced medium (Fig. 2A). However, hydrocortisone was not the only supplement found to improve barrier properties: addition of 1 μm insulin to the serum-reduced differentiation medium showed further improvement of TER (∼50%) at days 14 and 21 as compared with cells grown in 2% FCS alone. In combination with hydrocortisone, insulin improved TER values by approximately an extra 20% as compared with hydrocortisone supplementation only, reaching values up to 1000 Ωcm2.
Figure 2. Glucocorticoids and insulin induce barrier properties by upregulation of occludin.
A, influence of serum reduction, and the addition of hydrocortisone and insulin, on the electrical barrier properties (transendothelial electrical resistance, TER) of cEND monolayers. Growth medium (10% fetal calf serum, FCS) was changed after 5 days in culture to differentiation medium (2% FCS, ±additions), and analysis of the TER was performed after 7, 14 and 21 days in vitro. Incubation medium: (1) with 10% (v/v) FCS, without hydrocortisone (HC); (2) with 2% (v/v) FCS, without HC; (3) with 2% (v/v) FCS, 110 nm HC; (4) with 2% (v/v) FCS, 1 μm insulin (Ins); (5) with 2% (v/v) FCS, 110 nm HC, 1 μm Ins; (6) with 2% (v/v) FCS, 110 nm HC, 100 nm RU486. Data are given as means ±s.d. (n = 6). B, flux of fluorescein and of 4, 10, 70 and 500 kDa fluorescein isothiocyanate (FITC)-dextrans was reduced in cEND cells seeded on Transwell filters (pore size 0.4 μm) maintained in serum-reduced medium (2% FCS) as compared with cells maintained in growth medium (10% FCS). Glucocorticoid (110 nm HC) treatment did result in further improvement of the barrier qualities for the small macromolecules fluorescein and 4 kDa FITC-dextran, pointing to a glucocorticoid-mediated closure of TJ-based small pores in cEND cells. FITC-dextrans were purified from unconjugated FITC by size exclusion chromatography. Data are given as means ±s.d. (n = 5). C, HC stimulation of occludin and claudin-5 protein levels in cEND BCECs. Confluent monolayers of cEND BCECs were grown in gelatin-coated cell culture flasks in the presence of 110 nm HC for 24 h. Cell lysates were analysed by Western blot for occludin and claudin-5. Densitometric evaluation resulted in an estimated threefold upregulation of occludin protein content, and a 1.5-fold increase in claudin-5 protein in the presence of HC. D–E, altered cellular morphology and increased localization of occludin at sites of cell-to-cell contact after in vitro maturation of cEND cells in response to serum reduction and HC treatment. Control cells, after 3 days in medium containing 2% FCS (D) and after 3 days of treatment with HC (E), were fixed in methanol. Treatment with HC led to an increased junctional localization of occludin. Cellular morphology changed from a spindle-like elongated shape to a more cobble-stone-like pattern. Junctional length per area was lowered by 57 ± 3% in HC-treated versus untreated cEND cells. Data are given as means ±s.d. (n = 38). Scale bars, 100 μm.
To further validate the cEND BBB model, the passage of macromolecules, such as noncharged FITC-dextrans of molecular masses 4, 10, 70 and 500 kDa, and of fluorescein (300 Da), was tested by assessing flux across the cEND monolayer after removal of unconjugated FITC from the uptake mix by size-exclusion chromatography, as described in Methods (Fig. 2B). The flux of fluorescein and all four FITC-dextrans across the monolayer of cEND cells in differentiation medium (2% FCS) over 4 h was decreased compared with control cells maintained in growth medium containing 10% FCS: paracellular flux was reduced to 30% of control cells for fluorescein, to 26% for FITC-dextran 4 kDa, to 10–15% for both the FITC-dextrans 10 kDa and 70 kDa, and flux was reduced to 4.5% of control cells for FITC-dextran 500 kDa (Fig. 2B). The relatively small amounts of FITC-dextrans 10–500 kDa passing through monolayers kept in serum-reduced medium might possibly be attributed to transcellular vesicular transport over the time course (4 h) of the experiment. Hydrocortisone did not further decrease passage of FITC-dextrans in the molecular range of 10–500 kDa relative to cells kept in 2% FCS-containing medium. However, for fluorescein and FITC-dextran-4 kDa, a significant further decrease of approximately 30% relative to cells kept in 2% differentiation medium was observed in response to glucocorticoids (Fig. 2B). These data show that glucocorticoid-induced enhanced resistance to electrical current appears to be mainly caused by further closure of small-sized pores responsible for the passage of fluorescein and the smallest of the dextrans tested (4 kDa). Taken together, in addition to the immunocytochemical evaluation of endothelial and BBB marker expression, bioelectric and paracellular flux measurements prove that these cells are well suited to constitute a legitimate model of the BBB.
