Abstract
In four conscious patients who had electrodes implanted in the cervical epidural space for the control of pain, we recorded corticospinal volleys evoked by single-pulse transcranial magnetic stimulation (TMS) over the motor cortex before and after a 20 s period of continuous theta-burst stimulation (cTBS). It has previously been reported that this form of repetitive TMS reduces the amplitude of motor-evoked potentials (MEPs), with the maximum effect occurring at 5–10 min after the end of stimulation. The present results show that cTBS preferentially decreases the amplitude of the corticospinal I1 wave, with approximately the same time course. This is consistent with a cortical origin of the effect on the MEP. However, other protocols that lead to MEP suppression, such as short-interval intracortical inhibition, are characterized by reduced excitability of late I waves (particularly I3), suggesting that cTBS suppresses MEPs through different mechanisms, such as long-term depression in excitatory synaptic connections.
In recent years, several authors have used repeated pulses of transcranial magnetic stimulation (TMS) to produce effects on the excitability of the corticospinal system that outlast the period of stimulation for several minutes or even hours (Pascual-Leone et al. 1994, 1998; Chen et al. 1997; Tergau et al. 1997; Berardelli et al. 1998; Peinemann et al. 2000; Maeda et al. 2000; Huang et al. 2005). Thus 30 min of 1 Hz repetitive TMS (rTMS) decreases the amplitude of the motor-evoked potential (MEP) evoked by single-pulse stimulation for the next 30 min (Chen et al. 1997), whereas higher frequencies may increase MEPs (Berardelli et al. 1998; Peinemann et al. 2000; Maeda et al. 2000; Huang et al. 2005). Since spinal H–reflexes are unaffected by such conditioning, it is usually assumed that these after-effects are due to changes in neural circuits in the cortex, perhaps involving processes such as long-term depression (LTD) or potentiation (LTP) at cortical synapses. In two recent studies, we provided further evidence for the cortical origin of the effects of rTMS by direct recording of the corticospinal volleys evoked by single-pulse TMS in conscious human subjects who had received an implanted epidural stimulator for the control of pain (Di Lazzaro et al. 2002a, b). We found that suprathreshold 5 Hz stimulation of motor cortex is accompanied by a gradual increase in the size and number of descending corticospinal volleys evoked by each TMS pulse that parallels the increase in the MEP (Di Lazzaro et al. 2002a). Subthreshold 5 Hz stimulation (50 total stimuli at active motor threshold, AMT) has no effect on MEPs, but reduces short-interval intracortical inhibition (SICI), as evaluated with EMG measures (Di Lazzaro et al. 2002b). Previous studies with epidural recording have shown that this MEP suppression is accompanied by a reduction in the size and number of the later (I3, I4) I waves (Di Lazzaro et al. 1998). After 5 Hz rTMS, we found that this effect was abolished, consistent with the hypothesis that reduced SICI was due to effects at the motor cortex (Di Lazzaro et al. 2002b).
Huang et al. (2005) have recently described a rapid method of reducing excitability in the motor cortex termed continuous theta-burst conditioning. This uses a short burst of low-intensity (80% AMT), high-frequency (50 Hz) pulses repeated at 5 Hz, the frequency of the theta rhythm in the EEG. Twenty seconds of continuous theta-burst stimulation (cTBS) reduces the amplitude of MEPs for 20–30min.
In the present study, we present recordings of corticospinal activity evoked by single-pulse TMS before and after cTBS over motor cortex in four conscious subjects who had cervical spinal electrodes implanted chronically for control of pain. Previous experiments in which the MEP to a test pulse has been suppressed by a single conditioning stimulus, such as SICI (Di Lazzaro et al. 1998) or transcallosal inhibition (Di Lazzaro et al. 1999), have shown that there is a preferential reduction in the excitability of the circuits that produce the I3 and later waves. In contrast, the present study shows that cTBS preferentially reduces the amplitude of the I1 wave.
