Abstract
We tested the hypothesis that an acute decrease in muscle TCA cycle intermediates during contraction would compromise aerobic energy delivery. Male Wistar rats were anaesthetized and the gastrocnemius–plantaris–soleus (GPS) muscle complex from one leg was isolated and perfused with a red cell medium containing either saline (Con) or cycloserine (Cyclo; 0.05 mg g−1), an inhibitor of alanine aminotransferase (AAT). After 1 h of perfusion, the GPS muscle was either snap frozen (Con-Rest, n = 11; Cyclo-Rest, n = 9) or stimulated to contract for 10 min (1 Hz, 0.3 ms, 2 V) with blood flow fixed at 30 ml min−1 (100 g)−1 and then snap frozen (Con-Stim, n = 10; Cyclo-Stim, n = 10). Maximal AAT activity was > 80% lower (P < 0.001) in both Cyclo-treated groups (Rest: 0.61 ± 0.02; Stim: 0.63 ± 0.01 mmol (kg wet wt)−1 min−1; mean ± s.e.m.) compared to Con (Rest: 3.56 ± 0.16; Stim: 3.92 ± 0.29). The sum of five measured TCAI (ΣTCAI) was reduced by 23% in Cyclo-Rest versus Con-Rest but this was not different (P = 0.08). However, after 10 min of contraction, the ΣTCAI was 25% lower (P = 0.006) in Cyclo-Stim compared to Con-Stim (1.88 ± 0.15 versus 2.48 ± 0.11 mmol (kg dry wt)−1). Despite the acute decrease in TCAI after Cyclo treatment, the contraction-induced changes in markers of non-oxidative energy provision (phosphocreatine, ATP and lactate) and the decline in tension after 10 min of stimulation were similar compared to Con. These data do not support the hypothesis that the total muscle concentration of TCAI is causally linked to the rate of mitochondrial respiration during contraction.
The regulation of tricarboxylic acid (TCA) cycle flux in various tissues is extremely complex but one of the regulatory factors involved may be changes in the concentrations of TCA cycle intermediates (TCAI) (Williamson & Cooper, 1980). For example, in myocardium, depletion of TCAI results in a rapid decline in contractile function, which is reversed by addition of anaplerotic substrates that promote flux into the TCAI pool (Taegtmeyer et al. 1980; Russel & Taegtmeyer, 1991). In skeletal muscle, the total concentration of TCAI has been shown to increase above resting levels during electrically evoked contractions in rats (Aragon & Lowenstein, 1980) and strenuous exercise in humans (Sahlin et al. 1990; Gibala et al. 1997b); however, the physiological significance of this phenomenon is controversial. Some investigators have proposed that the increase in muscle TCAI is crucial in order to achieve high rates of mitochondrial respiration during exercise (Wagenmakers et al. 1990; Sahlin et al. 1990). Alternatively, we have suggested fluctuations in the size of the muscle TCAI pool during exercise are not causally related to TCA cycle flux or the capacity for aerobic energy provision (Gibala et al. 1998; Constantin-Teodosiu et al. 1999).
In an effort to discern the physiological significance of the contraction-induced increase in muscle TCAI, several studies have attempted to manipulate the TCAI pool during exercise and determine the effect that this has on aerobic energy metabolism and exercise performance. Two studies that augmented the rate of TCAI expansion during exercise using glutamine ingestion (Bruce et al. 2001) or altering muscle glycogen availability (Gibala et al. 2002) reported no effect on aerobic energy provision, as judged by changes in phosphocreatine degradation and lactate accumulation during exercise. Recently, we employed exercise training as a model in order to attenuate the rate of muscle TCAI expansion for the first time in humans (Dawson et al. 2003; Howarth et al. 2004). The results from these studies showed that, despite a ∼40% decrease in TCAI expansion during exercise, there was no impairment of aerobic energy provision or performance (Dawson et al. 2003; Howarth et al. 2004). However, a limitation inherent to training studies is that a wide array of variables may be altered, including possibly the impact of [TCAI] on metabolic control. A far more compelling strategy would be to acutely reduce the total muscle concentration of TCAI during contraction and determine the effect on muscle metabolism and function.
The purpose of the present study therefore was to determine whether an acute decrease in the muscle TCAI pool would compromise aerobic energy delivery and/or contractile function. We employed an isolated rat hindlimb model and treated animals with either cycloserine (4-amino-3-isoxazolidinone) or saline (control) prior to a 10 min stimulation protocol. Cycloserine is a structural analogue of alanine and potent inhibitor of alanine aminotransferase (Edmondson et al. 1977), which is believed to be the key enzyme responsible for the rapid increase in muscle TCAI upon contraction (Sahlin et al. 1990; Gibala et al. 1997a).