Hydrocortisone alters the cellular morphology and the expression levels of TJ integral membrane proteins
We examined the expression of occludin and claudin-5 protein constituting TJs in endothelial cells by immunoblotting in cEND cells in the presence and absence of hydrocortisone. Consistent with previous observations of increase in junctional staining of zonula occludens proteins in endothelial and epithelial cells (Fig. 2C) (Woo et al. 2000; Antonetti et al. 2002; Kurzen et al. 2002), we observed an increase in claudin-5 and occludin levels in cEND cells: threefold for occludin, and 1.6-fold for claudin-5, as determined by densitometric analysis (Fig. 2C). In confluent cEND cells maintained in 2% FCS, junctional labelling for occludin was rather feint and partly discontinuous (Fig. 2D). In contrast, hydrocortisone-treated cells displayed continuous and strong staining for occludin, which was visible as brightly fluorescent continuous lines surrounding individual cells. Cellular morphology changed from a spindle-like elongated shape to a more cobble-stone-like pattern, as commonly observed in primary BCECs and epithelial cells (Lossinsky & Shivers, 2004) (Fig. 2E). Digital image processing confirmed that the proportion of junctional length per area reflected by occludin immunoreactive signal at cell borders was lowered by 57 ± 3% in hydrocortisone-treated (cell area 4900 ± 1100 μm2) versus untreated (cell area 2130 ± 530 μm2) cEND cells (P < 0.005, Mann–Whitney U-test): the mean junctional length was estimated to be 5.7 × 106± 0.6 × 106μm cm−2 in HC-treated and 1.4 × 107± 0.4 × 107μm cm−2 in untreated cells (P < 0.0001, Mann–Whitney U-test).
Effects of glucocorticoids on brain EC resistance
The effects of glucocorticoids, like hydrocortisone and dexamethasone, are known to be mediated by the GR (Beato, 1989). GR can bind to specific DNA sequences (glucocorticoid-responsive element, GRE) in the 5′ flanking region of target genes, and transactivate gene transcription (Beato, 1989). To follow up the role of glucocorticoid signalling in the induction of the BBB phenotype in BCECs, we analysed transcriptional activation of the occludin gene by RT-PCR. Upregulation of occludin mRNA in confluent cEND cells was detected after 24 h of hydrocortisone (10−6m) treatment. This effect was not detected in the presence of the GR antagonist RU-386 (Fig. 3A), pointing to a GR-mediated transcriptional activation of the occludin promoter by hydrocortisone.
For determination of the dose–response of the glucocorticoid upregulation of occludin mRNA in cEND BCECs, confluent cEND cells were treated with two different glucocorticoids, the natural ligand hydrocortisone and the synthetic glucocorticoid dexamethasone (which has been shown to bind the GR with a threefold higher affinity than hydrocortisone, and to better stabilize the active GR receptor conformation; Hellal-Levy et al. 1999), both at concentrations between 10−6 and 10−12m (Fig. 3B). The maximal effect of both hydrocortisone and dexamethasone on the induction of occludin mRNA was observed at 10−7m (Fig. 3B), with higher concentrations of glucocorticoids having less effect on the induction of occludin mRNA: the induction of occludin mRNA in cEND cells was shown reach its peak value (about threefold) at 10−7m hydrocortisone, and 10−7m of the synthetic specific GR ligand dexamethasone (Fig. 3B). We could not detect an enhancement of occludin transcription below 2 h of GC treatment (data not shown), thus ruling out nongenomic glucocorticoid effects by signalling via other transcription factors. This observation is consistent with previously observed effects describing downregulation of the GR gene and activity by glucocorticoids as a means of negative autoregulation under high hormone concentrations (Bodwell et al. 1998).
Glucocorticoid hormones and insulin synergistically induce expression of transfected human occludin gene promoter constructs
Several studies have identified GR-binding sites in a number of target genes (Nakabayashi et al. 2001; Chen et al. 2003; Pascussi et al. 2003). These GREs are not always identical, but show some variability in several nucleotide positions. Nevertheless, Nakabayashi et al. (2001) determined the GRE consensus sequence to six nucleotides in a palindromic repeat separated by three unspecific nucleotides: TCY TGT nnn ACA RGA (Nakabayashi et al. 2001). Kraus et al. (1999) discussed a pentamer GRE core sequence, TGT NC, and showed GR binding to an imperfect GRE in the somatostatin receptor type 2. Therefore, we next examined whether the gene transcription of occludin integral membrane protein is directly regulated by glucocorticoids. In 2000, Mankertz et al. (2000) described the sequence of a 1853 bp human occludin promoter fragment. Searching for putative GR binding sites in the human occludin promoter, we revealed several pentamer sequences, which might belong to imperfect GREs (Fig. 3C).
