Abstract
In heart muscle the amplification and shaping of Ca2+ signals governing contraction are orchestrated by recruiting a variable number of Ca2+ sparks. Sparks reflect Ca2+ release from the sarcoplasmic reticulum (SR) via Ca2+ release channels (ryanodine receptors, RyRs). RyRs are activated by Ca2+ influx via L-type Ca2+ channels with a specific probability that may depend on regulatory mechanisms (e.g. β-adrenergic stimulation) or diseased states (e.g. heart failure). Changes of RyR phosphorylation may be critical for both regulation and impaired function in disease. Using UV flash photolysis of caged Ca2+ and short applications of caffeine in guinea-pig ventricular myocytes, we found that Ca2+ release signals on the cellular level were largely governed by global SR content. During β-adrenergic stimulation resting myocytes exhibited smaller SR Ca2+ release signals when activated by photolysis (62.3% of control), resulting from reduced SR Ca2+ content under these conditions (58.6% of control). In contrast, local signals triggered with diffraction limited two-photon photolysis displayed the opposite behaviour, exhibiting a larger Ca2+ release (164% of control) despite reduced global and local SR Ca2+ content. This apparent paradox implies changes of RyR open probabilities after β-adrenergic stimulation, enhancing local regenerativity and reliability of Ca2+ signalling. Thus, our results underscore the importance of phosphorylation of RyRs (or of a related protein), as a regulatory physiological mechanism that may also provide new therapeutic avenues to recover impaired Ca2+ signalling during cardiac disease.
Depolarization of cardiac myocytes during an action potential leads to the opening of voltage-dependent L-type Ca2+ channels. The subsequent elevation of the Ca2+ concentration ([Ca2+]i) in the dyadic cleft activates Ca2+ release channels (referred to as ryanodine receptors or RyRs) forming macromolecular complexes located in the sarcoplasmic reticulum (SR) membrane and in the dyadic cleft. This Ca2+-induced Ca2+ release (CICR) process amplifies and scales the Ca2+ signal via summation of discrete elementary Ca2+ signalling events termed Ca2+ sparks. In order for the Ca2+ signal to be transient the Ca2+ release from the SR has to terminate. Furthermore, cytosolic Ca2+ needs to be pumped back into the SR by the SR Ca2+ pump (SERCA) and transported out of the cell via the Na+–Ca2+ exchange (for review see Bers, 2002).
The discovery of Ca2+ sparks as elementary Ca2+ signalling events has fundamentally changed the view of excitation–contraction coupling (EC coupling) on the cellular and subcellular level (Cheng et al. 1993; Niggli, 1999). Contrary to the notion of systems in which released Ca2+ has access to a common cytosolic pool (Stern, 1992), amplification and shaping of the Ca2+ signal is now understood to be fine-tuned by recruiting a variable number of functionally independent Ca2+ sparks, each of which is an all-or-none event with a high degree of positive feedback. After opening of an L-type Ca2+ channel each SR Ca2+ release site capable of generating a Ca2+ spark is activated with a specific probability, which may depend on a variety of variables, including regulatory mechanisms and pathophysiological or diseased states. Many of these variables can thus affect EC coupling by changing the Ca2+ spark trigger probability. Indeed, a reduced Ca2+ spark trigger probability has been identified as a cause underlying impaired EC coupling in myocytes from hypertrophied and failing rat hearts (Gomez et al. 1997).
Among possible mechanisms that may affect the Ca2+ sensitivity of the RyRs, and thus the Ca2+ spark trigger probability, variations of SR Ca2+ content and regulatory changes due to protein phosphorylation after β-adrenergic stimulation have received much attention recently. On the basis of receptor number, the β1-adrenergic receptor (β1AR) is the predominant β-receptor subtype in cardiac ventricular myocytes and couples to stimulatory Gs proteins, which leads to activation of adenylyl cyclase after receptor activation. Adenylyl cyclase synthesizes the second messenger cyclic adenosine monophosphate (cAMP), which increases the activity of protein kinase A (PKA). PKA phosphorylates many substrates, several of which play an essential role in Ca2+ signalling: (1) phosphorylation of L-type Ca2+ channels enhances the Ca2+ current, thus increasing both the trigger signal for CICR and the extent of SR loading with Ca2+ (Reuter, 1983); (2) phosphorylation of phospholamban (PLB) relieves its inhibitory effect on the SERCA, subsequently stimulating the Ca2+ pump, again increasing the Ca2+ load of the SR (Lindemann et al. 1983; James et al. 1989; Shannon et al. 2001; Bers, 2002); (3) it has recently been reported that PKA could also directly phosphorylate the RyRs, possibly inducing dissociation of calstabin-2 (formerly called FKBP12.6) and increasing RyR open probability (Lu et al. 1995; Valdivia et al. 1995; Marx et al. 2000). Dissociation of calstabin-2 may subsequently disrupt coupled gating of the RyRs (Marx et al. 2001). Generally, PKA-mediated phosphorylation of the RyRs is thought to increase the Ca2+ sensitivity of the release channels. Conversely, several research groups found that RyRs were not phosphorylated (Jiang et al. 2002), or, when phosphorylated, the RyR open probability appeared to remain unchanged (Li et al. 2002). In addition, others have found that RyR phosphorylation by Ca2+-calmodulin-dependent kinase II (CaMKII) induced stimulatory or inhibitory effects in lipid bilayers and profound stimulatory effects in freshly isolated myocytes (Takasago et al. 1991; Hain et al. 1995; Lokuta et al. 1995; Li et al. 1997; Wang et al. 2004).
In intact cells changes of CICR after β-adrenergic stimulation could also result from increased SR Ca2+ load. Increased Ca2+ load leads to more Ca2+ release by the law of mass action (Fabiato, 1985; Bassani et al. 1995; Tripathy & Meissner, 1996; Santana et al. 1997; Satoh et al. 1997; Sitsapesan & Williams, 1997; Györke & Györke, 1998; Frank et al. 2000; Lukyanenko et al. 2001). In addition, the Ca2+ concentration inside the SR may have a regulatory effect on CICR by modulating the Ca2+ sensitivity of the RyRs, either via a Ca2+ receptor directly located on the luminal side of the RyRs (Györke & Györke, 1998; but see Tripathy & Meissner, 1996) or by involving a signalling pathway comprising calsequestrin as a Ca2+ sensor and one or more small accessory junctional SR proteins, such as triadin and junctin (Guo & Campbell, 1995; Zhang et al. 1997; Györke et al. 2004). In the failing heart, Ca2+ regulation is proposed to be one of several key players in the altered contractility (Houser et al. 2000; Hasenfuss & Pieske, 2002), but many other processes are affected too, such as structural changes, altered protein expression and decreased PKA-dependent phosphorylation of several proteins (Marks et al. 2002). Furthermore, heart failure may be associated with RyR hyperphosphorylation, possibly leading to dissociation of calstabin-2 from the RyRs and resulting in an increased SR Ca2+ leak, which may finally decrease SR Ca2+ content (Shou et al. 1998; Marx et al. 2000; Prestle et al. 2001). Ultimately, diminished SR Ca2+ content may then cause smaller Ca2+ transients and reduced cardiac force.
In the present study we found a strong correlation between global cellular Ca2+ release amplitude and SR Ca2+ load when the trigger signal was a spatially homogeneous photolytic Ca2+ transient induced by a UV flash. In contrast, highly localized Ca2+ release signals generated with diffraction-limited two-photon photolysis were less sensitive to SR Ca2+ load. In particular, larger localized Ca2+ release signals were recorded after β-adrenergic stimulation, even in conditions where the SR Ca2+ content was reduced. Based on this discrepancy between global and local Ca2+ signals we conclude that, in addition to the secondary effect via changes of luminal Ca2+, β-adrenergic stimulation may change the gating properties of the RyRs themselves, either directly or indirectly. These findings have been presented in preliminary form to the Biophysical Society (Lindegger & Niggli, 2002).