Methods
As described in previous publications (Di Lazzaro et al. 1998), we recorded descending corticospinal activity evoked by TMS of the motor cortex directly from the high cervical epidural space of four conscious patients (ages 50, 72, 51 and 47 years). These patients had no abnormality of central nervous system and had electrodes inserted for control of intractable dorso–lumbar pain. All patients gave their written informed consent. The study was performed according to the Declaration of Helsinki, and approved by the ethics committee of the Medical Faculty of the Catholic University of Rome. None of the patients was taking centrally acting medication at the time of the experiments. Recordings were made simultaneously from the epidural electrode and from the relaxed first dorsal interosseous muscle (FDI) of the left hand. MEPs and the corticospinal volleys were amplified and filtered (bandwidth 3 Hz–3 kHz) by D360 amplifiers (Digitimer, Welwyn Garden City, Hertfordshire, UK). Data were collected on a computer and stored for later analysis using a CED 1401 A/D converter (Cambridge Electronic Design, Cambridge, UK).
Magnetic stimulation was performed with a high-power Magstim 200 (Magstim Co., Whitland, Dyfed, UK). A figure-of-eight coil with external loop diameters of 9 cm was held over the right motor cortex at the optimum scalp position to elicit motor responses in the contralateral FDI. Intensities were expressed as a percentage of the maximum output of the stimulator. Resting motor threshold (RMT) was defined according to the recommendations of the IFCN Committee (Rossini et al. 1994) as the minimum stimulus intensity that produced a liminal MEP (>50 µV in 50% of 10 trials) with the tested muscle at rest.
Two different orientations of the stimulating coil over the motor strip were used, with the induced current flowing either in a latero–medial (LM) or in a posterior–anterior (PA) direction. RMT was determined separately for LM and PA stimulation. LM magnetic stimulation was used to identify the latency of the earliest (D wave) descending volley (Di Lazzaro et al. 2004). The responses to 20 stimuli at an intensity of RMT (subjects 1 and 2), and 150% RMT (subjects 3 and 4), were averaged at rest.
Epidural recordings were made between the most proximal and distal of the four electrode contacts on the epidural electrode. These had a surface area of 2.54 mm2 and were 30 mm apart. The distal contact was connected to the reference input of the amplifier. Motor responses and epidural activity were band-pass filtered (bandwidth 3Hz–3 kHz; Digitimer D360 amplifiers), and each single trial was recorded on computer for later analysis using a CED 1401 A/D converter and associated software. Amplitude of the volleys was measured from onset to peak, where onset was defined either as the immediately preceding trough, or as the initial deflection from baseline.
In order to better characterize the descending waves evoked by LM magnetic stimulation, the latencies of the earliest potentials evoked by LM magnetic stimulation in these four patients were compared with the mean values of the earliest potentials evoked by electrical anodal and LM magnetic stimulation in 10 patients (mean age (±s.d.) 60 ± 9.4 years) with a high cervical epidural electrode who had previously been studied.
Repetitive TMS was delivered using a MagPro (Medtronic A/S Denmark) stimulator. The initial direction of the current induced in the brain was anterior to posterior. The magnetic stimulus had a biphasic waveform with a pulse width of about 280 µs. The stimulation intensity was defined in relation to the AMT evaluated using this machine as the minimum single-pulse intensity required to produce a MEP greater than 200 µV on more than five out of 10 trials from the contralateral FDI while the subject was maintaining a voluntary contraction of about 20% of maximum using visual feedback.
Repetitive TMS was performed using the continuous cTBS pattern (Huang et al. 2005) in which three pulses of stimulation are given at 50 Hz, repeated every 200 ms for a total of 300 pulses delivered over the right motor cortex. The stimulus intensity was set at 80% of AMT. This protocol of stimulation leads to a pronounced and prolonged suppression of MEPs recorded in hand muscles that reaches a maximum about 5–10 min after the end of the protocol.
We compared the corticospinal volleys evoked by a standard TMS pulse before and after rTMS. We averaged the responses to 20 PA magnetic stimuli at an intensity of 150% RMT delivered immediately before rTMS, and to six sets of 20 stimuli delivered after the end of rTMS. In subject 4, we recorded the epidural volleys evoked by only 10 PA magnetic stimuli at the fourth interval, and together with these responses we also recorded the epidural volleys evoked by 10 LM magnetic stimuli at 150% RMT in order to compare the baseline D wave with the D wave recorded at this interval. In subject 3, we also repeated epidural recording 1 h after the end of rTMS.