The main findings analysing this human occludin promoter fragment are as follows (Fig. 3C). (1) 332–336, 361–365, 399–403, 448–452, 781–785, 1073–1077, 1325–1329 and 1427–1431 bp of the 5′ flanking region, the human occludin promoter contains pentamer sequences, which might belong to imperfect GREs, and on which glucocorticoids can influence gene expression (Kraus et al. 1999; Nakabayashi et al. 2001). (2) Half of these putative GRE half-site response elements are located within the proximal 452 bp upstream of the transcription initiation site, as assessed in this study (Fig. 3C).
Induction of the occludin promoter by glucocorticoids was assessed after treatment of HEK 293 and Cos-7 cells with both the natural GR ligand hydrocortisone and the synthetic glucocorticoid dexamethasone at physiological (100 nm) and supraphysiological (1 μm) concentrations. By treatment with dexamethasone, which binds with high affinity and specifically to the GR, effects of glucocorticoid binding to other nuclear receptors (i.e. androgen receptor, progesterone receptor) and subsequent transcriptional activation of these receptors can be excluded (Hellal-Levy et al. 1999). Transient transfection experiments with the occludin promoter region (OCLN-Proluc7; Mankertz et al. 2000) demonstrated responsiveness to dexamethasone or hydrocortisone induction in human GR (hGR)-cotransfected Cos-7 cells which do not contain endogenous functional GR (Hoeck & Groner, 1990) (Fig. 3D). Treatment of Cos-7 cells cotransfected with hGR with 1 μm (100 nm) dexamethasone stimulated reporter gene expression about 3.6-fold (3.4-), indicating that the observed transcriptional activation by dexamethasone is mediated via the GR and is detectable at physiological glucocorticoid concentrations (Fig. 3D). A lower enhancement of occludin gene expression (2.8-fold induction) was obtained with 1 μm of the natural ligand hydrocortisone (data not shown). Transcriptional upregulation by dexamethasone was nearly completely abolished in the presence of the GR antagonist RU486 which interferes with the binding of the steroid–receptor complex to the major groove in the DNA (Agarwai, 1996) (Fig. 3D). The glucocorticoid response was recapitulated in HEK 293 cells expressing endogenous GR (Oakley et al. 1999), showing 2.8- (2.7-) and 2.6-fold transactivation of occludin promoter activity in the presence of 1 μm (100 nm) dexamethasone and 1 μm hydrocortisone, respectively. Interestingly, when transfected cells were costimulated with low-dose dexamethasone or hydrocortisone (10−7m) plus 1 μm insulin, a marked increase in glucocorticoid-induced reporter activity was observed, leading to 4.1-fold induction of transactivation by both substances in HEK 293 cells versus the 2.6- or 2.8-fold induction observed in HEK 293 cells treated with either hydrocortisone or dexamethasone (Fig. 3D). On the other hand, insulin alone (data not shown), or insulin in combination with high concentrations of glucocorticoid hormone (1 μm) (Fig. 3D), was less effective in HEK 293 cells. However, this effect of insulin was not observed in Cos-7 cells lacking endogenous functional GR (Hoeck & Groner, 1990).
Insulin potentiates endothelial GR activity via GR induction
The biological effects of insulin are mediated by a family of receptors which include the insulin IGF-I and IGF-II receptors with tyrosine kinase activity. Since mouse glucocorticoid receptor phosphorylation status influences multiple functions of the receptor protein, we assessed whether insulin addition to the differentiation medium causes hyperphosphorylation of the mouse GR in cEND cells. In contrast to hyperphosphorylation by the ligand dexamethasone, as previously described (Weigel, 1996), hyperphosphorylation of the GR could not be detected by addition of 1 μm insulin to the differentiation medium (data not shown). Surprisingly, however, a more than threefold induction of the GR protein was observed by insulin in the absence of glucocorticoids (Fig. 4A), which could be recapitulated by RT-PCR analysis: addition of insulin caused an approximate 1.5-fold induction of GR transcript as assessed by densitometric analysis. Collectively, these data strongly suggest that insulin signalling to the GR does not result in receptor phosphorylation, but rather in an increase in or stabilization of GR protein. Insulin supplementation of the growth medium is thus a useful tool for in vitro differentiation of cEND cells to acquire high TER as displayed by the BBB in vivo.
Discussion
A major difficulty in the study of the mechanism of differentiation of BCECs into a tight barrier has been the lack of a suitable in vitro barrier model. Although primary cultured brain ECs from rat, bovine or porcine origin represent a well-differentiated phenotype, they appear to be unable to maintain functional TJs as displayed in vivo (Rubin et al. 1991; Takakura et al. 1991; Grant et al. 1998; de Boer et al. 1999). In order to overcome this problem, immortalized rat brain EC lines have been developed (Risau et al. 1990; Regina et al. 1999). These cell lines display low resistance but are of certain value for drug-transport studies. However, we believe that understanding the permeability regulation of endothelial cells constituting the BBB in the murine model is becoming increasingly important because the mouse is the mammalian model animal of choice for genetic modifications.
