Abstract
Insulin-mediated glucose clearance (GC) is diminished in type 2 diabetes. Skeletal muscle has been estimated to account for essentially all of the impairment. Such estimations were based on leg muscle and extrapolated to whole body muscle mass. However, skeletal muscle is not a uniform tissue and insulin resistance may not be evenly distributed. We measured basal and insulin-mediated (1 pmol min−1 kg−1) GC simultaneously in the arm and leg in type 2 diabetes patients (TYPE 2) and controls (CON) (n = 6 for both). During the clamp arterio-venous glucose extraction was higher in CON versus TYPE 2 in the arm (6.9 ± 1.0 versus 4.7 ± 0.8%; mean ± s.e.m.; P = 0.029), but not in the leg (4.2 ± 0.8 versus 3.1 ± 0.6%). Blood flow was not different between CON and TYPE 2 but was higher (P < 0.05) in arm versus leg (CON: 74 ± 8 versus 56 ± 5; TYPE 2: 87 ± 9 versus 43 ± 6 ml min−1 kg−1 muscle, respectively). At basal, CON had 84% higher arm GC (P = 0.012) and 87% higher leg GC (P = 0.016) compared with TYPE 2. During clamp, the difference between CON and TYPE 2 in arm GC was diminished to 54% but maintained at 80% in the leg. In conclusion, this study shows that glucose clearance is higher in arm than leg muscles, regardless of insulin resistance, which may indicate better preserved insulin sensitivity in arm than leg muscle in type 2 diabetes.
Skeletal muscle, which makes up ∼40% of the body mass, is the major tissue involved in glucose metabolism and an important site of insulin resistance in obesity and type 2 diabetes (DeFronzo et al. 1992).
Glucose transport and uptake are diminished in skeletal muscle in type 2 diabetes (Dela et al. 1995; Zierath et al. 1996). The reduced muscle glucose uptake has been estimated to account for ∼55% (Basu et al. 2000) or even up to ∼100% (DeFronzo et al. 1992) of the decrease in whole body glucose disappearance during a clamp compared to healthy individuals. These numbers are, however, calculated from leg muscle glucose uptake and extrapolated to the whole muscle mass, based on the assumption that glucose uptake is similar in upper and lower body muscles.
The possibility exists that there is a difference in glucose uptake between muscles, as for example glucose uptake capacity is larger in red oxidative than in white glycolytic muscle fibres (Lillioja et al. 1987; Goodyear et al. 1991). Although differences in fibre type composition may be larger in other species than man, they could contribute to a difference in insulin sensitivity between arm and leg muscles (Johnson et al. 1973; Schantz et al. 1983). Moreover, the preferential substrate oxidation differs between arm and leg muscles, and in the fasted state during normoinsulinaemia glucose uptake rates are higher in arm compared with leg muscles (Möller-Loswick et al. 1991; Ahlborg & Jensen-Urstad, 1991). Whether upper extremity muscles also maintain their insulin sensitivity better with age than the leg muscles remains to be studied. Recent reports have shown that the same tissue at different areas are not metabolically equal (Enevoldsen et al. 2001; Hagstrom-Toft et al. 2002).
We investigated arm and leg glucose uptake in a basal condition and during a physiological isoglycaemic insulin clamp in patients with type 2 diabetes (TYPE 2) and age-matched controls (CON). The hypothesis was that the insulin resistance in TYPE 2 is primarily located in the leg muscles.
Methods
Subjects
The study included six TYPE 2 and six CON (Table 1). All subjects were male. None of the subjects were engaged in any regular physical activities and none had hypertension. The patients were treated with diet (n = 2), insulin (n = 1), metformin (n = 2) and with metformin and sulphonylurea (n = 1). Two patients received a cholesterol-lowering drug (simvastatin) and one low-dose acetylsalicylic acid. In CON all had a normal medical record and oral glucose tolerance test (OGTT); none received medication or had a family history of type 2 diabetes. The study was approved by the local ethical committee and was conducted in accordance with the Declaration of Helsinki. All subjects gave their informed written consent.
Table 1.
Anthropometric measures
| Age (years) | Body weight(kg) | BMI (kg m−2) | Total body fat (%) | Arm muscle mass (kg) | Thigh muscle mass (kg) | |
|---|---|---|---|---|---|---|
| CON (n = 6) | 50 ± 4 | 93 ± 8 | 29 ± 2 | 29 ± 2 | 3.3 ± 0.2 | 8.1 ± 0.7 |
| TYPE 2 (n = 6) | 58 ± 2 | 105 ± 6 | 33 ± 2 | 29 ± 3 | 3.9 ± 0.2 | 9.0 ± 0.3 |
CON: six healthy control subjects. TYPE 2: six patients with type 2 diabetes. BMI: body mass index. Total body fat and muscle mass is estimated from a DEXA scan. There was no difference in percentage fat content in the arms or legs between the groups or between the two extremities (data not shown).