Statistics
Statistical analysis was performed using a repeated-measures ANOVA on the log-transformed peak measures of I wave and MEP amplitudes. Because of the small number of subjects, we averaged data from the post-TBS time points 0–200 s, 201–400 s and 401–600 s before performing the ANOVA. Amplitude data were log transformed in order to normalize their spread.
Results
LM magnetic stimulation evoked the earliest negative potential in all subjects (Fig. 1). It had a latency of 2.8 ms in subject 1, 2.6 ms in subject 2, and 3.2 ms in subject 4. The short latency of this wave is consistent with direct activation of corticospinal axons. We have therefore termed this volley D wave (Di Lazzaro et al. 2004). In the present patients we did not have the opportunity to compare this LM latency with the latency of the earliest volley produced by transcranial electrical stimulation. Nevertheless, we have previously examined 10 patients using both electrical and magnetic transcranial stimuli. In those individuals, electrical anodal stimulation evoked the shortest latency potential with a mean latency of 2.6 ± 0.1 (s.d.) (range 2.4–2.8 ms). The latency of this potential was the same as that of the earliest potential evoked by LM magnetic stimulation in the present study, consistent with our assumption that it was a D wave.
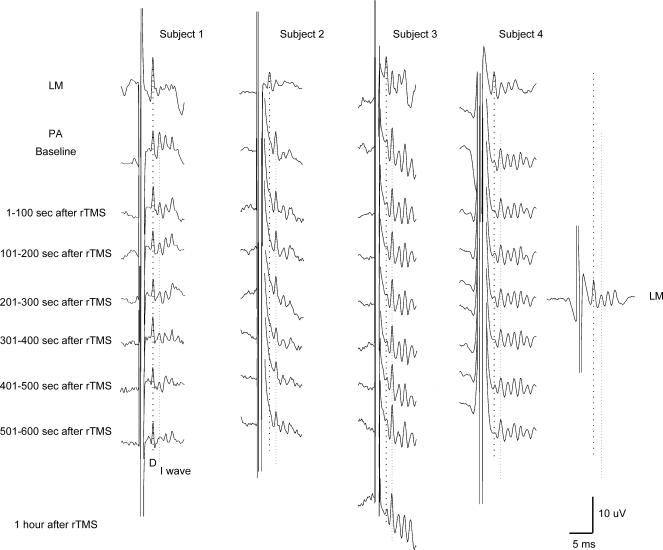
Figure 1. Epidural volleys evoked by magnetic stimulation in baseline and at different intervals after the end of repetitive magnetic stimulation in four subjects.
Epidural volleys evoked by latero–medial (LM) magnetic stimulation (upper traces) and by posterior–anterior (PA) magnetic stimulation (lower traces) in baseline, and at different intervals after the end of repetitive transcranial magnetic stimulation (rTMS). LM magnetic stimulation evokes the earliest wave, i.e. the D wave that is indicated by the vertical dotted line. PA magnetic stimulation evokes a series of descending waves (I waves) and, in subject 1 only, a large D wave. The earlier I wave, the I1, is indicated by the second vertical dotted line. The amplitude of the I1 wave is reduced after rTMS. The most pronounced inhibition is observed 301–500 s after the end of repetitive stimulation. The D wave evoked by PA magnetic stimulation in subject 1, and the D wave evoked by LM magnetic stimulation in subject 4, are not substantially modified by repetitive stimulation.
PA magnetic stimulation evoked a series of descending waves, the largest of these waves had a latency which was 1.2–1.4 ms longer than the earlier volley recruited by LM magnetic stimulation. Since the earliest volley elicited by LM magnetic stimulation is probably a D wave, we have termed the later volleys recruited by PA magnetic stimulation as I waves, numbered in order of their appearance. At the stimulation intensity used, a large D wave was also recorded in subject 1 after PA magnetic stimulation (Fig. 1).