The studies presented here offer a novel isolation and culture procedure for obtaining immortalized murine BCECs that retain expression, and appropriate localization, of endothelial and BBB markers. Specifically, the adherens junction-associated protein VE-cadherin, and the TJ-associated proteins claudin-5 and occludin, were all demonstrated to show appropriate localization along intercellular borders in these cells. Claudin-1 expression at endothelial cell contacts, which could be demonstrated in sections in vivo, was not detected in cEND cells (data not shown). Claudin-3 expression is restricted to large pial vessels in vivo in 129Sv mice (data not shown), and can therefore not be expected to be expressed in cEND BCECs, since meninges and large pial vessels were removed during the preparation procedure.
While other immortalized lines of murine BCECs have also been described (Wijsman & Shivers, 1998; Hosoya et al. 2000; Omidi et al. 2003), none have been shown by immunocytochemistry to express claudin-5 and occludin at intercellular borders. bEND.3 BCECs, which were immortalized by the same virus construct as cEND cells (Polyoma Middle T antigen), displayed a clear lack in the correct localization of the TJ-associated proteins Cld-5, occludin and ZO-1 (Omidi et al. 2003), so that differences in preparation technique (i.e. separation from non-BBB ECs and other cell types) and/or culture conditions could be assumed. Thus, so far, only nontransformed primary murine BCECs (Song & Pachter, 2003) offered a suitable tool to address BBB permeability regulation by means of genetic screens and modifications.
Despite expression of TJ proteins at intercellular borders, the barrier properties of cEND cells maintained in growth medium (10% FCS) were only moderate, as expected: correct cellular environment is required for the differentiation of cells to bearing tissue-specific qualities. Numerous studies therefore utilized conditioned medium generated from astrocytes, or even employed coculture techniques between astrocytes and BCECs (Rubin et al. 1991; Rist et al. 1997). In general, these techniques are cumbersome, and results did not lead to establishment of suitable barrier models. In this study, we now present the development of an in vitro culture system, in which the endogenous occludin gene can be induced by glucocorticoids in vitro, without any requirements for cocultivation with other cell types, leading to barrier formation. Hydrocortisone was the only natural supplement found to significantly induce barrier properties. The maximal effect of HC was reached at a concentration of 110 nm or above, which is well in the physiological concentration range of HC in mammalian blood between 70 and 550 nm (Karlson, 1994).
Hydrocortisone is a glucocorticoid, known to affect probably every organ in the mammalian body, yet many of the effects are specific for certain cell types or tissues. In cEND cells, hydrocortisone treatment was shown to induce a noticeable morphology change from an elongated spindle-shaped morphology to an epitheloid cobble-stone morphology. By this morphology change, the calculated junctional length available for paracellular diffusion across the monolayer was shortened by about 50% as compared with untreated cells. Since GC treatment triples TER values across cEND monolayers by up to 1000 Ωcm2, this decrease in junctional length must be assumed to be chiefly responsible for permeability reduction. Comparable TER values have been previously reported under hydrocortisone treatment for primary porcine BCECs (Hoheisel et al. 1998) and cells from epithelial origin (Nguyen & Neville, 1998; Woo et al. 2000), and are comparable to the TER of brain capillaries in vivo, which was estimated to average out at 2000 Ωcm2 (Crone & Olesen, 1982; Rubin et al. 1991).
The remaining portion of permeability decrease could point at a role for glucocorticoids in closure of a small pore system between cEND cells, probably constituted by TJs: while serum reduction alone lowered permeability for fluorescein and uncharged macromolecules across cEND monolayers to only 5–30% from cells grown in high-serum medium, additional treatment with a combination of hydrocortisone and insulin lowered the permeability for fluorescein and the smallest uncharged macromolecule (4 kDa dextran) by an additional 20%. In contrast, permeability of higher-molecular-mass dextrans was already significantly reduced by serum reduction, and did not further decrease in response to treatment with hydrocortisone and insulin. These data show that serum reduction mainly closed the large pore system that allowed the passage of large macromolecules, whereas glucocorticoids are responsible for closing the small pore system known to be critically controlled by TJs. These data are well in accordance with clinical reports describing the barrier-closing effects of glucocorticoids on MRI gadolinium enhancement in acute demyelinating lesions: gadolinium (Gd) enhancement of brain lesions by MRI is a marker of active BBB damage secondary to an inflammatory process. Glucocorticoid treatment was shown to lead to complete suppression of Gd enhancement after short courses (4–8 days) of intravenous glucocorticoid treatment (Burnham et al. 1991).
