Abstract
In young normotensive subjects, parental hypertension is associated with stiffening of the carotid artery and reduction in cardiovagal outflow and baroreflex gain. In subjects without parental hypertension regular exercise training was found to attenuate age-related reduction in carotid compliance and baroreflex gain. The aim of the present study was to test the hypothesis that regular physical activity is associated with better parameters of carotid artery elasticity, increased cardiovagal outflow and higher baroreflex gain in normotensive offspring of hypertensive parents. We studied 98 healthy, sedentary or endurance exercise trained subjects (49 men, 18–27 years of age) with or without family history of hypertension (FH+ and FH−, respectively) in a cross-sectional design. In the sedentary group spontaneous baroreflex indices (sequence method and spectral techniques) were lower in FH+ subjects than in their FH− peers, while in trained subjects these indices were not different between FH+ and FH−. Furthermore, in the FH+ group trained subjects had higher baroreflex indices than their sedentary peers, while in the FH− group no significant differences were found. Carotid compliance and distensibility coefficient (echo-tracking ultrasound and applanation tonometry) were not different in FH− sedentary and trained subjects, but were higher in FH+ trained subjects as compared to their sedentary peers. Significant but modest relationships were found between spontaneous baroreflex indices and carotid artery elastic parameters across all subjects. Our present data indicate that in subjects with parental hypertension aerobic exercise training is associated with higher levels of cardiovagal outflow and baroreflex gain, which finding, however, is not explained by greater elasticity of the carotid artery.
Essential hypertension is associated with impaired arterial baroreflex regulation of heart rate (Bristow et al. 1969; Mancia et al. 1978). The precise mechanism underlying this abnormality is not known, but stiffening of the barosensory vessel wall was suggested to play a role by reducing baroreceptor responsiveness to changes in pressure (Andresen, 1984; Lage et al. 1993). In normotensive offspring of hypertensive parents several abnormalities characteristic of hypertension have been reported. They are heavier (Blonde et al. 1981), have elevated blood pressure (Blonde et al. 1981; Burke et al. 1991) and produce exaggerated blood pressure responses to exercise (Matthews et al. 1998). There are also observations that indicate decreased carotid elasticity (Meaney et al. 1999) and impaired baroreflex sensitivity (Iwase et al. 1984; Parmer et al. 1992) in such offspring.
Several epidemiological studies have found that exercise training lowers the incidence of cardiovascular disease (American College of Sports Medicine, 1993). Recent data suggest that one of the mechanisms underlying this protective effect may be the attenuation of the age-related decline in both cardiovagal baroreflex gain (Monahan et al. 2000) and carotid artery elasticity (Tanaka et al. 2000; Monahan et al. 2001) by aerobic exercise. The aim of the present study was to test the hypothesis that regular physical activity is associated with better parameters of carotid artery elasticity and with higher levels of cardiovagal autonomic indices in normotensive offspring of hypertensive parents. We therefore compared carotid artery elastic parameters and time- and frequency-domain spontaneous indices of autonomic function in young sedentary and trained subjects with or without parental hypertension.
Methods
Subjects
We studied 98 subjects aged 18–27 years, who were normotensive (brachial pressure < 130/80 mmHg), non-obese (BMI < 25 kg m−2), non-smokers, without medication, and free of cardiovascular disease. The subjects were students of two faculties of the Semmelweis University: ‘sedentary’ subjects were recruited from the Faculty of Medicine, and ‘trained’ subjects from the Faculty of Physical Education and Sport Sciences. Trained subjects performed aerobic exercise, such as football, handball, basketball, volleyball, waterpolo, tennis, swimming, running, kayak-canoeing and cycling. They had been training in aerobic sports for 10.4 ± 1.1 years during 5.3 ± 0.3 sessions/week for ≥ 60 min session−1 (mean ± 1SD). The subjects had either a positive family history of hypertension (FH+), where one or both parents were hypertensive and/or on antihypertensive treatment, or a negative family history (FH−), where both parents were normotensive. From our 48 FH+ subjects 43 had one and five had two parents with hypertension. The maternal and paternal health status and blood pressure were verified by general practice records, which were not older than 3 months at the time of our measurements. None of the parents had a record of any other cardiovascular disease or diabetes mellitus. There was no difference between the parents' ages in the groups. Subjects gave written consent to participate in the study, which was approved by the Ethical Committee of the Semmelweis University and conformed to the Declaration of Helsinki.
Peak oxygen consumption
Aerobic capacity was determined in each subject by measurement of peak oxygen consumption (peak 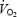 ) by online computer-assisted open-circuit spirometry (Jaeger Dataspir Analyser, Firma Jaeger, Aachen, Germany) during incremental treadmill exercise (Jaeger 6000 EL, Firma Jaeger, Aachen, Germany).
