Abstract
Four-week-old, Fischer–Brown Norway F1-generation male rats were given access to voluntary running wheels for 21 days, and then the wheels were locked for 5 (WL5), 10 (WL10), 29 (WL29), or 53 (WL53) hours. Two other groups (SED5 and SED10) had no access to voluntary running wheels and were killed at the same time as WL5 and WL10, respectively. Absolute and relative epididymal fat mass, mean cell volume, and amount of lipid per cell increased in WL53 relative to all other groups, with no change in cell number. C/EBPα protein levels in epididymal fat were 30% greater in SED5 than in WL5. The rate of triacylglycerol synthesis in epididymal fat was 4.2-fold greater in SED5 than in WL5, increased 14-fold between WLS and WL10, and was 79% lower in SED10 than in WL10. Triacylglycerol synthesis remained at this elevated level (at least 3.5-fold greater than SED5) through WL53. Thus, the rapid increase in epididymal fat mass with the cessation of voluntary wheel running is associated with a prolonged overshoot in epididymal fat triacylglycerol synthesis. Moreover, rats without running wheels had a 9.4% lower body mass after 21 days than those with running wheels. The individual mass of seven different muscles from the hindlimb, upper forelimb, and back were each lower in animals without running wheels, suggesting that physical activity in rapidly growing rats may be requisite for optimal muscle development.
Obesity is a burgeoning problem in the United States and throughout the world (Popkin, 1998; Flegal et al. 2002), with the most disturbing increase occurring among children and adolescents (U.S. Department of Health & Human Services, 2001; Ogden et al. 2002). It is generally agreed that the rampant rise in obesity is due to a change in lifestyle factors interacting with a genetic predisposition to accumulate body fat (Maffeis, 2000; Booth et al. 2002; Shuldiner & Munir, 2003; Webber, 2003; Thibault et al. 2004). Although several lifestyle factors have been suggested to contribute to the development of obesity, decreasing levels of physical activity are likely to be a major underlying cause (Saris, 1998; Hu, 2003; Webber, 2003; Swinburn et al. 2004). One of the complications frequently associated with obesity is insulin resistance (Despres, 1993; Vollenweider, 2003); this relationship is particularly true for abdominal obesity (Despres, 1993; Frayn, 2000; Gan et al. 2003; Rattarasarn et al. 2004). There exist strong associations between low physical activity and obesity, between low physical activity and insulin resistance, and between obesity and insulin resistance (Booth et al. 2002; Ryan, 2003). Despite the strength of these triadic associations, relatively few studies have investigated their integration.
One approach to studying the effects of reduced physical activity is to observe changes that occur when physically active animals return to their regular sedentary cage activity. Some (Dohm et al. 1977; Craig et al. 1983; Lambert et al. 1994), but not all (Applegate & Stern, 1987), studies have reported indices of fat content increasing 4–14 days after ending physical training in rats. For example, there is a 41% increase in epididymal fat mass (Dohm et al. 1977), a 53% increase in parametrial adipocyte volume (Craig et al. 1983), and a 23% increase in epididymal adipocyte diameter (Lambert et al. 1994) 14, 4 and 7 days, respectively, following the cessation of exercise training. None of these three studies reported indices of fat content earlier than 4 days following the last bout of exercise. Recently, we reported that rats that have had access to voluntary running wheels for 21 days exhibit a decline in submaximal insulin-stimulated 2-deoxyglucose transport into the epitrochlearis muscle after their wheels have been locked for 53 h (Kump & Booth, 2005). Because of the strong inverse association between insulin sensitivity and abdominal fat (Despres, 1993; Frayn, 2000; Gan et al. 2003; Rattarasarn et al. 2004), one purpose of the current study was to test the hypothesis that an increase in the mass and cellularity of epididymal adipose tissue would occur 53 h after reducing physical activity, coincident with the previously reported loss of enhanced insulin sensitivity in the epitrochlearis muscle (Kump & Booth, 2005).
Methods
Materials
Dulbecco's phosphate-buffered saline (PBS) was from Gibco (Grand Island, NY, USA). Nylon mesh was from Sefar America (Kansas City, MO, USA). 2-[1,2-3H(N)]Deoxyglucose and [carboxyl-14C]trioleoylglycerol were from American Radiolabeled Chemicals (St Louis, MO, USA). [9,10-3H]Palmitic acid was from Sigma (St Louis, MO, USA). Mouse anti-CCAAT/enhancer binding protein-α (C/EBPα) monoclonal and rabbit antiperoxisome proliferator-γ (PPARγ) polyclonal antibodies were from Affinity Bioreagents (Golden, CO, USA). All other reagents were either from Sigma or Fisher.
Animal protocol
The animal protocol was approved by the Institutional Animal Care and Use Committee at the University of Missouri–Columbia. Male Fischer 344 × Brown Norway F1 hybrid rats (Harlan) were obtained at age 21–23 days and allowed to acclimatize for 1 week. The animals were housed in approved temperature-controlled animal quarters (21°C) with a 04.00–16.00 h light : 16.00–04.00 h dark cycle that was maintained throughout the experimental period.
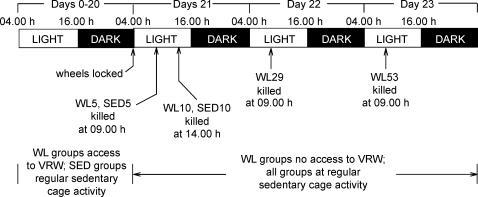
After 1 week (day 0), the rats were randomly assigned to one of six experimental groups: wheel lock 5 (WL5), wheel lock 10 (WL10), wheel lock 29 (WL29), or wheel lock 53 (WL53), which consisted of 21 days of voluntary wheel access followed by 5, 10, 29, or 53 h of wheel lock that prevented access to voluntary running, respectively; or SED5 or SED10, which were sedentary groups without running wheel access for the entire 21 days, killed at the same time points as WL5 and WL10, respectively (Fig. 1). At this time (on day 0), the animals were separated into individual cages of the same dimensions; cages for animals in the running groups (WL5, WL10, WL29 and WL53) were equipped with a voluntary running wheel outfitted with a Sigma Sport BC 800 bicycle computer (Cherry Creek Cyclery, Foster Falls, VA, USA) for measuring running activity.
Figure 1. Experimental design.
The top two lines represent the time line. The bar represents the light : dark cycle. Preliminary studies showed that > 99% of the running occurred during the dark cycle (data not shown). Wheel lock (WL) groups were given access to voluntary running wheels (VRW) at the start of day 0. Groups with regular sedentary cage activity (SED) were housed without VRW. For WL groups, wheels were locked at 04.00 h on day 21 (bottom lines); arrows pointing to bar indicate the time of kill. Access to food was denied at 04.00 h on the day of kill for a given group. See Methods for more detail.
All animals were provided with 200 g of standard rodent chow (Formulab 5008, Purina Mills, St Louis, MO, USA) at the beginning of each week (days 0, 7 and 14) when cages were changed. Running activity (for groups with wheel access), body mass and food intake were measured daily between 04.00 and 05.00 h. On day 21 (after 21 days of running activity), the wheels were locked for all running groups at 04.00 h. SED5 and WL5 were killed at 09.00 h, and SED10 and WL10 were killed at 14.00 h. The WL29 group remained in their cages with locked wheels before being killed the following day (on day 22) at 09.00 h (after 29 h of wheel lock), and WL53 remained in their cages with locked wheels until day 23 when they were killed at 09.00 h (after 53 h of wheel lock). All animals had ad libitum access to food at all times until the day of kill, when food was removed at 0400 h.
At the time of kill, the animals were anaesthetized with an intraperitoneal injection of 60 mg pentobarbital per kg body mass. The animals were exsanguinated by removal of the heart. Sixty-four animals (16 each from WL5, WL29, WL53 and SED5) were used in a previous study (Kump & Booth, 2005); epididiymal adipose tissue from these animals was weighed and used for cellularity experiments or for determination of 2-deoxyglucose uptake as described below, and the mass of the soleus muscle was obtained from 10 animals each in WL5 and SED5. A second set of rats was used exclusively for the current report (10 each from WL5, WL10, WL29, WL53, SED5 and SED10); epididymal and omental adipose tissue from these animals were weighed, quickly frozen in liquid N2, and stored at −80°C until homogenized as described below and used for immunoblot analysis or measurement of triacylglycerol synthesis. Masses of seven skeletal muscles were also obtained from 7–10 animals each in WL5 and SED5.
Adipocyte isolation
Adipocytes from epididymal fat pads were isolated by a modification of the Rodbell method (Rodbell, 1964); see Supplemental material). Packed isolated adipocytes were used in experiments as described below.
Adipocyte size and number
Preparation of epididymal adipose tissue for determination of cell size and number was performed essentially as described by Cartwright (1987); see Supplemental material.