Pre-experimental procedures
All subjects reported to the laboratory at 08.00 h after an overnight fast (12 h). All medication was discontinued 24 h before the study, and all subjects were instructed to refrain from any strenuous physical activity or special diets for the preceding 72 h. Under local anaesthesia a 20G Teflon catheter was introduced into the left femoral vein in the retrograde direction and into the left femoral artery using the Seldinger technique. To sample venous blood representative of the whole upper extremity, a 14G subclavian catheter was introduced into a cubital vein in the left arm and advanced in the antegrade direction to the subclavian vein. A thoracic X-ray was performed to ensure correct position with the tip of the catheter in the mid portion of the subclavian vein. For glucose and insulin infusion a 16G catheter was inserted in a cubital vein in the right arm. During the trial all catheters were kept patent by flushing with 0.9% saline. Subjects rested in a supine position during the experiment.
Experimental design
Both TYPE 2 and CON were investigated at their habitual fasting plasma glucose concentrations. At ∼09.30 h a basal period of 5 h was started. Arterial blood and leg and arm venous blood for substrate analysis were obtained at baseline and then every 60 min. At every sample point blood flow was measured in the subclavian and femoral artery using Doppler ultrasound. After the basal period a low dose insulin infusion (1 pmol (0.167 mU) min−1 kg−1) was commenced. Glucose was clamped at the value measured at the end of the 5 h basal period (Fig. 1). During the clamp an arterial blood sample was taken every 10 min and analysed for glucose and potassium concentrations (ABL 715, Radiometer, Denmark). Potassium was infused to maintain baseline values. Arterial and leg and arm venous blood were sampled every 60 min throughout the clamp period with subsequent measurement of blood flow (in triplicate). During blood sampling cuffs placed below the knee and at the wrist were inflated to above systolic blood pressure.
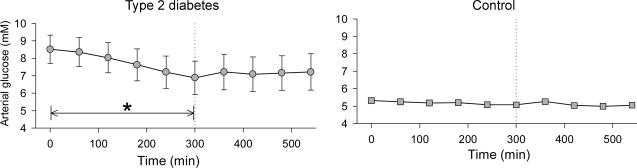
Figure 1. Arterial plasma glucose concentrations during 5 h basal period followed by a 4 h isoglycaemic, hyperinsulinaemic clamp in six patients with type 2 diabetes and six healthy control subjects.
*Decrease with time, P < 0.05 (one-way ANOVA for repeated measures). Values are means ± s.e.m.
Analytical procedures
Blood for insulin and C-peptide analysis (Elisa technique, DAKO, Glostrup, Denmark) was collected in prechilled tubes containing 0.3 m EDTA (10 µl ml−1 blood) and immediately centrifuged for 10 min at 4000 r.p.m. and 4°C. The plasma was frozen and stored at −80°C until analysis.
Expression of GLUT4 protein was measured by Western blot in a muscle biopsy obtained with the percutaneous needle biopsy technique with suction from both arm (deltoid) and leg (vastus lateralis) muscle during the fasting condition. The muscle biopsies were quickly cleaned from visible blood and/or fat, frozen in liquid nitrogen and stored at −80°C. The muscle tissue was subsequently homogenized with a Polytron PT 3100 (Kinematica AG, Littau-Luzern, Switzerland) at maximum speed for ∼10 s in 10 vol of ∼55°C buffer (4% SDS, 10 mm pyrophosphate, 2 mm sodium orthovanadate, 10 mm EDTA, 25 mm Tris-HCl, pH 6.8). Samples were sonicated for ∼5 s to break DNA strands and total protein concentrations were determined by the bicinchoninic acid (BCA) method using BSA as standard. For Western blot 10 µg of protein was separated by SDS-PAGE on 10% gels and electrophoretically transferred to PVDF membranes for 30 min at 20 V using a semidry system (Bio-Rad, Hercules, CA, USA). Transfer buffer contained 48 mm Tris, 39 mm glycine, 0.019% SDS and 5% methanol. Membranes were blocked in 2.5% defatted milk powder plus 5% BSA in TS buffer (10 mm Tris (pH 7.4), 150 mm NaCl), incubated for 90 and 60 min with primary and horseradish peroxidase (HRP)-labelled secondary antibodies diluted in blocking solution. Antigen–antibody complexes were visualized and quantified by a LAS 3000 imaging system (Fuji Film). Monoclonal antibody F-27 was used for detection of GLUT4.
