A reduced sensitivity of baroreflex control of heart rate (BRS) characterizes arterial hypertension and several cardiovascular diseases (Eckberg et al. 1971; La Rovere et al. 1998), carrying an adverse prognosis, while interventions which improve BRS, including physical training (Hull et al. 1994), may reduce the risk of cardiovascular events. A number of issues related to BRS modulation are still a matter of debate, however, such as the possible interaction between genetic and environmental factors in determining BRS levels, and the choice of the most suitable method to assess BRS in a given condition.
In this issue of The Journal of Physiology, Lenard et al. (2005) provide us with interesting observations on both these topics, by exploring BRS in young healthy sedentary or endurance exercise trained subjects with and without a family history of hypertension (FH+ and FH−, respectively). Indices of spontaneous BRS were derived from computer analysis of systolic blood pressure and heart interval variability, carried out in the time and in the frequency domain through the ‘sequence’ and the ‘spectral’ method, respectively.
A reduced BRS appears to characterize not only patients with established hypertension, but also normotensive offspring of hypertensive parents, who may display a slight blood pressure increase and a decreased carotid elasticity. This was confirmed by studies carried out in twins, which showed a strong influence of genetic variance on BRS in healthy humans. The study by Lenard et al. (2005) provides additional evidence by demonstrating that in sedentary FH+ subjects spontaneous BRS is lower than in their FH− peers. It also shows, however, that exercise training may overcome this genetic predisposition, leading to an improvement in the BRS of FH+ offspring. These findings are in line with the evidence obtained in previous papers on the association between exercise training and an increased BRS, possibly due to a concomitant increase in the compliance of arterial vessels where baroreceptors are located, to an increased efficiency of the neural components of the baroreflex arch, and/or to changes at the effector organ level. The reduced heart rate accompanying endurance exercise training might indeed be associated with an improved neurotransmission in the sinoatrial node. However, it has to be considered that, given the known hyperbolic relation between heart rate and heart interval, in the case of training-induced bradycardia, the same reflex change in heart rate (beats min−1), corresponds to a wider change in heart interval (ms) than in untrained subjects, which might lead to the spurious conclusion on an increased BRS, expressed in ms mmHg−1.
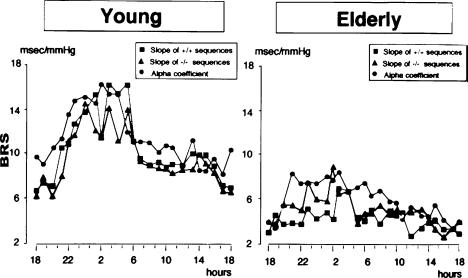
The conventional approach to BRS assessment has consisted for years of laboratory tests, based on the application of controlled and graded stimulation to the cardiovascular system (drug injections) or the baroreceptor areas (neck chamber devices). These methods allow exploration of the sigmoidal baroreflex stimulus–response curve over a wide range of stimulus intensities, approaching threshold and saturation. Although their results carry clinically relevant information, some limitations of this approach have to be acknowledged. The stimulus delivered to baroreceptors is often poorly comparable to physiological blood pressure variations, either because of drug interference or because of artificial stimulation. Moreover, laboratory methods only offer a ‘spot’ quantification of a highly dynamic function such as baroreflex cardiac modulation, known to undergo important changes in different behavioural conditions. In the eighties new methods were described, based on time or frequency domain analysis of spontaneous blood pressure fluctuations coupled with reflex changes in heart interval (Parati et al. 1988, 2000). The methods assessing ‘spontaneous’ BRS do not require any external intervention on the cardiovascular system, and can be used not only in standardized laboratory conditions, but also to investigate the dynamic features of BRS in daily life, thus identifying not only differences in BRS ‘levels’, but also differences in baroreflex cardiac modulation over time, as exemplified by the different 24 h BRS profile in young and elderly individuals (Fig. 1) (Parati et al. 1995). Finally, they allow BRS to be explored around the baroreflex ‘set point’, i.e. at the blood pressure levels where the baroreflex usually works in real life. Even if the actual ability of ‘spontaneous’ BRS assessment to offer new insights into neural cardiovascular regulation, over and above the solid evidence provided by laboratory tests, has recently stimulated some debate, a number of papers have supported the pathophysiological and clinical relevance of spontaneous BRS estimates. Indeed, the information yielded by laboratory and spontaneous methods appears to be complementary, when exploring the complexity inherent in baroreflex cardiovascular modulation.
Figure 1. 24 h BRS profiles in young and elderly subjects.
Different symbols refer to different methods to assess spontaneous baroreflex sensitivity (modified from Parati et al. 1995, used with permission) slope of hypertension-bradycardia (+/+) sequences and of hypotension-tachycardia (−/−) sequences; alpha coefficient (square root of the ratio between systolic blood pressure and heart interval spectral powers in the frequency regions where these signals are coherent). (Modified from Parati et al. 1995, used with permission.)
In spite of its interest, the study by Lenard et al. (2005) is not immune to limitations. This paper is based on data collected from four groups of subjects according to a cross-sectional design, including a relatively small number of subjects for such multiple comparison. The effect of exercise training on the BRS of subjects with different genetic background would have been probably better explored by studying the same subjects before and after endurance exercise training. Moreover, the influence of a family history of hypertension was investigated by including also subjects with only one parent hypertensive, in spite of the complex genetic background of arterial hypertension. Finally the authors, when comparing BRS values of the four groups, failed to account for the lower heart rate of trained subjects, which might result in spuriously higher BRS values (see above).
Although, due to these problems, some caution is needed in extrapolating the authors' conclusions to other groups of subjects, the evidence provided by this paper seems to suggest that regular endurance exercise may help overcoming the negative effects of a genetic predisposition to hypertension on reflex cardiac modulation. Whether such a desirable effect of exercise also has prognostic relevance for future development of cardiovascular complications needs to be assessed by longitudinal larger studies.
References
- Eckberg D, Drabinski M, Braunwald E. N Engl J Med. 1971;285:877–883. doi: 10.1056/NEJM197110142851602. [DOI] [PubMed] [Google Scholar]
- Hull SS, Vanoli E, Adamson PB, Verrier RL, Foreman RD, Schwartz PJ. Circulation. 1994;89:548–552. doi: 10.1161/01.cir.89.2.548. [DOI] [PubMed] [Google Scholar]
- La Rovere MT, Bigger TJ, Marcus FI, Mortara A, Schwartz PJ. Lancet. 1998;351:478–484. doi: 10.1016/s0140-6736(97)11144-8. [DOI] [PubMed] [Google Scholar]
- Lenard Z, Studinger P, Mersich B, Pavlik G, Kollai M. J Physiol. 2005;565:1031–1038. doi: 10.1113/jphysiol.2005.083386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M, Bertinieri G, Pomidossi G, Casadei R, Groppelli A, Pedoti A, Zanchetti A, Mancia G. Hypertension. 1988;12:214–222. doi: 10.1161/01.hyp.12.2.214. [DOI] [PubMed] [Google Scholar]
- Parati G, Di Rienzo M, Mancia G. J Hypertens. 2000;18:7–19. [PubMed] [Google Scholar]
- Parati G, Frattola A, Di Rienzo M, Castiglioni P, Pedotti A, Mancia G. Am J Physiol. 1995;268:H1606–H1612. doi: 10.1152/ajpheart.1995.268.4.H1606. [DOI] [PubMed] [Google Scholar]