Abstract
The genioglossus (GG) muscle of the tongue, innervated by the hypoglossal motor nucleus (HMN), helps maintain an open airway for effective breathing. In vitro studies in neonatal rodents have separately characterized muscarinic and nicotinic receptor influences at the HMN but the net effects of combined nicotinic and muscarinic receptor activation and increased endogenous acetylcholine have not been determined in adult animals in vivo. Urethane-anaesthetized, tracheotomized and vagotomised rats were studied. Microdialysis perfusion of acetylcholine into the HMN significantly decreased respiratory-related GG activity (28.5 ± 11.0% at a threshold dose of 0.1 mm). Application of the cholinergic agonists carbachol and muscarine have similar suppression effects (GG activity was decreased 11.8 ± 4.3 and 20.5 ± 5.8%, respectively, at 0.01 μm). Eserine, an acetylcholinesterase inhibitor, also decreased the amplitude of respiratory-related GG activity (36.4 ± 11.3% at 1.0 μm) indicating that endogenous acetylcholine modulates GG activity. Although these results showed that suppression of GG activity predominates during cholinergic stimulation at the HMN, application of the nicotinic receptor agonist dimethyl-4-phenylpiperazinium iodide significantly increased tonic and respiratory-related GG activity (156 ± 33% for respiratory activity at 1.0 mm) showing that excitatory responses are also present. Consistent with this, 100 μm carbachol decreased GG activity by 44.2 ± 7.5% of control, with atropine (10 μm) reducing this suppression to 13.8 ± 4.0% (P < 0.001). However, the nicotinic receptor antagonist dihydro-β-erythroidine (100 μm) increased the carbachol-mediated suppression to 69.5 ± 5.9% (P = 0.011), consistent with a role for nicotinic receptors in limiting the overall suppression of GG activity during cholinergic stimulation. Application of eserine to increase endogenous acetylcholine also showed that inhibitory muscarinic and excitatory nicotinic receptors together determine the net level of GG activity during cholinergic stimulation at the HMN. The results suggest that acetylcholine has mixed effects at the HMN with muscarinic-mediated GG suppression masking nicotinic excitation.
Acetylcholine (ACh) acts at a variety of central nervous system sites to modulate respiratory activity. ACh modulates neurones involved in the generation of respiratory frequency and pattern (Shao & Feldman 2000, 2001) and influences chemoreceptor responses (Nattie & Li, 1990; Nattie, 1999). Cholinergic stimulation of the pontine reticular formation alters respiratory regulation (Kubin & Fenik, 2004), slows respiratory rate (Lydic & Baghdoyan, 1993, 2003) and suppresses ventilatory responses (Lydic et al. 1991) via connections of this cholinoceptive region to respiratory nuclei (Lee et al. 1995). Although motoneurones are the final common pathway determining motor outflow, characterization of cholinergic effects on motoneurones, especially respiratory motoneurones, is scarce (Rekling et al. 2000). No studies have been performed in adult animals in vivo to characterize cholinergic effects at the hypoglossal motor nucleus (HMN), the source of motor outflow to the genioglossus (GG) muscle of the tongue, and the interaction of such mechanisms with chemoreceptor stimulation. Understanding these mechanisms is important because the tension of GG, in conjunction with other pharyngeal muscles, helps maintain an open airspace for effective breathing (Remmers et al. 1978; Fuller et al. 1999) and prevent obstructive sleep apnoea in humans, a common and serious sleep-related breathing problem (Young et al. 1993).
In neonatal rodents in vitro, nicotinic agonists cause post-synaptic excitation of hypoglossal motoneurones (Zaninetti et al. 1999; Chamberlin et al. 2002; Robinson et al. 2002) and increase tonic and respiratory-related hypoglossal nerve activity (Shao & Feldman, 2001; Robinson et al. 2002). These excitatory responses are antagonized by dihydro-β-erythroidine which has selectivity for α4β2 subunits of nicotinic ACh receptors (nAChRs), so implicating these subunits in the excitatory responses (Zaninetti et al. 1999; Chamberlin et al. 2002). Although binding of [3H]epibatidine (a nicotinic agonist with high affinity for α4-subunits) is prominent at the HMN in neonatal rats, it is not detectable in adult rats (Zaninetti et al. 1999). It is important to characterize the effects of nAChR activation at the HMN on GG muscle activity in adult animals in vivo to determine if a similar robust excitation occurs.
The effects of muscarinic ACh receptor (mAChR) activation at the HMN have also only been studied in vitro. In neonatal hypoglossal motoneurones, mAChR activation causes depolarization and facilitation of spike firing (Bellingham & Funk, 2000; Lape & Nistri, 2000), whereas in juvenile animals it suppresses activity via pre-synaptic inhibition of excitatory inputs (Bellingham & Berger, 1996). The effects of mAChR activation at the HMN on motor output to GG have not been determined in adult animals in vivo, and it is important to determine if excitatory or inhibitory events predominate.
The aim of the present study is to characterize nAChR and mAChR effects at the HMN on GG activity in adult rats in vivo, and to determine their interactions with chemoreceptor stimulation. Based on previous in vitro studies, the present study tests the hypotheses that activation of nAChRs at the HMN increases GG activity and that activation of mAChRs produces overall suppression of GG activity. Furthermore, given the suggestion that expression of certain nAChR subunits involved in excitatory responses at the HMN may decline from neonatal to adult animals (Zaninetti et al. 1999), we also sought to determine if mAChR-mediated suppression of GG activity would dominate over nAChR-mediated excitation during application to the HMN of cholinergic agonists or eserine, which increases endogenous levels of ACh (Bellingham & Berger, 1996). Characterizing cholinergic influences at the HMN is of significance since cholinergic innervations of the HMN originate from mesopontine cholinergic neurones (Connaughton et al. 1986; Woolf & Butcher, 1989; Woolf, 1991; Dobbins & Feldman, 1995) whose activity is minimal in non-REM sleep and maximal in wakefulness and/or REM sleep (Vertes, 1979; el Mansari et al. 1989; Steriade et al. 1990a, b; Thakkar et al. 1998) and may therefore contribute to state-dependent modulation of GG activity.
Methods
Studies were performed on adult male Wistar rats (mean body weight, 295 g; range, 230–350 g, Charles River). The numbers used are listed for each individual study as appropriate. All procedures conformed to the recommendations of the Canadian Council on Animal Care and the University of Toronto Animal Care Committee approved the experimental protocols.
Surgical preparation
The rats were anaesthetized with intraperitoneal (i.p.) urethane (1 g kg−1) and given dexamethasone (0.2 mg i.p.) to reduce brain oedema. Following the onset of surgical anaesthesia, as judged by abolition of hind limb withdrawal and corneal blink reflexes, the rats were tracheotomized, the femoral artery and vein cannulated, and cervical vagotomy was performed bilaterally. The rats spontaneously breathed a 50 : 50 mixture of room air and oxygen, and additional anaesthesia (halothane, typically 0.2–1%) was administered as necessary by inhalation. Once halothane was initiated within an animal only minor adjustments were necessary across the experiment to maintain stable electroencephalogram (EEG) and respiratory muscle activities. Core body temperature was monitored with a rectal probe and maintained between 36 and 38°C with a water pump and heating pad (T/Pump-Heat Therapy System, Gaymar, NY, USA). The rats received continuous intravenous fluid (0.4 ml h−1) containing 7.6 ml saline, 2 ml 5% dextrose and 0.4 ml of 1 m NaHCO3.
For electromyogram (EMG) recordings, bipolar electrodes were inserted into GG (Morrison et al. 2002; Liu et al. 2003) and sutured onto the costal diaphragm via an abdominal approach. The rats were then placed in a stereotaxic apparatus (Kopf Model 962, Tujunga, CA, USA) with blunt ear bars. To ensure consistent positioning between rats, the flat skull position was achieved with an alignment tool (Kopf model 944). To record the cortical EEG two stainless steel screws attached to insulated wire were implanted in the skull over the frontal-parietal cortex (Morrison et al. 2002; Liu et al. 2003).
Microdialysis
Microdialysis probes (CMA/11 14/01, CSC, St Laurent, QC, CA, USA) were placed through a small hole drilled at the junction of the interparietal and occipital bones. The probes were implanted into the HMN at the following co-ordinates: 13.8 ± 0.1 mm (s.e.m.) posterior to bregma (range, 11.7–15.4 mm), 0.3 ± 0.01 mm lateral to the midline (range, 0.1–0.5 mm) and 9.7 ± 0.1 mm ventral to bregma (range, 8.8–11.2). In each rat a brief burst of GG activity was observed when the probe initially penetrated the HMN, and then the probe was advanced another 0.5 mm before being left at the final co-ordinates. This burst of GG activity during probe insertion was transient (< 5 min) and did not affect diaphragm activity, blood pressure or respiratory rate, and was useful as a preliminary indication of probe placement (Jelev et al. 2001; Morrison et al. 2002). The rats stabilized for an average of 40.0 ± 5.3 min (range, 30–52 min) before any interventions.