Cellular lipid content
For determination of lipid content, 60 μl of packed isolated epididymal adipocytes were added to 2.75 ml of Dole's solution (isopropanol : heptane : 1 N H2SO4, 80 : 20 : 5, v:v:v) : heptane : H2O (27 : 18 : 10, v:v:v), vortexed, and allowed to sit for 60 min at room temperature. Samples were then re-vortexed and centrifuged at 450 g for 5 min; 2 ml of the upper phase was added to a preweighed glass scintillation vial and allowed to dry uncovered in a ventilated hood for 2 days. Vials were then weighed again to determine the amount of lipid.
2-Deoxyglucose uptake
A packed cell volume of 60 μl was added to 290 μl of Krebs-Ringer-HEPES buffer (KRHB: 130 mm NaCl, 4.7 mm KCl, 1.2 mm KH2PO4, 10 mm HEPES, 1 mm CaCl2, 1.2 mm MgSO4, 0.25 μg ml free fatty acid-free BSA, pH 7.4) containing either no additions, 0.4 nm insulin, 12.8 nm insulin, or 70 μm cytochalasin B and incubated at 37°C for 45 min. At this time, 10 μl of 2-[3H]deoxyglucose was added to a final concentration of 200 μm (1.5 μCi ml−1) and incubated at 37°C for 3 min; the reaction was stopped by adding 300 μl of ice-cold cytochalasin B in KRHB to a final concentration of 250 μm. The cells were separated from the medium by centrifugation through dinonyl phthalate oil at 1000 g for 10 s. The infranatant layer containing the media was removed by aspiration with a gel-loading tip, and the cells were washed 3 times with KRHB prior to scintillation counting. Values from the samples containing cytochalasin B, representing non-specific 2-deoxyglucose uptake, were subtracted from each of the other values from the same animal to yield carrier-mediated 2-deoxyglucose uptake. Non-specific 2-deoxyglucose uptake was < 10% of basal uptake in all samples and did not differ among groups (14% coefficient of variation; data not shown).
Triacylglycerol synthesis
Approximately 500 mg of frozen epididymal adipose tissue was homogenized in 1.5 volumes of homogenization buffer (50 mm Tris-HCl, 225 mm sucrose, 1 mm EDTA-Na4, pH 7.4, 1 mm benzamidine, 1 μg ml−1 pepstatin, 1 μg ml−1 leupeptin, 1 μg ml−1 aprotinin, 1 mm activated Na3VO4). The protein concentration of the samples was determined with 5 μl of homogenate using the CB-X protein assay kit (Genotech, St Louis, MO, USA) according to the manufacturer's instructions, except that only one-half of the recommended volume of each solution was used. Aliquots of samples were further diluted in homogenization buffer to a concentration of 2 μg protein μl−1. The incorporation of palmitic acid into triacylglycerol was used as a measure of triacylglycerol synthesis as adapted from Galton (1968); see Supplementary material.
Immunoblots
Twenty micrograms of homogenate protein from epididymal fat pads was subjected to immunoblotting as previously described (Kump & Booth, 2005), and probed for either PPARγ (1 : 6000 antibody dilution) or C/EBPα (1 : 1000 antibody dilution). The epididymal fat homogenate of three non-experimental animals was combined and used as a loading control, which was loaded in triplicate on each blot. All results are expressed as arbitrary densitometric units relative to the loading control.
Statistics
Statistical methods are described in the figure legends with further explanations in the Supplemental material. Significance for all tests was set at P < 0.05. All data are presented as the mean ± standard error of the mean (s.e.m.).
Results
Running activity
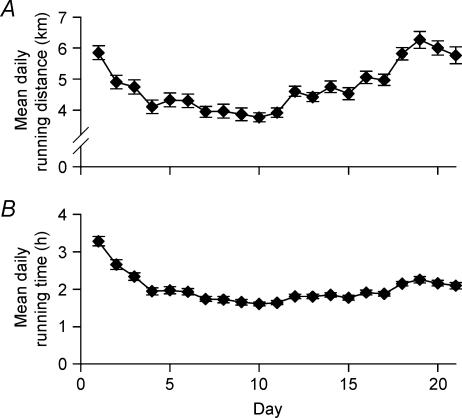
There was no difference among wheel lock groups undergoing voluntary running in their total 21-day running distance, in total 21-day time run, or in the distance or time run on the last night of running (Table 1). The average daily running distance for all groups is presented in Fig. 2. There was a significant negative correlation between the initial body mass (on day 0) and the total distance run (r=−0.332, P= 0.002; data not shown).
Table 1.
Comparison of body mass (BM), gth, running activity, and food intake variables across all groups
| WL5 | WL10 | WL29 | WL53 | SED5 | SED10 | |
|---|---|---|---|---|---|---|
| BM day 0 (g) | 75.9 ± 1.9 | 74.1 ± 2.6 | 70.6 ± 1.6 | 70.3 ± 1.3 | 75.3 ± 2.3 | 76.6 ± 3.2 |
| BM day 21 (g) | 211.0 ± 2.7 | 206.8 ± 5.9 | 205.2 ± 3.3 | 208.5 ± 2.5 | 187.2 ± 3.4*** | 192.3 ± 3.0* |
| BM on day of kill (g) | 211.0 ± 2.7 | 206.8 ± 5.9 | 208.3 ± 3.3 | 215.7 ± 2.6 | 187.2 ± 3.4*** | 192.3 ± 3.0* |
| Absolute increase in BM from day 0 to day 21 (g) | 135.1 ± 1.6 | 132.7 ± 4.9 | 134.6 ± 2.4 | 138.3 ± 1.8 | 111.9 ± 1.8*** | 115.7 ± 2.3*** |
| Fold- increase in BM from day 0 to day 21 | 2.81 ± 0.05 | 2.81 ± 0.09 | 2.92 ± 0.05 | 2.98 ± 0.04 | 2.51 ± 0.04** | 2.54 ± 0.08* |
| Total running distance (km) | 98.46 ± 5.28 | 98.61 ± 5.36 | 98.68 ± 5.78 | 102.79 ± 4.46 | — | — |
| Running distance on last night (km) | 5.87 ± 0.46 | 4.60 ± 0.8 | 5.53 ± 0.49 | 6.36 ± 0.52 | — | — |
| Total running time from day 0 to day 21 (h) | 41.5 ± 1.9 | 41.5 ± 2.1 | 41.6 ± 2.1 | 43.3 ± 1.7 | — | — |
| Running time on last night (h) | 2.1 ± 0.1 | 1.7 ± 0.3 | 2.0 ± 0.2 | 2.3 ± 0.2 | — | — |
| Total food intake from day 0 to day 21 | 373 ± 5 | 361 ± 9 | 364 ± 5 | 370 ± 4 | 313 ± 4*** | 315 ± 4*** |
| Food intake (g) per body mass (g) on day before kill × 1000 | 104 ± 1a | 102 ± 1a,b | 96 ± 1 | 100 ± 1b | 89 ± 1*** | 87 ± 1*** |
Data for all groups were compared using ANOVA, with the Student-Neuman-Keuls post hoc test. For body mass on day 21, initial body mass (body mass on day 0) was used as a covariate. Values are means ± s.e.m.. *, ** and ***, significantly different (P < 0.05, 0.005 and 0.001, respectively) versus WL5, WL10, WL29 and WL53. Wheel lock groups not sharing a letter for relative food intake on the last night are significantly different (P < 0.05). n = 26 each for WL5, WL29, WL53, and SED5. n = 10 each for WL10 and SED10.
Figure 2. Running activity.
The mean daily running distance (A) and time (B) of the combined running groups are shown. Data points represent the mean ± s.e.m. for each day's running activity. n = 88.
Adipose mass and cellularity
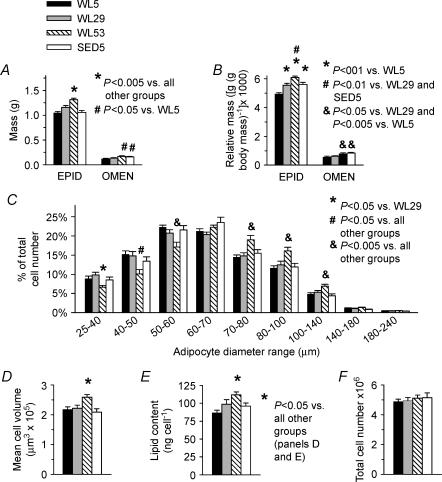
Epididymal fat pad mass was significantly higher in WL53 (1.311 ± 0.032 g) relative to all other groups (1.047 ± 0.030 g for WL5, 1.160 ± 0.039 g for WL29, and 1.054 ± 0.041 g for SED5; Fig. 3A). The relative mass of the epididymal fat pad ((g/g body mass) × 1000) was significantly greater in all other groups (5.548 ± 0.133 for WL29, 6.069 ± 0.111 for WL53, and 5.595 ± 0.140 for SED5) compared to WL5 (4.945 ± 0.088), and it was also significantly greater in WL53 relative to WL29 and SED5 (Fig. 3B). There was a significant negative correlation between total distance run and the relative epididymal fat mass in WL5 and WL10 (r=−0.42, P= 0.01, data not shown). Omental fat pad mass was greater in WL53 (0.172 ± 0.012 g) and SED5 (0.160 ± 0.010 g) than in WL5 (0.116 ± 0.016 g; Fig. 3A); WL29 (0.136 ± 0.009 g) was intermediate between, but not different from, WL5 and WL53. Relative omental fat mass ((g/g body mass) × 1000) was significantly higher in WL53 (0.800 ± 0.055) and SED (0.836 ± 0.054) than in WL5 (0.544 ± 0.072) or WL29 (0.616 ± 0.039; Fig. 3B).