Methods
Animals
Forty healthy male Wistar rats (Charles River, Margate, UK), housed in holding rooms at the University of Nottingham's Biomedical Services Unit, were exposed to ambient temperature and 12 h light–dark cycles. The animals had a mean body of mass 400 g and gastrocnemius–plantaris–soleus (GPS) muscle complex mass of ∼2.5 g. There were four experimental conditions as described further below: control–rest (Con-Rest; n = 11), control–stimulation (Con-Stim; n = 10), cycloserine–rest (Cyclo-Rest, n = 9), and cycloserine–stimulation (Cyclo-Stim, n = 10).
Experimental protocol
Animals were terminally anaesthetized (Inactin, 120 mg (kg body mass)−1, i.p.), and placed on a heated (37°C) surgical table in preparation for surgery. The musculature of the left hindlimb was exposed by removal of the skin from this region. The branches of the femoral artery and vein were ligated using silk ligatures or thermocautery up to the point where these vessels entered the gastrocnemius–plantaris–soleus (GPS) muscle complex. This ensured that blood flow to and from this muscle group was solely via the intact femoral artery and vein. The biceps femoris was then removed, and a length of thread was tied around the Achilles tendon and cut distal to the ligature. This resulted in the GPS muscle group remaining fixed to the limb on the dorsal side of the knee joint. The femoral artery and vein were then cannulated and heparinized saline (10 U ml−1) was slowly flushed through the vasculature of the GPS muscle group. An arterial cannula was then attached to a primed perfusion system which was contained in an enclosed chamber which maintained a temperature of 37°C, and the muscle group was perfused with previously prepared perfusion medium (see below for details). The animal was then killed humanely (according to UK Home Office guidelines) with an overdose of barbiturate by intracardiac injection and placed ventral surface down to enable the tibia to be secured using a clamp fixed to a stereological frame, after which the thread from the Achilles tendon was attached to an isometric force transducer (Grass/Astro-Med, Inc., West Warwick, RI, USA). Clamping the tibia facilitated the measurement of muscle force production during contraction by minimizing inertia generated by movement of the animal. Muscle contraction was achieved via direct electrical stimulation of the sciatic nerve using a hook electrode (Harvard Apparatus, Holliston, MA, USA) and isometric tension was recorded throughout (MacLab 400, ADInstruments, Australia). Muscle blood flow was increased 2-fold from the resting rate at the start of the 10 min of isometric contraction in order to simulate the contraction-induced hyperaemic response. This contracting flow rate remained constant throughout the remainder of the experiment.
The perfusion medium contained isolated porcine red blood cells suspended in a modified Krebs buffer (pH 7.4, Hct 47%, 6 mm glucose). dl-Cycloserine (Sigma C-7005), dissolved in saline, was added to the perfusion medium so that a total dose of 0.05 mg (g body wt)−1 was delivered to each animal in the cycloserine-treated groups (Blackshear et al. 1975), whereas control animals received perfusion medium containing an iso-volume of saline. Muscles were perfused with their respective treatment at a resting flow rate (15 ml min−1 (100 g of wet muscle)−1) for 60 min prior to undergoing, in a randomized order, one of the following: (i) excision of the GPS muscle group, which was then immediately snap frozen in liquid nitrogen (Con-Rest, Cyclo-Rest), or (ii) 10 min of isometric contraction (1 Hz, 0.3 ms, 2 V), followed by immediate excision of the GPS, which was then snap frozen in liquid nitrogen (Con-Stim; Cyclo-Stim).
Muscle analyses
All muscle samples were initially stored in liquid nitrogen and subsequently divided into two pieces. One piece was kept in liquid nitrogen for subsequent analysis of muscle enzymes, while the other piece was freeze-dried, powdered and dissected free of blood and connective tissue. Freeze-dried samples were stored at −86°C until analyses. One portion of freeze-dried muscle was extracted on ice using 0.5 m perchloric acid (containing 1 mm EDTA), neutralized with 2.2 m KHCO3, and the resulting supernatant was used for the determination of all metabolites except glycogen. ATP, phosphocreatine, creatine, lactate, malate, fumarate, citrate, isocitrate, 2-oxoglutarate, alanine and glutamate were measured using enzymatic assays adapted for fluorometry (Hitachi F-2500 fluorescence spectrophotometer, Hitachi High-Technologies Corp., Japan) (Harris et al. 1974; Passoneau & Lowry, 1993). For glycogen analysis, a ∼2 mg aliquot of freeze-dried muscle was incubated in 2.0 n HCl and heated for 2 h at 100°C to hydrolyse the glycogen to glucosyl units. The solution was subsequently neutralized with an equal volume of 2.0 n NaOH and analysed for glucose using an enzymatic assay adapted for fluorometry (Passoneau & Lowry, 1993). For enzyme analyses, frozen wet muscle samples were initially homogenized using methods described by Henriksson et al. (1986) to a 50 times dilution. The maximal activity of citrate synthase was determined on a spectrophotometer (Ultrospec 3000 pro UV/Vis) using a method described by Carter et al. (2001) and the maximal activity of alanine aminotransferase was determined on a fluorometer (Hitachi F-2500) using a method described by Passoneau & Lowry (1993).