Methods
Isolation of guinea-pig myocytes
Cardiac ventricular myocytes were isolated from adult male guinea-pigs using established enzymatic methods (DelPrincipe et al. 1999). All animal handling procedures were performed with the permission of the State Veterinary Administration and according to Swiss Federal Animal handling law. Guinea-pigs were killed by cervical dislocation, the hearts rapidly removed and mounted on a Langendorff system and retrogradely perfused with a Ca2+-free solution at 37°C for about 5 min (Mitra & Morad, 1985). For enzymatic digestion, collagenase type 2 (0.12 mg ml−1, Worthington, Switzerland) and protease type XIV (0.04 mg ml−1, Sigma, Switzerland) were added to the perfusion solution for another 3–5 min. After digestion, the ventricles were cut into small pieces, placed on a gently rotating shaker in a solution containing 200 μm Ca2+ and kept at room temperature until use.
Solutions
For the experiments cells were transferred into a chamber mounted on the stage of an inverted microscope. The extracellular superfusion solution contained (mm): NaCl 140, KCl 5, CaCl2 1.8, CsCl 1, BaCl2 0.5, Hepes 10, glucose 10, pH 7.4 (adjusted with NaOH). Cells were continuously superfused using a custom-made rapid superfusion system (t1/2 < 500 ms). Where indicated, isoproterenol (isoprenaline, Iso) 1 μm ([−]-N-iso-propyl-l-noradrenaline hydrochloride; Sigma) was added from a frozen stock (1 mm in 10% ascorbic acid). Cyclopiazonic acid (CPA, 10 μm; Sigma) was added to block the SERCA and to avoid loading of the SR. Short applications of 20 mm caffeine (Sigma) were used to estimate SR Ca2+ content. In some control experiments the SR function was inhibited using 1 μm thapsigargin and 10 μm ryanodine (both from Alamone Laboratories, Jerusalem, Israel). The pipette-filling solution contained (mm): caesium aspartate 120, TEA-Cl 20, Na4-DM-nitrophen 2 (Calbiochem, La Jolla, CA, USA), reduced glutathione (GSH) 1, CaCl2 0.5, K2-ATP 5, Hepes 10 and K5-fluo-3 0.05 (TefLabs, Austin, TX, USA), pH 7.2 (adjusted with CsOH). All experiments were carried out at room temperature (21°C).
Voltage clamp
Electrodes were pulled from filamented borosilicate glass capillaries (GC150F, Clark Electromedical Instruments, Pangbourne, UK) on a horizontal puller (DMZ, Zeitz Instrumente, Augsburg, Germany) to a series resistance of 1–2 MΩ. Cells were voltage-clamped in the whole-cell configuration of the patch-clamp technique and held at a resting potential of −70 mV or −40 mV using an Axopatch 200 amplifier (Axon Instruments, Foster City, CA, USA). Potentials were not corrected for the junction potential, which was calculated to be ∼12 mV for our pipette filling solution. The SR and cytosolic DM-nitrophen were loaded with Ca2+ by a variable number of L-type Ca2+ currents with voltage steps to 0 mV or +10 mV lasting 200 ms. Currents were digitized at 3 kHz using an A/D converter and custom-written data acquisition software developed by us under LabView (National Instruments, Ennetbaden, Switzerland) running on an Apple Macintosh G3 computer. Membrane current and voltage data were stored on a hard disk for later analysis using IgorPro software (WaveMetrics, Lake Oswego, OR, USA).
Confocal Ca2+ imaging
Cells were imaged with a 40 × oil-immersion objective lens (Fluor, N.A. = 1.3; Nikon) and loaded with fluo-3 by dialysis through the recording pipette. Fluo-3 was excited with the 488 nm line of an argon-ion laser (ILT-5000, Ion Laser Technology, Salt Lake City, UT, USA) at 50–150 μW on the cell. The fluorescence was detected at 540 ± 15 nm with a confocal laser-scanning microscope (MRC 1000, Bio-Rad, Glattbrugg, Switzerland). The amplitude and the time course of cytosolic Ca2+ transients were computed off-line using customized versions of either NIH Image or Image SXM and expressed as normalized fluorescence (F/F0). Resting [Ca2+] for experiments made in pipette filling solution (Fig. 1) was 20 nm and Kd was 400 nm (DelPrincipe et al. 1999). Mean Ca2+ concentration profiles were extracted from fluorescence images and calculated with IgorPro software using an established self-ratio calibration procedure (Cheng et al. 1993). Two-photon release signals were estimated as the maximal amplitude (mean of 5 points) minus the mean fluorescence during 100 ms prior to photolysis. Values were normalized to the maximal peak amplitude obtained in caffeine (Figs 5 and 6). Means of the normalized signals were plotted versus two-photon power and a sigmoidal function was fitted to the data. Under our conditions the fluo-3 fluorescence was not significantly quenched by the application of 20 mm caffeine (by 0.54 ± 0.16% of control, n = 2). Furthermore, the photolytic two-photon photolysis (TPP) Ca2+ signals remained unaffected by 20 mm caffeine.
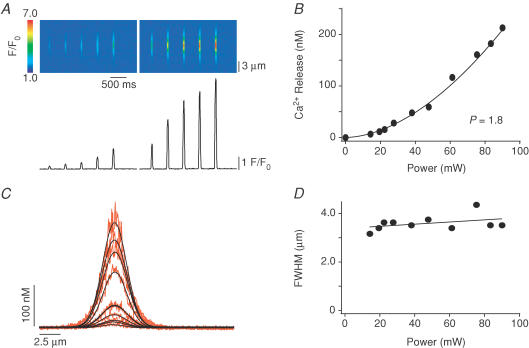
Figure 1. Two-photon photolysis in droplet.
A, characteristic TPP power–response relationship obtained in pipette filling solution. By increasing the TPP power, more Ca2+ is released from the caged compound. B, when plotting the amplitude of the Ca2+ signal versus the photolytical power, data displayed a power dependence with an exponent of 1.8, suggesting a two-photon excitation process. C, horizontal spreading by diffusion of local Ca2+ signals of increasing amplitude. D, no strong dependence of the full width at half-maximal amplitude (FWHM) of the local Ca2+ signals versus power was found.
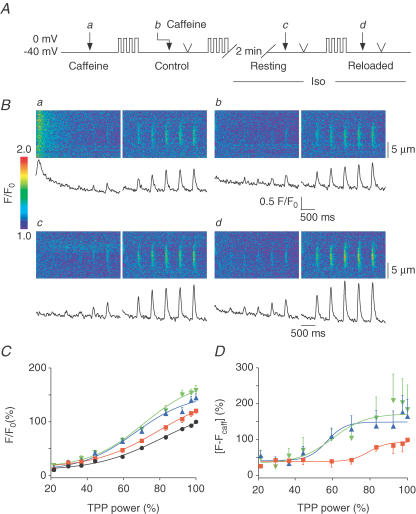
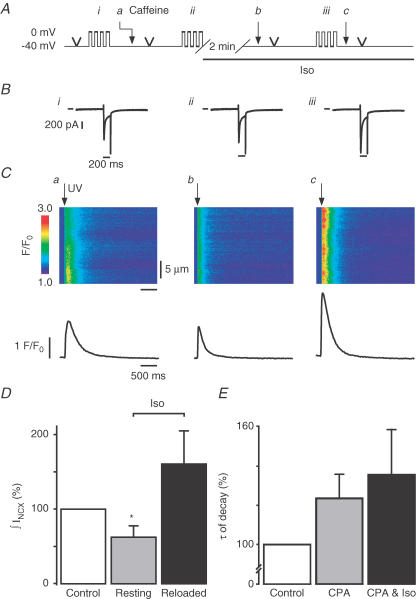
Figure 5. The amplitude of local Ca2+ signals is increased after β-adrenergic stimulation.