Figure 3. Adipose mass and cellularity.
Epididymal (EPID) and omental (OMEN) absolute (A) and relative (B) fat mass; C, relative epididymal adipocyte distribution based on cell diameter; D, mean epididymal adipocyte cell volume; E, epididymal fat cellular lipid content; and F, epididymal fat pad total cell number for both left and right pads. Bars represent the means ± s.e.m. Legends for significance are shown separately with each graph; D and E share a significance legend to the right of E. ANOVA with Student-Neuman-Keuls post hoc test was used for all comparisons. n = 26 for epididymal fat mass, 10 for omental fat, and 13–16 for cellularity measurements.
The percentage of epididymal adipocytes with a diameter between 25 and 60 μm was lower in WL53 than in the other groups, while the percent of cells with a diameter between 70 and 140 μm was greater in WL53 than in all other groups (Fig. 3C). The mean epididymal adipocyte volume (μm3× 105) was significantly higher in WL53 (2.58 ± 0.94) than all other groups (2.09 ± 1.11 for SED5, 2.18 ± 0.83 for WL5, and 2.22 ± 0.99 for WL29; Fig. 3D). Mean epididymal adipocyte diameter (μm) was also significantly higher (P < 0.005) in WL53 (70.6 ± 0.9) than all other groups (65.7 ± 0.7 for WL5, 65.9 ± 1.0 for WL29, and 65.7 ± 1.0 for SED; data not shown). The amount of lipid (ng) per cell was greater in WL53 (111.8 ± 4.2) than in all other groups (86.6 ± 3.8 for WL5, 98.7 ± 5.5 for WL29, and 96.2 ± 4.2 for SED5; Fig. 3E). Total epididymal adipocyte number (× 106) was not significantly different between groups (4.88 ± 0.17 for WL5, 4.95 ± 0.20 for WL29, 5.11 ± 0.21 for WL53, and 5.14 ± 0.32 for SED5; Fig. 3F).
2-Deoxyglucose uptake
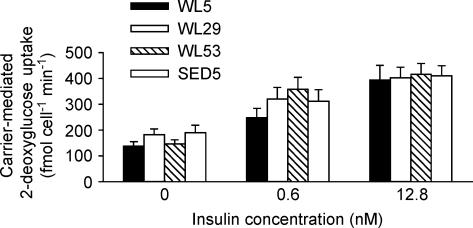
There were no significant differences between groups for 2-deoxyglucose uptake into isolated epididymal adipocytes with either no insulin or at 0.4 nm or 12.8 nm insulin (Fig. 4).
Figure 4. 2-Deoxyglucose uptake into isolated epididymal adipocytes.
2-Deoxyglucose uptake values in the presence of 70 μm cytochalasin B for each animal were subtracted from the other values measured in the absence of cytochalasin B (see Methods). Values within a given insulin concentration were compared using ANOVA with the Student-Neuman Keuls post hoc test. n = 12–16 per group.
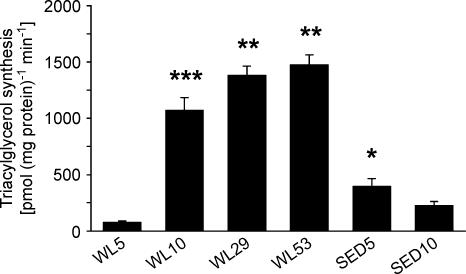
Triacylglycerol synthesis
Triacylglycerol synthesis was estimated by measuring the incorporation of palmitic acid into triacylglycerol (pmol (mg homogenate protein)−1 min−1). Triacylglycerol synthesis was greater in SED5 (395 ± 71) than in WL5 (76 ± 12; Fig. 5). Triacylglycerol synthesis was lower in WL5 and SED 5 than in both WL29 (1379 ± 86) and WL53 (1475 ± 91). Since triacylglycerol synthesis in WL5 was markedly suppressed relative to SED5, it was also measured in WL10 and SED10. WL5 and SED10 (226 ± 35) were less than WL10 (1072 ± 112); SED10 did not differ significantly from SED5.
Figure 5. Triacylglycerol synthesis in epididiymal fat.
The capacity for triacylglycerol synthesis was measured in epididymal fat homogenates and is expressed as pmol of palmitic acid incorporated into triacylglycerol per mg of homogenate protein per minute. Comparisons between WL5, WL29, WL53 and SED5 were performed using ANOVA; comparisons between WL5, WL10, SED5 and SED10 were performed using a 2-way ANOVA (see Methods). The Student-Neuman-Keuls post hoc test was used as follow-up for both comparisons. *P < 0.005 versus WL5. **P < 0.001 versus SED5 and WL5. ***P < 0.001 versus SED10 and WL5. n = 10 in each group.
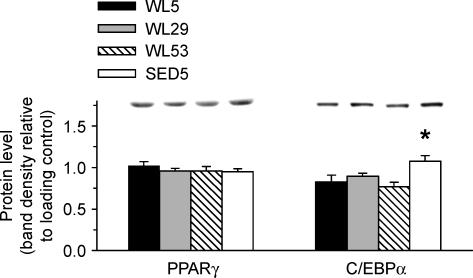
Immunoblots
PPARγ protein levels did not differ among groups (Fig. 6). C/EBPα protein levels were 30%, 20%, and 41% higher in SED5 than in WL5, WL29, and WL53, respectively (Fig. 6).
Figure 6. PPARγ and C/EBPα protein levels in epididymal fat.
Protein levels are relative to loading control (see Methods). A representative immunoblot for each protein is located above the graph. *Significantly different from all other groups (P < 0.05, ANOVA with Student-Neuman-Keuls post hoc test). n = 10 in each group.
Food intake
Food intake was lower in sedentary rats than in runners beginning on day 5
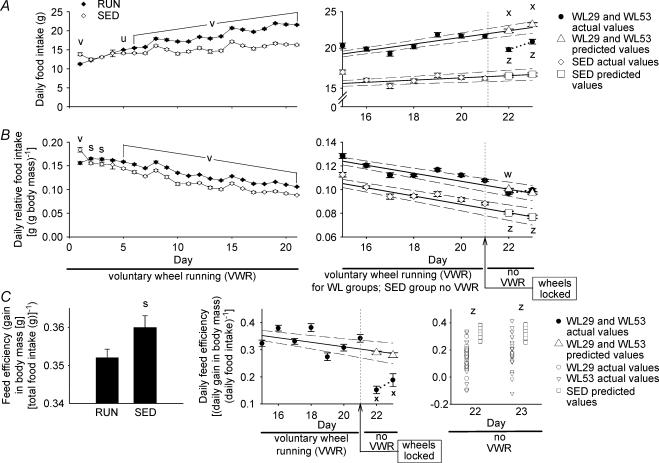
Absolute food intake was greater in the combined sedentary groups (SED) than the combined running groups (RUN) on day 1, but was lower in SED than RUN beginning on day 5 and extending through day 21 (Fig. 7A, left panel). Relative food intake was greater in SED than RUN on day 1, but was lower in SED than RUN on all other days except for day 4 (Fig. 7B, left panel), such that relative daily food intake was an average of 10.8% (P < 0.001) lower in SED than RUN from days 4 to 21. Feed efficiency, defined as the absolute increase in body mass (g) per total amount of food ingested (g), was higher in SED than in RUN (0.360 ± 0.003 and 0.352 ± 0.002, respectively, Fig. 7C, left panel). Food intake data for individual groups are presented in Table 1.
Figure 7. Food consumption.