The microdialysis probes were 240 μm in diameter with a 1 mm cuprophane membrane and a 6000 Dalton cut-off. The probes were connected to FEP Teflon tubing (inside diameter = 0.12 mm) and connected to 1.0 ml syringes via a zero dead space switch (Uniswitch, B.A.S., West Lafayette, IN, USA). The probes were continually flushed with artificial cerebrospinal fluid (ACSF) at a flow rate of 2.1 μl min−1 using a syringe pump and controller (MD-1001 and MD-1020, B.A.S.). The lag time for fluid to travel to the tip of the probe at this flow rate was 4 min 35 s. The composition (mm) of the ACSF was NaCl (125), KCl (3), KH2PO4 (1), CaCl2 (2), MgSO4 (1), NaHCO3 (25) and glucose (30). The ACSF was made fresh each day, warmed to 37°C and bubbled with CO2 to a pH of 7.40.
Recording
The electrical signals were amplified and filtered (Super-Z head-stage amplifiers and BMA-400 amplifiers/filters, CWE Inc., Ardmore, PA, USA). The EEG was bandpass filtered between 1 and 100 Hz, and the GG and diaphragm EMGs between 100 and 1000 Hz. The GG and diaphragm signals were recorded at the same amplification across all experiments. It was not necessary to alter the gain of the recording apparatus between experiments and baseline EMG activity was similar across rats. The electrocardiogram was removed from the diaphragm signal using an oscilloscope and electronic blanker (Model SB-1, CWE Inc.). The moving-time averages of the GG and diaphragm EMGs (time constant = 100 ms) were also obtained (Coulbourn S76-01, Lehigh Valley, PA, USA). Each signal, along with blood pressure (DT-XX transducer, Ohmeda, Madison, WI, USA and PM-1000 Amplifier, CWE Inc.) and inspired CO2 (CapStar-100, CWE Inc.) were recorded on chart (TA11, Gould, Valley View, OH, USA) and computer (Spike 2 software, 1401 interface, CED Ltd, Cambridge, UK).
Protocol
Three separate studies were performed. For each study, interventions were performed during steady-state periods with stable high-voltage low-frequency EEG activity and stable breathing.
Study 1: Effects of cholinergic receptor activation at the HMN with and without CO2 stimulation
Signals were monitored continuously during microdialysis perfusion of ACSF into the HMN (control condition) and then during perfusion of: (i) carbachol, a mixed muscarinic and nicotinic receptor agonist (n = 6 rats; 0, 0.01, 0.1, 1, 10 and 100 μm); (ii) ACh (n = 8 rats; 0, 0.01, 0.1, 1, 10 and 100 mm); (iii) eserine, an acetylcholinesterase inhibitor to increase endogenous levels of ACh (n = 6 rats; 0, 0.1, 1, 10, 100 and 1000 μm); (iv) muscarine (n = 6 rats; 0, 0.01, 0.1, 1, 10 and 100 μm); (v) dimethyl-4-phenylpiperazinium iodide (DMPP), a nicotinic receptor agonist (n = 6 rats; 0, 0.001, 0.01, 0.1, 1 and 10 mm). Drugs were purchased from Sigma (St Louis, MO, USA) and dissolved in ACSF. For each dose, 20–25 min were allowed to elapse after the initial switch of the perfusion medium and then the signals were recorded before, during and after steady-state (> 6 min) application of 7.5% inspired CO2. Accordingly, each dose took approximately 35 min to complete and the whole experiment for each drug lasted ∼210 min as there were six doses. We used 7.5% CO2 as it provides robust GG stimulation in anaesthetized (Morrison et al. 2002; Liu et al. 2003; Sood et al. 2003) as well as naturally sleeping animals (Horner et al. 2002; Morrison et al. 2003a, b).
Additional studies to examine time-dependent alterations in GG activity were completed in four rats. In these studies, ACSF was continually perfused into the HMN and responses to CO2 stimulation determined at 35 min intervals. Six ‘sham interventions’ were performed over ∼210 min, equivalent to the duration of cholinergic interventions.
Study 2: Responses to carbachol at the HMN and effects of cholinergic receptor antagonists
A separate study was performed to determine the effects of mAChR and nAChR antagonists at the HMN on the responses to cholinergic stimulation. Five separate experiments were performed. (i) After control perfusion of ACSF into the HMN (> 1 h), the response to a single dose of 100 μm carbachol was determined (n = 9 rats, carbachol applied for 20–25 min). This dose of carbachol at the HMN produced a robust suppression of GG activity (as predicted from the results of Study 1, see Results). (ii) To determine the role of mAChR in mediating this GG suppression, the response to the same dose of carbachol was determined following pre-application of 10 μm atropine to the HMN (n = 6 rats, atropine applied for 20–25 min). (iii) To determine the potential role of nAChR in modulating the overall GG responses to cholinergic stimulation at the HMN, the response to 100 μm carbachol was also determined following 20–25 min pre-application of the nicotinic receptor antagonists (a) mecamylamine (10 μm, n = 6 rats) and (b) dihydro-β-erythroidine (DβE) at a dose of 100 μm (n = 6 rats). (iv) In a final group of six rats, the response to 100 μm carbachol at the HMN was also determined following 20–25 min pre-application of combined 10 μm atropine and 10 μm mecamylamine.
We have found that delivery of drugs to brain tissue using in vivo microdialysis perfusion requires doses ∼10 times (or more) those used in vitro to produce the same robust effects (Jelev et al. 2001; Morrison et al. 2002, 2003a, b; Liu et al. 2003; Sood et al. 2003). This is likely due to reduced tissue access (Alessandri et al. 1996). Accordingly, the doses of mecamylamine and DβE used in these studies were at the upper end or higher than those used in vitro (Zaninetti et al. 1999; Chamberlin et al. 2002).
Study 3: Endogenous cholinergic mechanisms at the HMN
A separate study in six rats was performed to determine effects of increased endogenous ACh at the HMN on GG activity and the modulating effects of mAChRs and nAChRs. After control perfusion of ACSF into the HMN (> 1 h), the response to 1 mm eserine was determined. After 20–25 min of eserine, the perfusion medium was switched to 10 μm atropine combined with 1 mm eserine and this was also maintained for 20–25 min. Finally, in the same rats, the perfusion medium was then switched to combined delivery of 100 μm DβE, 10 μm atropine plus 1 mm eserine, i.e. increased endogenous ACh in the presence of combined nAChR and mAChR blockade.
Analyses
For each agent delivered to the HMN measurements were taken over 1-min periods. For Study 1, measurements were taken at each drug dose (i) immediately before CO2 application, (ii) in the last minute of CO2, and (iii) > 10 min after removal of CO2. For the time control experiments involving continual delivery of ACSF to the HMN measurements were taken ∼35 min apart encompassing each sham ACSF intervention, and for each intervention measurements were taken before, during and after CO2 stimulation as above. For Study 2, investigating the effect of perfusion of the HMN with mAChR and/or nAChR antagonists prior to a switch to carbachol, responses were measured 10 min after the switch to avoid potential washout of the antagonists. Previous studies using this approach have shown that washout can take > 30 min (Liu et al. 2003). For Study 3, measurements were taken in the last minute of each drug application.
Breath-by-breath measurements of GG and diaphragm activities were calculated and averaged in consecutive 5-s bins, and mean blood pressure and EEG frequencies were measured for these same bins. Values were written to a spreadsheet and matched to the corresponding intervention at the HMN and respective CO2 level to provide a grand mean for each variable, for each intervention, in each rat. The EMGs were analysed from the moving average signals (above electrical zero) and quantified in arbitrary units. Electrical zero was the voltage recorded with the amplifier inputs grounded. GG EMG was quantified as mean tonic activity (i.e. basal activity in expiration), peak inspiratory activity and phasic respiratory activity (i.e. peak inspiratory activity – tonic activity). Mean diaphragm EMG amplitudes, respiratory rates and blood pressure were also calculated for each 5-s period. The EEG was sampled by computer at 500 Hz and subjected to a fast Fourier transform for each 5-s bin (Morrison et al. 2002; Liu et al. 2003; Sood et al. 2003) and the power within frequency bands spanning the 0.5–30 Hz range was calculated. The ratio of high (β2, 20–30 Hz) to low (δ1, 2–4 Hz) frequency activity was calculated and used as a marker of EEG activation (Hamrahi et al. 2001; Morrison et al. 2003a; Sood et al. 2003). We also performed an additional analysis of EEG activity in the Θ (4–7.5 Hz), α (7.5–13.5 Hz) and β1 (13.5–20 Hz) frequency ranges because of the potential concern that additional halothane may have influenced pontine cholinergic mechanisms resulting in alterations of EEG or spindle activity (Keifer et al. 1994, 1996).