Statistical analyses
All muscle metabolites were analysed using a one-way analysis of variance owing to the four independent groups of rats. Significant effects were subsequently analysed using a Tukey's honestly significant difference post hoc test. Significance for all analysis was set at P < 0.05. All values are presented as means ± standard error of the mean (s.e.m.).
Results
Muscle enzymes
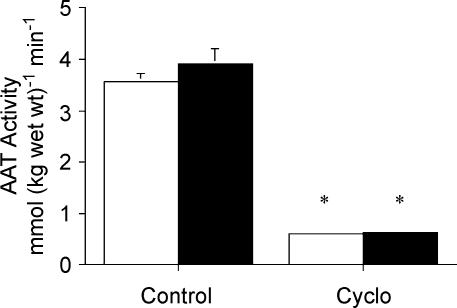
The maximal activity of alanine aminotransferase measured in the Cyclo-treated animals at rest and after stimulation was ∼80% lower (P < 0.001) compared to the respective control groups (Fig. 1). The maximal activity of citrate synthase was similar between all groups (Table 1).
Figure 1. Maximal activity of alanine aminotransferase measured at rest (open bars) and after stimulation (filled bars) following pretreatment with saline (control) or cycloserine (Cyclo).
Values are means ± s.e.m.*P < 0.05 versus saline-treated control at same time point.
Table 1.
Muscle metabolites and citrate synthase activity
| Control | Cycloserine | |||
|---|---|---|---|---|
| Metabolite | Rest | Stimulation | Rest | Stimulation |
| Glycogen | 152 ± 9 | 114 ± 5† | 152 ± 6 | 118 ± 5† |
| Pyruvate | 0.36 ± 0.06 | 0.72 ± 0.03† | 0.26 ± 0.03 | 0.60 ± 0.04† |
| Lactate | 12.7 ± 2.0 | 34.6 ± 2.9† | 14.8 ± 2.5 | 35.0 ± 1.2† |
| ATP | 25.6 ± 0.5 | 22.9 ± 0.6† | 25.4 ± 0.4 | 22.9 ± 0.4† |
| Creatine | 51.9 ± 4.3 | 88.6 ± 5.6† | 54.9 ± 4.4 | 87.4 ± 2.8† |
| Malate | 0.67 ± 0.08 | 1.21 ± 0.07† | 0.43 ± 0.03 | 0.68 ± 0.04*† |
| Fumarate | 0.10 ± 0.02 | 0.20 ± 0.02† | 0.06 ± 0.01 | 0.13 ± 0.01*† |
| 2-oxoglutarate | 0.09 ± 0.00 | 0.08 ± 0.00 | 0.08 ± 0.01 | 0.09 ± 0.00 |
| Citrate | 1.07 ± 0.12 | 0.89 ± 0.09 | 0.99 ± 0.11 | 0.96 ± 0.12 |
| Isocitrate | 0.17 ± 0.01 | 0.18 ± 0.01 | 0.06 ± 0.01* | 0.04 ± 0.01*† |
| Citrate synthase | 27.5 ± 1.0 | 23.3 ± 1.0 | 24.7 ± 1.1 | 24.8 ± 0.6 |
Values are means ± s.e.m. in mmol (kg dry wt)−1, except for citate synthase activity which is in mmol (kg wet wt)−1 min−1.
P < 0.05 versus saline-treated control at same time point.
P < 0.05 versus Rest within same condition.