) by online computer-assisted open-circuit spirometry (Jaeger Dataspir Analyser, Firma Jaeger, Aachen, Germany) during incremental treadmill exercise (Jaeger 6000 EL, Firma Jaeger, Aachen, Germany).
Blood pressure measurements
For determination of spontaneous indices of autonomic function, radial artery pressure was monitored continuously with an automated tonometric device (Colin CBM-7000, ADInstuments Ltd, Hastings, UK); this technology has been validated with intra-arterial pressure measurements (Zorn et al. 1997). During data collection the servo-reset mechanism of the Colin apparatus was turned off to permit continuous data acquisition. Systolic and diastolic blood pressure values measured on the brachial artery by an automatic microphonic sphygmomanometer built in the Colin device were used to calibrate the radial pressure pulse.
Common carotid artery pressure was measured by applanation tonometry (SPT-301, Millar Instruments, Houston, TX, USA) for determination of carotid elastic parameters. The carotid pulse wave recording was calibrated by using brachial diastolic and the electronically determined mean radial pressure values. Diastolic brachial pressure was assigned to the minimum value of the carotid pressure pulse wave and mean pressure to its electrically averaged value. This calibration of the tonometric signal was based on the assumption that mean pressure did not change in large conduit arteries and that diastolic pressure was not substantially different in the brachial and carotid arteries (Kelly & Fitchett, 1992).
Carotid ultrasonography
Common carotid artery diameter, its change with the arterial pressure pulse and carotid intima-media thickness (IMT) were measured 1.5 cm proximal to the bifurcation by ultrasonography using a vessel wall-tracking system (WTS) combined with a conventional ultrasound scanner (7.5 MHz linear array, Scanner 200 Pie Medical, Maastricht, the Netherlands) previously described in detail (Hoeks et al. 1990, 1997).
Pulse wave velocity
Aortic pulse wave velocity (PWV) was determined from carotid and femoral pressure waveforms obtained by applanation tonometry (SPT-301, Millar Instruments) using the SphygmoCor system (SCOR, PWV Medical, Sydney, Australia). Waveforms were referenced to a concurrent ECG and carotid to femoral transit time (ΔT) was computed from the foot to foot difference of their waveforms. After the waveform collection, the distance between carotid and femoral sampling sites was determined from three measurements: (1) midpoint of the sternal notch to the lower edge of the umbilicus, (2) lower edge of the umbilicus to the femoral artery sampling site, and (3) midpoint of the sternal notch to the carotid sampling site using a standard tape measure. The third value was subtracted from the sum of the first two. PWV was calculated by dividing the time component by the distance component.
Other measurements
RR-intervals were measured from R wave threshold crossings on continuously recorded ECGs and respiration was recorded with an inductive system (Respitrace System, Ambulatory Monitoring Inc., Ardsley, NY, USA).
Protocol
Subjects were studied in the morning hours under standardized conditions, in a quiet room at a comfortable temperature. All fasted at least 2 h before testing and were asked to refrain from strenuous exercise or consumption of alcohol- or caffeine-containing beverages for 24 h before the study. Upon arrival at the investigation unit the subjects were equipped with measurement devices, and then rested supine for about 15 min until heart rate and mean blood pressure trends demonstrated satisfactory baseline conditions. They were asked to synchronize their respiratory rate to a metronome beating at 0.25 Hz. RR-interval and radial artery pressure were recorded continuously for a 10-min period to determine spontaneous cardiovagal autonomic indices. Then carotid artery tonometric pressure on the right side and diameter and wall thickness on the left side were recorded simultaneously in five epochs, each containing four to eight distension pulses. The latter recordings were used to determine carotid artery elastic parameters.
Data analysis
Carotid artery elastic parameters
Carotid compliance (CC) and distensibility (DC) coefficients were calculated according to the following formulae:
where ΔD, D and ΔP represent pulsatile distension, end-diastolic diameter and carotid artery pulse pressure, respectively (van Bortel et al. 2002). Diameter and pressure data obtained during the recording epochs were averaged to give a mean value for each subject.
Cardiac-vagal activity (CVA)
Time and frequency domain measures of heart rate variability were used to assess the level of CVA. From 10 min recordings of RR-intervals the following CVA indices were calculated using the WinCPRS program (Absolute Aliens Oy, Turku, Finland): the standard deviation of RR-intervals (NNSD), the root mean square of successive differences (RMSSD), the percentage of successive RR-intervals which differed by more than 50 ms (pNN50), and low (0.05–0.15 Hz) and high frequency (0.15–0.4 Hz) power of RR-interval variability (LF and HF, respectively).