In an attempt to elucidate the molecular mechanisms of glucocorticoid-induced tightening of the barrier, we were able to show that glucocorticoid signals can directly act at the transcriptional level by interaction with specific cis-acting DNA sequence elements in the occludin gene promoter. In the present study, glucocorticoids dose-dependently increased transcription of occludin mRNA and protein in murine cEND BCECs. This effect was verified to be mediated specifically via the GR: we demonstrated using mifepristone (RU-38486), a potent and selective GR antagonist, that the effect of hydrocortisone and dexamethasone on occludin mRNA expression requires interaction of the glucocorticoid with its intracellular receptor. A similar finding has also been observed in other endothelial cell lines (Regina et al. 1999; Kurzen et al. 2002), as well as in the case of steroid receptor action in occludin induction in epithelial cell differentiation (Woo et al. 2000; Förster et al. 2002). Searching for putative GR-binding sites (Kraus et al. 1999; Nakabayashi et al. 2001) in the human occludin promoter revealed several pentamer sequences, which might belong to imperfect GREs (Fig. 3A). Further investigations need to be done to identify the exact GR binding sequence used in our target gene.
Further improvements of the barrier qualities across cEND monolayers could be achieved by insulin supplementation of the differentiation medium. Surprisingly, insulin signalling to the GR did not result in receptor phosphorylation, but in an increase in or stabilization of GR protein, manifested in an approximate 1.5-fold induction of GR transcript and more than threefold higher GR protein levels. Synergistic effects of glucocorticoids and insulin have been observed previously (Pan & Koontz, 1995).
To the best of our knowledge, this is the first report describing a possible role of glucocorticoids in direct regulation of the expression of TJ components. Moreover, the elucidation of a glucocorticoid–insulin signalling cross-talk in BCECs provides new ways for the induction of barrier qualities in BCECs in vitro. These observations might be of clinical significance, since they could open up new specific routes of treatment of CNS inflammatory diseases; at present, high-dose glucocorticoid therapy is used successfully in multiple sclerosis (MS) relapses. The prevailing opinion on the mode of glucocorticoid action is that it induces the inhibition of cytokine-induced barrier reduction and expression of CAMs (VCAM-1, ICAM-1, E-selectin and PECAM-1), which mediate T cell–BBB interaction and consequently chronic leucocyte recruitment across the BBB. However, this glucocorticoid action is believed to be indirect via repression of NF-kB signalling (Ray et al. 1997; Pitzalis et al. 2002), since the promoters of the CAMs investigated lack consensus GREs.
In contrast, our observations open up a new lead for an understanding of the beneficial effects of glucocorticoid action in a therapeutic regime: additionally to inhibition of cytokine-induced expression of cell adhesion molecules (CAMs), the mode of glucocorticoid action on brain microvascular endothelium appears to consist of tightening of the barrier, and probably by this reducing leucocyte recruitment across the BBB. In this context, the future design of cell- or tissue-specific steroidal drugs might be greatly beneficial for multiple sclerosis patients without the severe side-effects of classical glucocorticoid therapy.
Acknowledgments
This research was supported by SFB487 grant from the Deutsche Forschungsgemeinschaft to D.D., and the Reintegration grant MERG-CT-2004-510649 of the European Commission to C.F. The authors are grateful to Eva-Maria Klute for excellent technical assistance, and to Professor Dr Esther Asan, Dr Rainer Koob, Department of Anatomy and Cell Biology, University of Würzburg, Professor Dr Michael Gekle, Department of Physiology, University of Würzburg, and Dr Jörg Leers, Genetics Department, Justus-Liebig-University Giessen, for helpful discussions.