Muscle TCAI
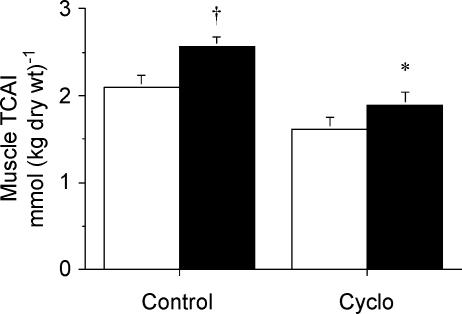
The total muscle concentration of the five measured TCAI (ΣTCAI) was 23% lower at rest in the Cyclo-treated group compared to Con, but this was not different (P = 0.08). However, after 10 min of contraction, the ΣTCAI was 25% lower (P = 0.006) in Cyclo-Stim compared to Con-Stim (Fig. 2). Notably, the ΣTCAI during contraction in the Cyclo-treated animals was not different compared to control group value at rest. The difference in ΣTCAI between groups after contraction was mainly due to a reduced concentration of malate (Table 1). The concentration of fumarate during contraction was also lower in the Cyclo-treated animals compared to controls, but the concentrations of citrate, isocitrate and 2-oxoglutarate were not different between groups (Table 1).
Figure 2. Sum concentration of 5 muscle TCAI measured at rest (open bars) and after stimulation (filled bars) following pretreatment with saline (control) or cycloserine (Cyclo).
Values are means ± s.e.m.*P < 0.05 versus saline-treated control at same time point. †P < 0.05 versus Rest within same condition.
Muscle amino acid
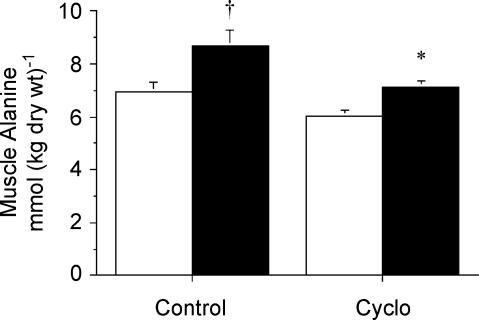
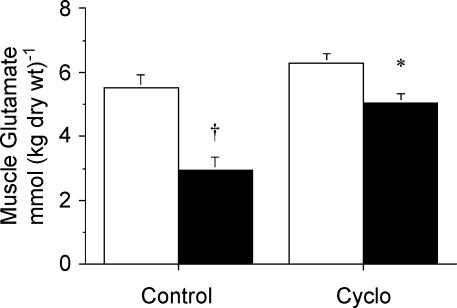
There were no significant differences between treatments in the resting muscle concentrations of alanine or glutamate. However, following contraction muscle [alanine] was lower (P = 0.03) in Con-Stim versus CycloStim (Fig. 3), whereas muscle [glutamate] was higher (P = 0.002) in the Cyclo-treated group compared to Con (Fig. 4).
Figure 3. Concentration of muscle alanine measured at rest (open bars) and after stimulation (filled bars) following pretreatment with saline (control) or cycloserine (Cyclo).
Values are means ± s.e.m.*P < 0.05 versus saline-treated control at same time point. †P < 0.05 versus Rest within same condition.
Figure 4. Concentration of muscle glutamate measured at rest (open bars) and after stimulation (filled bars) following pretreatment with saline (control) or cycloserine (Cyclo).
Values are means ± s.e.m.*P < 0.05 versus saline-treated control at same time point. †P < 0.05 versus Rest within same condition.
Other metabolites
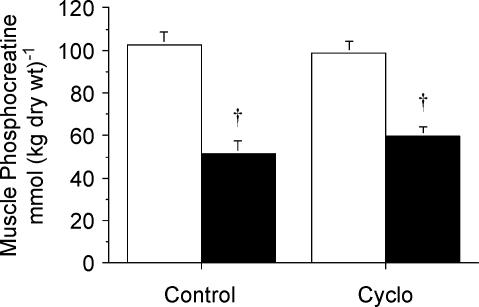
Muscle glycogen concentration was ∼25% lower (P < 0.001) after stimulation compared to rest in both the Cyclo-treated and control groups, but there were no differences between treatments (Table 1). Similarly, there were contraction-induced changes in the concentrations of muscle lactate, phosphocreatine and ATP but no differences between treatments. Muscle lactate concentration was higher (P < 0.001) after stimulation compared to rest in both conditions (Table 1), whereas the concentrations of muscle phosphocreatine (Fig. 5) and ATP (Table 1) were reduced (both P < 0.001).
Figure 5. Concentration of muscle phosphocreatine measured at rest (open bars) and after stimulation (filled bars) following pretreatment with saline (control) or cycloserine (Cyclo).
Values are means ± s.e.m.†P < 0.05 versus Rest within same condition.