Spontaneous baroreflex indices
The coupling between spontaneous fluctuations in heart rate and systolic pressure was determined by the sequence method and also by spectral analysis (Parati et al. 2000). Recordings of 10 min duration were digitized and analysed with the WinCPRS program using a sampling rate of 500 Hz and stored in a personal computer for subsequent off-line analysis. The software detected the ECG R-wave and computed RR-interval (RRI) and radial artery systolic blood pressure (SBP) time series and identified spontaneously occurring sequences in which SBP and RRI concurrently increased or decreased over three or more consecutive beats. Minimal accepted change was 1 mmHg for SBP and 5 ms for RRI. Sequence indices were calculated from up–up (Seq+) and down–down (Seq−) sequences as the slope of the regression line between SBP and RRI. Only sequences with a correlation coefficient > 0.85 were considered. To determine spectral indices, the signals were interpolated and resampled, and their power spectra were determined using FFT-based methods. The mean value of the α function (the square root of the ratio of the spectral powers of RRI and SBP), considering only those frequency components in the low frequency band (LF: 0.05–0.15 Hz) where the coherence was > 0.5, was taken as the α coefficient (LFα). We also determined low-frequency transfer function gain (LFgain), which expresses RRI and SBP cross-spectral magnitude in the 0.05–0.15 Hz frequency band, where coherence is greater than 0.5.
The investigators and technicians, who analysed the data, were blinded as they were presented only the code name of the computer files.
Statistical analysis
Data are expressed as mean ± 1 s.e.m. Group comparisons were made by two-way ANOVA (physical activity status and family history status) with post hoc Tukey analysis. Relationships between variables were determined by simple linear regression analysis. Significance was accepted at P < 0.05. Statistical analysis was performed by the SigmaStat for Windows version 2.03 program package (SPSS Inc., Chicago, IL, USA).
Results
Subjects' anthropometric and haemodynamic data are given in Table 1. Sex distribution, age, body mass index, and diastolic and systolic blood pressure were not different in the four groups due to appropriate subject selection. Trained subjects had significantly higher peak 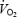 and lower heart rate as compared to sedentary subjects. Family history status had no influence on these parameters.
and lower heart rate as compared to sedentary subjects. Family history status had no influence on these parameters.
Table 1.
Anthropometric and haemodynamic parameters of sedentary and trained young subjects without (FH−) and with (FH+) a family history of hypertension
| Sedentary | Trained | |||
|---|---|---|---|---|
| FH− | FH+ | FH− | FH+ | |
| n | 26 | 24 | 24 | 24 |
| Male/female | 13/13 | 12/12 | 12/12 | 12/12 |
| Age (years) | 22 ± 1 | 22 ± 1 | 21 ± 1 | 21 ± 1 |
| BMI (kg m−2) | 21.0 ± 0.4 | 21.2 ± 0.6 | 20.8 ± 0.4 | 22.4 ± 0.3 |
| DBP (mmHg) | 65 ± 2 | 63 ± 2 | 65 ± 2 | 64 ± 2 |
| SBPb (mmHg) | 113 ± 3 | 112 ± 2 | 114 ± 2 | 110 ± 3 |
| SBPc (mmHg) | 105 ± 3 | 103 ± 2 | 104 ± 3 | 102 ± 2 |
| ΔP (mmHg) | 40 ± 1 | 40 ± 1 | 39 ± 1 | 38 ± 1 |
| HR (beats min−1) | 69 ± 3 | 72 ± 2 | 62 ± 2* | 62 ± 2* |
Peak 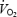 (ml kg−1 min−1) (ml kg−1 min−1) |
49.1 ± 1.5 | 45.4 ± 1.3 | 56.0 ± 1.7* | 56.6 ± 2.1* |
n, number of subjects; BMI, body mass index; DBP, carotid diastolic pressure; SBPb, brachial systolic pressure; SBPc, carotid systolic pressure; ΔP, carotid pulse pressure; HR, heart rate; peak 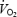 , peak oxygen consumption. Data are given as the mean ± 1 s.e.m.
, peak oxygen consumption. Data are given as the mean ± 1 s.e.m.
P < 0.05 versus sedentary subjects with the same family history.
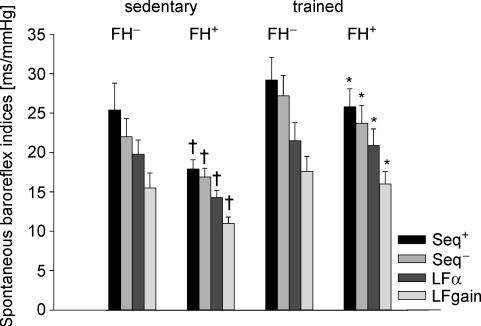
Differences in spontaneous baroreflex indices associated with physical activity and family history of hypertension are illustrated in Fig. 1. In the sedentary group baroreflex indices were lower in FH+ subjects than in their FH− peers. On the other hand, in the trained group no difference in the autonomic indices was found between FH+ and FH− subjects. Furthermore, in the FH+ group trained subjects had higher baroreflex indices than their sedentary peers, while in the FH− group no significant differences were found.