Absolute food intake (A), relative food intake (B), and feed efficiency (C). Leftmost panels: comparison of combined groups (WL5, WL10, WL29, and WL53) with unrestricted voluntary running wheel access (RUN, ♦) and sedentary groups (SED, ⋄). The group legend for the left panels of A and B is located at the top of A. Feed efficiency in the left panel of C is the gain in body mass from days 0 to 21 relative to the total food intake for the same period. Data points and bars represent the mean ± s.e.m. s, u and v; significant difference (P < 0.05, 0.005, and 0.001, respectively; Student's t test) between SED and RUN. n = 88 for RUN and 36 for SED. Right panels of A and B: food intake before and after running wheels were locked. Voluntary running wheels were locked beginning on day 21 (indicated by the arrow; see Supplemental material) so that the rats could not run. Predicted values for days 22 and 23 for WL29 and WL53 (▵) and for SED (□) were predicted from linear regression equations for each animal based on actual values from days 4 to 21 for WL29 and WL53 (•) and SED (⋄). Only data starting on day 15 are shown for clarity of the figure. The continuous lines represent the regression lines, and the dashed lines represent the 95% confidence intervals. The dotted line connects the observed values for days 22 and 23. Comparison 1 (see Supplemental material): the differences between the predicted and observed values for each animal in WL29 and WL53 on days 22 and 23 were compared using Student's t test. w and x, significant difference (P < 0.005 and P < 0.001, respectively) between predicted and actual values for WL29 and WL53. Comparison 2 (see Supplemental material): the differences between predicted values for SED and the observed WL29 and WL53 values were compared using Student's t test. z, significant difference (P < 0.001) between predicted values for SED and actual values for WL29 and WL53. Data points represent the mean ± s.e.m.n = 52 for both the actual and predicted values for the combined WL29 and WL53 on day 22, n = 26 for WL53 on day 23, and n = 36 for SED for both the actual and predicted values. Middle panel of C: same presentation as for right panels of A and B, except that SED values are not plotted and only Comparison 1 is made. Right panel of C: scatter plots of observed feed efficiency values for WL29 (○ in columns 1 and 3) and WL53 (▿ in columns 1 and 3) and predicted values for SED (□ in columns 2 and 4) during no voluntary wheel running (VWR) on days 22 and 23. Data points represent the individual values for each animal. Comparison 2 (see Supplemental material): values for SED were predicted for each animal using linear regression analysis and were compared to observed values for WL29 and WL53 with the Mann-Whitney rank-sum test. z, significant difference (P < 0.001) between predicted values for SED and actual values for WL29 and WL53. n = 26 each for WL29 and WL53 (total of 52 on day 22) and 36 for SED. For both Comparison 1 and Comparison 2, WL29 and WL53 were combined for analysis of day 22; only WL53 was used for analysis of day 23.
Absolute food intake and feed efficiency decreased in runners when voluntary running ceased
Comparison 1 (before and after wheel lock comparison for WL29 and WL53 groups; see Supplemental material): absolute food intake for wheel lock groups on days 22 and 23 after running wheels were locked was 11% and 10% lower, respectively, than their predicted values (Fig. 7A, right panel). Relative food intake for wheel lock groups on day 22 was 3.4% lower than their predicted values, but was not different from their predicted values on day 23 (Fig. 7B, right panel). Feed efficiency for wheel lock groups on days 22 and 23 were 48% and 33% lower, respectively, than their predicted values (Fig. 7C, middle panel).
Sedentary rats continued to eat less than runners when voluntary running ceased
Comparison 2 (between SED and the combined WL29 and WL53 groups after wheel lock; see Supplemental material): the predicted absolute food intake for SED on days 22 and 23 was 17% and 20% lower, respectively, than the observed values for the wheel lock groups (Fig. 7A, right panel). The predicted relative food intake for SED on days 22 and 23 was 17% and 23% lower, respectively, than the observed values for the wheel lock groups (Fig. 7B, right panel). The predicted feed efficiency for SED on days 22 and 23 was 109% and 66% greater, respectively, than the observed values in the wheel lock groups (Fig. 7C, right panel).
Growth rate
Sedentary rats grew more slowly than runners beginning on day 4
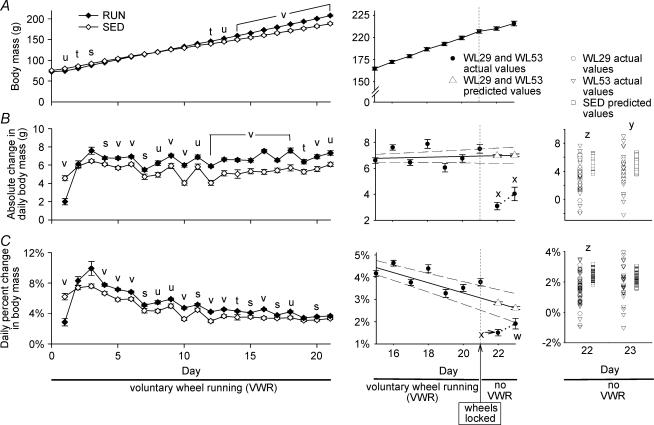
There was no difference in the initial body mass between RUN and SED (75.6 ± 1.8 and 72.5 ± 0.9 g, respectively; Fig. 8A, left panel). On days 1–3, SED had a greater absolute body mass than RUN (Fig. 8A, left panel). This was due to greater absolute and relative growth rates on day 1 in SED relative to RUN, but growth rates were not different on days 2 or 3 (Fig. 8B and C, left panels). However, beginning on day 4 both the absolute and relative growth rates were less in SED than in RUN (Fig. 8B and C, left panels); this was reflected in a significantly lower body mass in SED than RUN beginning on day 12 and remaining through day 21 (Fig. 8A, left panel), such that SED weighed 9.4% less (P < 0.001) on day 21 than did RUN. Over the 21-day experimental period, SED animals gained an average of 113.0 ± 8.8 g and RUN animals gained an average of 135.6 ± 10.7 g (P < 0.001 between groups). This represented a fold-increase in body mass of 2.52 ± 0.04 in SED and 2.89 ± 0.03 in RUN (P < 0.001 between groups). There was no correlation between total running distance and fold-increase in body mass (r= 0.15, P= 0.16; data not shown). Body mass and growth data for individual groups are presented in Table 1.
Figure 8. Growth characteristics.
A, growth curve; B, absolute growth rate; and C, relative growth rate. Absolute growth rate (B) is daily growth expressed as the absolute change from the previous day's mass. Relative growth rate (C) is the daily growth rate expressed as percentage change relative to the previous day's mass. Left panels: comparison of combined groups with unrestricted voluntary running wheel access (RUN; ♦) and groups with regular sedentary cage activity (SED; ⋄) during 21 days of voluntary wheel running. Data points represent the mean ± s.e.m. s, t, u and v, significant difference (P < 0.05, 0.01, 0.005 and 0.001, respectively, between SED and RUN; Student's t test). n = 88 for RUN and 36 for SED. Middle panels: growth characteristics for 7 days prior and 2 days after running wheels were locked. Voluntary running wheels were locked beginning on day 21 (indicated by arrow; see Supplemental material) so that the rats could no longer run. The legend for data points in the lower portion of A is for the middle panels of A, B and C. Middle panel of A: the continuous line connects actual values of the combined WL29 and WL53 groups for days 15–23. No predicted values are displayed in this panel. Middle panels for B and C: Comparison 1 (see Supplemental material): expected values for days 22 and 23 for WL29 and WL53 were predicted from linear regression equations for each animal based on values from days 4 to 21 (only data starting on day 15 are shown for clarity of the figure). ▵, predicted values on days 22 and 23; •, actual values from days 15 to 23. The continous line represents the regression line, and the dashed lines represent the 95% confidence intervals. The dotted line in the lower right-hand corner connects the observed values for days 22 and 23. The differences between the predicted and observed values for each animal were compared using Student's t test. Data points represent the mean ± s.e.m. w and x, significant difference (P < 0.05 and 0.001, respectively) between observed and predicted values. n = 52 for day 22, and n = 26 for day 23. Right panels: Comparison 2 (see Supplemental material): scatter plots of observed values for WL29 (○ in columns 1 and 3) and WL53 (▿ in columns 1 and 3) and predicted values for SED (□ in columns 2 and 4) for absolute and relative growth rates on days 22 and 23. The legend for data points is located above right panel B. Data points represent the individual values for each animal. Values for SED were predicted for each animal using linear regression analysis and were compared to observed values for WL29 and WL53 using the Mann-Whitney rank-sum test. y and z, significantly different (P < 0.005 and 0.001, respectively). n = 26 each for WL29 and WL53 (total of 52 on day 22) and 36 for SED. For both Comparison 1 and Comparison 2, WL29 and WL53 were combined for analysis of day 22; only WL53 was used for analysis of day 23.
Growth rate decreased on the first day of no voluntary running
Comparison 1 (before and after wheel lock comparison for WL29 and WL53 groups; see Supplemental material): upon the cessation of voluntary wheel running, the absolute increase in body mass on days 22 and 23 was 56% and 43% less, respectively, than their predicted growth values (Fig. 8B, middle panel). Relative increase in body mass for wheel lock groups on days 22 and 23 was 47% and 26% less, respectively, than their predicted values (Fig. 8C, middle panel).
Sedentary rats grew faster than runners when voluntary running ceased
Comparison 2 (between SED and the combined WL29 and WL53 groups after wheel lock; see Supplemental material): the predicted absolute increase in body mass for SED on days 22 and 23 was 73% and 33% greater, respectively, than the observed values for the wheel lock groups after running ceased (Fig. 8B, right panel). The predicted relative change in body mass for SED on day 22 was 65% greater than the observed value for the wheel lock groups, but on day 23, the predicted and observed values were not significantly different (Fig. 8C, right panel).