A, protocol used to obtain the power–response relationships in conditions similar to those of Fig. 4. The cells were held at −40 mV and diffraction-limited Ca2+ releases of increasing power were triggered in caffeine (Aa), in control (Ab), after a similar loading protocol and 2 min rest in Iso (Ac) and after 4 consecutive depolarizations in Iso (Ad ). B, line-scan images and averaged Ca2+ traces recorded during diffraction-limited releases. Ca2+ signals recorded in caffeine were used to estimate the amount of photolysis (Ba). Local Ca2+ signals were larger after 4 depolarizations in control (Bb) and further increased after Iso superfusion (Bc and Bd), independently of the global SR Ca2+ load (Fig. 4). C, normalized amplitude of Ca2+ signals for n = 6 cells in caffeine (•, 100% for the highest power), in control (▪, 120.4 ± 4.7%, n = 6), in Iso (▴, 144.5% ± 7.4%, n = 6) and after reloading in Iso (▾, 158.5% ± 9.0%, n = 6) are plotted versus power and fitted with sigmoidal functions. D, same data as in C but after subtraction of the photolytical component (signals in caffeine). CICR signals obtained in control (▪, 100%, n = 4, for the largest release) were significantly larger after Iso superfusion, whether cells were resting (▴, 164.6 ± 47.7%, n = 5) or being reloaded (▾, 184.2 ± 67.6%, n = 6).
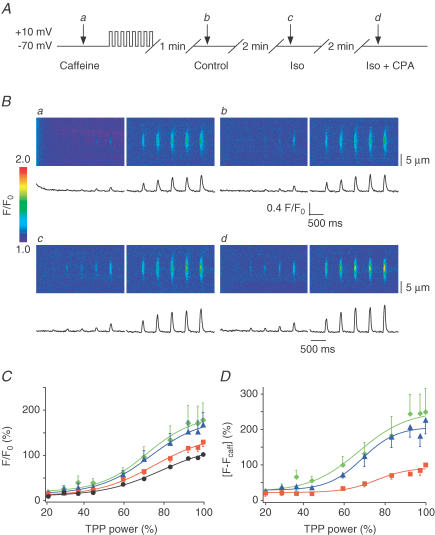
Figure 6. Enlargement of local Ca2+ signals is not prevented by superfusion of CPA.
A, cells were held at −70 mV and a first TPP power–response relationship was triggered in caffeine to determine the amplitude of photoreleased Ca2+ signals (Aa). Cells were then reloaded by eight 200 ms depolarizing steps to +10 mV and after 1 min rest in control, a TPP power–response relationship was recorded (Ab). After 2 min superfusion in Iso another power–response relationship was measured (Ac), which was followed by 2 min of superfusion with Iso and CPA (10 μm), after which a last TPP power–response relationship was elicited (Ad). B, data obtained from one cell: Ba, line-scan images and traces obtained in caffeine; Bb, in control; Bc, in Iso; and Bd, in Iso and CPA. C, plot of the normalized amplitude of Ca2+ release versus TPP power in caffeine (•, 100% for the maximal trigger, n = 14), control (▪, 126.8 ± 10.3%, n = 14), in Iso (▴, 163.7 ± 25.7%, n = 14) and in Iso with CPA (♦, 173.7 ± 36.8%, n = 11). D, same data as in C but after subtraction of the photolytical component (control: ▪, 100%, n = 11; Iso: ▴, 226.4 ± 39.3%, n = 10; and Iso + CPA: ♦, 249.2 ± 66.1%, n = 9).
Global UV flash and local two-photon photolysis of caged Ca2+ compounds
Photolytic Ca2+ concentration jumps were elicited with UV flashes from a xenon short-arc flash lamp (duration ∼400 μs, discharged energy up to 230 J) (Kaplan & Ellis-Davies, 1988). UV flashes were applied in an epi-illumination arrangement to trigger global and spatially homogenous Ca2+ releases from the entire SR (for details see DelPrincipe et al. 1999). In order to generate highly localized and diffraction limited photolytic Ca2+ sources, we used TPP of caged Ca2+ (DM-nitrophen) (Lipp & Niggli, 1998; DelPrincipe et al. 1999). The beam of a mode-locked titanium sapphire laser (Mira 900, Coherent) tuned to 710 nm, with < 100 fs pulse length at 80 MHz repetition rate, was guided through the camera port of the confocal microscope to produce a stationary diffraction-limited spot within the myocyte, parfocal with the plane of fluorescence detection. The excitation point spread function was determined to extend over ∼710 nm (full width at half-maximal amplitude; FWHM) in the x–y-direction and 1200 nm in the z-direction (Lipp & Niggli, 1998). Photolysis was elicited by the opening of a mechanical shutter (Uniblitz, Vincent Associates, Rochester, NY, USA). The shutter opening was set for a duration of 60 ms for all experiments, and the interval between subsequent shutter openings was 430 ms. Both the shutter and the UV flash were synchronized to the pixel-clock of the laser scanner to coerce synchronization with the image acquisition and the voltage-clamp recording system. The power of the two-photon laser was attenuated by means of a neutral density filter and an adjustable linear polarization filter, both placed in series. In order to rapidly estimate the Ca2+ dependence of the CICR process and to analyse signals of comparable amplitude before and after β-adrenergic stimulation, a filter wheel holding nine neutral density filters (Lambda 10-2, Sutter Instruments, Novato, CA, USA) was placed in the optical pathway. The filter wheel was controlled by a Motorola 68HC12 microcontroller, running software written by us under the LEMPS development system (GIBB, Bern, Switzerland). Using this system, a TPP power–response relationship could be measured within 6 s, as shown in an experiment where DM-nitrophen was photolysed in a droplet of pipette filling solution (Fig. 1). Ca2+ signals elicited by TPP in pipette solution did not saturate and displayed a power dependence with an exponent of 1.8, suggesting a two-photon process. In addition, this relationship implies that the amplitude of the photolytic Ca2+ release signals is proportional to the Ca2+ release flux. The width (FWHM) increased only slightly, consistent with the minimal power dependence of the volume excited by TPP, as determined by recording the fluorescent point spread function (PSF) in fluoresceine (not shown). Each power–response plot includes 10 different power levels (as a percentage of full power): 16, 22, 25, 31, 42, 53, 68, 84, 93 and 100, respectively.
Statistics
Data are expressed as mean ±s.e.m, and n represents the number of analysed cells. Significance was tested with Student's t test and is denoted as * (P < 0.05) or ** (P < 0.02). For estimates of CICR (Figs 5 and 6), recordings in which local Ca2+ signals at maximal photolytic power were not larger in control than in the presence of caffeine (i.e. contained only photolytic Ca2+ release and no CICR component) were excluded from the analysis. Furthermore, data where the photolytic signal amplitude at maximal laser power was less then 2 times the noise (s.e.m.) of the resting Ca2+ signal were not taken into account.
Results
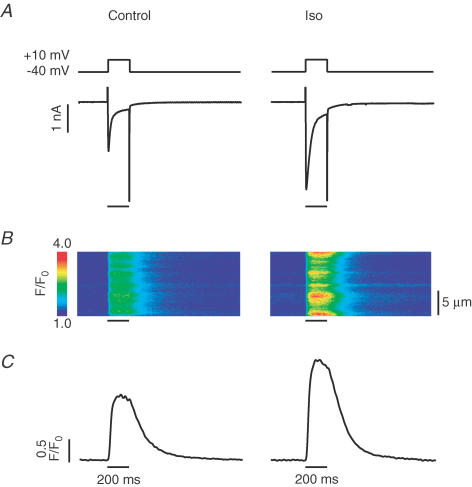
In initial experiments we verified that the entire β-adrenergic signalling and second messenger cascade were present and functional under the conditions of our experiments. Successful β-adrenergic stimulation was also confirmed at the end of each subsequent experiment. After 2 min of 1 μm isoproterenol (Iso) superfusion, the time needed to get a robust β-adrenergic stimulation in most cells, L-type Ca2+ currents elicited by depolarizations from −40 mV to +10 mV were considerably larger than in control (Fig. 2A). This resulted in an increase of the Ca2+ transient, as can be seen on the line-scan images and the traces averaged from the line-scans (Fig. 2B and C).