Contractile function
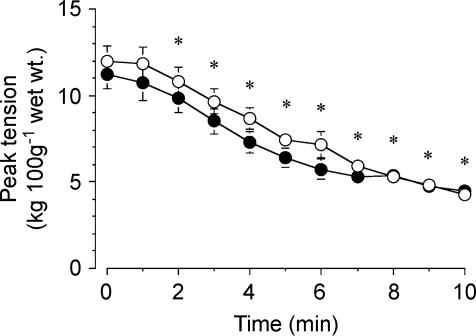
Absolute peak tension prior to stimulation was not different between groups (11.9 ± 0.9 and 11.2 ± 0.8 kg (100 g wet wt)−1 for Con and Cyclo, respectively, P = 0.40). Peak tension declined during stimulation in both groups (main effect for time, P = 0.001), but there were no differences between conditions (Fig. 6). At the end of the 10-min stimulation protocol, force production decreased to 37 ± 4 and 42 ± 4% of the peak value at baseline in the Con and Cyclo-treated groups, respectively.
Figure 6. Absolute peak tension over the course of 10 min stimulation protocol, following pretreatment with saline (open circles) or cycloserine (filled circles).
Values are means ± s.e.m.*P = 0.001 versus 0 min (main effect for time). There were no differences between treatments in absolute or relative peak tension at any time point.
Discussion
The major novel finding from the present study was that an acute reduction in the TCAI pool did not affect aerobic energy delivery or the decline in tension development after 10 min of electrically evoked contractions in rat muscle. We used cycloserine to inhibit alanine aminotransferase (AAT) since this enzyme is believed to be the primary mechanism responsible for the rapid increase in muscle TCAI during contraction (Gibala et al. 1997a; Sahlin et al. 1990). The effectiveness of our experimental intervention was evidenced by the fact that – as compared to saline-treated controls – the cycloserine-treated muscles showed (i) an ∼80% reduction in the maximal activity of AAT; (ii) a 25% reduction in the total concentration of five measured TCAI; and (iii) a lower alanine concentration and higher glutamate concentration following contraction. Despite the reduced AAT activity and blunted TCAI response in the cycloserine-treated group, the contraction-induced changes in muscle phosphocreatine, ATP and lactate were similar compared to controls. Resting absolute peak tension and the decline in force-generating capacity after 10 min of contraction were also similar between conditions. The present data therefore do not support the hypothesis proposed by others (Sahlin et al. 1990; Wagenmakers et al. 1990) that the total muscle concentration of TCAI is causally linked to the rate of mitochondrial respiration during contraction.
Significance of muscle TCAI expansion during contraction
Wagenmakers (1998) suggested that the size of the TCAI pool plays a central role in muscle energy metabolism and proposed that ‘the alanine aminotransferase reaction functions to establish and maintain high concentrations of TCA-cycle intermediates and a high TCA cycle flux in the first minutes of exercise.’ This theory remains prominent in the literature and a common interpretation is that a given concentration of TCAI is required in order to sustain a given rate of oxidative phosphorylation during exercise. For example, recent studies conducted on healthy athletes (Ivy et al. 2003) and patients with McArdle's disease (Vissing & Haller, 2003) suggested that improvements in exercise capacity after nutrient ingestion were attributable to an increase in muscle TCAI pool size that permitted a higher rate of oxidative metabolism. However, the present study demonstrates a clear dissociation between muscle [TCAI], energy metabolism and contractile function, since markers of non-oxidative metabolism and tension development were similar between treatments despite a 24% decrease in TCAI in the cycloserine-treated muscles. This is the first study to acutely reduce the size of the muscle TCAI pool during contraction; however, our data are consistent with two recent studies that used exercise training as an intervention to attenuate muscle TCAI expansion during exercise (Dawson et al. 2003; Howarth et al. 2004). In those two studies (Dawson et al. 2003; Howarth et al. 2004), the size of the muscle TCAI pool was reduced by ∼40% during exercise after training; however, a limitation of the work was that we could not rule out potential training-induced changes in the sensitivity of mitochondrial respiration to [TCAI]. The present data are more definitive with respect to the lack of effect of TCAI on mitochondrial respiration, since muscle oxidative potential – as reflected by the maximal activity of citrate synthase – was similar between groups.