References
- Agarwai MK. The antiglucocorticoid action of mifepristone. Pharmacol Ther. 1996;70:183–213. doi: 10.1016/0163-7258(96)00016-2. 10.1016/0163-7258(96)00016-2. [DOI] [PubMed] [Google Scholar]
- Almlof T, Wright AP, Gustafsson JA. Role of acidic and phosphorylated residues in gene activation by the glucocorticoid receptor. J Biol Chem. 1995;270:17535–17540. doi: 10.1074/jbc.270.29.17535. 10.1074/jbc.270.29.17535. [DOI] [PubMed] [Google Scholar]
- Antonetti DA, Wolpert EB, DeMaio L, Harhaj NS, Scaduto RC., Jr Hydrocortisone decreases retinal endothelial cell water and solute flux coincident with increased content and decreased phosphorylation of occludin. J Neurochem. 2002;80:667–677. doi: 10.1046/j.0022-3042.2001.00740.x. 10.1046/j.0022-3042.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- Aumailley M, Timpl R, Risau W. Differences in laminin fragment interactions of normal and transformed endothelial cells. Exp Cell Res. 1991;196:177–183. doi: 10.1016/0014-4827(91)90248-s. 10.1016/0014-4827(91)90248-S. [DOI] [PubMed] [Google Scholar]
- Beato M. Gene regulation by steroid hormones. Cell. 1989;56:335–344. doi: 10.1016/0092-8674(89)90237-7. [DOI] [PubMed] [Google Scholar]
- Bodwell JE, Webster JC, Jewell CM, Cidlowski JA, Hu JM, Munck A. Glucocorticoid receptor phosphorylation: overview, function and cell cycle-dependence. J Steroid Biochem Mol Biol. 1998;65:91–99. doi: 10.1016/s0960-0760(97)00185-4. [DOI] [PubMed] [Google Scholar]
- de Boer AG, Gaillard PJ, Breimer DD. The transference of results between blood–brain barrier cell culture systems. Eur J Pharm Sci. 1999;8:1–4. doi: 10.1016/s0928-0987(99)00003-2. 10.1016/S0928-0987(99)00003-2. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein–dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burnham JA, Wright RR, Dreisbach J, Murray RS. The effect of high-dose steroids on MRI gadolinium enhancement in acute demyelinating lesions. Neurology. 1991;41:1349–1354. doi: 10.1212/wnl.41.9.1349. [DOI] [PubMed] [Google Scholar]
- Chen Y, Ferguson SS, Negishi M, Goldstein JA. Identification of constitutive androstane receptor and glucocorticoid receptor binding sites in the CYP2C19 promoter. Mol Pharmacol. 2003;64:316–324. doi: 10.1124/mol.64.2.316. [DOI] [PubMed] [Google Scholar]
- Crone C, Olesen SP. Electrical resistance of brain microvascular endothelium. Brain Res. 1982;241:49–55. doi: 10.1016/0006-8993(82)91227-6. [DOI] [PubMed] [Google Scholar]
- Engelhardt B. Role of glucocorticoids on T cell recruitment across the blood–brain barrier. Z Rheumatol. 2000;59(suppl. 2):II/18–II/21. doi: 10.1007/s003930070012. [DOI] [PubMed] [Google Scholar]
- Fagart J, Wurtz JM, Souque A, Hellal-Levy C, Moras D, Rafestin-Oblin ME. Antagonism in the human mineralocorticoid receptor. EMBO J. 1998;17:3317–3325. doi: 10.1093/emboj/17.12.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Förster C, Makelä S, Becker D, Hultenby K, Warner M, Gustafsson J-Å. Involvement of estrogen receptor beta in terminal differentiation of mammary epithelium. Proc Natl Acad Sci U S A. 2002;99:15578–15583. doi: 10.1073/pnas.192561299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golenhofen N, Ness W, Wawrousek EF, Drenckhahn D. Expression and induction of the stress protein alpha-B-crystallin in vascular endothelial cells. Histochem Cell Biol. 2002;117:203–209. doi: 10.1007/s00418-001-0378-7. [DOI] [PubMed] [Google Scholar]
- Gotsch U, Borges E, Bosse R, Boggemeyer E, Simon M, Mossmann H, Vestweber D. VE-cadherin antibody accelerates neutrophil recruitment in vivo. J Cell Sci. 1997;110:583–588. doi: 10.1242/jcs.110.5.583. [DOI] [PubMed] [Google Scholar]
- Grant GA, Abbott NJ, Janigro D. Understanding the physiology of the blood–brain barrier. In Vitro models. News Physiol Sci. 1998;13:287–293. doi: 10.1152/physiologyonline.1998.13.6.287. [DOI] [PubMed] [Google Scholar]
- Gupta V, Wagner BJ. Expression of the functional glucocorticoid receptor in mouse and human lens epithelial cells. Invest Ophthalmol Vis Sci. 2003;44:2041–2046. doi: 10.1167/iovs.02-1091. [DOI] [PubMed] [Google Scholar]
- Hamm S, Dehouck B, Kraus J, Wolburg-Buchholz K, Wolburg H, Risau W, Cecchelli R, Engelhardt B, Dehouck MP. Astrocyte mediated modulation of blood–brain barrier permeability does not correlate with a loss of tight junction proteins from the cellular contacts. Cell Tissue Res. 2004;315:157–166. doi: 10.1007/s00441-003-0825-y. [DOI] [PubMed] [Google Scholar]