Figure 2. β-Adrenergic stimulation increases the amount of Ca2+ entry via L-type Ca2+ channels.
A, voltage protocol and recorded current. Left: cells were held at −40 mV and depolarized to +10 mV for 200 ms to activate L-type Ca2+ current. Right: same protocol after 2 min Iso superfusion. Entry of Ca2+ was larger thereby enhancing the subsequent CICR. B, line-scan images recorded during the depolarizing step. C, averages of the line-scan images in B, expressed as normalized fluorescence.
Activation of global cellular CICR with UV flash photolysis
In all subsequent experiments involving global cellular Ca2+ transients, we applied flash photolysis of caged Ca2+ to activate and examine CICR, since this is a trigger signal which itself is not affected by β-adrenergic stimulation, unlike the L-type Ca2+ current. To ensure a comparable and intermediate SR Ca2+ content, a specific loading protocol was carried out before each UV flash. It consisted of an initial SR emptying with a puff of caffeine, followed by a train of four L-type Ca2+ currents (depolarizing steps from −40 mV to 0 mV for 200 ms; Fig. 3A). To examine how CICR was affected by β-adrenergic stimulation while excluding the L-type Ca2+ current as the trigger signal, we applied UV flashes after 2 min at rest, either under control conditions or in the presence of Iso. Contrary to our expectations, the amplitude of the Ca2+ transient did not become larger after 2 min treatment with Iso. It even decreased to 62.3 ± 16.1% (n = 7, P < 0.05) of the control amplitude (Fig. 3Cb and D). This decline was even more surprising since loading protocols with trains of L-type Ca2+ currents were carefully kept identical for both, control and Iso-treated cells, to avoid any alterations of SR Ca2+ content (Fig. 3Bi and ii).
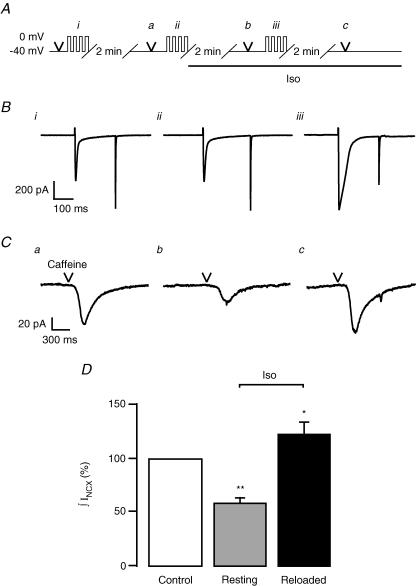
Figure 3. Whole-cell Ca2+ release signals are smaller after rest in Iso.
A, voltage protocol. Caffeine (20 mm; arrowhead), briefly applied to empty the SR, was followed by 4 consecutive depolarizations from −40 mV to 0 mV for 200 ms to moderately load the SR (Ai). After 2 min a UV flash was triggered to induce a global and homogenous Ca2+ release from the SR (Aa) and immediately followed by another short application of caffeine. Four similar depolarizations were again applied (Aii) and followed by superfusion of Iso. After 2 min rest another UV flash (Ab) was triggered, followed by a short application of caffeine. Finally, 4 depolarizations were applied during β-adrenergic stimulation (Aiii) and followed by a UV flash (Ac). B, the third L-type Ca2+ current of a train of four consecutive depolarizations: Bi, in control: Bii, in control and before Iso application for 2 min: and Biii, during β-adrenergic stimulation. Line indicates 0 pA. C, line-scan images acquired during the UV flashes. Ca, control; Cb, resting in Iso; and Cc, stimulated in Iso. D, normalized amount of extruded Ca2+ (∫INCX) in control (100%), after rest in Iso (62.3 ± 16.1%, n = 7, P < 0.05) and after reloading in Iso (160.4 ± 45.4%, n = 7). E, decays of global fluorescence signals were fitted with a monoexponential function and τ was normalized to control. As expected CPA prolonged the decay to 124 ± 12.5%, n = 3 and to 136 ± 23.7%, n = 4, in the continued presence of CPA and Iso.
Thus we wondered in what respect our flash photolytic experiments differed from the more physiological situation relying on L-type Ca2+ currents as triggers for CICR. First, our trigger signal was, on purpose, not increased by Iso. Second, we had chosen conditions to avoid exposing the SR to augmented Ca2+ currents during the loading protocol, in order to maintain the SR Ca2+ loads similar for both experiments. Could it be that the larger L-type Ca2+ currents are a prerequisite to obtain more release from the store, by either representing a more efficient trigger signal for CICR or by providing more Ca2+ influx for reloading the SR? To answer these questions and to distinguish between the two possibilities we slightly modified our loading protocol and started the application of Iso 2 min before performing the SR Ca2+ loading protocol. Or, in other words, we loaded the SR with larger Ca2+ currents (Fig. 3Biii) while keeping the trigger signal constant (i.e. the photolytic [Ca2+] jump). As it turned out, the amount of Ca2+ released was indeed larger than in control (160.4 ± 45.2%, n = 7; Fig. 3C and D). In addition to the increased L-type current after β-adrenergic stimulation used to reload the SR, SERCA stimulation could be seen during the decay of the signals. The maximal rate of decay increased to 137 ± 11.3% (n = 7) after 2 min rest in Iso and to 118 ± 12.6% (n = 7) after reloading in Iso. In guinea-pigs, cytosolic Ca2+ removal is more dependent on Na+–Ca2+ exchange than, for example, in rat (Sham et al. 1995). Thus the effect of SERCA stimulation is less pronounced. Finally, as reported recently (Ginsburg & Bers, 2004), the maximal rate of Ca2+ release was indeed larger when cells were reloaded in Iso (147.8 ± 53.4%, n = 7). As expected, superfusion of 10 μm CPA prolonged the decay of the Ca2+ signal to 124 ± 12.5% and to 136 ± 23.7% in the presence of CPA and CPA plus Iso, respectively (Fig. 3E). But how can we explain the reduced Ca2+ signal amplitude after β-adrenergic stimulation, despite the identical SR Ca2+ loading protocol? First, run-down of L-type Ca2+ channels, occuring within a time window of 20–30 min, could not be held responsible for the decreased loading as shown in Fig. 3Bi and ii. Smaller Ca2+ transients could also result from a decrease in the Ca2+ sensitivity of the RyRs or from an acceleration of the SR Ca2+ loss occurring during the 2 min rest in Iso. The first possibility seemed less likely after β-adrenergic stimulation. To evaluate changes of SR Ca2+ content occurring during the resting period, we used the same loading protocol as above, but instead of triggering CICR with UV flashes, short puffs of caffeine were applied to completely empty the SR (Fig. 4). The resulting membrane currents reflecting electrogenic Ca2+ removal via the Na+–Ca2+ exchanger (INCX) were integrated to estimate SR Ca2+ content (Trafford et al. 1998). Interestingly, the SR contained significantly less Ca2+ when resting for 2 min in Iso (58.6 ± 7.3%, n = 6, P < 0.02) than when resting in control solution (100%, n = 10; Fig. 4C and D). However, when the cells were Ca2+ loaded with L-type Ca2+ currents increased by β-adrenergic stimulation, the SR Ca2+ content was raised to 123.0 ± 11.7% (n = 10, P < 0.05). Thus, the amplitude of the global Ca2+ signals observed after triggering CICR with identical photolytic triggers mainly reflected the SR Ca2+ content prevailing under the various conditions. The reduced SR Ca2+ content after a resting period in Iso is an interesting finding by itself and could be the consequence of an increased SR Ca2+ leak, possibly resulting from an enhanced Ca2+ sensitivity of the RyRs after phosphorylation (Marx et al. 2000). This leak was presumably small since no significant change in resting [Ca2+] was measured between control and Iso (see below).