Figure 1. Time- and frequency-domain spontaneous baroreflex indices of sedentary and trained young subjects without (FH−) and with (FH+) a family history of hypertension.
Seq+, spontaneous sequence index calculated from up–up sequences; Seq−, spontaneous sequence index calculated from down–down sequences; LFα, α coefficient in the low frequency range; LFgain, cross-spectral transfer gain in the low-frequency range. Data are given as mean ± 1 s.e.m. *P < 0.05 versus sedentary subjects with the same family history; †P < 0.05 versus FH− subjects with the same physical activity status.
Differences in cardiac vagal activity (CVA) indices were similar to those in spontaneous baroreflex indices (Table 2). Baroreflex indices were closely related to CVA indices (adjusted r-values fell between 0.15 and 0.62, P < 0.001).
Table 2.
Measures of cardiac vagal activity of sedentary and trained young subjects without (FH−) and with (FH+) a family history of hypertension
| Sedentary | Trained | |||
|---|---|---|---|---|
| FH− | FH+ | FH− | FH+ | |
| NNSD (ms) | 62 ± 5 | 51 ± 3 | 78 ± 5* | 70 ± 5* |
| RMSSD (ms) | 63 ± 8 | 47 ± 4 | 77 ± 8 | 65 ± 6* |
| pNN50 (%) | 32 ± 5 | 21 ± 4 | 43 ± 5 | 37 ± 5* |
| LF (ms2) | 794 ± 107 | 541 ± 83 | 1067 ± 198 | 997 ± 168* |
| HF (ms2) | 1663 ± 386 | 862 ± 157 | 1965 ± 412 | 1485 ± 363 |
NNSD, standard deviation of RR-intervals; RMSSD, root mean square of successive RR-interval differences; pNN50, percent of RR-intervals, which differ more than 50 ms; HF, high (0.15–0.4 Hz) frequency power of RR-interval variability; LF, low (0.05–0.15 Hz) frequency power of RR-interval variability. Data are given as the mean ± 1 s.e.m.
P < 0.05 versus sedentary subjects with the same family history sedentary trained.
Carotid dimensions and elastic variables are given in Table 3. Carotid artery diameter and intima-media thickness (IMT) were not different in the four groups, while pulsatile distension was less in FH+ sedentary subjects as compared to the other three groups. In the sedentary group carotid and aortic elastic parameters were poorer in FH+ than in FH− with the difference being significant for compliance (CC) and approaching significance for distensibility (DC) (P = 0.072), while in the trained group no such differences were found. Furthermore, in the FH+ group the elastic parameters were better in trained subjects as compared to their sedentary peers, while in the FH− group no differences were found.
Table 3.
Carotid artery dimensions and elastic variables of sedentary and trained young subjects without (FH−) and with (FH+) a family history of hypertension
| Sedentary | Trained | |||
|---|---|---|---|---|
| FH− | FH+ | FH− | FH+ | |
| DD (µm) | 6228 ± 99 | 6013 ± 85 | 6314 ± 115 | 6199 ± 103 |
| ΔD (µm) | 780 ± 31 | 677 ± 32† | 777 ± 31 | 759 ± 32 |
| CC (µm mmHg−1) | 20.0 ± 0.9 | 17.3 ± 0.9† | 20.4 ± 0.9 | 20.4 ± 0.7* |
| DC (10−3 mmHg−1) | 6.4 ± 0.3 | 5.8 ± 0.3 | 6.4 ± 0.2 | 6.6 ± 0.2* |
| PWV (ms−1) | 6.0 ± 0.1 | 6.4 ± 0.3 | 6.0 ± 0.2 | 5.8 ± 0.1* |
| IMT (µm) | 519.0 ± 16 | 507 ± 11 | 517 ± 14 | 504 ± 13 |
DD, end-diastolic diameter; ΔD, pulsatile distension; CC, compliance coefficient; DC, distensibility coefficient; PWV, pulse wave velocity of the aorta; IMT, intima-media thickness. Data are given as the mean ± 1 s.e.m.
P < 0.05 versus sedentary subjects with the same family history;
P < 0.05 versus FH subjects with the same physical activity status.