Our statistical analyses revealed that there were no significant differences between treatments in either absolute or relative tension at rest or the decline in tension during stimulation. Although absolute peak tension was slightly lower at baseline and over the first few minutes of contraction in the cycloserine-treated animals (Fig. 6), if this was a ‘real’ effect of the drug then it should have also been detected in our chemical analyses performed after 10 min of stimulation. That is, if cycloserine compromised aerobic energy delivery during the early phase of contraction, then phosphocreatine degradation should have been greater at the end of the stimulation protocol. There are numerous examples in the literature showing that ‘early’ perturbations to mitochondrial respiration can be detected in chemical analyses performed at a ‘later’ time point during contraction. For example, in a recent study (Gibala et al. 2002), we showed that low muscle glycogen availability compromised aerobic energy delivery during the rest–work transition, as evidenced by greater phosphocreatine degradation after 1 min of contraction (difference of ∼10 mmol (kg dry wt)−1. compared to the high glycogen control trial). After an additional 9 min of contraction, the ‘early’ effect of reduced glycogen availability on mitochondrial respiration was still evident, since biopsies obtained after 10 min revealed a similar ∼10 mmol (kg dry wt)−1 difference in phosphocreatine utilization between the low and high glycogen conditions. Thus, based on our statistical analyses of tension development and chemical marker of mitochondrial respiration after 10 min of contraction, there is little evidence to suggest that cycloserine affected muscle function in the present study.
Muscle TCAI profile during contraction
The magnitude of TCAI expansion during contraction in the present study (∼50% increase compared to rest in the control animals) was lower than the 2- to 4-fold increase in [TCAI] that is typically observed during strenuous exercise in humans (Sahlin et al. 1990; Gibala et al. 1997a, b; Bruce et al. 2001). While it is difficult to make direct comparisons between species and experimental models, it could be interpreted that our ‘exercise’ protocol was rather modest based strictly on the TCAI response. However, the relative intensity of contraction was actually quite high as reflected by the ∼60% decline in peak tension over the 10 min stimulation period in both conditions. In addition, there were significant changes in several markers of non-oxidative energy provision including a ∼45% decrease in muscle phosphocreatine concentration. Moreover, it appears that the peak capacity for TCAI expansion in the rat hindlimb is lower than in the human vastus lateralis muscle and this may be related in part to fibre type differences (Hintz et al. 1982). We are aware of only one other study that attempted to measure the pool of muscle TCAI in rat skeletal muscle during stimulation (Aragon & Lowenstein, 1980), and the contraction-induced size of the TCAI pool in that study compares favourably with the control group data in the present study (∼2.4 versus 2.48 ± 0.11 mmol (kg dry wt)−1, respectively, for the same 5 TCAI after 10 min of stimulation). The relative intensity of contraction in the Aragon & Lowenstein (1980) study also appeared to be quite high, based on the 3-fold increase in inosine monophosphate after 10 min of stimulation, and the 50% decrease in muscle phosphocreatine concentration is similar to that observed in the present study. Finally, irrespective of absolute concentration changes, the TCAI profile during stimulation in our control animals compares favourably to previous studies conducted on rats (Aragon & Lowenstein, 1980) and humans (Sahlin et al. 1990; Sahlin et al. 1995; Gibala et al. 1997a, b; Bruce et al. 2001) in that changes in the concentration of malate accounted for the majority of the increase in the TCAI pool.
Cycloserine as an experimental intervention
Cycloserine has been used as an experimental intervention to study intermediary metabolism in numerous tissues including skeletal muscle (Ruderman & Berger, 1974; Blackshear et al. 1975; Wu et al. 1989). The compound exists in both natural d- and synthetic l-isomer forms and both have been shown to inhibit aminotransferase enzymes (Cornell et al. 1984). We modelled our dosing strategy after a study by Blackshear et al. (1975) who examined factors regulating the release of alanine from extrasplanchnic tissues in vivo following functional hepatectomy in rats. Following a single intravenous injection of 0.05 mg g−1 of cycloserine, blood alanine accumulation was reduced by 82% compared to control animals, suggesting there was a marked inhibition of alanine release from extrasplanchnic tissues due to inhibition of AAT (Blackshear et al. 1975). More specifically with respect to skeletal muscle, Ruderman & Berger (1974) showed that cycloserine added to a perfusion medium markedly attenuated alanine release from the isolated rat hindquarter. These authors cautioned, however, that the effect of cycloserine on alanine production could not be solely attributed to inhibition of AAT since cycloserine can inhibit several different aminotransferases (Cornell et al. 1984). Thus, while we show evidence of a marked inhibition of AAT in the present study, we cannot rule out an effect of cycloserine on other aminotransferases. Irrespective of the precise mechanism for the reduction in muscle TCAI in the cycloserine-treated animals, our data suggest that an acute reduction in the muscle TCAI pool during contraction does not adversely affect muscle oxidative energy metabolism or contractile function. The present data are consistent with a number of recent studies that have shown neither augmentation of TCAI expansion during contraction (Bruce et al. 2001; Gibala et al. 2002) nor inhibition of TCAI expansion (Dawson et al. 2003; Howarth et al. 2004) is causally linked to muscle energy metabolism.