Calculations
Data on glucose balance across the arm and leg are given as clearance rates per kilogram muscle mass (ml min−1 kg−1) and calculated as:
where [Glucose]venous is the leg or arm venous plasma glucose concentration and Hct is the haematocrit. Muscle mass is the lean thigh or arm muscle mass obtained from the DEXA scans (Table 1).
Insulin-mediated whole body glucose uptake was calculated as the steady state glucose infusion rate (GIR) averaged over a 10-min period.
When comparing the basal period with that of the insulin-stimulated period the last 4 h of the basal period was used for comparison.
Statistics
Results are presented as means ± s.e.m. Differences in glucose clearance rates and GIR were analysed with a two-way ANOVA for repeated measures. In the case of a statistically significant interaction between the variables, a pairwise multiple comparison procedure (Student-Newman-Keuls method) was used. Differences in parameters that were represented by single measurements were analysed with Student's t test. P < 0.05 in two-tailed testing was considered significant.
Results
Whole body
During the 5 h basal period, arterial plasma glucose decreased from 8.5 to 6.9 mm (P < 0.05) in TYPE 2, but remained stable in CON (Fig. 1). During insulin infusion arterial glucose concentrations were 7.3 and 5.1 mm in TYPE 2 and CON, respectively, with no significant change over time (Fig. 1).
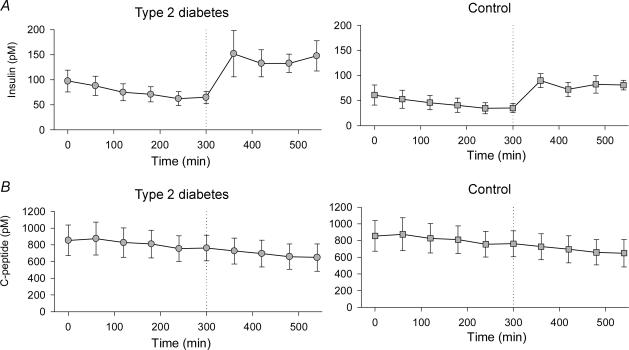
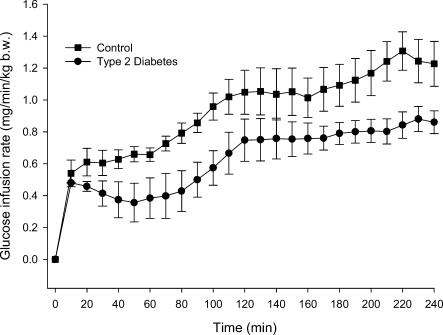
During the basal period plasma insulin concentrations were higher in TYPE 2 (P < 0.002) but decreased in both groups over the 5 h period (P < 0.001) (Fig. 2). During the clamp plasma insulin concentrations tended (P = 0.05) to be higher in TYPE 2 compared with CON (Fig. 2). Mean plasma insulin increased from 39 ± 6 pmol l−1 during the fasting period to 82 ± 7 pmol l−1 during the insulin clamp (P < 0.0001) in CON and from 68 ± 7 pmol l−1 to 141 ± 15 pmol l−1 (P < 0.0001) in TYPE 2 (Fig. 2A). Glucose infusion rates were lower in TYPE 2 compared with CON (P < 0.05) (Fig. 3).
Figure 2. Arterial plasma insulin (A) and C-peptide (B) concentrations during 5 h basal period followed by a 4 h isoglycaemic, hyperinsulinaemic clamp in six patients with type 2 diabetes and six healthy control subjects.
Insulin and C-peptide concentrations always decreased with time during the basal period (P < 0.05), and C-peptide further decreased during the clamp. During the clamp, insulin (but not C-peptide) concentrations tended (P = 0.054) to be higher in patients with type 2 diabetes. Values are means ± s.e.m.
Figure 3. Glucose infusion rates (GIR) during 4 h isoglycaemic, hyperinsulinaemic clamp in six patients with type 2 diabetes and six healthy control subjects.
GIR was significantly lower in the patients with type 2 diabetes (P < 0.05). Values are means ± s.e.m.