Skeletal and left ventricular muscle mass
Sedentary rats had smaller skeletal muscle mass than runners
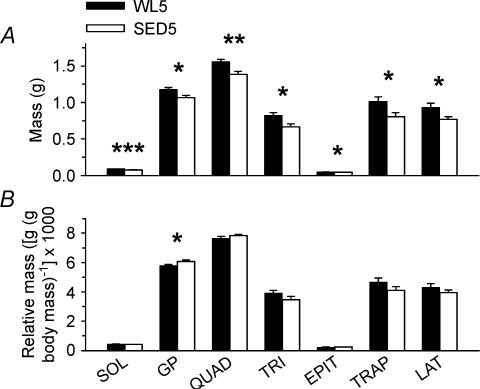
To determine whether the lower body mass in SED than in RUN (Fig. 8A, left panel) could be due to lower skeletal muscle mass, seven different muscle masses were obtained from WL5 and SED5 groups. The absolute wet mass of each muscle was significantly lower in SED5 than in WL5 (Fig. 9A). Muscle wet mass relative to body mass was not different for 6 of the 7 muscles (Fig. 9B). Left ventricular mass was 16% lower (P < 0.001) in SED5 than in WL5 (0.395 ± 0.017 and 0.472 ± 0.014 g, respectively; data not shown). This relationship remained true when left ventricular mass was expressed relative to body mass (2.063 ± 0.023 and 2.203 ± 0.030 ((g/g body mass) × 1000), respectively (P < 0.005; data not shown).
Figure 9. Skeletal muscle mass.
Absolute (A) and relative (B) wet masses for the soleus (SOL), gastrocnemius/plantaris complex (GP), quadriceps (QUAD), triceps (TRI), epitrochlearis (EPIT), acromiotrapezius (TRAP), and latissimus dorsi (LAT) muscles. Muscles were removed from the left side only; the values shown demonstrate only the unilateral masses. Bars represent the means ± s.e.m.*, ** and ***, significant difference (P < 0.05, 0.01 and 0.001, respectively; Student's t test) between WL5 and SED5 for a given muscle. n = 20 for SOL; 10 for GP, QUAD, TRI and EPIT; and 7 for TRAP and LAT.
Discussion
An important finding of the current study is that epididymal fat mass increases 25% in just 48 h, from 5 to 53 h after cessation of 21 days of voluntary running. The increase in epididymal adipose tissue led to a new hypothesis that, relative to 5 h of no voluntary running, triacylglycerol synthesis would be enhanced at 29 and 53 h after cessation of 21 days of voluntary running, which was then tested. The dramatic increase in triacylglycerol synthesis between the 5th and 10th hour of no voluntary running was unexpected, as well as the magnitude of the increased triacylglycerol synthesis, a 3.5–4.7-fold overshoot between 10 and 53 h of no voluntary running relative to the groups with regular sedentary cage activity. Other potentially important findings in the present study include the higher epididymal fat protein level of C/EBPα and the lower muscle mass in pubertal male rats with regular sedentary cage activity relative to 21 days of voluntary wheel running. Another novel observation is the quick decline in the accelerated growth rate of rapidly growing rats with voluntary running wheels following a return to regular sedentary cage activity.
Epididymal adipose tissue mass and cellularity
The epididymal and omental fat pads increased in mass by 25% and 48%, respectively, following 53 h of no voluntary running compared to 5 h of no voluntary running (Fig. 3A). During this same period, mean epididymal adipocyte volume increased 19% with no change in cell number (Fig. 3D and F), suggesting that there is an increase in the amount of cellular lipid. This implication was further supported by the finding that the amount of lipid per epididymal adipocyte increased 29% between 5 and 53 h of no voluntary running (Fig. 3E). Thus, increases in epididymal pad mass, adipocyte volume, and lipid per adipocyte occurred concurrently between 29 and 53 h of no voluntary running, a coincident time frame during which there is decreased submaximal insulin-stimulated glucose uptake into the epitrochlearis muscle relative to 5 h of no voluntary running (Kump & Booth, 2005).
Triacylglycerol levels in adipose tissue are a result of the balance between storage and lipolysis; increased storage could be affected by multiple factors, such as increases in substrate (plasma glucose, free fatty acids, and triacylglycerol) supply and uptake (increased adipocyte insulin sensitivity and lipoprotein lipase activity), enhanced de novo synthesis of fatty acids in adipocytes, and augmented incorporation of fatty acids or other substrates into triacylglycerol in adipocytes. Triacylglycerol levels could also be increased by enhanced re-incorporation rate of lipolysis-derived fatty acids into triacylglycerol. Of the possible mechanisms regulating triacylglycerol storage in adipose tissue when daily exercise stops, previous studies have reported that glucose uptake (Craig et al. 1983), lipoprotein lipase activity (Applegate & Stern, 1987; Lambert et al. 1994), the capacity for fatty acid synthesis (as measured by fatty acid synthase activity (Tsai et al. 1981)), and glycerolipid synthesis (Askew et al. 1973) are elevated 1–9 days, 84 h to 7 days, 12 days and 24 h, respectively, following the last exercise bout in exercise-trained animals relative to sedentary controls. Exercise training in rats enhances adrenaline-induced lipolysis (Askew et al. 1975), but basal lipolysis is unchanged (Kawanami et al. 2002) relative to sedentary controls up to 48 h after the last exercise bout, and thus decreased lipolysis is not suggested as a likely contributor to the increased epididymal fat upon cessation of voluntary exercise. In addition to these potential metabolic mechanisms, the transcription factors PPARγ and C/EBPα are important positive regulators of adipocyte differentiation and lipogenic metabolism (Gregoire, 2001). Of these prospective mechanisms, we considered that glucose uptake, triacylglycerol synthesis, PPARγ and C/EBPα protein levels, and food intake might be associated with the increased epididymal fat mass observed with the cessation of voluntary wheel running, although there are likely to be other mechanisms that could be involved. In addition, to ascertain whether the rapid increase in epididymal and omental fat masses upon cessation of voluntary running were paralleled by increases in body mass, we also determined the growth rates of the animals.
Epididymal triacylglycerol synthesis
A new hypothesis was generated that an increase in the capacity to synthesize triacylglycerol could contribute to the increased epididymal adipose mass. This hypothesis was based on findings showing that glycerolipid synthesis in epididymal fat increased 59% 24 h after the last exercise bout following 12 weeks of treadmill training in rats relative to untrained controls (Askew et al. 1973). Our findings (a) extend this earlier report (Askew et al. 1973) by filling in some of the time points within the first 24 h of ending voluntary running, and (b) suggest the existence of a daily cycle of triacylglycerol synthesis with daily running, both of which are discussed next.
Time points within the first 24 h of no voluntary running
At 5 h of regular sedentary cage activity after 21 days of voluntary running, triacylglycerol synthesis was 4.2-fold greater in the group with regular sedentary cage activity than in the 5-h wheel lock group. However, only 5 h later, at the 10th hour of no voluntary running, triacylglycerol synthesis rose to be 14-fold higher than after the 5th hour of no voluntary running, so that regular sedentary cage activity values were now only 21% of the triacylglycerol synthesis rates in 10-h wheel lock rats (Fig. 5). Thus, a dramatic rise and overshoot in the activity of the triacylglycerol synthetic pathway occurs between the 5th and 10th hour of no voluntary running, which remains elevated above regular sedentary cage activity values at the 53rd hour of no voluntary running. The 3.5- to 4.7-fold overshoot in triacylglycerol synthesis in epididymal fat at the 10th, 29th, and 53rd hours of no voluntary running could contribute to the concomitant increases in its mass and cell size.
Daily cycle of triacylglycerol synthesis with daily running
Since our observations were made in the first 12 h of no voluntary running (the light cycle), these observations imply what was likely to be occurring during each light cycle of the third week of voluntary wheel running. Triacylglycerol synthesis would have been ∼19% of the value of regular sedentary cage activity at the 5th hour of the light cycle, and then would have been ∼370% of the value of regular sedentary cage activity at the 10th hour of the light cycle, implying the potential existence of a cycle of epididymal triacylglycerol synthesis in reoccurrence with daily running. The triacylglycerol synthesis cycling conjecture is supported by the findings of Park et al. (2002), who demonstrated that the activity of the mitochondrial form of glycerol-3-phosphate acyltransferase (GPAT) in rat epididymal fat is repressed ∼50% following a single 30-min treadmill run. GPAT catalyses the initial and committed step of glycerolipid synthesis (Coleman & Lee, 2004). Thus, an observation emerges that epididymal triacylglycerol synthesis cycles when daily physical activity is employed. It is likely to be repressed during and up to at least 5 h after physical activity in the animals in the current report, and then overshoots (relative to regular sedentary cage activity) until the dark cycle begins and voluntary running resumes. The data from Park et al. (2002) showing that mitochondrial GPAT activity is repressed immediately after 30 min of exercise suggests that triacylglycerol synthesis could be repressed early in the dark cycle when nightly running first begins. However, if running does not occur, such as took place in the groups that had their wheels locked so that they could not run, then triacylglycerol synthesis is at least 3.5-fold greater than sedentary for 10–53 h post-voluntary running. We speculate that the overshoot in triacylglycerol synthesis continues until the next bout of physical activity or until enlargement of the adipocyte provides negative feedback on triacylglycerol synthesis. Collectively, an important concept materializes: daily physical activity is associated with cycling of triacylglycerol synthesis in the epididymal fat pad of rats, but the cycling vanishes when physical activity ceases and triacylglycerol synthesis stalls at its apparent zenith. The prolonged maintenance of high triacylglycerol synthesis is associated with increased deposition of lipid in epididymal fat.