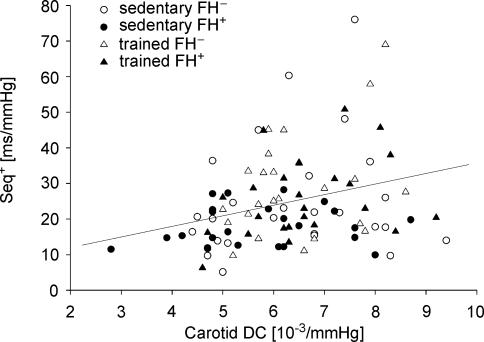
Significant, although modest, correlations were found between carotid artery elastic variables and spontaneous baroreflex indices across all subjects: CC was related to LFα and Seq− (r = 0.21 and r = 0.23, respectively, P < 0.05), while DC was related to LFα, Seq+ and Seq− (r = 0.27, r = 0.28 and r = 0.28, respectively, P < 0.01) (Fig. 2).
Figure 2. Relationship between carotid artery distensibility coefficient (DC) and spontaneous sequence index calculated from up-up sequences (Seq+) across sedentary and trained young subjects without (FH−) and with (FH+) a family history of hypertension.
Seq+ = 7.57 + 2.70 × DC, r = 0.28, P < 0.01.
Discussion
In the present study we compared spontaneous autonomic function indices and carotid artery elastic parameters in young, normotensive, sedentary and trained subjects with and without a family history of hypertension. The main new finding of the study is that in FH+ subjects regular aerobic exercise training is associated with higher levels of cardiovagal outflow and baroreflex gain as compared to the sedentary lifestyle, to the extent that no significant differences exist in spontaneous baroreflex indices between FH− and trained FH+ subjects. The higher values of spontaneous baroreflex indices were not importantly determined by increased carotid artery elastic parameters, but were significantly related to increased cardiovagal activity.
In a recent twin study, Tank et al. (2001) found that baroreflex sensitivity was strongly influenced by genetic variance in the healthy human. In hypertensive families Iwase et al. (1984) were the first to report reduced baroreflex sensitivity in FH+ children. Parmer et al. (1992) confirmed this observation, and suggested that the reduction in baroreflex sensitivity might have an aetiological role in the hypertension developing later on. It has also been shown that family history of hypertension was the strongest predictor of baroreflex sensitivity in both normotensive and hypertensive subjects determined by multiple linear regression analysis including age, mean arterial pressure, body weight and race as independent variables (Parmer et al. 1992). Our present data confirm this work, as in our sedentary subjects we observed lower spontaneous baroreflex indices in FH+ than in FH− subjects. However, our findings also indicate that reduced cardiovagal outflow and baroreflex gain found in FH+ offspring may improve with exercise training.
In our group of young healthy subjects exercise training was not associated with higher cardiovagal baroreflex sensitivity, presumably due to a ceiling effect (Monahan et al. 2000). On the other hand, both in elderly subjects (Monahan et al. 2000, 2001) and in cardiovascular patients (Iellamo et al. 2000; La Rovere et al. 2002) exercise training was associated with increased baroreflex gain. Hypertension and ageing are known to be associated with similar impairment in cardiovagal autonomic function (Eckberg & Sleight, 1992), and this may be related to our finding that in FH+ subjects physical exercise was associated with higher values of spontaneous baroreflex indices.
The mechanism by which exercise training is associated with higher levels of cardiovagal outflow and baroreflex gain in FH+ subjects is not clear. Improvement may occur through increased barosensory vessel compliance, leading to enhancement of facilitatory baroreceptor input to vagal motoneurones. Although a causative relationship between changes in carotid elasticity and in baroreflex gain has never been proven experimentally, several observations support this concept. In young, healthy subjects direct proportionality was found between carotid distensibility and baroreflex gain (Bonyhay et al. 1996), and exercise training was shown to attenuate age-related declines in both carotid elasticity and baroreflex gain (Monahan et al. 2000, 2001; Tanaka et al. 2000). In the present study we found that in the FH+ group carotid compliance and distensibility were higher and aortic pulse wave velocity was lower in trained than in sedentary subjects. Although changes in spontaneous baroreflex indices and carotid artery elasticity ran nearly parallel in the different groups of subjects, less than 10% of the variability in spontaneous baroreflex indices was explained by differences in carotid artery elastic parameters.