Histology
At the end of the study the rats were given an overdose of urethane and killed by intravenous injection of 3–5 ml of saturated KCl. The rats were then perfused intracardially with 0.9% saline and 10% formalin and the brain removed and fixed in 10% formalin. The medullary regions were blocked and transferred to a 30% sucrose solution and subsequently cut in 50 μm coronal sections using a cryostat (CM1850, Leica). Sections were mounted and stained with neutral red. The site of the lesion left by the microdialysis probe was localized and placed on a corresponding standard cross-section using an atlas of the rat brain (Paxinos & Watson, 1998).
Statistics
The statistical analyses performed for experimental data are included in the text where appropriate. For all comparisons, differences were considered significant if the null hypothesis was rejected at P < 0.05 using a two-tailed test. Where post hoc comparisons were performed after analysis of variance with repeated measures (ANOVA-RM), the Bonferroni corrected P value was used to infer statistical significance. Statistical analyses for Studies 1 and 3 were performed on the raw data in arbitrary units. In Study 2 comparisons of the percentage changes in carbachol-induced GG suppression in response to the cholinergic antagonists were performed. The onset of significant responses of GG muscle or other physiological parameters to the different doses of cholinergic agonists was determined using one-way ANOVA followed by Bonferroni t test for comparisons with a single control (i.e. ACSF). Analyses were performed using Sigmastat (SPSS Inc., Chicago, IL, USA). Data are presented as means ± s.e.m. unless otherwise indicated.
Results
Sites of microdialysis
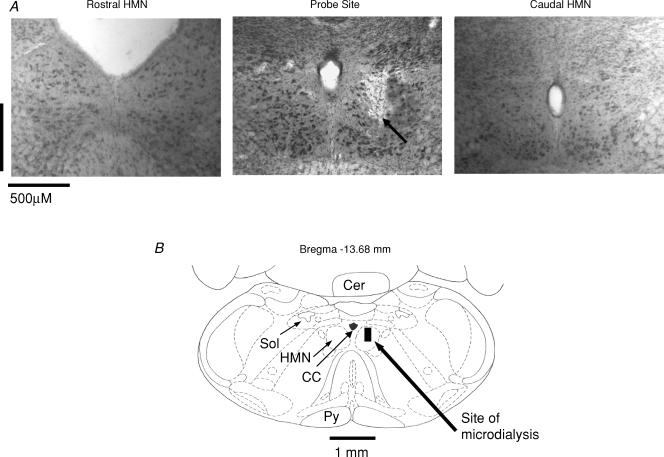
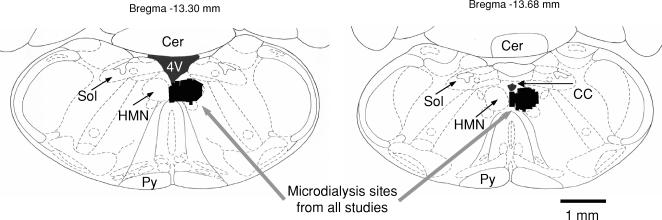
Figure 1 shows an example of a site of microdialysis perfusion into the HMN from one rat. Figure 2 shows the distribution of microdialysis sites in the HMN from all experiments.
Figure 1. Example showing location of microdialysis probe.
A, example of a lesion site made by the microdialysis probe in the hypoglossal motor nucleus (HMN). The arrow shows the location of the lesion site in the HMN. Sections both rostral and caudal to the probe site are also shown. B, schematic representation of the lesion site from the histological section. The size of the bar represents the apparent size of the lesion. Abbreviations: Cer, cerebellum; Sol, nucleus tractus solitarius; Py, pyramidal tract; CC, central canal.
Figure 2. Group data showing location of microdialysis probes.
Group data showing the distribution of microdialysis sites (illustrated by black lines) in the hypoglossal motor nucleus from all rats in Studies 1–3 (see text for the total number of rats). Abbreviations: 4 V, fourth ventricle; for other abbreviations see Fig. 1.
Study 1: Cholinergic receptor activation at the HMN with and without CO2 stimulation
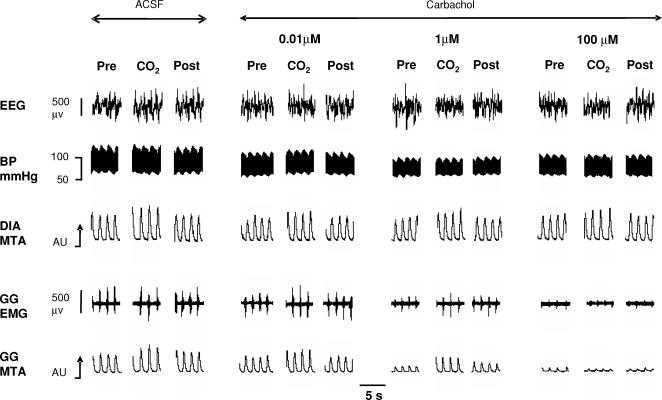
Figure 3 shows an example of the progressive decrease in GG activity with increasing carbachol at the HMN observed both with and without CO2-mediated respiratory stimulation.
Figure 3. Example showing GG motor suppression with carbachol at the HMN.
Example showing progressive suppression of genioglossus (GG) muscle activity with increasing carbachol concentration at the hypoglossal motor nucleus while breathing room air (Pre, Post) and 7.5% CO2. Also shown are the electroencephalogram (EEG), blood pressure (BP) and diaphragm (DIA) signals. The GG and DIA signals are displayed as their moving-time averages (MTA) in arbitrary units (AU). The baseline of the integrator (i.e. electrical zero) is shown for the GG MTA signal. The arrows on the MTA signals denote an increase in EMG activity.
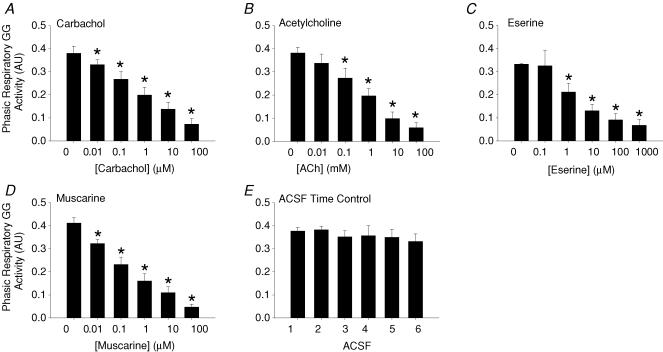
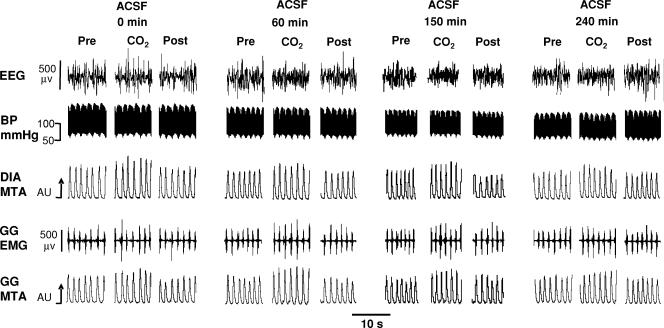
Figure 4 shows group data for the effects on GG activity of the cholinergic agonists applied to the HMN. (i) Carbachol: Fig. 4A shows that carbachol at the HMN produced a dose-dependent suppression of GG activity (F5,25 = 101.6, P < 0.001, 1-way ANOVA-RM) with significant decreases first observed at 0.01 μm compared with ACSF controls (0.380 ± 0.031 to 0.331 ± 0.020 arbitrary units (AU), P = 0.033, post hoc test, mean decrease = 11.8 ± 4.3%). (ii) ACh: Fig. 4B shows that ACh also produced dose-dependent decreases in GG activity (F5,35 = 29.7, P < 0.001) with significant suppression first observed at 0.1 mm (0.382 ± 0.025 to 0.273 ± 0.043 AU, P < 0.006, post hoc test, mean decrease = 28.5 ± 11.0%). (iii) Eserine: Fig. 4C shows that eserine at the HMN, to increase endogenous ACh, also led to dose-dependent decreases in GG activity (F5,25 = 16.0, P < 0.001) with significant effects first observed at 1 μm (0.333 ± 0.003 to 0.212 ± 0.038 AU, P = 0.035, post hoc test, mean decrease = 36.4 ± 11.3%). (iv) Muscarine: Fig. 4D shows that mAChR activation at the HMN also produced dose-dependent decreases in GG activity (F5,25 = 61.9, P < 0.001) with significant suppression first observed at 0.01 μm (0.412 ± 0.023 to 0.323 ± 0.017 AU, P = 0.006, post hoc test, mean decrease = 20.5 ± 5.8%). (v) Time control data for ACSF: Fig. 4E shows that there was no significant change in GG activity in response to the six sham ACSF interventions that were performed over the same time frame as the experiments with the cholinergic agonists described above (F5,15 = 1.5, P = 0.241, 1-way ANOVA-RM). Figure 5 shows an example of the stability of respiratory muscle activities in the time control experiments.
Figure 4. Group responses to AChR activation at the HMN and time control experiments with ACSF.