PPARγ and C/EBPα protein levels
PPARγ and C/EBPα are important regulators of adipocyte differentiation and lipid metabolism (Gregoire, 2001), and PPARγ activators lead to increased deposition of fat (Yamauchi et al. 2001). We therefore measured PPARγ and C/EBPα protein levels in epididymal fat to determine if they were associated with the increased fat mass upon cessation of voluntary wheel running. Although epididymal adipocyte number did not change in the present study (Fig. 3F), the increased C/EBPα protein levels in the sedentary group (Fig. 6) might suggest that if a longer period of running were employed, there could be an increase in epididymal adipocyte number with regular sedentary cage activity compared to access to running wheels. This concept is supported by data from Craig et al. (1987), who showed that male rats with regular sedentary cage activity had 108% more epididymal adipocytes than rats who had voluntary wheel access from 6 to 12 months of age, although the runners were subjected to an ∼8% food restriction for approximately the last 2 months. In addition, we have observed that 87-week-old female rats with regular sedentary cage activity have 123% more fat cells in the ovarian fat pad than do age-matched rats that have had access to running wheels beginning at 4 weeks of age (D. S. Kump and F. W. Booth, unpublished observation; n = 3 per group). It is also noteworthy that C/EBPα mRNA in human adipose tissue has been shown to higher in obese than in lean humans (Krempler et al. 2000).
Food intake
Another possible cause of the increased epididymal and omental fat mass when voluntary running ceases is the maintenance of both absolute and relative food intake above predicted sedentary values (Fig. 7A and B, right panels). This is further supported by the finding that on day 23, relative food intake in rats that had their wheels locked for 53 h was not different from what was predicted if they would have had continued unrestricted access to the running wheel (Fig. 7B, middle panel). Thus, despite a reduction in physical activity, relative food intake on the second day of no voluntary running did not decrease, suggesting a possible lingering increase in voluntary food intake upon the cessation of voluntary running.
Growth rate
Because of the rapid increase in epididymal and omental fat masses upon cessation of voluntary wheel running, we examined the growth rate of the different groups before wheel lock as a reference point for what occurred after wheel lock. Remarkably, we found that rapidly growing male rats with regular sedentary cage activity grew at a slower rate than those with access to voluntary running wheels, so that they gained 22.6 g less body mass than the runners when body mass approximately tripled over 21 days (Fig. 8A, left panel). This phenomenon has been observed in some (Yano et al. 1997), but not all (Collier et al. 1969; Hokama et al. 1997; Johnson et al. 1977; Zachwieja et al. 1997), studies using voluntary running wheels with male rats of a similar age. One probable contributing factor to the difference in body mass between rats with regular sedentary cage activity and those with running wheel access could be the lower food intake observed in rats with regular sedentary cage activity (Fig. 7A, left panel). Although a lower food intake might be expected of the animals with regular sedentary cage activity because of their lower body mass, their food intake per body mass was also lower (Fig. 7B, left panel); this is consistent with some (Yano et al. 1997; Zachwieja et al. 1997), but not all (Collier et al. 1969; Johnson et al. 1977) studies. Despite the lower food intake and body mass in the group with regular sedentary cage activity, there was a higher feed efficiency in this group relative to the one with wheel access (Fig. 7C, left panel); this is most likely a result of additional calories expended on the running wheel by the group that had wheel access. Intriguingly, in the current study the accelerated rise in body mass in young male rats with voluntary running was attenuated with 1 day of decreased physical activity, whereas it took 4 days to accelerate growth rate at the initiation of voluntary wheel running (Fig. 8B and C, left and middle panels); this attenuation in growth rate occurred, at least in part, as a result of a lower feed efficiency (Fig. 7C, middle panel). In addition, the attenuated growth rate (Fig. 8B and C, middle panels) fell to sedentary levels despite maintenance of food intake at levels higher than sedentary values (Fig. 7A and B, right panels). The attenuation in growth rate also occurred during the same time frame in which epididymal fat mass increased 25% and omental fat mass increased 48%, indicating that although whole-body growth rate was attenuated, these fat pad masses were increasing. Future investigations, including measurement of water intake, whole body composition analysis, and intestinal contents are needed to follow up these observations.
Skeletal muscle mass
To determine whether differences in skeletal muscle mass contributed to the difference in body mass between rats with and without wheel access, the masses of seven different muscles were measured (6 predominantly type II and 1 predominately type I muscle (Delp & Duan, 1996; Nesher et al. 1985)). A lower skeletal muscle mass in animals with regular sedentary cage activity accounts, at least partly, for the lower growth rate (Fig. 9A). Rodnick et al. (1989, 1990) have reported that 6 weeks of voluntary running is associated with larger soleus (predominantly type I), but not plantaris (predominantly type II), muscle mass in male rats with initial body masses of 180 g; thus, their time points did not encapsulate the period of body mass increase from 70 to 200 g in male rats as reported herein. The present report extends these findings to rapidly growing rats and demonstrates for the first time that type II muscles undergo increased growth in pubertal male rats in response to voluntary wheel running. Hypertrophy of the predominately type II extensor digitorum longus muscle in adult and old female rats who had 5 or 23 months of voluntary wheel running beginning at 4 months of age has also been reported (Brown et al. 1992).
Our presumption is that the lower muscle mass in animals with regular sedentary cage activity is a result of not having access to running wheels during what is a period of rapid growth. Human studies support a proposed concept that physical inactivity during whole body growth results in lower skeletal muscle mass. Physically inactive, 10-year-old children consumed 196 fewer kilocalaries per day, had 3.5% more of their body as fat, had a lower fat-free mass, and exhibited similar body mass index as compared to more physically active children (Deheeger et al. 1997). Wells (2003) cites reports that children in more current times are more physically inactive with lower fat-free mass as compared to the reference child (Fomon et al. 1982). Wells (2003) suggests further investigation to test the possibility that children are building up lower levels of fat-free mass during childhood due to declining physical activity levels, an idea supported by the current data. Taken together, a concept is offered that physical inactivity in childhood contributes to lesser growth of skeletal muscle mass.
Clinical significance
There are several findings of the current study that, if applicable to humans, might carry important implications for human health. One of these implications is the importance of regular physical activity to prevent increases in abdominal fat (Hill et al. 2003), especially given the strong evidence that regular physical activity protects against obesity (Swinburn et al. 2004; U.S. Department of Health & Human Services, 1996). This may be particularly true for already overweight or obese individuals who are trying to reduce body fat. It has been suggested that a consistent element of successful weight loss programmes is regular exercise (Saris et al. 2003; U.S. Department of Health & Human Services (1996). Indeed, it is difficult for overweight or obese individuals to reduce body weight and prevent future weight regain. The current results may provide one possible mechanism by which difficulty in weight loss with irregular daily exercise might occur, i.e. with regular exercise there is a cyclic repression of triacylglycerol synthesis in adipose tissue; however, if exercise frequency is irregular, an elevation above baseline of triacylglycerol synthesis occurs that might tend to offset any progress in body fat reduction. Additionally, it has been suggested that pharmacological inhibition of components of triacylglycerol synthesis may be a treatment for obesity (Subauste & Burant, 2003; Thuresson, 2004). The findings of the current study, in conjunction with those of Park et al. (2002), would suggest that daily physical activity/exercise may be an effective non-pharmacological intervention to suppress triacylglycerol synthesis in adipose tissue. Another implication is the further development and elucidation of a model of physical inactivity and its concomitant health implications, with multiple changes occurring within 53 h of no voluntary running (decline in insulin sensitivity in the epitrochlearis muscle (Kump & Booth, 2005), increase in epididymal and omental adipose tissue mass and epididymal adipocyte size, and overshoot in triacylglycerol synthesis in epididymal fat). The rapid increase in fat mass and cell size observed upon cessation of voluntary running is analogous to the decreased physical activity and resulting increase in adiposity occurring in modern humans. As obesity is slow in developing, the mechanisms responsible may only have small differences between physically active and inactive humans, making it difficult to detect the underlying pathophysiology. However, with the locking of running wheels in highly physically active rats, as undertaken in the current study, a more rapid and pronounced increase in epididymal fat allows for future mechanistic studies. The potential implications have tremendous relevance to human health, as ‘A shortage of credible information exists on physical activity patterns that have the potential to reverse the national obesity epidemic and reduce the risk of chronic diseases’ (Prentice et al. 2004).
The observation that there is a lower skeletal muscle mass with regular sedentary cage activity compared to voluntary wheel running in rapidly growing rats may also have potential important implications for human health. One potential implication is the possibility that decreased physical activity during childhood and/or adolescent growth might contribute to suboptimal muscular development. Another implication of the lower muscle mass in rats without wheel access is that skeletal muscle accounts for 75–95% of insulin-mediated glucose disposal in humans (Baron et al. 1988), so a smaller mass of skeletal muscle would result in less tissue for insulin-mediated glucose disposal. The lower mass of the predominately type I soleus muscle in rats without running wheel access may also have an important health implication as a lower percentage of type I fibres in humans is associated with lower measures of insulin sensitivity (Lillioja et al. 1987; Hickey et al. 1995; Kriketos et al. 1996) and greater adiposity (Lillioja et al. 1987; Wade et al. 1990; Hickey et al. 1995; Kriketos et al. 1996; Tanner et al. 2002).