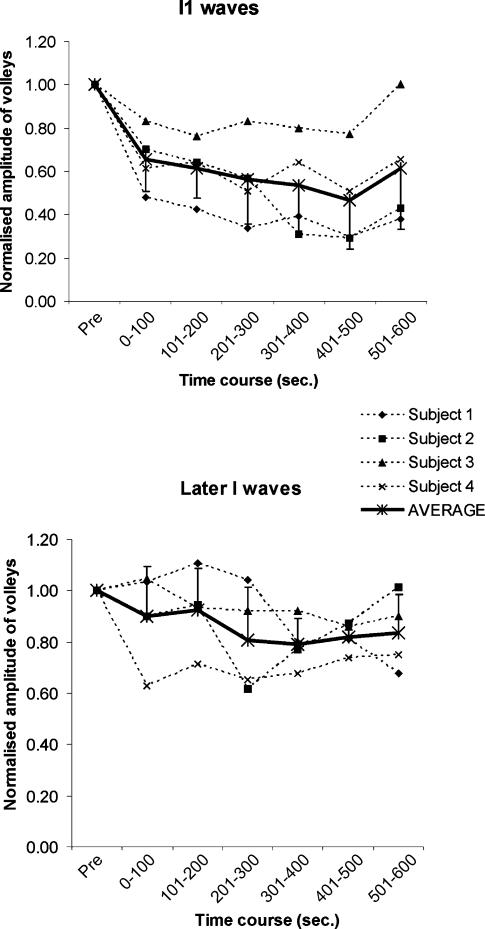
Figures 1 and 2 show the time course of the effect of TBS on the amplitudes of the I1 or later I waves (the sum of the amplitudes of all the waves following the I1 wave). A repeated-measures ANOVA on the log-transformed data with time and I wave (I1 or later I waves) as main factors showed a significant effect of both time (F3,9 = 11.1, P < 0.005) and I wave (F1,3 = 11.1, P < 0.05), but no significant interaction. Follow-up one-factor ANOVAs showed that this was because there was a significant effect of time (F3,9 = 9, P < 0.005) on the I1 wave, but this was not the case for the later I waves (F3,9 = 2.7, P > 0.05), with significant suppression of I1 at all but the last post-rTMS intervals. The maximal effect on I1 wave amplitude occurred 7–8 min after the end of rTMS when the amplitude of the I1 wave decreased by more than 50% (Fig. 2A).
Figure 2. Effects of repetitive megnetic stimulation on the amplitude of waves.
A, effects of rTMS on the mean amplitude of the I1 wave in individual subjects (dashed lines), the continuous line represents the mean from the same subjects. Error bars indicate standard deviations. rTMS has a pronounced effect on the amplitude of the I1 wave (F3,9 = 9, P < 0.005). B, effects of rTMS on the mean amplitude of later I waves.
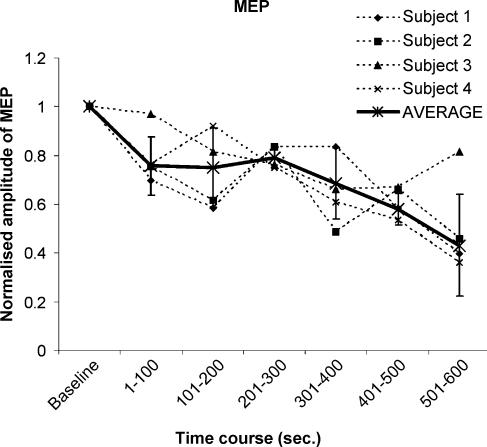
The consequence of these changes can be observed in MEPs we recorded in the FDI muscle. These were reduced to about 60% of their pre-rTMS size 7–8 min after the end of rTMS (Fig. 3) (one-factor ANOVA on the log-transformed MEP amplitudes: F3,9 = 11.7, P ≤ 0.005).
Figure 3. Effects of repetitive magnetic stimulation on the amplitude of motor evoked potentials.
Effects of rTMS on the mean amplitude of motor-evoked potentials (MEPs) in individual subjects (dashed lines); the continuous line represents the mean from the same subjects. Error bars indicate standard deviations. rTMS has a pronounced effect on the amplitude of MEPs (F3,9 = 11.7, P ≤ 0.005).