Group data showing the response of GG muscle to microdialysis perfusion into the HMN of: A, carbachol, a non-selective mAChR and nAChR agonist; B, ACh; C, eserine, an acetylcholinesterase inhibitor that increases levels of endogenous ACh; D, muscarine; and E, time control experiments with ACSF. *Significant difference compared with the respective ACSF controls.
Figure 5. Example showing a time control experiment with ACSF.
Example showing stable respiratory muscle activities, blood pressure and EEG during time control experiments with ACSF at the HMN with and without CO2 stimulation. Abbreviations as for Fig. 3.
Nicotinic receptor activation with DMPP
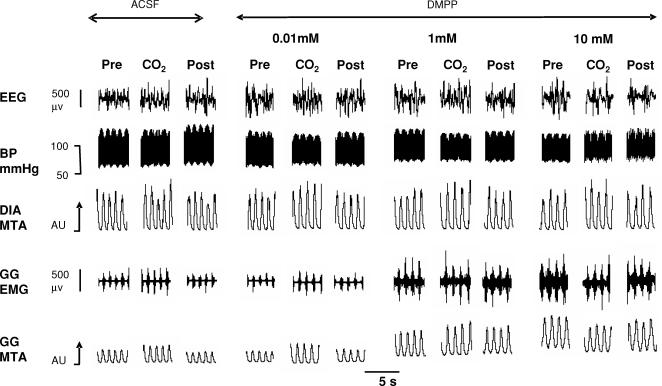
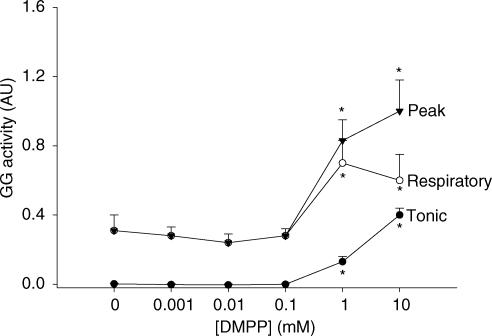
In contrast to the suppressant effects on GG activity with the cholinergic agonists shown in Figs 3 and 4, selective nAChR activation at the HMN with DMPP increased GG activity. An example of such a response is shown in Fig. 6. In Fig. 7 the group data from all animals showed that DMPP increased tonic, respiratory-related, as well as peak GG activities (all F5,25 > 7.4, P < 0.001, 1-way ANOVA-RM) with statistically significant effects first observed at 1 mm compared with ACSF controls (all P < 0.005, post hoc test, mean increase in peak and respiratory-related GG activities = 219 ± 45% and 156 ± 36%, respectively, at this threshold dose). Since there was minimal tonic GG activity with ACSF at the HMN the percentage increase with DMPP was not calculated.
Figure 6. Example showing GG motor activation with DMPP at the HMN.
Example showing increases in GG activity by increasing concentrations of DMPP, a nAChR agonist, at the HMN with and without CO2 stimulation. Note increases in both tonic and respiratory-related GG activity at the higher doses of DMPP. Abbreviations as for Fig. 3.
Figure 7. Group responses to DMPP at the HMN.
Group data showing the response of GG muscle to microdialysis perfusion of DMPP into the HMN. Note that tonic, respiratory-related and peak GG activities are all increased at the higher doses of DMPP compared with baseline GG activity with ACSF. *Significant difference compared with ACSF controls.
Specificity of GG responses and responses of other variables
Table 1 shows that there were no significant changes in respiratory rate, diaphragm amplitude, blood pressure or EEG activity at the doses of cholinergic agonists that significantly altered GG activity. As shown below, changes in these physiological parameters occurred in response to administration of cholinergic agonists to the HMN at doses 100–1000 times higher than those affecting GG activities.
Table 1.
Values of physiological variables during control perfusion of artificial cerebrospinal fluid (ACSF) into the hypoglossal motor nucleus and at the threshold doses of the various cholinergic agonists that significantly altered genioglossus activity
| Values during ACSF control | Values at threshold dose | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Experimental group for cholinergic agonists | Respiratory rate (breaths min−1) | Diaphragm amplitude (AU) | Blood pressure (mmHg) | EEG (%β2/%δ1) | Threshold dose of cholinergic agonist | Respiratory rate (breaths min−1) | Diaphragm amplitude (AU) | Blood pressure (mmHg) | EEG (%β2/%δ1) |
| Carbachol | 50.1 (2.8) | 0.78 (0.17) | 98.7 (5.1) | 0.71 (0.10) | 0.01 μm carbachol | 49.1 (2.7) | 0.74 (0.16) | 95.7 (5.4) | 0.75 (0.10) |
| ACh | 48.0 (2.5) | 0.69 (0.06) | 95.9 (6.9) | 0.50 (0.07) | 0.1 mmACh | 47.0 (2.0) | 0.63 (0.05) | 81.4 (7.2)* | 0.50 (0.09) |
| Eserine | 50.6 (1.0) | 0.95 (0.12) | 96.0 (4.4) | 0.81 (0.16) | 1 μmeserine | 48.7 (0.8) | 0.90 (0.11) | 90.5 (4.5) | 0.77 (0.09) |
| Muscarine | 53.3 (3.4) | 0.59 (0.05) | 102.6 (4.8) | 1.10 (0.09) | 0.01 μm muscarine | 52.5 (3.3) | 0.58 (0.05) | 100.7 (5.4) | 0.85 (0.13) |
| DMPP | 47.1 (2.8) | 0.64 (0.13) | 95.9 (3.8) | 0.79 (0.08) | 1 mm DMPP | 43.4 (3.6) | 0.56 (0.09) | 92.0 (6.3) | 0.73 (0.11) |
Values shown are means (± s.e.m.).
Significant difference from ACSF control.
(i) Carbachol at 0.01 μm significantly decreased GG activity (Fig. 4A) whereas a dose 100 times higher was required to decrease respiratory rate (50.1 ± 2.8 to 46.1 ± 2.2 breaths min−1 at 1 μm) and 1000 times higher decreased blood pressure (98.7 ± 5.1 to 88.6 ± 6.9 mmHg at 10 μm, both P < 0.03, post hoc test). Carbachol, however, had no significant effects at any dose on other physiological parameters, i.e. diaphragm amplitude or markers of EEG activity (β2/δ1, Θ, α or β1 activity). (ii) ACh at 0.1 mm significantly decreased GG activity (Fig. 4B) and also decreased blood pressure (95.9 ± 6.9 to 81.4 ± 7.2 mmHg), whereas a dose 100 times higher decreased diaphragm amplitude (0.69 ± 0.06 to 0.61 ± 0.06 AU, both P < 0.006, post hoc test). However, there were no effects of ACh, at any dose, on other physiological parameters. (iii) Eserine at 1 μm significantly decreased GG activity (Fig. 4C) but a dose 100 times higher was required to decrease respiratory rate and blood pressure (mean decreases = 4.8 ± 1.6 breaths min−1 and 22.0 ± 7.1 mmHg, respectively, at 100 μm; see Table 1 for baseline values with ACSF, both P < 0.001, post hoc test). Nonetheless, there were no effects of eserine at any dose on other physiological parameters. (iv) Muscarine at 0.01 μm significantly decreased GG activity (Fig. 4D) whereas a dose 1000 times higher was required to decrease respiratory rate and blood pressure (mean decreases = 4.2 ± 1.9 breaths min−1 and 18.1 ± 7.0 mmHg, respectively, at 10 μm, both P < 0.05, post hoc test). However, there were no effects of muscarine at any dose on other physiological parameters. (v) DMPP at 1 mm significantly increased GG activity (Fig. 7) whereas a dose 10 times higher was required to alter diaphragm amplitude (0.64 ± 0.13 to 0.59 ± 0.10 AU at 10 mm, P < 0.05, post hoc test). Nonetheless, in the group of animals DMPP caused no significant changes in mean respiratory rate, blood pressure or markers of EEG activity at any dose. (vi) Time control data for ACSF: GG activity did not change during the sham ACSF interventions at the HMN (Fig. 4E). Likewise, there was no change in respiratory rate or EEG frequencies in these time control experiments. A significant decrease in blood pressure was observed at the fifth sham ACSF intervention compared with the beginning of the study (101.6 ± 4.9 to 90.6 ± 2.5 mmHg, P = 0.033, post hoc test), i.e. after a prolonged period of time compared with the changes in GG activity observed with first or second doses of the cholinergic agonists (Fig. 4A–D). A decrease in diaphragm amplitude was also not observed until the fifth ACSF intervention (0.72 ± 0.09 to 0.61 ± 0.08 AU, P < 0.001, post hoc test).
Responses to CO2 stimulation during mAChR and nAChR activation
With ACSF at the HMN there was a significant increase in GG activity with CO2 stimulation (F1,27 = 13.14, P = 0.001, 2-way ANOVA-RM), with average increases from 0.358 ± 0.021 to 0.412 ± 0.016 AU in those experiments with ACSF before application of the cholinergic agonists in Study 1. There was no subsequent effect of the dose of applied carbachol, ACh, eserine or muscarine on the change in GG activity from before to during CO2 stimulation (all F5,25 < 2.25, all P > 0.08, 2-way ANOVA-RM), i.e. despite the decrease in baseline GG activity with administration of cholinergic agonists the response to CO2 was preserved (e.g. Fig. 3). In contrast, DMPP at the HMN significantly affected the GG responses to CO2 (F5,25 = 5.50, P = 0.002, 2-way ANOVA-RM) with respiratory-related GG activity being significantly decreased at the highest dose of DMPP in response to CO2 application (0.602 ± 0.152 to 0.440 ± 0.110 AU, P < 0.05, post hoc t test, see Fig. 6).