- Hatashita S, Hoff JT. Brain edema and cerebrovascular permeability during cerebral ischemia in rats. Stroke. 1990;21:582–588. doi: 10.1161/01.str.21.4.582. [DOI] [PubMed] [Google Scholar]
- Hellal-Levy C, Couette B, Fagart J, Souque A, Gomez-Sanchez C, Rafestin-Oblin M. Specific hydroxylations determine selective corticosteroid recognition by human glucocorticoid and mineralocorticoid receptors. FEBS Lett. 1999;464:9–13. doi: 10.1016/s0014-5793(99)01667-1. [DOI] [PubMed] [Google Scholar]
- Hoeck W, Groner B. Hormone-dependent phosphorylation of the glucocorticoid receptor occurs mainly in the amino-terminal transactivation domain. J Biol Chem. 1990;265:5403–5408. [PubMed] [Google Scholar]
- Hoheisel D, Nitz T, Franke H, Wegener J, Hakvoort A, Tilling T, Galla HJ. Hydrocortisone reinforces the blood–brain properties in a serum-free cell culture system. Biochem Biophys Res Commun. 1998;247:312–315. [PubMed] [Google Scholar]
- Hosoya K, Tetsuka K, Nagase K, Tomi M, Saeki S, Ohtsuki S, Takanaga H, Yanai N, Obinata M, Kikuchi A, Okano T, Terasaki T. Conditionally immortalized brain capillary endothelial cell lines established from a transgenic mouse harboring temperature-sensitive simian virus 40 large T-antigen gene. AAPS Pharmsci. 2000;2:E27. doi: 10.1208/ps020328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlson P, Doenecke D, Koolman J. Stuttgart: Georg Thieme Verlag; 1994. Bioechmie für Mediziner und Naturwissenschaftler. [Google Scholar]
- Kraus J, Woltje M, Hollt V. Regulation of mouse somatostatin receptor type 2 gene expression by glucocorticoids. FEBS Lett. 1999;459:200–204. doi: 10.1016/s0014-5793(99)01236-3. [DOI] [PubMed] [Google Scholar]
- Kurzen H, Manns S, Dandekar G, Schmidt T, Pratzel S, Kraling BM. Tightening of endothelial cell contacts: a physiologic response to cocultures with smooth-muscle-like 10T1/2 cells. J Invest Dermatol. 2002;119:143–153. doi: 10.1046/j.1523-1747.2002.01792.x. [DOI] [PubMed] [Google Scholar]
- Kyhse-Andersen J. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J Biochem Biophys Methods. 1984;10:203–209. doi: 10.1016/0165-022x(84)90040-x. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lossinsky AS, Shivers RR. Structural pathways for macromolecular and cellular transport across the blood–brain barrier during inflammatory conditions. Rev Histol Histopathol. 2004;19:535–564. doi: 10.14670/HH-19.535. [DOI] [PubMed] [Google Scholar]
- Mankertz J, Tavalali S, Schmitz H, Mankertz A, Riecken EO, Fromm M, Schulzke JD. Expression from the human occludin promoter is affected by tumor necrosis factor alpha and interferon gamma. J Cell Sci. 2000;113:2085–2090. doi: 10.1242/jcs.113.11.2085. [DOI] [PubMed] [Google Scholar]
- McDonald WI. Rachelle Fishman–Matthew Moore Lecture. The pathological and clinical dynamics of multiple sclerosis. J Neuropathol Exp Neurol. 1994;53:338–343. doi: 10.1097/00005072-199407000-00003. [DOI] [PubMed] [Google Scholar]
- Nakabayashi H, Koyama Y, Sakai M, Li HM, Wong NC, Nishi S. Glucocorticoid stimulates primate but inhibits rodent alpha-fetoprotein gene promoter. Biochem Biophys Res Commun. 2001;287:160–172. doi: 10.1006/bbrc.2001.5564. [DOI] [PubMed] [Google Scholar]
- Nguyen DA, Neville MC. Tight junction regulation in the mammary gland. J Mammary Gland Biol Neoplasia. 1998;3:233–246. doi: 10.1023/a:1018707309361. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Webster JC, Jewell CM, Sar M, Cidlowski JA. Immunocytochemical analysis of the glucocorticoid receptor alpha isoform (GRalpha) using GRalpha-specific antibody. Steroids. 1999;64:742–751. doi: 10.1016/s0039-128x(99)00059-8. [DOI] [PubMed] [Google Scholar]
- Omidi Y, Campbell L, Barar J, Connell D, Akhtar S, Gumbleton M. Evaluation of the immortalised mouse brain capillary endothelial cell line, b.End3, as an in vitro blood–brain barrier model for drug uptake and transport studies. Brain Res. 2003;990:95–112. doi: 10.1016/s0006-8993(03)03443-7. [DOI] [PubMed] [Google Scholar]
- Pan L, Koontz J. Insulin enhances glucocorticoid receptor-mediated induction of gene expression independent of a specific insulin response element. Arch Biochem Biophys. 1995;316:886–892. doi: 10.1006/abbi.1995.1119. [DOI] [PubMed] [Google Scholar]