Arms and legs
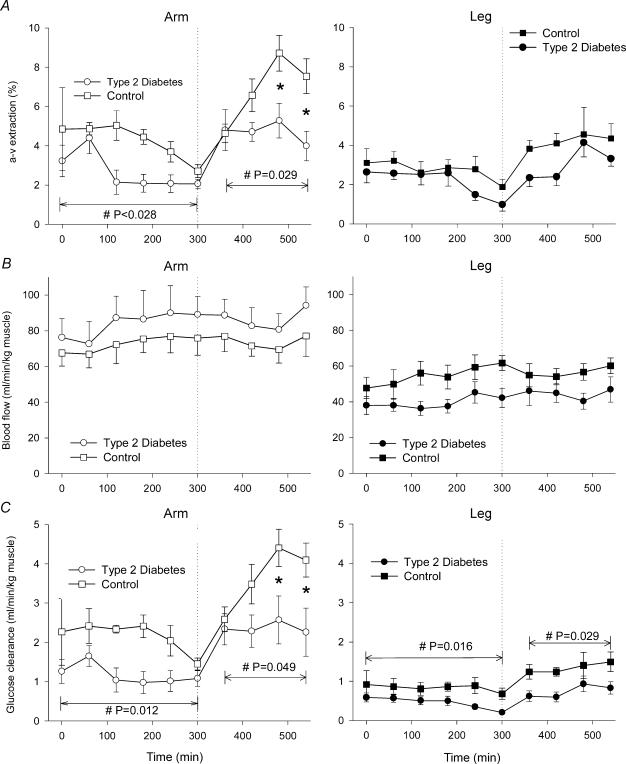
The arterio-venous glucose extraction was higher (P < 0.05) in CON compared with TYPE 2 in the arm, but not in the legs (Fig. 4A).
Figure 4. Arterio-venous (a-v) glucose extraction (A), blood flow (B) and glucose clearance rates (C) in an arm (left panel) and a leg (right panel) is six patients with type 2 diabetes and in six healthy, control subjects.
Values are means ± s.e.m.#Significant difference (main effect) between the two study groups. Interaction between the variables was seen in arm a-v extraction (A) (P = 0.017) and glucose clearance rates (C) (P = 0.038) during insulin infusion and an asterisk (*) indicates the location of the significant difference between the groups (Student-Newman-Keuls method).
Blood flow did not differ between TYPE 2 and CON in the arms or in the legs, and no effect of insulin infusion could be detected (Fig. 4B). Arm blood flow was always higher (P < 0.05) than leg blood flow. Arm and leg blood flow values measured in the subclavian and femoral artery, respectively, were comparable to values obtained by others using either thermodilution or Doppler ultrasound techniques (Volianitis & Secher, 2002; Huonker et al. 2003).
During the basal period CON had on average 84% higher glucose clearance rate in the arm (P = 0.01) and 87% higher glucose clearance rate in the leg (P < 0.02) compared with TYPE 2 (Fig. 4C). During insulin infusion, glucose clearance rates were 54% (P < 0.05) and 80% (P < 0.03) higher in the arm and leg, respectively, compared with TYPE 2 (Fig. 4C).
Within each group glucose clearance rates in the arm were higher than in the leg in the basal period (CON: +157% (P = 0.0001); TYPE 2: +158% (P = 0.0005)) and during insulin infusion (CON: +171% (P = 0.0002); TYPE 2: +217% (P = 0.007) (Fig. 4C).
In the arm the average glucose clearance during insulin infusion was higher compared with the basal period in both CON (+69%, P < 0.0003) and TYPE 2 (+102%, P < 0.05). In the leg average glucose clearance during insulin infusion was higher compared with the basal period in CON (+ 60%, P < 0.04) but not in TYPE 2 (NS) (Fig. 4C).
There was no difference in arm or leg GLUT4 protein content between CON and TYPE 2 or between arm and leg in both groups (data not shown).
Discussion
The main findings of the present study are (1) higher muscle glucose clearance in the arm than in the leg, regardless of insulin resistance; (2) relatively better preserved insulin sensitivity in arm muscle in TYPE 2. Thus, this study demonstrates that skeletal muscle is not a uniform tissue and that previous extrapolations of leg muscle insulin sensitivity to the whole body is not always appropriate particularly under conditions of low insulin concentrations.
The present findings have several implications. Most intriguingly is the demonstration of a non-homogeneous distribution of insulin resistance in arm and leg muscles of TYPE 2. Impaired insulin-stimulated muscle glucose uptake (MGU) is a hallmark of type 2 diabetes (DeFronzo et al. 1992), and reduced MGU has been consistently shown in studies using arterio-venous differences across the leg (e.g. Dela et al. 1995). However, in studies using the forearm arterio-venous balance technique, reduced glucose uptake has been less consistent (Avogaro et al. 1996, 1997; Blaak & Wagenmakers, 2002). This is in line with our findings. It is possible that the difficulties in demonstrating a reduced MGU in the forearm model are due to relatively preserved insulin sensitivity in arm muscles in TYPE 2. This should be considered when using the forearm arterio-venous balance technique.