In the time control experiments GG activity also increased with CO2 stimulation (F2,6 = 6.0, P = 0.037, 2-way ANOVA-RM, see Fig. 5). This response was not affected by the repeated sham ACSF interventions (F10,30 = 1.3, P = 0.283, 2-way ANOVA-RM) confirming that GG activities were responsive throughout the perfusion of ACSF into the HMN.
Study 2: Responses to carbachol at the HMN and effects of cholinergic receptor antagonists
In control conditions, a switch from ACSF to 100 μm carbachol produced significant suppression of GG activity (0.340 ± 0.021 to 0.192 ± 0.030 AU, t8 = 5.76, P < 0.001, paired t test, mean decrease = 44.2 ± 7.5%). The time taken for the onset of this decrease was 97.9 ± 13.3 s after allowing for the lag time for the drug to travel from the switch to the tip of the microdialysis probe in the HMN.
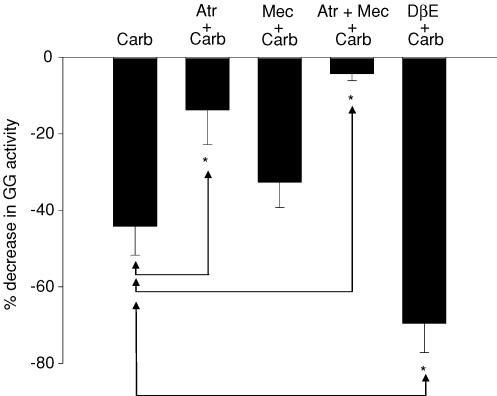
Figure 8 shows that pre-application of cholinergic antagonists to the HMN had significant effects on the magnitude of carbachol-induced GG suppression (F4,31 = 18.0, P < 0.001, 1-way ANOVA). Although carbachol decreased GG activity in the presence of the mAChR antagonist atropine at the HMN (0.366 ± 0.030 to 0.314 ± 0.029 AU, t5 = 3.08, P = 0.027, paired t test), the magnitude of this suppression was reduced compared with when carbachol was applied alone (13.8 ± 4.0 versus 44.2 ± 7.5%, P = 0.006, post hoc t test after 1-way ANOVA). In contrast, pre-application of the nAChR antagonist mecamylamine did not significantly affect the carbachol-induced GG suppression (mean decrease = 32.7 ± 6.6%) compared with carbachol alone (P = 0.784, post hoc t test after 1-way ANOVA, Fig. 8); in the presence of mecamylamine GG activity decreased from 0.319 ± 0.040 to 0.215 ± 0.027 AU after carbachol (t5 = 3.46, P = 0.018, paired t test).
Figure 8. Effects of cholinergic antagonists on carbachol-induced GG motor suppression.
Group data showing the effects of pre-application of cholinergic antagonists into the HMN on GG muscle suppression produced by 100 μm carbachol (Carb, see left bar on graph). Pre-application of 10 μm atropine significantly reduced this carbachol-induced GG muscle suppression (Atr + Carb) indicating a contribution of mAChRs. In contrast, pre-application of the nAChR antagonist mecamylamine (10 μm) did not significantly affect this carbachol-induced GG suppression (Mec + Carb). Combined administration of atropine and mecamylamine (Atr + Mec + Carb) also reduced GG suppression compared with when carbachol was applied alone, consistent with mAChR-induced GG suppression. However, pre-application of DβE (100 μm) increased the magnitude of the carbachol-induced GG suppression (DβE + Carb). See text for further details. *Significant difference compared with application of carbachol alone (Carb).
Carbachol also decreased GG activity in the presence of combined atropine and mecamylamine at the HMN (0.384 ± 0.049 to 0.371 ± 0.052 AU, t5 = 2.73, P = 0.041, paired t test). The magnitude of this GG suppression with atropine and mecamylamine was reduced compared with when carbachol was applied alone (Fig. 8; 4.3 ± 1.8 versus 44.2 ± 7.5%, P < 0.001, post hoc t test after 1-way ANOVA) but not compared with when atropine was applied alone (Fig. 8; 4.3 ± 1.8 versus 13.8 ± 4.0%, P = 0.784, post hoc t test after 1-way ANOVA). Overall these results showed that mAChRs were primarily responsible for the net suppression of GG activity following cholinergic stimulation at the HMN.
However, since the data in Figs 6 and 7 showed that nAChR activation at the HMN can increase GG activity, the results of Study 2 did not exclude a contribution of residual nAChR effects to the level of GG activity after carbachol application if mecamylamine did not effectively antagonize those subunits on the nAChRs mediating the excitatory responses. Consequently the effect of pre-application of DβE, an antagonist with selectivity for the α4β2 nAChR subunits on hypoglossal motoneurones (Zaninetti et al. 1999; Chamberlin et al. 2002), on the magnitude of the carbachol-induced GG motor suppression was also determined. Carbachol decreased GG activity in the presence of DβE at the HMN (0.330 ± 0.027 to 0.105 ± 0.025 AU, t8 = 10.21, P < 0.001, paired t test). The magnitude of this carbachol-induced GG suppression with DβE was larger than when carbachol was applied alone (Fig. 8; 69.5 ± 5.9 versus 44.2 ± 7.5%, P = 0.011, post hoc t test after 1-way ANOVA), consistent with the notion that carbachol (a non-selective mAChR and nAChR agonist) decreased GG muscle activity primarily due to mAChR effects, but that concomitant nAChR activation limited this GG suppression, an effect which is revealed by effective nAChR antagonism with DβE.
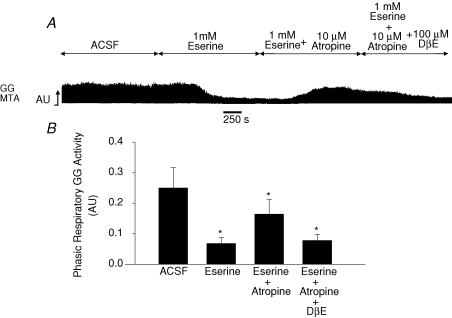
Study 3: Increased endogenous ACh at the HMN
To determine if increased endogenous ACh at the HMN modulates GG activity, the responses to atropine applied either alone or in combination with DβE were determined in the presence of eserine at the HMN. Figure 9 shows an example from one rat and the corresponding group data from six animals. The data showed a significant effect of drug intervention at the HMN on GG activity (F3,15 = 9.02, P = 0.001, 1-way ANOVA-RM). Post hoc analyses showed that eserine at the HMN decreased GG activity compared with ACSF (0.251 ± 0.067 to 0.069 ± 0.022 AU, t5 = 4.55, P = 0.001), in agreement with the results shown in Fig. 4C. Atropine at the HMN antagonized this effect and increased GG activity toward the ACSF control level (0.164 ± 0.049 AU, t5 = 2.17, P = 0.140, post hoc t test versus ACSF). However, as suggested from the results of Study 2, residual nAChR activation at the HMN may also account for a component of the increased GG activity after atropine application in the presence of increased endogenous ACh, an effect that was confirmed when GG activity decreased following addition of the nAChR antagonist DβE to the HMN (0.079 ± 0.022 AU with DβE, t5 = 4.30, P = 0.002, post hoc t test versus ACSF).
Figure 9. Opposing mAChR and nAChR modulation of GG activity in vivo.
Raw trace (A) and group data (B) showing opposing mAChR- and nAChR-mediated modulation of hypoglossal motor output to GG muscle. Microdialysis perfusion of eserine into the HMN produced suppression of GG activity. Additional application of atropine increased GG activity consistent with a contribution of mAChR to this suppression. However, further addition of the nAChR antagonist DβE decreased GG activity, suggesting that the cholinergic effects observed after atropine in the presence of eserine were due to both mAChR blockade as well as unopposed excitatory nAChR activation, an effect which was blocked by addition of DβE. See text for further details. *Significant difference compared with ACSF.
Discussion
The present study shows that mAChR activation at the HMN produced dose-dependent suppression of respiratory-related GG activity whereas nAChR activation increased tonic and respiratory GG activity. Since co-activation of mAChRs and nAChRs with ACh or carbachol produced overall suppression of GG activity, the results suggest that inhibitory muscarinic effects predominated and masked the excitatory nicotinic effects. Importantly, the use of the acetylcholinesterase inhibitor eserine confirmed that the primary effect of increased endogenous ACh at the HMN was suppression of GG activity and that activation of mAChR and nAChR determined the net effect in vivo.