Exercise training may affect the neural component of the baroreflex arc, which encompasses central integration of baroreceptor input, efferent vagal outflow and sinoatrial node responsiveness. The neural component can be assessed directly as the change in RR-interval in response to unit change in barosensory vessel diameter during phenylephrine-induced pressor responses (Hunt et al. 2001a). It was not feasible to employ this method in the present study, but we may draw conclusions with respect to changes in the neural component, by comparing differences in carotid compliance and baroreflex indices – an approach that has been used before (Lénárd et al. 2004). In our FH+ subjects exercise training was associated with 50% increase in baroreflex indices, but only with 10% increase in carotid compliance. Others and we, as well, have shown earlier that changes in carotid artery compliance are linearly associated with changes in baroreflex gain (Bonyhay et al. 1996; Lipman et al. 2002). Since the product of mechanical and neural components gives the integral cardiovagal baroreflex gain (Hunt et al. 2001a), the greater increase in baroreflex indices than in carotid compliance with physical exercise may indicate improved autonomic neural function. In middle-aged subjects, physical activity has been shown to preserve baroreflex function from age-related decline by maintaining neural function (Hunt et al. 2001b). Another possible mechanism is related to neurotransmission in the sinoatrial node. It could be that the slower baseline heart rate in exercising subjects caused each bolus of acetylcholine to arrive at a more propitious time in relation to the susceptibility of the sinoatrial node to inhibition.
A few experimental considerations should be noted. First, subjects' family history of hypertension was defined in the present study based on the presence of hypertension in only one of the two parents. Considering the complex genetic background of essential hypertension, this definition of family history of hypertension is an apparent simplification, which is acknowledged as a limitation. Second, because of the cross-sectional design of the present study, we cannot exclude the possibility that arterial biomechanics and cardiovagal function are regulated by the same variable, which led our FH+ subjects to become physically active. We decided to use the cross-sectional design because of the simpler logistics that are involved in collecting cross-sectional data. We feel, however, that even the cross-sectional design yielded important new findings, namely that cardiovagal outflow and baroreflex gain of those subjects who have a family history of hypertension, but who perform regular physical exercise, is not different from that of those subjects who are without a family history of hypertension, and that differences in cardiovagal autonomic indices, related to parental hypertension and physical exercise, are not importantly explained by differences in carotid artery elasticity. We believe that this new information may provide a strong rationale for a prospective study. Third, in the present study we used the non-invasive method of relating spontaneous oscillations in blood pressure and heart rate to characterize cardiovagal baroreflex sensitivity, and assumed that these fluctuations are partly linked through the baroreflex. This assumption is supported by reported correlations between baroreflex gain and spontaneous baroreflex indices (Pellizzer et al. 1996; Maestri et al. 1998; Pitzalis et al. 1998). Validation of spontaneous baroreflex indices was obtained not only through comparison with drug-induced responses (Parlow et al. 1995), but also through experimental studies based on arterial baroreceptor denervation (Bertinieri et al. 1988). We are aware that agreement between the absolute values of pharmacologically determined baroreflex gain and spontaneous baroreflex indices is weak (Pitzalis et al. 1998), but our conclusion focuses on changes in spontaneous baroreflex indices rather than absolute values. Others and we, in the present study, have shown that spontaneous baroreflex indices are closely related to cardiac vagal activity, and considering that the dominant excitatory input to cardiac vagal motoneurones arises from arterial baroreceptors (Parati et al. 2000), changes in spontaneous indices are likely to be related to changes in baroreflex gain. This notion is further supported by the evidence that spontaneous modulation of baroreflex gain over 24 h is directly related to the 24-h variability of pulse interval (Parati et al. 1995).
Perspectives
The present results support the idea that regular, aerobic physical activity can attenuate the impairment of cardiovagal autonomic function and stiffening of the carotid artery in young subjects with a family history of hypertension. As such, this may be an effective lifestyle intervention for minimizing negative effects of a family history of hypertension on autonomic circulatory control. Improved cardiovagal autonomic function and central arterial elasticity may contribute to the lower incidence of hypertension observed in individuals who exercise regularly.
Acknowledgments
The Hungarian National Scientific Research Fund Grant OTKA-49690 supported this work. The authors acknowledge the skilled technical assistance of M. Petrekanits and Zs. Kovats.