Subject 3 was studied again 1 h after the end of rTMS. At this interval the amplitude of the I1 wave had completely recovered to pre-rTMS level.
In subjects 1 and 4, we also had the opportunity to evaluate the effects of rTMS on the amplitude of the presumed D wave. In subject 1, this wave was not substantially modified by rTMS (Fig. 1): after rTMS the amplitude of this wave ranged between 107 and 88% of baseline response. Seven to eight minutes after the end of rTMS, the amplitude of this wave was about 88% of baseline response, whereas the amplitude of the I1 wave at this interval was about 30% of baseline response. The amplitude of the D wave in subject 4 was also not modified by rTMS; 4–5 min after the end of rTMS, the amplitude of the D wave was approximately the same as the baseline response, whereas the amplitude of the I1 wave at this interval was about 80% of baseline response.
Discussion
The present results demonstrate that continuous theta-burst rTMS leads to a pronounced decrease in the excitability of cortical circuits generating the I1 wave, whilst later I waves are affected much less. The D wave was unaffected by cTBS in the two patients in whom it could be identified. Since the D wave is due to direct excitation of the axons of the corticospinal tract (Di Lazzaro et al. 2004), this implies that cTBS produces its effect by influencing the intrinsic circuitry of the motor cortex. The constant D wave is also confirmation that there was no change in the effectiveness of the stimulation throughout the trials. The slow build-up of the effect on the I1 wave over the first 5–8 min after the end of the conditioning period parallels the time course of MEP suppression that was described by Huang et al. (2005) who used exactly the same parameters of cTBS in their study. Although the I waves that we record may be destined for muscles other than the FDI, it seems likely that at least some of the MEP effect is explained by suppression of the I1 wave.
The effect on the I1 wave contrasts with other TMS protocols that suppress MEPs. For example, the suppression seen in SICI and the suppression produced via transcallosal inhibition both preferentially affect the I3 wave, and leave the I1 wave virtually unchanged (Di Lazzaro et al. 1998, 1999). This specificity has been interpreted as indicating that these inhibitory effects do not change the overall excitability of the corticospinal neurones, since that would lead to suppression of all I–wave inputs. Instead the data are consistent with the idea that there can be specific inhibitory effects on the intracortical circuits that generate late I waves.
In the present case, the I1 wave was suppressed, but not later I waves. Again, this suggests that cTBS does not have a general effect on the excitability of the corticospinal neurones, but instead has a specific effect on I1–wave inputs. A differential sensitivity of earlier and later I waves to changes in cortical excitability has already been suggested by Sugawara et al. (2005) while evaluating the remote effects of voluntary teeth clenching. Interestingly, the short latency of the I1 wave is usually taken to indicate that it is produced by a monosynaptic input to corticospinal neurones. Thus the implication is that cTBS has its major effect on the synapse between the I1 inputs and the corticospinal neurones. Although we have no direct evidence this would be consistent with the idea that cTBS had produced long-term depression (LTD) at the excitatory synapse between I1 input and the corticospinal neurone.
Like Huang et al. (2005), we applied cTBS at an intensity of only 80% AMT. The I1 input usually has the lowest threshold of all I–wave inputs, and may have been activated at this intensity. In addition, the stimulus will also recruit activity in circuits that suppress the I3 inputs, since 80% AMT is above the threshold for recruiting SICI. Repeated activation of these two elements by the cTBS protocol may result in depression of their synaptic connections, and hence explain why both the MEP and SICI are depressed. Indeed, results would be consistent with a model in which TBS produces LTD at the I1 input to corticospinal neurones as well as LTD at the excitatory synapses onto inhibitory interneurones normally activated by low-intensity conditioning pulses.
In conclusion, we found that theta-burst rTMS at an intensity of 80% AMT leads to a rapid decrease in the excitability of cortical mechanisms generating the I1 wave. This suggests that cTBS may reduce the responsiveness of pyramidal cells to excitatory stimuli.
Acknowledgments
This work was supported by Programma di ricerca cofinanziato MIUR anno 2003: Strategie Innovative Bio-Inspirate per il Controllo di Sistemi di Movimentazione.