Figure 4. SR Ca2+ content governs the amplitude of whole-cell Ca2+ signals.
A, protocol used to estimate total SR Ca2+ content. Short puffs of caffeine were applied in control (Aa), after a similar loading protocol and 2 min rest in Iso (Ab) and after reloading during β-adrenergic stimulation (Ac). B, third L-type Ca2+ current of a train of four consecutive depolarizations: Bi, in control; Bii, in control and before Iso application for 2 min; and Biii, during β-adrenergic stimulation. C, caffeine-induced NCX currents recorded in the 3 different conditions described above (Ca, Cb and Cc, respectively) were integrated to estimate the SR Ca2+ load. D, normalized ∫INCX: control (100%, n = 10), resting in Iso (58.6 ± 7.3%, n = 6, P < 0.02) and stimulated in Iso (123 ± 11.7%, n = 10, P < 0.05).
Local activation of CICR with two-photon photolysis
We have previously observed that Ca2+ refilling of the SR occurs much faster after local activation than after global activation of CICR, presumably because a rapid redistribution of Ca2+ within the SR network takes place after functional Ca2+ depletion of a highly confined Ca2+ release unit only (i.e. one junctional SR structure near a dyad) (DelPrincipe et al. 1999). Thus, localized Ca2+ release might be expected to behave differently than global cellular signals, possibly by being less dependent on the global SR Ca2+ content. Thus, local signals might be more sensitive to changes of RyR gating. Therefore, we next elicited highly localized photolytic Ca2+ signals by TPP of DM-nitrophen (Fig. 5), while following an SR loading protocol analogous to the one used for UV flash experiments (Fig. 3). TPP power–response relationships in six cells (Fig. 5B) were recorded in order to obtain local Ca2+ signals of varying amplitudes under each condition (i.e. in caffeine, in the presence or absence of Iso, at low and high SR Ca2+ load). Signals of various amplitudes were required for later comparison of the signals and their properties, which may depend on the signal amplitude itself. As in our previous studies using two-photon photolytic activation of CICR (Lipp & Niggli, 1998; DelPrincipe et al. 1999), the first series of TPP power–response relationships was obtained in caffeine to estimate the photolytic component of the Ca2+ signals (normalized to the highest two-photon power applied, n = 6). Caffeine, in our hands, did not interfere with the TPP signals and the quench of fluo-3 fluorescence was negligible (−0.5 ± 0.2%, n = 2). After reloading the SR with four depolarizing steps another TPP power–response relationship was recorded in control solution (120.4 ± 4.7%, n = 6) and immediately followed by a short caffeine application to empty the SR. After SR reloading and a subsequent resting period of 2 min in Iso, another TPP power–response relationship was recorded. During these 2 min at rest, the SR Ca2+ content was known to decay more in Iso than in control solution, as we found above (see Fig. 4). Surprisingly however, despite reduced SR Ca2+ content and much unlike global Ca2+ signals, the local Ca2+ release signals were larger during β-adrenergic stimulation than in control (144.5 ± 7.4%, n = 6). When the SR was reloaded during β-adrenergic stimulation to an extent that was above control, only a small and not significant further increase of the signal amplitude was found, despite the higher SR Ca2+ load under these conditions (to 158.5 ± 9.0%, n = 6). The weak effect of the reloaded SR on the local signals was expected since cells were kept at low SR Ca2+ load to avoid the appearance of regenerative Ca2+ signal spreading. A more prominent and significant increase in the signal amplitude has been observed with higher SR Ca2+ loads, such as after 12 prepulses (Lipp & Niggli, 1998). To estimate the Iso effect on SR Ca2+ release the photolytical component obtained in caffeine was subtracted from the total signals obtained for each trigger in control and in Iso at low and high SR Ca2+ load. After normalization to the CICR amplitude obtained for the maximal trigger in control (100%, n = 4), data were plotted versus power (Fig. 5D) and fitted with a sigmoidal function. Interestingly, CICR started at lower power levels and was larger in Iso whether the SR was depleted (164.6 ± 47.7%, n = 5) or reloaded (184.2 ± 67.6%, n = 6). Taken together, these findings suggest that β-adrenergic stimulation with Iso enhanced local SR Ca2+ release irrespective of global SR Ca2+ content, much unlike the behaviour of global Ca2+ transients triggered with UV flashes.
What could be responsible for this discrepancy between global and local Ca2+ signals? Could we derive conclusions regarding the underlying mechanism from this peculiar behaviour? First we excluded possible Ca2+ sources other than the SR by confirming the ryanodine sensitivity of the signal amplification by Iso (data not shown). Given that the source of the Ca2+ seemed to be the SR, only two basic possibilities remained: (1) either the SR Ca2+ content was elevated above the remainder of the SR network at the site where we performed photolysis or (2) local Ca2+ release from the SR was larger despite the reduced SR Ca2+ content and regardless of the smaller Ca2+ gradient across the SR membrane. The latter possibility would require a change of the gating or Ca2+ permeability of the RyRs. Because of the slow kinetics of the TPP (Ca2+ source lasting for 60 ms) one could imagine that some of the photoreleased Ca2+ could be locally transported into the SR. The extent of this local uptake would depend on the activity of the SERCA. When more Ca2+ is pumped into the SR, the local SR Ca2+ content could increase above the remainder of the SR network, which could explain our observations of enhanced local Ca2+ releases from the SR in the presence of Iso. To test for the possibility of local Ca2+ uptake we applied CPA to inhibit the SERCA. CPA would prevent the local amplification of the TPP Ca2+ signals if it were mediated by the SERCA. Thus, the following experiments were carried out (Fig. 6): a first TPP power–response relationship was obtained during superfusion of caffeine. After SR reloading another series of Ca2+ signals was recorded, and this was repeated after treatment with Iso. Again, local Ca2+ signals were larger during β-adrenergic stimulation than in control and the signals obtained in the presence of functioning CICR were significantly larger than in caffeine, as expected. Finally, the SERCA was blocked by 2 min superfusion of 10 μm CPA in the presence of Iso. As it turned out, CPA could not prevent the amplification of CICR by β-adrenergic stimulation (Fig. 6B). Figure 6C presents data obtained from 14 cells and normalized to the local release signal recorded in caffeine for the maximal TPP power (100%). The Ca2+ signal recorded in control was 126.8 ± 10.3%, demonstrating the presence of the biological signal amplification by CICR. The signal obtained during Iso superfusion had an amplitude of 163.7 ± 25.7% (n = 14), and in the presence of both CPA and Iso, the amplitude was 173.7 ± 36.8% (n = 11). After subtraction of the photolytical Ca2+ release component, it became evident that CICR was increased to 226.4 ± 39.3% (n = 10) by Iso and remained unaffected at 249.2 ± 66.1% (n = 9) when the SERCA was inhibited (Fig. 6D). This set of data suggests that CPA could not suppress the amplification of local CICR by β-adrenergic stimulation. To confirm successful inhibition of the SERCA by CPA in these experiments, decay rates after UV flash-triggered Ca2+ transients were analysed (Fig. 3E) confirming successful inhibition of the SERCA by CPA.