References
- American College of Sports Medicine. Position Stand. Physical activity, physical fitness and hypertension. Med Sci Sports Exerc. 1993;25:i–x. [PubMed] [Google Scholar]
- Andresen MC. Short- and long-term determinants of baroreceptor function in aged normotensive and spontaneously hypertensive rats. Circ Res. 1984;54:750–759. doi: 10.1161/01.res.54.6.750. [DOI] [PubMed] [Google Scholar]
- Bertinieri G, Di Rienzo M, Cavallazzi A, Ferrari AU, Pedotti A, Mancia G. Evaluation of baroreceptor reflex by blood pressure monitoring in unanesthetized cats. Am J Physiol. 1988;254:H377–H383. doi: 10.1152/ajpheart.1988.254.2.H377. [DOI] [PubMed] [Google Scholar]
- Blonde CV, Webber LS, Foster TA, Berenson GS. Parental history and cardiovascular disease risk factor variables in children. Prev Med. 1981;10:25–31. doi: 10.1016/0091-7435(81)90003-7. [DOI] [PubMed] [Google Scholar]
- Bonyhay I, Jokkel G, Kollai M. Relation between baroreflex sensitivity and carotid artery elasticity in healthy humans. Am J Physiol. 1996;271:H1139–H1144. doi: 10.1152/ajpheart.1996.271.3.H1139. [DOI] [PubMed] [Google Scholar]
- van Bortel LM, Duprez D, Starsman-Kool MJ, Safar ME, Giannattassio C, Cockcroft J, Kaiser DR, Thuillez C. Clinical applications of arterial stiffness, task force III: recommendations for user procedures. Am J Hypertens. 2002;15:445–452. doi: 10.1016/s0895-7061(01)02326-3. [DOI] [PubMed] [Google Scholar]
- Bristow JD, Honour AJ, Pickering GW, Sleight P, Smyth HS. Diminished baroreflex sensitivity in high blood pressure. Circulation. 1969;39:48–54. doi: 10.1161/01.cir.39.1.48. [DOI] [PubMed] [Google Scholar]
- Burke GL, Savage PJ, Sprafka JM, Selby JV, Jacobs DR, Jr, Perkins LL, Roseman JM, Hughes GH, Fabsitz RR. Relation of risk factor levels in young adulthood to parental history of disease. The CARDIA study. Circulation. 1991;84:1176–1187. doi: 10.1161/01.cir.84.3.1176. [DOI] [PubMed] [Google Scholar]
- Eckberg DL, Sleight P. Human Baroreflexes in Health and Disease. Oxford, UK: Clarendon; 1992. [Google Scholar]
- Hoeks AP, Brands PJ, Smeets F, Reneman RS. Assessment of the distensibility of superficial arteries. Ultrasound Med Biol. 1990;16:121–128. doi: 10.1016/0301-5629(90)90139-4. [DOI] [PubMed] [Google Scholar]
- Hoeks APG, Willekes C, Boutouyrie P, Brands PJ, Willigers JM, Reneman RS. Automated detection of local artery wall thickness based on M-line signal processing. Ultrasound Med Biol. 1997;23:1017–1023. doi: 10.1016/s0301-5629(97)00119-1. [DOI] [PubMed] [Google Scholar]
- Hunt BE, Fahy L, Farquhar WB, Taylor JA. Quantification of mechanical and neural components of vagal baroreflex in humans. Hypertension. 2001a;37:1362–1368. doi: 10.1161/01.hyp.37.6.1362. [DOI] [PubMed] [Google Scholar]
- Hunt BE, Farquhar WB, Taylor JA. Does reduced vascular stiffening fully explain preserved cardiovagal baroreflex function in older, physically active men? Circulation. 2001b;103:2424–2427. doi: 10.1161/01.cir.103.20.2424. [DOI] [PubMed] [Google Scholar]
- Iellamo F, Legramante JM, Massaro M, Raimondi G, Galante A. Effects of a residential exercise training on baroreflex sensitivity and heart rate variability in patients with coronary artery disease: a randomized, controlled study. Circulation. 2000;102:2588–2592. doi: 10.1161/01.cir.102.21.2588. [DOI] [PubMed] [Google Scholar]
- Iwase N, Takata S, Okuwa H, Ogawa J, Ikeda T, Hattori N. Abnormal baroreflex control of heart rate in normotensive young subjects with a family history of essential hypertension. J Hypertension. 1984;2:409–411. [PubMed] [Google Scholar]
- Kelly R, Fitchett D. Non-invasive determination of aortic input impedance and external left ventricular power output: a validation and repeatability study of a new technique. J Am Coll Cardiol. 1992;20:952–963. doi: 10.1016/0735-1097(92)90198-v. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bersano C, Gnemmi M, Specchia G, Schwartz PJ. Exercise-induced increase in baroreflex sensitivity predicts improved prognosis after myocardial infarction. Circulation. 2002;106:945–949. doi: 10.1161/01.cir.0000027565.12764.e1. [DOI] [PubMed] [Google Scholar]
- Lage SG, Polak JF, O'Leary DH, Creager MA. Relationship of arterial compliance to baroreflex function in hypertensive patients. Am J Physiol. 1993;265:H232–H237. doi: 10.1152/ajpheart.1993.265.1.H232. [DOI] [PubMed] [Google Scholar]