References
- Berardelli A, Inghilleri M, Rothwell JC, Romeo S, Curra A, Gilio F, Modugno N, Manfredi M. Facilitation of muscle-evoked responses after repetitive cortical stimulation in man. Exp Brain Res. 1998;122:79–84. doi: 10.1007/s002210050493. [DOI] [PubMed] [Google Scholar]
- Chen R, Classen J, Gerloff C, Celnik P, Wassermann EM, Hallett M, Cohen LG. Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology. 1997;48:1398–1403. doi: 10.1212/wnl.48.5.1398. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Berardelli A, Mazzone P, Insola A, Pilato F, Saturno E, Dileone M, Tonali PA, Rothwell JC. Direct demonstration of the effects of repetitive transcranial magnetic stimulation on the excitability of the human motor cortex. Exp Brain Res. 2002a;144:549–553. doi: 10.1007/s00221-002-1106-9. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Mazzone P, Pilato F, Saturno E, Dileone M, Insola A, Tonali PA, Rothwell JC. Short-term reduction of intracortical inhibition in the human motor cortex induced by repetitive transcranial magnetic stimulation. Exp Brain Res. 2002b;147:108–113. doi: 10.1007/s00221-002-1223-5. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Pilato F, Saturno E, Dileone M, Mazzone P, Insola A, Tonali PA, Rothwell JC. The physiological basis of transcranial motor cortex stimulation in conscious humans. Clin Neurophysiol. 2004;115:255–266. doi: 10.1016/j.clinph.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Oliviero A, Profice P, Insola A, Mazzone P, Tonali P, Rothwell JC. Direct demonstration of interhemispheric inhibition of the human motor cortex produced by transcranial magnetic stimulation. Exp Brain Res. 1999;124:520–524. doi: 10.1007/s002210050648. [DOI] [PubMed] [Google Scholar]
- Di Lazzaro V, Restuccia D, Oliviero A, Profice P, Ferrara L, Insola A, Mazzone P, Tonali P, Rothwell JC. Magnetic transcranial stimulation at intensities below active motor threshold activates intracortical inhibitory circuits. Exp Brain Res. 1998;119:265–268. doi: 10.1007/s002210050341. [DOI] [PubMed] [Google Scholar]
- Huang YZ, Edwards MJ, Rounis E, Bhatia KP, Rothwell JC. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Maeda F, Keenan JP, Tormos JM, Topka H, Pascual-Leone A. Interindividual variability of the modulatory effects of repetitive transcranial magnetic stimulation on cortical excitability. Exp Brain Res. 2000;133:425–430. doi: 10.1007/s002210000432. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Valls-Solè J, Wassermann EM, Hallett M. Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain. 1994;117:847–858. doi: 10.1093/brain/117.4.847. [DOI] [PubMed] [Google Scholar]
- Pascual-Leone A, Tormos JM, Keenan J, Tarazona F, Canete C, Catala MD. Study and modulation of human cortical excitability with transcranial magnetic stimulation. J Clin Neurophysiol. 1998;15:333–343. doi: 10.1097/00004691-199807000-00005. [DOI] [PubMed] [Google Scholar]
- Peinemann A, Lehner C, Mentschel C, Munchau A, Conrad B, Siebner HR. Subthreshold 5-Hz repetitive transcranial magnetic stimulation of the human primary motor cortex reduces intracortical paired-pulse inhibition. Neurosci Lett. 2000;296:21–24. doi: 10.1016/s0304-3940(00)01616-5. [DOI] [PubMed] [Google Scholar]
- Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijevic MR, Hallett M, Katayama Y, Lucking CH, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Sugawara K, Furubayashi T, Takahashi M, Ni Z, Ugawa Y. Remote effects of voluntary teeth clenching on excitability of the human hand motor area induced by different oriented currents. Neurosci Lett. 2005;377:25–30. doi: 10.1016/j.neulet.2004.11.059. [DOI] [PubMed] [Google Scholar]
- Tergau F, Tormos JM, Paulus W, Pascual-Leone A, Ziemann U. Effects of repetitive transcranial magnetic stimulation (rTMS) on cortico-spinal and cortico-cortical excitability. Neurology. 1997;48:A107. [Google Scholar]