Conclusion
In summary, there is a rapid increase and overshoot in triacylglycerol synthesis between 5 and 10 h of reduced physical activity that is sustained at 29 and 53 h of reduced physical activity. It is likely that the sustained overshoot could contribute to an increase in epididymal fat mass and adipocyte size between 29 and 53 h of no voluntary running, although other mechanisms, such as increased fatty acid synthesis (Tsai et al. 1981), and lipoprotein lipase activity (Applegate & Stern, 1987; Lambert et al. 1994) may also be involved. Further research is needed to investigate mechanisms responsible for the increase and overshoot in triacylglycerol synthesis and other possible mechanisms responsible for increased body fat with reduced physical activity.
Acknowledgments
We thank Dr Boyd O'Dell for the use of the Coulter counter, Tsghe Abraha for help with the harvesting of tissues, Dr Richard Madsen for consultation on statistics, and Drs Andrew Shanely, Craig Stump, and Sean Newcomer for helpful comments on the manuscript. This work was supported by the following grants: American Heart Association (Heartland chapter) predoctoral fellowship 0135202Z (DSK), NIH R21AR48368 (FWB), and anonymous gifts.
Supplemental material
The online version of this paper can be accessed at: 10.1113/jphysiol.2005.084525 http://jp.physoc.org/cgi/content/full/jphysiol.2005.084525/DC1 and contains supplemental material consisting of more detailed methods, including statistical analysis.
This material can also be found as part of the full-text HTML version available from http://www.blackwell-synergy.com
References
- Applegate EA, Stern JS. Exercise termination effects on food intake, plasma insulin, and adipose lipoprotein lipase activity in the Osborne-Mendel rat. Metabolism. 1987;36:709–714. doi: 10.1016/0026-0495(87)90104-1. [DOI] [PubMed] [Google Scholar]
- Askew EW, Huston RL, Dohm GL. Effect of physical training on esterification of glycerol-3-phosphate by homogenates of liver, skeletal muscle, heart, and adipose tissue of rats. Metabolism. 1973;22:473–480. doi: 10.1016/0026-0495(73)90039-5. [DOI] [PubMed] [Google Scholar]
- Askew EW, Huston RL, Plopper CG, Hecker AL. Adipose tissue cellularity and lipolysis. Response to exercise and cortisol treatment. J Clin Invest. 1975;56:521–529. doi: 10.1172/JCI108120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron AD, Brechtel G, Wallace P, Edelman SV. Rates and tissue sites of non-insulin- and insulin-mediated glucose uptake in humans. Am J Physiol. 1988;255:E769–E774. doi: 10.1152/ajpendo.1988.255.6.E769. [DOI] [PubMed] [Google Scholar]
- Booth FW, Chakravarthy MV, Gordon SE, Spangenburg EE. Waging war on physical inactivity: using modern molecular ammunition against an ancient enemy. J Appl Physiol. 2002;93:3–30. doi: 10.1152/japplphysiol.00073.2002. [DOI] [PubMed] [Google Scholar]
- Brown M, Ross TP, Holloszy JO. Effects of ageing and exercise on soleus and extensor digitorum longus muscles of female rats. Mech Ageing Dev. 1992;63:69–77. doi: 10.1016/0047-6374(92)90017-8. [DOI] [PubMed] [Google Scholar]
- Cartwright AL. Determination of adipose tissue cellularity. In: Hausman GJ, Von Martin R, editors. Biology of the Adipocyte: Research Approaches. New York: Nostrand Reinhold Co; 1987. [Google Scholar]
- Coleman RA, Lee DP. Enzymes of triacylglycerol synthesis and their regulation. Prog Lipid Res. 2004;43:134–176. doi: 10.1016/s0163-7827(03)00051-1. [DOI] [PubMed] [Google Scholar]
- Collier G, Leshner AI, Squibb RL. Dietary self-selection in active and non-active rats. Physiol Behav. 1969;4:79–82. [Google Scholar]
- Craig BW, Garthwaite SM, Holloszy JO. Adipocyte insulin resistance: effects of aging, obesity, exercise, and food restriction. J Appl Physiol. 1987;62:95–100. doi: 10.1152/jappl.1987.62.1.95. [DOI] [PubMed] [Google Scholar]
- Craig BW, Thompson K, Holloszy JO. Effects of stopping training on size and response to insulin of fat cells in female rats. J Appl Physiol. 1983;54:571–575. doi: 10.1152/jappl.1983.54.2.571. [DOI] [PubMed] [Google Scholar]
- Deheeger M, Rolland-Cachera MF, Fontvieille AM. Physical activity and body composition in 10 year old French children: linkages with nutritional intake? Int J Obes Relat Metab Disord. 1997;21:372–379. doi: 10.1038/sj.ijo.0800415. [DOI] [PubMed] [Google Scholar]
- Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol. 1996;80:261–270. doi: 10.1152/jappl.1996.80.1.261. [DOI] [PubMed] [Google Scholar]
- Despres JP. Abdominal obesity as important component of insulin-resistance syndrome. Nutrition. 1993;9:452–459. [PubMed] [Google Scholar]
- Dohm GL, Barakat HA, Tapscott EB, Beecher GR. Changes in body fat and lipogenic enzyme activities in rats after termination of exercise training. Proc Soc Exp Biol Medical. 1977;155:157–159. doi: 10.3181/00379727-155-39764. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288:1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Fomon SJ, Haschke F, Ziegler EE, Nelson SE. Body composition of reference children from birth to age 10 years. Am J Clin Nutr. 1982;35:1169–1175. doi: 10.1093/ajcn/35.5.1169. [DOI] [PubMed] [Google Scholar]
- Frayn KN. Visceral fat and insulin resistance – causative or correlative? Br J Nutr. 2000;83(Suppl. 1):S71–S77. doi: 10.1017/s0007114500000982. [DOI] [PubMed] [Google Scholar]
- Galton DJ. Lipogenesis in human adipose tissue. J Lipid Res. 1968;9:19–26. [PubMed] [Google Scholar]
- Gan SK, Kriketos AD, Poynten AM, Furler SM, Thompson CH, Kraegen EW, Campbell LV, Chisholm DJ. Insulin action, regional fat, and myocyte lipid: altered relationships with increased adiposity. Obes Res. 2003;11:1295–1305. doi: 10.1038/oby.2003.176. [DOI] [PubMed] [Google Scholar]
- Gregoire FM. Adipocyte differentiation: from fibroblast to endocrine cell. Exp Biol Med (Maywood) 2001;226:997–1002. doi: 10.1177/153537020122601106. [DOI] [PubMed] [Google Scholar]
- Hickey MS, Carey JO, Azevedo JL, Houmard JA, Pories WJ, Israel RG, Dohm GL. Skeletal muscle fiber composition is related to adiposity and in vitro glucose transport rate in humans. Am J Physiol. 1995;268:E453–E457. doi: 10.1152/ajpendo.1995.268.3.E453. [DOI] [PubMed] [Google Scholar]
- Hill JO, Wyatt HR, Reed GW, Peters JC. Obesity and the environment: where do we go from here? Science. 2003;299:853–855. doi: 10.1126/science.1079857. [DOI] [PubMed] [Google Scholar]
- Hokama JY, Streeper RS, Henriksen EJ. Voluntary exercise training enhances glucose transport in muscle stimulated by insulin-like growth factor I. J Appl Physiol. 1997;82:508–512. doi: 10.1152/jappl.1997.82.2.508. [DOI] [PubMed] [Google Scholar]
- Hu FB. Sedentary lifestyle and risk of obesity and type 2 diabetes. Lipids. 2003;38:103–108. doi: 10.1007/s11745-003-1038-4. [DOI] [PubMed] [Google Scholar]
- Johnson PB, Kochan R, Graves D. Voluntary exercise, food intake pattern, and early growth in young male albino rats. Med Sci Sports. 1977;9:54. [Google Scholar]
- Kawanami H, Nomura S, Sakurai T, Sakurai T, Yamagishi H, Komabayashi T, Izawa T. Possible role of nitric oxide on adipocyte lipolysis in exercise-trained rats. Jpn J Physiol. 2002;52:343–352. doi: 10.2170/jjphysiol.52.343. [DOI] [PubMed] [Google Scholar]
- Krempler F, Breban D, Oberkofler H, Esterbauer H, Hell E, Paulweber B, Patsch W. Leptin, peroxisome proliferator-activated receptor-gamma, and CCAAT/ enhancer binding protein-alpha mRNA expression in adipose tissue of humans and their relation to cardiovascular risk factors. Arterioscler Thromb Vasc Biol. 2000;20:443–449. doi: 10.1161/01.atv.20.2.443. [DOI] [PubMed] [Google Scholar]