Muscarinic receptor effects
The cholinergic-induced suppression of respiratory-related GG activity in this study was mimicked by muscarine (Fig. 4) and attenuated by atropine (Figs 8 and 9), supporting a role for mAChR mechanisms at the HMN. Although this in vivo preparation cannot be used to determine a pre- or post-synaptic site of action for the mAChR effects, the decrease in respiratory-related GG activity (Figs 3 and 4) is consistent with pre-synaptic mAChR-mediated suppression of excitatory glutamatergic inputs to the HMN (Bellingham & Berger, 1996), i.e. those inputs involved in the transmission of respiratory drive (Greer et al. 1991; Funk et al. 1993). Post-synaptic M2 receptors on adult rat hypoglossal motoneurones (Hellstrom et al. 2003) may also contribute to the decreased GG activity.
It was not an aim of this study to characterize the mAChR subtypes responsible for suppression of GG activity. Such an analysis in juvenile rats has already suggested a role of inhibitory M2 receptors (Bellingham & Berger, 1996). Importantly, GG motor suppression was the dominant effect observed with mAChR activation suggesting it overrides any potential post-synaptic excitatory mAChR effects as occur in neonatal hypoglossal motoneurones in vitro (Bellingham & Funk, 2000; Lape & Nistri, 2000). Whether developmental changes in the composition or quantity of different mAChR subtypes at the HMN are responsible for the predominantly excitatory muscarinic effects in neonates (Bellingham & Funk, 2000; Lape & Nistri, 2000) but suppressant effects in older animals (Bellingham & Berger, 1996) remains to be determined. Of relevance, the medulla contains high densities of mAChR with the majority (> 70%) being of the M2 type (Wamsley et al. 1981; Li et al. 1991; Mallios et al. 1995). In adult cats the levels of the inhibitory M2 receptor at the HMN is also markedly higher than excitatory M1 and M3 receptors (Mallios et al. 1995).
Nicotinic receptor effects
Although mixed mAChR and nAChR activation at the HMN produced net suppression of GG motor activity (Fig. 4), selective nAChR activation with DMPP produced robust increases in GG activity (Figs 6 and 7). The increased GG activity with DMPP is most simply explained by nAChRs on hypoglossal motoneurones (Zaninetti et al. 1999; Chamberlin et al. 2002; Robinson et al. 2002). That mAChR-induced GG suppression masked nAChR-induced excitation during co-activation with ACh, carbachol or eserine in Study 1 was shown by Studies 2 and 3 using the nAChR antagonist DβE. In Study 2, a single application of the mixed nAChR and mAChR agonist carbachol produced overall suppression of GG activity, as predicted from the results of Study 1 (Fig. 4A), with a significant component of this suppression being antagonized by atropine, showing a major contribution of mAChRs (Fig. 8, second bar). After carbachol application, however, the net level of GG motor activity could have been determined by a balance of mAChR-mediated suppression and a prevailing level of nAChR-mediated excitation, with the net balance in favour of the former. If so, nAChR blockade with DβE would be expected to remove any lingering excitation of hypoglossal motoneurones leading to greater reductions in GG activity after carbachol due to now unopposed mAChR activation. Since such an effect was observed (Fig. 8, far right bar), the results suggest that with cholinergic stimulation at the HMN, nAChR-mediated excitation limits the GG motor suppression caused by mAChR activation.
Study 3 confirmed that the prevailing level of GG motor activity during cholinergic stimulation of the HMN was due to a balance between opposing excitatory and inhibitory effects, but importantly extended this result to the effects of increased endogenous ACh at the HMN. Eserine caused a decrease in GG activity (Fig. 9). The increased GG activity following combined administration of eserine and atropine (Fig. 9) is again consistent with antagonism of mAChR-mediated inhibition (Bellingham & Berger, 1996) and compatible with the results of Study 2 (Fig. 8). Again, however, a component of the increased GG activity after atropine application could have been due to now unopposed excitation of nAChRs by increased endogenous ACh. The subsequent decrease in GG activity following DβE application in the combined presence of eserine and atropine suggests this was the case (Fig. 9).
The nAChR agonist DMPP was chosen for these studies as it activates neonatal hypoglossal motoneurones in vitro (Zaninetti et al. 1999; Chamberlin et al. 2002), and in the present study it similarly increased GG activity. DβE and mecamylamine were chosen as antagonists as both block DMPP-induced post-synaptic inward currents on hypoglossal motoneurones (Zaninetti et al. 1999; Chamberlin et al. 2002). In our study, DβE but not mecamylamine significantly modulated GG responses to carbachol at the HMN (Fig. 8). The potency of DβE in blocking excitation of HMN is larger than mecamylamine (Chamberlin et al. 2002) such that our dose of mecamylamine may have been insufficient to increase the magnitude of carbachol-mediated GG suppression compared with application of carbachol alone (Fig. 8). Nevertheless, it is also possible that significant alterations in carbachol-mediated GG suppression with DβE but not mecamylamine may have been due to the subunit composition of nAChR on adult hypoglossal motoneurones. Overall, however, the significant effects of DβE (Figs 8 and 9) show a role of nAChRs at the HMN in increasing GG activity in vivo.
Responses to CO2
Although GG activity decreased with cholinergic stimulation at the HMN, GG muscle was still able to respond to excitatory inputs as shown by the absence of a statistically significant effect of carbachol, ACh, eserine or muscarine on the change in GG activity from before to during CO2 stimulation. These results suggest that the progressive reductions in GG activity during hypercapnia in the presence of mAChR activation were not due to an effect on CO2 responsiveness of the hypoglossal motoneurone pool per se as it was still able to increase activity, albeit from progressively reduced baseline levels. Similar CO2-mediated effects on GG muscle activity occur following GG motor suppression produced by glycine and GABAA receptor agonists at the HMN (Morrison et al. 2002; Liu et al. 2003). In contrast to the stimulatory effects of CO2 on GG activity during mAChR activation, respiratory-related GG activity was actually decreased by CO2 at the highest levels of nAChR activation (Fig. 6). Although the mechanisms underlying this effect were not determined in this study, a similar reduction in respiratory GG activity has been observed at high levels of tonic drive produced by serotonin (Kubin et al. 1992; Sood et al. 2003).
Critique of preparation
The current experiments were performed under anaesthesia rather than in freely behaving animals because without this initial controlled characterization of the cholinergic effects at the HMN, the added complexity of sleep and awake states occurring at unpredictable times, and the different effects of these states on GG activity (Jelev et al. 2001; Morrison et al. 2003a, b) and responses to CO2 (Horner et al. 2002) would have severely complicated data interpretation and precluded observation of the opposing nature of the nAChR and mAChR responses at the HMN.
The experiments were performed in vagotomised rats in order to allow measurement of GG and diaphragm activities un-confounded by vagal reflexes due to CO2-mediated changes in breathing rate and depth. Vagotomy was also performed to relieve GG muscle from reflex inhibition originating in vagal afferents (Bailey et al. 2001), such that any suppressant effects of cholinergic stimulation could be readily observed. In this preparation also, GG activity during vagotomy is stable over time (Fig. 4E) such that changes following interventions at the HMN can be readily observed.
As in previous studies (Horner et al. 2002; Morrison et al. 2002; Liu et al. 2003), experiments were performed under urethane anaesthesia, with addition of halothane as required. It has been shown, however, that increasing halothane from 1 to 3% in cats decreases GG activity (Ochiai et al. 1989), such that there is a concern that a component of the cholinergic-induced GG motor suppression (Fig. 4A–D) may have been due to additional halothane during an experiment. The range of halothane in our studies, however, was 0.2–1%, and once initiated within an animal it required only minor adjustment. Moreover, the time control experiments with ACSF showed stability of the preparation, especially GG activity, over time (Fig. 4E), suggesting that the GG responses (excitatory and inhibitory) to interventions at the HMN were most likely due to the cholinergic agonists per se and not halothane. Indeed, the suppressant and excitatory effects of mAChR and nAChR activation at the HMN confirm previous in vitro studies obtained in the absence of anaesthetics (Bellingham & Berger, 1996; Chamberlin et al. 2002; Robinson et al. 2002).
Halothane has also been observed to alter pontine cholinergic activity, of which changes in the EEG or spindle activity can be a marker (Keifer et al. 1994; Keifer et al. 1996). However, there was no change in EEG activity during the experiments, providing additional support for the stability of the preparation over time. However, the absence of blood gas measurements or end-tidal CO2 levels cannot ultimately exclude pH imbalances or blood gas disturbances in contributing to a decline in respiratory muscle activities, despite the observations of stable GG activity during the time control experiments (Fig. 4E). Indeed, although most physiological variables were stable for the duration of the time control experiments, decreases in blood pressure and diaphragm amplitude did occur at the end of the sham ACSF interventions, and as such these changes were probably due to the prolonged duration of the studies. Nevertheless, as shown in Table 1, there were typically no changes in any physiological variables at the doses of cholinergic agonists that caused significant changes in GG activity. Those changes that were observed occurred at doses 100–1000 times higher than the doses causing changes in GG activity. Such responses at high doses are probably due to spread of perfusate to neighbouring regions of medulla, e.g. the decreases in blood pressure may involve modulation of autonomic activities or cardiovascular reflexes by effects at the nucleus tractus solitarius (Tsukamoto et al. 1994).