Conclusion
In summary, the main finding of the present study was that cycloserine administration reduced the muscle TCAI pool in rodent skeletal muscle during contraction by 25% compared to saline-treated controls. However, the acute decrease in [TCAI] did not compromise aerobic energy provision during contraction, as judged by changes in the concentrations of muscle phosphocreatine, ATP and lactate, which were similar to the control condition. Moreover, resting absolute peak tension and the decline in force-generating capacity after 10 min of contraction were also similar between conditions. These data do not support the hypothesis that the magnitude of muscle TCAI pool expansion is causally linked to rate of mitochondrial respiration during contraction.
Acknowledgments
This project was supported by the University of Nottingham and an operating grant to M.J.G. from the Natural Sciences and Engineering Research Council of Canada.
References
- Aragon JJ, Lowenstein JM. The purine-nucleotide cycle. Comparison of the levels of citric acid cycle intermediates with the operation of the purine nucleotide cycle in rat skeletal muscle during exercise and recovery from exercise. Eur J Biochem. 1980;110:371–377. doi: 10.1111/j.1432-1033.1980.tb04877.x. 10.1111/j.1432-1033.1980.tb04877.x. [DOI] [PubMed] [Google Scholar]
- Blackshear PJ, Holloway PA, Alberti KG. Factors regulating amino acid release from extrasplanchnic tissues in the rat. Interactions of alanine and glutamine. Biochem J. 1975;150:379–387. doi: 10.1042/bj1500379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce M, Constantin-Teodosiu D, Greenhaff PL, Boobis LH, Williams C, Bowtell JL. Glutamine supplementation promotes anaplerosis but not oxidative energy delivery in human skeletal muscle. Am J Physiol Endocrinol Metab. 2001;280:E669–E675. doi: 10.1152/ajpendo.2001.280.4.E669. [DOI] [PubMed] [Google Scholar]
- Carter S, Rennie CD, Hamilton SJ, Tarnopolsky M. Changes in skeletal muscle in males and females following endurance training. Can J Physiol Pharmacol. 2001;79:386–392. [PubMed] [Google Scholar]
- Constantin-Teodosiu D, Simpson EJ, Greenhaff PL. The importance of pyruvate availability to PDC activation and anaplerosis in human skeletal muscle. Am J Physiol. 1999;276:E472–E478. doi: 10.1152/ajpendo.1999.276.3.E472. [DOI] [PubMed] [Google Scholar]
- Cornell NW, Zuurendonk PF, Kerich MJ, Straight CB. Selective inhibition of alanine aminotransferase and aspartate aminotransferase in rat hepatocytes. Biochemistry. 1984;220:707–716. doi: 10.1042/bj2200707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson KD, Howarth KR, Tarnopolsky MA, Wong ND, Gibala MJ. Short-term training attenuates muscle TCA cycle expansion during exercise in women. J Appl Physiol. 2003;95:999–1004. doi: 10.1152/japplphysiol.01118.2002. [DOI] [PubMed] [Google Scholar]
- Edmondson JW, Lumeng L, Li TK. Direct measurement of active transport systems for alanine in freshly isolated rat liver cells. Biochem Biophys Res Commun. 1977;76:751–757. doi: 10.1016/0006-291x(77)91564-9. 10.1016/0006-291X(77)91564-9. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, MacLean DA, Graham TE, Saltin B. Anaplerotic processes in human skeletal muscle during brief dynamic exercise. J Physiol. 1997a;502:703–713. doi: 10.1111/j.1469-7793.1997.703bj.x. 10.1111/j.1469-7793.1997.703bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, MacLean DA, Graham TE, Saltin B. Tricarboxylic acid cycle intermediate pool size and estimated cycle flux in human muscle during exercise. Am J Physiol. 1998;275:E235–E242. doi: 10.1152/ajpendo.1998.275.2.E235. [DOI] [PubMed] [Google Scholar]
- Gibala MJ, Peirce N, Constantin-Teodosiu D, Greenhaff PL. Exercise with low muscle glycogen augments TCA cycle anaplerosis but impairs oxidative energy provision in humans. J Physiol. 2002;540:1079–1086. doi: 10.1113/jphysiol.2001.012983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, Tarnopolsky MA, Graham TE. Tricarboxylic acid cycle intermediates in human muscle at rest and during prolonged cycling. Am J Physiol Endocrinol Metab. 1997b;272:E239–E244. doi: 10.1152/ajpendo.1997.272.2.E239. [DOI] [PubMed] [Google Scholar]