Finally changes in resting Ca2+ were not significant between control and Iso (2.5 ± 5.3%, n = 14) or between control and Iso + CPA (7.7 ± 10.5%, n = 11), suggesting that cytosolic Ca2+ did not interfere with the increased local signals. In summary, these findings indicate that local loading of the SR with Ca2+ did not play an important role in the amplification of the TPP release signals by β-adrenergic stimulation. Thus, the increased TPP Ca2+ fluorescent signals were resulting from more Ca2+ being released from the SR despite reduced Ca2+ content.
Discussion
The notion of a cardiac Ca2+ signalling system relying on the recruitment of functionally independent elementary signalling events (Ca2+ sparks) has opened the door for a new concept of EC coupling incorporating a probabilistic paradigm, whereby each Ca2+ spark event is triggered under local control by an L-type Ca2+ channel with a specific non-zero probability (Cheng et al. 1993; Lipp & Niggli, 1998; Niggli, 1999). Such an arrangement not only allows for a new understanding of modulatory changes of EC coupling, but also for a novel mechanism of EC coupling failure under pathological conditions, whereby the Ca2+ spark trigger probability could be altered by modulatory, metabolic and pathophysiological mechanisms (Gomez et al. 1997; Marx et al. 2000; Pacher et al. 2002; Isaeva & Shirokova, 2003). Two mechanisms are thought to be very important for this type of regulation: the Ca2+ content of the SR (Terentyev et al. 2003) and the phosphorylation of various Ca2+ signalling proteins (Takasago et al. 1991; Simmerman & Jones, 1998; Marx et al. 2000). These two regulatory pathways are expected to exhibit a high degree of cross-talk because of at least two reasons: (1) functional changes of Ca2+ signalling proteins after phosphorylation will affect SR Ca2+ content (Hussain & Orchard, 1997); (2) changes of Ca2+ concentrations may alter the extent of phosphorylation, for example through the CaMKII pathway.
In the present study we used a combination of biophysical techniques in an attempt to dissect these two regulatory pathways, for example by using photolysis of caged compounds as a trigger for CICR to remove some variables (e.g. changes of ICa after phosphorylation of L-type Ca2+ channels). With this combination of approaches we were able to simplify the system, eliminate some variables while controlling and measuring others. By comparing features of global (i.e. whole-cell) Ca2+ fluorescent signals with those of subcellularly localized signals (i.e. diffraction-limited two-photon photorelease signals) we could derive conclusions about the relative significance of either mechanism for CICR (i.e. the SR Ca2+ load or the phosphorylation of Ca2+ signalling proteins) under the two conditions.
Whole-cell signals are dominated by SR Ca2+ load
It is known that an increase in luminal Ca2+ can enhance the Ca2+ sensitivity on the cytosolic side of RyRs reconstituted in lipid bilayers (Sitsapesan & Williams, 1997). This finding may also be related to the generation of spontaneous Ca2+ sparks and Ca2+ waves in isolated cardiac myocytes under conditions of SR Ca2+ overload (Wier et al. 1987; Lukyanenko et al. 1999; Terentyev et al. 2003). In contrast, a decrease of SR Ca2+ content is thought to control termination and refractory behaviour of CICR in cardiac myocytes (DelPrincipe et al. 1999; Sobie et al. 2002; Terentyev et al. 2002; Szentesi et al. 2004). Our data show that whole-cell Ca2+ signals are largely governed by the SR Ca2+ load, a feature which possibly masks subtle effects due to changes of RyR Ca2+ sensitivity arising from phosphorylation. Indeed, irrespective of how many RyRs open before or after their phosphorylation, only a specific releasable fraction of SR Ca2+ will be liberated into the cytosol (previously determined to be typically 57% under our conditions (DelPrincipe et al. 1999)). Thus, the number of opening RyRs will only affect the kinetics of SR Ca2+ release, but not increase the amount of Ca2+ that can be released (unless CICR would be more exhaustive after RyR phosphorylation, for which there is little evidence). Thus, with or without β-adrenergic stimulation, the extent of whole-cell SR Ca2+ release is dominated (i.e. limited) by SR Ca2+ content and by processes which terminate CICR by functional SR Ca2+ depletion.
Local signals reveal effects of β-adrenergic stimulation
Interestingly, the present study revealed that subcellularly localized SR Ca2+ signals exhibited a strikingly different behaviour than whole-cell signals. Even though local Ca2+ signals also depend somewhat on the SR Ca2+ load, particularly at more elevated SR Ca2+ loads (Lipp & Niggli, 1998; DelPrincipe et al. 1999), the localized Ca2+ releases are not expected to significantly lower the global SR Ca2+ content. Since SR Ca2+ release only occurs from one or a few functional units of the SR, we think that local depletion of intra-SR Ca2+ will be less pronounced, since neighbouring SR sites do not release and Ca2+ can, in fact, rapidly diffuse to the active sites within the SR network. Thus, under these conditions more open channels allow more Ca2+ to be released (Sobie et al. 2002), even at reduced SR Ca2+ content. Thus, the declining global SR Ca2+ load no longer terminates the release flux, contrary to the whole-cell activation. Thus, TPP signals might be a suitable technique to allow observation of localized SR Ca2+ release process modulation.
Estimates of CICR
Subtraction of fluorescent signals obtained in caffeine from signals in control, Iso or Iso and CPA was used to estimate the amount of CICR. As seen in Figs 5D and 6D, a threshold seems to appear at about 70–80% of the maximal trigger power in control, while it is shifted toward smaller triggers in Iso or in Iso and CPA. For the maximal trigger, Iso approximately doubled the caffeine-insensitive component (165 to 249%); as for the lowest trigger power range no clear separation could be made. This suggests that very low photolytic power may not detectably increase the open probability of the RyRs at the low SR Ca2+ load used in this study, thereby triggering no CICR. Finally, the sigmoidal function fit to the data point tends to become more shallow and saturate. In the droplet, saturation occurs at very high powers and is thought to result from depletion of DM-nitrophen within the diffraction limited excited volume. In a droplet this is expected to occur at higher photolytic power levels than in cells, because DM-nitrophen diffusion may not be entirely free in the cytosol. In addition, inside living cells many other factors may also contribute to saturation and shallow release functions, including mobile and stationary Ca2+ buffers and saturation of CICR.
Conclusion
In conclusion, we found that when CICR was activated in a synchronized way throughout the cell, the Ca2+ signals were predominantly governed by SR Ca2+ content, thus defining the amplitude of the resulting Ca2+ transient. Although β-adrenergic stimulation may lead to synchronization of SR Ca2+ release channels (Song et al. 2001; Viatchenko-Karpinski & Györke, 2001; Ginsburg & Bers, 2004), this will not necessarily affect the amount of global Ca2+ release from the SR. This observation on the global level is therefore consistent with the notion that whole-cell SR Ca2+ release is terminated by emptying of the Ca2+ store, leading to deactivation of the RyRs by reducing their Ca2+ sensitivity (DelPrincipe et al. 1999; Terentyev et al. 2002; Szentesi et al. 2004). However, when CICR was activated on a local level (i.e. with TPP), the system behaved in a completely different and apparently opposite way, that cannot be explained by global changes in SR Ca2+ content. Insensitivity of these unexpected signals to inhibitors of the SERCA also suggested that these effects were not mediated by increased local SR Ca2+ loading via the SERCA that could, in principle, occur before and during the photorelease process. Increased local Ca2+ releases were thus observed regardless of the decreased SR content, suggesting a direct modulatory effect of the β-adrenergic signalling cascade on the SR Ca2+ release pathway. Increased Ca2+ release from the SR despite reduced Ca2+ content can only occur when the open probability of the RyRs is somehow increased, either by activation of more channels or by changing the gating properties of the channels (or both). Based on these observations, we conclude that increased local Ca2+ release signals are the result of the PKA-dependent phosphorylation of a Ca2+ signalling protein other than the L-type Ca2+ channels, phospholamban and the SR Ca2+ pump, and possibly the ryanodine receptors themselves (or other SR proteins).