- Kriketos AD, Pan DA, Lillioja S, Cooney GJ, Baur LA, Milner MR, Sutton JR, Jenkins AB, Bogardus C, Storlien LH. Interrelationships between muscle morphology, insulin action, and adiposity. Am J Physiol. 1996;270:1332–1339. doi: 10.1152/ajpregu.1996.270.6.R1332. [DOI] [PubMed] [Google Scholar]
- Kump DS, Booth FW. Alterations in insulin receptor signaling in the rat epitrochlearis muscle upon cessation of voluntary exercise. J Physiol. 2005;562:829–838. doi: 10.1113/jphysiol.2004.073593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert EV, Wooding G, Lambert MI, Koeslag JH, Noakes TD. Enhanced adipose tissue lipoprotein lipase activity in detrained rats: independent of changes in food intake. J Appl Physiol. 1994;77:2564–2571. doi: 10.1152/jappl.1994.77.6.2564. [DOI] [PubMed] [Google Scholar]
- Lillioja S, Young AA, Culter CL, Ivy JL, Abbott WG, Zawadzki JK, Yki-Jarvinen H, Christin L, Secomb TW, Bogardus C. Skeletal muscle capillary density and fiber type are possible determinants of in vivo insulin resistance in man. J Clin Invest. 1987;80:415–424. doi: 10.1172/JCI113088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffeis C. Aetiology of overweight and obesity in children and adolescents. Eur J Pediatr. 2000;159(Suppl. 1):S35–S44. doi: 10.1007/pl00014361. [DOI] [PubMed] [Google Scholar]
- Nesher R, Karl IE, Kipnis DM. Dissociation of effects of insulin and contraction on glucose transport in rat epitrochlearis muscle. Am J Physiol. 1985;249:C226–C232. doi: 10.1152/ajpcell.1985.249.3.C226. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288:1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- Park H, Kaushik VK, Constant S, Prentki M, Przybytkowski E, Ruderman NB, Saha AK. Coordinate regulation of malonyl-CoA decarboxylase, sn-glycerol-3-phosphate acyltransferase, and acetyl-CoA carboxylase by AMP-activated protein kinase in rat tissues in response to exercise. J Biol Chem. 2002;277:32571–32577. doi: 10.1074/jbc.M201692200. [DOI] [PubMed] [Google Scholar]
- Popkin BM. The nutrition transition and its health implications in lower-income countries. Public Health Nutr. 1998;1:5–21. doi: 10.1079/phn19980004. [DOI] [PubMed] [Google Scholar]
- Prentice RL, Willett WC, Greenwald P, Alberts D, Bernstein L, Boyd NF, Byers T, Clinton SK, Fraser G, Freedman L, Hunter D, Kipnis V, Kolonel LN, Kristal BS, Kristal A, Lampe JW, McTiernan A, Milner J, Patterson RE, Potter JD, Riboli E, Schatzkin A, Yates A, Yetley E. Nutrition and physical activity and chronic disease prevention: research strategies and recommendations. J Natl Cancer Inst. 2004;96:1276–1287. doi: 10.1093/jnci/djh240. [DOI] [PubMed] [Google Scholar]
- Rattarasarn C, Leelawattana R, Soonthornpun S, Setasuban W, Thamprasit A. Gender differences of regional abdominal fat distribution and their relationships with insulin sensitivity in healthy and glucose-intolerant Thais. J Clin Endocrinol Metab. 2004;89:6266–6270. doi: 10.1210/jc.2004-0209. [DOI] [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. J Biol Chem. 1964;239:375–380. [PubMed] [Google Scholar]
- Rodnick KJ, Holloszy JO, Mondon CE, James DE. Effects of exercise training on insulin-regulatable glucose-transporter protein levels in rat skeletal muscle. Diabetes. 1990;39:1425–1429. doi: 10.2337/diab.39.11.1425. [DOI] [PubMed] [Google Scholar]
- Rodnick KJ, Reaven GM, Haskell WL, Sims CR, Mondon CE. Variations in running activity and enzymatic adaptations in voluntary running rats. J Appl Physiol. 1989;66:1250–1257. doi: 10.1152/jappl.1989.66.3.1250. [DOI] [PubMed] [Google Scholar]
- Ryan DH. Diet and exercise in the prevention of diabetes. Int J Clin Pract Supplement. 2003:28–35. [PubMed] [Google Scholar]
- Saris WH. Fit, fat and fat free: the metabolic aspects of weight control. Int J Obes Relat Metab Disord. 1998;22(Suppl. 2):S15–S21. [PubMed] [Google Scholar]
- Saris WH, Blair SN, van Baak MA, Eaton SB, Di Davies PSPL, Fogelholm M, Rissanen A, Schoeller D, Swinburn B, Tremblay A, Westerterp KR, Wyatt H. How much physical activity is enough to prevent unhealthy weight gain? Outcome of the IASO 1st Stock Conference and consensus statement. Obes Rev. 2003;4:101–114. doi: 10.1046/j.1467-789x.2003.00101.x. [DOI] [PubMed] [Google Scholar]
- Shuldiner AR, Munir KM. Genetics of obesity: more complicated than initially thought. Lipids. 2003;38:97–101. doi: 10.1007/s11745-003-1037-5. [DOI] [PubMed] [Google Scholar]
- Subauste A, Burant CF. DGAT: novel therapeutic target for obesity and type 2 diabetes mellitus. Curr Drug Targets Immune Endocr Metabol Disord. 2003;3:263–270. doi: 10.2174/1568008033340081. [DOI] [PubMed] [Google Scholar]
- Swinburn BA, Caterson I, Seidell JC, James WP. Diet, nutrition and the prevention of excess weight gain and obesity. Public Health Nutr. 2004;7:123–146. doi: 10.1079/phn2003585. [DOI] [PubMed] [Google Scholar]
- Tanner CJ, Barakat HA, Dohm GL, Pories WJ, MacDonald KG, Cunningham PR, Swanson MS, Houmard JA. Muscle fiber type is associated with obesity and weight loss. Am J Physiol Endocrinol Metab. 2002;282:E1191–E1196. doi: 10.1152/ajpendo.00416.2001. [DOI] [PubMed] [Google Scholar]
- Thibault L, Woods SC, Westerterp-Plantenga MS. The utility of animal models of human energy homeostasis. Br J Nutr. 2004;92(Suppl. 1):S41–S45. doi: 10.1079/bjn20041141. [DOI] [PubMed] [Google Scholar]
- Thuresson ER. Inhibition of glycerol-3-phosphate acyltransferase as a potential treatment for insulin resistance and type 2 diabetes. Curr Opin Invest Drugs. 2004;5:411–418. [PubMed] [Google Scholar]
- Tsai AC, Bach J, Borer KT. Somatic, endocrine, and serum lipid changes during detraining in adult hamsters. Am J Clin Nutr. 1981;34:373–376. doi: 10.1093/ajcn/34.3.373. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Physical Activity and Health. A report of the Surgeon General. Rockville,MD,USA: U.S. Department of Health and Human Services, Public Health Service, Office of the Surgeon General; 1996. [Google Scholar]
- U.S. Department of Health and Human Services. The surgeon General's Call to Action to Prevent and Decrease Overweight and Obesity. Rockville,MD,USA: U.S. Department of Health and Human Services, Public. Health Service, Office of the Surgeon General; 2001. [PubMed] [Google Scholar]
- Vollenweider P. Insulin resistant states and insulin signaling. Clin Chem Laboratory Med. 2003;41:1107–1119. doi: 10.1515/CCLM.2003.173. [DOI] [PubMed] [Google Scholar]
- Wade AJ, Marbut MM, Round JM. Muscle fibre type and aetiology of obesity. Lancet. 1990;335:805–808. doi: 10.1016/0140-6736(90)90933-v. [DOI] [PubMed] [Google Scholar]
- Webber J. Energy balance in obesity. Proc Nutr Soc. 2003;62:539–543. doi: 10.1079/pns2003256. [DOI] [PubMed] [Google Scholar]
- Wells JC. Body composition in childhood: effects of normal growth and disease. Proc Nutr Soc. 2003;62:521–528. doi: 10.1079/pns2003261. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Murakami K, Motojima K, Komeda K, Ide T, Kubota N, Terauchi Y, Tobe K, Miki H, Tsuchida A, Akanuma Y, Nagai R, Kimura S, Kadowaki T. The mechanisms by which both heterozygous peroxisome proliferator-activated receptor gamma (PPARgamma) deficiency and PPARgamma agonist improve insulin resistance. J Biol Chem. 2001;276:41245–41254. doi: 10.1074/jbc.M103241200. [DOI] [PubMed] [Google Scholar]
- Yano H, Yano L, Kinoshita S, Tsuji E. Effect of voluntary exercise on maximal oxygen uptake in young female Fischer 344 rats. Jpn J Physiol. 1997;47:139–141. doi: 10.2170/jjphysiol.47.139. [DOI] [PubMed] [Google Scholar]
- Zachwieja JJ, Hendry SL, Smith SR, Harris RB. Voluntary wheel running decreases adipose tissue mass and expression of leptin mRNA in Osborne-Mendel rats. Diabetes. 1997;46:1159–1166. doi: 10.2337/diab.46.7.1159. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.