- Harris RC, Hultman E, Nordesjo LO. Glycogen, glycolytic intermediates and high-energy phosphates determined in biopsy samples of musculus quadriceps femoris of man at rest. Scand J Clin Laboratory Invest. 1974;33:109–120. [PubMed] [Google Scholar]
- Henriksson J, Chi MM, Hintz CS, Young DA, Kaiser KK, Salmons S, Lowry OH. Chronic stimulation of mammalian muscle: changes in enzymes of six metabolic pathways. Am J Physiol Cell Physiol. 1986;251:C614–C632. doi: 10.1152/ajpcell.1986.251.4.C614. [DOI] [PubMed] [Google Scholar]
- Hintz CS, Chi MM-Y, Fell RD, Ivy JL, Kaiser KK, Lowry CV, Lowry OH. Metabolite changes in individual rat muscle fibers during stimulation. Am J Physiol Cell Physiol. 1982;242:C218–C228. doi: 10.1152/ajpcell.1982.242.3.C218. [DOI] [PubMed] [Google Scholar]
- Howarth KR, LeBlanc PJ, Heigenhauser GJF, Gibala MJ. The effect of endurance training on muscle TCA cycle metabolism during exercise in humans. J Appl Physiol. 2004;97:579–584. doi: 10.1152/japplphysiol.01344.2003. 10.1152/japplphysiol.01344.2003. [DOI] [PubMed] [Google Scholar]
- Ivy JL, Res PT, Sprague RC, Widzer MO. Effect of a carbohydrate-protein supplement on endurance performance during exercise of varying intensity. Int J Sport Nutr Exerc Metab. 2003;13:382–395. doi: 10.1123/ijsnem.13.3.382. [DOI] [PubMed] [Google Scholar]
- Passoneau JV, Lowry OH. Enzymatic Analysis: A Practical Guide. Totowa NJ USA: Humana Press; 1993. [Google Scholar]
- Ruderman NB, Berger M. The formation of glutamine and alanine in skeletal muscle. J Biol Chem. 1974;249:5500–5506. [PubMed] [Google Scholar]
- Russell RR, Taegtmeyer H. Pyruvate carboxylation prevents the decline in contractile function of rat hearts oxidizing acetoacetate. Am J Physiol Heart Circ Physiol. 1991;261:H1756–H1762. doi: 10.1152/ajpheart.1991.261.6.H1756. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Jorfeldt L, Henriksson K-G, Lewis SR, Haller RG. Tricarboxylic acid cycle intermediates during incremental exercise in healthy subjects and in patients with McArdle's disease. Clin Sci. 1995;88:687–693. doi: 10.1042/cs0880687. [DOI] [PubMed] [Google Scholar]
- Sahlin K, Katz A, Broberg S. Tricarboxylic acid cycle intermediates in human muscle during prolonged exercise. Am J Physiol Endocrinol Metab. 1990;259:C834–C841. doi: 10.1152/ajpcell.1990.259.5.C834. [DOI] [PubMed] [Google Scholar]
- Taegtmeyer H, Hems R, Krebs HA. Utilization of energy providing substrates in the isolated working rat heart. Biochem J. 1980;186:701–711. doi: 10.1042/bj1860701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vissing J, Haller RG. The effect of oral sucrose on exercise tolerance in patients with McArdle's disease. N Engl J Med. 2003;349:2503–2509. doi: 10.1056/NEJMoa031836. 10.1056/NEJMoa031836. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJM. Protein and amino acid metabolism in human muscle. Adv Exp Med Biol. 1998;441:307–319. doi: 10.1007/978-1-4899-1928-1_28. [DOI] [PubMed] [Google Scholar]
- Wagenmakers AJM, Coakley JH, Edwards RHT. Metabolism of branched-chain amino acids and ammonia during exercise: clues from McArdle's disease. Int J Sports Med. 1990;11:S101–S113. doi: 10.1055/s-2007-1024861. [DOI] [PubMed] [Google Scholar]
- Williamson JR, Cooper RH. Regulation of the citric acid cycle in mammalian systems. FEBS Lett. 1980;117:K73–K85. doi: 10.1016/0014-5793(80)80572-2. 10.1016/0014-5793(80)80572-2. [DOI] [PubMed] [Google Scholar]
- Wu G, Thompson JR, Sedgwick GW, Drury M. Formation of alanine and glutamine in chick (Gallus domesticus) skeletal muscle. Comp Biochem Physiol B. 1989;93:609–613. doi: 10.1016/0305-0491(89)90384-2. 10.1016/0305-0491(89)90384-2. [DOI] [PubMed] [Google Scholar]