- Pardridge WM. Recent advances in blood–brain barrier transport. Annu Rev Pharmacol Toxicol. 1988;28:25–39. doi: 10.1146/annurev.pa.28.040188.000325. [DOI] [PubMed] [Google Scholar]
- Pascussi JM, Busson-Le Coniat M, Maurel P, Vilarem MJ. Transcriptional analysis of the orphan nuclear receptor constitutive androstane receptor (NR1I3) gene promoter: identification of a distal glucocorticoid response element. Mol Endocrinol. 2003;17:42–55. doi: 10.1210/me.2002-0244. [DOI] [PubMed] [Google Scholar]
- Pitzalis C, Pipitone N, Perretti M. Regulation of leukocyte–endothelial interactions by glucocorticoids. Ann N Y Acad Sci. 2002;966:108–118. doi: 10.1111/j.1749-6632.2002.tb04208.x. [DOI] [PubMed] [Google Scholar]
- Qizilbash N, Lewington SL, Lopez-Arrieta JM. Corticosteroids for acute ischaemic stroke. Cochrane Database Syst Rev. 2002;2 doi: 10.1002/14651858.CD000064. CD000064. [DOI] [PubMed] [Google Scholar]
- Ray KP, Farrow S, Daly M, Talabot F, Searle N. Induction of the E-selectin promoter by interleukin 1 and tumour necrosis factor alpha, and inhibition by glucocorticoids. Biochem J. 1997;328:707–715. doi: 10.1042/bj3280707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regina A, Koman A, Piciotti M, El Hafny B, Center MS, Bergmann R, Couraud PO, Roux F. Mrp1 multidrug resistance-associated protein and P-glycoprotein expression in rat brain microvessel endothelial cells. J Neurochem. 1998;71:705–715. doi: 10.1046/j.1471-4159.1998.71020705.x. [DOI] [PubMed] [Google Scholar]
- Regina A, Romero IA, Greenwood J, Adamson P, Bourre JM, Couraud PO, Roux F. Dexamethasone regulation of P-glycoprotein activity in an immortalized rat brain endothelial cell line, GPNT. J Neurochem. 1999;73:1954–1963. [PubMed] [Google Scholar]
- Risau W, Engelhardt B, Wekerle H. Immune function of the blood–brain barrier: incomplete presentation of protein (auto-) antigens by rat brain microvascular endothelium in vitro. J Cell Biol. 1990;110:1757–1766. doi: 10.1083/jcb.110.5.1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risau W, Wolburg H. Development of the blood–brain barrier. Trends Neurosci. 1990;13:174–178. doi: 10.1016/0166-2236(90)90043-a. [DOI] [PubMed] [Google Scholar]
- Rist RJ, Romero IA, Chan MW, Couraud PO, Roux F, Abbott NJ. F-actin cytoskeleton and sucrose permeability of immortalised rat brain microvascular endothelial cell monolayers: effects of cyclic AMP and astrocytic factors. Brain Res. 1997;768:10–18. doi: 10.1016/s0006-8993(97)00586-6. [DOI] [PubMed] [Google Scholar]
- Rubin LL, Hall DE, Porter S, Barbu K, Cannon C, Horner HC, Janatpour M, Liaw CW, Manning K, Morales J, et al. A cell culture model of the blood–brain barrier. J Cell Biol. 1991;115:1725–1735. doi: 10.1083/jcb.115.6.1725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Pachter JS. Culture of murine brain microvascular endothelial cells that maintain expression and cytoskeletal association of tight junction-associated proteins. In Vitro Cell Dev Biol Anim. 2003;39:313–320. doi: 10.1290/1543-706X(2003)039<0313:COMBME>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Takakura Y, Audus KL, Borchardt RT. Blood–brain barrier: transport studies in isolated brain capillaries and in cultured brain endothelial cells. Adv Pharmacol. 1991;22:137–165. doi: 10.1016/s1054-3589(08)60034-4. [DOI] [PubMed] [Google Scholar]
- Wang F, Daugherty B, Keise LL, Wei Z, Foley JP, Savani RC, Koval M. Heterogeneity of claudin expression by alveolar epithelial cells. Am J Respir Cell Mol Biol. 2003;29:62–70. doi: 10.1165/rcmb.2002-0180OC. [DOI] [PubMed] [Google Scholar]
- Weigel NL. Steroid hormone receptors and their regulation by phosphorylation. Biochem J. 1996;319:657–667. doi: 10.1042/bj3190657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman JA, Shivers RR. Immortalized mouse brain endothelial cells are ultrastructurally similar to endothelial cells and respond to astrocyte-conditioned medium. In Vitro Cell Dev Biol Anim. 1998;34:777–784. doi: 10.1007/s11626-998-0032-y. [DOI] [PubMed] [Google Scholar]
- Woo PL, Cercek A, Desprez PY, Firestone GL. Involvement of the helix–loop–helix protein Id-1 in the glucocorticoid regulation of tight junctions in mammary epithelial cells. J Biol Chem. 2000;275:28649–28658. doi: 10.1074/jbc.M910373199. [DOI] [PubMed] [Google Scholar]