Another implication of the present finding is the muscle fractional glucose uptake. Basu et al. (2000) have estimated that reduced muscle glucose uptake may account for ∼55% of the reduced whole body glucose disposal, while DeFronzo et al. (1992) estimated that muscle accounts for essentially all of the impairment in insulin-mediated glucose uptake. These estimations were calculated from leg muscle and extrapolated to whole body muscle mass. As insulin resistance may not be evenly distributed, it should be taken into account that at least the arm and shoulder muscles of the upper body have a higher glucose uptake in both healthy subjects and in type 2 diabetes.
The present findings raise the question of why arm and leg muscles are different in glucose clearance during resting conditions and why insulin resistance is less pronounced in the arm muscles in type 2 diabetes. In healthy individuals GLUT4 density has been shown to be higher in slow compared to fast muscle fibres (Goodyear et al. 1991; Gaster et al. 2000; Daugaard & Richter, 2004). In contrast GLUT4 density in type 2 diabetes has been shown to be higher in fast compared with slow fibres (Gaster et al. 2001), and it has been proposed that type 2 diabetes is primarily a disease of the slow type 1 muscle fibres. Given a higher proportion of fast type 2 muscle fibres in the upper body, this could have explained the preserved glucose uptake and insulin sensitivity in the arm muscle in type 2 diabetes. We did not measure the fibre type composition, however; as it has been shown previously that only the triceps brachii muscle is dominated by type 2 fibres (Johnson et al. 1973; Schantz et al. 1983), this is hardly the explanation. In line with this, GLUT4 measurements in our study did not reveal any differences between arm (deltoideus) and leg (vastus lateralis) muscles. Recently it has been shown in animal studies that partial knock-out of GLUT4 preserves insulin sensitivity and MGU when hexokinase levels are normal, and only when the glucose phosphorylation barrier is lowered by hexokinase overexpression does GLUT4 availability becomes a limit to insulin stimulated MGU (Fueger et al. 2004).
Physical inactivity is one of the major predisposing factors in developing insulin resistance and an elevated level of physical activity can prevent type 2 diabetes in individuals at risk (Tuomilehto et al. 2001; Knowler et al. 2002; Pan et al. 1997). It may be hypothesized that through evolution, the upper body/arm muscles, have adapted to be less dependent on muscle usage. The issue of disuse of the muscles and the ‘thrifty’ gene concept have recently been discussed (Chakravarthy & Booth, 2004). This concept highlights that leg skeletal muscle needs not only a high muscle glycogen content, but also a high capacity to rapidly replenish these stores to be prepared for fast, but short bursts of running for survival. In addition, the leg muscles have to develop various glycogen sparing mechanisms during walking over long distances. It is true that arm muscles also have to perform explosive contractions developing high power, but this effort is brief, primarily using the energy rich phosphagen stores. The possibility then exists that legs, in contrast to arm muscles, depend upon regular endurance type exercise to maintain their insulin sensitivity whereas arm muscles have adapted to inactivity as a consequence of the upright posture with only the legs being used for movement. If this were true, muscle insulin resistance resulting in type 2 diabetes would be primarily a disease of the lower body muscles and possibly also the muscles of the torso.
A possible explanation for the difference in arm and leg insulin sensitivity found here could result from differences in vascular responsiveness between upper and lower limbs. Recently Newcomer et al. (2004) have demonstrated, that arm vasodilator response compared to the legs is higher to both pharmacological and physiological vasodilator stimuli, caused either by a decreased NO production or decreased NO responsiveness of the leg. An important action of insulin is vasodilatation through the generation of NO (Scherrer et al. 1994), contributing to its overall effect on glucose and hormone delivery to muscle (Clark et al. 2003). A reduced vasodilator response to insulin in the leg with a diminished recruitment of nutritive capillaries as compared to the arm would therefore be likely to result in a reduced glucose clearance in the lower limb. At low physiological doses as used in our study, insulin may stimulate capillary recruitment, without a concomitant increase in limb blood flow (Zhang et al. 2004). This probably explains why we did not see an increase in either leg or arm total blood flow during the insulin clamp.
That differences between upper and lower limbs exist is seen in the development of atherosclerosis. Although not unseen in the arms (Royster & Older, 1966; Sorensen et al. 1997), artherosclerotic plaques develops primarily and more severely in the legs (Bucciarelli et al. 2002). This is also consistent with the common clinical experience of ischaemic symptoms.