Cholinergic neuromodulation of the HMN – implications
Cholinergic neurones of the pedunculopontine tegmental nucleus (PPT) are retrogradely labelled following injection of fluorescent tracers into the trigeminal, facial and hypoglossal motor nuclei (Woolf & Butcher, 1989). Numerous cholinergic axons make synaptic contact with hypoglossal motoneurones while other cholinergic endings are opposed to non-cholinergic terminals (Connaughton et al. 1986; Davidoff & Irintchev, 1986; Hellstrom et al. 2003). The former provide the anatomical substrate for post-synaptic modulation of hypoglossal motoneurones (Zaninetti et al. 1999; Bellingham & Funk, 2000; Lape & Nistri, 2000; Chamberlin et al. 2002; Robinson et al. 2002) whereas the latter may provide the basis for pre-synaptic modulation of inputs to HMN (Bellingham & Berger, 1996). Since the activity of PPT neurones varies across sleep and awake states, with minimal activity in non-REM sleep and maximal activity in wakefulness and/or REM sleep (Vertes, 1979; el Mansari et al. 1989; Steriade et al. 1990a, b; Thakkar et al. 1998), there is appropriate circuitry for alterations in cholinergic inputs to the HMN to potentially contribute to sleep-state-dependent modulation of GG activity (Sauerland et al. 1981; Wiegand et al. 1991; Worsnop et al. 1998; Fogel et al. 2003). However, the role of nAChR and mAChR mechanisms at the HMN modulating GG activity across sleep–wake states remains to be determined in natural sleep.
Characterizing mAChR and nAChR effects at the HMN in vivo has clinical relevance because modulation of central cholinergic activity has been attempted as a pharmacological treatment of obstructive sleep apnoea in humans (Gothe et al. 1985; Hedner et al. 2003), although in those studies the sites and mode of action of the cholinergic agents were not known. The reduction of obstructive apnoea severity following nicotine-containing chewing gum (Gothe et al. 1985) is consistent with central stimulation of pharyngeal motoneurones as observed in this study, although measures of muscle activity were not obtained in that study and other sites of action cannot be ruled out. Also in agreement with central stimulation of pharyngeal motoneurones, nicotine injected either intravenously, into the lateral ventricle or onto the ventral medullary surface, increases hypoglossal nerve and GG activity in adult cats in vivo (Haxhiu et al. 1984a, b). In contrast, reductions in obstructive apnoeas with intravenous eserine in humans (Hedner et al. 2003) cannot be explained based on the effect of increased ACh at the HMN since suppression of pharyngeal muscle activity would be expected to worsen obstructive apnoeas. In that human study, however, eserine was applied intravenously such that it may have acted at multiple sites within the central nervous system thereby indirectly affecting inputs to the HMN, although there is also the possibility that the balance of mAChR-mediated suppression versus nAChR-mediated excitation may, unlike rats, be balanced in favour of the latter in humans.
Acknowledgments
This work was supported by funds from the Canadian Institutes of Health Research (CIHR, Grant MT-15563). X.L. was a recipient of a Merck Frosst Fellowship, Division of Respirology, University of Toronto.
References
- Alessandri B, Landolt H, Langemann H, Gregorin J, Hall J, Gratzl O. Application of glutamate in the cortex of rats: a microdialysis study. Acta Neurochirurgica – Suppl. 1996;67:6–12. doi: 10.1007/978-3-7091-6894-3_2. [DOI] [PubMed] [Google Scholar]
- Bailey EF, Jones CL, Reeder JC, Fuller DD, Fregosi RF. Effect of pulmonary stretch receptor feedback and CO2 on upper airway and respiratory pump muscle activity in the rat. J Physiol. 2001;532:525–534. doi: 10.1111/j.1469-7793.2001.0525f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellingham MC, Berger AJ. Presynaptic depression of excitatory synaptic inputs to rat hypoglossal motoneurons by muscarinic M2 receptors. J Neurophysiol. 1996;76:3758–3770. doi: 10.1152/jn.1996.76.6.3758. [DOI] [PubMed] [Google Scholar]
- Bellingham MC, Funk GD. Cholinergic modulation of respiratory brain-stem neurons and its function in sleep-wake state determination. Clin Exp Pharmacol Physiol. 2000;27:132–137. doi: 10.1046/j.1440-1681.2000.03192.x. [DOI] [PubMed] [Google Scholar]
- Chamberlin NL, Bocchiaro CM, Greene RW, Feldman JL. Nicotinic excitation of rat hypoglossal motoneurons. Neuroscience. 2002;115:861–870. doi: 10.1016/s0306-4522(02)00454-2. [DOI] [PubMed] [Google Scholar]
- Connaughton M, Priestley JV, Sofroniew MV, Eckenstein F, Cuello AC. Inputs to motoneurones in the hypoglossal nucleus of the rat: light and electron microscopic immunocytochemistry for choline acetyltransferase, substance P and enkephalins using monoclonal antibodies. Neuroscience. 1986;17:205–224. doi: 10.1016/0306-4522(86)90237-x. [DOI] [PubMed] [Google Scholar]
- Davidoff MS, Irintchev AP. Acetylcholinesterase activity and type C synapses in the hypoglossal, facial and spinal-cord motor nuclei of rats. An electron-microscope study. Histochemistry. 1986;84:515–524. doi: 10.1007/BF00482985. [DOI] [PubMed] [Google Scholar]
- Dobbins EG, Feldman JL. Differential innervation of protruder and retractor muscles of the tongue in rat. J Comparative Neurol. 1995;357:376–394. doi: 10.1002/cne.903570305. [DOI] [PubMed] [Google Scholar]
- el Mansari M, Sakai K, Jouvet M. Unitary characteristics of presumptive cholinergic tegmental neurons during the sleep-waking cycle in freely moving cats. Exp Brain Res. 1989;76:519–529. doi: 10.1007/BF00248908. [DOI] [PubMed] [Google Scholar]
- Fogel RB, White DP, Pierce RJ, Malhotra A, Edwards JK, Dunai J, Kleverlaan D, Trinder J. Control of upper airway muscle activity in younger versus older men during sleep onset. J Physiol. 2003;553:533–544. doi: 10.1113/jphysiol.2003.045708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Williams JS, Janssen PL, Fregosi RF. Effect of co-activation of tongue protrudor and retractor muscles on tongue movements and pharyngeal airflow mechanics in the rat. J Physiol. 1999;519:601–613. doi: 10.1111/j.1469-7793.1999.0601m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Gothe B, Strohl KP, Levin S, Cherniack NS. Nicotine: a different approach to treatment of obstructive sleep apnea. Chest. 1985;87:11–17. doi: 10.1378/chest.87.1.11. [DOI] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrahi H, Chan B, Horner RL. On-line detection of sleep-wake states and application to produce intermittent hypoxia only in sleep in rats. J Appl Physiol. 2001;90:2130–2140. doi: 10.1152/jappl.2001.90.6.2130. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Mitra J, van Lunteren E, Bruce EN, Cherniack NS. Hypoglossal and phrenic responses to cholinergic agents applied to ventral medullary surface. Am J Physiol. 1984a;247:R939–R944. doi: 10.1152/ajpregu.1984.247.6.R939. [DOI] [PubMed] [Google Scholar]
- Haxhiu MA, Van Lunteren E, Van de Graaff WB, Strohl KP, Bruce EN, Mitra J, Cherniack NS. Action of nicotine on the respiratory activity of the diaphragm and genioglossus muscles and the nerves that innervate them. Respir Physiol. 1984b;57:153–169. doi: 10.1016/0034-5687(84)90090-2. [DOI] [PubMed] [Google Scholar]
- Hedner J, Kraiczi H, Peker Y, Murphy P. Reduction of sleep-disordered breathing after physostigmine. Am J Respir Crit Care Med. 2003;168:1246–1251. doi: 10.1164/rccm.200211-1344OC. [DOI] [PubMed] [Google Scholar]
- Hellstrom J, Oliveira AL, Meister B, Cullheim S. Large cholinergic nerve terminals on subsets of motoneurons and their relation to muscarinic receptor type 2. J Comp Neurol. 2003;460:476–486. doi: 10.1002/cne.10648. [DOI] [PubMed] [Google Scholar]
- Horner RL, Liu X, Gill H, Nolan P, Liu H, Sood S. Effects of sleep-wake state on the genioglossus vs. diaphragm muscle response to CO2 in rats. J Appl Physiol. 2002;92:878–887. doi: 10.1152/japplphysiol.00855.2001. [DOI] [PubMed] [Google Scholar]
- Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep–wake states in rats. J Physiol. 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer JC, Baghdoyan HA, Becker L, Lydic R. Halothane decreases pontine acetylcholine release and increases EEG spindles. Neuroreport. 1994;5:577–580. doi: 10.1097/00001756-199401000-00011. [DOI] [PubMed] [Google Scholar]