Acknowledgments
We would like to thank the Swiss National Science Foundation (grant 61344 to E.N.), the Swiss Cardiovascular Research and Training Network (SCRTN) and the Swiss Federal Office for Science and Technology (B.B.W.) for support. We also appreciate the comments on the manuscript by Christophe Pignier and technical assistance by Daniel Lüthi.
References
- Bassani JW, Yuan W, Bers DM. Fractional SR Ca2+ release is regulated by trigger Ca2+ and SR Ca2+ content in cardiac myocytes. Am J Physiol. 1995;268:C1313–1319. doi: 10.1152/ajpcell.1995.268.5.C1313. [DOI] [PubMed] [Google Scholar]
- Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- Cheng H, Lederer WJ, Cannell MB. Calcium sparks: Elementary events underlying excitation-contraction coupling in heart muscle. Science. 1993;262:740–744. doi: 10.1126/science.8235594. [DOI] [PubMed] [Google Scholar]
- DelPrincipe F, Egger M, Niggli E. Calcium signalling in cardiac muscle: Refractoriness revealed by coherent activation. Nat Cell Biol. 1999;1:323–329. doi: 10.1038/14013. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J General Physiol. 1985;85:291–320. doi: 10.1085/jgp.85.2.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank K, Tilgmann C, Shannon TR, Bers DM, Kranias EG. Regulatory role of phospholamban in the efficiency of cardiac sarcoplasmic reticulum Ca2+ transport. Biochemistry. 2000;39:14176–14182. doi: 10.1021/bi001049k. [DOI] [PubMed] [Google Scholar]
- Ginsburg KS, Bers DM. Modulation of excitation–contraction coupling by isoproterenol in cardiomyocytes with controlled SR Ca2+ load and ICa trigger. J Physiol. 2004;556:463–480. doi: 10.1113/jphysiol.2003.055384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez AM, Valdivia HH, Cheng H, Lederer MR, Santana LF, Cannell MB, et al. Defective excitation-contraction coupling in experimental cardiac hypertrophy and heart failure. Science. 1997;276:800–806. doi: 10.1126/science.276.5313.800. [DOI] [PubMed] [Google Scholar]
- Guo W, Campbell KP. Association of triadin with the ryanodine receptor and calsequestrin in the lumen of the sarcoplasmic reticulum. J Biol Chem. 1995;270:9027–9030. doi: 10.1074/jbc.270.16.9027. [DOI] [PubMed] [Google Scholar]
- Györke I, Györke S. Regulation of the cardiac ryanodine receptor channel by luminal Ca2+ involves luminal Ca2+ sensing sites. Biophys J. 1998;75:2801–2810. doi: 10.1016/S0006-3495(98)77723-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Györke I, Hester N, Jones LR, Györke S. The role of calsequestrin, triadin, and junctin in conferring cardiac ryanodine receptor responsiveness to luminal calcium. Biophys J. 2004;86:2121–2128. doi: 10.1016/S0006-3495(04)74271-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hain J, Onoue H, Mayrleitner M, Fleischer S, Schindler H. Phosphorylation modulates the function of the calcium release channel of sarcoplasmic reticulum from cardiac muscle. J Biol Chem. 1995;270:2074–2081. doi: 10.1074/jbc.270.5.2074. [DOI] [PubMed] [Google Scholar]
- Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- Houser SR, Piacentino V, Weisser J. Abnormalities of calcium cycling in the hypertrophied and failing heart. J Mol Cell Cardiol. 2000;32:1595–1607. doi: 10.1006/jmcc.2000.1206. [DOI] [PubMed] [Google Scholar]
- Hussain M, Orchard CH. Sarcoplasmic reticulum Ca2+ content, L-type Ca2+ current and the Ca2+ transient in rat myocytes during β-adrenergic stimulation. J Physiol. 1997;505:385–402. doi: 10.1111/j.1469-7793.1997.385bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaeva EV, Shirokova N. Metabolic regulation of Ca2+ release in permeabilized mammalian skeletal muscle fibres. J Physiol. 2003;547:453–462. doi: 10.1113/jphysiol.2002.036129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James P, Inui M, Tada M, Chiesi M, Carafoli E. Nature and site of phospholamban regulation of the Ca2+ pump of sarcoplasmic reticulum. Nature. 1989;342:90–92. doi: 10.1038/342090a0. [DOI] [PubMed] [Google Scholar]
- Jiang MT, Lokuta AJ, Farrell EF, Wolff MR, Haworth RA, Valdivia HH. Abnormal Ca2+ release, but normal ryanodine receptors, in canine and human heart failure. Circ Res. 2002;91:1015–1022. doi: 10.1161/01.res.0000043663.08689.05. [DOI] [PubMed] [Google Scholar]
- Kaplan JH, Ellis-Davies GC. Photolabile chelators for the rapid photorelease of divalent cations. Proc Natl Acad Sci U S A. 1988;85:6571–6575. doi: 10.1073/pnas.85.17.6571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Kranias EG, Mignery GA, Bers DM. Protein kinase a phosphorylation of the ryanodine receptor does not affect calcium sparks in mouse ventricular myocytes. Circ Res. 2002;90:309–316. doi: 10.1161/hh0302.105660. [DOI] [PubMed] [Google Scholar]
- Li L, Satoh H, Ginsburg KS, Bers DM. The effect of Ca2+-calmodulin-dependent protein kinase II on cardiac excitation–contraction coupling in ferret ventricular myocytes. J Physiol. 1997;501:17–31. doi: 10.1111/j.1469-7793.1997.017bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindegger N, Niggli E. Estimation of EC-coupling efficiency in vivo. Biophys J. 2002;82:72A. [Google Scholar]
- Lindemann JP, Jones LR, Hathaway DR, Henry BG, Watanabe AM. β-adrenergic stimulation of phospholamban phosphorylation and Ca2+-ATPase activity in guinea pig ventricles. J Biol Chem. 1983;258:464–471. [PubMed] [Google Scholar]
- Lipp P, Niggli E. Fundamental calcium release events revealed by two-photon excitation photolysis of caged calcium in guinea-pig cardiac myocytes. J Physiol. 1998;508:801–809. doi: 10.1111/j.1469-7793.1998.801bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokuta AJ, Rogers TB, Lederer WJ, Valdivia HH. Modulation of cardiac ryanodine receptors of swine and rabbit by a phosphorylation–dephosphorylation mechanism. J Physiol. 1995;487:609–622. doi: 10.1113/jphysiol.1995.sp020904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Xu L, Meissner G. Phosphorylation of dihydropyridine receptor II–III loop peptide regulates skeletal muscle calcium release channel function. Evidence for an essential role of the beta-OH group of Ser687. J Biol Chem. 1995;270:18459–18464. doi: 10.1074/jbc.270.31.18459. [DOI] [PubMed] [Google Scholar]
- Lukyanenko V, Subramanian S, Györke I, Wiesner TF, Györke S. The role of luminal Ca2+ in the generation of Ca2+ waves in rat ventricular myocytes. J Physiol. 1999;518:173–186. doi: 10.1111/j.1469-7793.1999.0173r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukyanenko V, Viatchenko-Karpinski S, Smirnov A, Wiesner TF, Györke S. Dynamic regulation of sarcoplasmic reticulum Ca2+ content and release by luminal Ca2+-sensitive leak in rat ventricular myocytes. Biophys J. 2001;81:785–798. doi: 10.1016/S0006-3495(01)75741-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marks AR, Reiken S, Marx SO. Progression of heart failure: Is protein kinase a hyperphosphorylation of the ryanodine receptor a contributing factor? Circulation. 2002;105:272–275. [PubMed] [Google Scholar]