The subjects were studied at their habitual glucose and insulin concentrations (isoglycaemic clamp). Also the small difference in glucose and insulin concentration might represent a limitation to our study; this does not affect our main finding that glucose clearance rates are higher in the arms compared to legs in both healthy subjects and in patients with type 2 diabetes, as arms and legs were exposed to the same level of glucose and insulin in the same individual. At the low insulin infusion used, glucose clearance rates in the leg did not show a statistical significant increase while this was the case in the arm, indicating a better sensitivity to insulin in this extremity in type 2 diabetes. This difference between the arm and leg muscles may not have been detected if we had used a high grade insulin infusion rate, eliciting plasma insulin concentrations outside the physiological range. The low insulin concentration obtained unmasked a difference in insulin sensitivity between the upper and lower extremity. We cannot rule out, that a higher insulin infusion rate, resulting in near maximal glucose metabolism, would have yielded a different result. Webber et al. (1999) have shown that with regard to glucose and fatty acid metabolism in patients with type 2 diabetes, glucose metabolism parallels glycerol and non-esterified fatty acid metabolism at low physiological insulin concentrations, but not at concentrations obtained during a supraphysiological insulin clamp, indicating that different aspects of insulin action are being measured by the two methodologies. In accordance with this we have recently disclosed by the use of a low insulin infusion rate that in type 2 diabetes arm and leg differ in fatty acid (FA) metabolism with the leg but not the arm showing an impairment of FA metabolism both during basal and hyperinsulinaemic conditions (Sacchetti et al. 2005).
A limitation to the present study relates to the fact that subjects were investigated in the supine position during the whole experiment. Apart from when in bed humans spend much of the time walking or sitting. Lying down for a long period is not a true physiological situation, and therefore our results may only apply to the supine, resting position.
In conclusion this study has shown that glucose clearance and insulin sensitivity are relatively preserved in arm muscles in type 2 diabetes, and that in both CON and TYPE 2 arm muscle glucose clearance is higher compared to leg glucose clearance. Whether this relates to fibre type composition, inherent metabolic differences developed through evolution or is the result of different vasodilator response to insulin is not known. Future studies examining GLUT4 translocation and the phosphorylating steps may give an insight not only into why this regional difference in insulin sensitivity exists between skeletal muscles in the human body, but also into why insulin resistance develops.
Acknowledgments
Gerda Hau is thanked for skilfull technical assistence. The study was supported by The Danish National Research Foundation (504-14), Novo Nordisk A/S, The Foundation of 1870, Jacob and Olga Madsens Foundation, and Else and Mogens Wedell-Wedellsborg Foundation.
References
- Ahlborg G, Jensen-Urstad M. Metabolism in exercising arm vs. leg muscle. Clin Physiol. 1991;11:459–468. doi: 10.1111/j.1475-097x.1991.tb00818.x. [DOI] [PubMed] [Google Scholar]
- Avogaro A, Piarulli F, Valerio A, Miola M, Calveri M, Pavan P, Vicini P, Cobelli C, Tiengo A, Calo L, Del Prato S. Forearm nitric oxide balance, vascular relaxation, and glucose metabolism in NIDDM patients. Diabetes. 1997;46:1040–1046. doi: 10.2337/diab.46.6.1040. [DOI] [PubMed] [Google Scholar]
- Avogaro A, Toffolo G, Miola M, Valerio A, Tiengo A, Cobelli C, Del Prato S. Intracellular lactate- and pyruvate interconversion rates are increased in muscle tissue of non-insulin-dependent diabetic individuals. J Clin Invest. 1996;98:108–115. doi: 10.1172/JCI118754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Basu R, Shah P, Vella A, Johnson CM, Nair KS, Jensen MD, Schwenk WF, Rizza RA. Effects of type 2 diabetes on the ability of insulin and glucose to regulate splanchnic and muscle glucose metabolism: evidence for a defect in hepatic glucokinase activity. Diabetes. 2000;49:272–283. doi: 10.2337/diabetes.49.2.272. [DOI] [PubMed] [Google Scholar]