- Lénárd Z, Studinger P, Mersich B, Kocsis L, Kollai M. Maturation of cardiovagal autonomic function from childhood to young adult age. Circulation. 2004;110:2307–2312. doi: 10.1161/01.CIR.0000145157.07881.A3. [DOI] [PubMed] [Google Scholar]
- Lipman RD, Grossman P, Bridges SE, Hamner JW, Taylor JA. Mental stress response, arterial stiffness, and baroreflex sensitivity in healthy aging. J Gerontol Biol Sci Med Sci. 2002;57:B279–B284. doi: 10.1093/gerona/57.7.b279. [DOI] [PubMed] [Google Scholar]
- Maestri R, Pinna GD, Mortara A, La Rovere MT, Tavazzi L. Assessing baroreflex sensitivity in post-myocardial infarction patients: comparison of spectral and phenylephrine techniques. J Am Coll Cardiol. 1998;31:344–351. doi: 10.1016/s0735-1097(97)00499-3. [DOI] [PubMed] [Google Scholar]
- Mancia G, Ludbrook J, Ferrari A, Gregorini L, Zanchetti A. Baroreceptor reflexes in human hypertension. Circ Res. 1978;43:170–177. doi: 10.1161/01.res.43.2.170. [DOI] [PubMed] [Google Scholar]
- Matthews CE, Pate RR, Jackson KL, Ward DS, Macera CA, Kohl HW, Blair SN. Exaggerated blood pressure response to dynamic exercise and risk of future hypertension. J Clin Epidemiol. 1998;51:29–35. doi: 10.1016/s0895-4356(97)00223-0. [DOI] [PubMed] [Google Scholar]
- Meaney E, Samaniego V, Alva F, Valdovinos RA, Marrufo R, Vela A, Allen T, Misra A, Madsen R. Increased arterial stiffness in children with a parental history of hypertension. Pediatric Cardiol. 1999;20:203–205. doi: 10.1007/s002469900441. [DOI] [PubMed] [Google Scholar]
- Monahan KD, Dinenno FA, Tanaka H, Clevenger CM, DeSouza CA, Seals DR. Regular aerobic exercise modulates age-associated declines in cardiovagal baroreflex sensitivity in healthy men. J Physiol. 2000;529:263–271. doi: 10.1111/j.1469-7793.2000.00263.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monahan KD, Tanaka H, Dinenno FA, Seals DR. Central arterial compliance is associated with age- and habitual exercise-related differences in cardiovagal baroreflex sensitivity. Circulation. 2001;104:1627–1632. doi: 10.1161/hc3901.096670. [DOI] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M, Mancia G. How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J Hypertens. 2000;18:7–19. [PubMed] [Google Scholar]
- Parati G, Frattola A, Di Rienzo M, Castiglioni P, Pedotti A, Mancia G. Effects of aging on 24-h dynamic baroreceptor control of heart rate in ambulant subjects. Am J Physiol. 1995;268:H1606–H1612. doi: 10.1152/ajpheart.1995.268.4.H1606. [DOI] [PubMed] [Google Scholar]
- Parlow J, Viale JP, Annat G, Hughson R, Quintin L. Spontaneous cardiac baroreflex in humans. Comparison with drug-induced responses. Hypertension. 1995;25:1058–1068. doi: 10.1161/01.hyp.25.5.1058. [DOI] [PubMed] [Google Scholar]
- Parmer RJ, Cervenka JH, Stone RA. Baroreflex sensitivity and heredity in essential hypertension. Circulation. 1992;85:497–503. doi: 10.1161/01.cir.85.2.497. [DOI] [PubMed] [Google Scholar]
- Pellizzer AM, Kamen PW, Jackman G, Brazzale D, Krum H. Non-invasive assessment of baroreflex sensitivity and relation to measures of heart rate variability in man. Clin Exp Pharmacol Physiol. 1996;23:621–644. doi: 10.1111/j.1440-1681.1996.tb02800.x. [DOI] [PubMed] [Google Scholar]
- Pitzalis MV, Mastropasqua F, Passantino A, Massari F, Ligurgo L, Forleo C, Balducci C, Lombardi F, Rizzon P. Comparison between noninvasive indices of baroreceptor sensitivity and the phenylephrine method in post-myocardial infarction patients. Circulation. 1998;97:1362–1367. doi: 10.1161/01.cir.97.14.1362. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Dinenno FA, Monahan KD, Clevenger CM, DeSouza CA, Seals DR. Aging, habitual exercise, and dynamic arterial compliance. Circulation. 2000;102:1270–1275. doi: 10.1161/01.cir.102.11.1270. [DOI] [PubMed] [Google Scholar]
- Tank J, Jordan J, Diedrich A, Stoffels M, Franke G, Faulhaber HD, Luft FC, Busjahn A. Genetic influences on baroreflex function in normal twins. Hypertension. 2001;37:907–910. doi: 10.1161/01.hyp.37.3.907. [DOI] [PubMed] [Google Scholar]
- Zorn EA, Wilson MB, Angel JJ, Zanella J, Alpert BS. Validation of an automated arterial tonometry monitor using association for the advancement of medical instrumentation standards. Blood Press Monit. 1997;2:185–188. [PubMed] [Google Scholar]