- Keifer JC, Baghdoyan HA, Lydic R. Pontine cholinergic mechanisms modulate the cortical electroencephalographic spindles of halothane anesthesia. Anesthesiology. 1996;84:945–954. doi: 10.1097/00000542-199604000-00023. [DOI] [PubMed] [Google Scholar]
- Kubin L, Fenik V. Pontine cholinergic mechanisms and their impact on respiratory regulation. Respir Physiol Neurobiol. 2004;143:235–249. doi: 10.1016/j.resp.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Kubin L, Tojima H, Davies RO, Pack AI. Serotonergic excitatory drive to hypoglossal motoneurons in the decerebrate cat. Neuroscience Lett. 1992;139:243–248. doi: 10.1016/0304-3940(92)90563-m. [DOI] [PubMed] [Google Scholar]
- Lape R, Nistri A. Current and voltage clamp studies of the spike medium afterhyperpolarization of hypoglossal motoneurons in a rat brain stem slice preparation. J Neurophysiol. 2000;83:2987–2995. doi: 10.1152/jn.2000.83.5.2987. [DOI] [PubMed] [Google Scholar]
- Lee LH, Friedman DB, Lydic R. Respiratory nuclei share synaptic connectivity with pontine reticular regions regulating REM sleep. Am J Physiol. 1995;268:L251–L262. doi: 10.1152/ajplung.1995.268.2.L251. [DOI] [PubMed] [Google Scholar]
- Li M, Yasuda RP, Wall SJ, Wellstein A, Wolfe BB. Distribution of m2 muscarinic receptors in rat brain using antisera selective for m2 receptors. Mol Pharmacol. 1991;40:28–35. [PubMed] [Google Scholar]
- Liu X, Sood S, Liu H, Nolan P, Morrison JL, Horner RL. Suppression of genioglossus muscle tone and activity during reflex hypercapnic stimulation by GABA-A mechanisms at the hypoglossal motor nucleus in-vivo. Neuroscience. 2003;116:249–259. doi: 10.1016/s0306-4522(02)00564-x. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Pedunculopontine stimulation alters respiration and increases ACh release in the pontine reticular formation. Am J Physiol. 1993;264:R544–R554. doi: 10.1152/ajpregu.1993.264.3.R544. [DOI] [PubMed] [Google Scholar]
- Lydic R, Baghdoyan HA. Neurochemical evidence for the cholinergic modulation of sleep and breathing. In: Carley DW, Radulovacki M, editors. Sleep-Related Breathing Disorders: Experimental Models and Therapeutic Potential. New York: Dekker; 2003. pp. 57–91. [Google Scholar]
- Lydic R, Baghdoyan HA, Wertz R, White DP. Cholinergic reticular mechanisms influence state-dependent ventilatory response to hypercapnia. Am J Physiol. 1991;261:R738–R746. doi: 10.1152/ajpregu.1991.261.3.R738. [DOI] [PubMed] [Google Scholar]
- Mallios VJ, Lydic R, Baghdoyan HA. Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am J Physiol. 1995;268:L941–L949. doi: 10.1152/ajplung.1995.268.6.L941. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu X, Liu H, Park E, Nolan P, Horner RL. Glycine at the hypoglossal motor nucleus: genioglossus activity, CO2 responses and the additive effects of GABA. J Appl Physiol. 2002;93:1786–1796. doi: 10.1152/japplphysiol.00464.2002. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. GABAA receptor antagonism at the hypoglossal motor nucleus increases genioglossus muscle activity in NREM but not REM sleep. J Physiol. 2003a;548:569–583. doi: 10.1113/jphysiol.2002.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JL, Sood S, Liu H, Park E, Nolan P, Horner RL. Role of inhibitory amino acids in control of hypoglossal motor outflow to genioglossus muscle in naturally sleeping rats. J Physiol. 2003b;552:975–991. doi: 10.1113/jphysiol.2003.052357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nattie E. CO2, brainstem chemoreceptors and breathing. Prog Neurobiol. 1999;59:299–331. doi: 10.1016/s0301-0082(99)00008-8. [DOI] [PubMed] [Google Scholar]
- Nattie EE, Li AH. Ventral medulla sites of muscarinic receptor subtypes involved in cardiorespiratory control. J Appl Physiol. 1990;69:33–41. doi: 10.1152/jappl.1990.69.1.33. [DOI] [PubMed] [Google Scholar]
- Ochiai R, Guthrie RD, Motoyama EK. Effects of varying concentrations of halothane on the activity of the genioglossus, intercostals, and diaphragm in cats: an electromyographic study. Anesthesiology. 1989;70:812–816. doi: 10.1097/00000542-198905000-00018. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. San Diego: Academic Press; 1998. [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remmers JE, deGroot WJ, Sauerland EK, Anch AM. Pathogenesis of upper airway occlusion during sleep. J Appl Physiol Respiratory Environ Exercise Physiol. 1978;44:931–938. doi: 10.1152/jappl.1978.44.6.931. [DOI] [PubMed] [Google Scholar]
- Robinson DM, Peebles KC, Kwok H, Adams BM, Clarke LL, Woollard GA, Funk GD. Prenatal nicotine exposure increases apnoea and reduces nicotinic potentiation of hypoglossal inspiratory output in mice. J Physiol. 2002;538:957–973. doi: 10.1113/jphysiol.2001.012705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauerland EK, Orr WC, Hairston LE. EMG patterns of oropharyngeal muscles during respiration in wakefulness and sleep. Electromyogr Clin Neurophysiol. 1981;21:307–316. [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBotzinger complex inspiratory neurons. J Neurophysiol. 2000;83:1243–1252. doi: 10.1152/jn.2000.83.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Mechanisms underlying regulation of respiratory pattern by nicotine in preBotzinger complex. J Neurophysiol. 2001;85:2461–2467. doi: 10.1152/jn.2001.85.6.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sood S, Liu X, Liu H, Nolan P, Horner RL. 5-HT at hypoglossal motor nucleus and respiratory control of genioglossus muscle in anesthetized rats. Respiratory Physiol Neurobiol. 2003;138:205–221. doi: 10.1016/j.resp.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. J Neurosci. 1990a;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steriade M, Pare D, Datta S, Oakson G, Curro Dossi R. Different cellular types in mesopontine cholinergic nuclei related to ponto-geniculo-occipital waves. J Neurosci. 1990b;10:2560–2579. doi: 10.1523/JNEUROSCI.10-08-02560.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakkar MM, Strecker RE, McCarley RW. Behavioral state control through differential serotonergic inhibition in the mesopontine cholinergic nuclei: a simultaneous unit recording and microdialysis study. J Neurosci. 1998;18:5490–5497. doi: 10.1523/JNEUROSCI.18-14-05490.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukamoto K, Yin M, Sved AF. Effect of atropine injected into the nucleus tractus solitarius on the regulation of blood pressure. Brain Res. 1994;648:9–15. doi: 10.1016/0006-8993(94)91898-8. [DOI] [PubMed] [Google Scholar]
- Vertes RP. Brain stem gigantocellular neurons: patterns of activity during behavior and sleep in the freely moving rat. J Neurophysiol. 1979;42:214–228. doi: 10.1152/jn.1979.42.1.214. [DOI] [PubMed] [Google Scholar]
- Wamsley JK, Lewis MS, Young WS, III, Kuhar MJ. Autoradiographic localization of muscarinic cholinergic receptors in rat brainstem. J Neurosci. 1981;1:176–191. doi: 10.1523/JNEUROSCI.01-02-00176.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand L, Zwillich CW, Wiegand D, White DP. Changes in upper airway muscle activation and ventilation during phasic REM sleep in normal men. J Appl Physiol. 1991;71:488–497. doi: 10.1152/jappl.1991.71.2.488. [DOI] [PubMed] [Google Scholar]
- Woolf NJ. Cholinergic systems in mammalian brain and spinal cord. Prog Neurobiol. 1991;37:475–524. doi: 10.1016/0301-0082(91)90006-m. [DOI] [PubMed] [Google Scholar]
- Woolf NJ, Butcher LL. Cholinergic systems in the rat brain. IV. Descending projections of the pontomesencephalic tegmentum. Brain Res Bull. 1989;23:519–540. doi: 10.1016/0361-9230(89)90197-4. [DOI] [PubMed] [Google Scholar]
- Worsnop C, Kay A, Pierce R, Kim Y, Trinder J. Activity of respiratory pump and upper airway muscles during sleep onset. J Appl Physiol. 1998;85:908–920. doi: 10.1152/jappl.1998.85.3.908. [DOI] [PubMed] [Google Scholar]
- Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- Zaninetti M, Tribollet E, Bertrand D, Raggenbass M. Presence of functional neuronal nicotinic acetylcholine receptors in brainstem motoneurons of the rat. Eur J Neurosci. 1999;11:2737–2748. doi: 10.1046/j.1460-9568.1999.00689.x. [DOI] [PubMed] [Google Scholar]