- Blaak EE, Wagenmakers AJ. The fate of [U-13C]palmitate extracted by skeletal muscle in subjects with type 2 diabetes and control subjects. Diabetes. 2002;51:784–789. doi: 10.2337/diabetes.51.3.784. [DOI] [PubMed] [Google Scholar]
- Bucciarelli P, Sramek A, Reiber JH, Rosendaal FR. Arterial intima-media thickness and its relationship with cardiovascular disease and atherosclerosis: a possible contribution of medium-sized arteries. Thromb Haemost. 2002;88:961–966. [PubMed] [Google Scholar]
- Chakravarthy MV, Booth FW. Eating, exercise, and ‘thrifty’ genotypes: connecting the dots toward an evolutionary understanding of modern chronic diseases. J Appl Physiol. 2004;96:3–10. doi: 10.1152/japplphysiol.00757.2003. [DOI] [PubMed] [Google Scholar]
- Clark MG, Wallis MG, Barrett EJ, Vincent MA, Richards SM, Clerk LH, Rattigan S. Blood flow and muscle metabolism: a focus on insulin action. Am J Physiol Endocrinol Metab. 2003;284:E241–E258. doi: 10.1152/ajpendo.00408.2002. [DOI] [PubMed] [Google Scholar]
- Daugaard JR, Richter EA. Muscle- and fibre type-specific expression of glucose transporter 4, glycogen synthase and glycogen phosphorylase proteins in human skeletal muscle. Pflugers Arch. 2004;447:452–456. doi: 10.1007/s00424-003-1195-8. 10.1007/s00424-003-1195-8. [DOI] [PubMed] [Google Scholar]
- DeFronzo RA, Bonadonna RC, Ferrannini E. Pathogenesis of NIDDM – A balanced overview. Diabetes Care. 1992;15:318–368. doi: 10.2337/diacare.15.3.318. [DOI] [PubMed] [Google Scholar]
- Dela F, Mikines KJ, Larsen JJ, Ploug T, Petersen LN, Galbo H. Insulin stimulated muscle glucose clearance in patients with type 2 diabetes mellitus. Effects of one-legged physical training. Diabetes. 1995;44:1010–1020. doi: 10.2337/diab.44.9.1010. [DOI] [PubMed] [Google Scholar]
- Enevoldsen LH, Simonsen L, Stallknecht B, Galbo H, Bulow J. In vivo human lipolytic activity in preperitoneal and subdivisions of subcutaneous abdominal adipose tissue. Am J Physiol Endocrinol Metab. 2001;281:E1110–E1114. doi: 10.1152/ajpendo.2001.281.5.E1110. [DOI] [PubMed] [Google Scholar]
- Fueger PT, Hess HS, Bracy DP, Pencek RR, Posey KA, Charron MJ, Wasserman DH. Regulation of insulin-stimulated muscle glucose uptake in the conscious mouse: role of glucose transport is dependent on glucose phosphorylation capacity. Endocrinology. 2004;145:4912–4916. doi: 10.1210/en.2004-0465. 10.1210/en.2004-0465. [DOI] [PubMed] [Google Scholar]
- Gaster M, Poulsen P, Handberg A, Schroder HD, Beck-Nielsen H. Direct evidence of fiber type-dependent GLUT-4 expression in human skeletal muscle. Am J Physiol. 2000;278:E910–E916. doi: 10.1152/ajpendo.2000.278.5.E910. [DOI] [PubMed] [Google Scholar]
- Gaster M, Staehr P, Beck-Nielsen H, Schrøder DD, Handberg A. GLUT4 is reduced in slow muscle fibers of type 2 diabetic patients: is insulin resistance in type 2 diabetes a slow, type 1 fiber disease? Diabetes. 2001;50:1324–1329. doi: 10.2337/diabetes.50.6.1324. [DOI] [PubMed] [Google Scholar]
- Goodyear LJ, Hirshman MF, Smith RJ, Horton ES. Glucose transporter number, activity, and isoform content in plasma membranes of red and white skeletal muscle. Am J Physiol. 1991;261:E556–E561. doi: 10.1152/ajpendo.1991.261.5.E556. [DOI] [PubMed] [Google Scholar]
- Hagstrom-Toft E, Qvisth V, Nennesmo I, Ryden M, Bolinder H, Enoksson S, Bolinder J, Arner P. Marked heterogeneity of human skeletal muscle lipolysis at rest. Diabetes. 2002;51:3376–3383. doi: 10.2337/diabetes.51.12.3376. [DOI] [PubMed] [Google Scholar]