- Marx SO, Gaburjakova J, Gaburjakova M, Henrikson C, Ondrias K, Marks AR. Coupled gating between cardiac calcium release channels (ryanodine receptors) Circ Res. 2001;88:1151–1158. doi: 10.1161/hh1101.091268. [DOI] [PubMed] [Google Scholar]
- Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): Defective regulation in failing hearts. Cell. 2000;101:365–376. doi: 10.1016/s0092-8674(00)80847-8. [DOI] [PubMed] [Google Scholar]
- Mitra R, Morad M. A uniform enzymatic method for dissociation of myocytes from hearts and stomachs of vertebrates. Am J Physiol. 1985;249:H1056–1060. doi: 10.1152/ajpheart.1985.249.5.H1056. [DOI] [PubMed] [Google Scholar]
- Niggli E. Localized intracellular calcium signaling in muscle: Calcium sparks and calcium quarks. Annu Rev Physiol. 1999;61:311–335. doi: 10.1146/annurev.physiol.61.1.311. [DOI] [PubMed] [Google Scholar]
- Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: Miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci U S A. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestle J, Janssen PM, Janssen AP, Zeitz O, Lehnart SE, Bruce L, et al. Overexpression of FK506-binding protein FKBP12.6 in cardiomyocytes reduces ryanodine receptor-mediated Ca2+ leak from the sarcoplasmic reticulum and increases contractility. Circ Res. 2001;88:188–194. doi: 10.1161/01.res.88.2.188. [DOI] [PubMed] [Google Scholar]
- Reuter H. Calcium channel modulation by neurotransmitters, enzymes and drugs. Nature. 1983;301:569–574. doi: 10.1038/301569a0. [DOI] [PubMed] [Google Scholar]
- Santana LF, Kranias EG, Lederer WJ. Calcium sparks and excitation–contraction coupling in phospholamban deficient mouse ventricular myocytes. J Physiol. 1997;503:21–29. doi: 10.1111/j.1469-7793.1997.021bi.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh H, Blatter LA, Bers DM. Effects of [Ca2+]i, SR Ca2+ load, and rest on Ca2+ spark frequency in ventricular myocytes. Am J Physiol. 1997;272:H657–668. doi: 10.1152/ajpheart.1997.272.2.H657. [DOI] [PubMed] [Google Scholar]
- Sham JS, Hatem SN, Morad M. Species differences in the activity of the Na+–Ca2+ exchanger in mammalian cardiac myocytes. J Physiol. 1995;488:623–631. doi: 10.1113/jphysiol.1995.sp020995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon TR, Chu G, Kranias EG, Bers DM. Phospholamban decreases the energetic efficiency of the sarcoplasmic reticulum Ca2+ pump. J Biol Chem. 2001;276:7195–7201. doi: 10.1074/jbc.M007085200. [DOI] [PubMed] [Google Scholar]
- Shou W, Aghdasi B, Armstrong DL, Guo Q, Bao S, Charng MJ, et al. Cardiac defects and altered ryanodine receptor function in mice lacking FKBP12. Nature. 1998;391:489–492. doi: 10.1038/35146. [DOI] [PubMed] [Google Scholar]
- Simmerman HK, Jones LR. Phospholamban: Protein structure, mechanism of action, and role in cardiac function. Physiol Rev. 1998;78:921–947. doi: 10.1152/physrev.1998.78.4.921. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R, Williams AJ. Regulation of current flow through ryanodine receptors by luminal Ca2+ J Membr Biol. 1997;159:179–185. doi: 10.1007/s002329900281. [DOI] [PubMed] [Google Scholar]
- Sobie EA, Dilly KW, dos Santos Cruz J, Lederer WJ, Jafri MS. Termination of cardiac Ca2+ sparks: An investigative mathematical model of calcium-induced calcium release. Biophys J. 2002;83:59–78. doi: 10.1016/s0006-3495(02)75149-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song LS, Wang SQ, Xiao RP, Spurgeon H, Lakatta EG, Cheng H. β-adrenergic stimulation synchronizes intracellular Ca2+ release during excitation-contraction coupling in cardiac myocytes. Circ Res. 2001;88:794–801. doi: 10.1161/hh0801.090461. [DOI] [PubMed] [Google Scholar]
- Stern MD. Theory of excitation-contraction coupling in cardiac-muscle. Biophys J. 1992;63:497–517. doi: 10.1016/S0006-3495(92)81615-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szentesi P, Pignier C, Egger M, Kranias EG, Niggli E. Sarcoplasmic reticulum Ca2+ refilling controls recovery from Ca2+-induced Ca2+ release refractoriness in heart muscle. Circ Res. 2004;95:807–813. doi: 10.1161/01.RES.0000146029.80463.7d. [DOI] [PubMed] [Google Scholar]
- Takasago T, Imagawa T, Furukawa K, Ogurusu T, Shigekawa M. Regulation of the cardiac ryanodine receptor by protein kinase-dependent phosphorylation. J Biochem (Tokyo) 1991;109:163–170. doi: 10.1093/oxfordjournals.jbchem.a123339. [DOI] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Györke I, Volpe P, Williams SC, Györke S. Calsequestrin determines the functional size and stability of cardiac intracellular calcium stores: Mechanism for hereditary arrhythmia. Proc Natl Acad Sci. 2003;100:11759–11764. doi: 10.1073/pnas.1932318100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terentyev D, Viatchenko-Karpinski S, Valdivia HH, Escobar AL, Györke S. Luminal Ca2+ controls termination and refractory behavior of Ca2+-induced Ca2+ release in cardiac myocytes. Circ Res. 2002;91:414–420. doi: 10.1161/01.res.0000032490.04207.bd. [DOI] [PubMed] [Google Scholar]
- Trafford AW, Diaz ME, Eisner DA. Measurement of sarcoplasmic reticulum Ca2+ content and sarcolemmal fluxes during the transient stimulation of the systolic Ca2+ transient produced by caffeine. Ann N Y Acad Sci. 1998;853:368–371. doi: 10.1111/j.1749-6632.1998.tb08302.x. [DOI] [PubMed] [Google Scholar]
- Tripathy A, Meissner G. Sarcoplasmic reticulum lumenal Ca2+ has access to cytosolic activation and inactivation sites of skeletal muscle Ca2+ release channel. Biophys J. 1996;70:2600–2615. doi: 10.1016/S0006-3495(96)79831-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdivia HH, Kaplan JH, Ellis-Davies GC, Lederer WJ. Rapid adaptation of cardiac ryanodine receptors – modulation by Mg2+ and phosphorylation. Science. 1995;267:1997–2000. doi: 10.1126/science.7701323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viatchenko-Karpinski S, Györke S. Modulation of the Ca2+-induced Ca2+ release cascade by β-adrenergic stimulation in rat ventricular myocytes. J Physiol. 2001;533:837–848. doi: 10.1111/j.1469-7793.2001.t01-1-00837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhu W, Wang S, Yang D, Crow MT, Xiao RP, et al. Sustained beta1-adrenergic stimulation modulates cardiac contractility by Ca2+/calmodulin kinase signaling pathway. Circ Res. 2004;95:798–806. doi: 10.1161/01.RES.0000145361.50017.aa. [DOI] [PubMed] [Google Scholar]
- Wier WG, Cannell MB, Berlin JR, Marban E, Lederer WJ. Cellular and subcellular heterogeneity of [Ca2+]i in single heart-cells revealed by fura-2. Science. 1987;235:325–328. doi: 10.1126/science.3798114. [DOI] [PubMed] [Google Scholar]
- Zhang L, Kelley J, Schmeisser G, Kobayashi YM, Jones LR. Complex formation between junctin, triadin, calsequestrin, and the ryanodine receptor. Proteins of the cardiac junctional sarcoplasmic reticulum membrane. J Biol Chem. 1997;272:23389–23397. doi: 10.1074/jbc.272.37.23389. [DOI] [PubMed] [Google Scholar]