- Huonker M, Schmid A, Schmidt-Trucksass A, Grathwohl D, Keul J. Size and blood flow of central and peripheral arteries in highly trained able-bodied and disabled athletes. J Appl Physiol. 2003;95:685–691. doi: 10.1152/japplphysiol.00710.2001. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, Appleton D. Data on the distribution of fibre types in thirty-six human muscles. An autopsy study. J Neurol Sci. 1973;18:111–129. doi: 10.1016/0022-510x(73)90023-3. 10.1016/0022-510X(73)90023-3. [DOI] [PubMed] [Google Scholar]
- Knowler WC, Barrett-Connor E, Fowler SE, Hamman RF, Lachin JM, Walker EA, Nathan DM. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med. 2002;346:393–403. doi: 10.1056/NEJMoa012512. 10.1056/NEJMoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WGH, Zawadzki JK, Yki-Järvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80:415–424. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller-Loswick AC, Bennegard K, Lundholm K. The forearm and leg perfusion techniques in man do not give the same metabolic information. Clin Physiol. 1991;11:385–395. doi: 10.1111/j.1475-097x.1991.tb00667.x. [DOI] [PubMed] [Google Scholar]
- Newcomer SC, Leuenberger UA, Hogeman CS, Handly BD, Proctor DN. Different vasodilator responses of human arms and legs. J Physiol. 2004;556:1001–1011. doi: 10.1113/jphysiol.2003.059717. 10.1113/jphysiol.2003.059717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan XR, Li GW, Hu YH, Wang JX, Yang WY, An ZX, Hu ZX, Lin J, Xiao JZ, Cao HB, Liu PA, Jiang XG, Jiang YY, Wang JP, Zheng H, Zhang H, Bennett PH, Howard BV. Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care. 1997;20:537–544. doi: 10.2337/diacare.20.4.537. [DOI] [PubMed] [Google Scholar]
- Royster TS, Older TM. Arteriosclerotic occlusive disease of the brachial artery. Case report. Am Surg. 1966;32:272–274. [PubMed] [Google Scholar]
- Sacchetti M, Olsen DB, Saltin B, Van Hall G. Heterogeneity in limb fatty acid kinetics in type 2 diabetes. Diabetologia. 2005 doi: 10.1007/s00125-005-1727-1. (in press) [DOI] [PubMed] [Google Scholar]
- Schantz P, Randall-Fox E, Hutchinson W, Tydén A, Åstrand P-O. Muscle fiber type distribution, muscle cross-sectional area and maximal voluntary strenght in humans. Acta Physiol Scand. 1983;117:219–226. doi: 10.1111/j.1748-1716.1983.tb07200.x. [DOI] [PubMed] [Google Scholar]
- Scherrer U, Randin D, Vollenweider P, Vollenweider L, Nicod P. Nitric oxide release accounts for insulin's vascular effects in humans. J Clin Invest. 1994;94:2511–2515. doi: 10.1172/JCI117621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen KE, Kristensen IB, Celermajer DS. Atherosclerosis in the human brachial artery. J Am Coll Cardiol. 1997;29:318–322. doi: 10.1016/s0735-1097(96)00474-3. 10.1016/S0735-1097(96)00474-3. [DOI] [PubMed] [Google Scholar]
- Tuomilehto J, Lindstrom J, Eriksson JG, Valle TT, Hamalainen H, Ilanne-Parikka P, Keinanen-Kiukaanniemi S, Laakso M, Louheranta A, Rastas M, Salminen V, Uusitupa M. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med. 2001;344:1343–1350. doi: 10.1056/NEJM200105033441801. 10.1056/NEJM200105033441801. [DOI] [PubMed] [Google Scholar]
- Volianitis S, Secher NH. Arm blood flow and metabolism during arm and combined arm and leg exercise in humans. J Physiol. 2002;544:977–984. doi: 10.1113/jphysiol.2002.023556. 10.1113/jphysiol.2002.023556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber J, Whitelaw D, Smith JM, Nattrass M. Glucose and fatty acid metabolism in type 2 diabetes mellitus: an assessment using low-dose insulin infusion and the hyperinsulinaemic euglycaemic clamp. Diabetes Obes Metab. 1999;1:173–178. doi: 10.1046/j.1463-1326.1999.00011.x. 10.1046/j.1463-1326.1999.00011.x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Vincent MA, Richards SM, Clerk LH, Rattigan S, Clark MG, Barrett EJ. Insulin sensitivity of muscle capillary recruitment in vivo. Diabetes. 2004;53:447–453. doi: 10.2337/diabetes.53.2.447. [DOI] [PubMed] [Google Scholar]
- Zierath JR, He L, Guma A, Ödegaard Wahlström E, Klip A, Wallberg-Henriksson H. Insulin action on glucose transport and plasma membrane GLUT4 content in skeletal muscle from patients with NIDDM. Diabetologia. 1996;39:1180–1189. doi: 10.1007/BF02658504. [DOI] [PubMed] [Google Scholar]